Summary
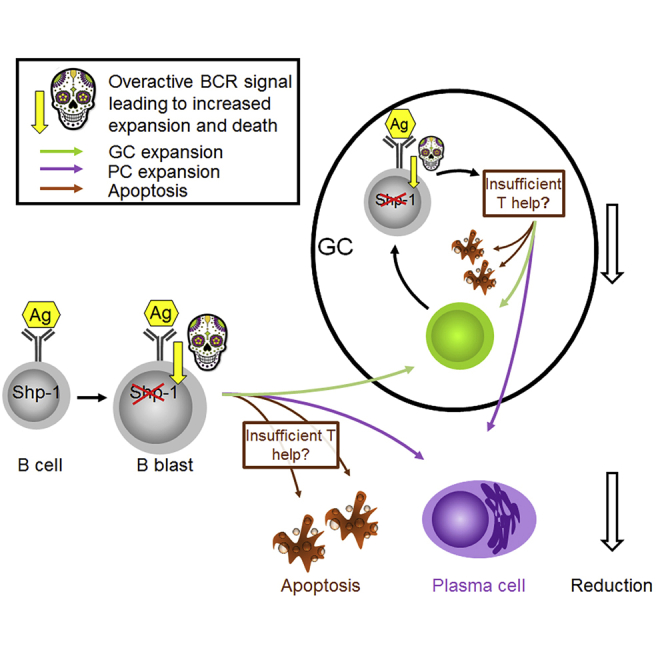
It is still not clear how B cell receptor (BCR) signaling intensity affects plasma cell (PC) and germinal center (GC) B cell differentiation. We generated Cγ1Cre/wtPtpn6fl/fl mice where SHP-1, a negative regulator of BCR signaling, is deleted rapidly after B cell activation. Although immunization with T-dependent antigens increased BCR signaling, it led to PC reduction and increased apoptosis. Dependent on the antigen, the early GC B cell response was equally reduced and apoptosis increased. At the same time, a higher proportion of GC B cells expressed cMYC, suggesting GC B cell-Tfh cell interactions may be increased. GC B cell numbers returned to normal at later stages, whereas affinity maturation was suppressed in the long term. This confirms that BCR signaling not only directs affinity-dependent B cell selection but also, without adequate further stimulation, can inflict cell death, which may be important for the maintenance of B cell tolerance.
Subject areas: Immunology, immune system, cell biology
Graphical Abstract
Highlights
-
•
BCR signaling without adequate further stimulation can inflict cell death
-
•
Inappropriate BCR signaling can inhibit extra-follicular PC differentiation
-
•
Increased BCR signaling induces apoptosis in early GC B cells
-
•
BCR signaling intensity affects affinity-dependent B cell selection
Immunology; immune system; cell biology
Introduction
Specific interaction between antigen and the B cell receptor (BCR) is the key signal for B cell selection and activation (Niiro and Clark, 2002; Yam-Puc et al., 2018). After initial activation in vivo, B cells may differentiate into plasma cells (PCs) through rapid extra-follicular expansion or become germinal center (GC) cells that will undergo BCR affinity maturation for antigen (MacLennan, 1994; MacLennan et al., 2003; Victora and Nussenzweig, 2012). GCs contribute to long-lived humoral responses by producing high-affinity antibody-forming PCs and memory B cells (MacLennan, 1994; Victora and Nussenzweig, 2012; Weisel et al., 2016). High-affinity neutralizing antibodies represent a crucial mechanism by which vaccines or natural infections confer sterilizing immunity protecting against on re-exposure to the same pathogen (Bachmann et al., 1994; Steinhoff et al., 1995). Two major signals regulate B cell activation leading to antibody production: signals from T helper cells have been studied intensely in recent years (Oropallo and Cerutti, 2014; Shulman et al., 2013; Victora et al., 2010), whereas less attention has been given to the impact of BCR signaling during selection of B cells by antigen (Khalil et al., 2012; Mueller et al., 2015). Although signals from pathogen recognition receptors may participate in B cell activation (Li et al., 2013; Pone et al., 2015), the interaction between antigen and BCR has been described as crucial to control whether activated B cells enter the GC or undergo rapid PC differentiation in extra-follicular proliferative foci. B cell clones undergoing a strong initial interaction with antigen can efficiently differentiate into extra-follicular PCs contributing to the rapid early phase of the antibody production (Paus et al., 2006). B cells expressing a wide range of BCR affinities become pre-GC B cells after T-B interaction (Dal Porto et al., 2002; Schwickert et al., 2011; Victora et al., 2010). Higher affinity BCRs can induce stronger signal transduction than lower affinity ones (Kouskoff et al., 1998). BCR occupancy is a product of BCR affinity and antigen concentration, and concentration of free antigen can be limited by antibody feedback (Toellner et al., 2018).
B cell activation upon BCR ligation can be amplified by Toll-like receptor (TLR) signaling (Castro-Dopico and Clatworthy, 2019; Pone et al., 2012), and this may act at very early stages before T cell help is available. However, these innate immune receptors may also have an inhibitory effect on B cell activation, such as TLR-9, which can inhibit antigen processing and presentation by B cells, inhibiting acquisition of T cell help (Akkaya et al., 2018a, 2018b). The effect of all this on cell fate decisions during B cell differentiation merits more attention.
The Src homology 2 (SH2) domain-containing protein-tyrosine phosphatase (PTP)-1 (SHP-1), encoded by the Ptpn6 gene, negatively regulates BCR signaling primarily via its binding to the immunoreceptor tyrosine-based inhibitory motif (ITIM)-containing receptors CD72, CD22, FcγRIIB, paired Ig-like receptor (PIR)-B, and FCRL3 (Adachi et al., 2001; D'Ambrosio et al., 1995; Kochi et al., 2009; Maeda et al., 1998; Nitschke and Tsubata, 2004). Although FCRL3 can mediate B cell activation through TLR-9 stimulation, this seems to be independent of SHP-1 (Li et al., 2013). SHP-1 is expressed and constitutively activated in all B cells, and its specific deletion on B cells results in systemic autoimmunity (Pao et al., 2007). SHP-1 is highly expressed and activated in GC B cells, suggesting that BCR signaling is negatively regulated during differentiation of GC B cells (Khalil et al., 2012). BCR signaling has been shown to be absent in dark zone (DZ) GC B cells (Stewart et al., 2018), whereas there is more signal transduction in light zone (LZ) B cells competing for selection signals through affinity-dependent activation of their BCR (Mueller et al., 2015).
