Abstract
Background
The clinical characteristics of atrial fibrillation (AF) resulting from non-pulmonary vein (PV) triggers remain unknown. This study aimed to evaluate the clinical characteristics of patients with AF caused by non-PV triggers, localization of non-PV foci, clinical differences, and clinical outcomes after catheter ablation in each AF focus.
Methods
A total of 2967 patients who underwent initial catheter ablation for paroxysmal or persistent AF were examined. After PV isolation, all patients underwent high-dose isoproterenol infusion to assess the existence of non-PV foci.
Results
Non-PV foci were identified in 564 patients (19.2%). The localization of successfully ablated non-PV foci in 514 patients were the superior vena cava (SVC: 213 cases), interatrial septum (IAS: 125 cases), coronary sinus (CS: 98 cases), right atrium (RA: 125 cases), left atrium (LA: 114 cases), and unmappable (50 cases). Multivariate analysis revealed that female gender, low body mass index (BMI), non-paroxysmal AF (PAF), and sick sinus syndrome were independent and significant indicators of non-PV foci. In the multivariate analysis of each AF focus, female gender, low BMI, and non-PAF were significant predictors of IAS and CS foci, RA and IAS foci, and CS foci, respectively. In addition, dilatation of the LA was significantly associated with LA foci, whereas RA, LA, IAS, and CS foci were associated with AF recurrence.
Conclusion
These findings could help to identify patients at a higher risk of AF caused by non-PV triggers and clarify the clinical difference according to the localization of non-PV foci.
Keywords: Catheter ablation, Atrial fibrillation, Pulmonary vein isolation, Non-pulmonary vein foci
1. Introduction
Pulmonary vein (PV) isolation is an established treatment for atrial fibrillation (AF) [1] because PVs are the major source of ectopic foci that initiate AF [2]. However, several studies reported the presentation of non-PV triggers and their clinical importance in paroxysmal AF (PAF), [3], [4], [5] as well as persistent and long-standing persistent AF [6]. The manifestation of non-PV AF foci has been documented in the superior vena cava (SVC), left atrium (LA), right atrium (RA), interatrial septum (IAS), coronary sinus (CS), and ligament of Marshall, among others [7]. However, there are few reports on the examination of non-PV AF foci that are divided into the aforementioned foci and that discuss the clinical difference in each AF focus. Therefore, this study was conducted to retrospectively evaluate the clinical characteristics of a large number of patients with AF caused by non-PV triggers, the localization of non-PV foci, the clinical differences, and clinical outcomes after catheter ablation in each AF focus.
2. Methods
2.1. Patient population
This study included 2967 consecutive patients who underwent an initial catheter ablation for paroxysmal or persistent AF at the Japanese Red Cross Saitama Hospital between January 2013 and June 2019 (2029 men; mean age, 64.4 ± 10.5 years). All patients underwent complete PV isolation with a cryoballoon (CB) or radiofrequency (RF) catheter. Among these patients, 1730 (1401 PAF, 329 non-PAF) underwent PV isolation using a second-generation CB catheter, whereas 1237 (557 PAF, 680 non-PAF) underwent PV isolation using an RF catheter. The method of ablation was determined based on the discretion of the operator and the patient’s request. AF was defined as paroxysmal when AF ended spontaneously and lasted for less than 7 days; persistent when AF lasted more than 7 days; and long-standing persistent when AF lasted more than 1 year.
Exclusion criteria were previous maze procedures, unidirectional or no PV isolation, and the exclusive manifestation of atrial tachycardia (AT).
The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the Ethics Committee of the Japanese Red Cross Saitama Hospital (approval date: 6/10/2020; reference number: 20-C). Written informed consent was obtained from all subjects before their enrollment in the study.
2.2. Intraprocedural management
All patients were administered anticoagulation therapy for more than 30 days, and antiarrhythmic drugs were discontinued for more than 7 days (amiodarone was discontinued for >1 month) before ablation. A 6-Fr, 20-pole, 4-site (2-pole for the SVC, 8-pole for the RA, 2-pole for the ostium of CS, and 8-pole for the CS) mapping catheter (BeeAT Cath; Japan-Life-Line, Tokyo, Japan) was inserted through the right subclavian vein or internal jugular vein and positioned in the CS for pacing, recording, and internal cardioversion. Two long sheaths (SL0 [AF Division, Abbott Medical, Minneapolis, MN, USA]) and another sheath (SL0 [AF Division, Abbott Medical, Minneapolis, MN, USA] for RF cases without LA dilatation, Agilis [AF Division, Abbott Medical, Minneapolis, MN, USA] for RF cases with an LA diameter >42 mm, or Flexcath Advance [Medtronic, Minneapolis, MN, USA] for CB cases were inserted through the right femoral vein. A temperature probe (SensiTherm Multi Probe; Abbott Medical) was placed within the esophagus to monitor the esophageal temperatures during the ablation procedure. A single transseptal puncture was performed using an RF-powered transseptal needle (RF needle; Japan Lifeline, Tokyo, Japan). Subsequently, a bolus and continuous infusion of heparin were administered to achieve an activated clotting time ranging between 300 and 350 s during the procedure.
