Abstract
Background
Human metapneumovirus (HMPV) is a leading cause of respiratory tract infections. Few studies have compared the clinical characteristics and severity of HMPV-associated pneumonia with other pathogens.
Methods
Active, population-based surveillance was previously conducted for radiographically confirmed, community-acquired pneumonia hospitalizations among children and adults in 8 United States hospitals. Clinical data and specimens for pathogen detection were systematically collected. We described clinical features of all HMPV-associated pneumonia and, after excluding codetections with other pathogen types, we compared features of HMPV-associated pneumonia with other viral, atypical, and bacterial pneumonia and modeled the severity (mild, moderate, and severe) and length of stay using multivariable proportional odds regression.
Results
HMPV was detected in 298/2358 (12.6%) children and 88/2320 (3.8%) adults hospitalized with pneumonia and was commonly codetected with other pathogens (125/298 [42%] children and 21/88 [24%] adults). Fever and cough were the most common presenting symptoms of HMPV-associated pneumonia and were also common symptoms of other pathogens. After excluding codetections in children (n = 1778), compared to HMPV (reference), bacterial pneumonia exhibited increased severity (odds ratio [OR], 3.66; 95% confidence interval [CI], 1.43–9.40), respiratory syncytial virus (RSV; OR, 0.76; 95% CI, .59–.99) and atypical (OR, 0.39; 95% CI, .19–.81) infections exhibited decreased severity, and other viral pneumonia exhibited similar severity (OR, 0.88; 95% CI, .55–1.39). In adults (n = 2145), bacterial (OR, 3.74; 95% CI, 1.87–7.47) and RSV pneumonia (OR, 1.82; 95% CI, 1.32–2.50) were more severe than HMPV (reference), but all other pathogens had similar severity.
Conclusions
Clinical features did not reliably distinguish HMPV-associated pneumonia from other pathogens. HMPV-associated pneumonia was less severe than bacterial and adult RSV pneumonia, but was otherwise as or more severe than other common pathogens.
Keywords: human metapneumovirus, community-acquired pneumonia, viral pneumonia
Clinical features did not reliably distinguish human metapneumovirus (HMPV) pneumonia from other pathogens. HMPV pneumonia was less severe than bacterial and adult respiratory syncytial virus pneumonia but was similar or more severe than other viral and atypical pathogens.
(See the Editorial Commentary by Papenburg and Alghounaim on pages 118–20.)
Human metapneumovirus (HMPV) is an important cause of acute respiratory illness (ARI) across all ages [1–7], with manifestations ranging from a mild upper respiratory tract infection to a lower respiratory tract infection (LRTI), including croup, bronchiolitis, and pneumonia. Younger children and older adults are most likely to experience severe disease [1, 2, 5, 6, 8, 9]. Previous studies indicate that HMPV and respiratory syncytial virus (RSV) exhibit similar clinical manifestations and seasonality [10], and are leading viral causes of LRTI [11–15]. However, few studies directly compare clinical features and outcomes of HMPV with other pathogens in cases of radiographically confirmed community-acquired pneumonia (CAP) [16].
The Centers for Disease Control and Prevention (CDC) Etiology of Pneumonia in the Community (EPIC) study was a prospective, multicenter, population-based, active surveillance study designed to determine the etiology of CAP in hospitalized children and adults [17, 18]. Systematic and prospective microbiological testing for respiratory pathogens was performed using multiple modalities, allowing for the detailed study of the clinical features and outcomes of HMPV-associated CAP across all ages. Our objectives were to determine the clinical features of HMPV pneumonia in children and adults, as compared with pneumonia caused by RSV, other viruses, atypical bacteria, and usual bacterial pathogens.
METHODS
Patient Enrollment and Data Collection
Children and adults were enrolled from January 2010 through June 2012 at 8 hospitals in Chicago, IL; Memphis, TN; Nashville, TN; and Salt Lake City, UT. Detailed study methods have been reported elsewhere [17, 18]. Briefly, patients were eligible if they were admitted to a study hospital, resided in the study catchment area, had evidence of an acute infection (fever, chills, hypothermia, leukocytosis, or leukopenia), had evidence of an ARI, and had radiographic findings compatible with pneumonia that were confirmed by independent study radiologists. Patients were excluded if they were recently hospitalized, had enrolled within the previous 28 days, or had an alternative respiratory diagnosis, tracheostomy, cystic fibrosis, cancer, a recent organ or stem cell transplant, graft-versus-host disease or bronchiolitis obliterans, or a human immunodeficiency virus (HIV) infection with CD4 <200 cells/mm3 or <14%. Children and adults residing in extended care facilities and unable to function independently were also excluded. Detailed demographic, epidemiologic, and clinical data were systematically collected. The study was approved by institutional review boards at each institution and by the CDC [17, 18].
Sample Collection and Laboratory Testing
Sera, blood, and nasopharyngeal/oropharyngeal (NP/OP) swabs were obtained as soon as possible after enrollment. Convalescent sera were collected 3–10 weeks later, when possible. Pleural fluid (PF), endotracheal (ET) aspirates, or bronchoalveolar (BAL) specimens, if obtained for routine clinical care, were evaluated. In adults, urine was collected, and sputum was obtained in those patients with a productive cough. Only samples collected ≤72 hours after admission were included, except for PF (≤7 days after admission) [17, 18].
Blood, PF, sputum, ET, and BAL specimens were cultured using standard techniques. Bacterial real-time polymerase chain reaction (PCR) was performed on PF for all ages, and whole-blood Streptococcus pneumoniae and Streptococcus pyogenes PCR was performed for children. For adults, Legionella pneumophila and S. pneumoniae urine antigen testing was performed. Legionella PCR assays were performed on sputum, regardless of the sputum quality [18].
NP/OP swabs were tested by a CDC-developed PCR assay [19] for the detection of HMPV; adenovirus (AdV); coronaviruses (CoV) 229E, HKU1, NL63, and OC43; human rhinovirus (HRV); influenza A/B viruses; parainfluenza viruses (PIV) 1, 2, and 3; RSV; Chlamydophila pneumoniae; and Mycoplasma pneumoniae. Serology for AdV, HMPV, influenza A/B, PIV, and RSV was performed on acute and convalescent sera [17, 18].
Pneumonia Definitions
HMPV-associated pneumonia was defined as pneumonia with HMPV virus detection by NP/OP swab PCR and/or a ≥4-fold rise in antibody titers between acute and convalescent sera. RSV-associated pneumonia was defined similarly. All other patients with pneumonia were considered negative for HMPV and/or RSV.
Some patients with HMPV-associated or RSV-associated pneumonia also had codetections with other pathogens. HMPV-only and RSV-only pneumonia were defined as the detection of only the single virus. Other viral pneumonia was defined as pneumonia with at least 1 other virus detected (AdV, CoV, influenza, HRV, or PIV, inclusive of codetections) by NP/OP swab PCR and/or a ≥4-fold rise in antibody titers between acute and convalescent sera without the codetection of HMPV, RSV, or bacteria [17, 18].
Bacterial pneumonia was defined as pneumonia with bacterial detection by blood, ET, BAL, PF culture, or PF PCR in any patient; bacterial detection by whole-blood PCR in children; or bacterial detection by urine antigen testing or sputum culture in adults [17, 18]. Patients in the “bacterial-only” category had only typical bacteria, such as S. pneumoniae, Staphylococcus aureus, and S. pyogenes, without atypical bacteria or any virus detected. Atypical bacterial pneumonia included detections of C. pneumoniae, M. pneumoniae, or L. pneumophila by NP/OP swab PCR or urine antigen without typical bacteria or viruses detected.
