Abstract
Purpose
To investigate whether live birth rates from euploid blastocyst frozen-thawed embryo transfer (FET) cycles are associated with infertility diagnosis or oocyte source.
Design
Retrospective analysis of FET cycles reported to SART CORS in 2014.
Methods
Data from fresh IVF cycles with preimplantation genetic testing for aneuploidy (PGT-A), linked to the first FET cycles, were collected from the 2014 SART CORS database for autologous and donor oocyte cycles. Inclusion criteria were patients undergoing FET with euploid embryos (n = 4148). Demographic data including age, BMI, prior fertility, and etiology of infertility were collected from the retrieval cycle and analyzed. Patients with uterine anomalies, preimplantation genetic testing-mutation (PGT-M) for genetic diseases, gender selection, HLA determination, or systemic and immunologic disorders were excluded. The primary outcome measure was live birth (LB) rate. Potential confounders such as age, prior fertility, and maximum baseline FSH values were analyzed with regression models as indicated.
Results
Though age, maximum baseline FSH, and infertility diagnosis were significantly different, LB was similar between patients undergoing autologous or donor oocyte FET cycles. Etiology of infertility was not significantly associated with LB in autologous cycles (p = 0.95). Potential confounders such as maternal age, prior fertility, and maximum baseline FSH were not associated with outcomes; however, maternal BMI was inversely related to LB in autologous cycles, with an odds ratio of 0.97 (95% CI: 0.96–0.98 (rho = − 0.08, p < 0.01)).
Conclusions
After controlling for confounding variables, a euploid embryo derived from a donor or autologous oocyte results in similar LB in women with different infertility diagnoses.
Keywords: Euploid, Live birth rate, Infertility, Frozen embryo transfer
Introduction
Transferring euploid embryos is one key for successful implantation and live birth following in vitro fertilization (IVF) treatment. Chromosomal abnormalities are believed to be the most common cause of embryonic non-viability [1]. Hence, preimplantation genetic testing for aneuploidy (PGT-A) was developed to select chromosomally normal embryos. It has been shown that cleavage-stage embryo blastomere aspiration combined with fluorescence in situ hybridization (FISH) of the chromosomes did not improve implantation rates [2]. Scott et al. suggest that this in part may be due to the large proportion of embryo content biopsied (1 out of 8 cells) or removal of a multipotent cell which may have been destined for cell lines necessary for embryo survival [2]. On the other hand, trophectoderm biopsy combined with comprehensive chromosome screening showed improved and sustained implantation rates [2–4]. Despite this improvement, pregnancy rates from transfers of euploid embryos do not approach 100% and are typically reported up to 70%. Implantation failure can approach 30% [5], and miscarriages after implantation occur despite embryo euploidy [6].
Why do euploid embryos implant up to 70% of the time on average, and not up to 100%? Endometrial receptivity issues represent a potential source of implantation failure [7]. Evidence suggests that in apparently normal endometria, recurrent implantation failure (RIF) may be associated with molecular and functional changes in the uterus such as abnormal endometrial microbiota, including the presence of chronic endometritis, poor synchronization between the blastocyst and endometrium, and/or excessive uterine peristalsis [8].
However, the evidence that live birth rate is only slightly higher in gestational carriers compared to their autologous counterparts suggests that some euploid embryos do not survive even in optimal endometrial environments; thus, endometrial receptivity issues may not be the only reason for implantation failure [9]. Other elements influencing embryo implantation have been suggested such as immunologic or inflammatory mediators of implantation failure [5, 10, 11]. Data are lacking on whether oocyte source (donor vs. autologous) or etiology of infertility is associated with significant differences in IVF outcomes once euploid status is established. A 2017 study showed no association between varying degrees of male factor and live birth rates from euploid blastocysts [12], and a lack of association has been demonstrated between patients with polycystic ovarian syndrome (PCOS) and embryonic aneuploidy [13]. However, it has not been shown whether euploid embryos from women with tubal disease, for example, result in the same live birth rates as euploid embryos from women with diminished ovarian reserve, or whether euploid embryos from donor oocytes implant at a higher rate than euploid embryos from autologous oocytes given that the donor oocytes come from fertile women.
The purpose of this study was to compare cycle outcomes from women with different etiologies of infertility when euploid embryos were transferred in an IVF cycle. We also looked at whether euploid embryos from donor oocytes would implant at a greater rate than euploid embryos from autologous cycles.
Materials and methods
This is a retrospective cohort study of nationally reported 2014 IVF data to SART CORS. 2014 was the first year that frozen-thawed embryo transfer (FET) cycle information could be linked to the original oocyte retrieval cycle data in SART CORS, making this study possible. The ability to link fresh to frozen cycles which are then reported in a national database allows for powerful data to be generated and leveraged for analysis; a single year of data was included in this study.
