Introduction
Generation of calcium ions (Ca2+) is a pre-requisite for the oocyte activation process. However, the pathways and factors governing in vivo as well as in vitro oocyte activation are complicated and still under intense research. Moreover, during in vitro fertilisation (IVF), the gonadotropin-induced stimulation for multi-follicular development entails a lag in oocyte cytoplasmic maturation vis-à-vis its nuclear maturation. This often results in cytoplasmic dysmorphism that hampers the oocyte activation (OA) process [1]. The effects are manifested in the form of total fertilisation failure (TFF) or low fertilisation rates, poor embryo development/implantation rates, and arrest at a new metaphase-like stage called MIII stage due to insufficient release of calcium from internal stores [2]. Thus, any disparity in the generation of calcium ions (Ca2+), maintenance of intracellular calcium homeostasis or function of mitochondria and the Ca2+ ATPase pumps plasma membrane calcium-pump activity (PMCA) and sarco-endoplasmic reticulum Ca2+-ATPases (SERCA) may adversely influence fertilisation and embryo development [3–5].
Pertinently, during IVF, such adverse impacts are usually observed in women with advanced age, diminished ovarian reserve (DOR), premature ovarian failure (POF) and polycystic ovary syndrome (PCOS). The observed detrimental effects compel a correlation with inadequate or no OA among these women. Therefore, several studies employed in vitro mechanical, physical and chemical artificial oocyte activation (AOA) methods like strontium chloride, thimerosal, calcium ionophores and human recombinant phospholipase C-zeta (PLCζ) injections [6]. Although few studies have indeed reported advantages of using these methods, their invasive nature and altered biological mode of action [7, 8] have raised doubts regarding the competence and long-term benefits, thereby questioning the practical usability of these artificial techniques [9–11].
It is remarkable that recent studies evaluating failed fertilisation post-ICSI have hinted at the possible involvement of defective oocyte cytoplasmic molecules [12]. A recent observation [13] of oocyte activation and fertilisation leading to live birth using PLCζ null sperm (PLCζ−/−) also lays credence to speculation regarding alternative oocyte activation mechanisms. Another study [14] that obtained “calcium signatures” by measuring calcium oscillations in mouse and donated human oocytes has also envisaged the role of an oocyte-related factor in oocyte activation deficiency (OAD). Among the so-called oocyte-related molecules, the steroid estradiol E2 has been reported to alter calcium oscillation via its receptors on the oocyte surface to bring about non-genomic maturation of oocyte [15, 16]. However, its role in the oocyte activation process is unexplored and poorly understood.
In this context, the steroid hormone dehydroepiandrosterone sulphate (DHEAS) holds immense relevance. Primarily, in humans, 15% of dehydroepiandrosterone (DHEA) is produced by the ovaries and 99% of the systemic DHEA exists as DHEAS, which makes DHEAS the most abundant circulating as well as localised steroid in humans [17]. Moreover, during IVF, the in vitro exogenously supplemented DHEA exerts its effects in vivo by getting converted to DHEAS [18]. The ovarian site for DHEAS synthesis is the theca and cumulus-granulosa cells of oocytes and it is also present in follicular fluid [19]. Although present in cells surrounding the oocyte (therefore our term ‘oocyte-related’), the negatively charged sulphate group (SO42−) may help DHEAS exert its effect either by acting via putative oocyte receptors [20, 21] or by transporting across oocyte membrane through sodium-dependent organic anion transporters (SOAT) [22, 23]. Most notably, in certain human excitable cell types, DHEAS is known to regulate the same calcium-pumps (PMCA and SERCA) [24] which maintain calcium oscillations and cause OA in mice [5]. Importantly, it has been observed that DHEAS levels are either too low (in elderly, DOR, POF women) or very high (in women with PCOS) among women known to exhibit hampered fertilisation, embryo development and pregnancy outcomes. We hypothesised that any deviation from endogenous DHEAS concentrations may be detrimental for oocyte/embryo development: low DHEAS levels might prevent generation of adequate Ca2+ oscillations whereas high DHEAS levels may probably result in single spikes of Ca2+ rather than an expected Ca2+ wave pattern, thereby affecting OA and influencing IVF outcomes.
Objective
To evaluate if clinical treatment causes rectification of abnormal DHEAS levels to normal, thereby improving embryologic and clinical outcomes. The aim thus was to implicate DHEAS as a potential, innate ‘oocyte-related factor’ (related to oocyte and distinct from the ‘sperm-factor’) that affects IVF outcomes by influencing oocyte activation.
Material and methods
This prospective closed-cohort study recruited reproductive age women (n = 750, age: 25–42 years, BMI: 18–36 kg/m2) undergoing IVF treatment cycles between July 2016 and June 2019. Indications for IVF were, previous 2–3 attempts of failed IVF cycles with no/low fertilisation rates, previous 3–4 failed intra-uterine-insemination cycles in women with unexplained infertility and fresh IVF cycles in women with bilateral tubal block/hydrosalpinx (for normal control group). Details of patient characteristics and indications for IVF are shown in Table 1.
Table 1.
