Abstract
Osteochondral autograft transfer (OAT) allows for the treatment of focal chondral lesions of the femoral condyles. Patients undergoing OAT have been shown to have the greatest rate and quickest return to sport of any cartilage-restoration procedure. Disadvantages encountered with the OAT procedure include limited donor sources, small treatable lesion size, and donor-site morbidity. Here, we describe our preferred technique of open OAT with donor-site back-filling using precut fresh osteochondral allograft plugs and micronized extracellular cartilage augmentation. Advantages to this technique include single-stage transfer of living autologous osteochondral grafts allowing for early ambulation, predictable return to sport, enhanced long-term graft survival, and decreased donor-site morbidity secondary to fresh osteochondral allograft back-fill.
Focal cartilage defects in the knee are common, with a prevalence of roughly 36% in athletes and 63% in the general population.1 Consequently, cartilage-restoration surgery has developed as a way to treat pain, preserve physical function, and prevent arthritis progression. Osteochondral autograft transfer (OAT) allows for the treatment of 2- to 5-cm2 focal chondral lesions of the femoral condyles with or without underlying bony involvement (Table 1). A recent systematic review by Krych et al.2 showed that patients undergoing OAT have a 93% return-to-sport at a minimum of 2-year follow-up compared with 88% for osteochondral allograft transplantation (OCA) and 58% for microfracture (MFx).2 This same study showed that patients undergoing OAT had the quickest return-to-sport at an average of 5.2 ± 1.8 months, compared with 9.6 ± 3 months for OCA and 9.1 ± 2.2 months for MFx.2 Furthermore, long-term studies have shown that patients undergoing OAT have superior physical function and patient reported-outcome measures at 5, 10, and 15 years postoperatively compared with MFx.3,4 The main disadvantage of OAT is donor-site morbidity caused by the harvest of autograft plugs from the medial/lateral trochlea or intercondylar notch. Although harvested from areas of minimal weight-bearing, the donor sites may result in a persistent source of pain or mechanical irritation. Some studies have shown that failure of recipient sites on the lateral trochlea to fill in with fibrocartilage can result in mechanical irritation during patellofemoral tracking.5 Moreover, a case report by LaPrade and Botker6 showed that the donor sites may become overfilled with hypertrophic fibrocartilage, leading to knee pain and occasional locking. Both of these potential donor-site complications may be treated and/or prevented by back-fill of the donor-sites with precut fresh osteochondral allografts at the time of index surgery.
Table 1.
Surgical Indications and Contraindications for OAT With BioCartilage Augmentation and Donor-Site Back-Fill
| Indications | Contraindications |
|---|---|
| Focal cartilage defects of the femoral condyles (2-5 cm2) | Uncorrectable meniscal deficiency |
| Young high-demand patients with persistent symptoms despite conservative treatment | Uncorrectable mechanical malalignment |
| Prior unsuccessful surgical treatment of grade 3 or 4 cartilage lesions | Severe obesity Generalized osteoarthritis |
| Active infection or bone tumor | |
| Bipolar lesions (tibia and femur) |
OAT, osteochondral autograft transfer.
Surgical Technique (With Video Illustration)
Please see the narrated video for visual demonstration of the surgical technique described herein (Video 1).
Patient Positioning
The patient is placed in the supine position on a standard operating room table, and a regional nerve block is performed. A tourniquet is placed on the proximal thigh but is not inflated. A lateral leg post is placed at the level of the tourniquet to assist with visualization of the medial compartment of the knee during diagnostic arthroscopy. The operative extremity is then prepped and draped in usual sterile fashion and an examination of the knee under anesthesia is performed documenting range of motion and ligamentous stability.
Diagnostic Arthroscopy
Standard anterolateral and anteromedial portals are established, and a diagnostic arthroscopy is performed to determine lesion characteristics (location, morphology, grade, size) and assess the knee for additional intra-articular pathology (Fig 1). Once the size of the lesion is determined, the size and number of autograft plugs needed to fill the defect can be calculated. The authors prefer to use no more than 3 to 4 autograft plugs depending on the patient's anatomy. It is important that the surgeon determine the depth of bony involvement of the lesion on preoperative magnetic resonance imaging (MRI), as you do not want to leave behind any diseased bone (i.e. osteochondritis dissecans lesions) that may weaken graft fixation.