Inclusion and exclusion criteria
Inclusion criteria were all patients undergoing transfer of frozen-thawed autologous euploid blastocysts or frozen-thawed euploid blastocysts derived from donor oocytes. Blastocysts from days 5–7 were biopsied and cryopreserved, and trophectoderm cells were sent for genetic analysis. Euploidy was determined by the genetics lab chosen by each SART member clinic, either using array-CGH or next-generation sequencing (NGS). For each patient, only the outcome of the first FET cycle was included. Transfer of the best quality euploid embryo was assumed. Exclusion criteria included PGT-A cycles with embryo banking, fertility preservation, use of donor embryos, gestational carriers, PGT-M cycles for single gene disorders, gender selection, or HLA determination and patients with systemic and immunologic disorders.
Variables and outcomes
Demographic data, including patient age, maximum baseline FSH, BMI, the number of embryos transferred, and infertility etiology (male factor (MF), endometriosis, PCOS, diminished ovarian reserve (DOR), tubal factor, uterine factors, and unexplained infertility), were collected and analyzed from the oocyte retrieval cycle. Only cycles with a single etiology were included to allow for meaningful and direct associations with cycle outcomes.
The primary outcome measure was live birth (LB) rate; the secondary outcome measures included clinical pregnancy rate, miscarriage rate, and ectopic pregnancy rate, as defined by SART CORS. Clinical pregnancy is defined as intrauterine gestational sac by transvaginal ultrasound. Miscarriage is defined as pregnancy loss before 20 weeks of gestation. Live birth is defined by the presence of a heartbeat at the time of delivery after 24 weeks of gestation.
Statistics and data collection
STATA version 15 was used to perform statistical analyses. The chi-square test was used for categorical variables, and t test and ANOVA were used for continuous data when appropriate. Logistic regression models were used to control for confounding variables and reported as odds ratios and 95% confidence intervals (CI). Trend analysis and Bonferroni correction were used to evaluate the trend and association of outcomes with BMI. p value < 0.05 was considered statistically significant.
Data were collected and verified by SART and reported to the Centers for Disease Control and Prevention in compliance with the Fertility Clinic Success Rate and Certification Act of 1992 (Public Law 102-493). The data in the SART CORS are validated annually with some clinics having on-site visits for chart review based on an algorithm for clinic selection [14]. During each visit, data reported by the clinic were compared with information recorded in patients’ charts. Ten out of 11 data fields selected for validation were found to have discrepancy rates of ≤ 5%. This study was approved by the institutional review board of Albert Einstein College of Medicine.
Results
Autologous vs. donor oocyte cycles
This study included 3828 autologous oocyte FET cycles (92.3%), and 320 donor oocyte FET cycles (7.7%). The demographic characteristics of participants classified according to oocyte source are presented in Table 1. As anticipated, mean maternal age (41.7 vs. 36.8 years, p < 0.001) and maximum FSH (12.2 vs. 7.9 IU/mL, p < 0.01) were higher in women who used donor oocytes than autologous oocytes. This correlated to a higher degree of DOR patients in the donor oocyte group while male factor, tubal factor, PCOS, and unexplained infertility were more prevalent in the autologous group. Mean maternal BMI and the average number of embryos transferred were similar between the groups (Table 1).
Table 1.
Demographic and cycle parameters for autologous and donor cycles
| Autologous (n = 3828) | Donor (n = 320) | p value | |
|---|---|---|---|
| Age (years) | 36.8 ± 4.1 | 41.7 ± 5.5 | p < 0.001 |
| BMI (kg/m2) | 24.4 ± 5.0 | 24.0 ± 5.1 | p = 0.212 |
| Gravidity | 1.7 ± 1.7 | 1.8 ± 2.0 | p = 0.38 |
| Max FSH (IU/mL) | 7.9 ± 4.0 | 12.2 ± 12.4 | p < 0.001 |
| Etiology | p = 0.001 | ||
| DOR | 390 (10.2%) | 121 (37.8%) | |
| Tubal | 101 (2.6%) | 1 (0.3%) | |
| Endometriosis | 64 (1.7%) | 8 (2.5%) | |
| PCOS | 226 (5.9%) | 2 (0.6%) | |
| Male | 384 (10.0%) | 10 (3.1%) | |
| Uterine | 65 (1.7%) | 0 | |
| Unexplained | 495 (12.9%) | 8 (2.5%) | |
| Other | 671 (17.5%) | 82 (25.6%) | |
| Mixed | 1432 (37.4%) | 88 (27.5%) | |
| Live birth rate* | 2062 (53.9%) | 160 (50%) | p = 0.183 |
| Clinical pregnancy rate* | 2408 (62.9%) | 187 (58.4%) | p = 0.113 |
| Ectopic pregnancy rate* | 15 (0.4%) | 0 | p = 0.623 |
| Miscarriage rate* | 340 (8.9%) | 27 (8.4%) | p = 0.788 |
| Multiple birth rate* | 284 (7.4%) | 26 (8.1%) | p = 0.213 |
Data are represented as mean ± standard deviated for discrete and continuous variables and as n (%) for categorical variables. Italic emphasis denote statistically significant findings
*Per embryo transfer cycle
Live birth rate was not significantly different between autologous and donor cycles (53.9% vs. 50.0%, p = 0.18). In addition, there were no statistical differences between clinical pregnancy rates (62.9% vs. 58.4%), miscarriage rates (8.9% vs. 8.4%), ectopic pregnancy rates (0.4% vs. 0%), and multiple pregnancy rates (7.4% vs. 8.1%) from autologous oocyte vs. donor oocyte FET cycles of euploid embryos, respectively (p > 0.05) (Table 1).