To test how BCR signaling inhibition by SHP-1 affects antigen-induced B cell differentiation, we generated Cγ1Cre/wtPtpn6fl/fl mice, in which the T-dependent B cell activation induces SHP-1 deletion in most B cells (Roco et al., 2019). Most induction of immunoglobulin class switch recombination (CSR) happens during the initial phase of cognate T cell-B cell interaction before GCs are formed, which is accompanied by rapid strong induction of IgG1 germline transcripts (Marshall et al., 2011; Roco et al., 2019; Toellner et al., 1998). Although CD40 ligation and interleukin (IL)-4 are strong inducers of IgG1 germline transcripts (Stavnezer et al., 2008), their expression is not necessarily followed by CSR. Here we use Cre recombinase located inside the IgG1 heavy chain locus as a reporter for successful T-dependent B cell activation (Casola et al., 2006; Roco et al., 2019). Using Cγ1Cre mice that contain a Cre-deletable version of SHP-1 (Ptpn6), we show that Cγ1Cre/wtPtpn6fl/fl B cells exhibit stronger BCR signaling. Paradoxically this leads to a smaller extra-follicular IgG1+ PC response and to death of GC B cells, resulting in reduced affinity maturation in the GC.
Results
Increased apoptosis in extra-follicular plasma cells of Cγ1Cre/wtPtpn6fl/fl mice
B cells binding antigen with higher affinity are more likely to differentiate into extra-follicular PCs (O'Connor et al., 2006; Paus et al., 2006). To test whether deletion of the negative regulator of BCR signaling, SHP-1, affects the early extra-follicular PC response to immunization, Cγ1Cre/wtPtpn6fl/wt and Cγ1Cre/wtPtpn6fl/fl, in the following abbreviated as Shp1fl/wt and Shp1fl/fl mice, were immunized with sheep red blood cells (SRBCs) intravenously. The Cγ1Cre allele reports expression of IgG1 germline transcripts (Casola et al., 2006), which are strongly induced after the initial interaction of B cells with T helper cells before PCs or GCs appear (Marshall et al., 2011; Roco et al., 2019; Zhang et al., 2018). This should lead to efficient deletion of SHP-1 in extra-follicular PCs and GC founder B cells. Spleens were analyzed 5 days post immunization, when the extra-follicular PC response peaks and early GCs have formed (Zhang et al., 2018).
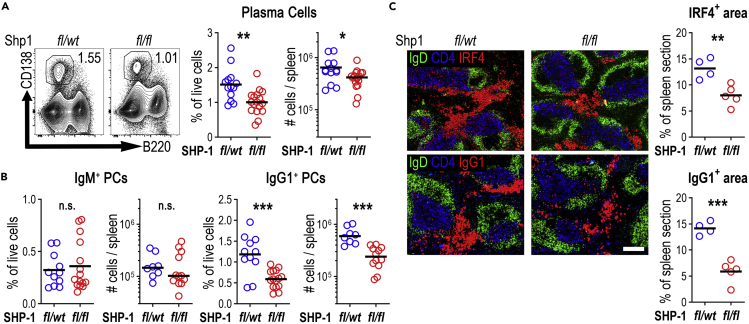
Against expectation, flow cytometry showed that Shp1fl/fl PC numbers were reduced by 50% (Figure 1A). This primarily affected IgG1-switched PCs, whereas non-switched IgM PCs developed in similar numbers as in Shp1fl/wt control animals (Figure 1B). Testing deletion of SHP-1 in PCs by flow cytometry showed that Shp1fl/wt and Shp1fl/fl PC expressed similar amounts of SHP-1 (Figures S1A and S1B), suggesting that the surviving PCs had not deleted SHP-1. Immunohistology, using IRF4 as a marker for PCs, confirmed reduced PC foci in the splenic red pulp, primarily in the IgG1-switched PCs of Shp1fl/fl mice (Figure 1C). PCs emerging from GCs at the GC-T zone interface (GTI) (Zhang et al., 2018) were unaffected at this point (Figure S1C). These data indicate that increased BCR signaling after initial B cell activation inhibits extra-follicular PC differentiation.
Figure 1.
Plasma cells are reduced in Cγ1Cre/wtPtpn6fl/fl mice post SRBC immunization
Mouse spleens were analyzed 5 days post intravenous immunization with SRBCs
(A) Representative contour plots gating PCs (lymphocytes/singlets/live/B220−CD138+, numbers indicate percentage of cells within live lymphocyte gate). Right: % of live cells and total numbers per spleen; data combined from three independent experiments.
(B) IgM+ and IgG1+ PCs (% of live cells and total numbers per spleen; data combined from three independent experiments).
(C) Splenic sections from Shp1fl/wt (fl/wt) and Shp1fl/fl (fl/fl) mice staining B cell follicles (IgD, green), T cell zone (CD4, blue) and PCs (top, IRF4 in red), or IgG1+ cells (bottom, IgG1 in red); scale bar, 200 μm. IRF4 and IgG1 area is shown as a percentage of the total spleen area. Data are representative of one of two independent experiments.
Each symbol corresponds to one animal; horizontal lines indicate the mean. n.s. not significant, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 (two-tailed t-test).
