Abstract
Purpose
To identify disease-causing genes involved in female infertility.
Methods
Whole-exome sequencing and Sanger DNA sequencing were used to identify the mutations in disease-causing genes. We performed subcellular protein localization, western immunoblotting analysis, and co-immunoprecipitation analysis to evaluate the effects of the mutation.
Results
We investigated 17 families with female infertility. Whole-exome and Sanger DNA sequencing were used to characterize the disease gene in the patients, and we identified a novel heterozygous mutation (p.Ser173Cys, c.518C > G) in the ZP3 gene in a patient with empty follicle syndrome. When we performed co-immunoprecipitation analysis, we found that the S173C mutation affected interactions between ZP3 and ZP2.
Conclusions
We identified a novel mutation in the ZP3 gene in a Chinese family with female infertility. Our findings thus expand the mutational and phenotypical spectrum of the ZP3 gene, and they will be helpful in precisely diagnosing this aspect of female infertility.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10815-020-01995-0.
Keywords: Female infertility, Empty follicle syndrome (EFS), Zona pellucida (ZP), Mutation
Introduction
In mammals (including humans), the zona pellucida (ZP) is an extracellular glycoprotein coat that surrounds the oocyte [1], and is vital for oocyte development [2] and the protection of early embryos prior to implantation [3]. During fertilization, the ZP plays a critical role in sperm recognition, induction of the acrosome reaction, and prevention of polyspermy [4]. Mammalian ZP glycoproteins contain an N-terminal signal peptide, a ZP domain, a C-terminal peptide with a consensus furin cleavage site (CFCS), and a transmembrane domain (TMD) [1]. ZP glycoproteins are synthesized in the oocyte, and the proteins are secreted extracellularly after the C-terminal CFCS is cleaved by proprotein convertase. The mature ZP glycoproteins are then assembled into a fiber network [5].
There are three types of ZP glycoproteins (ZP1, ZP2, and ZP3) in mice and four types (ZP1, ZP2, ZP3, and ZP4) in humans. In a ZP1 knock-out mouse model, the zona pellucida was more loosely organized than in normal oocytes. Although ZP1-null female mice were fertile, their fertility was below that of normal controls [6]. In humans, Huang et al. reported that a homozygous frameshift mutation in ZP1 resulted in oocyte maturation defect-1 (OOMD1, 615774) [7]. Mutations in ZP2 also caused female infertility in both mice and humans due to a defective oocyte zona pellucida [8–10]. ZP3 defects were reported to lead to the loss of the ZP (ZP-free) and female infertility in both mice and humans [11, 12]. Chen et al. in 2017 first reported a heterozygous missense mutation in ZP3 caused empty follicle syndrome (EFS) and female infertility [13].
Cumulus-oocyte complexes (COCs) consist of cumulus granulosa cells that surround a centrally located oocyte that is coated by the zona pellucida, and these complexes are routinely isolated from the follicular fluid. During in vitro fertilization (IVF), oocytes can be retrieved from mature ovarian follicles after ovarian stimulation. However, in some cases, follicular development is normal but no oocytes are collected which is referred to as empty follicle syndrome (EFS) [14]. EFS can be classified as “false” EFS and “genuine” EFS: “false” EFS is often caused by pharmacologic issues with the HCG used [15], while “genuine” EFS is known to be caused by genetic factors such as mutations in LHCGR and ZP3 [13, 16].
In the present study, we characterized a woman from a Chinese family with infertility. The patient was diagnosed as having “genuine” EFS, and we identified a novel mutation (p.Ser173Cys, c.518C > G) in her ZP3 gene, with her oocytes shown to be ZP-free. Co-immunoprecipitation analysis suggested that the S173C mutation affected interactions between ZP3 and ZP2, which may lead to abnormal assembly of the zona pellucida fiber network and result in EFS and ZP-free oocytes.
Materials and methods
Clinical samples
We recruited patients from the Reproductive Medicine Center, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, with all women from 17 families having been diagnosed with infertility and exhibiting an abnormal zona pellucida or empty follicle syndrome (Table 1). Male infertility in these families was excluded after semen analysis or in vitro fertilization (IVF). We extracted genomic DNA samples from all of the peripheral blood samples. Our studies involving human participants were in accordance with the ethical standards of the Ethics Committee on Human Subject Research at Huazhong University of Science and Technology, and all of the participants signed the informed consent form of this study.
Table 1.