Etiology of infertility and association with outcomes in autologous cycles
In autologous cycles restricted to a single primary diagnosis, we were able to assess the relationship of etiology of infertility with cycle outcome (Table 2). Unexpectedly, uterine factor had the highest LB rate (55.4%) and tubal factor the lowest (48.5%); however, these differences did not reach statistical significance (p = 0.95) after adjusting for maternal BMI (Table 2).
Table 2.
Association between etiology of infertility and IVF outcomes in autologous cycles
| Etiology (n) | Clinical pregnancy rate** N (%) |
p value | Live birth rate** N (%) |
p value | Miscarriage rate** N (%) |
p value | Multiple birth rate** N (%) |
p value | Adjusted p value*** |
|---|---|---|---|---|---|---|---|---|---|
| DOR* (390) | 235 (60.3%) | 0.68 | 201 (51.5) | 0.95 | 33 (8.5%) | 0.83 | 25 (6.4%) | 0.91 | 0.29 |
| TF* (101) | 60 (59.4%) | 49 (48.5%) | 11 (10.9%) | 6 (6.0%) | 0.58 | ||||
| Endo* (64) | 36 (56.3%) | 32 (50%) | 4 (6.3%) | 6 (9.4%) | 0.78 | ||||
| PCOS* (226) | 137 (60.6%) | 117 (51.8%) | 19 (8.4%) | 18 (8.0%) | 0.11 | ||||
| MF* (384) | 230 (59.9%) | 202 (52.6%) | 28 (7.3%) | 34 (8.9%) | 0.36 | ||||
| Uterine (65) | 45 (69.2%) | 36 (55.4%) | 8 (12.3%) | 4 (6.2%) | 0.32 | ||||
| Unexpl* (495) | 312 (63.0%) | 267 (53.9%) | 45 (9.1%) | 39 (7.9%) | 0.63 | ||||
| Other (671) | 426 (63.5%) | 363 (54.1%) | 63 (9.4%) | 39 (9.0%) | 0.96 |
*DOR, diminished ovarian reserve; TF, tubal factor; Endo, endometriosis; PCOS, polycystic ovarian syndrome; MF, male factor; Unexpl, unexplained
**Per embryo transfer cycle
***p values adjusted for BMI
Etiology of infertility and association with other patient demographic parameters in autologous cycles
We assessed whether other patient demographic parameters were significantly associated with etiologies of infertility (Table 3). As anticipated, DOR patients were older than the mean (39.3 vs. 36.8 years, p < 0.01) and had higher FSH levels (9.5 ± 4.9 IU/mL, p < 0.01) than the other cohorts while PCOS and endometriosis patients were slightly younger (35 and 34.9 years, p < 0.01). PCOS patients had higher BMI (24.8 vs. 24.1 kg/m2, p < 0.01) and lower FSH levels (6.7 vs. 7.9 IU/mL, p < 0.01) when compared to the mean BMI and FSH levels of all patients without PCOS. The highest BMI was found in uterine factor patients (25.7 vs. 24.4 kg/m2, p < 0.01). This finding is consistent with prior data that obesity, independent of fibroid size, is a risk factor for the development of uterine myomas [15, 16]. Tubal factor patients were more likely to have had prior children compared to the mean including PCOS patients (79.2% vs. 68.9%, p < 0.01). Age, BMI, history of multiparity, and max FSH values all had a statistically significant relationship with etiology of infertility (p < 0.01) (Table 3), but the number of embryos transferred did not (p = 0.42).
Table 3.