2.3. RF-based PV isolation
Two long sheaths were introduced in both superior PVs after the transseptal puncture via the same transseptal insertion site, and contrast esophagography and pulmonary venography were performed to determine the anatomical relationships of the esophagus, LA, and PV ostia. Mapping was performed with the CARTO 3D system (Biosense Webster) or EnSite Navix system (Abbott Medical). A 20-polar circular mapping catheter (Inquiry Optima, Abbott Medical; or Lasso, Biosense Webster, Diamond Bar, California, USA) and an irrigated ablation catheter with a 3.5-mm tip (Thermocool, Biosense Webster; Flexability, Abbott Medical; CoolPath, Abbott Medical; Tacti-cath, Abbot Medical) were delivered to the LA. Ablation energy was set at a maximum power of 25–35 W for 30–40 s for each lesion and maximum catheter tip temperature of 42 °C. Energy delivery was further reduced when the baseline esophageal temperature increased and discontinued when the temperature reached 41 °C. PV isolation was extensively performed in all cases, and 71.6% (886 cases) of RF cases underwent PV isolation with an adjunct LA posterior wall isolation, the creation of the right posterior wall line adjacent to the left posterior wall line (called the touching rings method), or additional LA roof and floor line connecting the wide antral PV isolation line [8].
2.4. CB-based PV isolation
One long sheath (FlexCath Advance; Medtronic and SL0; Abbott Medical) was introduced into the LA and RA after the transseptal puncture. A 10-polar circular mapping catheter (Inquiry; AF Division, Abbott Medical, Minneapolis, MN, USA) was positioned at the SVC. All patients underwent PV isolation using a second-generation 28-mm CB catheter inserted through the FlexCath Advance (Medtronic). Balloon positioning and the degree of PV occlusion were evaluated by the injection of a contrast medium diluted at a 1:1 ratio with 0.9% saline. All cryo applications were performed for 180 or 240 s per application alongside one or more additional cryo applications, as determined by the Achieve catheter (Medtronic). During cryo applications to the right PV, continuous phrenic nerve stimulation (10 V for 2.9 ms) was performed using the circular mapping catheter. The delivery of cryo energy was immediately terminated during the loss of phrenic capture [9]. Subsequently, PVs were carefully mapped with an Achieve catheter (Medtronic). In the event PV isolation was not achieved, touch-up ablation was performed using an RF catheter (Thermocool, Biosense Webster; Flexability, Abbott Medical; CoolPath, Abbott Medical; or Ablaze, Japan Lifeline, Tokyo, Japan).
2.5. Diagnosis and treatment of non-PV AF foci
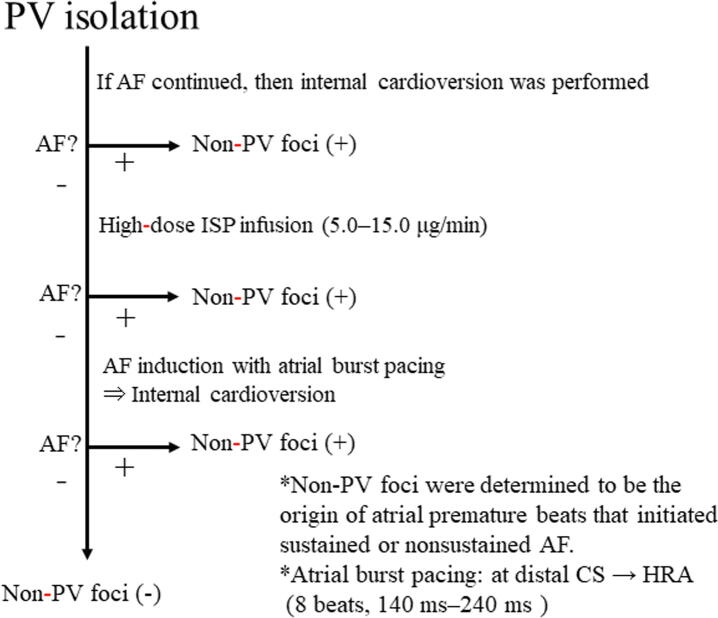
We attempted to determine the existence of non-PV foci. After PV isolation, isoproterenol (0.06–0.2 mg per hour for 1 min) was intravenously injected, and atrial burst pacing (140–240 ms) was performed to induce non-PV AF foci. Non-PV AF foci were determined using our protocol [10] (Fig. 1). In previous study, the isoproterenol dose for induction of non-PV foci varied from 0.012 mg to 0.25 mg per hour [4], [5], but the appropriate isoproterenol dose was unclear. In this study, we used high-dose isoproterenol up to 0.2 mg per hour, which exceeded the physiological isoproterenol dose to unmask the non-PV foci. In this study, non-PV foci were determined to be the origin of atrial premature beats that initiated sustained or non-sustained AF.
Fig. 1.
Protocol for the non-PV AF foci induction test. Non-PV foci were determined to be the origin of atrial premature beats that initiated sustained or non-sustained AF. If AF continued after PV isolation, internal cardioversion was performed to convert the AF to sinus rhythm. If AF could not be stopped by internal cardioversion, CFAE-guided catheter ablation was performed until AF termination was achieved spontaneously or by internal cardioversion. These patients had unmappable non-PV foci. After AF termination, continuous infusion of isoproterenol was performed at a dose of approximately 0.06 to 0.2 mg per hour for 1 min. In the event AF could not be provoked, atrial burst pacing was performed at the distal CS for 240 ms per 8 beats, and the time was reduced by 20 ms at every time point until 140 ms for AF induction. Similarly, the pacing site was changed to the HRA if AF could not be provoked. If AF occurred, internal cardioversion was performed, and the presence of non-PV foci was assessed after AF stopped. PV, pulmonary vein; AF, atrial fibrillation; CFAE, complex fractionated atrial electrogram; CS, coronary sinus; HRA, high right atrium; PV, pulmonary vein.
Catheter positions for mapping of non-PV foci were as follows. We used a 20-pole, 4-site mapping catheter (BeeAT), a 20-polar circular mapping catheter, and an ablation catheter to find non-PV triggers. A circular mapping catheter was positioned into the IAS and the ablation catheter was delivered to the LA’s posterior or bottom surface. We estimated the approximate location of non-PV foci using the endocardial atrial activation sequences from these catheters, and the circular mapping catheter was manipulated to map their detailed location. Finally, mapping and ablation of non-PV foci was performed using an irrigation catheter.