Outcomes: Disease Severity and Length of Stay
For children and adults, illness severity was categorized in HMPV, RSV, and bacterial mutually exclusive groups according to the following hierarchy: (1) severe pneumonia, defined as an in-hospital death, need for extracorporeal membrane oxygenation, acute respiratory distress syndrome, shock, and/or invasive mechanical ventilatory support; (2) moderate pneumonia, defined as an intensive care unit (ICU) admission without meeting the criteria for severe pneumonia; and (3) mild pneumonia, defined as all other hospitalized patients that did not require ICU admission [20]. We defined length of stay (LOS) as the time from admission to hospital discharge.
Statistical Analysis
Pediatric and adult analyses were performed separately and stratified by age group. We described patients with HMPV-associated pneumonia (any case in which HMPV was detected, including codetections with other pathogens). We compared features of HMPV-only pneumonia to those with RSV-only, other viral (sole or codetection of any combination of AdV, CoV, influenza, HRV, and/or PIV), bacterial-only, and atypical bacterial-only pneumonia, as well as pneumonia with no pathogens detected. Categories were mutually exclusive; viral-bacterial, viral-atypical, and atypical-bacterial codetections were excluded from these comparisons. Any viral codetection, including HMPV or RSV, was excluded. This analysis was limited to patients with at least 1 specimen available for both viral and bacterial pathogen detection, as previously described [17, 18]. Chi-square and Kruskal-Wallis tests were used to compare categorical and continuous variables, respectively, between and among groups, with P values ≤ .05 considered statistically significant. We performed a multivariable proportional odds regression to evaluate the association between the pneumonia etiologic category (HMPV only [reference], RSV only, other viral, atypical bacterial, bacterial, or no pathogen detected) and clinical disease severity by the 3-level scale. We used a multivariable proportional hazards regression to evaluate the association between the etiologic category and LOS. Covariates for the regression models were selected a priori and included site, year, age, race, sex, insurance status, influenza and pneumococcal vaccination history, antibiotic use and symptom duration prior to admission, cigarette smoke exposure, and presence of comorbidities. For all subjects, comorbidities included chronic kidney or liver disease, neurologic disorder, diabetes mellitus, immunosuppression, HIV infection (CD4 count > 200/mm3), and splenectomy. Comorbidities in children also included asthma/reactive airway disease, chromosomal disorders, and preterm birth (<2 years). Adult comorbidities included chronic lung or heart disease. Clustering of observations at the hospital level was accounted for using the Huber-White method for variance calculations in the regression models. Analyses were conducted in Stata Version 14.2 (StataCorp LLC, College Station, TX) and R (version 3.5.0).
RESULTS
Epidemiological Features of Human Metapneumovirus–Associated Community-acquired Pneumonia
Children
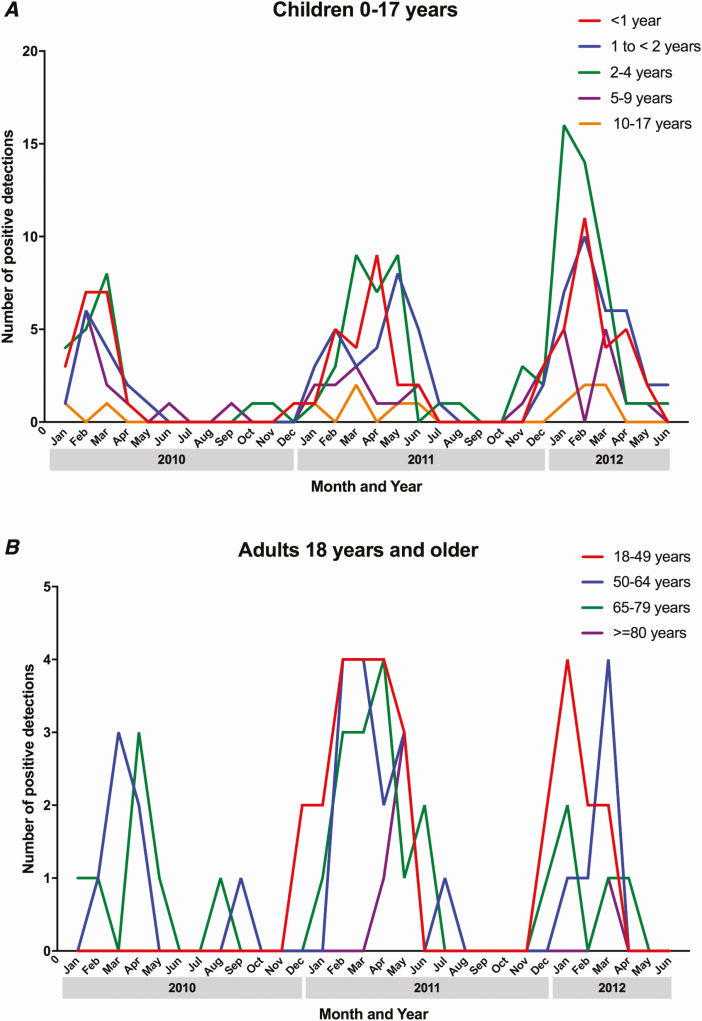
Overall, HMPV was detected in 298/2358 (12.6%) children (median age, 23 months) with pneumonia. In 173/298 (58%) cases, HMPV was the sole pathogen detected. HMPV was most commonly codetected with other viruses (97/125; 78%), including RSV, AdV, and/or HRV [18]; bacterial pathogens (13/125; 10%); or both bacterial and viral pathogens (8/125; 6%). Peak HMPV detections occurred in the winter and spring (Figure 1A). For HMPV, among those with specimens available for both viral and bacterial testing (n = 285), 252 (88%) were detected by PCR, 108 (38%) by serology, and 75 (26%) by both modalities [18, 21].
Figure 1.
Detection of human metapneumovirus by month and year among US (A) children and (B) adults with community-acquired pneumonia requiring hospitalization.
Adults
Human metapneumovirus was detected in 88/2320 (3.8%) adults with pneumonia (median age, 56.5 years). HMPV-associated pneumonia cases were relatively well-distributed among adult age groups, with the exception of the group ≥80 years old, in which only 5/316 (1.6%) cases of HMPV-associated pneumonia occurred. Of 88 HMPV cases in adults, HMPV was the sole pathogen detected in 67 (76%) cases. HMPV was most commonly codetected with other viruses (10/21, 48%), including RSV and/or PIV [17], or bacterial pathogens (9/21, 43%). Seasonal detection patterns were similar to those in children (Figure 1B). For HMPV pneumonia, 63 (72%) were detected by PCR, 9 (10%) by serology, and 16 (18%) by both modalities [17, 21].
Clinical Features of Human Metapneumovirus–Only Pneumonia Compared to Respiratory Syncytial Virus–Only Pneumonia
Children
When limited to children with samples tested for both viruses and bacteria, and after the exclusion samples from children with the codetection of other pathogen types, 1778 pediatric pneumonia cases were analyzed. In unadjusted comparisons, children with HMPV pneumonia were older than children with RSV (Table 1). Children with HMPV pneumonia were significantly less likely to have a cough (96%), dyspnea (64%), retractions (42%), documented wheezing (39%), and a white blood cell count >15 × 103 (13%) than those with RSV pneumonia (99%, 77%, 53%, 51%, and 17%, respectively; P ≤ .05 for all comparisons). Of HMPV pneumonia cases, 24% were characterized as moderate or severe, compared to 20% in children with RSV (P = .501; Table 3).
Table 1.