Association between etiology of infertility and other patient demographics in autologous cycles
| Etiology | Age (years) (mean ± SD) |
BMI (kg/m2) (mean ± SD) |
Gravidity (% multiparous) |
Max FSH (IU/mL) (mean ± SD) |
# embryos to uterus |
|---|---|---|---|---|---|
| DOR* | 39.3 ± 3.5 | 23.8 ± 4.4 | 70.8 | 9.5 ± 4.9 | 1.2 ± 0.5 |
| TF* | 36.1 ± 3.6 | 24.8 ± 5.7 | 79.2 | 7.2 ± 2.1 | 1.2 ± 0.5 |
| Endo* | 34.9 ± 3.8 | 23.3 ± 3.9 | 59.4 | 8.3 ± 3.2 | 1.3 ± 0.5 |
| PCOS* | 35.0 ± 4.0 | 24.8 ± 5.6 | 70.4 | 6.7 ± 2.7 | 1.3 ± 0.5 |
| MF* | 35.2 ± 3.7 | 24.1 ± 4.8 | 54.7 | 7.2 ± 2.4 | 1.2 ± 0.5 |
| Uterine | 37.0 ± 3.6 | 25.7 ± 5.5 | 67.7 | 7.1 ± 2.0 | 1.3 ± 0.5 |
| Unexplained | 36.3 ± 3.5 | 23.5 ± 4.5 | 66.9 | 7.8 ± 3.7 | 1.2 ± 0.4 |
| Other | 35.6 ± 4.3 | 24.6 ± 4.9 | 74.8 | 7.6 ± 4.5 | 1.2 ± 0.4 |
| p values | < 0.01 | < 0.01 | < 0.01 | < 0.01 | 0.42 |
Italic emphasis denote statistically significant findings
*DOR, diminished ovarian reserve; TF, tubal factor; Endo, endometriosis; PCOS, polycystic ovarian syndrome; MF, male factor
These potential cofounders underwent analysis separately to assess possible individual or group effects on cycle outcome (Table 4). Age was not associated with clinical pregnancy (p = 0.69), live birth (p = 0.38), or miscarriage rates (p = 0.53) once PGT-A tested embryos were transferred, in contrast to prior studies from untested embryos [17, 18].
Table 4.
Association between patient demographics and autologous cycle outcomes
| Patient demographics | Clinical pregnancy* | p value | Live birth* | p value | Miscarriage* | p value |
|---|---|---|---|---|---|---|
| BMI category (n) | (%) | < 0.01 | (%) | < 0.01 | (%) | 0.58 |
| 0–24.9 (1987) | 65.7% | 56.6% | 9.0% | |||
| 25–29.9 (666) | 59.5% | 50.5% | 9.0% | |||
| 30–34.9 (219) | 58.0% | 45.2% | 12.3% | |||
| 35–39.9 (108) | 50.9% | 41.7% | 8.3% | |||
| > 40 (42) | 66.7% | 54.7% | 9.5% | |||
| Gravidity (multiparity) | 62.6% | 0.86 | 53.1% | 0.35 | 9.4% | 0.09 |
| Age (years) (mean ± SD) | 36.8 ± 4.0 | 0.69 | 36.8 ± 4.0 | 0.38 | 36.9 ± 4.1 | 0.53 |
| Max FSH (IU/mL ± SD) | 7.9 ± 4.2 | 0.86 | 7.9 ± 4.4 | 0.77 | 7.8 ± 2.9 | 0.53 |
Italic emphasis denote statistically significant findings
*Per embryo transfer
BMI is inversely associated with outcomes in autologous cycles
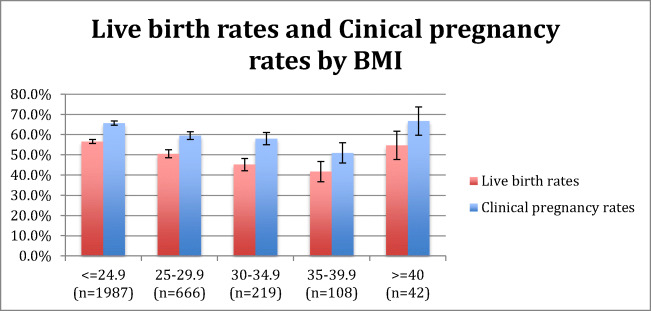
Of the demographics analyzed in the original univariate model (age, BMI, history of prior delivery and maximum baseline FSH), only BMI was significantly associated with LB rate in autologous cycles (Table 4). The number of obese patients in donor oocyte recipient FET cycles was too small; therefore, it was not included in analysis. On regression analysis, BMI was inversely associated with live birth rate, with an OR of 0.97 (95% CI: 0.96–0.98 (rho = − 0.08, p < 0.01). Women with BMI < 25 had the highest clinical pregnancy (65.7%) and live birth (56.6%) rates. Clinical pregnancy and live birth rates were 59.5% and 50.5% in BMI group 25–29.99; 58.0% and 45.2% in BMI group 30–34.99; 50.9% and 41.7% in BMI group 35–39.99; and 66.7% and 54.7% in BMI group > 40 (Fig. 1). Of those that had a live birth, 69.1% had a BMI < 25; this number approached 90% for BMI < 30. A trend test was performed for the association between BMI and live birth rate and demonstrated statistical significance (p < 0.001). On the other hand, BMI was not a predictor of miscarriage (p = 0.58) (Table 4) or ectopic pregnancy (p = 0.72) (data not shown).
