Abstract
This paper traces the history of noncommunicable disease public health research and programming at the World Health Organization. Specifically, it investigates the origins of the now pervasive 4 × 4 framework focusing on four sets of diseases (cardiovascular diseases, diabetes, chronic respiratory diseases, and cancers) caused by four behavioral risk factors (tobacco use, harmful use of alcohol, unhealthy diets, and physical inactivity). We have found that the 4 × 4 framework developed as a generalization from strategies to control epidemics of cardiovascular disease and stroke in high-income countries during the second half of the twentieth century. These strategies, which were narrowly focused on interventions to address behavioral “lifestyle” risk factors as well as pharmacotherapy for physiologic risk factors, were ultimately packaged as an integrated approach initially in high-income countries and subsequently extended to low- and middle-income countries, where they have failed to address much of the burden among very poor populations.
Keywords: Noncommunicable Diseases (NCDs), WHO, 4 × 4 framework, Global health
Highlights
-
•
The WHO has used the 4 × 4 framework as a dominant framing for NCDs and recommended interventions since at least 2011.
-
•
The framework is based upon epidemiological research on risk factors for cardiovascular disease in high-income countries.
-
•
The 4 × 4 framework has failed to effectively address the NCD burden in low- and middle-income countries.
1. Introduction
When the Global Burden of Disease (GBD) Study brought the term “noncommunicable disease” (NCD) into common usage in global health statistics in the early 1990s, it was composed of 12 major groups of causes: malignant neoplasms, diabetes mellitus, nutritional and endocrine, neuropsychiatric, sense-organ, cardiovascular, respiratory, digestive, genitourinary, musculoskeletal, congenital abnormalities, and oral health (Murray et al., 1992). In an attempt to simplify the International Classification of Diseases, and in a nod to Omran's (1971) theory of epidemiologic transition, GBD grouped these disparate conditions together based on their relatively slow rate of mortality decline with socioeconomic development as compared with more rapid reductions in mortality due to communicable and reproductive diseases. This broad meaning of NCDs continues to apply both in GBD and in the World Health Organization's Global Health Estimates (Mathers and Ho, 2017).
By the time of the 2011 United Nations (UN) High-Level Meeting on NCDs, however, NCDs had been reduced to a “4 × 4” model focusing on four sets of diseases (cardiovascular diseases, diabetes, chronic respiratory diseases, and cancers) caused by four behavioral risk factors (tobacco use, harmful use of alcohol, unhealthy diets, and physical inactivity) (United Nations General Assembly, 2011). As noted in a UN news bulletin from September 20th, 2011, “Delegates … today considered strategies to combat the preventable, mostly lifestyle- and diet-related illnesses that have become the major killers across the world” (United Nations, 2011).
Theoretically, this narrowly defined framing for NCDs amounted to a simpler governance tool for the World Health Organization (WHO). The 4 × 4 framework enabled the WHO to more easily advise and consult with ministries of health around the world on their emerging NCD policies and programs. This framework also attempted to consolidate and more clearly conceptualize the NCD burden. By mapping this conception of NCD burden onto conceptual and operational levers for policy action—lifestyle modifiable risk factors—the WHO could make straightforward recommendations for organizing NCD control and public health programming. Practically, the 4 × 4 framework for NCDs has been highly influential with respect to subsequent global monitoring frameworks and indicators for disease control, as well as the way that NCDs are understood as part of Universal Health Coverage and the post-2015 Sustainable Development Goals (United Nations, 2017; World Health Organization, 2013, 2018).
The 4 × 4 framework has been criticized variously for (1) excluding specific diseases such as mental illnesses, neurological conditions, hemoglobinopathies, and musculoskeletal conditions, for example; (2) its limited explanatory power and focus on vertical disease control; and, (3) neglecting the diversity of the diseases and infectious and environmental risk factors that affect the global poor (Binagwaho et al., 2014; Birbeck, 2011; Mensah & Mayosi, 2012; Raviola et al., 2011; World Health Organization, 2013).
More recently, there are signs that the WHO's commitment to the rigidly narrow 4 × 4 framework may be softening. Indeed, the third UN High-Level Meeting on NCDs, held in September of 2018, broadened commitments to action on environmental risk factors such as air pollution, and to treatment of mental illnesses, marking a shift to a “5 × 5” agenda (United Nations General Assembly, 2018). This shift is also reflected in the WHO Independent High-Level Commission on NCDs (World Health Organization, 2018). Finally, The Lancet Commission on Reframing NCDs and Injuries (NCDIs) for the Poorest Billion has also characterized the NCDI burden in populations living in extreme poverty and made recommendations regarding pro-poor NCD control strategies that address a broader range of severe conditions among children and young adults (http://www.ncdipoverty.org) (Bukhman et al., 2020).
The following history aims to contribute to these ongoing efforts to move beyond the 4 × 4 framework by developing a critical understanding of the circumstances within which it emerged, as well as its subsequent simplification and generalization to settings around the world. Specifically, we detail how the 4 × 4 framework developed as a generalization of strategies to control epidemics of ischemic cardiovascular disease and stroke in high-income countries during the second half of the 20th century, and was then subsequently applied to low- and middle-income countries. This process of simplification and generalization—and the exclusions it produced—has had dire implications for the prevention and treatment of NCDs and injuries amongst the poorest billion people around the world.
2. Materials and methods
To track the evolution of the 4 × 4 framework at WHO, we examined approximately 500 archival documents from WHO Archives in Geneva, Switzerland. We also drew from more than 450 published documents from WHO, including official histories and technical report series. All documents were then categorized and reviewed using Evernote software; salient themes and excerpts were tagged according to topic and geographic region by authors during the review process, creating a library from which to source material for the manuscript. Finally, we conducted semi-structured interviews with four directors of the Division of Noncommunicable Diseases at WHO.
3. Theory
Knowledge of the social world and, more precisely, the categories that make it possible, are the stakes, par excellence, of political struggle, the inextricably theoretical and practical struggle for the power to conserve or transform the social world by conserving or transforming the categories through which it is perceived.