Families with female infertility
| Family | Symptom | Gene |
|---|---|---|
| 1 | Empty follicle syndrome | ZP3 mutation |
| 2 | Empty follicle syndrome | Unknown |
| 3 | Abnormal zona pellucida | Unknown |
| 4 | Abnormal zona pellucida | Unknown |
| 5 | Thin zona pellucida | Unknown |
| 6 | Empty follicle syndrome | Unknown |
| 7 | Abnormal zona pellucida | Unknown |
| 8 | Abnormal zona pellucida | Unknown |
| 9 | Empty follicle syndrome | Unknown |
| 10 | Abnormal zona pellucida | Unknown |
| 11 | Abnormal zona pellucida | Unknown |
| 12 | Abnormal zona pellucida | Unknown |
| 13 | Abnormal zona pellucida | Unknown |
| 14 | Abnormal zona pellucida | Unknown |
| 15 | Abnormal zona pellucida | Unknown |
| 16 | Abnormal zona pellucida | ZP3 mutation |
| 17 | Abnormal zona pellucida | Unknown |
Family 1: ZP3 S173C heterozygous mutation
Family 16: ZP3 mutation, data need to be confirmed
Genetic studies
We performed whole-exome sequencing to identify the disease-causing mutations in the patients (details of the procedure are described in a previous paper) [17]. Genomic DNA was randomly fragmented by Covaris, adapters were ligated to both ends of the resulting fragments, and the adapter-ligated templates were purified by Agencourt AMPure SPRI beads. Extracted DNA was then amplified by ligation-mediated PCR (LM-PCR), purified, and hybridized to the Agilent SureSelect Human All Exon V4 library kit for exome enrichment. Captured LM-PCR products were subjected to an Agilent 2100 Bioanalyzer to estimate the magnitude of enrichment, and each captured library was then loaded onto a HiSeq2000 sequencer. We processed raw-image files using the Illumina base-calling software 1.7 for base calling with default parameters.
After performing quality control of the reads, we used variant annotation (our databases included NCBI CCDS, RefSeq, Ensembl, and Encode) and variant filtration (using dbSNP 135, the HapMap database [HapMap project phases 1, 2&3], 1000 Genome Project database, and the YH database) to narrow down the variants.
Polymerase chain reaction (PCR) was used to confirm the mutation. Our 50-μl reaction volume consisted of 20 ng of genomic DNA, 1 unit of Taq DNA polymerase, 5 μl of 10 × Taq buffer, 0.2 mM dNTP, 0.5 μM forward/ reverse primers, and H2O.
Bioinformatic analysis
We predicted the functional effects of the mutations with PolyPhen-2, SIFT, and the PROVEAN software. The structures of wild-type and S173C mutant ZP3 proteins were predicted by SWISS-MODEL based on homology-modeling (PDB ID: 3nk3.1.A), and the PYMOL software was used to analyze the wild-type and mutant ZP3 proteins structures. We used the DISULFIND software to analyze the disulfide bonds in the ZP domain of the ZP3 protein.
Construction of ZP3, ZP1, and ZP2 expression plasmids
The human wild-type and S173C mutant ZP3 cDNAs were constructed and inserted into a pEGFP-C1 or pCMV-MYC vector. The human full-length ZP1 and ZP2 cDNAs were constructed and inserted into a p3xFLAG-CMV10 and pEGFP-C1 vector, respectively. The ZP1, ZP2, and ZP3 expression plasmids were verified by enzyme digestion and DNA sequencing (the cDNAs for the human ZP1, ZP2, and ZP3 genes were gifts from the Wang Laboratory at Fudan University).
Cell culture and subcellular localization of ZP3 in HEK293T cells
HEK293T cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Hyclone, USA) supplemented with 10% fetal bovine serum (FBS) (Gibco, USA), in Glass Bottom Cell Culture Dishes (NEST, CHN), and transiently transfected with pEGFP-C1-WT ZP3 or pEGFP-C1-MUT ZP3 vector. Twenty-four hours after transfection, the cells were washed with PBS, fixed in 4% paraformaldehyde, and then again washed with PBS. We stained the nuclei with DAPI (BIOSHARP, CHN), and ultimately analyzed the cells under a confocal microscope (Olympus FV1000, JP).
Western immunoblotting analysis
Forty-eight hours after transfection, we extracted total proteins from HEK293T cells; lysed cells by RIPA lysis buffer (Beyotime, CHN); collected supernatants by centrifugation at 12000 g for 30 min; and then added 5 × loading buffer heating for 10 min at 95 °C. Total proteins were analyzed in 10% SDS-PAGE gels and then transferred to polyvinylidene difluoride (PVDF) membranes. The primary antibodies we used were directed against GFP (1:2000 dilution; ABclonal, Cat: AE012) and GAPDH (1:2000 dilution, ABclonal, Cat: AC033). The secondary antibody was HRP-conjugated goat anti-mouse IgG (1:10000 dilution; CWbio, Cat: 01325).