Patient characteristics in the three study groups/subgroups
| No DHEAS Supplementation A1 (n = 128) | DHEAS supplementation A2 (n = 137) | Normal control B (n = 145) | No metformin treatment C1 (n = 137) | Metformin treatment C2 (n = 134) | |
|---|---|---|---|---|---|
| Female age (years) | 35.31 ± 3.7 | 34.47 ± 3.7# | 32.12 ± 2.5 | 33.45 ± 2.1 | 32.28 ± 2.3* |
| Infertility duration (years) | 5.2 ± 1.3 | 5.5 ± 2# | 4.8 ± 1.5 | 5.1 ± 1.8 | 6 ± 2.5* |
| BMI (kg/m2) | 24.3 ± 2.5 | 23.08 ± 4.0# | 24.69 ± 3.3 | 25.5 ± 2.7 | 25.4 ± 3.1* |
| AMH (ng/ml) | 1.03 ± 0.08 | 1.2 ± 0.06# | 2.8 ± 0.9 | 6.8 ± 1.1 | 7.1 ± 1.0* |
| AFC | 5.1 ± 0.7 | 5.8 ± 1.0# | 12.2 ± 2.5 | 20.45 ± 1.9 | 21.21 ± 2.3* |
| Baseline D3 DHEAS (μg/dl) | 60 ± 18 | 69 ± 16# | 145 ± 28 | 332 ± 46 | 348 ± 42* |
| Indications for IVF | |||||
| Previous 2–3 failed IVF cycles of no/poor fertilisation | 83 | 80# | 00 | 94 | 98* |
| Fresh IVF with previous 3–4 failed IUI due to unexplained infertility | 45 | 57# | 36 | 43 | 36* |
| Fresh IVF cycles with bilateral tubal block/hydrosalpinx | 00 | 00 | 109 | 00 | 00 |
Values are mean ± SD
#A2 vs. A1, *C2 vs. C1 = p > 0.05 = non-significant
Donor oocyte cycles, cryopreservation cycles and in vitro maturation cycles were excluded from this study. Women with endometriosis and/or adrenal patho-physiology (e.g. adrenal or ovarian cancers); female/male partners that are hypertensive, diabetic or habitual to smoking and/or alcohol; male partners with oligo/astheno/terato-zoospermia; all confounding factors known to potentially affect fertilisation and embryo quality were excluded from the study. From among the patient population recruited, women whose baseline DHEAS levels did not revert to the optimum post-treatment, were not included in the present study (please refer to “Classification into subgroups as per clinical treatment” under “Study design” regarding details of individual study subgroups A2 and C2). All females were frequency matched; embryo culture conditions were maintained throughout and embryo transfer was performed using same technique over the entire study duration. Written informed consent was obtained from all couples for participation in the study. Study protocol was approved by our local hospital Review Board and Ethics Committee.
Sample collection for analysis
On day 2/3 of the menstrual cycle, fasting blood sample was drawn by venipuncture and centrifuged to obtain serum from all females recruited for the study. Radio-immunoassay-based diagnostic kits supplied by Beckman Coulter were used to measure baseline serum DHEAS (Immunotech IM0729, analytical/functional sensitivity 2.64 μg/dL, intra-assay and inter-assay coefficient of variation ≤ 4.93% and ≤ 9.32% respectively) and Estradiol E2 (Immunotech-A21854, analytical/functional sensitivity 9.58/13.11 pg/ml, intra-assay and inter-assay coefficient of variations 14.4% and 14.5% respectively) in serum on day of injection-hCG administration as well as in follicular fluid. Anti-mullerian hormone (AMH) in baseline serum was measured by ELISA method (Ansh Labs AL-105, analytical measure range 0.08–14.2 ng/ml; sensitivity 23 pg/ml). Antral follicle count (AFC) was recorded at baseline scan by ultrasonography. DHEAS levels were also measured in follicular fluid of each woman, obtained on day of oocyte pick-up (OPU).
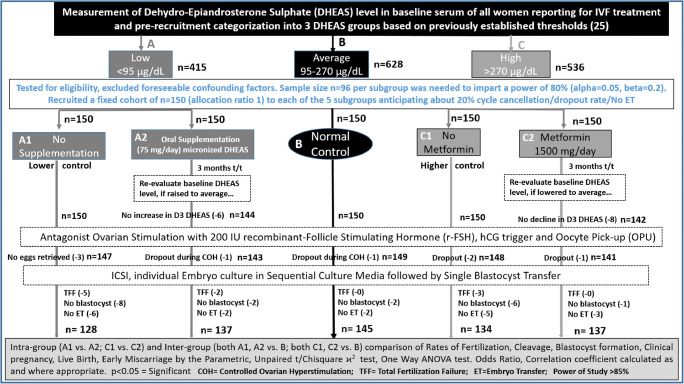
Study design (Fig. 1)
Fig. 1.
Study design flowchart for closed cohort study evaluating impact on embryologic and clinical outcomes following clinical treatments
Sample size and power of study
Sample size n = 96 per study subgroup (allocation ratio 1) was required (ClinCalc.comhttps://clincalc.com/stats/samplesize.aspx) to generate a power of 80% (alpha 0.05, beta 0.2) to detect independent difference between means. We recruited a fixed cohort of n = 150 women per subgroup (overall sample-size n = 750) so that even after accounting for about 20% incidence of dropout/cycle cancellation/no embryo-transfer; a power > 80% would still be imparted to the study.
Pre-recruitment categorisation of women into D3-DHEAS groups
All women reporting for IVF treatment at our private fertility clinic undergo baseline D3 serum DHEAS measurement as a routine procedure of baseline endocrine profiling. Depending on D3-DHEAS levels, women are categorised into low (A: < 95 μg/dl), average (B: 95–270 μg/dl) and high (C: > 270 μg/dl) DHEAS groups in lieu with our previous study [25] where three groups were formed based on 25th and 75th centiles of measurement. Women in each of these groups were evaluated for eligibility criteria and, after excluding foreseeable confounding factors, were prospectively recruited as a fixed cohort (n = 150) in each of the five subgroups in our present study.
Classification into subgroups as per clinical treatment (Fig. 1)
A1 subgroup: No DHEAS supplementation (n = 150): Lower control subgroup.