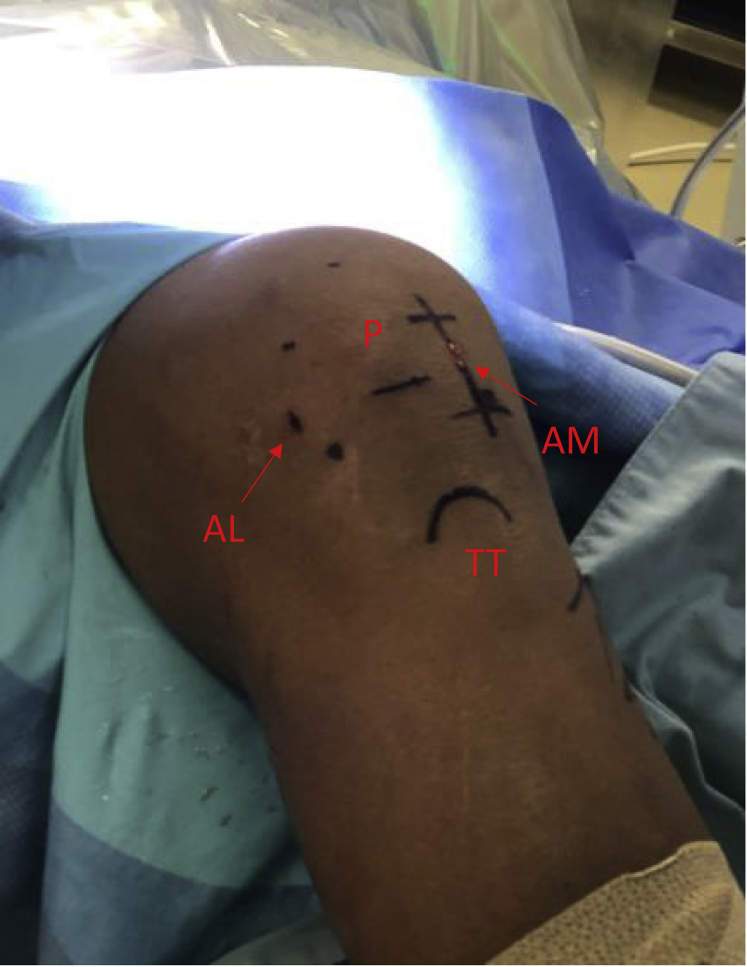
Fig 1.
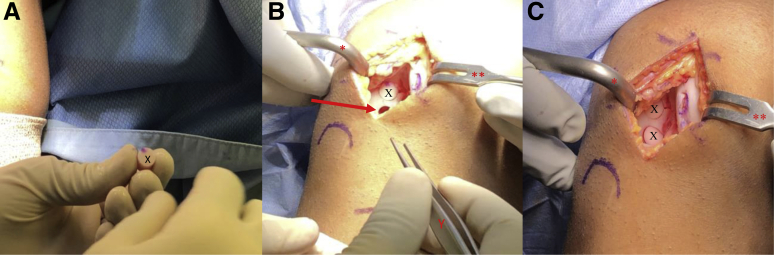
Surface markings for diagnostic arthroscopy and osteochondral autograft transfer of a right knee in the supine position. (P) Patella with a line at its inferior pole and dots medially, laterally, and superiorly. AL, anterolateral portal; AM, anteromedial portal with proximal and distal extension for mini-medial parapatellar arthrotomy; TT, tibial tubercle.).
