Main Text
Antibiotic resistance continues to be a growing concern worldwide, with increasing rates of multidrug-resistant (MDR) bacteria and an overall lack of new antibiotic development (1). Antimicrobial peptides (AMPs) are an innate immune defense with generally broad spectrum potential for bacterial membrane disruption and have been shown to synergize with common antibiotics to eliminate MDR bacterial species (2). In addition to this well-established antibiotic synergy, AMPs can also synergize with other AMPs, as is the case with LL-37 (LLGDFFRKSKEKIGKEFKRIVQRIKDFLRNLVPRTES), a cathelicidin-derived peptide, and human neutrophil peptide 1 (HNP1), an α-defensin peptide (ACYCRIPACIAGERRYGTCIYQGRLWAFCC; disulfide bridges: 2–30, 4–19, 9–29) (3). LL-37 has been further engineered into potent antimicrobials that can eliminate ESKAPE pathogens, even resensitizing strains of vancomycin-resistant Staphylococcus aureus, suggesting its usefulness for the treatment of a variety of MDR bacterial species (4,5). What has not been fully understood is how host eukaryotic cells themselves avoid the disruptive properties of LL-37 and HNP1—the answer to which may lie in a “cooperativity switch” between these AMPs, despite their synergy against bacteria.
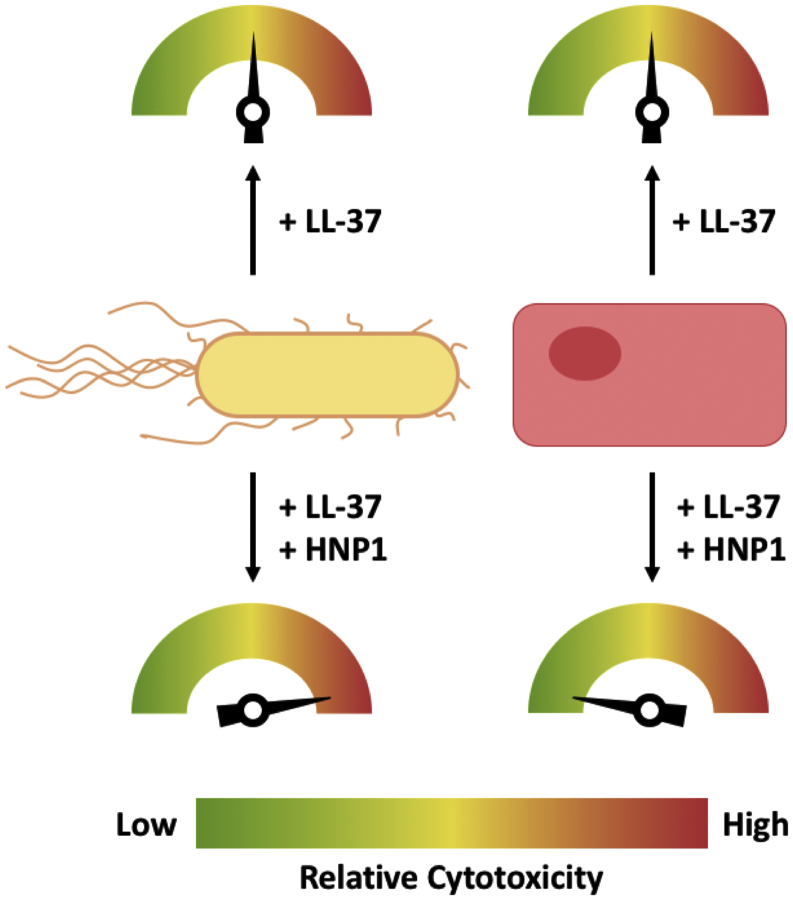
In this issue of Biophysical Journal, Drab and Sugihara (6) make important advances in our understanding of AMP function and cooperativity. To investigate this apparent eukaryotic avoidance with regards to LL-37-mediated cytotoxicity, the authors challenged multiple eukaryotic cell lines and associated membrane mimics with LL-37, HNP1, or a combination of the two peptides. When treated individually, LL-37 and HNP1 induced pore formation, as well as membrane protrusion in the case of HNP1, in each eukaryotic cell model, which was deemed cytotoxic in the case of LL-37 by calcium-sensitive dye assays. These results were confirmed by fluorescence recovery after photobleaching, in which treatment with LL-37 compromised the integrity of the bilayer, whereas treatment with HNP1 induced membrane protrusions without disrupting the bilayer, providing an explanation for respective cytotoxicity. However, LL-37/HNP1 combination treatment largely abolished pore formation and associated cytotoxicity, as demonstrated by the aforementioned methods, in addition to quartz crystal microbalance with dissipation, conceptually depicted in Fig. 1. Particularly interesting is the finding that HNP1 seemingly neutralizes LL-37-mediated cytotoxicity, despite these peptides displaying no apparent direct interaction in solution or in membranes. Such a lack of peptide-peptide interaction is well explained in solution, given that their respective oligomeric states would confer net positive charges (LL-37 monomer = +6; HNP-1 monomer = +3) that would inhibit their interaction (7,8). The authors’ overall results led them to a schematic model in which protrusions of HNP1 from the membrane apparently contact LL-37 in a manner that prevents the usual detergent-type membrane disruption by LL-37 alone.