A2 subgroup: Oral supplementation of DHEAS (n = 150): Women received 3 months oral supplementation of 75 mg/day micronised DHEAS [26]. D3-DHEAS level was re-evaluated after 3 months of treatment to check if there was a rise in levels to optimal average. For this study, only those women whose DHEAS levels increased to average post-treatment were considered for stimulation protocol and outcome evaluation.
B group: (n = 150) Normal control group: This group comprised of women with optimal levels of baseline DHEAS, which as per our previous study [25], had demonstrated standard optimal rates of fertilisation, embryo development and live- birth. Thus, it constituted our ‘normal control group’ against which the low and high DHEAS group results were inter-compared.
C1 Subgroup: No metformin treatment (n = 150): Higher control subgroup.
C2 Subgroup: Metformin treatment (n = 150): This subgroup women received 1500 mg/day (500 mg TDS) oral metformin treatment for 3 months [27]. Metformin treatment was offered to this subgroup women in agreement with a report suggesting that metformin significantly lowers DHEAS as well as insulin levels in PCOS women with raised baseline DHEAS whereas it apparently has no effect on DHEAS levels in women with normal baseline DHEAS concentrations [27]. D3-DHEAS level was re-evaluated post-treatment to check if there was a drop in the levels to optimal average. Only those women, whose DHEAS reduced to average levels post-treatment, were considered for stimulation protocol and outcome evaluation in this study.
Classification of Follicular-Fluid (FF) DHEAS groups
All cycles were also classified into low (< 760 ng/ml), medium (760–2100 ng/ml) and high (>2100 ng/ml) FF-DHEAS groups as per 25th and 75th centile of FF DHEAS levels, for inter-comparison with serum groups.
Stimulation protocol and OPU
Standard antagonist ovarian stimulation with 200 IU recombinant FSH (Gonal F, Merck-Serono, India) starting from day 2 and daily administration of antagonist (cetrorelix acetate 0.25 mg) from day 6 until the day of injection hCG trigger (Ovitrelle 250 mcg, Merck, India) when ≥ 2 follicles reached a size of 18 mm. Transvaginal ultrasound–guided oocyte pick-up (OPU) was done under patient sedation between 34 and 36 h after hCG administration. Micronised progesterone (Injection Susten 100 mg daily) was provided as luteal phase support starting from day OPU until day14 of embryo transfer (ET). Luteal phase support was continued until 12-week gestation if βhCG tested positive and discontinued if βhCG was negative on day 14 ET.
Sperm preparation
Sperm sample was prepared by swim-up/density gradient centrifugation method. Only normozoospermic males with semen analysis and sperm function parameters within normal range as per the World Health Organization (WHO 2010) criteria were recruited in the study to rule out any influence on oocyte activation due to sperm factor/contribution of defective PLC-ζ. An ICSI-all protocol was followed because our patient cohort included women with idiopathic and/or unexplained infertility, poor oocyte yield and previous no/poor fertilisation cycles with conventional insemination. ICSI was also done to ensure entry of single sperm and to rule out polyspermia; a factor that would otherwise influence fertilisation and embryo development rates.
Embryo culture
Sequential media (Cooks, Sydney IVF, Australia) and individual culture in 30-μl microdrops under mineral oil overlay method were adopted for embryo culture. Embryos were cultured at 37 °C temperature in fertilisation media from day/OPU to zygote stage, cleavage media for culture until day 3 and finally in blastocyst media until day 5 blastocyst formation, in a bench-top incubator with triple-gas mixture (5% O2, 6% CO2 and 89% N2). Fixed time assessment of embryo development was done daily until day/ET and micrographic images of the same were stored. Record of fertilisation, cleavage, blastocyst formation rates and embryo grades were maintained.
Embryo transfer
All women underwent trans-cervical single blastocyst transfer using Cook’s soft-tipped ET catheter. Record of dropout rates and cycle cancellation was maintained. β-hCG > 20 mIU/ml on day14/ET was considered indicative of pregnancy. Presence of gestational sac with cardiac activity on ultrasound at 8th week of gestation confirmed clinical pregnancy. Record of clinical pregnancy, early pregnancy loss and live birth rates was maintained. Excess blastocysts, if available, were cryopreserved.
Data analysis
Intra-group (A1 vs. A2 and C1 vs. C2) and inter-group (both A1/A2 independently vs. B and both C1/C2 independently vs. B; low/medium/high groups) comparisons in serum and FF for differences in rates of fertilisation, cleavage, blastocyst formation, clinical pregnancy, live-birth and early miscarriages were done by the parametric, unpaired Student’s t test/chi-square χ2 and one-way ANOVA statistical analysis tests assuming a normal data distribution with same variances. Odds ratio and correlation coefficients were calculated as appropriate, using Graph Pad Prism VI software. p < 0.05 was considered statistically significant. Values expressed as mean ± SD.
Results
Our earlier studies [25, 28], involving measurement of DHEAS in FF and D3 serum, had reported significantly higher incidences of fertilisation and embryo development in the average group. DHEAS levels correlated with the number of mature MII oocytes (Pearson r = 0.41) and fertilisation rates (Pearson r = 0.52). In the present study, we have explored the possible involvement of DHEAS in the oocyte activation process.
An intra-group comparison of results between the subgroups of low DHEAS (A) group showed that the DHEAS supplementation A2 subgroup (n = 137) women whose DHEAS levels increased to optimal/average after 3-month oral DHEAS supplementation had significantly improved embryologic and outcome parameters compared to no-supplementation A1 subgroup (n = 128) (Table 2a) despite being matched (n > 0.05) for all other patient characteristics (Table 1). Our study also reports a concomitant significant rise in FF-E2 levels post-DHEAS exposure in A2 subgroup compared to no-exposure A1 subgroup (FF E2: 179842 ± 4136 vs. 100,037 ± 7420 pg/ml; p = 0.002).