Select Characteristics of Hospitalized Children with Human Metapneumovirus–Only Pneumonia Compared with Respiratory Syncytial Virus, Other Viral, Bacterial, and Atypical Pneumonia
| HMPV-Only Pneumonia, n = 165, % (n) | RSV-Only Pneumonia, n = 358, % (n) | Other Viral Pneumonia, n = 665, % (n) | Atypical-Only Pneumonia, n = 133, % (n) | Bacterial-Only Pneumonia, n = 41, % (n) | No Pathogen Detected, n = 416, % (n) | P Value | |
|---|---|---|---|---|---|---|---|
| Female sex | 56% (92) | 53% (190) | 43% (284) | 38% (51) | 34% (14) | 43% (178) | <.001a |
| Median age, months (IQR) | 28.0 | 17.0 | 32.0 | 104.0 | 55.0 | 57.5 | <.001a |
| (14.0–51.0) | (7.0–30.5) | (13.0–72.0) | (67.0–140.0) | (16.0–104.0) | (18.0–113.8) | ||
| Age group | … | *** | … | … | … | … | <.001a |
| <1 year | 19% (31) | 35% (125) | 22% (144) | 2% (2) | 15% (6) | 15% (62) | |
| 1 to < 2 years | 25% (41) | 31% (110) | 21% (138) | 6% (8) | 12% (5) | 13% (56) | |
| 2–4 years | 37% (61) | 28% (99) | 26% (172) | 14% (19) | 29% (12) | 22% (92) | |
| 5–9 years | 14% (23) | 4% (13) | 21% (141) | 39% (52) | 29% (12) | 27% (113) | |
| 10–17 years | 5% (9) | 3% (11) | 11% (70) | 39% (52) | 15% (6) | 22% (93) | |
| Race/ethnicity | … | … | … | … | … | … | <.001a |
| Hispanic | 28% (46) | 22% (79) | 17% (111) | 14% (19) | 17% (7) | 13% (55) | |
| Non-Hispanic Black | 33% (54) | 34% (121) | 42% (276) | 16% (21) | 17% (7) | 35% (144) | |
| Non-Hispanic White | 28% (47) | 35% (125) | 34% (224) | 65% (87) | 59% (24) | 47% (194) | |
| Other | 11% (18) | 9% (33) | 8% (54) | 5% (6) | 7% (3) | 6% (23) | |
| Insurance | … | … | … | … | … | … | <.001a |
| None | 0% (0) | 1% (5) | 2% (12) | 3% (4) | 0% (0) | 1% (6) | |
| Public | 71% (117) | 70% (250) | 67% (444) | 44% (58) | 49% (20) | 51% (214) | |
| Private | 27% (45) | 28% (102) | 31% (207) | 53% (71) | 51% (21) | 47% (196) | |
| Other/unknown | 2% (3) | 0% (1) | 0% (2) | 0% (0) | 0% (0) | 0% (0) | |
| Received influenza vaccine | 44% (72) | 39% (139) | 33% (221) | 31% (41) | 22% (9) | 36% (151) | .057 |
| Received PCV or PPSV | 95% (156) | 88% (316) | 87% (577) | 68% (91) | 80% (33) | 78% (326) | <.001a |
| Daycare attendance, <6 years, n = 1404 | 23% (34) | 29% (102) | 33% (179) | 20% (11) | 24% (7) | 25% (70) | .295 |
| Any underlying comorbidityb | 47% (77) | 46% (165) | 55% (367) | 41% (54) | 27% (11) | 49% (204) | <.001a |
| Asthma | 30% (49) | 30% (107) | 43% (285) | 28% (37) | 20% (8) | 29% (119) | <.001a |
| Household smoke exposure | 36% (60) | 38% (135) | 39% (259) | 26% (34) | 39% (16) | 30% (126) | .051 |
| Symptom duration prior to admission, days (IQR) | 4.0 | 3.0 | 2.0 | 7.0 | 4.0 | 3.0 | <.001a |
| (2.0–5.0) | (2.0–5.0) | (1.0–4.0) | (5.0–9.0) | (2.0–8.0) | (1.0–7.0) | ||
| Antibiotics prior to admission | 21% (34) | 24% (85) | 20% (131) | 55% (73) | 32% (13) | 29% (121) | <.001a |
| Clinical presentation | |||||||
| Fever/feverish | 97% (160) | 96% (342) | 87% (576) | 93% (124) | 95% (39) | 90% (375) | <.001a |
| Cough | 96% (159) | 99% (356)** | 94% (622) | 95% (127) | 90% (37) | 89% (371) | <.001a |
| Dyspnea | 64% (106) | 77% (274)** | 72% (480) | 62% (82) | 76% (31) | 65% (271) | <.001a |
| Retractions | 42% (69) | 53% (191)* | 45% (299) | 24% (32) | 44% (18) | 39% (163) | <.001a |
| Wheezing | 39% (64) | 51% (181)* | 49% (328) | 25% (33) | 12% (5) | 28% (115) | <.001a |
| Rhinorrhea | 78% (129) | 81% (291) | 72% (478) | 41% (54) | 54% (22) | 53% (222) | <.001a |
| Abdominal pain | 14% (23) | 12% (44) | 23% (150) | 45% (60) | 41% (17) | 29% (121) | <.001a |
| Oxygen requirement | 75% (123) | 72% (257) | 58% (387) | 64% (85) | 59% (24) | 50% (209) | <.001a |
| WBC count > 15 × 103 | 13% (22) | 17% (60)* | 34% (226) | 18% (24) | 54% (22) | 44% (183) | <.001a |
| Radiographic features | … | … | … | … | … | … | <.001a |
| Single lobar | 20% (33) | 14% (51) | 23% (154) | 26% (35) | 34% (14) | 33% (139) | |
| Multi lobar | 21% (34) | 22% (78) | 23% (151) | 20% (26) | 37% (15) | 25% (104) | |
| Other consolidation | 1% (1) | 7% (24) | 7% (48) | 3% (4) | 5% (2) | 5% (19) | |
| Other infiltrate | 50% (83) | 49% (176) | 41% (271) | 44% (58) | 20% (8) | 30% (126) | |
| Mixed infiltrate | 8% (14) | 8% (29) | 6% (41) | 8% (10) | 5% (2) | 7% (28) | |
| Empyema | 7% (12) | 4% (15) | 6% (42) | 21% (28) | 66% (27) | 19% (77) | <.001a |
| Length of stay, hr, median (IQR) | 68.0 | 70.5 | 55.0 | 58.0 | 160.0 | 62.5 | <.001a |
| (46.0–111.0) | (46.2–114.8) | (38.0–89.0) | (41.0–88.0) | (87.0–240.0) | (38.0–107.0) |
n = 1778. “HMPV-only” indicates the detection of HMPV without the codetection of any other virus or bacteria. “RSV-only” indicates the detection of RSV without the codetection of any other virus or bacteria. “Other viral” indicates pneumonia with at least 1 other virus detected (adenovirus, coronaviruses, influenza, human rhinovirus, or parainfluenza viruses, inclusive of codetections of more than 1 of these viruses). “Atypical-only” indicates that Chlamydophila pneumoniae, Mycoplasma pneumoniae, or Legionella pneumophila was detected without bacteria or viruses detected. “Bacterial-only” indicates the detection of typical bacteria without atypical bacteria or any virus detected. Asterisks indicate statistical significance (P < .05) in a bivariate comparison (HMPV vs RSV; *P < .05; **P < .01; ***P < .001).
Abbreviations: HMPV, human metapneumovirus; IQR, interquartile range; PCV, pneumococcal conjugate vaccine; PPSV, pneumococcal polysaccharide vaccine; RSV, respiratory syncytial virus; WBC, white blood cell.
a P < .05 was considered statistically significant and represents P values for comparisons across all groups.
bAny comorbidity, including asthma, congenital heart disease, diabetes, chronic renal disease, chronic liver disease, immunosuppression, splenectomy, prematurity (assessed if <2 years), or neurological disorder.
Table 3.