The locations of non-PV AF foci were classified into 5 mappable foci as follows: SVC, RA, LA, IAS, and CS. Undetectable or multi-changing non-PV foci were defined as unmappable foci. IAS included the right- and left-side septa, and the CS included the internal CS and the ostium of the CS. Following the location of non-PV foci in the SVC or LA posterior wall, the SVC or LA posterior wall was electrically isolated. Other non-PV foci were focally ablated. Successful ablation was determined as the absence of spontaneous AF with or without isoproterenol infusion.
2.6. Follow-up
Patients underwent in-hospital electrocardiography (ECG) monitoring for 3 days following the procedure. The first outpatient clinic visit occurred 2–3 weeks after the procedure. Subsequent follow-up visits consisted of a clinical interview and ECG every 2–3 months and a 24-hour Holter monitoring, or a 2-week cardiac event recording at 3 and 12 months after the procedure. When patients experienced symptoms suggestive of arrhythmic events, they were subjected to ECG, 24-hour Holter monitoring, or a 2-week cardiac event recording to clarify the cause of their symptoms at our hospital or neighboring medical clinics. In the event antiarrhythmic drugs were prescribed during the 3-month blanking period, these drugs were discontinued 3 months after the procedure. Recurrence was defined as any atrial tachyarrhythmia lasting longer than 30 s after the 3-month blanking period. In the case of recurrence, antiarrhythmic drugs were prescribed, cardioversion was performed, and a repeat ablation procedure was considered.
2.7. Statistical analysis
All continuous variables, except for brain natriuretic peptide (BNP), were expressed as the mean ± standard deviation; BNP was expressed as the median and interquartile range. Student’s t-test or the Mann-Whitney U test was used for comparisons between groups. Categorical and continuous variables were expressed as absolute and relative frequencies, respectively. The chi-square test was used to evaluate differences in categorical variables between the two groups. Arrhythmia-free survival curves for each group were presented as Kaplan-Meier plots, and the time-to-event analysis was performed using the log-rank test. A multivariate logistic regression analysis was used to determine the independent predictor of the existence of non-PV foci, and variables with P values <0.10 in the univariate analysis were included in the multivariate analysis. Similarly, a multivariate logistic regression analysis was used to identify the independent predictor of each SVC, RA, LA, IAS, and CS focus. Variables such as age, female gender, body mass index (BMI), BNP level >100 pg/mL, non-PAF, ejection fraction (EF) < 50%, hypertension, alcohol consumption, sick sinus syndrome (SSS), and LA diameter were included in the multivariate analysis, and odds ratios, hazard ratios, and 95% confidence intervals were calculated. In all analyses, P values <0.05 were considered statistically significant. All statistical analyses were conducted with SPSS Statistics version 19.0 (IBM Corp, Armonk, NY, USA).
3. Results
3.1. Baseline and clinical characteristics of non-PV AF foci
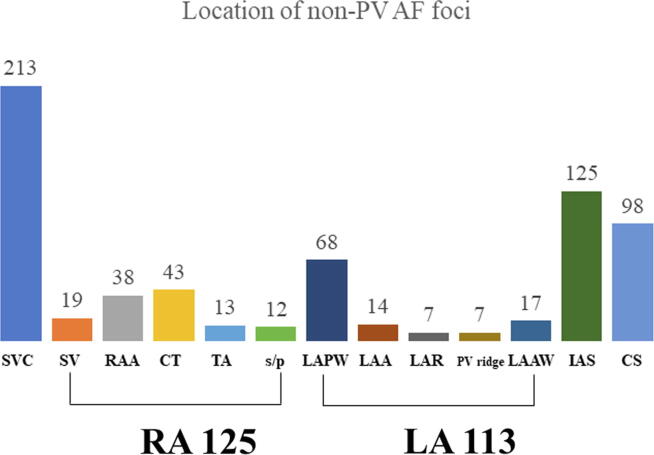
Additional non-PV ablation was performed in 952 of 2967 total patients (32%); of these 952 patients, ablation was performed in 388 patients exclusively due to premature atrial contraction (PAC) with a short-run pattern. Non-PV AF foci were identified in 564 patients (19.2%). The baseline and electrophysiological findings are shown in Table 1. In 514 patients, the localization of successfully ablated non-PV foci included the following: SVC (213 cases), IAS (125 cases), CS (98 cases), RA (125 cases), LA (114 cases), and unmappable (50 cases). The detailed distribution of non-PV AF foci is illustrated in Fig. 2. Patients with non-PV AF foci when compared to patients without non-PV AF foci were older (65.6 ± 9.8 vs. 64.2 ± 10.7; P = 0.003), more likely to be female (40.6% vs. 29.3%; P < 0.001) with non-PAF (40.6% vs. 32.5%; P < 0.001) and SSS (7.3% vs. 4.9%; P < 0.028), had higher BNP levels (60.6 vs. 49.5 pg/mL; P < 0.001), had lower BMI (23.5 ± 3.7 vs. 24.2 ± 3.7; P < 0.001), and had longer LA diameter (37.7 ± 7.7 vs. 37.0 ± 7.2; P = 0.023). In addition, the procedure time was longer in patients with non-PV AF foci than that in patients without non-PV AF foci (187.7 ± 58.3 vs. 135.2 ± 56.1 min; P < 0.001). Multivariate analysis revealed that female gender, BMI, non-PAF, and SSS were independent and significant indicators of non-PV AF foci.