Table 2.
Intra-comparison of data between subgroups of Low (A) and high (C) baseline serum DHEAS groups. (2a) A2 vs. A1 and (2b) C2 vs. C1
| Parameters | 2a | 2b | ||
|---|---|---|---|---|
| Oral DHEAS supplementation subgroup A2 (n = 137) | No DHEAS supplementation lower control subgroup A1 (n = 128) | Metformin treatment subgroup C2 (n = 137) | No metformin treatment higher control subgroup C1 (n = 134) | |
| Mean eggs retrieved | 7.76 ± 3.0 | 4.83 ± 2.0a | 22.62 ± 5.4 | 20.59 ± 5.6d |
| Fertilisation, % | 79.83 ± 3.3 | 55.68 ± 3.14a | 79.04 ± 2.9 | 53.04 ± 2.9a |
| Cleavage, % | 78.5 ± 2.86 | 53.39 ± 3.16a | 76.24 ± 4.01 | 52.09 ± 4.9a |
| Blastocyst formation, % | 42.61 ± 5.14 | 22.26 ± 3.09a | 40.58 ± 4.3 | 20.17 ± 3.04a |
| Live birth (n), % | (51) 37.23 ± 4.8 | (21) 16.41 ± 3.7b | (48) 35.04 ± 4.9 | (20) 14.93 ± 3.5b |
| Early miscarriage, % | 3.65 ± 0.19 | 10.16 ± 0.3c | 3.65 ± 0.19 | 9.7 ± 0.3e |
Values are mean ± SD (standard deviation) p < 0.05 = significant
ap < 0.0001; bp = 0.0002; cp = 0.035; dp = 0.0025; ep = 0.04
Our results in the subgroups of the high DHEAS (C) group demonstrated that the metformin-treated C2 subgroup of women (n = 137), whose DHEAS levels reduced to optimal/average after 3-month clinical treatment with metformin, displayed significantly enhanced embryologic and clinical outcome parameters compared to no metformin-treated C1 subgroup (n = 134) (Table 2b) inspite of comparable, non-significant differences in all other patient characteristics (Table 1).
We also report an overall rise in the odds of fertilisation (A1 vs. A2: percent rise: 43%, odds ratio 0.32, 95% CI: 0.25–0.39, χ2 p < 0.0001; C1 vs. C2: percent rise: 49%, odds ratio 0.3, 95% CI: 0.27–0.33, χ2 p < 0.0001) and live birth (A1 vs. A2: percent rise: > 100%, odds ratio 0.33, 95% CI: 0.18–0.59, χ2 p = 0.0001; C1 vs. C2: percent rise: > 100%, odds ratio 0.32, 95% CI: 0.18–0.58, χ2 p = 0.0001) in the post-treatment subgroups compared to the untreated control groups.
Furthermore, an inter-group comparison of results showed that, whereas the post-treatment improvement in parameters in both A2 and C2 were comparable (χ2 p > 0.05) with those observed in the normal control B group (n = 145); the untreated subgroups A1 and C1 displayed significantly lower values of assessment parameters compared to average B group (Table 3). Moreover, few women, whose DHEAS levels had not become ‘normal’ post-3-month treatment and therefore were not included in present study, had undergone OPU and ET. These women presented with a marginal but statistically non-significant improvement in all outcome parameters indicating that requisite impetus for oocyte activation was probably still missing.
Table 3.
Inter-comparison of data between the three baseline serum study groups
| Parameter | B (normal control n = 145) | B vs. A2 χ2 p value |
B vs. C2 χ2 p value |
B vs. A1 χ2 p value |
B vs. C1 χ2 p value |
|---|---|---|---|---|---|
| Fertilisation, % | 81.94 ± 6.6 | 0.68 | 0.47 | < 0.0001 | < 0.0001 |
| Cleavage, % | 81.62 ± 7.36 | 0.52 | 0.17 | < 0.0001 | < 0.0001 |
| Blastocyst formation, % | 45.61 ± 7.01 | 0.36 | 0.05 | <0.0001 | < 0.0001 |
| Live birth (n, )% | (58) 40.00 ± 4.9 | 0.75 | 0.56 | < 0.0001 | < 0.0001 |
| Early miscarriage, % | 2.76 ± 0.16 | 0.68 | 0.68 | 0.011 | 0.015 |
Table 4 depicts the semen parameters of all the normozoospermic males included in our study. There was no statistically significant difference observed among all compared subgroups.
Table 4.