Distribution of Cases of Mild, Moderate, and Severe Pneumonia by Etiology in Children and Adults
| HMPV-Only Pneumonia, % (n) | RSV-Only Pneumonia, % (n) | Other Viral Pneumonia, % (n) | Atypical-Only Pneumonia, % (n) | Bacterial-Only Pneumonia, % (n) | No Pathogen Detected, % (n) | P Value | |
|---|---|---|---|---|---|---|---|
| Children | n = 165 | n = 358a | n = 665 | n = 133 | n = 41 | n = 416 | <.001b |
| Mild | 76% (125) | 80% (286) | 80% (534) | 90% (120) | 56% (23) | 79% (327) | |
| Moderate | 18% (30) | 14% (51) | 13% (85) | 9% (12) | 15% (6) | 13% (55) | |
| Severe | 6% (10) | 6% (21) | 7% (46) | 1% (1) | 29% (12) | 8% (34) | |
| Adults | n = 67 | n = 55a | n = 391 | n = 74 | n = 169 | n = 1389 | <.001b |
| Mild | 81% (54) | 71% (39) | 81% (315) | 85% (63) | 52% (88) | 79% (1093) | |
| Moderate | 13% (9) | 11% (6) | 13% (50) | 12% (9) | 22% (37) | 12% (164) | |
| Severe | 6% (4) | 18% (10) | 7% (26) | 3% (2) | 26% (44) | 10% (132) |
Mild indicates hospitalized patients not meeting the criteria for moderate or severe disease. Moderate indicates ICU admission without meeting the severe disease criteria. Severe indicates an in-hospital death, a need for extracorporeal membrane oxygenation, acute respiratory distress syndrome, and/or requiring mechanical ventilatory support. The P values shown in the table represent the comparison among all etiologic groups listed.
Abbreviations: HMPV, human metapneumovirus; ICU, intensive care unit; RSV, respiratory syncytial virus.
aBivariate comparisons of HMPV vs RSV were performed; P = .501 (children) and P = .108 (adults).
b P < .05 was considered statistically significant.
Adults
When limited to adults with samples tested for both viruses and bacteria, and after the exclusion of codetections with other pathogen types, 2145 pneumonia cases were analyzed. In adults, only 4% of HMPV pneumonia cases occurred in the ≥80 age group, while 20% of RSV infections occurred in adults ≥80 (P = .023; Table 2). The prevalences of cough, dyspnea, and documented wheezing did not differ significantly between adults with HMPV and RSV, but those with HMPV were less likely to exhibit rhinorrhea (43%) than those with RSV (62%; P = .042). Of adult HMPV pneumonia cases, 19% were characterized as moderate or severe, compared to 29% in adults with RSV (P = .108; Table 3).
Table 2.
Select Characteristics of Hospitalized Adults with Human Metapneumovirus–Only Pneumonia Compared with Respiratory Syncytial Virus, Other Viral, Bacterial, and Atypical Pneumonia
| HMPV-Only Pneumonia, n = 67, % (n) | RSV-Only Pneumonia, n = 55, % (n) | Other Viral Pneumonia, n = 391, % (n) | Atypical-Only Pneumonia, n = 74, % (n) | Bacterial-Only Pneumonia, n = 169, % (n) | No Pathogen Detected, n = 1389, % (n) | P Value | |
|---|---|---|---|---|---|---|---|
| Female sex | 69% (46) | 62% (34) | 54% (211) | 43% (32) | 50% (84) | 50% (694) | .012a |
| Median age, years (IQR) | 56.0 | 64.0 | 55.0 | 51.0 | 61.0 | 58.0 | .002a |
| (44.5–70.5) | (52.0–75.5) | (44.0–70.0) | (38.2–64.0) | (50.0–71.0) | (47.0–71.0) | ||
| Age group | * | .001a | |||||
| 18–49 years | 34% (23) | 18% (10) | 36% (139) | 46% (34) | 22% (38) | 29% (402) | |
| 50–64 years | 28% (19) | 33% (18) | 31% (123) | 30% (22) | 36% (60) | 36% (494) | |
| 65–79 years | 33% (22) | 29% (16) | 19% (74) | 18% (13) | 30% (50) | 22% (304) | |
| 80 + years | 4% (3) | 20% (11) | 14% (55) | 7% (5) | 12% (21) | 14% (189) | |
| Race/ethnicity | … | … | … | … | … | … | .075 |
| Hispanic | 6% (4) | 7% (4) | 12% (48) | 18% (13) | 8% (14) | 10% (134) | |
| Non-Hispanic Black | 46% (31) | 36% (20) | 39% (151) | 31% (23) | 32% (54) | 40% (557) | |
| Non-Hispanic White | 43% (29) | 51% (28) | 45% (177) | 51% (38) | 57% (97) | 46% (636) | |
| Other | 4% (3) | 5% (3) | 4% (15) | 0% (0) | 2% (4) | 4% (62) | |
| Insurance | … | … | … | … | … | … | .057 |
| None | 19% (13) | 7% (4) | 17% (67) | 20% (15) | 12% (20) | 15% (210) | |
| Public | 48% (32) | 42% (23) | 52% (202) | 35% (26) | 53% (89) | 48% (668) | |
| Private | 33% (22) | 51% (28) | 29% (114) | 43% (32) | 34% (57) | 36% (495) | |
| Other/unknown | 0% (0) | 0% (0) | 2% (8) | 1% (1) | 2% (3) | 1% (16) | |
| Received influenza vaccine | 36% (24) | 44% (24) | 40% (158) | 32% (24) | 35% (59) | 38% (529) | .684 |
| Received PPSV | 33% (22) | 42% (23)* | 40% (158) | 32% (24) | 38% (65) | 38% (524) | .367 |
| Any underlying comorbidityb | 81% (54) | 73% (40) | 77% (302) | 58% (43) | 83% (141) | 79% (1099) | <.001a |
| Current smoker | 15% (10) | 29% (16) | 32% (125) | 24% (18) | 28% (48) | 25% (346) | .158 |
| Symptom duration prior to admission | 4.0 | 3.0 | 4.0 | 5.0 | 3.0 | 4.0 | .102 |
| (2.0, 7.0) | (2.0, 4.5) | (2.0, 7.0) | (3.0, 7.0) | (1.0, 7.0) | (1.0, 8.0) | ||
| Antibiotics prior to admission | 22% (15) | 27% (15) | 17% (67) | 22% (16) | 11% (18) | 21% (293) | .027a |
| Clinical presentation | |||||||
| Fever/feverish | 79% (53) | 78% (43) | 72% (283) | 86% (64) | 66% (112) | 65% (909) | <.001a |
| Cough | 96% (64) | 98% (54) | 94% (367) | 92% (68) | 79% (134) | 86% (1190) | <.001a |
| Dyspnea | 81% (54) | 76% (42) | 81% (318) | 66% (49) | 75% (126) | 78% (1085) | .074 |
| Wheezing | 49% (33) | 51% (28) | 39% (151) | 19% (14) | 27% (45) | 25% (342) | <.001a |
| Rhinorrhea | 43% (29) | 62% (34)* | 51% (199) | 22% (16) | 36% (61) | 34% (473) | <.001a |
| Abdominal pain | 19% (13) | 18% (10) | 23% (89) | 14% (10) | 21% (36) | 20% (279) | .566 |
| O2 requirement, n = 2144 | 49% (33) | 65% (36) | 56% (220) | 47% (35) | 66% (112) | 52% (717) | .002a |
| WBC count > 15 × 103 | 13% (9) | 27% (15) | 19% (75) | 19% (14) | 39% (66) | 25% (343) | <.001a |
| Radiographic features | … | … | … | … | … | … | <.001a |
| Single lobar | 34% (23) | 18% (10) | 27% (104) | 49% (36) | 30% (50) | 27% (375) | |
| Multi lobar | 25% (17) | 40% (22) | 24% (93) | 27% (20) | 28% (48) | 27% (372) | |
| Other consolidation | 4% (3) | 0% (0) | 5% (21) | 1% (1) | 8% (13) | 4% (56) | |
| Other infiltrate | 31% (21) | 38% (21) | 38% (147) | 14% (10) | 28% (47) | 35% (492) | |
| Mixed infiltrate | 4% (3) | 4% (2) | 7% (26) | 9% (7) | 7% (11) | 7% (94) | |
| Empyema | 3% (2) | 5% (3) | 7% (27) | 8% (6) | 20% (34) | 8% (105) | <.001a |
| Length of stay, days, median (IQR) | 3.0 | 3.0 | 3.0 | 3.0 | 6.0 | 3.0 | <.001a |
| (2.0–6.0) | (2.0–5.5) | (2.0–5.0) | (2.0–4.0) | (3.0–11.0) | (2.0–6.0) |
n = 2145. “HMPV-only” indicates the detection of HMPV without the codetection of any other virus or bacteria. “RSV-only” indicates the detection of RSV without the codetection of any other virus or bacteria. “Other viral” indicates pneumonia with at least 1 other virus detected (adenovirus, coronaviruses, influenza, human rhinovirus, or parainfluenza viruses, inclusive of codetections of more than 1 of these viruses). “Atypical-only” indicates that Chlamydophila pneumoniae, Mycoplasma pneumoniae, or Legionella pneumophila was detected without bacteria or viruses detected. “Bacterial-only” indicates the detection of typical bacteria without atypical bacteria or any virus detected. Asterisks indicate statistical significance (P < .05) in a bivariate comparison (HMPV vs RSV; *P < .05).