Table 1.
Clinical data of all patients and the localization of non-PV AF foci.
| All patients (n = 2967) |
Non-PV AF foci (−) (n = 2403) |
Non-PV AF foci (+) (n = 564) |
Univariate analysis P |
Multivariate analysis P |
|
|---|---|---|---|---|---|
| Observation period (day) | 769 ± 531 | 756 ± 527 | 826 ± 542 | 0.004* | |
| Procedure time (min) | 145.2 ± 60.2 | 135.2 ± 56.1 | 187.7 ± 58.3 | <0.001* | |
| Cryoballoon (n) | 1730 (58.3%) | 1404 (58.4%) | 326 (57.8%) | 0.280 | |
| Non-PV AF foci (successful ablated) | 514 (91.1%) | ||||
| SVC foci | 213 (37.8%) | ||||
| RA foci | 125 (22.2%) | ||||
| LA foci | 114 (20.2%) | ||||
| IAS foci | 125 (22.2%) | ||||
| CS foci | 98 (17.3%) | ||||
| Unmappable foci | 50 (8.9%) | ||||
| Age (years) | 64.4 ± 10.5 | 64.2 ± 10.7 | 65.6 ± 9.8 | 0.003* | 0.181 |
| Female (n) | 938 (31.6%) | 703 (29.3%) | 229 (40.6%) | <0.001* | 0.001* |
| BNP (pg/mL) | 51.4 (20.7, 108.6) | 49.5 (19.5, 104.9) | 60.6 (27.7, 130.7) | <0.001* | 0.289 |
| Non-PAF (n) | 1009 (34.0%) | 780 (32.5%) | 229 (40.6%) | <0.001* | 0.014* |
| HT (n) | 1646 (55.5%) | 1319 (54.92%) | 327 (57.48%) | 0.171 | |
| Habitual alcoholic drinker (n) | 982 (33.0%) | 798 (33.2%) | 174 (30.9%) | 0.079 | 0.774 |
| SSS (n) | 158 (5.3%) | 117 (4.9%) | 41 (7.3%) | 0.028* | 0.037* |
| BMI (kg/m2) | 24.1 ± 3.7 | 24.2 ± 3.7 | 23.5 ± 3.7 | <0.001* | 0.002* |
| EF (%) | 64.4 ± 11.4 | 64.6 ± 11.3 | 63.6 ± 12.0 | 0.069 | 0.308 |
| LA diameter (mm) | 37.1 ± 7.3 | 37.0 ± 7.2 | 37.7 ± 7.7 | 0.033* | 0.127 |
Values (except BNP) are expressed as mean ± standard deviation. BNP is expressed as medians and interquartile ranges.
Abbreviations: AF, atrial fibrillation; PV, pulmonary vein; SVC, superior vena cava; IAS, interatrial septum; CS, coronary sinus; RA, right atrium; LA, left atrium; BNP, brain natriuretic peptide; PAF, paroxysmal atrial fibrillation; HT, hypertension; SSS, sick sinus syndrome; BMI, body mass index; EF, ejection fraction.
P < 0.05.
Fig. 2.
Location of non-PV AF foci. SVC, superior vena cava; SV, sinus venarum; RAA, right atrial appendage; CT, crista terminalis; TA, tricuspid valve annulus; s/p, slow pathway; LAPW, left atrial posterior wall; LAA, left atrial appendage; LAR, left atrial roof; PV, pulmonary vein; LAAW, left atrial anterior wall; IAS, interatrial septum; CS, coronary sinus.
3.2. Clinical differences according to the localization of non-PV AF foci
The predictors of non-PV AF foci were analyzed in a multivariate analysis for SVC, RA, LA, IAS, and CS foci. The variables included age, gender, BMI, BNP level > 100 pg/mL, non-PAF, EF < 50%, hypertension, alcohol consumption, SSS, and large LA diameter (Table 2). In the multivariate analysis of each AF focus, female gender, low BMI, and non-PAF were significant predictors of IAS and CS foci, RA and IAS foci, and CS foci, respectively. On the contrary, SSS was not a significant predictor of any AF focus. In addition, large LA diameter was significantly associated with LA foci, and there was no significant main risk factor in SVC foci.
Table 2.
Predictors of each non-PV AF focus by multivariate logistic regression analysis.