Co-immunoprecipitation
HEK293T cells were co-transfected with plasmids containing MYC-ZP3 (wild-type ZP3 or ZP3 S173C) and FLAG-ZP1 (p3xFLAG-CMV10-ZP1) or GFP-ZP2 (pEGFP-ZP2). After transfection for 48 h, cells were lysed for 20 min on a flip-shaking table at 4 °C using 1 ml of cell-lysis buffer (Beyotime). The supernatants were collected by centrifugation at 12000 g for 20 min and a 100-ul aliquot was removed as an input control; equal amounts of the remaining supernatants were then incubated with a primary antibody to Myc (1:100 dilution; Proteintech, Cat: 16286-1-AP) and IgG overnight at 4 °C. We transferred 40 μL of Protein A/G Magnetic Beads to a 1.5-mL tube containing the supernatants and resuspended them (MCE, Cat: HY-K0202). We then added 400 μL of binding/wash buffer (PBS + 0.5% Triton X-100) to the beads and gently mixed the solution. The tube was placed on a magnetic stand to collect the beads, and we discarded the supernatant, twice repeating this procedure. The protein-antibody complex was added to the tube with the beads and incubated for 4 h at 4 °C. We then discarded the supernatant, and we added 400 μL of binding/wash buffer, washing 4 times. Fifty μL of 1 × sample buffer was added and the mixture was heated for 10 min at 95 °C. We allowed magnetic separation to take place and collected the supernatant for western blotting (WB).
The proteins were analyzed in 8% SDS-PAGE gels. The primary antibodies were directed against GFP (1:2000 dilution; ABclonal, Cat: AE012), GAPDH (1:2000 dilution; ABclonal, Cat: AC033), FLAG (1:2000 dilution; ABclonal, Cat: AE005), and MYC (1:2000 dilution; ORIGENE, Lot: F005). The secondary antibody was HRP-conjugated goat anti-mouse IgG (1:10000 dilution; CWbio, Cat: 01325) and HRP-conjugated AffiniPure goat anti-mouse IgG light chain (1:10000 dilution; ABclonal, Cat: AS062).
Statistical analysis
Data are shown as mean values ± SEM. Two-tailed Student’s t tests were used to compare means, and significance was set at a p value of < 0.05. All of the data were analyzed using Prism 6.01 (GraphPad).
Results
Clinical characteristics of the patient
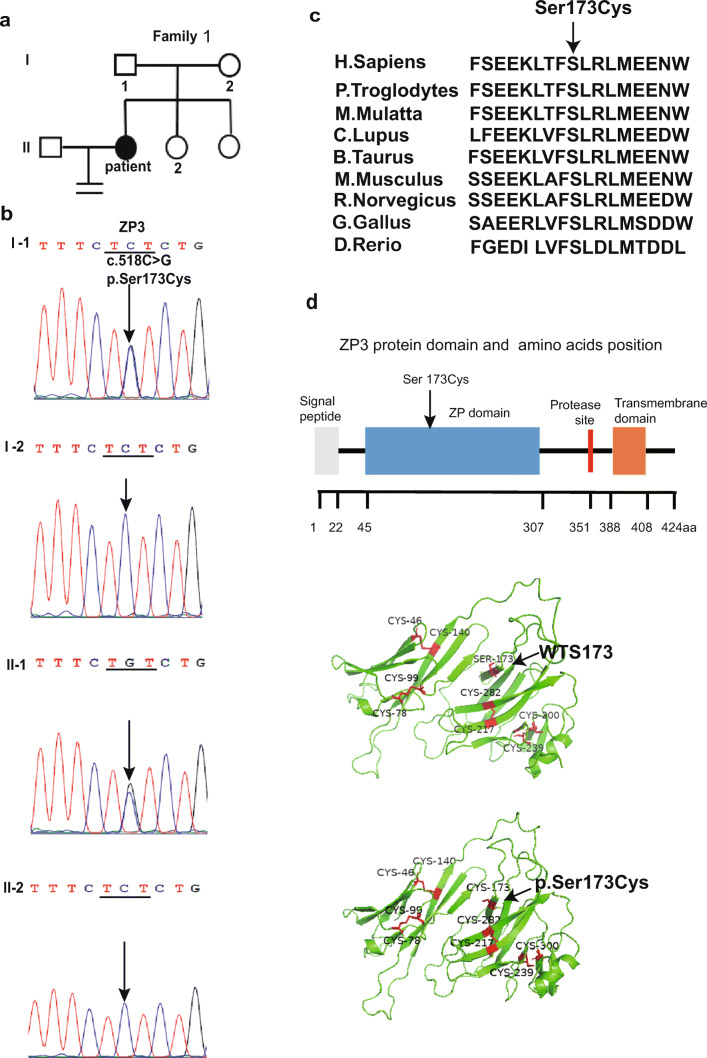
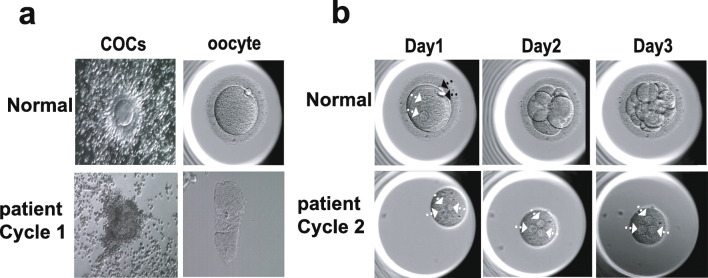