Initial Recipient Site Preparation
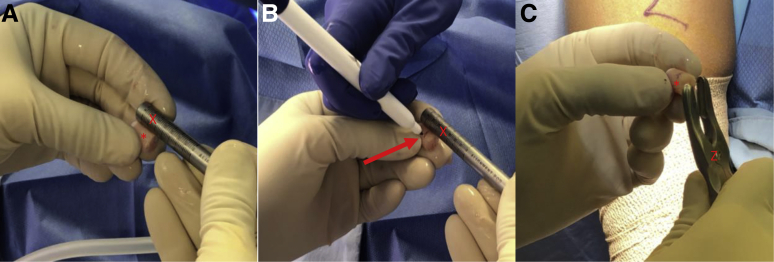
Following diagnostic arthroscopy, the authors prefer to make a small ∼6-cm medial or lateral vertical skin incision (depending on the location of the lesion) in-line with the previous arthroscopic portal (Fig 1). A corresponding arthrotomy is performed and a combination of bent Hohmann (placed along the intercondylar notch) and Paulsen retractor (placed on the outside of the affected condyle) are used to retract the patella and soft tissues exposing the affected condyle (Fig 2). The patient's knee is placed in the appropriate amount of flexion for visualization of the lesion and stabilized by the surgeon's assistant. A metal sizing tamp of the desired diameter (6, 8, or 10 mm) is then placed in the area of the chondral lesion to determine the exact location and number of the autograft plugs needed. The end of the metal sizing tamp is then colored with a marking pen and pressed against the chondral lesion, marking the area for future recipient site harvest (Fig 3, A and B). Care is taken to leave a 3- to 5-mm osteochondral bridge between future graft sites to ensure adequate graft fixation (Fig 3B). The authors then proceed with harvest of the donor autograft plugs.
Fig 2.
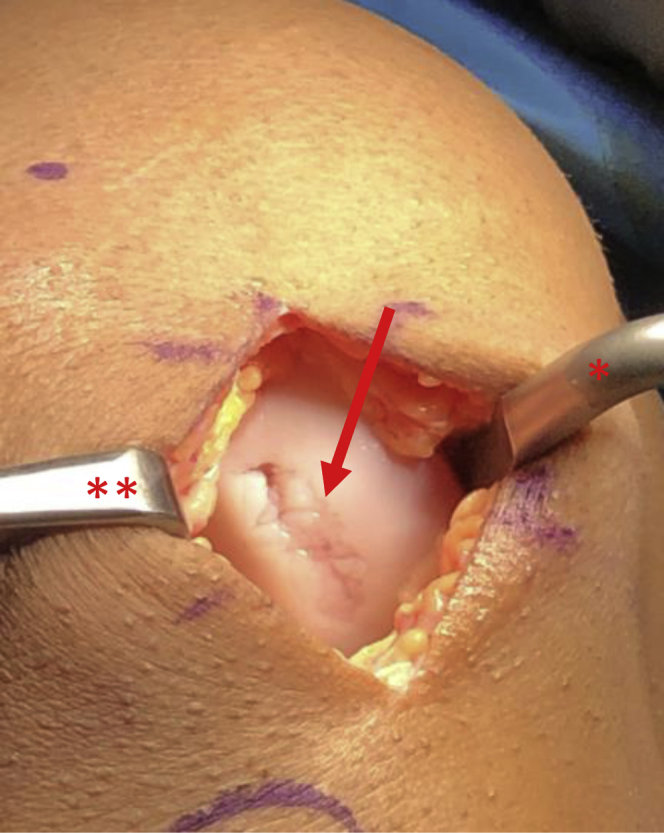
View through mini-medial parapatellar arthrotomy of the right knee in the supine position showing a 20 x 25-mm cartilage lesion (arrow). A Paulsen retractor (∗) is placed along the medial aspect of the medial femoral condyle retracting the soft tissues and a sharp bent Hohman (∗∗) is placed in the intercondylar notch in order to retract the patella.
