Figure 1.
A human gephyrin missense mutation associated with epileptic encephalitis alters multimer binding stability
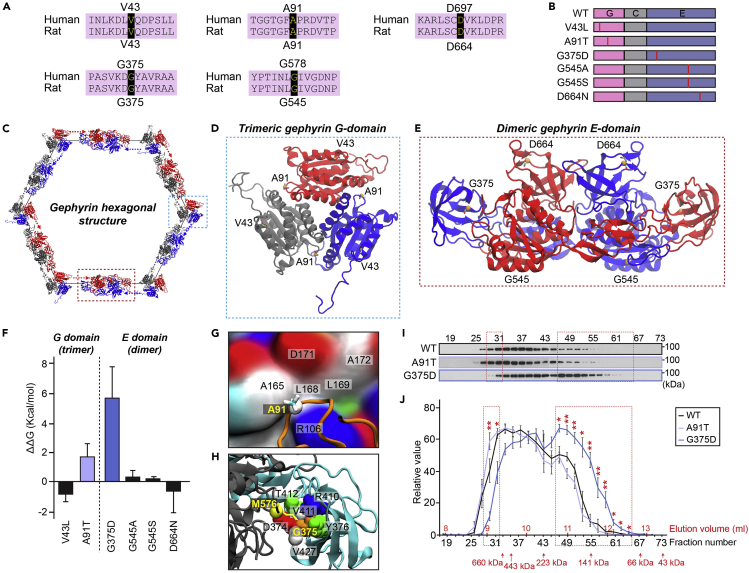
(A) Alignment and conservation between human gephyrin- and rat gephyrin residues that are mutated in human patients with epileptic encephalopathy or ASDs.
(B) Schematic diagrams of gephyrin WT and its mutants. Abbreviations: G, G-domain; C, C-domain; E, E-domain.
(C) Schematic depiction of gephyrin hexagonal lattices.
(D) Structure of G-domain trimers and position of point mutations in gephyrin.
(E) Structure of E-domain dimers and position of point mutations in gephyrin.
(F) Multimer binding free energy difference between gephyrin WT and the indicated point mutants, as calculated by thermodynamic integration analyses. Numerical values per mutation points were averaged. Data are represented as means ± SDs.
(G) A91 and hydrophobic pockets in the binding interface between gephyrin G-domains.
(H) G375 positioned inside of the β-strand bundle at the binding interface between chains. Bead and surface colors indicate the following: white, hydrophobic; green, hydrophilic; red, negatively charged; blue, positively charged; and yellow, methionine.
(I) Extracts of HEK293T cells transfected with untagged gephyrin WT or its mutants (A91T or G375D) were fractionated on a gel filtration column. Fractions were analyzed by immunoblotting using anti-gephyrin antibodies.
(J) Quantification of expression levels of gephyrin WT and its mutants (A91T or G375D) in each fraction. Data are means ± SEMs (∗p < 0.05, ∗∗p < 0.01; WT versus G375D; Mann-Whitney U test; n = 5/group).