Fig. 1.
Live birth and clinical pregnancy rate stratified by BMI
Discussion
This data demonstrates that once euploidy is established, oocyte source and etiology of infertility are not associated with cycle outcome. As shown previously in the literature, maternal BMI was found to be a significant and negative predictor of live birth [19, 20]. In this analysis, maternal age at time of oocyte retrieval was not significantly associated with FET cycle outcome.
An analysis of FETs from donor oocyte-derived euploid embryo vs. autologous oocyte-derived euploid embryo allows for independent exploration of patient demographics that may impact cycle outcomes. We found that patients who utilized donor oocytes were significantly older and had higher baseline FSH levels. However, these factors did not affect pregnancy outcomes, demonstrating that age in and of itself does not contribute to cycle success once euploid embryos are utilized. Interestingly, there was an increased number of embryos transferred in donor cycles (1.3 vs. 1.2, p = 0.001). It is unclear why this occurred as many SART member clinics have moved towards elective single embryo transfers, particularly in cycles using donor oocytes. More embryos transferred did not increase pregnancy or live birth rates in this cohort, adding support to the recommendation for elective single embryo transfer [21].
We also focused on outcomes from autologous oocytes cycles and possible associations to patient etiology of infertility. In order to analyze this as cleanly as possible, we included only those cycles with a single etiology. We found that etiology was not associated with live birth following transfer of euploid embryos. Other reports have shown similar data; a multinational cohort of 22,000 women showed no difference in ART outcomes from untested embryos when looking at endometriosis patients vs. tubal factor/endocrine disorders or unexplained infertility [22]. Similarly, a study looking at the relationship between age and ovarian reserve as independent predictors of euploidy noted that while age was inversely related to euploidy, no other factors were predictive of ploidy status, including etiology of infertility [23]. Our data demonstrates no significant impact of age on cycle outcome once euploidy is established. In contrast, a recent study suggests that age negatively correlates with implantation rate of euploid embryos in patients older than 38 years old. Furthermore, they did not find any relationship between BMI and outcome [24]. Interestingly, embryo morphology and AMH were independent predictors of outcome, indicating that IVF success involves more than embryo euploidy [24].
Our data demonstrates that BMI is significantly related to live birth and clinical pregnancy rates following autologous oocyte-derived euploid FETs in an inverse bimodal pattern, which has been established previously in the literature. The number of obese patients in donor oocyte recipient FET cycles was too small (n = 22); therefore, our analysis was restricted to autologous cycles. Obese patients are known to have poorer ART outcomes [19]. However, this in part is due to poorer stimulation and poor oocyte quality [25, 26]. Our current study demonstrates that once euploid embryos are utilized, there is still an adverse effect of increasing BMI on outcome. This finding is in parallel with other research showing an increased time to conception in obese ovulatory, non-infertile patients [27]. Perhaps the inflammatory state induced by obesity [28, 29] has an effect on implantation or endometrial receptivity [10, 30, 31]. Of note there was an increase in clinical pregnancy and live birth rates in the highest BMI group > 40 kg/m2. A similar U-shaped relationship to BMI has been noted in relation to the number of embryos achieved [32] as well as miscarriage rates [33], contrary to our finding of increased live birth rates in this subpopulation. It is unclear what causes this bimodal pattern although we note this cohort was our smallest sample size of only 42 patients. A larger cohort of patients with BMI > 40 kg/m2 may elucidate whether this relationship still has significance. A recent SART study also noted a negative correlation, although linear, between BMI and ART outcomes [34]. That study, despite being a robust analysis of a large cohort, included fresh IVF cycles with untested embryos. Our data of euploid embryos being transferred in an FET setting allows for a more focused view of BMI and pregnancy rates.
The strengths of this study include the large sample size of national data giving our study external validity. In addition, exclusion criteria were strictly applied to the data to achieve as accurate a comparison as possible between study groups; confounders were appropriately accounted for as needed. We were selective in analyzing first euploid FET cycles only, as well as excluding those who underwent PGT for reasons other than ploidy testing, in order to minimize embryo selection bias, though the highest quality euploid embryo was likely chosen in most if not all cycles. This additionally prevents selection bias of patients with recurrent implantation failure who require multiple euploid FET cycles.