The patient (Fig. 2a) from family 1 was 30 years old and had been diagnosed with primary infertility for 2 years; her husband was normally fertile (Table 2). The woman’s chromosomal karyotype was 46, XX, and her menstrual cycles were normal. Her basal follicle-stimulating hormone (FSH) level was 4.76 IU/L, and luteinizing hormone (LH) and estradiol (E2) levels were 3.15 IU/L and 53.21 ng/L, respectively. She received two cycles of IVF treatment (Table 3). During the first IVF cycle (08 Jul 2017), we performed a gonadotropin-releasing hormone (GnRH) agonist protocol and obtained 14 follicles with diameters > 14 mm from the patient, with her estradiol concentration at 5095 pg/mL. Only 6 COCs were collected, and we verified all 6 oocytes as degenerated, without a surrounding zona pellucida (Fig. 1a), after being denuded of cumulus cells and in vitro fertilization. In the second ICSI cycle (04 Aug 2019), a GnRH antagonist protocol was performed, and we aspirated 5 follicles, but we collected only 2 COCs. After removal of the granulosa cells, we found that 1 oocyte was degenerated, while the other oocyte was ZP-free and approximately ½–1/3 normal volume. After ICSI the zygote formed 3 pronuclei (3PN) and did not cleave (Fig. 1b) in subsequent culture. The patient’s two sisters, in contrast, had normal pregnancy histories.
Fig. 2.
Identification of a mutation in ZP3. a The pedigree of a Chinese family with primary infertility (family 1). b DNA sequencing results of the ZP3 gene from the patient and her unaffected family members. c The S173 arginine residue in the ZP3 protein is highly conserved during evolution. d ZP3 (NM_001110354.2) protein domain, and the structural prediction of the WT and S173C mutant ZP3 protein; the 4 disulfide bonds are linked by 8 conserved cysteines (Cys46/Cys140, Cys78/Cys99, Cys217/Cys282, and Cys239/Cys300)
Table 2.
Semen analysis of patient’s husband (family 1)
| Patient’s husband | Normal value | |
|---|---|---|
| Semen volume (mL) | 3.40 | ≥ 1.5 |
| pH | 7.4 | ≥ 7.2 |
| Ratio of normal sperm (%) | 12 | ≥ 4 |
| Sperm concentration (× 106/mL) | 110.35 | ≥ 15 |
| Sperm motility (PR) (%) | 56.78 | ≥ 32 |
| Curvilinear velocity(VCL) (μm/s) | 48.85 | |
| Straight-line velocity (VSL) (μm/s) | 29.46 | |
| Average path velocity (VAP) (μm/s) | 32.39 | |
| Linearity (LIN) (%) | 59.54 |
Table 3.
Cycles of IVF/ICSI and oocytes from patient (family 1)
| Cycle 1 | Cycle 2 | |
|---|---|---|
| No. of follicles | 14 | 5 |
| No. of COCs | 6 | 2 |
| No. of oocytes | 0 | 1 oocyte with ZP-free |
Fig. 1.