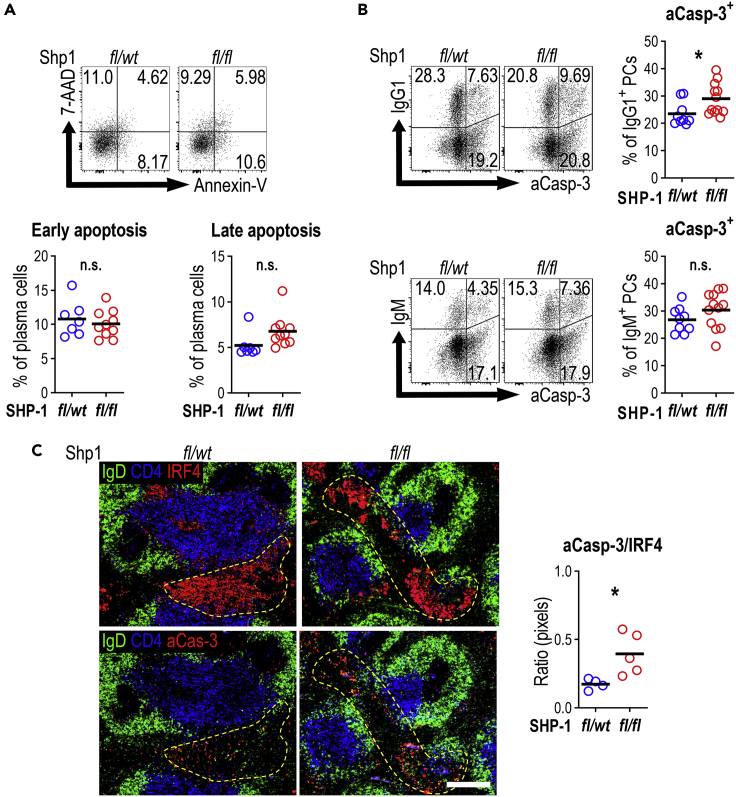
Hyper-activation of B cells through BCR signaling can lead to programmed cell death (Akkaya et al., 2018b; Parry et al., 1994; Tsubata et al., 1994b; Watanabe et al., 1998). To test whether cell death was responsible for the smaller extra-follicular PC response, apoptotic cells were detected using Annexin V and 7-AAD staining. This showed a minor increase in the proportion of apoptotic PCs (Annexin V+ve and 7-AAD+ve) in Shp1fl/fl mice (Figure 2A). Also, the expression of active caspase-3 on different isotypes of PCs showed that IgG1+ PCs of Shp1fl/fl animals were more likely to express active caspase-3 (Figure 2B). Immunohistology confirmed an increase in active caspase-3+ cells in the IRF-4+ extra-follicular splenic foci of Shp1fl/fl mice (Figure 2C). Interestingly, apoptosis was increased despite the similar SHP-1 protein expression in Shp1fl/fl PCs that survived to this stage (Figure S1A). Therefore, it is likely that at earlier stages the differences in apoptosis rates were even more pronounced. Taken together, this indicates that an inappropriate increase in BCR signaling can negatively affect extra-follicular PC generation through increased cell death.
Figure 2.
Plasma cell apoptosis is increased in SRBC immunized Cγ1Cre/wtPtpn6fl/fl mice
Apoptosis rate on PCs was analyzed 5 days post SRBCs immunization in Cγ1Cre/wtPtpn6fl/wt and Cγ1Cre/wtPtpn6fl/fl mice.
(A) Representative dot plots show apoptosis rate based on the binding of Annexin V and the dead cell dye 7-AAD (pregated on PCs; top panel). Annexin V+ 7-AAD- cells were considered as early apoptotic cells, and Annexin V+ 7-AAD+, cells as late apoptotic cells. Summary data (bottom panel; % of plasma cells; results are combined from two independent experiments).
(B) Active caspase-3 expression on IgG1+ or IgM+ PCs. Graphs on the right show the percentage of active caspase-3+ cells within IgG1+ or IgM+ plasma cells. Results are combined from three independent experiments.
(C) Spleen sections from Shp1fl/wt and Shp1fl/fl mice staining for B cell follicles (IgD, green), T cell zone (CD4, blue), and PCs (IRF4 in red) in the top, or active caspase-3+ cells (Caspase-3 in red) in the bottom. Ratio of active caspase-3+ pixel/IRF4+ pixel, representative of one of two independent experiments. Each symbol corresponds to one animal; horizontal lines indicate the mean. n.s. not significant, ∗p < 0.05 (two-tailed t test).
SRBC-induced GC formation of Cγ1Cre/wtPtpn6fl/fl mice is unaffected
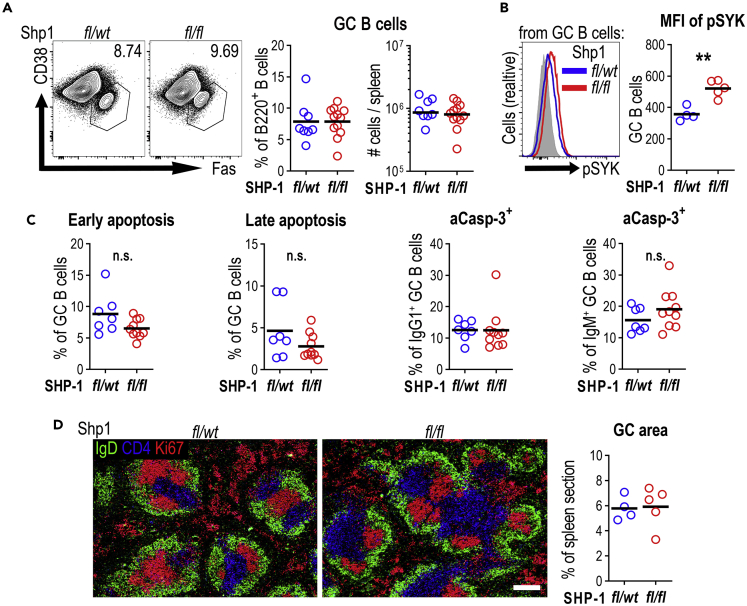
In established GCs, BCR signaling is limited by SHP-1 hyper-phosphorylation, and this is important for GC maintenance (Khalil et al., 2012). To test how SHP-1 deletion, starting from the earliest stages of GC development, affects the GC response we followed GC B cell differentiation in Cγ1Cre/wtPtpn6fl/fl mice 5 days after SRBC immunization. Surprisingly, at this early stage there was no significant change in the number of GC B cells in Shp1fl/fl mice (Figure 3A). Flow cytometry confirmed a reduction of SHP-1 staining intensity in all GC B cells (Figure S2A), indicating that most GC B cells had deleted the gene. The increase in SYK phosphorylation seen in GC B cells in Shp1fl/fl mice confirmed that SHP-1 deletion does increase BCR signaling in this system (Figure 3B). In contrast to what was seen in extra-follicular PCs, cell death in GC B cells, evaluated by flow cytometric analysis of Annexin V/7-AAD and active caspase-3 staining, was not increased at this stage (Figure 3C). Immunohistology confirmed that there were no obvious changes in GCs in Shp1fl/fl compared with Shp1fl/wt mice (Figure 3D). These data indicate that increased BCR signaling after initial B cell activation does not affect the formation of GCs.
Figure 3.
SRBC-induced GC formation in Cγ1Cre/wtPtpn6fl/fl mice is largely unaffected
Germinal center response was analyzed 5 days post intravenous SRBC immunization of Cγ1Cre/wtPtpn6fl/wt (fl/wt) and Cγ1Cre/wtPtpn6fl/fl (fl/fl) mice.