| Age | Female gender | BMI | BNP > 100 pg/mL | Non-PAF | EF < 50 | HT | Alcohol consumption | SSS | LAD | |
|---|---|---|---|---|---|---|---|---|---|---|
| SVC | 1.01(0.98–1.03) P = 0.46 | 1.44(0.95–2.17) P = 0.082 | 0.97(0.91–1.03) P = 0.28 | 0.85(0.53–1.35) P = 0.49 | 0.82(0.53–1.28) P = 0.39 | 1.04(0.54–1.97) P = 0.91 | 0.91(0.62–1.31) P = 0.60 | 1.35(0.91–2.0) P = 0.12 | 1.45(0.74–2.82) P = 0.27 | 0.99(0.96–1.02) P = 0.49 |
| RA | 0.98(0.96–1.01) P = 0.17 | 1.36(0.81–2.27) P = 0.24 | 0.92(0.85–0.99) P = 0.032* | 1.44(0.82–2.51) P = 0.19 | 1.00(0.57–1.72) P = 0.99 | 0.76(0.33–1.74) P = 0.51 | 1.01(0.62–1.64) P = 0.96 | 0.75(0.45–1.23) P = 0.25 | 1.03(0.40–2.66) P = 0.94 | 1.00(0.96–1.04) P = 0.95 |
| LA | 1.03(0.99–1.06) P = 0.07 | 1.69(0.99–2.86) P = 0.052 | 1.01(0.94–1.08) P = 0.76 | 0.87(0.50–1.49) P = 0.60 | 1.07(0.62–1.82) P = 0.80 | 0.93(0.43–1.97) P = 0.83 | 1.40(0.84–2.33) P = 0.19 | 1.09(0.65–1.82) P = 0.73 | 1.86(0.87–3.84) P = 0.10 | 1.06(1.02–1.10) P = 0.001* |
| IAS | 1.00(0.97–1.03) P = 0.89 | 2.39(1.39–4.09) P = 0.002* | 0.89(0.82–0.96) P = 0.004* | 0.76(0.43–1.36) P = 0.35 | 1.32(0.75–2.29) P = 0.32 | 1.33(0.63–2.79) P = 0.44 | 1.69(0.99–2.85) P = 0.051 | 1.14(0.66–1.95) P = 0.63 | 1.85(0.84–4.08) P = 0.12 | 1.04(0.99–1.08) P = 0.064 |
| CS | 1.00(0.97–1.03) P = 0.85 | 2.01(1.13–3.57) P = 0.018* | 0.96(0.89–1.04) P = 0.31 | 1.21(0.66–2.18) P = 0.53 | 2.06(1.14–3.72) P = 0.017* | 1.73(0.86–3.47) P = 0.12 | 1.36(0.78–2.37) P = 0.27 | 0.91(0.51–1.61) P = 0.74 | 1.34(0.51–3.50) P = 0.54 | 1.00(0.95–1.04) P = 0.88 |
Values are expressed as odds ratio (95% confidence interval).
Abbreviations: AF, atrial fibrillation; PV, pulmonary vein; SVC, superior vena cava; IAS, interatrial septum; CS, coronary sinus; RA, right atrium; LA, left atrium; BNP, brain natriuretic peptide; PAF, paroxysmal atrial fibrillation; HT, hypertension; SSS, sick sinus syndrome; BMI, body mass index; EF, ejection fraction.
P < 0.05.
3.3. Clinical outcomes after catheter ablation
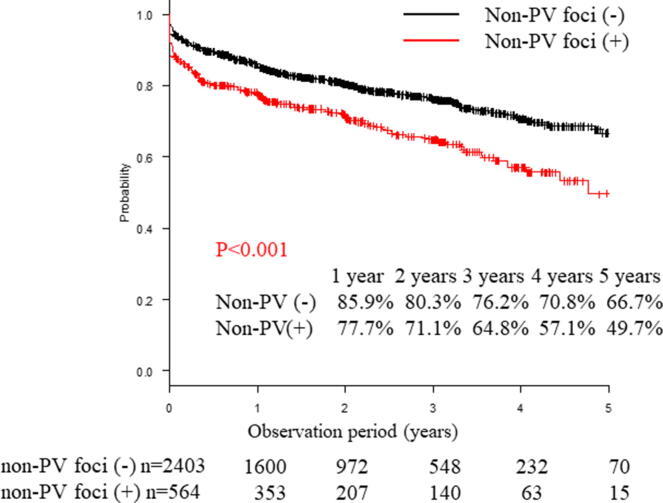
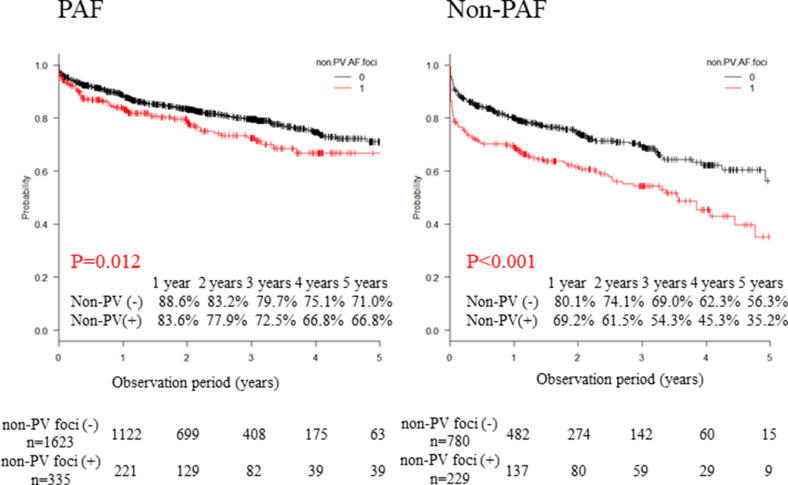
AF-free survival rates during a mean follow-up period of 769 ± 531 days in patients without and with non-PV AF foci were 85.9% and 77.7% at 1 year, 80.3% and 71.1% at 2 years, 76.2% and 64.8% at 3 years, 70.8% and 57.1% at 4 years, and 66.7% and 49.7% at 5 years, respectively (P < 0.001, log-rank test, Fig. 3). Next, we analyzed the AF-free survival rates separately in patients with paroxysmal and persistent disease while stratifying them into with or without non-PV AF foci groups (paroxysmal: n = 1958, mean follow-up period = 772 ± 525 days/persistent: n = 1009, mean follow-up period = 764 ± 540 days). With respect to paroxysmal AF, AF-free survival rates in patients without and with non-PV AF foci were 88.6% and 83.6% at 1 year, 83.2% and 77.9% at 2 years, 79.7% and 72.5% at 3 years, 75.1% and 66.8% at 4 years, and 71.0% and 66.8% at 5 years, respectively (P = 0.012, log-rank test; Fig. 4). Additionally, AF-free survival rates in persistent AF patients without and with non-PV AF foci were 80.1% and 69.2% at 1 year, 74.1% and 61.5% at 2 years, 69.0% and 54.3% at 3 years, 62.3% and 45.3% at 4 years, and 56.3% and 35.2% at 5 years, respectively (P < 0.001, log-rank test; Fig. 4).