Public health categories—like the category of NCDs, traced here—are social constructions (Berger & Luckmann, 1966) used by governments and other civil society entities for the purpose of improving specific forms of population level health (Foucault, 1976, pp. 237–264). The social construction of pragmatically coherent and useful categories within public health science and governance has been investigated and theorized by anthropologists, sociologists, and historians of science and medicine (Brown, 1995; Conrad & Barker, 2010; Gieryn, 1983; Lamont & Molnár, 2002; Sangaramoorthy & Benton, 2012). More recent research into the practices of “knowing governance” explores the increasingly technical nature of political struggle within scientific epistemic communities who vie for the legitimate authority to carve, name, and frame the natural and social world using their choice of categories, frameworks, evidence, and data (Haas, 1992; Voss & Freeman, 2016). Public health science is often a form of political priority setting and implicit hierarchy-making, pursued by technical and bureaucratic means and thus not acknowledged as such.
This paper offers a brief historical narrative of the construction and crystallization of one such framework—the 4 × 4 framework—as deployed by WHO in its attempt to rationalize and govern the wide-ranging, unruly, and newly identified (as of 1976) health problems associated with NCDs. In what follows, we trace how early models used to understand and intervene in population-level cardiovascular disease lifestyle-modifiable risk factors were generalized to a much wider swath of problematic chronic health conditions.
Beginning in the mid-1970s, WHO officials formally linked ideas of shared lifestyle-modifiable risk factors to the pragmatic inclusion or exclusion in what came to be known as the “main four” NCDs. The result of this history has been that the principal drivers of NCDs amongst the poorest populations in the world have become rendered less visible to policy makers and ministries of health, leading to very little political attention and funding (Bukhman et al., 2020).
4. Findings and discussion
Our findings and discussion of this history are laid out chronologically in sections 1.4.1 through 1.4.4. In the first section, we begin with a description of the early genesis of NCD epidemiology through the Framingham Heart Study and its influence on the WHO. We then describe the rise and simplification of NCD governance at the WHO through the emergence of a shared modifiable risk factor framework and demonstration projects that informed the creation of the 4 × 4 framework between 1978 and 2000. In section 1.4.3 we illustrate two examples of NCD conditions—J-type diabetes and rheumatic heart disease—that were left on the chopping block as a result of the narrowing 4 × 4 framework taken up by the WHO during this period. Finally, we conclude with the relatively recent policy crystallization of the 4 × 4 framework beginning with the release of the Global Burden of Disease findings and the WHO's World Health Assembly resolution on the prevention and control of NCDs in 2000.
1.4.1. 1948–1978: from Framingham to Finland: disease specific programming and the rise of cardiovascular disease epidemiology
WHO was created in 1948 in the wake of World War II as the United Nations technical agency for deploying scientific and governance authority for international public health. From its birth, the agency's agenda was broad and ambitious. Early WHO reports anticipated impending changes in disease patterns as developing countries became industrialized and urbanized—in short, more like developed countries in diet and lifestyle (WHO Expert Committee on Cancer Control & World Health Organization, 1963). Initial interest in NCDs developed out of the WHO European Regional Office (EURO), which hosted the Consultative Committee for Europe at its first session in May 1951.
There, the Committee recommended that specific WHO programs might be developed in “non-infectious chronic diseases of adult populations,” “dental hygiene,” and “nutrition,” among others (World Health Organization, 1951). Still, during this formative period, the organization of NCD activities at WHO was overwhelmingly broken into disease-specific programming and clinical focus areas, including distinct programs in cardiovascular diseases, diabetes, cancer, chronic respiratory diseases, rheumatic diseases, congenital abnormalities, renal diseases, occupational health, mental health, and malnutrition.
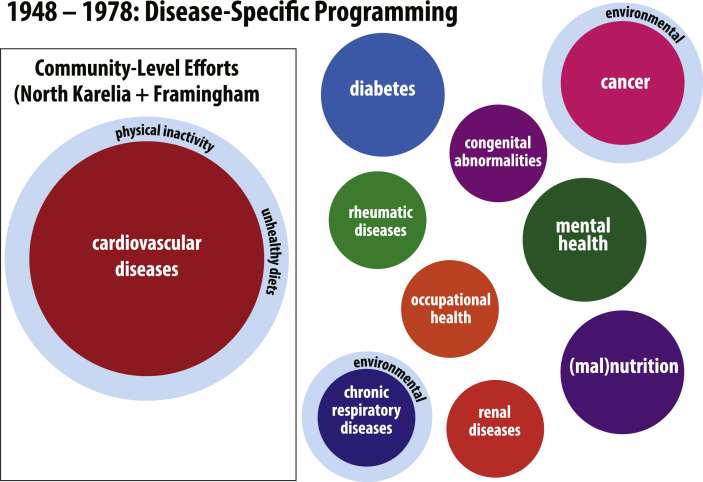
The notion that NCDs could or should be defined by a delimited set of shared risk factors has its roots in the 1947 launch of the Framingham Heart Study by the United States National Heart Institute and Public Health Service. In the post-World War II years, the etiological causes and possible techniques for control of cardiovascular diseases (CVD) were largely speculative. The Framingham Heart Study represented a remarkable move to deploy the tools and techniques of epidemiology – historically used in the study of infectious diseases – to analyze the distribution and causes of chronic and noncommunicable disease (Oppenheimer et al., 2011). This study, initiated in the wake of the death of U.S. President Franklin D. Roosevelt from a sudden stroke, first quantified various “lifestyle risk factors” that appeared to strongly correlate with the likelihood of developing CVD (Mahmood et al., 2014). These new statistical tools and the explanatory power of “risk factor analysis” became central to the increasingly refined domain of public health research and practice. Fig. 1 graphically represents this early disease-specific programing and emerging CVD epidemiology at the WHO.
Fig. 1.
Disease-specific programming at the WHO and the emergence of community-level epidemiology characterization of CVD between 1948 and 1978.