(A) Representative contour plots gating GC B cells from spleen (lymphocytes/singlets/live/CD138-B220+CD38−Fas+). Right panel shows percentage of B220+ B cells and total numbers per spleen. Data are combined from two independent experiments.
(B) pSYK expression in GC B cells. Right panel shows pSYK median fluorescence intensity (MFI) in GC B cells; results are representative of two independent experiments. Gray histogram shows the fluorescence minus one (FMO) control for pSYK staining.
(C) Apoptosis rate based on the binding of Annexin V and 7-AAD, active caspase-3 in IgG1+, or IgM+ cells as in Figure 2. % of GC B cells; data are combined from two independent experiments.
(D) Spleen sections from Shp1fl/wt (fl/wt) and Shp1fl/fl (fl/fl) mice staining for B cell follicles (IgD, green), T cell zone (CD4, blue), and proliferating cells (Ki67 in red); scale bar, 200 μm. Positive area of Ki67+IgD- was calculated as percentage of total splenic area. Each symbol corresponds to one animal; horizontal lines indicate the mean. n.s. not significant, ∗∗p < 0.01 (two-tailed t test).
Germinal center B cell responses and affinity maturation to hapten protein are impaired in Cγ1Cre/wtPtpn6fl/fl mice
While SRBC immunization rapidly induces B cell activation and differentiation (Zhang et al., 2018), it also has a T-independent component. Primary foot immunization with 4-hydroxy-3-nitrophenyl acetyl coupled to chicken γ-globulin (NP-CGG) in alum induces strong IL-4 expression in T cells, Th2 type B cell activation, and rapid differentiation of extra-follicular PCs as well as GC in the draining popliteal lymph nodes (Toellner et al., 1998). Furthermore, this antigen allows the identification of antigen-specific antibodies.
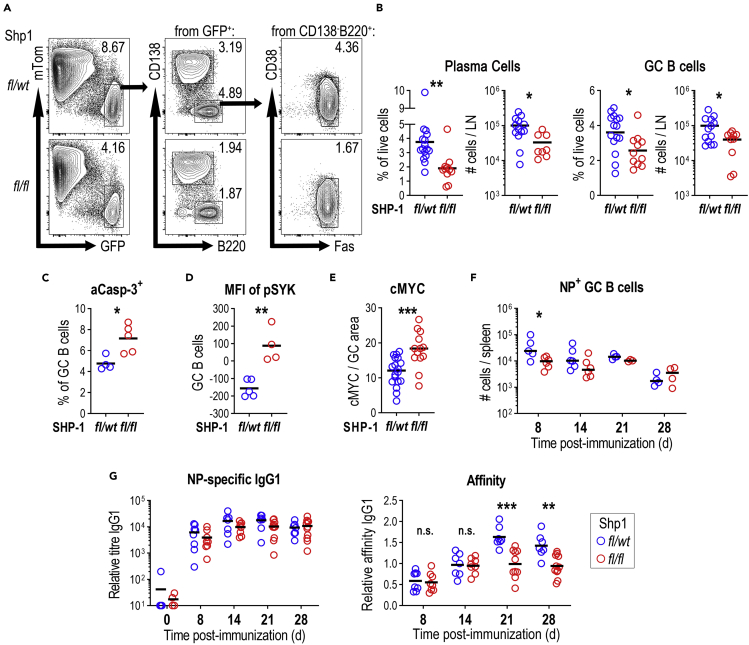
To better estimate the response in B cells that had an actual history of Cre-recombinase expression, we generated Cγ1Cre/wtPtpn6fl/wt and Cγ1Cre/wtPtpn6fl/fl on the ROSAmTmG background, which contain a Cre-inducible membrane-tagged version of eGFP (Muzumdar et al., 2007). Eight days post subcutaneous immunization with NP-CGG, Shp1fl/fl draining lymph nodes showed a reduced numbers of PCs (Figures 4A and 4B), similar to what was seen in Shp1fl/fl spleens after SRBC immunization. Again, PCs surviving to this stage contained normal amounts of SHP-1 (Figures S2B and S2C). GCs developing in Shp1fl/fl mice were smaller (Figure S2D) and numbers of GC B cells were reduced (Figures 4A and 4B). This went along with increased GC B cell apoptosis (Figure 4C). Again, SHP-1 deletion (Figure S2E) resulted in increased BCR signaling, as detected by increased SYK phosphorylation (Figure 4D).
Figure 4.
Germinal center B cell responses and affinity maturation to NP-CGG are impaired in Cγ1Cre/wtPtpn6fl/fl mice
(A) Germinal center B cell responses in popliteal lymph nodes (PLN) 8 days post NP-CGG immunization of Cγ1Cre/wtPtpn6fl/wt ROSAmTmG and Cγ1Cre/wtPtpn6fl/fl ROSAmTmG mice. Sequential gating strategy for the identification of PCs (lymphocytes/singlets/live/tomato−GFP+B220−CD138+) and GC B cells (lymphocytes/singlets/live/tomato−GFP+CD138-B220+CD38−Fas+).
(B) Summary of PC and GC B cell numbers (% of live cells and total numbers per LN); three independent experiments.
(C) Active caspase-3 on GC B cells from PLNs 8 days post NP-CGG immunization (% of GC B cells; results are representative of one experiment).
(D) SYK phosphorylation in GC B cells (MFI on GC B cells).
(E) Relative percentage of GC area containing cMYC-expressing cells. Each symbol represents a different GC. Data combined from two independent experiments.
(F) Splenic NP-specific GC B cells at different time points after immunization (total cell numbers per spleen; results are from one to two independent experiments).
(G) Serum antibody titers for NP-specific IgG1 (left panel) and relative affinity of NP-specific IgG1 (right panel).
Results are from two to three independent experiments; horizontal lines indicate the mean. n.s. not significant, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 (B–E two-tailed t test and F–G two-way ANOVA).
To test the hypothesis that B cell are dying by apoptosis because they are not receiving sufficient T cell help, we evaluated the expression of cMYC, which is induced after GC B cell stimulation by Tfh cells (Calado et al., 2012; Dominguez-Sola et al., 2012; Luo et al., 2018). Despite expectation, cMYC was found in a larger number of Shp1fl/fl GC B cells (Figure 4E), suggesting more efficient Tfh help. These results suggest that, similar to what is seen in extra-follicular PCs, overstimulation of GC B cells leads to increased cell death. Deletion of C-terminal Src kinase (Csk), another downstream inhibitor of BCR signaling, led to a similar reduction of the GC B cell response in Cγ1Cre/wtCskfl/fl mice after 8 and 14 days of NP-CGG immunization (Figures S3A and S3B).