Morphology of patients’ oocytes. a Cumulus-oocyte complexes (COCs) and oocytes from the controls and patient in their first IVF/ICSI cycle. b The oocyte of the patient was ZP-free, and after ICSI the zygote formed 3 pronuclei (3PN) and did not cleave (the zygote was from the second IVF/ICSI cycle). The white arrowhead indicates pronuclei, the black arrowhead indicates the polar body. The patient from family 1
Identification of a mutation in the ZP3 gene
We performed whole-exome sequencing on the patient from family 1 and her parents, and after variant annotation and filtration to narrow the variants, 1001 variants were found in the patient. Among these, there were 53 homozygous mutations. We priority-checked all of the known disease-causing genes of female infertility or oocyte development (including ZP1, ZP2, ZP3, ZP4, and LHCGR), but only identified the mutation in ZP3. To reiterate, there were no mutations identified in any other gene related to female infertility or oocyte development; the patient did the CNVs analysis and exclude the CNVs. Sanger DNA sequencing was used to confirm the mutation, and it showed that the patient carried a ZP3 heterozygous S173C (p.Ser173Cys, c.518C > G) mutation (Fig. 2b). The S173 residue of the ZP3 protein is highly conserved during evolution (Fig. 2c), and the S173C mutation shows a very low frequency in gnomAD (among 56,761 non-Finnish Europeans, only 1 individual was heterozygous for the S173C; the S173C mutation was, however, not reported for other populations). The patient inherited the S173C mutation from her father, while her mother and sister did not carry this mutation in their ZP3 genes. Bioinformatic analysis using Polyphen-2 (Polyphen2 HVAR predicted: Damaging), SIFT (Prediction: Damaging), and the PROVEAN (Prediction: Deleterious) software showed that mutations were harmful. The S173C mutation was located at the ZP domain in ZP3, and the ZP domain is the most highly conserved of all of the ZP proteins. The 4 disulfide bonds are linked by 8 conserved cysteines (Cys46/Cys140, Cys78/Cys99, Cys217/Cys282, and Cys239/Cys300), and they play an important role in zona pellucida assembly [18] (Fig. 2d). DISULFIND analysis demonstrated that the S173C mutation may affect the stability of the disulfide bonds and affect interactions between ZP3 and other ZP proteins.
Expression and localization of ZP3 in HEK293T cells
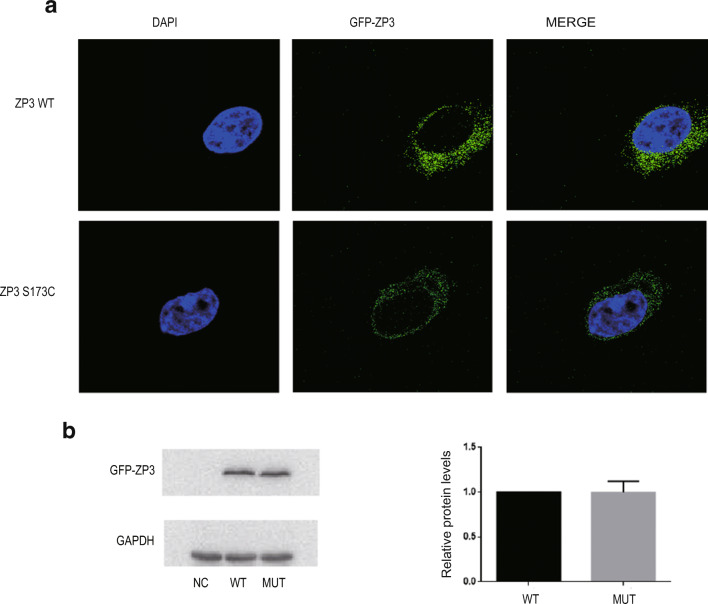
To further evaluate the effects of the S173C mutant in ZP3, we constructed a GFP-ZP3 expression plasmid and transfected it into HEK293T cells. Both wild-type protein and the S173C mutant ZP3 protein were localized to the cytoplasm (Fig. 3a), indicating that the S173C mutation in ZP3 did not alter ZP3 protein localization in HEK293T cells. When we extracted total cytoplasmic proteins from HEK293T cells and performed western blotting analysis (WB), we determined (as shown in Fig. 3b) that the expression level of the S173C mutant ZP3 protein in HEK293T cells was not obviously changed compared with that of WT ZP3. These results suggested that the S173C mutation did not alter the expression level of ZP3 in cellular lysates.
Fig. 3.