Allusions to the emerging literature and epistemological frameworks developed by CVD epidemiologists can be seen at WHO as early as 1955 in the Recommendations of the Fifth Session of the Regional Committee of Europe (WHO Regional Committee for Europe, 1955). The meeting participants also discussed the possible utility of grouping these discrete clinical issues into a shared category of public health practice, stating, “Effective international work at regional level and in close co-operation with Headquarters appears possible in a group of chronic diseases amongst which the public health aspects of cardiovascular diseases, diabetes, cancer and rheumatism are examples which need immediate consideration by the Regional Organization” (Executive Board 17, 1955).
In October and November 1957, public health and ministry of health officials gathered for the Symposium on the Public Health Aspects of Chronic Disease in Amsterdam, Netherlands. In the published report that followed, the four example “degenerative diseases” (CVD, diabetes, respiratory illnesses, and cancer), which were becoming more visible throughout Europe and the United States, were grouped within the headings and etiological explanations of certain “risks,” “behaviors,” and “modes of life” (WHO Regional Office for Europe, 1957). In being taken up by global policymakers at WHO, the epistemological precision of risk factor analysis, first developed through the Framingham Heart Study, was becoming universalized and globalized. The tools of quantitative risk-factor analysis continued to migrate the globe providing evidence for the causal mechanisms of CVD.
Indeed, the Framingham Heart Study served as a valuable template for WHO to study CVD epidemiology in other industrialized countries. Most notable was Finland's North Karelia Project, initiated in 1978 by the charismatic and enthusiastic leadership of Pekka Puska, a Finnish physician and public health practitioner (Puska, 2002). The North Karelia Project explicitly drew from the intellectual tools of NCD risk factor analysis and extended them, with the aim of developing community-based interventions to reduce the prevalence of lifestyle modifiable risk factors within populations. As Puska and colleagues explain, “Since major NCD epidemics are due to unhealthy lifestyles, which often arise during periods of economic transition, a significant reduction in NCD rates should be possible by promoting general changes in the known NCD-related lifestyles” (Nissinen et al., 2001).
4.2. 1978–2000: from cardiovascular disease risk factors to the shared risk factor paradigm at WHO
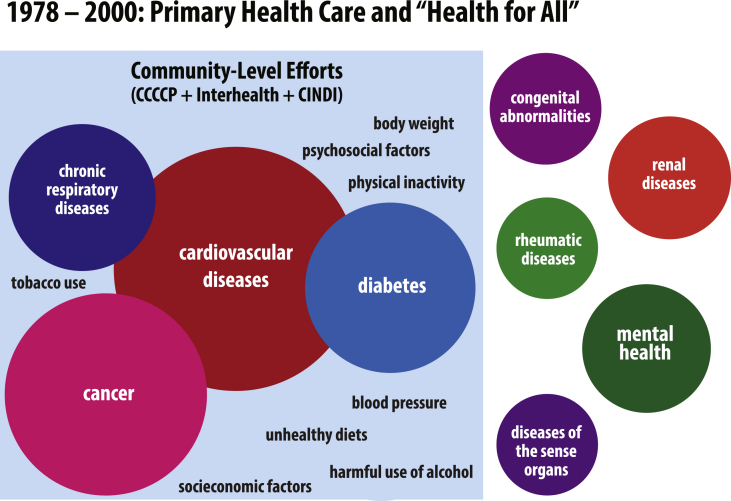
Following successes in Framingham and Finland, WHO sought to strengthen its NCD programming, leading to the development of several major programs in the early 1980s. Initially targeted only toward CVD prevention, these programs ultimately adopted an integrated approach to NCD prevention, a shift facilitated by the growing adoption of the shared risk factor framework. However, because this framework was initially developed to explain the causes of NCD burden among affluent populations with a high CVD burden, WHO's NCD programming took a sharp turn toward a small set of diseases that appeared to fit the shared risk factor framework, and away from the diseases that did not. As a result, diseases that did not fit within the shared risk factor framework—often, those most prevalent among the poor—became increasingly occluded from the view of policymakers. Fig. 2 graphically illustrates the early grouping of NCDs that came from notions of risk factor control efforts emerging from community NCD control pilot programs.
Fig. 2.
Illustration of the emergence of shared risk factors as categorizing schema for NCDs between 1978 and 2000.
Just two years after the launch of the North Karelia Project, WHO initiated the Comprehensive Cardiovascular Community Control Programme (CCCCP). This new program's main objective was “to apply scientific knowledge in a well-conceived way to attain maximum control of CVD in the entire community and to evaluate the experience for wider use,” and consisted of pilot projects across Europe, including in Czechoslovakia, Finland, Germany, Hungary, Italy, Norway, Switzerland, the USSR, and Yugoslavia (Puska et al., 1988). With the CCCCPs formalized and coordinated by WHO, the North Karelia Project (now considered the “CCCCP pilot project” in Finland) grew in global importance.
In fact, by 1981, the National Public Health Institute of Finland in Helsinki, which served as the coordinating center of the North Karelia Project and which was led by Puska, was formally appointed a WHO collaborating center for community control programmes in CVD (Puska et al., 1988). Meanwhile, with the CCCCP underway, WHO launched MONICA in 1982, another major international project with a focus on monitoring trends in CVD. Notably, although MONICA eventually included 38 populations in 21 countries on four continents, the biggest contributor by far was Europe (Gutziller, 1993).
Not long into the course of community prevention programmes on CVD, epidemiologists began examining whether cardiovascular risk factors also had an impact on other disease manifestations in their study populations. The realization that cancer, diabetes, and respiratory diseases shared some of the same modifiable risk factors as CVD provided cardiologists with immediate rhetorical support for integrated NCD programming and policy. A 1982 WHO Expert Committee on the Prevention of CHD concluded, “Preventive efforts against CHD should be seen as part of a more general programme against noncommunicable diseases. Control of CHD risk factors could lead at the same time to the reduction of respiratory disease, some cancers, diabetes, etc. Policy-makers should see this as an argument reinforcing the case for efforts against CHD, and the different components of prevention should effectively complement and strengthen each other” (WHO Expert Committee on Prevention of Coronary Heart Disease & World Health Organization, 1982).