There are several limitations to this study design. The data was collected and analyzed retrospectively, and the data is self-reported by SART member clinics, allowing for reporting bias and inaccurate data collection. Although data is validated, it is not validated for every clinic for every submission year; therefore, data may be skewed. A single year of data was analyzed, which was sufficiently powered to answer the question we originally asked. However, with additional years of data, the study findings could be different. 2014 was the first year linking fresh to frozen cycles. In 2014, genetics labs were doing both NGS and array-CGH to determine ploidy status. This genetic data is not collected by SART CORS, so we could not control for differences in technology platforms in the analysis. Euploid status of the embryos was therefore determined based on the current testing chosen by each clinic. NGS technology is able to detect more mosaic embryos than aCGH and we acknowledge that is it possible that undiagnosed mosaic or aneuploid embryos were transferred. We also acknowledge that any testing platform have limits of detection and possible diagnostic inaccuracies, although NGS technology has been reported to have a specificity > 95% [35]. All cycles included in this study were blastocyst FET; however, we cannot confirm whether the embryos were biopsied on day 5, 6, or 7 of culture. We did not limit cycles to elective single embryo transfer FET cycles, so there is a possible confounding effect of single vs. double embryo transfer on our analysis. However, the mean number of embryos transferred in both autologous and donor cycles was significantly below 2 and there were no differences in outcomes. Many cycles with multiple diagnoses of infertility were collected in SART CORS but were eliminated from this study in order to more directly associate cycle outcome with a single infertility diagnosis. However, this filter may have altered our finding of no association of infertility diagnosis to cycle outcome. Certain demographics such as race, ethnicity, and smoking status were sparsely populated in self-reported SART CORS data and were therefore not included in this analysis. Embryo quality at the time of frozen-thawed embryo transfer is not provided in SART CORS, so we could not comment on embryo quality in this study. However, we assume that the best quality euploid embryo was selected for the first FET cycle. It is possible that the maternal BMI during the stimulation cycle impacted the gonadotropin dose used and therefore impacted the ovarian response. Finally, we do not know if the donor oocyte source was from fresh oocyte donation or frozen oocytes.
In summary, our data indicates that neither oocyte source nor etiology of infertility impacts live birth rates when euploid embryos are transferred, while BMI remains a significant negative predictor of success. Euploid embryos derived from donor or autologous oocytes result in similar live birth rates in women with different infertility diagnoses.
Acknowledgments
SART wishes to thank all of its members for providing clinical information to the SART CORS database for use by patients and researchers. Without the efforts of our members, this research would not have been possible.
Authors’ contributions
FM, SJ, EW, and EB contributed to the study conception and design. EW assisted with data acquisition and material preparation. FM, MG, EB, and SJ performed data analysis, interpretation, and writing of the manuscript. All authors edited and approved the final manuscript.
Data availability
SART CORS database available with application to SART.
Compliance with ethical standards
This study was approved by the institutional review board of Albert Einstein College of Medicine.
Conflict of interest
FM, MG, EB, and SJ with nothing to disclose. EW employed by Redshift technologies, the data vendor for SART.
Footnotes
F. Meng and M. Goldsammler share first authorship equally.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hassold T, Abruzzo M, Adkins K, Griffin D, Merrill M, Millie E, Saker D, Shen J, Zaragoza M. Human aneuploidy: incidence, origin, and etiology. Environ Mol Mutagen. 1996;28(3):167–175. doi: 10.1002/(sici)1098-2280(1996)28:3<167::Aid-em2>3.0.Co;2-b. [DOI] [PubMed] [Google Scholar]
- 2.Scott RT, Jr, Upham KM, Forman EJ, Zhao T, Treff NR. Cleavage-stage biopsy significantly impairs human embryonic implantation potential while blastocyst biopsy does not: a randomized and paired clinical trial. Fertil Steril. 2013;100(3):624–630. doi: 10.1016/j.fertnstert.2013.04.039. [DOI] [PubMed] [Google Scholar]