Abbreviations: HMPV, human metapneumovirus; IQR, interquartile range; PPSV, pneumococcal polysaccharide vaccine; RSV, respiratory syncytial virus; WBC, white blood cell.
a P < .05 was considered statistically significant and represents P values for comparisons across all groups.
bAny comorbidity, including chronic lung disease, chronic heart disease, diabetes, chronic renal disease, chronic liver disease, immunosuppression, splenectomy, and neurological disorder.
Clinical Features of Human Metapneumovirus–Only Pneumonia Compared to Other Viral and Bacterial Pathogens
Children
In unadjusted comparisons, among 1778 cases of pediatric pneumonia, excluding codetection with other pathogen types, children with HMPV-only pneumonia (165/1778; 9%) were younger (median 28 months) than children with other viral, atypical-only, and bacterial-only pneumonia (medians, 32, 104, and 55 months, respectively; Table 1) [18]. Gender also differed among pathogens, with HMPV exhibiting a slight female predominance. The proportion with an underlying comorbidity was higher in HMPV cases (47%) than in children with bacterial pneumonia (27%) and was similar to cases with other viral and atypical bacterial pneumonia (41–55%). Wheezing was more common in HMPV (39%) and other viral infections (49%) than in atypical bacterial (25%) or bacterial (12%) infections. The proportion of children requiring supplemental oxygen was highest in HMPV infections (123/165; 75%), and more common than in children with other viral, atypical bacterial, or bacterial infections (50–64%). Single or multi-lobar infiltrate patterns were seen on chest X-rays in 41% of HMPV infections, less commonly than in bacterial pneumonia cases (71%) and similar to in cases with other pathogen types (36–46%). Parapneumonic effusion was less common among children with HMPV (7%) and other viral (6%) infections than those with atypical bacterial (21%) or bacterial (66%) pneumonia. Pneumonia of moderate severity occurred in 18% of HMPV-only cases, slightly higher than the rates of pneumonia caused by bacteria (15%), other viruses (13%), and atypical bacteria (9%), while severe pneumonia occurred less commonly in HMPV (6%) and other types of pneumonia (1–8%) than in bacterial pneumonia cases (29%).
Adults
In unadjusted comparisons of pathogen types excluding codetection with other pathogens, HMPV was detected in 67/2145 (3.0%) adult patients with pneumonia. Females comprised the majority of HMPV (69%) pneumonia cases; gender was more equally distributed among other viral, atypical bacterial, and bacterial pneumonias (female, 43–54%; Table 2) [17]. The proportion with an underlying comorbidity in HMPV infections (81%) was similar to the rates in other viral (77%), and bacterial (83%) infections but more common than in atypical bacterial infections (58%). In contrast to pediatric cases, oxygen use was less common in patients with HMPV (49%) than in those with bacterial (66%) pneumonia. Single or multi-lobar consolidation was seen on chest X-rays in 59% of HMPV cases, less commonly than in atypical pneumonia (76%), but similar to other pathogens. The presence of a parapneumonic effusion was less common among adults with HMPV (3%) than in those with bacterial (20%) pneumonia. Severe pneumonia occurred less commonly with HMPV (6%) than in bacterial pneumonia cases (28%).
Clinical Severity and Length of Stay in Human Metapneumovirus Pneumonia Compared to Other Pathogens
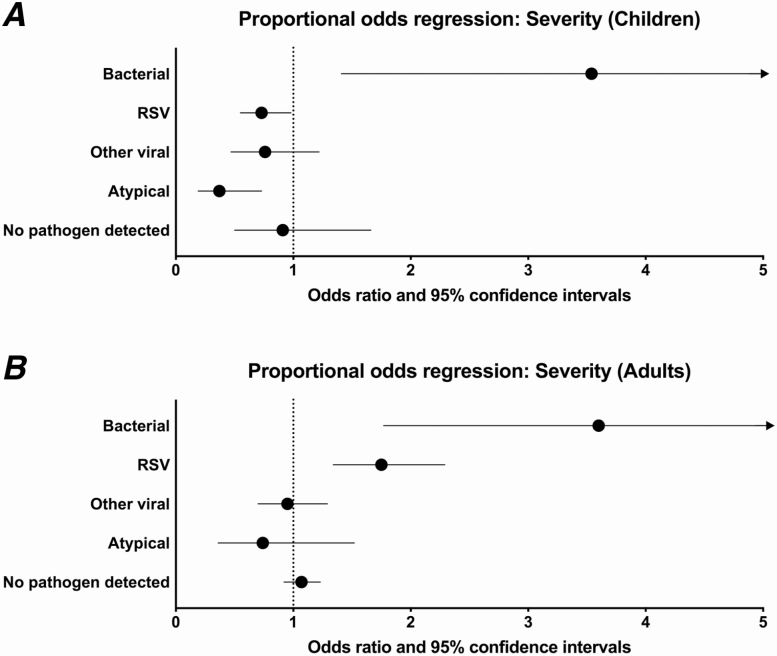
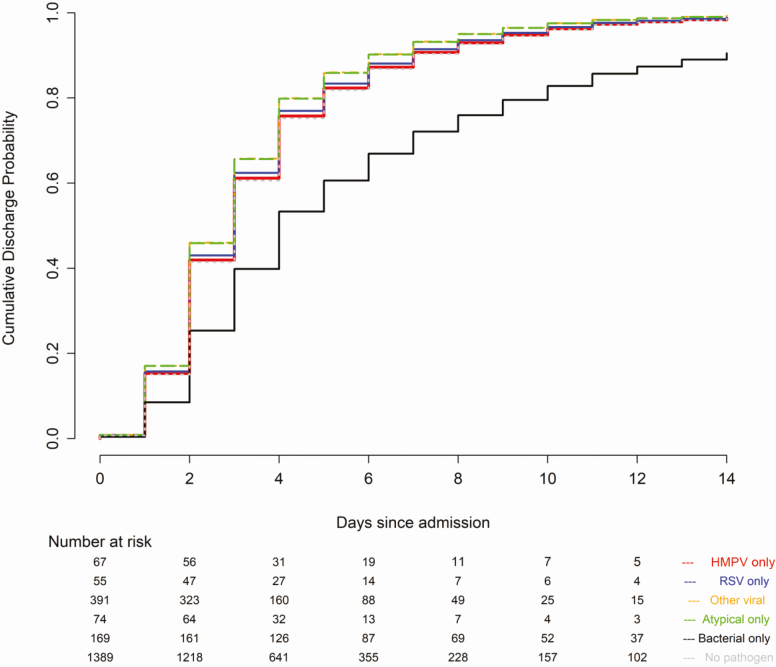
In a multivariable analysis in children, compared to HMPV pneumonia (reference), bacterial pneumonia was associated with increased severity (odds ratio [OR], 3.66; 95% confidence interval [CI], 1.43–9.40), while atypical bacterial pneumonia (OR, 0.39; 95% CI, .19–.81) and RSV (OR, 0.76; 95% CI, .59–.99) were associated with decreased disease severity (Figure 2A). The severity did not significantly differ between HMPV pneumonia and either other viruses (OR, 0.88; 95% CI, .55–1.39) or pneumonia with no pathogen detected (OR, 1.00; 95% CI, .55–1.82). Compared to HMPV, bacterial pneumonia was associated with a longer LOS (lower probability of discharge at a given time; adjusted hazard ratio [aHR], 0.40; 95% CI, .27–.61; Figure 3), whereas pneumonia caused by atypical bacteria (aHR, 1.21; 95% CI, 1.07–1.37) was associated with a decreased LOS. The lengths of stay in HMPV, RSV (aHR, 1.02; 95% CI, .83–1.24), other viral cases (aHR, 1.18; 95% CI, .97–1.43), and pneumonia with no pathogen detected (aHR, 1.03; 95% CI, .75–1.40) were similar (Figure 3).