The adoption of this framework by the WHO NCD Division is also apparent in a request for funding written by the Division's director at the time, Dr. Igor Glasunov: “The evaluation of the experience accumulated in the field of chronic disease control in the community is leading to a growing interest in and acceptance of the idea of a more comprehensive and integrated approach to chronic diseases instead of the existing traditional specialty-oriented approach. One of the prerequisites advocating an integrated approach is the universality of a group of risk factors to chronic diseases (environmental, nutritional, behavioural), which might be combined under the “life-style” definition” (Glasunov, 1981). Both the WHO expert committee's conclusions and Dr. Glasunov's funding requests make clear that WHO leadership saw pragmatic utility in uniting NCDs under the banner of a delimited set of lifestyle modifiable risk factors.
In 1984, EURO formally invited countries to incorporate their CCCCPs into the new WHO Countrywide Integrated Noncommunicable Diseases Intervention (CINDI) program. CINDI differed from the CCCCPs in several important ways. Most significantly, CINDI called for an integrated approach to NCDs. This was motivated, in part, by a World Health Assembly resolution in 1985 that explicitly called for “community studies with a view to arriving at population-centered measures to prevent and control cardiovascular diseases, lung cancer, diabetes mellitus, chronic respiratory and other noncommunicable diseases” (Thirty-Eighth World Health Assembly, 1985). Moreover, while the CCCCPs and MONICA focused on small regions in a handful of countries, CINDI consisted of several national demonstration programs in a more diverse network of countries, though all still within Europe. Ultimately, though, CINDI maintained a narrow vision of NCD prevention; a summary of surveys conducted at sixteen CINDI sites reported: “The areas of programme emphasis most commonly mentioned were: (a) the major risk factors: tobacco control, high blood pressure, diabetes, lipids; (b) selected target groups: worksite populations, children and youth; and (c) the population at large” (Regional Office for Europe &World Health Organization, 1996).
CINDI's successor was formally launched in 1990 as the WHO Integrated Programme for Community Health in Noncommunicable Diseases (INTERHEALTH), which aimed to improve health through a community-based approach to combat the common risk factors for four major NCDs (World Health Organization, 1993). In total, twelve countries, representing every WHO region, joined the program (Berrios et al., 1997). In this way, INTERHEALTH played an important role in exporting WHO's prevailing NCD agenda beyond the EURO region. For example, in 1996, while the Pan American Health Organization (PAHO) was taking initial steps to implement INTERHEALTH, it also collaborated with CINDI to develop the CARMEN Initiative, a comparable program specific to the needs of Latin American and Caribbean countries (The CARMEN Network, 2002). Though more diverse in their geographic scope, these programs perpetuated the 4 × 4 framework, in that they remained narrowly focused on the “big four” diseases.
4.3. The consequences of exclusion
As the WHO NCD Division pushed forward with a shared risk factor framework, its focus significantly shifted away from many NCDs, particularly those afflicting the poor. Two noteworthy examples include J-type diabetes and rheumatic heart disease. By further exploring the trajectory of these diseases within WHO's NCD programming, it becomes clear not only how dominant the shared risk factor framework was, but also how influential the European template for NCD prevention became.
First taken up by WHO due to an insulin shortage following World War II, diabetes was initially conceived as a metabolic disorder with varying etiologies that cut across social classes (WHO Expert Committee on Diabetes Mellitus & World Health Organization, 1965). Indeed, in addition to what we now call Type I and Type II diabetes, the latter of which was conceived as associated with overnutrition in affluent societies, WHO recognized a third sub-type known as malnutrition-related diabetes or J-type diabetes (J for Jamaica, where it was first documented) (Abu-Bakare et al., 1986).
Despite recognizing the important distinction in etiology, as late at 1980, there was still little consensus on the problem of diabetes in developing countries. In its Second Report, the WHO Expert Committee on Diabetes Mellitus wrote: “Although a more prominent health problem in developed countries, it is erroneous to consider diabetes a disease of affluent societies. Epidemiological studies indicate high rates universally, but little is known of the real extent of diabetes and its sequelae in developing countries …. In some societies obesity is a major association of diabetes; in others malnutrition is probably a more important determinant” (WHO Expert Committee on Diabetes Mellitus & World Health Organization, 1980). Because WHO's NCD programming was formulated on the basis of shared modifiable lifestyle risk factors, it is hardly surprising that malnutrition-related diabetes is not represented in the 4 × 4.
WHO's dealings with rheumatic fever is similarly illustrative of the ways in which the poor were excluded from major NCD prevention efforts. At the 1957 Conference on the Public Health Aspects of Chronic Disease, the conference report authors rightfully acknowledge the different burden of chronic disease among the poor: “Poverty and its attendant circumstances seem to prevent atherosclerosis, diabetes and the like rather than to promote them. On the other hand, rheumatic fever is more relevant in families in the lower income groups” (WHO Regional Office for Europe, 1957). Despite the early inclusion of developing countries within various NCD policy arenas, policymakers were keenly aware that the modifiable risk-factor framework was not applicable to those who did not live affluent lifestyles.