Fig. 3.
Freedom from AF/AT recurrence after the first ablation. Recurrence-free was defined as AT/AF-free without any antiarrhythmic drugs. The Kaplan-Meier curve of AF recurrence between patients with and without non-PV foci. AF, atrial fibrillation; AT, atrial tachycardia; PV, pulmonary vein.
Fig. 4.
Freedom from AF/AT recurrence after the first ablation separately in patients with paroxysmal and persistent AF. Recurrence-free was defined as AT/AF-free without any antiarrhythmic drugs. The Kaplan-Meier curve of AF recurrence between patients with and without non-PV foci. AF, atrial fibrillation; AT, atrial tachycardia; PV, pulmonary vein.
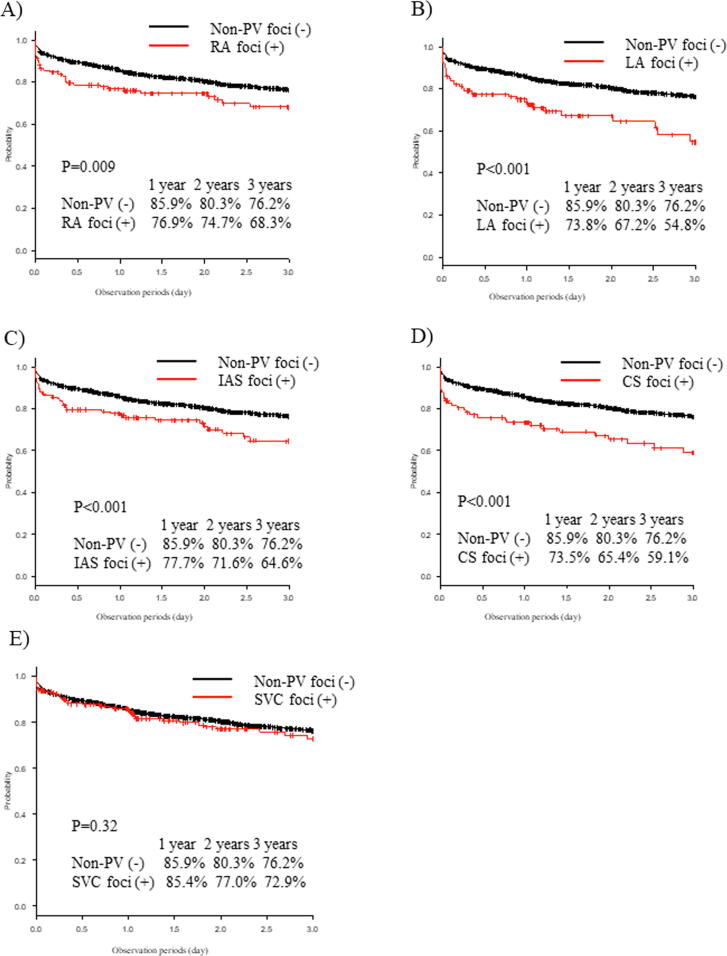
Concerning each aforementioned non-PV AF focus, the AF recurrence rate was significantly higher in patients with RA, LA, IAS, and CS foci than that in patients without non-PV AF foci (P = 0.009, <0.001, <0.001, and < 0.001, respectively; Fig. 5); however, no significant difference in the AF recurrence rate was observed between patients with SVC AF foci and those without non-PV AF foci (P = 0.32; Fig. 5).
Fig. 5.
Freedom from AF/AT recurrence after the first ablation with respect to each non-PV AF focus. (A) The Kaplan-Meier curve of AF recurrence between patients with RA foci and without non-PV foci. (B) Patients with LA foci and without non-PV foci. (C) Patients with IAS foci and without non-PV foci. (D) Patients with CS foci and without non-PV foci. (E) Patients with SVC foci and without non-PV foci. AF, atrial fibrillation; AT, atrial tachycardia; PV, pulmonary vein; RA, right atrium; LA, left atrium; IAS, interatrial septum; CS, coronary sinus; SVC: superior vena cava.
4. Discussion
4.1. Major findings
The major findings of this study are as follows: (1) the incidence of non-PV AF foci in patients with AF was 19.2%; (2) female gender, non-PAF, SSS, and low BMI were associated with non-PV AF foci; (3) female gender, low BMI, and non-PAF were significant predictors of IAS and CS foci, RA and IAS foci, and CS foci, respectively, and SSS was not a significant predictor of any AF focus; (4) concerning each non-PV AF focus, RA, LA, IAS, and CS AF foci were associated with AF recurrence, even after additional non-PV foci ablation.
4.2. Clinical differences between non-PV AF foci
The incidence and detailed distribution of non-PV foci after CB ablation in patients with PAF have been previously reported in the evaluation of the relationship between non-PV foci after CB ablation and AF recurrence [5], [11]. In these reports, non-PV foci were observed in about 32% of patients with PAF, and this reported incidence was relatively higher than that observed in our study. This discrepancy could be due to the difference in the definition of non-PV foci, which includes both patients with PAC-triggered AF and PAC with a short-run pattern (with or without AF). In this study, non-PV foci were observed in 32% of all patients with AF if non-PV foci included PAC with a short-run pattern; this result is not significantly different from the earlier report.