Comparison of semen parameters between subgroups of low (A), medium (B) and high (C) baseline serum DHEAS groups
| All samples were collected by masturbation and allowed to liquefy at 370 C for 20 min before examination | |||||
|---|---|---|---|---|---|
| Parameters | Subgroup A2 (n = 137) | Subgroup A1 (n = 128) | Normal control group B (n = 145) | Subgroup C2 (n = 137) | Subgroup C1 (n = 134) |
| Patient age | 36.50 ± 3.2 | 37.22 ± 3.9 | 35.68 ± 2.7 | 37.12 ± 2.5 | 36.92 ± 3.1 |
| Semen volume | 2.5 ± 0.08 | 2.8 ± 0.05 | 3.0 ± 0.1 | 2.9 ± 0.25 | 2.7 ± 0.15 |
| Sperm concentration (× 106/ml) | 37 ± 8.5 | 43 ± 6.2 | 46 ± 10.1 | 38 ± 12.4 | 40 ± 5.6 |
| Total motility, % | 78 ± 3.0 | 83 ± 3.5 | 87 ± 2.5 | 81 ± 2.8 | 85 ± 3.2 |
| Progressive motility, % | 45 ± 4 | 53 ± 6 | 58 ± 4 | 62 ± 5 | 52 ± 9 |
| Morphology % normal (Kruger’s strict criteria) | 9.2 ± 2.3 | 8.1 ± 3.8 | 12 ± 2.6 | 9.8 ± 3.0 | 11 ± 1.9 |
All parameters, except morphology, evaluated by the WHO (2010) criteria
Values are mean ± SD (standard deviation)
All intra-group/inter-group comparisons: p > 0.05 = non-significant
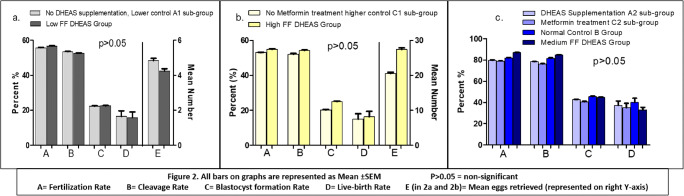
Finally, to evaluate and validate the effects of altered baseline serum DHEAS levels on the follicular microenvironment, we estimated DHEAS levels in FF of all women in this study. An inter-comparison of embryologic and clinical parameters in the low (< 760 ng/ml, n = 133), medium (760–2100 ng/ml, n = 407) and high (> 2100 ng/ml, n = 141) FF-DHEAS groups displayed significant differences in the evaluated parameters (Table 5). Although the overall difference (one-way ANOVA) between the three groups was statistically significant, it was observed that the low vs. high group had comparable results (p > 0.05) except for mean number of eggs retrieved and FF-E2 levels which, understandably, differed significantly. However, both these groups had significantly lower values compared to medium FF-DHEAS group. Moreover, a remarkable analogous result between the serum and FF groups was discernible. As depicted in Fig. 2, the parameters in A1 serum subgroup were similar to low FF group (Fig. 2a), C1 comparable with high FF group (Fig. 2b) and those in A2, C2 and B serum subgroups corroborated with medium FF group (Fig. 2c) (p > 0.05). As with serum DHEAS levels, the follicular fluid DHEAS levels strongly correlated with fertilisation rates (Pearson r = 0.78).
Table 5.
Inter-comparison of data between low, medium and high follicular fluid (FF) DHEAS groups
| Parameter | Low FF DHEAS < 760 ng/ml (n = 133) | Medium FF DHEAS 760–2100 ng/ml (n = 407) | High FF DHEAS > 2100 ng/ml (n = 141) | One-way ANOVA (Kruskal-Wallis test) p value |
|---|---|---|---|---|
| Age (years) | 33.96 ± 2.9 | 32.53 ± 2.1 | 32.81 ± 3.0 | 0.091 |
| day of hCG E2 (pg/ml) | 780 ± 26.31 | 2250 ± 75.43 | 5500 ± 77.58 | 0.029* |
| FF E2 (pg/ml) | 78,250 ± 5525 | 114,700 ± 4870 | 181,380 ± 9950 | 0.0002 |
| Mean no. of eggs retrieved | 4.2 ± 1.98 | 12.5 ± 2.7 | 27.4 ± 3.1 | 0.001* |
| Eggs fertilised, % | 56.67 ± 2.9 | 86.84 ± 6.0 | 54.97 ± 3.7 | < 0.0001 |
| Embryos cleaved, % | 52.58 ± 2.4 | 84.57 ± 6.2 | 54.29 ± 3.5 | < 0.0001 |
| Blastocysts formed, % | 22.38 ± 3.17 | 45.51 ± 7.6 | 24.97 ± 4.2 | < 0.0001 |
| Live birth (n), % |
(21) 15.79 ± 3.66 |
(154) 37.84 ± 4.8 |
(23) 16.31 ± 3.7 |
< 0.0001 |
Fig. 2.
Inter-comparison of parameters between baseline serum DHEAS groups and follicular fluid DHEAS groups. a Lower control (A1) serum DHEAS subgroup vs. lower FF DHEAS group. b Higher control (C1) serum DHEAS subgroup vs. higher FF DHEAS group. c Treatment subgroups (A2 and C2) and normal control (B) serum DHEAS groups vs. medium FF DHEAS group
Discussion
Our results in the low serum DHEAS group apparently corroborate with those of previous studies reporting beneficial effects of DHEAS supplementation [26, 29–31]. However, it may be noted that those studies involved DHEAS supplementation to women with poor ovarian response to gonadotropin stimulation, e.g. women with advanced reproductive age, DOR and POF, whereas in our study, DHEAS was supplemented to women with low baseline DHEAS levels, irrespective of whether they were poor responders or not. In fact, we observed that not all poor responder females had low levels of baseline DHEAS. On the contrary, few elderly PCOS women who had low baseline DHEAS also benefitted from clinical treatment with DHEAS. Thus, it is relevant to emphasise that whereas empirical DHEAS supplementation to all poor responders is ill advised, it may actually be advisable to supplement DHEAS to few elderly PCOS women with low baseline levels. Obviously therefore, our study is NOT about, nor restricted to, DHEAS supplementation to poor responders.