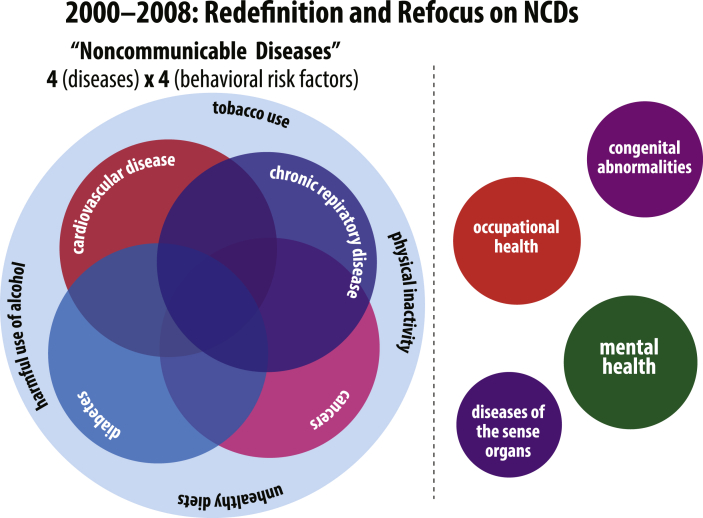
In a 1986 report from the WHO Expert Committee on Community Prevention and Control of CVD (1986), the authors detail those aspects of Western lifestyles that contribute to important risk factors for CVD, noting, “… higher mortality rates in the less affluent groups cannot be fully explained by the standard cardiovascular risk factors. The recommendations for prevention in this report are compatible with a high standard of living.” Given that NCD programming was so fundamentally informed by the “standard cardiovascular risk factors,” rheumatic heart disease, with its divergent risk factors, was effectively misaligned with WHO priorities and, in turn, rendered invisible. The consequences of the framework's exclusionary nature can be directly observed in the fact that, in an effort to revive funding for the still-prevalent disease, the World Health Assembly passed a resolution in April 2018 to invigorate rheumatic heart disease prevention efforts. The resolution notes that 30 million people continue to be affected by rheumatic heart disease each year, primarily in low- and middle-income countries (Seventy-first World Health Assembly, 2018). These exclusions are graphically represented in Fig. 3.
Fig. 3.
Illustration of key NCD exclusions crystalized by the 4 × 4 framework between 2000 and 2008.
4.4. 2000–2008: crystallizing the 4 × 4 framework
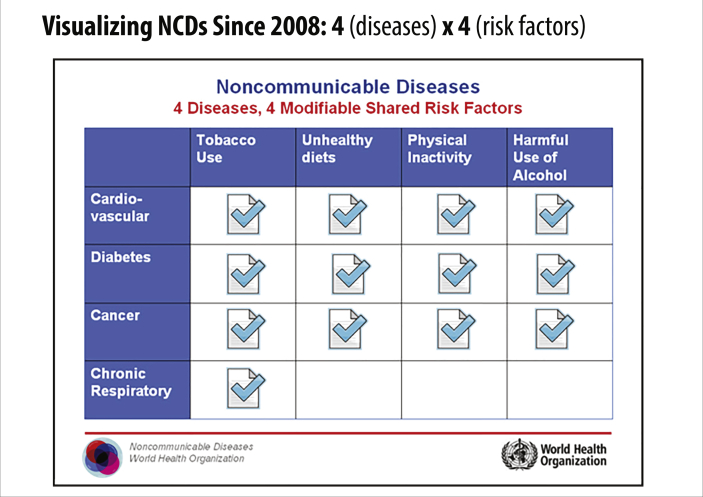
Following the release of the GBD study findings in May 2000, WHO's 52nd World Health Assembly passed resolution WHA51.18 calling for the implementation of the Global Strategy for the Prevention and Control of Noncommunicable Diseases. Both the resolution and the Global Strategy explicitly focused on what had by then become the characteristic “big four” diseases: cardiovascular disease, cancer, chronic pulmonary disease, and diabetes; similarly, they both referenced the most prominent risk factors: tobacco use, alcohol abuse, unhealthy diet, and physical inactivity (however, alcohol abuse was absent from the 2000 Global Strategy) (World Health Organization, 2000). The WHO's 4 × 4 framework as published in the 2008 WHO Action Plan for the Global Strategy is shown below in Fig. 4.
Fig. 4.
WHO's rendering of the 4 × 4 policy framework for NCDs.
While the 4 × 4 has endured and serves as an emblem of WHO's current NCD policies, its hegemonic status within the NCD sphere has resulted from targeted actions in the decades following its emergence. In 2003, the WHA endorsed the Framework Convention on Tobacco Control, a landmark treaty intended to protect populations from the harmful effects of tobacco consumption and exposure to tobacco smoke through regulation of tobacco pricing, taxing, packaging, and labeling (World Health Organization, 2003). Just one year later, in 2004, WHO released the Global Strategy on Diet, Physical Activity and Health (World Health Organization, 2004). Despite some pressure to take a strong regulatory approach, it was decided that, unlike its tobacco counterpart, the food industry had to be treated as a partner rather than an adversary (Weisz & Vignola-Gagné, 2015).
In a span of just four years, WHO managed to lay out policy strategy for three of the four risk factors in the 4 × 4 framework. Notably missing, of course, was alcohol abuse; Derek Yach attributes this to a successful effort on the part of the alcohol industry to lobby against its inclusion (Yach, 2018). In 2008, however, the industry's efforts ultimately failed when the WHA passed resolution WHA61.4 on Strategies to Reduce the Harmful Use of Alcohol (World Health Organization, 2010). That same year, WHO released the Action Plan for the Global Strategy, which prominently featured the 4 × 4 on its cover (World Health Organization, 2008).
As the 4 × 4 grew in prominence, criticism arose from those frustrated by the apparent narrowing in scope of WHO efforts around NCDs. In what appears to be an explicit attempt to address those concerns and to justify its promotion of the 4 × 4 framework, the authors of WHO's 2008 Action Plan mentioned by name a list of other NCDs, including osteoporosis, renal diseases, and oral diseases. The authors explain their exclusion by pointing out that those diseases do not share the same risk factors as the “big four” and, therefore, require different intervention strategies (World Health Organization, 2008).