ZP3 expression and localization in HEK293T cells. a Wild-type ZP3 proteins were localized to the cytoplasm, and the S173C mutant ZP3 proteins were also primarily located in the cytoplasm. b Expression levels of wild-type and mutant ZP3 in cytoplasm. Data are expressed as means ± SEM of 5 independent experiments
Mutation in ZP3 affects the interactions between ZP3 and ZP2
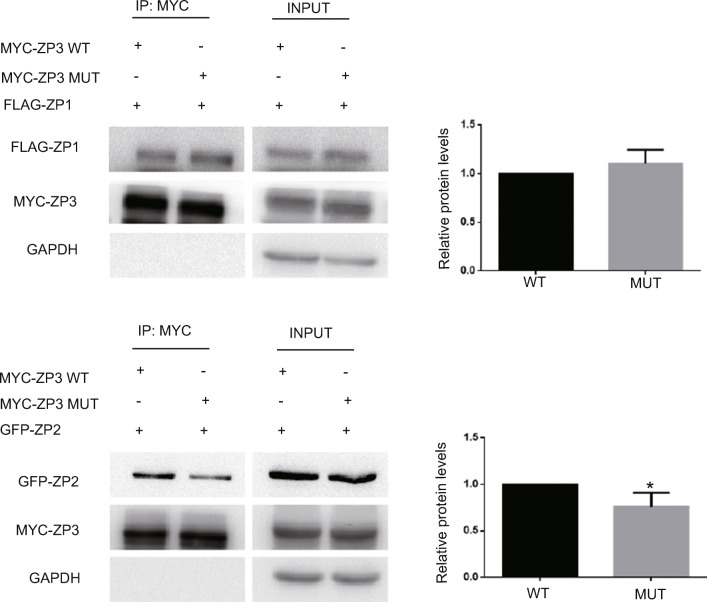
As the patient exhibited an abnormal zona pellucida (ZP) and the S173C mutation was located in the ZP domain of ZP3, we speculated that the mutation in ZP3 may affect interactions between ZP glycoproteins. The encoding human full-length FLAG-ZP1 expression plasmid and MYC-ZP3 or MYC-ZP3S173C expression plasmid were thus co-transfected into HEK293T cells, and we performed co-immunoprecipitation (Co-IP) to analyze interactions between ZP3 and ZP1. Our results showed that the interactions between MYC-ZP3S173C and FLAG-ZP1 were not changed compared with MYC-ZP3 and FLAG-ZP1 (Fig. 4a). We then also co-transfected a GFP-ZP2 and MYC-ZP3 or MYC-ZP3S173C expression plasmid into HEK293T cells and performed Co-IP analysis, which revealed that the interaction between GFP-ZP2 and MYC-ZP3S173C was significantly reduced relative to that of GFP-ZP2 and MYC-ZP3 (Fig. 4b). These results suggested that the S173C mutation affected interactions between ZP3 and ZP2, which may lead to abnormal assembly of the zona pellucida fiber network.
Fig. 4.
The mutation in ZP3 affects interactions between ZP3 and ZP2. a MYC-ZP3 (WT) or MYC-ZP3S173C (MUT) and FLAG-ZP1 co-transfected into HEK293T cells, and analyzed by Co-IP assays. Lanes 1 and 2 show co-immunoprecipitation by anti-MYC antibody, and Lanes 3 and 4 show input. b MYC-ZP3 (WT) or MYC-ZP3S173C (MUT) and GFP-ZP2 co-transfected into HEK293T cells and analyzed by Co-IP assays. Lanes 1 and 2 show co-immunoprecipitation by anti-MYC antibody, and Lanes 3 and 4 show input. Data are expressed as means ± SEM of more than 3 independent experiments. Single asterisk represents P < 0.05
Discussion
Herein, we performed a genetic analysis on 17 Chinese families with female infertility (including four families with empty follicle syndrome. Table S1). The patient from family 1 was diagnosed with “genuine” empty follicle syndrome in the first IVF cycle, and she failed to become pregnant after two cycles of IVF/ICSI. We executed whole-exome sequencing and Sanger DNA sequencing, and we identified a novel mutation (p.Ser173Cys, c.518C > G) of the ZP3 gene in the patient. The mutation was inherited from the woman’s father, as her mother and sister did not carry the mutation. Bioinformatic analysis indicated that the mutation might affect the stability of the disulfide bonds in the ZP domain of the ZP3 protein, and potentially affect interactions between ZP3 and other ZP proteins. The results of western blotting analysis suggested that the S173C mutation did not alter the expression of ZP3 in the cell lysates. However, Co-IP analysis revealed that the S173C mutation affected the linkage between ZP3 and ZP2.The mutation of the ZP3 gene in the patient from family 16 needs to be further confirmed; as we did not have the blood samples of the parents of the patient, we do not know the mutation is inherited from the mother or the father of the patient, or a de novo mutation. In other 15 families, we did not found any mutation of the known disease-causing genes indicate new disease-causing genes may cause the disease in these families.
The human zona pellucida (ZP) consists of four glycoproteins (ZP1, ZP2, ZP3, and ZP4). The cytoplasmic-tail cleavage sites of ZP proteins are cleaved by proteolytic enzymes, and the ZPs are secreted outside the oocyte, where they are disassembled into monomers. ZP3 then interacts with ZP1, ZP2, and ZP4 to form three types of heterodimers [5, 19] that are assembled into ZP filaments.