Our focus was to evaluate the probable involvement of DHEAS in influencing calcium oscillations and oocyte activation. It may be argued that lower DHEAS levels might cause insufficient release of calcium from the internal mitochondrial stores to elicit the requisite spikes for optimal calcium oscillation. Our basic premise was based on a pioneering study [17] which concluded that DHEAS, rather than DHEA, plays a significant role in ovarian follicular steroidogenesis. It has been reckoned that DHEAS is not only a significant, active precursor for oestrogen production, but the formation of oestradiol and androstenedione is also sensitive to the dose of DHEAS supplemented [15]. In lieu with this, a rise in FF E2 levels as reported in our study therefore cannot completely rule out the possibility that DHEAS, by virtue of oestrogen production, may aid in the generation of Ca2+ waves via its receptors and/or transporters.
Our findings in the high serum DHEAS group are also remarkable. As already mentioned earlier, differential levels of baseline DHEAS influence the effect of metformin and that metformin treatment lowers high DHEAS levels observed in PCOS women [27]. Lowering of DHEAS levels post-metformin treatment in C2 subgroup in our study thus substantiates those findings. At the same time, we interestingly observed that not all hyper-responder/PCOS women displayed high DHEAS levels or hampered fertilisation and embryo development rates. On the contrary, our normal-control group B women included both the so-called poor responders (women with advanced age/DOR/POF) and hyper-responders (women with PCOS), with normal levels of DHEAS yielding good-quality oocytes, optimal rates of fertilisation, embryo development to blastocyst stage and successful live births. Therefore, we are justified in our classification of cycles based on DHEAS levels rather than on the responder status and in ascribing an additional presumptive role to DHEAS in oocyte activation.
Attempts have earlier been made to evaluate role of higher DHEAS in women with polycystic ovary syndrome (PCOS) [32]. Whereas one study [33] linked higher than threshold levels of DHEAS with reduced stimulatory effect; another study [32] measuring DHEAS in cumulus cells reckoned that increased incidences of degenerated oocytes and early miscarriage rates in PCOS women can be attributed to adverse effects of higher DHEAS in these women. In mouse oocytes, very high DHEAS levels have been speculated to result in single sharp spikes of Ca2+ rather than an expected Ca2+ steady wave pattern and can cause oocyte fragmentation and apoptosis [34]. Undoubtedly, even in our study, we observed significantly higher incidences of poor grade embryos in the untreated C1 compared to the metformin-treated C2 subgroup (26.2 ± 2.8 vs. 11.7 ± 1.9%; p = 0.005). However, ours is the only study that reports improvement in all evaluated parameters post-metformin treatment, i.e. after reduction of high DHEAS levels to a ‘normal’ range, as in the C2 subgroup. Can we therefore attribute the improvement in parameters to the correction in calcium spikes and oscillations as a result of stabilization in DHEAS levels? Of course, at this stage, our results may be considered only indicative and more focused studies are warranted to explore and establish such a correlation.
Interestingly, the lower incidences of early miscarriages observed in our treatment subgroups A2 and C2 may also be attributed to the rectification in DHEAS levels, in conformity with a study [19] that reports an association between DHEAS concentration, mitochondrial enzyme activity and miscarriage rates. These results further consolidate our proposition that both clinical treatments, i.e. DHEAS supplementation and metformin treatment, not only facilitate a rectification of irregular DHEAS levels but also subsequently manifest in the form of overall improvement in embryologic and clinical parameters. Thus, from our overall statistically significant and strongly suggestive results, it may be appropriate to safely presume that DHEAS exerts its effects by influencing the calcium oscillation pattern. Similarly, an inter-comparison of results in the follicular fluid samples further substantiates our postulation that DHEAS levels probably influence the follicular micro-environment, thereby affecting oocyte and embryo development. Whether DHEAS conceivably does this by regulating the calcium oscillation pattern remains to be consolidated by more studies.
At the moment, it may seem farfetched to presume that DHEAS regulates the Ca2+ transport across membranes to maintain homeostasis and induce oocyte activation. However, it was necessary to verify our concept with basic studies before employing oocyte-destructive methods for directly measuring calcium oscillations. Theoretically, it does seem quite credible because DHEAS is among the steroids that bimodally regulates the PMCA and SERCA in human erythrocytes and neuronal cells by modulating the ATP-dependent transport across membranes [24, 33]. As already mentioned earlier, these pumps (PMCA and SERCA) are involved in maintaining calcium oscillations in mouse oocytes [5, 35]. Therefore, we reiterate our claim that DHEAS is the most probable candidate which may correlate with maintenance of calcium homeostasis via regulation of calcium pumps for oocyte activation.
Having said that, we do accept that the greatest limitation of our study is that instead of directly measuring Ca2+oscillations to evaluate OA, we indirectly assessed embryologic and clinical outcome parameters among women with differential baseline DHEAS levels. However, ethical considerations currently prevent the use of oocyte-destructive methods. Also, measuring Ca2+oscillations in mice or any other mammalian species is impractical as results cannot be extrapolated to humans. Nevertheless, our future aim is to measure Ca2+oscillations directly in donated human oocytes from consenting couples.