To test the effects of SHP-1 deletion on later stages of the response to NP-CGG, splenic GC response and affinity maturation were monitored after intraperitoneal primary immunization of Shp1fl/fl and Shp1fl/wt mice with NP-CGG. Similar to what was seen in lymph nodes, increased BCR signaling led to a reduced early GC response 8 days after immunization, but this effect was lost at later stages of the response (Figures 4F and S4A). NP-specific IgG1 was marginally reduced, whereas NP-specific antibody affinity in Shp1fl/fl mice did not increase at late stages after immunization (Figure 4G), showing that despite the normalization in GC B cell numbers, there is a long-term effect on the efficiency of affinity-dependent B cell selection. A similar reduction of NP-specific IgG and IgG1 titers and antibody affinity were seen in Cγ1Cre/wtCskfl/fl animals (Figures S3C and S3D).
Discussion
Enhanced BCR signaling due to specific deletion of SHP-1 in all B cells during development leads to B1a B cell subset expansion and results in autoimmunity (Pao et al., 2007). Few studies have tested the effects of artificially enhanced BCR signaling in mature B cells that had undergone normal B cell development (Davidzohn et al., 2020; Li et al., 2014). The model presented here allows normal B cell development and increased BCR signaling by deletion of SHP-1 only after mature and naive B cells are activated by signals that may induce class-switching to IgG1.
SHP-1 inhibits BCR signaling through SYK (Adachi et al., 2001), and activated B cells in the current model show clear signs of pSYK overexpression after B cell activation.
After antigen-mediated BCR stimulation and T cell help, B cells may differentiate into extra-follicular PCs (MacLennan et al., 2003) or become GC precursor cells to start GC reactions (Victora and Nussenzweig, 2012). The exact mechanisms that control this fate decision is still controversial and under scrutiny. It has been shown that B cells experiencing a strong initial interaction with antigen more efficiently differentiate into extra-follicular PCs (Paus et al., 2006). Here, we show that higher signaling through the BCR affects PC differentiation in unexpected ways. Cγ1 germline transcripts are induced during the initial B cell activation before extra-follicular or GC B cell differentiation (Marshall et al., 2011; Roco et al., 2019). Therefore, early B blasts differentiating into extra-follicular plasmablasts would be the first to encounter Cre-mediated deletion of SHP-1 and increased BCR signaling. As increased BCR signaling should enhance extra-follicular PC differentiation (Paus et al., 2006), it was surprising to see reduced numbers of extra-follicular plasmablasts. It is very likely that BCR signaling, without adequate regulation, is inflicting cell death, which may be important for the maintenance of B cell tolerance.
Although the frequency of apoptosis in the PC compartment seems high, this appears to fit with the substantial PC death during the contraction of the immune response (McCarron et al., 2017; Smith et al., 1996; Sze et al., 2000). Kinetics of cell death in extra-follicular foci in the primary response are scarce, but it has been estimated that the daily cell loss should be over 20%, enough to account for their massive decline (Smith et al., 1996). PC death in the extra-follicular response is sharp and short (Sze et al., 2000), whereas GC responses and apoptosis therein are happening over longer periods, and apoptotic GC B cells are efficiently removed by tingible body macrophages. Therefore, it seems quite possible that removal of apoptotic cells in the extra-follicular response is less efficient.
Owing to the low number of B cells activated in a non-BCR transgenic animal it was not possible to test whether the number of B cells initially activated to enter plasmablast differentiation was changed. Stronger BCR-mediated activation may have led to larger numbers of B cells entering plasmablast differentiation, however, stronger activation in the absence of co-stimulation from T cells can also promote activation-induced cell death (Akkaya et al., 2018b; Parry et al., 1994; Tsubata et al., 1994a, 1994b; Watanabe et al., 1998). This would suggest that after activation, SHP-1-deficient B cells are not maintained because they do not receive timely co-stimulatory signals needed for full activation (Akkaya et al., 2018b). These results are in line with data from an earlier study (Li et al., 2014) that showed a modest reduction in PC production in the response to primary immunization with TD antigens in mice where Ptpn6 is deleted by Cre expressed under the control of the Aicda promoter. Aicda is also induced during primary B cell activation before GCs form; however, its expression is at lower levels than Cγ1 germline transcripts (Roco et al., 2019), which may explain the more subtle changes.
The effect of SHP-1 deletion on GC size is only transient, which could be due to the expansion of a minority of cells with incomplete deletion. The longer-term change in affinity maturation, however, makes it more likely that the complex balance between affinity-dependent GC B cell selection, proliferation, output, and death reaches a new equilibrium, filling GC B cell niches to normal occupancy levels. This may explain differences seen to an earlier study, where tamoxifen-induced deletion of SHP-1 during the peak of the GC response resulted in a rapid loss of GC B cells within a short period (Khalil et al., 2012).
Although the effect on the size of the GC compartment in NP-CGG immunized mice was only transient, the higher pSYK levels clearly indicate considerably increased signal transduction in GC B cells. pSYK levels were also increased in GC B cells induced by SRBC immunization, although there was less obvious effect on GC size. This may be explained by the fact that the response to SRBC immunization is less dependent on T cell help, and that GC B cells are able to survive and expand for a limited time without T cell help (de Vinuesa et al., 2000). A recent study testing the inhibition of pSYK degradation in GC B cells using mixed bone marrow chimeras (Davidzohn et al., 2020) showed that increased SYK signaling led to an increase in the GC LZ compartment. Further differentiation of these LZ B cells depended on Tfh cell help (Davidzohn et al., 2020; de Vinuesa et al., 2000; Gitlin et al., 2015; Shulman et al., 2013). We show here that SHP-1 deletion in the GC leads to higher levels of pSYK. Many of these GC B cells are able to recruit efficient Tfh cell help, indicated by the increased expression of cMYC (Calado et al., 2012; Dominguez-Sola et al., 2012). However, many GC B cells undergo apoptosis. BCR overstimulation and inadequate expression of cMYC can induce cell death (Akkaya et al., 2018b; Meyer and Penn, 2008; Watanabe et al., 1998), and this may be responsible for the reduced viability of B cells at the early stages of the GC response.