Chen et al. reported a heterozygous mutation (c.400G > A) in ZP3 that caused empty follicle syndrome where the A134T mutant amino acid in ZP3 impeded the interaction between ZP3 and ZP2, destroying the assembly of the ZP proteins and leading to oocyte degeneration and empty COCs [13]. Cao et al. also reported that the same heterozygous mutation (c.400G > A) in ZP3 caused a formation disorder of the ZP and induced female infertility in women, with their patient manifesting ZP-free oocytes or lacking oocytes entirely in her IVF cycles. Their A134T mutant was shown to affect the interaction between ZP3 and ZP2, thus eradicating ZP assembly [20]. Zhou et al. reported a heterozygous mutation (c.763C > G) in ZP3 that caused primary infertility due to ZP-free oocytes, and they hypothesized that the mutant ZP3 proteins competed with wild-type ZP3 proteins for binding to the other 3 wild-type ZP proteins in vivo [12]. Liu et al. described mutations in ZP2 (c.2092C > T) and ZP3 (c.1045_1046insT) that occurred in a woman with primary infertility who produced oocytes with abnormal ZP morphologies [10]. In our investigation, we identified a novel heterozygous S173C mutation in ZP3 in a patient with empty follicle syndrome, where the Ser173 was located in the ZP domain of ZP3 glycoproteins. The function of the ZP domain is to interact with other ZP glycoproteins, and our bioinformatic analysis showed that the mutation damaged the interaction between ZP3 and other ZP proteins. Co-IP analysis also showed that the S173C mutation reduced the interaction between ZP3 and ZP2, which may then affect the formation of the zona pellucida fiber network. The abnormal zona pellucida will then lead to ZP-free or degenerate oocytes, resulting in female infertility.
In conclusion, we identified a novel mutation in the ZP3 gene in a patient with female infertility. Our findings thus expand the mutational spectrum of the ZP3 gene, and they will be helpful in precisely diagnosing aspects of female infertility.
Supplementary Information
(DOCX 15 kb)
Funding
This work is supported by the National Natural Science Foundation of China grants (81000079, 81170165, and 81870959 to X.Z.), the Program for HUST Academic Frontier Youth Team (2016QYTD02), and the Fundamental Research Funds for the Central Universities (HUST: 2019JYCXJJ035).
Compliance with ethical standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of the ethics committee on human subject research at Huazhong University of Science and Technology and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Written informed consent was obtained from the participants.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Dazhi Zhang and Lixia Zhu contributed equally to this work.
Contributor Information
Zhou Li, Email: Lizhou618@hotmail.com.
Xianqin Zhang, Email: xqzhang04@hust.edu.cn.
References
- 1.Wassarman PM. Zona pellucida glycoproteins. J Biol Chem. 2008;283:24285–24289. doi: 10.1074/jbc.R800027200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matzuk MM, Burns KH, Viveiros MM, Eppig JJ. Intercellular communication in the mammalian ovary: oocytes carry the conversation. Science (New York, NY) 2002;296:2178–2180. doi: 10.1126/science.1071965. [DOI] [PubMed] [Google Scholar]
- 3.Conner SJ, Lefievre L, Hughes DC, Barratt CL. Cracking the egg: increased complexity in the zona pellucida. Hum Reprod (Oxford, England) 2005;20:1148–1152. doi: 10.1093/humrep/deh835. [DOI] [PubMed] [Google Scholar]
- 4.Gupta SK. Role of zona pellucida glycoproteins during fertilization in humans. J Reprod Immunol. 2015;108:90–97. doi: 10.1016/j.jri.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 5.Kiefer SM, Saling P. Proteolytic processing of human zona pellucida proteins. Biol Reprod. 2002;66:407–414. doi: 10.1095/biolreprod66.2.407. [DOI] [PubMed] [Google Scholar]
- 6.Rankin T, Talbot P, Lee E, Dean J. Abnormal zonae pellucidae in mice lacking ZP1 result in early embryonic loss. Development (Cambridge, England) 1999;126:3847–3855. doi: 10.1242/dev.126.17.3847. [DOI] [PubMed] [Google Scholar]