- 3.Forman EJ, Hong KH, Ferry KM, Tao X, Taylor D, Levy B, et al. In vitro fertilization with single euploid blastocyst transfer: a randomized controlled trial. Fertil Steril. 2013;100(1):100–7.e1. doi: 10.1016/j.fertnstert.2013.02.056. [DOI] [PubMed] [Google Scholar]
- 4.Dahdouh EM, Balayla J, Garcia-Velasco JA. Comprehensive chromosome screening improves embryo selection: a meta-analysis. Fertil Steril. 2015;104(6):1503–1512. doi: 10.1016/j.fertnstert.2015.08.038. [DOI] [PubMed] [Google Scholar]
- 5.Franasiak JM, Scott RT. Contribution of immunology to implantation failure of euploid embryos. Fertil Steril. 2017;107(6):1279–1283. doi: 10.1016/j.fertnstert.2017.04.019. [DOI] [PubMed] [Google Scholar]
- 6.Luo L, Gu F, Jie H, Ding C, Zhao Q, Wang Q, Zhou C. Early miscarriage rate in lean polycystic ovary syndrome women after euploid embryo transfer - a matched-pair study. Reprod BioMed Online. 2017;35(5):576–582. doi: 10.1016/j.rbmo.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 7.Lessey BA, Young SL. What exactly is endometrial receptivity? Fertil Steril. 2019;111(4):611–617. doi: 10.1016/j.fertnstert.2019.02.009. [DOI] [PubMed] [Google Scholar]
- 8.Bellver J, Simón C. Implantation failure of endometrial origin: what is new? Curr Opin Obstet Gynecol. 2018;30(4):229–236. doi: 10.1097/gco.0000000000000468. [DOI] [PubMed] [Google Scholar]
- 9.Perkins KM, Boulet SL, Jamieson DJ, Kissin DM. Trends and outcomes of gestational surrogacy in the United States. Fertil Steril. 2016;106(2):435–42.e2. doi: 10.1016/j.fertnstert.2016.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galgani M, Insabato L, Cali G, Della Gatta AN, Mirra P, Papaccio F, et al. Regulatory T cells, inflammation, and endoplasmic reticulum stress in women with defective endometrial receptivity. Fertil Steril. 2015;103(6):1579–86.e1. doi: 10.1016/j.fertnstert.2015.03.014. [DOI] [PubMed] [Google Scholar]
- 11.Geva E, Yaron Y, Lessing JB, Yovel I, Vardinon N, Burke M, Amit A. Circulating autoimmune antibodies may be responsible for implantation failure in in vitro fertilization. Fertil Steril. 1994;62(4):802–806. doi: 10.1016/S0015-0282(16)57008-3. [DOI] [PubMed] [Google Scholar]
- 12.Mazzilli R, Cimadomo D, Vaiarelli A, Capalbo A, Dovere L, Alviggi E, et al. Effect of the male factor on the clinical outcome of intracytoplasmic sperm injection combined with preimplantation aneuploidy testing: observational longitudinal cohort study of 1,219 consecutive cycles. Fertil Steril. 2017;108(6):961–72.e3. doi: 10.1016/j.fertnstert.2017.08.033. [DOI] [PubMed] [Google Scholar]
- 13.Weghofer A, Munne S, Chen S, Barad D, Gleicher N. Lack of association between polycystic ovary syndrome and embryonic aneuploidy. Fertil Steril. 2007;88(4):900–905. doi: 10.1016/j.fertnstert.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 14.Center for Disease Control and Prevention ASRM, and Society for Assisted Reproductive Technology. 2012 assisted reproductive technology success rates: national summary and fertility clinic reports US Dept of Health and Human Services. 2014. http://www.cdc.gov/art/pdf/2012-report/national-summary/art_2012_national_summary_report.pdf.
- 15.Sparic R, Mirkovic L, Malvasi A, Tinelli A. Epidemiology of uterine myomas: a review. Int J Fertil Steril. 2016;9(4):424–435. doi: 10.22074/ijfs.2015.4599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dandolu V, Singh R, Lidicker J, Harmanli O. BMI and uterine size: is there any relationship? Int J Gynecol Pathol Off J Int Soc Gynecol Pathol. 2010;29(6):568–571. doi: 10.1097/PGP.0b013e3181e8ae64. [DOI] [PubMed] [Google Scholar]
- 17.Pal L, Santoro N. Age-related decline in fertility. Endocrinol Metab Clin N Am. 2003;32(3):669–688. doi: 10.1016/S0889-8529(03)00046-X. [DOI] [PubMed] [Google Scholar]
- 18.Malizia BA, Hacker MR, Penzias AS. Cumulative live-birth rates after in vitro fertilization. N Engl J Med. 2009;360(3):236–243. doi: 10.1056/NEJMoa0803072. [DOI] [PubMed] [Google Scholar]
- 19.Bellver J, Ayllon Y, Ferrando M, Melo M, Goyri E, Pellicer A, et al. Female obesity impairs in vitro fertilization outcome without affecting embryo quality. Fertil Steril. 2010;93(2):447–454. doi: 10.1016/j.fertnstert.2008.12.032. [DOI] [PubMed] [Google Scholar]