GCs are not only sites of affinity maturation. B cell selection in the GC also guarantees peripheral tolerance (Goodnow et al., 1989; Russell et al., 1991). The data shown here could reflect the deletion of autoreactive GC B cells that encounter inadequate BCR signaling and are not able to recruit adequate Tfh cell help in time. In the same way, higher affinity SHP-1-deficient GC B cells may be deleted because they are not recruiting sufficient Tfh cell help. This would indicate that affinity-dependent BCR signaling not only is important for affinity-dependent B cell selection but also that the balance of BCR signaling and Tfh cell-mediated rescue may regulate tolerance during GC B cell responses.
Limitations of the study
Although CSR occurs during the initial T cell:B cell interaction before GC formation or extra-follicular plasmablasts differentiation, very early signals influencing B cell fate decisions might have been overlooked. Furthermore, it must be considered that SHP-1 gene deletion may not necessarily translate into immediate reduction of SHP-1 levels due to the half-life of existing kinases inside the cell, making some delays in our observations. On the other hand, our interpretations are limited to Th2-type responses, further evaluation of BCR signals to other type of responses would be needed for more general conclusions.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by Lead Contact, Kai-Michael Toellner (k.m.toellner@bham.ac.uk).
Materials availability
Materials generated in this study are available upon request. Materials and resources are summarized in Table S1.
Data and code availability
No data sets or code were generated or analyzed in this study. All software is commercially available.
Methods
All methods can be found in the accompanying Transparent methods supplemental file.
Acknowledgments
We are grateful to Benjamin G. Neel (NYU School of Medicine) for Ptpn6fl/wt mice and S. Casola (IFOM, Milan, Italy) for Cγ1Cre/wt mice. We thank Mark J. Shlomchik and Wei Luo for helpful discussion. In addition, we would like to thank at the Biomedical Service Unit, Flow Cytometry Services, and Microscopy and Imaging Services at the University of Birmingham. This work was supported by grants from the BBSRC BB/M025292/1 to K.-M.T. and post-doctoral fellowship program from National Council of Science and Technology, Mexico (CONACYT-Mexico) to J.C.Y.-P.
Author contributions
Conceptualization, J.C.Y-P. and K.-M.T. Investigation, J.C.Y.P., L.Z., R.A.M.-A., L.G.-I., and Y.Z. Formal analysis J.C.Y-P. Resources, Y.A.S., M.S., and K.-M.T. Writing – Original Draft. J.C.Y.-P. Writing – Review & Editing, J.C.Y.-P. and K.-M.T. All authors reviewed and edited the final version of the manuscript. Supervision and Funding Acquisition, K.-M.T.
Declaration of interests
The authors declare no personal, professional, or financial conflict of interest.
Published: February 19, 2021
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2021.102038.
Contributor Information
Juan Carlos Yam-Puc, Email: j.c.yam-puc@bham.ac.uk.
Kai-Michael Toellner, Email: k.m.toellner@bham.ac.uk.
Supplemental information
References
- Adachi T., Wienands J., Wakabayashi C., Yakura H., Reth M., Tsubata T. SHP-1 requires inhibitory co-receptors to down-modulate B cell antigen receptor-mediated phosphorylation of cellular substrates. J. Biol. Chem. 2001;276:26648–26655. doi: 10.1074/jbc.M100997200. [DOI] [PubMed] [Google Scholar]
- Akkaya M., Akkaya B., Kim A.S., Miozzo P., Sohn H., Pena M., Roesler A.S., Theall B.P., Henke T., Kabat J. Toll-like receptor 9 antagonizes antibody affinity maturation. Nat. Immunol. 2018;19:255–266. doi: 10.1038/s41590-018-0052-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akkaya M., Traba J., Roesler A.S., Miozzo P., Akkaya B., Theall B.P., Sohn H., Pena M., Smelkinson M., Kabat J. Second signals rescue B cells from activation-induced mitochondrial dysfunction and death. Nat. Immunol. 2018;19:871–884. doi: 10.1038/s41590-018-0156-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann M.F., Kundig T.M., Odermatt B., Hengartner H., Zinkernagel R.M. Free recirculation of memory B cells versus antigen-dependent differentiation to antibody-forming cells. J. Immunol. 1994;153:3386–3397. [PubMed] [Google Scholar]
- Calado D.P., Sasaki Y., Godinho S.A., Pellerin A., Kochert K., Sleckman B.P., de Alboran I.M., Janz M., Rodig S., Rajewsky K. The cell-cycle regulator c-Myc is essential for the formation and maintenance of germinal centers. Nat. Immunol. 2012;13:1092–1100. doi: 10.1038/ni.2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casola S., Cattoretti G., Uyttersprot N., Koralov S.B., Seagal J., Hao Z., Waisman A., Egert A., Ghitza D., Rajewsky K. Tracking germinal center B cells expressing germ-line immunoglobulin gamma1 transcripts by conditional gene targeting. Proc. Natl. Acad. Sci. U S A. 2006;103:7396–7401. doi: 10.1073/pnas.0602353103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Dopico T., Clatworthy M.R. IgG and fcgamma receptors in intestinal immunity and inflammation. Front. Immunol. 2019;10:805. doi: 10.3389/fimmu.2019.00805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Ambrosio D., Hippen K.L., Minskoff S.A., Mellman I., Pani G., Siminovitch K.A., Cambier J.C. Recruitment and activation of PTP1C in negative regulation of antigen receptor signaling by Fc gamma RIIB1. Science. 1995;268:293–297. doi: 10.1126/science.7716523. [DOI] [PubMed] [Google Scholar]