Conclusion
Our results demonstrate the adverse impacts of irregular (lower as well as higher than threshold) DHEAS levels on embryologic and clinical outcomes. Our findings also establish that post-treatment rectification in DHEAS levels causes significant improvement in parameters at par with those exhibited by normal DHEAS thresholds. These robust results impart substantial degree of internal and external validity to our study. Thus, DHEAS apparently is the most potent, innate oocyte-related factor that affects embryologic and clinical IVF outcomes possibly by influencing calcium oscillations and OA. This study thus sets new paradigms by introducing DHEAS as a potential non-invasive, endogenous and scientifically more pragmatic oocyte-related factor in OA.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
5/4/2021
A Correction to this paper has been published: 10.1007/s10815-021-02215-z
Contributor Information
Bindu N. Chimote, Email: bindunm10@yahoo.com
Natachandra M. Chimote, Email: nmchimote@yahoo.co.in
References
- 1.Balaban B, Urman B. Effect of oocyte morphology on embryo development and implantation. Reprod BioMed Online. 2006;12(Suppl 5):608–615. doi: 10.1016/S1472-6483(10)61187-X. [DOI] [PubMed] [Google Scholar]
- 2.Tripathi A, Kumar KV, Chaube SK. Meiotic cell cycle arrest in mammalian oocytes. J Cell Physiol. 2010;223(Suppl 3):592–600. doi: 10.1002/jcp.22108. [DOI] [PubMed] [Google Scholar]
- 3.Ducibella T, Huneau D, Angelichio E, Xu Z, Schultz RM, Kopf GS, Fissore R, Maoux S, Ozil JP. Egg-to-embryo transition is driven by differential responses to Ca(2+) oscillation number. Dev Biol. 2002;250(Suppl 2):280–291. doi: 10.1006/dbio.2002.0788. [DOI] [PubMed] [Google Scholar]
- 4.Ozil JP, Banrezes B, Toth S, Pan H, Schultz RM. Ca2+ oscillatory pattern in fertilized mouse eggs affects gene expression and development to term. Dev Biol. 2006;300(Suppl 2):534–544. doi: 10.1016/j.ydbio.2006.08.041. [DOI] [PubMed] [Google Scholar]
- 5.Wakai T, Fissore RA. Ca(2+) homeostasis and regulation of ER Ca(2+) in mammalian oocytes/eggs. Cell Calcium. 2013;53(Suppl 1):63–67. doi: 10.1016/j.ceca.2012.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen J, Qian Y, Tan Y, Mima H. Successful pregnancy following oocyte activation by strontium in normozoospermic patients of unexplained infertility with fertilization failure during previous intracytoplasmic sperm injection treatment. Reprod Fertil Dev. 2010;22(Suppl 5):852–855. doi: 10.1071/RD09268. [DOI] [PubMed] [Google Scholar]
- 7.Nomikos M. Novel signaling mechanism and clinical applications of sperm specific PLCζ. Biochem Soc Trans. 2015;43(Suppl 3):371–376. doi: 10.1042/BST20140291. [DOI] [PubMed] [Google Scholar]
- 8.Amdani SN, Yeste M, Jones C, Coward K. Phospholipase C zeta (PLCζ) and male infertility: clinical update and topical developments. Adv Biol Regul. 2016;61:58–67. doi: 10.1016/j.jbior.2015.11.009. [DOI] [PubMed] [Google Scholar]
- 9.Human Fertilization and Embryology Authority (HFEA) Treatment Add ons: available at: https://www.hfea.gov.uk/treatments/explore-all-treatments/treatment-add-ons/. Accessed 10 July 2020
- 10.Santella L, Dale B. Assisted yes, but where do we draw the line? Reprod BioMed Online. 2015;31(Suppl 4):476–478. doi: 10.1016/j.rbmo.2015.06.013. [DOI] [PubMed] [Google Scholar]
- 11.Bos-Mikich A, Bressan FF, Ruggeri RR, Watanabe Y, Meirelles FV. Parthenogenesis and human assisted reproduction. Stem Cells Int. 2015;2016:1–8. doi: 10.1155/2016/1970843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neri QV, Lee B, Rosenwaks Z, Machaca K, Palermo GD. Understanding fertilization through intracytoplasmic sperm injection (ICSI) Cell Calcium. 2014;55(Suppl 1):24–37. doi: 10.1016/j.ceca.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hachem A, Godwin J, Ruas M, Lee HC, Buitrago MF, Ardestani G, Bassett A, Fox S, Navarete F, de Sutter P, Heindryckx B, Fissore R, Parrington J. PLCζ is the physiological trigger of the Ca2+ oscillations that induce embryogenesis in mammals but offspring can be conceived in its absence. Development. 2017;144(Suppl 16):2914–2924. doi: 10.1242/dev.150227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferrer-Buitrago M, Dhaenens L, Lu Y, Bonte D, Meerschaut FV, de Sutter P, Leybaert L, Heindryckx B. Human oocyte calcium analysis predicts the response to assisted oocyte activation in patients experiencing fertilization failure after ICSI. Hum Reprod. 2018;33(Suppl 3):416–425. doi: 10.1093/humrep/dex376. [DOI] [PubMed] [Google Scholar]
- 15.Bonser J, Walker J, Purohit A, Reed MJ, Potter BV, Willis DS, Franks S, Mason HD. Human granulosa cells are a site of sulphatase activity and are able to utilize dehydroepiandrosterone sulphate as a precursor for oestradiol production. J Endocrinol. 2000;167(Suppl 3):465–471. doi: 10.1677/joe.0.1670465. [DOI] [PubMed] [Google Scholar]