- 20.Provost MP, Acharya KS, Acharya CR, Yeh JS, Steward RG, Eaton JL, Goldfarb JM, Muasher SJ. Pregnancy outcomes decline with increasing body mass index: analysis of 239,127 fresh autologous in vitro fertilization cycles from the 2008–2010 Society for Assisted Reproductive Technology registry. Fertil Steril. 2016;105(3):663–669. doi: 10.1016/j.fertnstert.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 21.Guidance on the limits to the number of embryos to transfer: a committee opinion. Fertil Steril. 2017;107(4):901–3. 10.1016/j.fertnstert.2017.02.107. [DOI] [PubMed]
- 22.Gonzalez-Comadran M, Schwarze JE, Zegers-Hochschild F, Souza MD, Carreras R, Checa MA. The impact of endometriosis on the outcome of assisted reproductive technology. Reprod Biol Endocrinol: RB&E. 2017;15(1):8. doi: 10.1186/s12958-016-0217-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.La Marca A, Minasi MG, Sighinolfi G, Greco P, Argento C, Grisendi V, et al. Female age, serum antimullerian hormone level, and number of oocytes affect the rate and number of euploid blastocysts in in vitro fertilization/intracytoplasmic sperm injection cycles. Fertil Steril. 2017;108(5):777–83.e2. doi: 10.1016/j.fertnstert.2017.08.029. [DOI] [PubMed] [Google Scholar]
- 24.Reig A, Franasiak J, Scott RT, Jr, Seli E. The impact of age beyond ploidy: outcome data from 8175 euploid single embryo transfers. J Assist Reprod Genet. 2020;37(3):595–602. doi: 10.1007/s10815-020-01739-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shah DK, Missmer SA, Berry KF, Racowsky C, Ginsburg ES. Effect of obesity on oocyte and embryo quality in women undergoing in vitro fertilization. Obstet Gynecol. 2011;118(1):63–70. doi: 10.1097/AOG.0b013e31821fd360. [DOI] [PubMed] [Google Scholar]
- 26.Maheshwari A, Stofberg L, Bhattacharya S. Effect of overweight and obesity on assisted reproductive technology--a systematic review. Hum Reprod Update. 2007;13(5):433–444. doi: 10.1093/humupd/dmm017. [DOI] [PubMed] [Google Scholar]
- 27.Zaadstra BM, Seidell JC, Van Noord PA, te Velde ER, Habbema JD, Vrieswijk B, et al. Fat and female fecundity: prospective study of effect of body fat distribution on conception rates. BMJ: Br Med J. 1993;306(6876):484–487. doi: 10.1136/bmj.306.6876.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thaler JP, Schwartz MW. Minireview: inflammation and obesity pathogenesis: the hypothalamus heats up. Endocrinology. 2010;151(9):4109–4115. doi: 10.1210/en.2010-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nteeba J, Ganesan S, Keating AF. Progressive obesity alters ovarian folliculogenesis with impacts on pro-inflammatory and steroidogenic signaling in female mice. Biol Reprod. 2014;91(4):86. doi: 10.1095/biolreprod.114.121343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bellver J, Melo MA, Bosch E, Serra V, Remohi J, Pellicer A. Obesity and poor reproductive outcome: the potential role of the endometrium. Fertil Steril. 2007;88(2):446–451. doi: 10.1016/j.fertnstert.2006.11.162. [DOI] [PubMed] [Google Scholar]
- 31.Orostica L, Astorga I, Plaza-Parrochia F, Vera C, Garcia V, Carvajal R, et al. Proinflammatory environment and role of TNF-alpha in endometrial function of obese women having polycystic ovarian syndrome. Int J Obes. 2016;40(11):1715–1722. doi: 10.1038/ijo.2016.154. [DOI] [PubMed] [Google Scholar]
- 32.Pinborg A, Gaarslev C, Hougaard CO, Nyboe Andersen A, Andersen PK, Boivin J, Schmidt L. Influence of female bodyweight on IVF outcome: a longitudinal multicentre cohort study of 487 infertile couples. Reprod BioMed Online. 2011;23(4):490–499. doi: 10.1016/j.rbmo.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 33.Veleva Z, Tiitinen A, Vilska S, Hyden-Granskog C, Tomas C, Martikainen H, et al. High and low BMI increase the risk of miscarriage after IVF/ICSI and FET. Human Reprod (Oxf, Engl) 2008;23(4):878–884. doi: 10.1093/humrep/den017. [DOI] [PubMed] [Google Scholar]
- 34.Provost MP, Acharya KS, Acharya CR, Yeh JS, Steward RG, Eaton JL, Goldfarb JM, Muasher SJ. Pregnancy outcomes decline with increasing recipient body mass index: an analysis of 22,317 fresh donor/recipient cycles from the 2008-2010 Society for Assisted Reproductive Technology Clinic Outcome Reporting System registry. Fertil Steril. 2016;105(2):364–368. doi: 10.1016/j.fertnstert.2015.10.015. [DOI] [PubMed] [Google Scholar]
- 35.Fiorentino F, Bono S, Biricik A, Nuccitelli A, Cotroneo E, Cottone G, et al. Application of next-generation sequencing technology for comprehensive aneuploidy screening of blastocysts in clinical preimplantation genetic screening cycles. Hum Reprod (Oxf, Engl) 2014;29(12):2802–2813. doi: 10.1093/humrep/deu277. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
SART CORS database available with application to SART.