Figure 2.
Proportional odds regression of severity (mild, moderate, severe) by pathogen type in (A) children and (B) adults (reference values: human metapneumovirus infection, white race, male sex, single lobar chest radiographic pattern, public insurance). Circles indicate odds ratios; lines represent 95% confidence intervals. Covariates include sex, age, race, year, study site, insurance status, chest X-ray pattern, antibiotics prior to admission, duration of symptoms prior to admission, influenza and pneumococcal vaccination status, smoke exposure (household smoke exposure for children, current smoker for adults), presence of an underlying comorbidity, and presence of empyema. Abbreviation: RSV, respiratory syncytial virus.
Figure 3.
Proportional hazards regression of time to hospital discharge in children (hours). Covariates include sex, age, race, year, study site, insurance status, chest X-ray pattern, antibiotics prior to admission, duration of symptoms prior to admission, influenza and pneumococcal vaccination status, household smoke exposure, presence of an underlying comorbidity, and presence of empyema. Abbreviations: HMPV, human metapneumovirus; RSV, respiratory syncytial virus.
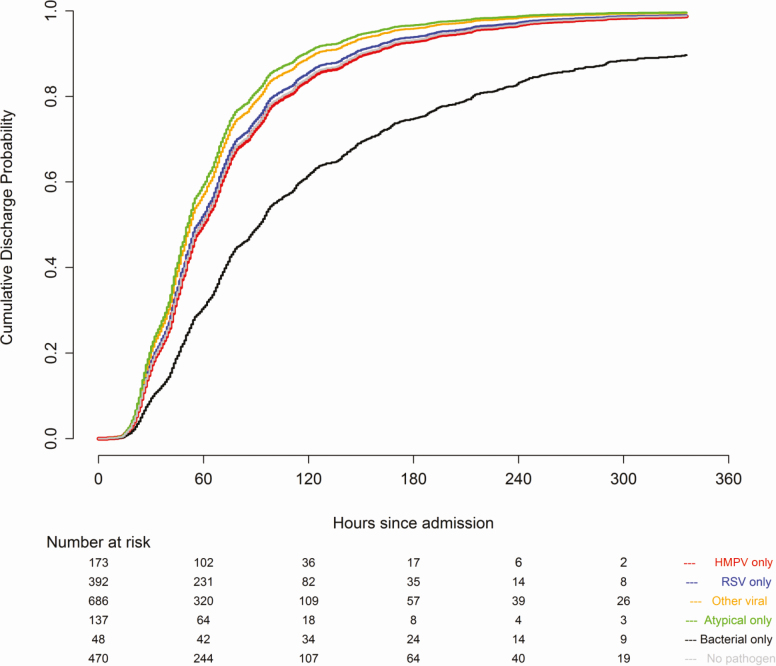
In adults, compared to HMPV (reference), both bacterial pneumonia (OR, 3.74; 95% CI, 1.87–7.47) and RSV (OR, 1.82; 95% CI, 1.32–2.50) were associated with an increased severity of illness, while HMPV pneumonia was similar in severity to infections caused by other viruses (OR, 0.96; 95% CI, .73–1.26), atypical bacterial pathogens (OR, 0.77; 95% CI, .38–1.55), and pneumonia with no pathogen detected (OR, 1.10; 95% CI, .98–1.24; Figure 2B). Compared to HMPV, bacterial pneumonia was associated with a longer LOS (lower probability of discharge; aHR, 0.48; 95% CI, .32–.73), while the LOS was similar among HMPV pneumonia and RSV (aHR, 1.02; 95% CI, .79–1.31), other viral (aHR, 1.11; 95% CI, .85–1.44), atypical bacterial (aHR, 1.13; 95% CI, .82–1.57), and no pathogen detected (aHR, 0.95; 95% CI, .72–1.27) pneumonia (Figure 4).
Figure 4.
Proportional hazards regression of time to hospital discharge in adults (days). Covariates include sex, age, race, year, study site, insurance status, chest X-ray pattern, antibiotics prior to admission, duration of symptoms prior to admission, influenza and pneumococcal vaccination status, current smoking status, presence of an underlying comorbidity, and presence of empyema. Abbreviations: HMPV, human metapneumovirus; RSV, respiratory syncytial virus.
DISCUSSION
In this multi-site, population-based surveillance study, HMPV infections contributed to 12.6% and 3.8% of radiographically confirmed CAP in hospitalized children and adults, respectively. HMPV detections peaked in the late winter and early spring, and we did not observe clear evidence that HMPV detections occurred earlier the season in children than adults. No clinical features could reliably distinguish HMPV-associated pneumonia from other pneumonia etiologies. In both children and adults, HMPV infections were associated with reduced severity relative to bacterial infections. In children, HMPV infections were associated with increased severity relative to RSV and atypical bacterial infections; in adults, HMPV pneumonia was less severe than RSV and similar to other viruses.
In studies of LRTI and CAP, the prevalence of HMPV infections typically ranges from 4–10% [1, 14, 16, 22–24], although some studies have reported prevalences as high as 17–18% [25, 26]. In the CDC New Vaccine Surveillance Network study of ARI, the annual hospitalization rate associated with HMPV infections was 1:1000 children <5 years, similar to rates for influenza and parainfluenza but lower than rates of RSV [1]. In a international study of severe childhood pneumonia, HMPV was among the 10 most common pathogens at each site and was estimated to account for 7.5% of pneumonia cases [27]. In our study, the annual incidence was 1.9 pneumonia hospitalizations per 10 000 children, with the highest incidence in the youngest age group (<2 years, 8.5 per 10 000) [18]. A previous study of adults presenting to emergency departments or hospitalized with ARI found a 2.6% prevalence of HMPV, with annual hospitalization rates of 10:10 000 in those ≥50 and 2:10 000 among those 18–49 years [5]. In adults in the EPIC study, the annual incidence was 1.9 hospitalizations per 10 000 adults, with the highest incidence in the older age groups [17]. Although most previous studies focused on LRTI or ARI and our study included only patients hospitalized with radiographically confirmed pneumonia, our range of prevalences is similar to these reports.