Figure 1.
Concept for the cooperativity switch between LL-37 and HNP1. Escherichia coli (beige) or human endothelial cells (pink) treated with LL-37 alone or LL-37 and HNP1 results in low to high relative cytotoxicity, as indicated by the position of the arrow on the dial. When treated with both LL-37 and HNP1, E. coli are synergistically destroyed, whereas human endothelial cells are protected. To see this figure in color, go online.
Previously, it was demonstrated that lipid composition can affect the membrane topology of two other synergistic AMPs: PGLa and magainin 2. Using 15N solid-state nuclear magnetic resonance spectroscopy, the authors showed that magainin 2 altered the bilayer thickness (or hydrophobic thickness) in such a way that PGLa was inserted deeper in the bilayer, providing a structural explanation for the synergistic pore-forming properties of the AMP mixture (9). In the case of LL-37, the oligomerization of the peptide has also been shown to affect antimicrobial activity and host membrane-interaction (7). Together, these findings may suggest fundamental explanations for the LL-37/HNP1 cooperativity switch when coembedded within bacterial and eukaryotic membranes, respectively. In this context, future investigations would benefit from similar topological analyses, along with a corresponding analysis of any observed synergistic effects in a 25% anionic (bacterial) membrane in addition to pure POPC membranes. Comparing the lipid-mediated interactions within both lipid environments could point to specific components contributing to these contrasting outcomes, thereby unraveling the cause of the membrane-destructive-to-membrane-protective switch of LL-37/HNP1.
Drab and Sugihara have provided an exciting first step toward realizing the clinical potential of LL-37 and its derivatives. Elucidation of the detailed mechanism by which this cooperativity switch occurs may guide the development of new peptide-based therapeutics with improved specificity for bacterial membranes while self-suppressing off-target cytotoxicity.
Acknowledgments
This work was supported, in part, by a grant to C.M.D. from the Canadian Institutes of Health Research (Project Grant #376666).
Editor: Claudia Steinem.
References
- 1.Schäberle T.F., Hack I.M. Overcoming the current deadlock in antibiotic research. Trends Microbiol. 2014;22:165–167. doi: 10.1016/j.tim.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 2.Zharkova M.S., Orlov D.S., Shamova O.V. Application of antimicrobial peptides of the innate immune system in combination with conventional antibiotics-a novel way to combat antibiotic resistance? Front. Cell. Infect. Microbiol. 2019;9:128. doi: 10.3389/fcimb.2019.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nagaoka I., Hirota S., Hirata M. Synergistic actions of antibacterial neutrophil defensins and cathelicidins. Inflamm. Res. 2000;49:73–79. doi: 10.1007/s000110050561. [DOI] [PubMed] [Google Scholar]
- 4.Wang G., Narayana J.L., Wang X. Design of antimicrobial peptides: progress made with human cathelicidin LL-37. Adv. Exp. Med. Biol. 2019;1117:215–240. doi: 10.1007/978-981-13-3588-4_12. [DOI] [PubMed] [Google Scholar]
- 5.Shurko J.F., Galega R.S., Lee G.C. Evaluation of LL-37 antimicrobial peptide derivatives alone and in combination with vancomycin against S. aureus. J. Antibiot. (Tokyo) 2018;71:971–974. doi: 10.1038/s41429-018-0090-7. [DOI] [PubMed] [Google Scholar]
- 6.Drab E., Sugihara K. Cooperative function of LL-37 and HNP1 protects mammalian cell membranes from lysis. Biophys. J. 2020;119:2440–2450. doi: 10.1016/j.bpj.2020.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xhindoli D., Pacor S., Tossi A. Native oligomerization determines the mode of action and biological activities of human cathelicidin LL-37. Biochem. J. 2014;457:263–275. doi: 10.1042/BJ20131048. [DOI] [PubMed] [Google Scholar]
- 8.Pazgier M., Wei G., Lu W. Sometimes it takes two to tango: contributions of dimerization to functions of human α-defensin HNP1 peptide. J. Biol. Chem. 2012;287:8944–8953. doi: 10.1074/jbc.M111.332205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salnikov E.S., Bechinger B. Lipid-controlled peptide topology and interactions in bilayers: structural insights into the synergistic enhancement of the antimicrobial activities of PGLa and magainin 2. Biophys. J. 2011;100:1473–1480. doi: 10.1016/j.bpj.2011.01.070. [DOI] [PMC free article] [PubMed] [Google Scholar]