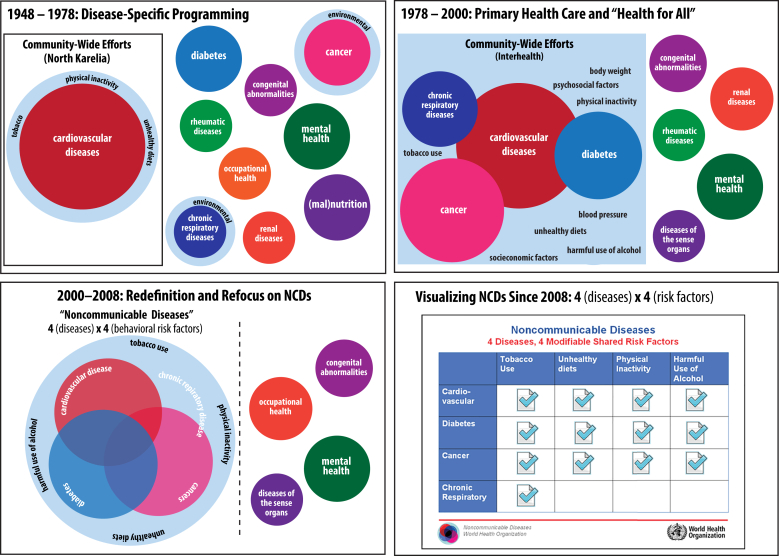
Fig. 5 summarizes this history graphically. The upper-left panel shows the period between 1948 and 1978. That period was marked by the emergence of a public health epistemology rooted in the correlation of lifestyle-modifiable risk factors as deployed to understand and intervene in cardiovascular disease developed first in Framingham, MA and translated into community-based intervention through the North Karelia Project in Finland. Importantly, this early public health research and intervention involved the initial boundary-work (Gieryn, 1983; Lamont & Molnár, 2002) that shaped early distinctions between conditions sharing lifestyle-modifiable risk factors and those with environmental, social, congenital, and other epidemiological drivers.
Fig. 5.
A graphical summary of the history of the evolution of the 4 × 4 framework for NCDs at the WHO.
Between 1978 and 2000, as depicted in the upper-right panel in Fig. 5, the boundary between conditions deemed practically linked through shared modifiable risk factors was sharpened by the growing evidence produced by community-led efforts of the INTERHEALTH studies. The bottom-left panel in Fig. 5 shows the crystallization of NCDs as a public health scientific and governance category which became synonymous with the narrowed 4 × 4 framework's lifestyle-modifiable risk factors and associated health conditions. The 4 × 4 framework's formal representation, drawn from the 2008 WHO Action Plan, is shown in the bottom-right panel of Fig. 5.
Despite its gaps, the 4 × 4 framework has been featured in every major WHO NCD policy and publication since its emergence in 2000. And, while WHO itself has taken steps to promote it, NGOs and allied organizations aided in its publicization. One notable example is that of the Oxford Health Alliance's (OxHA) 3Four50 model (three risk factors lead to four diseases, which contribute to more than 50% of the global disease burden), launched in 2003 (Suhrcke et al., 2006). With Yach both an original member of OxHA and an official at WHO at the time, the model, both catchy and succinct, underscored the utility of effective communication and influenced the global NCD agenda.
More recently, the United Nations has taken up the cause of NCDs, beginning in 2011 with the first UN High-Level Meeting on NCDs, followed by a second in 2014, and a third in 2018 (the first of such meetings on a health topic since the auspicious UN High-Level Meeting on HIV/AIDS in 2000). The NCD High-Level Meetings have generated a flurry of research, political statements, economic analyses, and public declarations, but have yet to galvanize much in the way of new resources for the poorest countries (Bukhman et al., 2020).
Very few multilateral, bilateral, or private philanthropic donors have been committed to funding evidence-based NCD care and health systems strengthening programs (Bukhman et al., 2020). The percent of all global health financing (official development assistance, or ODA) for NCDs has remained constant over the last 15 years at approximately 1–2% of the total, despite NCDs contributing more than 55% of the total global burden of illness and disability and 70% of all deaths globally (World Health Organization, 2018).
Additionally, there is a striking disparity in the investments made by global health and development financing agencies between infectious killers and NCDs: in 2010, a measly $.78 per disability adjusted life year (DALY) averted was invested in NCD care, treatment, and prevention programs. This is compared to a more robust (if still inadequate) investment of $23.90 per DALY averted invested in HIV, tuberculosis, and malaria care, treatment, and prevention programs (Nugent & Feigl, 2010).
5. Conclusion
This paper has traced the public health research and institutional factors that have led to the dominance of the 4 × 4 framework for NCDs at WHO. We have found that the 4 × 4 framework emerged as a generalization from strategies to control epidemics of cardiovascular disease in Europe and North America during the second half of the twentieth century. These strategies were focused on interventions to address behavioral “lifestyle” risk factors as well as pharmacotherapy for physiologic risk factors such as hypertension and hyperlipidemia. These cardiovascular strategies were noted to be important as well for some kinds of malignancies and chronic respiratory diseases, as well as Type II diabetes, and were packaged as an integrated approach initially in high-income countries and subsequently extended to low- and middle-income countries, where they have failed to address much of the NCD burden among the poor.
This research contributes novel understanding of the evolution of the 4 × 4 framework for NCDs at the WHO. The currently dominant, narrowly defined framing for NCDs amounted to a simpler governance tool for the WHO. It is a potent theoretical example of how public health categories are social constructions (Berger & Luckmann, 1966), used by governments and other civil society entities for the intend purpose of improving specific forms of population level health, often at the exclusion of others. The result of this history has been that the principal drivers of NCDs amongst the poorest populations have become rendered less visible to policy makers and ministries of health, leading to limited political attention and funding (Bukhman et al., 2020).
The third UN High-Level Meeting on NCDs, held in September of 2018, marked a significant departure from the shared risk factor framework that has dominated NCD framing at WHO since the 1980s (United Nations General Assembly, 2018). The third Meeting's inclusion of mental health and environmental risks, and emphasis on country-driven priority setting and people-centered care, opened the door to a more inclusive paradigm consistent with the aspirations of “precision public health” (Horton, 2018). We hope that future global NCD action plans will bring even greater attention to the true etiology and burden of NCDs and injuries amongst the poorest populations globally.
Authors’ statement
GB developed the initial idea for the paper. LNS wrote the initial draft of the manuscript and undertook the initial archival research. GB, LNS, and JDS conducted related interviews. All authors contributed significantly to subsequent revisions.
Ethical statement
The research for this manuscript was funded by Leona M. and Harry B. Helmsley Charitable Trust.
It was approved under the Harvard Medical School Institutional Review Board.
We have no financial conflicts of interest to disclose.
Acknowledgements
The authors would like to thank Laura Drown, Alma Alder, and Andrew Marx for their support with completing this project. We would like to thank Nicole Bassoff for her organization and initial analysis of the archival materials underpinning this research. We would also like to thank those individuals who contributed their time to be interviewed for this work.