The median ages of children with HMPV-only and RSV-only pneumonia were 27.0 and 16.0 months, respectively, consistent with other reports that disease and hospitalizations for HMPV infections occur in slightly older children than RSV infections [15, 28–30]. In adults in our study, hospitalizations for HMPV-associated pneumonia were evenly distributed across age groups, except that adults >80 years were less often infected with HMPV.
In children and adults, HMPV pneumonia was associated with decreased severity relative to bacterial infections. In children, HMPV pneumonia was associated with increased severity relative to RSV infections. Other studies that have compared HMPV to RSV infections in children have generally reported similar severities between these 2 viruses [14, 29, 31–35], although 1 study of severe ARI reported a significantly longer duration of mechanical ventilation with HMPV compared to RSV [36], and another study of hospitalized children with an acute respiratory illness reported higher severities associated with RSV infections than HMPV [37]. Varying design and case definitions of respiratory illness (which may have been inclusive of bronchiolitis and not restricted to radiographically confirmed CAP) in other studies may limit direct comparisons to our findings. Currently, no licensed vaccines exist for HMPV, but several candidates are being evaluated [29]. Findings from our study and others support the need for additional efforts to develop a safe and effective vaccine.
Strengths of our study include active surveillance to systematically capture eligible patients using a rigorous case definition, comprehensive clinical data and sample collection, and the use of multiple modes of diagnostic testing to identify pathogens. The study also has several limitations. Not all patients had all diagnostic tests performed, including many who contributed acute but not convalescent serologies. The diagnostic tests applied have limited sensitivity, especially for bacterial detection. Moreover, bacteria may be more readily detected in cases with a complicated disease that warrants invasive procedures, thus biasing detections of bacterial pneumonia toward more severe cases. Milder, uncomplicated bacterial pneumonia cases may be underrepresented in the bacterial pneumonia group. Lower respiratory tract specimens were infrequently obtained. Antibiotic use prior to hospitalization may have reduced the yield of bacterial cultures [38]. Some pathogens were detected infrequently, making a multivariable analysis challenging; many direct comparisons of clinical features were unadjusted. Finally, our findings may be difficult to generalize to other health-care settings or geographical regions outside the United States.
In summary, no clinical characteristics were identified that could distinguish HMPV from other pathogen types at presentation, although patients with bacterial pneumonia exhibited more severe disease than those with HMPV pneumonia in both children and adults. Among children, HMPV pneumonia was associated with increased severity relative to RSV.
Notes
Acknowledgments. The authors thank the patients who graciously consented to participate in this study; Associated Regional and University Pathologists Laboratories, including Heather London and Torrance Meyer; BioFire Diagnostics, including Mark A. Poritz; the Centers for Disease Control and Prevention, including Suzette Bartley, Bernie Beall, Nicole Burcher, Robert Davidson, Michael Dillon, Barry Fields, Phalasy Juieng, and Shelley Magill; Le Bonheur Children’s Hospital, including Jody Cockroft, John Devincenzo, Tonya Galloway, Vivian Lebaroff, Moses Lockhart, Lakesha London, Tekita McKinney, Amanda Nesbit, Chirag Patel, Tina Pitt, Shante Richardson, Naeem Shaikh, Davida Singleton, and Mildred Willis; Monroe Carell Jr Children’s Hospital, including Thomas Abramo, Gretchen Edwards, Regina Ellis, Angela Harbeson, Deborah Hunter, Romina Libster, Angela Mendoza, Renee Miller, Deborah Myers, Natalee Rathert, Becca Smith, Bob Sparks, Kristy Spilman, Tanya Steinback, Scott Taylor, and Sandy Yoder; Primary Children’s Hospital, including Trenda Barney and Patrick Morris; St. Jude Children’s Research Hospital, including Edwina Anderson, Nancy Foster, Donna Nance, Ryan Heine, Amanda-Anderson Green, Amy Iverson, Shane Gansebom, Pat Flynn, Randall Hayden, and Kim Allison; and the University of Utah, including Fumiko Alger, Alexandra Burringo, Christopher Carlson, Lacey Collom, Gabriel Cortez, Kristina Grim, Keith Gunnerson, David Halladay, Caroline Heyrend, Jarrett Killpack, Kevin Martin, Brittany McDowell, Francesca Nichols, Parker Plant, Margaret Reid, Joshua Shimizu, Luke Schunk, Melanie Sperry, John Sweeley, and Lucy Williams.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention or the National Institutes of Health.
Financial support. This work was supported by the Influenza Division in the National Center for Immunizations and Respiratory Diseases at the Centers for Disease Control and Prevention, which funded the Etiology of Pneumonia in the Community (EPIC) study through cooperative agreements with each study site and was based on a competitive research funding opportunity. Work by L.M.H. has been performed as a Young Investigator Award recipient of the IDSA Education and Research Foundation (ERF) supported by Pfizer. L. M. H. is supported by the National Institutes of Health under award number 1K23AI141621.
Potential conflicts of interest. L. M. H. reports receiving grant support from Pfizer. W. S. receiving fees for serving on advisory boards for Pfizer, Merck, Cempra Pharmaceuticals, Ferring Pharmaceuticals, and BioTest AG, grant support through his institution from BioAegis, Pfizer, Merck, Rapid Pathogen Screening, BioMerieux, Ferring Pharmaceuticals and Gilead Sciences. D. J. W. receiving non-financial support from Biomerieux. R. G. W. receiving consulting fees from Roche, the Medicines Company, Vical, Cubist Pharmaceuticals, Bayer, Cerexa, and Visterra. C. G. G. has received consulting fees from Pfizer, Sanofi and Merck and received research support from Sanofi-Pasteur, Campbell Alliance, the Centers for Disease Control and Prevention, National Institutes of Health, The Food and Drug Administration, and the Agency for Healthcare Research and Quality. E. J. A. receiving grant support through his institution from MedImmune, Pfizer, Merck, Sanofi Pasteur, PaxVax, Novavax, GSK, and Micron Biomedical, consulting fees from AbbVie, and personal fees from Pfizer. S. R. A. receiving grant support from GlaxoSmithKline. K. A. receiving fees through his institution from GlaxoSmithKline and Cubist Pharmaceuticals for the enrollment of patients in other studies, collaborating with BioFire Diagnostics on grants funded by the National Institutes of Health, and receiving consultant fees from Merck. A. T. P. receiving fees for serving on an advisory board from BioFire Diagnostics, fees for the preparation of educational material from Medscape, royalties from Antimicrobial Therapy Inc., personal fees from WebMD, Genentech, Merck, and grants from the NIH. K. M. E. serving on a data and safety monitoring board for Novartis for which her institution receives fees, receiving other fees from Bionet, Roche, Sanofi, Sequiras, X4 Pharmaceuticals, and grants from the NIH. No other potential conflict of interest relevant to this article was reported. All other authors have no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Edwards KM, Zhu Y, Griffin MR, et al. ; New Vaccine Surveillance Network. Burden of human metapneumovirus infection in young children. N Engl J Med 2013; 368:633–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Williams JV, Harris PA, Tollefson SJ, et al. . Human metapneumovirus and lower respiratory tract disease in otherwise healthy infants and children. N Engl J Med 2004; 350:443–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Falsey AR, Erdman D, Anderson LJ, Walsh EE. Human metapneumovirus infections in young and elderly adults. J Infect Dis 2003; 187:785–90. [DOI] [PubMed] [Google Scholar]