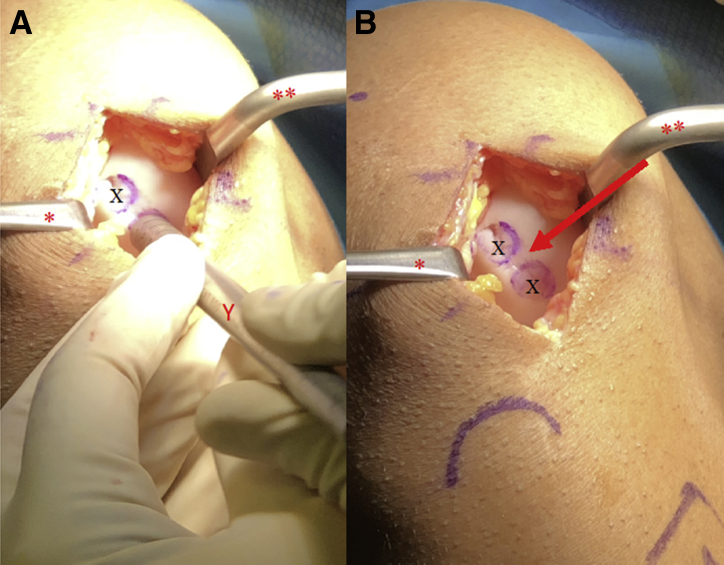
Fig 3.
View through a mini-medial parapatellar arthrotomy of the right knee in the supine position showing a 2 × 2.5-cm cartilage lesion. A Paulsen retractor (∗) is placed along the medial aspect of the medial femoral condyle retracting the soft tissues and a sharp bent Hohman (∗∗) is placed in the intercondylar notch in order to retract the patella. (A-B) A 10-mm diameter metal sizing tamp (Y) with its end colored by a marking pen is used to mark the locations of the recipient site harvests (Χ). Care is taken to leave a 5-mm osteochondral bridge (arrow) between graft sites.
Donor Graft Harvest
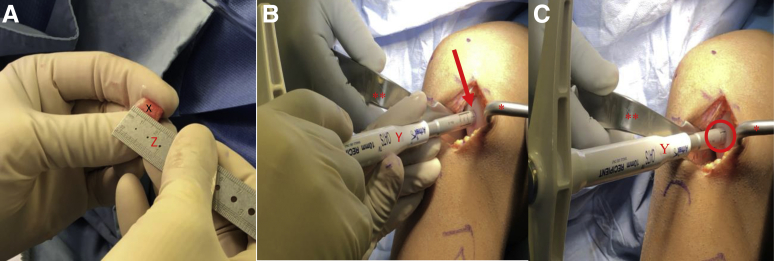
The authors' preferred graft harvest site is the lateral aspect of the intercondylar notch. If the chondral lesion is large, additional osteochondral plugs may be taken from the medial intercondylar notch or medial/lateral trochlea (non–weight-bearing zone). A disposable, commercially available, single-use OAT system harvester is used for donor graft harvest as well as recipient site preparation. Using this system, 6-, 8-, and 10-mm osteochondral plugs can be harvested. The appropriately sized OAT system harvester is then placed over the desired harvest site (Fig 4A). Care is taken to make sure that the harvester is placed perpendicular to the articular surface. The harvester is then impacted to the desired depth (usually at least 8-10 mm) (Fig 4B). While applying an axial load, the harvester handle is twisted 90° clockwise and then 90° counterclockwise to disengage the graft from the underlying subchondral bone (Fig 4C).7 The extruder stylus is then reinserted into the harvester handle to release the osteochondral plug from the harvester. These steps are repeated if more than one osteochondral plug is needed. If consecutive osteochondral plugs are taken from the same location, it is important to provide 3-5 mm of space between sites as to avoid convergence of the donor sites and truncation of the subchondral bone plugs (Fig 5).
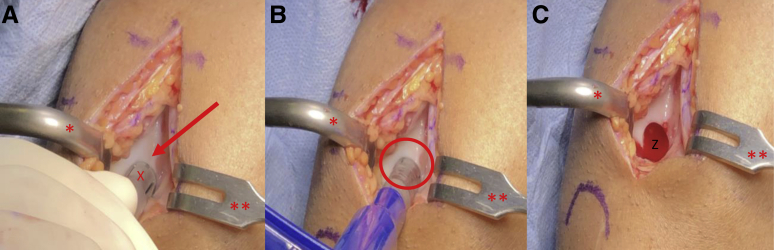
Fig 4.