Contributor Information
Leah N. Schwartz, Email: leah_schwartz@hms.harvard.edu.
Jonathan D. Shaffer, Email: jshaffer@bu.edu.
Gene Bukhman, Email: gene_bukhman@hms.harvard.edu.
References
- Abu-Bakare A., Gill G.V., Taylor R., Alberti K.G.M.M. Tropical or malnutrition-related diabetes: A real syndrome? Lancet. 1986;1(8490):1135–1138. doi: 10.1016/s0140-6736(86)91846-5. [DOI] [PubMed] [Google Scholar]
- Berger P.L., Luckmann T. Doubleday; Garden City, N.Y.: 1966. The social construction of reality: A treatise in the sociology of knowledge (Anchor books) [Google Scholar]
- Berrios X., Koponen T., Huiguang T., Khaltaev N., Puska P., Nissinen A. Distribution and prevalence of major risk factors of noncommunicable diseases in selected countries: The WHO inter-health programme. Bulletin of the World Health Organization. 1997;75(2):99–108. [PMC free article] [PubMed] [Google Scholar]
- Binagwaho A., Muhimpundu M.A., Bukhman G., NCD Synergies Group 80 under 40 by 2020: An equity agenda for NCDs and injuries. Lancet (London, England) 2014;383(9911):3–4. doi: 10.1016/S0140-6736(13)62423-X. [DOI] [PubMed] [Google Scholar]
- Birbeck G.L. Where did all the other non-communicable diseases go? BMJ. 2011;343:d5785. doi: 10.1136/bmj.d5785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourdieu P. The social space and the genesis of groups. Theory and Society. 1985;14(6) doi: 10.1007/BF00174048. [DOI] [Google Scholar]
- Brown P. Naming and framing: The social construction of diagnosis and illness. Journal of Health and Social Behavior. 1995:34–52. [PubMed] [Google Scholar]
- Bukhman G., Mocumbi A.O., Atun R., Becker A.E., Bhutta Z., Binagwaho A.…Mayosi B, Lancet NCDI Poverty Commission Study Group The Lancet NCDI poverty commission: Bridging a gap in universal health coverage for the poorest billion. The Lancet. 2020;396(10256):991–1044. doi: 10.1016/s0140-6736(20)31907-3. In this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad P., Barker K.K. The social construction of illness: Key insights and policy implications. Journal of Health and Social Behavior. 2010;51(S):S67–S79. doi: 10.1177/0022146510383495. [DOI] [PubMed] [Google Scholar]
- Executive Board 17 . World Health Organization; 1955. Regional committee for Europe: Report on fifth session.http://www.who.int/iris/handle/10665/130906 [Google Scholar]
- Foucault M. 1976. “Chapter 11: 17 march 1976” in society must be defended: Lectures at the College de France. 1975-6. [Google Scholar]
- Gieryn T.F. Boundary-work and the demarcation of science from non-science: Strains and interests in professional ideologies of scientists. American Sociological Review. 1983;48(6):781–795. [Google Scholar]
- Glasunov V. World Health Organization Archives; 1981. Request for financial support for the integrated NCD prevention and control programme for 1981. [Google Scholar]
- Gutziller F. Seminars and symposia on noncommunicable diseases – general. World Health Organization Archives; 1993, Sep. 29. Monitoring of cardiovascular disease and risk factor trends: Experiences from the WHO/MONICA project. [Google Scholar]
- Haas P.M. Introduction: Epistemic communities and international policy coordination. International Organization. 1992;46(1):1–35. [Google Scholar]
- Horton R. Offline: In defence of precision public health. Lancet. 2018;392(10157):1504. doi: 10.1016/S0140-6736(18)32741-7. [DOI] [PubMed] [Google Scholar]
- Lamont M., Molnár V. The study of boundaries in the social sciences. Annual Review of Sociology. 2002;28:167–195. doi: 10.1146/annurev.soc.28.110601.141107. 2002. [DOI] [Google Scholar]
- Mahmood S.S., Levy D., Vasan R.S., Wang T.J. The Framingham heart study and the epidemiology of cardiovascular disease: A historical perspective. Lancet (London, England) 2014;383(9921):999–1008. doi: 10.1016/S0140-6736(13)61752-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathers C., Ho J. World Health Organization; 2017. WHO methods and data sources for global burden of disease Estimates 2000-2015.https://www.who.int/healthinfo/global_burden_disease/GlobalDALYmethods_2000_2015.pdf?ua=1 [Google Scholar]
- Mensah G.A., Mayosi B.M. The 2011 united Nations high-level meeting on non-communicable diseases: The Africa agenda calls for a 5-by-5 approach. South African Medical Journal. 2012;103(2):77–79. doi: 10.7196/samj.6347. [DOI] [PubMed] [Google Scholar]
- Murray C.J., Yang G., Qiao X. Adult mortality: Levels, patterns and causes. In: Feachem R.G.A., Kjellstrom T., Murray C.J.L., Over M., Phillips M.A., editors. The health of adults in the developing world. Oxford University Press; New York: 1992. pp. 23–111. [Google Scholar]
- Nissinen A., Berrios X., Puska P. Community-based noncommunicable disease interventions: Lessons from developed countries for developing ones. Bulletin of the World Health Organization. 2001;79(10):963–970. [PMC free article] [PubMed] [Google Scholar]
- Nugent R., Feigl A. Where have all the donors gone? Scarce donor funding for non-communicable diseases. SSRN Electronic Journal. 2010 doi: 10.2139/ssrn.1824392. [DOI] [Google Scholar]
- Omran A.R. The epidemiologic transition: A theory of the epidemiology of population change. Milbank Memorial Fund Quarterly. 1971;49(4):509–538. doi: 10.2307/3349375. [DOI] [PubMed] [Google Scholar]
- Oppenheimer G.M., Blackburn H., Puska P. From Framingham to North Karelia to U.S. community-based prevention programs: Negotiating research agenda for coronary heart disease in the second half of the 20th century. Public Health Reviews. 2011;33(2):450–483. doi: 10.1007/BF03391646. [DOI] [Google Scholar]
- Puska P. Successful prevention of non-communicable diseases: 25 year experiences with North Karelia project in Finland. Public Health Medicine. 2002;4(1):507. [Google Scholar]
- Puska P., Lepariski E., Lamm G., Heine H., Pereira J., Pisa Z., Thelle D. World Health Organization; 1988. Comprehensive cardiovascular community control programmes in Europe.https://apps.who.int/iris/handle/10665/260476 [Google Scholar]