Similarly, female gender, advanced age, and lower BMI were reported as independent predictors of non-PV foci for PAF [5]. In this study, female gender, lower BMI, non-PAF, and SSS were predictors of non-PV foci for paroxysmal, persistent, and long-standing persistent AF. Our results are in line with those reported in previous studies. Several studies have reported the relationship between non-PV foci and female gender [5]. Magnami et al. and Sharma et al. reported an association between sex hormone and the progression of AF [12], [13]. Several studies reported an association between female gender and SVC foci [14]. In our clinical data, several female patients tended to experience SVC AF foci, although not significantly (P = 0.082). Lower BMI similarly increased the incidence of non-PV foci in a previous study [5]. Obesity is a well-known risk factor of AF occurrence; similarly, the data described thinness as a risk factor of non-PV triggered AF [5], [15], especially RA and LA foci. Non-PAF is well known to be the result of non-PV AF foci. Hung Y et al. reported the incidence of non-PV triggers as 44.7% in long-standing persistent AF [16]. In our data, non-PAF was a significant indicator of CS foci. SSS is equally associated with non-PV foci. Hayashi et al. reported that PAF and SSS were associated with a higher prevalence of non-PV AF foci [17]. However, our data showed that SSS was not a significant predictor of any AF focus.
CB ablation is excellent in achieving PV isolation durability and, therefore, we performed CB ablation in many cases [10]. However, CB ablation could not be used to treat non-PV triggers. If AF caused by non-PV triggers was observed after CB ablation, additional RF ablation would be necessary, which would incur an excessive medical cost. Our findings could help to identify patients at a higher risk of AF caused by non-PV triggers in advance and aid in the selection of the PV isolation method.
4.3. Catheter ablation for non-PV triggers
Patients with extra PV triggers may have a higher AF recurrence rate even after ablation for the additional non-PV triggers. Our study showed that the patients with an SVC trigger were the only ones who did not have a higher recurrence rate compared to those without non-PV AF foci. This result suggests that the focal ablation method might be insufficient to eliminate non-PV triggers and that anatomical isolation of the triggering structure might be expected to achieve better outcomes. The following may explain why focal ablation could not achieve better outcomes. First, several patients exhibited multiple foci, leading to difficulty in identification of the focus and making a wide-spread ablation challenging. Additionally, widespread ablation may cause novel ATs. Second, several areas were difficult to map or treat because of anatomical problems, such as epicardial side or regions adjacent to the sinus/atrioventricular node. Third, mechanical PAC caused by the mapping catheter, which ought to be distinguished, is mixed in clinical non-PV triggers and makes mapping non-PV AF triggers difficult. On the other hand, several studies reported that isolation of AF triggers improved clinical results. Satomi et al. reported that RA-free wall isolation has the potential of abolishing multiple and unstable macroreentrant ATs from the RA-free wall [18]. This method could be useful in the treatment of multiple RA-free wall foci. Di Biase et al. reported that LA appendage isolation resulted in optimal results and safety for multiple LA appendage triggers [19]. They also reported that empirical electrical isolation of the LA appendage could improve long-term freedom from ATs without increasing complications [20]. Isolation method might improve clinical results if we could isolate in safety.
4.4. Limitations
This study has several limitations. First, this study was a nonrandomized, retrospective, single-center study; thus, there was a potential confounder in patient selection, which is a major limitation of this study. Second, both contact-force and non-contact-force catheters were used in this study; similarly, a non-irrigated catheter was used in PV touch-up ablation in a small number of CB cases, which might have influenced the results. Third, the three-dimensional mapping system was not used for the CB group. Therefore, additional ablation without three-dimensional mapping would be necessary if non-PV foci were documented, which might have equally influenced the results. Fourth, in the persistent and long-standing persistent AF, a large number of RF cases underwent PV isolation with some LA posterior wall isolation. This method may have treated LA posterior wall AF foci before the non-PV provocation test and caused a reduction in LA AF foci. Fifth, direct cannulation from the CS to the vein of Marshall was not used with a small multipolar catheter. Thus, in the data, the foci of the ostium of the vein of Marshall included CS foci, and the foci of the ligament of Marshall included LA foci because the earliest activation site was observed at the ridge between the LA appendage and the left superior PV. Sixth, cryoballoon ablation was consistently used among these patients, but when non-PV triggers were documented, an additional RF catheter was used to treat them. In such cases, a significantly higher procedure cost was required. Finally, continuous cardiac rhythm monitoring was not performed to evaluate AF recurrence; thus, the recurrence rate could have been underestimated.
4.5. Conclusions
The incidence of non-PV AF foci in patients with AF was 19.2%. Female gender, low BMI, non-PAF, and SSS were independent and significant indicators of non-PV AF foci. Catheter ablation in patients with non-PV foci, except SVC, is not expected to yield better long-term outcomes than that in patients without non-PV foci. These findings could help to identify patients at a higher risk of AF caused by non-PV triggers and clarify clinical differences according to the localization of non-PV AF foci. These clinical assessments need to be further investigated in large-scale, randomized prospective studies.
Declaration of Competing Interest
The authors report no relationships that could be construed as a conflict of interest.
Acknowledgments
We would like to thank Editage (www.editage.jp) for English language editing.