- 7.Huang HL, Lv C, Zhao YC, Li W, He XM, Li P, Sha AG, Tian X, Papasian CJ, Deng HW, Lu GX, Xiao HM. Mutant ZP1 in familial infertility. N Engl J Med. 2014;370:1220–1226. doi: 10.1056/NEJMoa1308851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rankin TL, O'Brien M, Lee E, Wigglesworth K, Eppig J, Dean J. Defective zonae pellucidae in Zp2-null mice disrupt folliculogenesis, fertility and development. Development (Cambridge, England) 2001;128:1119–1126. doi: 10.1242/dev.128.7.1119. [DOI] [PubMed] [Google Scholar]
- 9.Dai C, Hu L, Gong F, Tan Y, Cai S, Zhang S, et al. ZP2 pathogenic variants cause in vitro fertilization failure and female infertility. Genet Med. 2019;21:431–40. doi: 10.1038/s41436-018-0064-y. [DOI] [PubMed] [Google Scholar]
- 10.Liu W, Li K, Bai D, Yin J, Tang Y, Chi F, Zhang L, Wang Y, Pan J, Liang S, Guo Y, Ruan J, Kou X, Zhao Y, Wang H, Chen J, Teng X, Gao S. Dosage effects of ZP2 and ZP3 heterozygous mutations cause human infertility. Hum Genet. 2017;136:975–985. doi: 10.1007/s00439-017-1822-7. [DOI] [PubMed] [Google Scholar]
- 11.Rankin T, Familari M, Lee E, Ginsberg A, Dwyer N, Blanchette-Mackie J, et al. Mice homozygous for an insertional mutation in the Zp3 gene lack a zona pellucida and are infertile. Development (Cambridge, England) 1996;122:2903–2910. doi: 10.1242/dev.122.9.2903. [DOI] [PubMed] [Google Scholar]
- 12.Zhou Z, Ni C, Wu L, Chen B, Xu Y, Zhang Z, Mu J, Li B, Yan Z, Fu J, Wang W, Zhao L, Dong J, Sun X, Kuang Y, Sang Q, Wang L. Novel mutations in ZP1, ZP2, and ZP3 cause female infertility due to abnormal zona pellucida formation. Hum Genet. 2019;138:327–337. doi: 10.1007/s00439-019-01990-1. [DOI] [PubMed] [Google Scholar]
- 13.Chen T, Bian Y, Liu X, Zhao S, Wu K, Yan L, Li M, Yang Z, Liu H, Zhao H, Chen ZJ. A recurrent missense mutation in ZP3 causes empty follicle syndrome and female infertility. Am J Hum Genet. 2017;101:459–465. doi: 10.1016/j.ajhg.2017.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coulam CB, Bustillo M, Schulman JD. Empty follicle syndrome. Fertil Steril. 1986;46:1153–1155. doi: 10.1016/S0015-0282(16)49898-5. [DOI] [PubMed] [Google Scholar]
- 15.Zegers-Hochschild F, Fernandez E, Mackenna A, Fabres C, Altieri E, Lopez T. The empty follicle syndrome: a pharmaceutical industry syndrome. Hum Reprod (Oxford, England) 1995;10:2262–2265. doi: 10.1093/oxfordjournals.humrep.a136281. [DOI] [PubMed] [Google Scholar]
- 16.Yuan P, He Z, Zheng L, Wang W, Li Y, Zhao H, et al. Genetic evidence of 'genuine’ empty follicle syndrome: a novel effective mutation in the LHCGR gene and review of the literature. Hum Reprod (Oxford, England) 2017;32:944–953. doi: 10.1093/humrep/dex015. [DOI] [PubMed] [Google Scholar]
- 17.Zhang D, Yuan C, Liu M, Zhou X, Ge S, Wang X, Luo G, Hou M, Liu Z, Wang QK, Wang X, Li H, Tan Y, Jia W, Wang J, Wu Y, Wang A, Yang X, Zhang X. Deficiency of SCAMP5 leads to pediatric epilepsy and dysregulation of neurotransmitter release in the brain. Hum Genet. 2020;139:545–555. doi: 10.1007/s00439-020-02123-9. [DOI] [PubMed] [Google Scholar]
- 18.Zhao M, Boja ES, Hoodbhoy T, Nawrocki J, Kaufman JB, Kresge N, Ghirlando R, Shiloach J, Pannell L, Levine RL, Fales HM, Dean J. Mass spectrometry analysis of recombinant human ZP3 expressed in glycosylation-deficient CHO cells. Biochemistry. 2004;43:12090–12104. doi: 10.1021/bi048958k. [DOI] [PubMed] [Google Scholar]
- 19.Litscher ES, Qi H, Wassarman PM. Mouse zona pellucida glycoproteins mZP2 and mZP3 undergo carboxy-terminal proteolytic processing in growing oocytes. Biochemistry. 1999;38:12280–12287. doi: 10.1021/bi991154y. [DOI] [PubMed] [Google Scholar]
- 20.Cao Q, Zhao C, Zhang X, Zhang H, Lu Q, Wang C, Hu Y, Ling X, Zhang J, Huo R. Heterozygous mutations in ZP1 and ZP3 cause formation disorder of ZP and female infertility in human. J Cell Mol Med. 2020;24:8557–8566. doi: 10.1111/jcmm.15482. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 15 kb)