- Dal Porto J.M., Haberman A.M., Kelsoe G., Shlomchik M.J. Very low affinity B cells form germinal centers, become memory B cells, and participate in secondary immune responses when higher affinity competition is reduced. J. Exp. Med. 2002;195:1215–1221. doi: 10.1084/jem.20011550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidzohn N., Biram A., Stoler-Barak L., Grenov A., Dassa B., Shulman Z. Syk degradation restrains plasma cell formation and promotes zonal transitions in germinal centers. J. Exp. Med. 2020;217 doi: 10.1084/jem.20191043. jem.20191043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vinuesa C.G., Cook M.C., Ball J., Drew M., Sunners Y., Cascalho M., Wabl M., Klaus G.G., MacLennan I.C. Germinal centers without T cells. J. Exp. Med. 2000;191:485–494. doi: 10.1084/jem.191.3.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez-Sola D., Victora G.D., Ying C.Y., Phan R.T., Saito M., Nussenzweig M.C., Dalla-Favera R. The proto-oncogene MYC is required for selection in the germinal center and cyclic reentry. Nat. Immunol. 2012;13:1083–1091. doi: 10.1038/ni.2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitlin A.D., Mayer C.T., Oliveira T.Y., Shulman Z., Jones M.J., Koren A., Nussenzweig M.C. HUMORAL IMMUNITY. T cell help controls the speed of the cell cycle in germinal center B cells. Science. 2015;349:643–646. doi: 10.1126/science.aac4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodnow C.C., Crosbie J., Jorgensen H., Brink R.A., Basten A. Induction of self-tolerance in mature peripheral B lymphocytes. Nature. 1989;342:385–391. doi: 10.1038/342385a0. [DOI] [PubMed] [Google Scholar]
- Khalil A.M., Cambier J.C., Shlomchik M.J. B cell receptor signal transduction in the GC is short-circuited by high phosphatase activity. Science. 2012;336:1178–1181. doi: 10.1126/science.1213368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochi Y., Myouzen K., Yamada R., Suzuki A., Kurosaki T., Nakamura Y., Yamamoto K. FCRL3, an autoimmune susceptibility gene, has inhibitory potential on B-cell receptor-mediated signaling. J. Immunol. 2009;183:5502–5510. doi: 10.4049/jimmunol.0901982. [DOI] [PubMed] [Google Scholar]
- Kouskoff V., Famiglietti S., Lacaud G., Lang P., Rider J.E., Kay B.K., Cambier J.C., Nemazee D. Antigens varying in affinity for the B cell receptor induce differential B lymphocyte responses. J. Exp. Med. 1998;188:1453–1464. doi: 10.1084/jem.188.8.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F.J., Schreeder D.M., Li R., Wu J., Davis R.S. FCRL3 promotes TLR9-induced B-cell activation and suppresses plasma cell differentiation. Eur. J. Immunol. 2013;43:2980–2992. doi: 10.1002/eji.201243068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y.F., Xu S., Ou X., Lam K.P. Shp1 signalling is required to establish the long-lived bone marrow plasma cell pool. Nat. Commun. 2014;5:4273. doi: 10.1038/ncomms5273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo W., Weisel F., Shlomchik M.J. B cell receptor and CD40 signaling are rewired for synergistic induction of the c-myc transcription factor in germinal center B cells. Immunity. 2018;48:313–326 e315. doi: 10.1016/j.immuni.2018.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLennan I.C. Germinal centers. Annu. Rev. Immunol. 1994;12:117–139. doi: 10.1146/annurev.iy.12.040194.001001. [DOI] [PubMed] [Google Scholar]
- MacLennan I.C., Toellner K.M., Cunningham A.F., Serre K., Sze D.M., Zuniga E., Cook M.C., Vinuesa C.G. Extrafollicular antibody responses. Immunol. Rev. 2003;194:8–18. doi: 10.1034/j.1600-065x.2003.00058.x. [DOI] [PubMed] [Google Scholar]
- Maeda A., Kurosaki M., Ono M., Takai T., Kurosaki T. Requirement of SH2-containing protein tyrosine phosphatases SHP-1 and SHP-2 for paired immunoglobulin-like receptor B (PIR-B)-mediated inhibitory signal. J. Exp. Med. 1998;187:1355–1360. doi: 10.1084/jem.187.8.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall J.L., Zhang Y., Pallan L., Hsu M.C., Khan M., Cunningham A.F., MacLennan I.C., Toellner K.M. Early B blasts acquire a capacity for Ig class switch recombination that is lost as they become plasmablasts. Eur. J. Immunol. 2011;41:3506–3512. doi: 10.1002/eji.201141762. [DOI] [PubMed] [Google Scholar]
- McCarron M.J., Park P.W., Fooksman D.R. CD138 mediates selection of mature plasma cells by regulating their survival. Blood. 2017;129:2749–2759. doi: 10.1182/blood-2017-01-761643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer N., Penn L.Z. Reflecting on 25 years with MYC. Nat. Rev. Cancer. 2008;8:976–990. doi: 10.1038/nrc2231. [DOI] [PubMed] [Google Scholar]
- Mueller J., Matloubian M., Zikherman J. Cutting edge: an in vivo reporter reveals active B cell receptor signaling in the germinal center. J. Immunol. 2015;194:2993–2997. doi: 10.4049/jimmunol.1403086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzumdar M.D., Tasic B., Miyamichi K., Li L., Luo L. A global double-fluorescent Cre reporter mouse. Genesis. 2007;45:593–605. doi: 10.1002/dvg.20335. [DOI] [PubMed] [Google Scholar]
- Niiro H., Clark E.A. Regulation of B-cell fate by antigen-receptor signals. Nat. Rev. Immunol. 2002;2:945–956. doi: 10.1038/nri955. [DOI] [PubMed] [Google Scholar]
- Nitschke L., Tsubata T. Molecular interactions regulate BCR signal inhibition by CD22 and CD72. Trends Immunol. 2004;25:543–550. doi: 10.1016/j.it.2004.08.002. [DOI] [PubMed] [Google Scholar]
- O'Connor B.P., Vogel L.A., Zhang W., Loo W., Shnider D., Lind E.F., Ratliff M., Noelle R.J., Erickson L.D. Imprinting the fate of antigen-reactive B cells through the affinity of the B cell receptor. J. Immunol. 2006;177:7723–7732. doi: 10.4049/jimmunol.177.11.7723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oropallo M.A., Cerutti A. Germinal center reaction: antigen affinity and presentation explain it all. Trends Immunol. 2014;35:287–289. doi: 10.1016/j.it.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pao L.I., Lam K.P., Henderson J.M., Kutok J.L., Alimzhanov M., Nitschke L., Thomas M.L., Neel B.G., Rajewsky K. B cell-specific deletion of protein-tyrosine phosphatase Shp1 promotes B-1a cell development and causes systemic autoimmunity. Immunity. 2007;27:35–48. doi: 10.1016/j.immuni.2007.04.016. [DOI] [PubMed] [Google Scholar]
- Parry S.L., Hasbold J., Holman M., Klaus G.G. Hypercross-linking surface IgM or IgD receptors on mature B cells induces apoptosis that is reversed by costimulation with IL-4 and anti-CD40. J. Immunol. 1994;152:2821–2829. [PubMed] [Google Scholar]