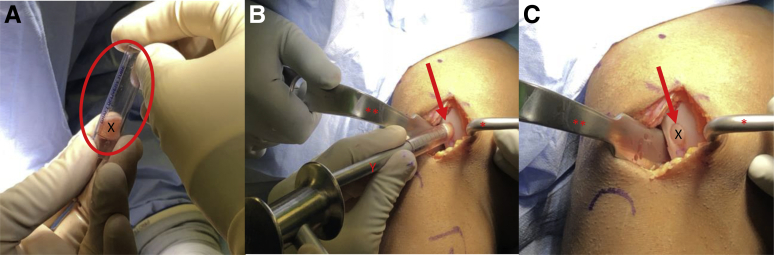
View through a mini-medial parapatellar arthrotomy of the right knee in the supine position. (A-C) A Paulsen retractor (∗) is placed on the lateral aspect of the lateral femoral condyle retracting the patella and a 2-prong rake (∗∗) is used to retract the medial soft tissues exposing the lateral aspect of the intercondylar notch (arrow). (A-C) A single-use osteochondral autograft transfer donor-site harvester (X) is placed along the lateral intercondylar ridge perpendicular to the articular surface. The harvester is impacted to the desired depth (oval) and removed, resulting in the extraction of a donor graft and a subsequent defect in the lateral intercondylar notch (Z).
Fig 5.
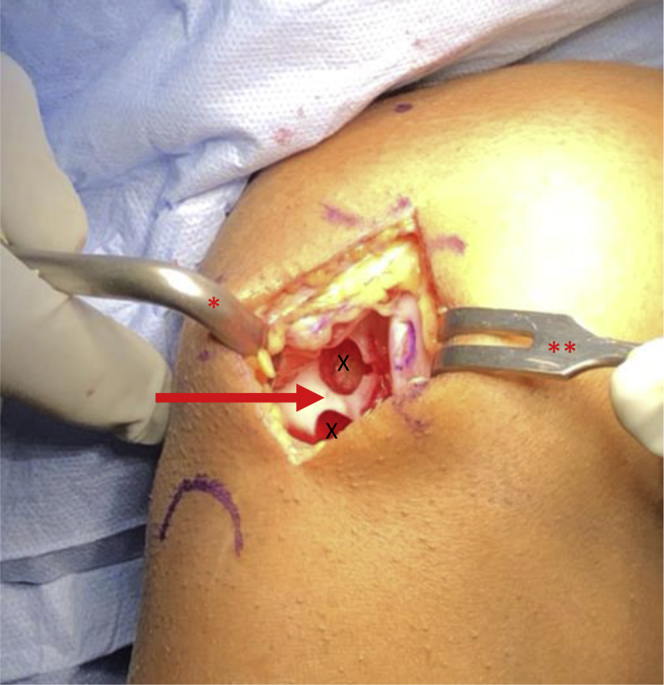
View through a mini-medial parapatellar arthrotomy of the right knee in the supine position. A Paulsen retractor (∗) is placed on the lateral aspect of the lateral femoral condyle retracting the patella and a 2-prong rake (∗∗) is used to retract the medial soft tissues exposing the lateral aspect of the intercondylar notch showing two empty donor graft harvest sites (X) with a 3- to 5-mm osteochondral bridge (arrow).