References
- 1.Oral H., Knight B.P., Ozaydin M. Segmental ostial ablation to isolate the pulmonary veins during atrial fibrillation: Feasibility and mechanistic insights. Circulation. 2002;106:1256–1262. doi: 10.1161/01.cir.0000027821.55835.00. [DOI] [PubMed] [Google Scholar]
- 2.Haїssaguerre M., Jaїs P., Shah D.C. Spontaneous initiation of atrial fibrillation by ectopic beats originating from the pulmonary veins. N. Engl. J. Med. 1998;339:659–666. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 3.Lin W.S., Tai C.T., Hsieh M.H. Catheter ablation of paroxysmal atrial fibrillation initiated by non-pulmonary vein ectopy. Circulation. 2003;107:3176–3183. doi: 10.1161/01.CIR.0000074206.52056.2D. [DOI] [PubMed] [Google Scholar]
- 4.Chang H.Y., Lo L.W., Lin Y.J. Long-term outcome of catheter ablation in patients with atrial fibrillation origination from nonpulmonary vein ectopy. J. Cardiovasc. Electrophysiol. 2013;24:250–258. doi: 10.1111/jce.12036. [DOI] [PubMed] [Google Scholar]
- 5.Kato N., Nitta J., Sato A. Characteristics of the nonpulmonary vein foci induced after second-generation cryoballoon ablation for paroxysmal atrial fibrillation. J. Cardiovasc. Electrophysiol. 2020;31:174–184. doi: 10.1111/jce.14314. [DOI] [PubMed] [Google Scholar]
- 6.Santangeli P., Zado E.S., Hutchinson M.D. Prevalence and distribution of focal triggers in persistent and long-standing persistent atrial fibrillation. Heart Rhythm. 2016;13:374–382. doi: 10.1016/j.hrthm.2015.10.023. [DOI] [PubMed] [Google Scholar]
- 7.Santangeli P., Marchilinski F.E. Techniques for the provocation, localization, and ablation of non-pulmonary vein triggers for atrial fibrillation. Heart Rhythm. 2017;14:1087–1096. doi: 10.1016/j.hrthm.2017.02.030. [DOI] [PubMed] [Google Scholar]
- 8.Sugumar H., Thomas S.P., Prabhu S., Vaskoboinik A., Kistler P.M. How to perform posterior wall isolation in catheter ablation for atrial fibrillation. J. Cardiovasc. Electrophysiol. 2018;29:345–352. doi: 10.1111/jce.13397. [DOI] [PubMed] [Google Scholar]
- 9.Sacher F., Monahan K.H., Thomas S.P. Phrenic nerve injury after atrial fibrillation catheter ablation: characterization and outcome in a multicenter study. J. Am. Coll. Cardiol. 2006;47:2498–2503. doi: 10.1016/j.jacc.2006.02.050. [DOI] [PubMed] [Google Scholar]
- 10.Inamura Y., Nitta J., Inaba O. Differences in the electrophysiological findings of repeat ablation between patients who first underwent cryoballoon ablation and radiofrequency catheter ablation for paroxysmal atrial fibrillation. J. Cardiovasc. Electrophysiol. 2019;30:1792–1800. doi: 10.1111/jce.14065. [DOI] [PubMed] [Google Scholar]
- 11.Watanabe K., Nitta J., Inaba O. Predictors of non-pulmonary vein foci in paroxysmal atrial fibrillation. J. Interv. Card. Electrophysiol. 2020 doi: 10.1007/s10840-020-00779-x. [DOI] [PubMed] [Google Scholar]
- 12.Magnani J.W., Moser C.B., Murabito J.M. Association of sex hormones, aging, and atrial fibrillation in men: the Framingham Heart Study. Circ. Arrhythm. Electrophysiol. 2014;7:307–312. doi: 10.1161/CIRCEP.113.001322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharma R., Oni O.A., Gupta K. Normalization of testosterone levels after testosterone replacement therapy is associated with decreased incidence of atrial fibrillation. J. Am. Heart Assoc. 2017;9(6(5)):e004880. doi: 10.1161/JAHA.116.004880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee S.H., Tai C.T., Hsieh M.H. Predictors of non-pulmonary vein ectopic beats initiating paroxysmal atrial fibrillation: implication for catheter ablation. J. Am. Coll. Cardiol. 2005;46:1054–1059. doi: 10.1016/j.jacc.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 15.Takigawa M., Takahashi A., Kuwahara T. Impact of Non-pulmonary vein foci on the outcome of the second session of catheter ablation for paroxysmal atrial fibrillation. J. Cardiovasc. Electrophysiol. 2015;26:739–746. doi: 10.1111/jce.12681. [DOI] [PubMed] [Google Scholar]
- 16.Hung Y., Lo L.W., Lin Y.J. Characteristics and long-term catheter ablation outcome in long-standing persistent atrial fibrillation patients with non-pulmonary vein triggers. Int. J. Cardiol. 2017;241:205–211. doi: 10.1016/j.ijcard.2017.04.050. [DOI] [PubMed] [Google Scholar]
- 17.Hayashi K., Fukunaga M., Yamaji K. Impact of catheter ablation for paroxysmal atrial fibrillation in patients with sick sinus syndrome – Important role of non-pulmonary vein foci. Circ. J. 2016;80:887–894. doi: 10.1253/circj.CJ-15-1384. [DOI] [PubMed] [Google Scholar]
- 18.Satomi K., Chun K.R., Tilz R. Catheter ablation of multiple unstable macroreentrant tachycardia within the right atrium free wall in patients without previous cardiac surgery. Circ. Arrhythm. Electrophysiol. 2010;3:24–31. doi: 10.1161/CIRCEP.109.879015. [DOI] [PubMed] [Google Scholar]
- 19.Di Biase L., Burkhardt J.D., Mohanty P. Left appendage: an underrecognized trigger site of atrial fibrillation. Circulation. 2010;122:109–118. doi: 10.1161/CIRCULATIONAHA.109.928903. [DOI] [PubMed] [Google Scholar]
- 20.Di Biase L., Burkhardt J.D., Mohanty P. Left atrial appendage isolation in patients with longstanding persistent AF undergoing catheter ablation: BELIEF trial. J. Am. Coll. Cardiol. 2016;68:1929–1940. doi: 10.1016/j.jacc.2016.07.770. [DOI] [PubMed] [Google Scholar]