- Paus D., Phan T.G., Chan T.D., Gardam S., Basten A., Brink R. Antigen recognition strength regulates the choice between extrafollicular plasma cell and germinal center B cell differentiation. J. Exp. Med. 2006;203:1081–1091. doi: 10.1084/jem.20060087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pone E.J., Lou Z., Lam T., Greenberg M.L., Wang R., Xu Z., Casali P. B cell TLR1/2, TLR4, TLR7 and TLR9 interact in induction of class switch DNA recombination: modulation by BCR and CD40, and relevance to T-independent antibody responses. Autoimmunity. 2015;48:1–12. doi: 10.3109/08916934.2014.993027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pone E.J., Zhang J., Mai T., White C.A., Li G., Sakakura J.K., Patel P.J., Al-Qahtani A., Zan H., Xu Z. BCR-signalling synergizes with TLR-signalling for induction of AID and immunoglobulin class-switching through the non-canonical NF-kappaB pathway. Nat. Commun. 2012;3:767. doi: 10.1038/ncomms1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roco J.A., Mesin L., Binder S.C., Nefzger C., Gonzalez-Figueroa P., Canete P.F., Ellyard J., Shen Q., Robert P.A., Cappello J. Class-switch recombination occurs infrequently in germinal centers. Immunity. 2019;51:337–350 e337. doi: 10.1016/j.immuni.2019.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell D.M., Dembic Z., Morahan G., Miller J.F., Burki K., Nemazee D. Peripheral deletion of self-reactive B cells. Nature. 1991;354:308–311. doi: 10.1038/354308a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwickert T.A., Victora G.D., Fooksman D.R., Kamphorst A.O., Mugnier M.R., Gitlin A.D., Dustin M.L., Nussenzweig M.C. A dynamic T cell-limited checkpoint regulates affinity-dependent B cell entry into the germinal center. J. Exp. Med. 2011;208:1243–1252. doi: 10.1084/jem.20102477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman Z., Gitlin A.D., Targ S., Jankovic M., Pasqual G., Nussenzweig M.C., Victora G.D. T follicular helper cell dynamics in germinal centers. Science. 2013;341:673–677. doi: 10.1126/science.1241680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K.G., Hewitson T.D., Nossal G.J., Tarlinton D.M. The phenotype and fate of the antibody-forming cells of the splenic foci. Eur. J. Immunol. 1996;26:444–448. doi: 10.1002/eji.1830260226. [DOI] [PubMed] [Google Scholar]
- Stavnezer J., Guikema J.E., Schrader C.E. Mechanism and regulation of class switch recombination. Annu. Rev. Immunol. 2008;26:261–292. doi: 10.1146/annurev.immunol.26.021607.090248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhoff U., Muller U., Schertler A., Hengartner H., Aguet M., Zinkernagel R.M. Antiviral protection by vesicular stomatitis virus-specific antibodies in alpha/beta interferon receptor-deficient mice. J. Virol. 1995;69:2153–2158. doi: 10.1128/jvi.69.4.2153-2158.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart I., Radtke D., Phillips B., McGowan S.J., Bannard O. Germinal center B cells replace their antigen receptors in dark zones and fail light zone entry when immunoglobulin gene mutations are damaging. Immunity. 2018;49:477–489 e477. doi: 10.1016/j.immuni.2018.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sze D.M., Toellner K.M., Garcia de Vinuesa C., Taylor D.R., MacLennan I.C. Intrinsic constraint on plasmablast growth and extrinsic limits of plasma cell survival. J. Exp. Med. 2000;192:813–821. doi: 10.1084/jem.192.6.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toellner K.M., Luther S.A., Sze D.M., Choy R.K., Taylor D.R., MacLennan I.C., Acha-Orbea H. T helper 1 (Th1) and Th2 characteristics start to develop during T cell priming and are associated with an immediate ability to induce immunoglobulin class switching. J. Exp. Med. 1998;187:1193–1204. doi: 10.1084/jem.187.8.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toellner K.M., Sze D.M., Zhang Y. What are the primary limitations in B-cell affinity maturation, and how much affinity maturation can we drive with vaccination? A role for antibody feedback. Cold Spring Harb. Perspect. Biol. 2018;10:a028795. doi: 10.1101/cshperspect.a028795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsubata T., Murakami M., Honjo T. Antigen-receptor cross-linking induces peritoneal B-cell apoptosis in normal but not autoimmunity-prone mice. Curr. Biol. 1994;4:8–17. doi: 10.1016/s0960-9822(00)00003-8. [DOI] [PubMed] [Google Scholar]
- Tsubata T., Murakami M., Nisitani S., Honjo T. Molecular mechanisms for B lymphocyte selection: induction and regulation of antigen-receptor-mediated apoptosis of mature B cells in normal mice and their defect in autoimmunity-prone mice. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1994;345:297–301. doi: 10.1098/rstb.1994.0109. [DOI] [PubMed] [Google Scholar]
- Victora G.D., Nussenzweig M.C. Germinal centers. Annu. Rev. Immunol. 2012;30:429–457. doi: 10.1146/annurev-immunol-020711-075032. [DOI] [PubMed] [Google Scholar]
- Victora G.D., Schwickert T.A., Fooksman D.R., Kamphorst A.O., Meyer-Hermann M., Dustin M.L., Nussenzweig M.C. Germinal center dynamics revealed by multiphoton microscopy with a photoactivatable fluorescent reporter. Cell. 2010;143:592–605. doi: 10.1016/j.cell.2010.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe N., Nomura T., Takai T., Chiba T., Honjo T., Tsubata T. Antigen receptor cross-linking by anti-immunoglobulin antibodies coupled to cell surface membrane induces rapid apoptosis of normal spleen B cells. Scand. J. Immunol. 1998;47:541–547. doi: 10.1046/j.1365-3083.1998.00346.x. [DOI] [PubMed] [Google Scholar]
- Weisel F.J., Zuccarino-Catania G.V., Chikina M., Shlomchik M.J. A temporal switch in the germinal center determines differential output of memory B and plasma cells. Immunity. 2016;44:116–130. doi: 10.1016/j.immuni.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yam-Puc J.C., Zhang L., Zhang Y., Toellner K.M. Role of B-cell receptors for B-cell development and antigen-induced differentiation. F1000Res. 2018;7:429. doi: 10.12688/f1000research.13567.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Tech L., George L.A., Acs A., Durrett R.E., Hess H., Walker L.S.K., Tarlinton D.M., Fletcher A.L., Hauser A.E. Plasma cell output from germinal centers is regulated by signals from Tfh and stromal cells. J. Exp. Med. 2018;215:1227–1243. doi: 10.1084/jem.20160832. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No data sets or code were generated or analyzed in this study. All software is commercially available.