- Raviola G., Becker A.E., Farmer P. A global scope for global health--including mental health. Lancet (London, England) 2011;378(9803):1613–1615. doi: 10.1016/S0140-6736(11)60941-0. [DOI] [PubMed] [Google Scholar]
- Regional Office for Europe and World Health Organization . World Health Organization; 1996. Annual meeting of CINDI programme directors: Report of a WHO meeting, Casteldefels, Spain, 2-3 June 1995. [Google Scholar]
- Sangaramoorthy T., Benton A. 2012. Enumeration, identity, and health. Medical Anthropology: Cross cultural studies in health and illness. [DOI] [PubMed] [Google Scholar]
- Seventy-first World Health Assembly . World Health Assembly; 2018. Rheumatic fever and rheumatic heart disease: Report by the Director General. [Google Scholar]
- Suhrcke M., Nugent R.A., Stuckler D., Rocco L. Oxford Health Alliance; 2006. Chronic disease: An economic perspective. [Google Scholar]
- The CARMEN Network . Pan-American Health Association; 2002. CARMEN: An initiative for integrated prevention of noncommunicable diseases in the Americas. [Google Scholar]
- Thirty-Eighth World Health Assembly . World Health Organization; 1985. Resolution of the world health assembly 28.30: Prevention and control of noncommunicable diseases. [Google Scholar]
- United Nations . UN News; 2011. September 20). UN gathering on non-communicable diseases considers ways to combat scourge.https://news.un.org/en/story/2011/09/387532 [Google Scholar]
- United Nations . 2017. The sustainable development Goals report 2017.https://unstats.un.org/sdgs/report/2017/ [Google Scholar]
- United Nations General Assembly . 2011. Political declaration of the high-level meeting of the General Assembly on the prevention and control of non-communicable diseases.https://digitallibrary.un.org/record/710899/?ln=en [Google Scholar]
- United Nations General Assembly . 2018. Political declaration of the 3rd high-level meeting of the General Assembly on the prevention and control of non-communicable diseases.https://digitallibrary.un.org/record/1648984?ln=en [Google Scholar]
- Voss J.-P., Freeman R. Palgrave Macmillan in the UK is an imprint of Macmillan Publishers Limited; Basingstoke, Hampshire: 2016. Knowing governance: The epistemic construction of political order. Houndmills. [Google Scholar]
- Weisz G., Vignola- Gagné E. The World Health Organization and the globalization of chronic noncommunicable diseases. Population and Development Review. 2015;41(3):507–532. doi: 10.1111/j.1728-4457.2015.00070.x. [DOI] [Google Scholar]
- WHO Expert Committee on Cancer Control & World Health Organization . 1963. Cancer control: First report of an expert committee [meeting held in Geneva from 12 to 17 October 1962]http://www.who.int/iris/handle/10665/40552 [Google Scholar]
- WHO Expert Committee on Community Prevention and Control of Cardiovascular Disease & World Health Organization . World Health Organization; 1986. Community prevention and control of cardiovascular diseases: Report of a WHO expert committee. [PubMed] [Google Scholar]
- WHO Expert Committee on Diabetes Mellitus & World Health Organization . World Health Organization; 1965. Diabetes mellitus: Report of a WHO expert committee. [Google Scholar]
- WHO Expert Committee on Diabetes Mellitus & World Health Organization . World Health Organization; 1980. WHO expert committee on diabetes mellitus [meeting held in Geneva from 25 September to 1 October 1979: Second report. [PubMed] [Google Scholar]
- WHO Expert Committee on Prevention of Coronary Heart Disease & World Health Organization . World Health Organization; 1982. Prevention of coronary heart disease: Report of a WHO expert committee [meeting held in Geneva from 30 November to 8 December 1981]https://apps.who.int/iris/handle/10665/39293 [Google Scholar]
- WHO Regional Committee for Europe . World Health Organization; 1955. Regional committee for Europe: Report on fifth session.http://www.who.int/iris/handle/10665/130906 [Google Scholar]
- WHO Regional Office for Europe . Collaboration with the Government of The Netherlands. World Health Organization; 1957. The public health aspects of chronic disease: Report of a symposium sponsored by the regional Office for Europe of the world health organization. [Google Scholar]
- World Health Organization . 1951. WHO programmes in Europe: Guiding principles: Proposed four-year programme of work.http://www.who.int/iris/handle/10665/128073 [Google Scholar]
- World Health Organization . 1993. Non-communicable diseases – past, present, and future activities: An evaluation report.http://www.who.int/iris/handle/10665/171792 [Google Scholar]
- World Health Organization . 2000. Global strategy for the prevention and control of noncommunicable diseases. [Google Scholar]
- World Health Organization . 2003. WHO framework convention on tobacco control. [Google Scholar]
- World Health Organization . 2004. Global Strategy on Diet, Physical Activity and Health. [Google Scholar]
- World Health Organization . 2008. 2008-2013 action plan for the global strategy for the prevention and control of noncommunicable diseases: Prevent and control cardiovascular diseases, cancers, chronic respiratory diseases and diabetes. [Google Scholar]
- World Health Organization . 2010. Global strategy to reduce the harmful use of alcohol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . 2013. Draft comprehensive global monitoring framework and targets for the prevention and control of noncommunicable diseases.https://apps.who.int/iris/handle/10665/105633 [Google Scholar]
- World Health Organization . 2018. Time to deliver: Report of the WHO independent high-level commission on noncommunicable diseases.https://apps.who.int/iris/handle/10665/272710 [Google Scholar]
- Yach D. 2018, February 27. Personal communication [telephone interview] [Google Scholar]