- 4. Walsh EE, Peterson DR, Falsey AR. Human metapneumovirus infections in adults: another piece of the puzzle. Arch Intern Med 2008; 168:2489–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Widmer K, Griffin MR, Zhu Y, Williams JV, Talbot HK. Respiratory syncytial virus- and human metapneumovirus-associated emergency department and hospital burden in adults. Influenza Other Respir Viruses 2014; 8:347–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Widmer K, Zhu Y, Williams JV, Griffin MR, Edwards KM, Talbot HK. Rates of hospitalizations for respiratory syncytial virus, human metapneumovirus, and influenza virus in older adults. J Infect Dis 2012; 206:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Howard LM, Edwards KM, Zhu Y, et al. . Clinical features of human metapneumovirus infection in ambulatory children aged 5–13 years. J Pediatric Infect Dis Soc 2017; 7:165–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Caracciolo S, Minini C, Colombrita D, et al. . Human metapneumovirus infection in young children hospitalized with acute respiratory tract disease: virologic and clinical features. Pediatr Infect Dis J 2008; 27:406–12. [DOI] [PubMed] [Google Scholar]
- 9. Foulongne V, Guyon G, Rodière M, Segondy M. Human metapneumovirus infection in young children hospitalized with respiratory tract disease. Pediatr Infect Dis J 2006; 25:354–9. [DOI] [PubMed] [Google Scholar]
- 10. Papenburg J, Boivin G. The distinguishing features of human metapneumovirus and respiratory syncytial virus. Rev Med Virol 2010; 20:245–60. [DOI] [PubMed] [Google Scholar]
- 11. da Silva ER, Pitrez MC, Arruda E, et al. . Severe lower respiratory tract infection in infants and toddlers from a non-affluent population: viral etiology and co-detection as risk factors. BMC Infect Dis 2013; 13:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Homaira N, Luby SP, Petri WA, et al. . Incidence of respiratory virus-associated pneumonia in urban poor young children of Dhaka, Bangladesh, 2009-2011. PLOS One 2012; 7:e32056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Singleton RJ, Bulkow LR, Miernyk K, et al. . Viral respiratory infections in hospitalized and community control children in Alaska. J Med Virol 2010; 82:1282–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wolf DG, Greenberg D, Kalkstein D, et al. . Comparison of human metapneumovirus, respiratory syncytial virus and influenza A virus lower respiratory tract infections in hospitalized young children. Pediatr Infect Dis J 2006; 25:320–4. [DOI] [PubMed] [Google Scholar]
- 15. Wu A, Budge PJ, Williams J, et al. . Incidence and risk factors for respiratory syncytial virus and human metapneumovirus infections among children in the remote highlands of Peru. PLOS One 2015; 10:e0130233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Feikin DR, Fu W, Park DE, et al. ; PERCH Study Group. Is higher viral load in the upper respiratory tract associated with severe pneumonia? Findings from the Pneumonia Etiology Research for Child Health Study. Clin Infect Dis 2017; 64:337–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jain S, Self WH, Wunderink RG, et al. ; Centers for Disease Control and Prevention (CDC) Etiology of Pneumonia in the Community (EPIC) Study Team. Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med 2015; 373:415–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jain S, Williams DJ, Arnold SR, et al. ; Centers for Disease Control and Prevention (CDC) Etiology of Pneumonia in the Community (EPIC) Study Team. Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med 2015; 372:835–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Weinberg GA, Schnabel KC, Erdman DD, et al. . Field evaluation of TaqMan Array Card (TAC) for the simultaneous detection of multiple respiratory viruses in children with acute respiratory infection. J Clin Virol 2013; 57:254–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Williams DJ, Zhu Y, Grijalva CG, et al. Predicting severe pneumonia outcomes in children. Pediatrics. 2016; 138:e20161019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhang Y, Sakthivel SK, Bramley A, et al. . Serology enhances molecular diagnosis of respiratory virus infections other than influenza in children and adults hospitalized with community-acquired pneumonia. J Clin Microbiol 2017; 55:79–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wolf DG, Greenberg D, Shemer-Avni Y, Givon-Lavi N, Bar-Ziv J, Dagan R. Association of human metapneumovirus with radiologically diagnosed community-acquired alveolar pneumonia in young children. J Pediatr 2010; 156:115–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Johnstone J, Majumdar SR, Fox JD, Marrie TJ. Viral infection in adults hospitalized with community-acquired pneumonia: prevalence, pathogens, and presentation. Chest 2008; 134:1141–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Johnstone J, Majumdar SR, Fox JD, Marrie TJ. Human metapneumovirus pneumonia in adults: results of a prospective study. Clin Infect Dis 2008; 46:571–4. [DOI] [PubMed] [Google Scholar]
- 25. Choi SH, Hong SB, Ko GB, et al. . Viral infection in patients with severe pneumonia requiring intensive care unit admission. Am J Respir Crit Care Med 2012; 186:325–32. [DOI] [PubMed] [Google Scholar]
- 26. Jonnalagadda S, Rodríguez O, Estrella B, Sabin LL, Sempértegui F, Hamer DH. Etiology of severe pneumonia in Ecuadorian children. PLOS One 2017; 12:e0171687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pneumonia Etiology Research for Child Health Study Group. Causes of severe pneumonia requiring hospital admission in children without HIV infection from Africa and Asia: the PERCH multi-country case-control study. Lancet 2019; 394:757–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Henderson FW, Collier AM, Clyde WA Jr, Denny FW. Respiratory-syncytial-virus infections, reinfections and immunity. A prospective, longitudinal study in young children. N Engl J Med 1979; 300:530–4. [DOI] [PubMed] [Google Scholar]
- 29. Shafagati N, Williams J. Human metapneumovirus - what we know now. F1000Res 2018; 7:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Anderson EJ, Simões EA, Buttery JP, et al. . Prevalence and characteristics of human metapneumovirus infection among hospitalized children at high risk for severe lower respiratory tract infection. J Pediatric Infect Dis Soc 2012; 1:212–22. [DOI] [PubMed] [Google Scholar]
- 31. Paget SP, Andresen DN, Kesson AM, Egan JR. Comparison of human metapneumovirus and respiratory syncytial virus in children admitted to a paediatric intensive care unit. J Paediatr Child Health 2011; 47:737–41. [DOI] [PubMed] [Google Scholar]
- 32. Morrow BM, Hatherill M, Smuts HE, Yeats J, Pitcher R, Argent AC. Clinical course of hospitalised children infected with human metapneumovirus and respiratory syncytial virus. J Paediatr Child Health 2006; 42:174–8. [DOI] [PubMed] [Google Scholar]
- 33. Wang Y, Ji W, Chen Z, Yan YD, Shao X, Xu J. Comparison of severe pneumonia caused by human metapneumovirus and respiratory syncytial virus in hospitalized children. Indian J Pathol Microbiol 2014; 57:413–7. [DOI] [PubMed] [Google Scholar]
- 34. Jroundi I, Mahraoui C, Benmessaoud R, et al. . A comparison of human metapneumovirus and respiratory syncytial virus WHO-defined severe pneumonia in Moroccan children. Epidemiol Infect 2016; 144:516–26. [DOI] [PubMed] [Google Scholar]
- 35. Akhras N, Weinberg JB, Newton D. Human metapneumovirus and respiratory syncytial virus: subtle differences but comparable severity. Infect Dis Rep 2010; 2:e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Eggleston HA, Gunville CF, Miller JI, Sontag MK, Mourani PM. A comparison of characteristics and outcomes in severe human metapneumovirus and respiratory syncytial virus infections in children treated in an intensive care unit. Pediatr Infect Dis J 2013; 32:1330–4. [DOI] [PubMed] [Google Scholar]
- 37. Papenburg J, Hamelin MÈ, Ouhoummane N, et al. . Comparison of risk factors for human metapneumovirus and respiratory syncytial virus disease severity in young children. J Infect Dis 2012; 206:178–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Harris AM, Bramley AM, Jain S, et al. . Influence of antibiotics on the detection of bacteria by culture-based and culture-independent diagnostic tests in patients hospitalized with community-acquired pneumonia. Open Forum Infect Dis 2017; 4:ofx014. [DOI] [PMC free article] [PubMed] [Google Scholar]