- 16.Virant-Klun I, Skutela T, Kubista M, Vogler A, Sinkovec J, Meden-Vrtovec H. Expression of pluripotency and oocyte related genes in single putative stem cells from human adult ovarian surface epithelium cultured in vitro in the presence of follicular fluid. Biomed Res Int. 2013;2013:86148–86166. doi: 10.1155/2013/861460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neunzig J, Bernhardt R. Dehydroepiandrosterone sulfate (DHEAS) stimulates the first step in the biosynthesis of steroid hormones. PLoS One. 2014;92:e89727. doi: 10.1371/journal.pone.0089727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maninger N, Capitanio JP, Mason WA, Ruys JD, Mendoza SP. Acute and chronic stress increase DHEAS concentrations in rhesus monkeys. Psychoneuroendocrinology. 2010;35(Suppl 7):1055–1062. doi: 10.1016/j.psyneuen.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ford JH. Reduced quality and accelerated follicle loss with female reproductive ageing-does decline in theca dehydroepiandrosterone (DHEA) underlie the problem? J Biomed Sci. 2013;20 Suppl 93:9. doi: 10.1186/1423-0127-20-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu D, Dillon JS. Dehydroepiandrosterone activates endothelial cell nitric-oxide synthase by a specific plasma membrane receptor coupled to Gαi2,3. J Biol Chem. 2002;277:21379–21388. doi: 10.1074/jbc.M200491200. [DOI] [PubMed] [Google Scholar]
- 21.Chen F, Knecht K, Birzin E, Fisher J, Wilkinson H, Mojena M, Moreno CT, Schmidt A, Harada SI, Freedman LP, Reszka AA. Direct agonist/antagonist functions of dehydroepiandrosterone. Endocrinology. 2005;146(Suppl 11):4568–4576. doi: 10.1210/en.2005-0368. [DOI] [PubMed] [Google Scholar]
- 22.Geyer J, Godoy JR, Petzinger E. Identification of a sodium-dependent organic anion transporter from rat adrenal gland. Biochem Biophys Res Commun. 2004;316(Suppl 2):300–306. doi: 10.1016/j.bbrc.2004.02.048. [DOI] [PubMed] [Google Scholar]
- 23.Webb SJ, Geoghegan TE, Prough RA, Michael Miller KK. The biological actions of dehydroepiandrosterone involves multiple receptor. Drug Metab Rev. 2006;38(Suppl 1–2):89–116. doi: 10.1080/03602530600569877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zylinska L, Kowalska I, Ferene B. Calmodulin effects on steroids-regulated plasma membrane calcium pump activity. Cell Biochem Funct. 2009;27(Suppl 2):111–117. doi: 10.1002/cbf.1543. [DOI] [PubMed] [Google Scholar]
- 25.Chimote BN, Nath N, Chimote NM. Dehydroepiandrosterone sulphate (DHEAs) supplementation: a promising non-invasive futuristic strategy for oocyte activation in IVF cycles. Hum Reprod. 2017;32(Suppl 1):i190. [Google Scholar]
- 26.Fu J, Jiang HF, Li L, Xin AJ, Sun YJ, Sun XX. Effects of dehydroepiandrosterone on embryo quality and follicular-fluid markers in patients with diminished ovarian reserves. Reprod Dev Med. 2017;1((Suppl) 1):1–8. doi: 10.4103/2096-2924.210696. [DOI] [Google Scholar]
- 27.Kolodziejczyk B, Duleba AJ, Spaczynski RZ, Pawelczyk L. Metformin therapy decreases hyperandrogenism and hyperinsulinemia in women with polycystic ovary syndrome. Fertil Steril. 2000;73(Suppl 6):1149–1154. doi: 10.1016/S0015-0282(00)00501-X. [DOI] [PubMed] [Google Scholar]
- 28.Chimote NM, Nath NM, Chimote NN, Chimote NM. Follicular fluid dehydroepiandrosterone sulphate is a credible marker of oocyte maturity and pregnancy outcome in conventional in-vitro fertilization cycles. J Hum Reprod Sci. 2015;8 Suppl 4:209–213. doi: 10.4103/0974-1208.170397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin LT, Wang PH, Wen ZH, Li CJ, Chen SN, Tsai EM, Cheng JT, Tsui KH. The application of dehydroepiandrosterone on improving mitochondrial function and reducing apoptosis of cumulus cells in poor ovarian responders. Int J Med Sci. 2017;14(Suppl 6):585–594. doi: 10.7150/ijms.18706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gleicher N, Ryan E, Weghofer A, Blanco-Mejia S, Barad DH. Miscarriage rates after dehydro-epiandrosterone supplementation in women with diminished ovarian reserve: a case-control study. Reprod Biol Endocrinol. 2009;7:108. doi: 10.1186/1477-7827-7-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sciard C, Berthiller J, Brosse A, Bartra NR, Hadj S, Bordes A, Du Mesnildot P, Lornage J, Lejeune H, Plotton I, Salle B. Preliminary results of DHEA in poor responders in IVF. Open J Obstet Gynecol. 2016;6:396–403. doi: 10.4236/ojog.2016.67052. [DOI] [Google Scholar]
- 32.Jimenez PT, Frolova AI, Chi MM, Grindler NM, Willcockson R, Reynolds KA, Zhao Q, Moley KH. DHEA-mediated inhibition of the pentose-phosphate pathway alters oocyte lipid metabolism in mice. Endocrinology. 2013;154(Suppl 12):4835–4844. doi: 10.1210/en.2012-2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zylinska L, Kowalska I, Kozaczuk A. Fast action of neuroactive steroids on plasma membrane calcium pump in PC12 cells. Ann N Y Acad Sci. 2008;1148:515–519. doi: 10.1196/annals.1410.077. [DOI] [PubMed] [Google Scholar]
- 34.Ozil JP, Markoulaki S, Toth S, Matson S, Banrezes B, Knott JG, Schultz RM, Huneau D, Ducibella T. Egg activation events are regulated by the duration of a sustained [Ca2+]cyt signal in the mouse. Dev Biol. 2005;282(Suppl 1):39–54. doi: 10.1016/j.ydbio.2005.02.035. [DOI] [PubMed] [Google Scholar]
- 35.Wakai T, Zang N, Vangheluwe P, Fissore RA. Regulation of endoplasmic reticulum Ca2+ oscillations in mammalian eggs. J Cell Sci. 2013;126(Suppl 24):5714–5724. doi: 10.1242/jcs.136549. [DOI] [PMC free article] [PubMed] [Google Scholar]