Precut Allograft Plug Preparation and Implantation
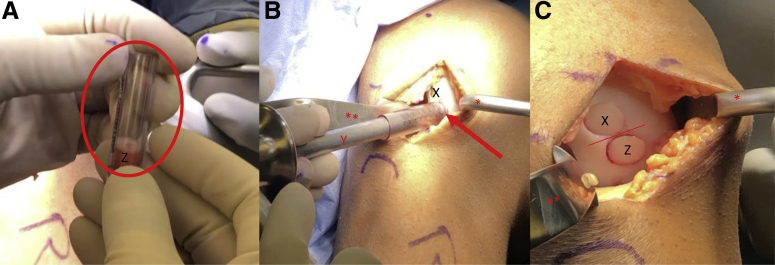
At this time, the desired number of fresh precut osteochondral allograft plug(s) may be removed from their media and placed on a back table for preparation. Fresh precut osteochondral allograft plugs from JRF Ortho (Centennial, CO) are available in 10 × 12-mm and 15 × 12-mm sizes. If plugs smaller than 10 mm in diameter are required, the same size disposable single-use OAT harvester used for the donor graft(s) can be used to downsize the plugs according to the technique previously described by the senior author.8 A metal sizing tamp is placed in each donor site, and the arthroscope is used to confirm the depth of the donor site at the 3-, 6-, 9-, and 12-o'clock positions (Fig 6, A and B). A skin marker and metal ruler or sizing tamp are then used to mark the appropriate lengths at the 12-, 9-, 6-, and 3-o'clock positions (Fig 7, A and B). A rongeur or sagittal saw may then be used to trim the bone plug to the desired length (Fig 7C). A skin marker is used to place a mark on the cartilaginous surface at the 12-o'clock position to assist with graft orientation (Fig 8A). Bone marrow elements are then flushed from the graft using 1 to 2 minutes of pulsatile lavage. The prepared osteochondral plug(s) may be soaked in bone marrow aspirate concentrate (BMAC) at this time if the surgeon desires. The grafts are then manually inserted into the donor-harvest sites (Fig 8B). A tamp and mallet may be used to gently impact the plug(s) so that the articular surface of each graft is sitting flush or slightly recessed to the existing articular surface (Fig 8C).
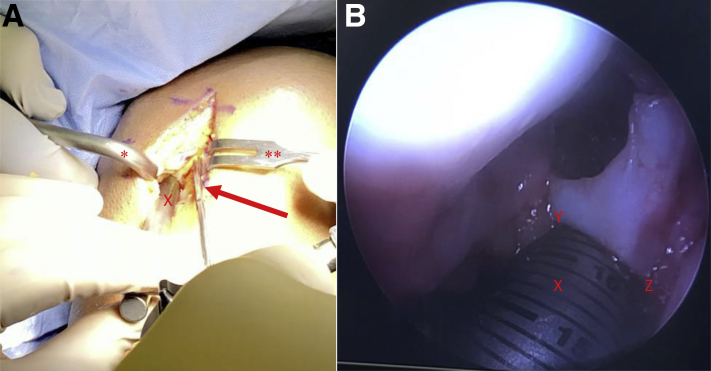
Fig 6.
View through a mini-medial parapatellar arthrotomy of the right knee in the supine position. (A) A Paulsen retractor (∗) is placed on the lateral aspect of the lateral femoral condyle retracting the patella and a 2-prong rake (∗∗) is used to retract the medial soft tissues exposing the lateral aspect of the intercondylar notch. (B) A metal sizing tamp (X) is placed in each donor site and the arthroscope (arrow) is used to confirm the depth of the donor site at the 3 (Z)-, 6-, 9-, and 12-o'clock (Y) positions.
Fig 7.
View of the preparation of the precut 10-mm diameter osteochondral allograft dowels (∗) on the operative field. Bone marrow elements are flushed from the grafts via 1-2 minutes of pulsatile lavage. (A-C) The lengths of the grafts are measured using a metal sizing tamp (X). The desired lengths of each graft were previously determined by inserting the metal sizing tamp into each donor site and confirming the depth at the 3-, 6-, 9-, and 12-o'clock positions using the arthroscope. These lengths are marked on the graft with a marking pen (arrow). A rongeur (Z) is used to remove excess bone until the grafts are the appropriate length.
Fig 8.
View through a mini-medial parapatellar arthrotomy of the right knee in the supine position. (A) A Paulsen retractor (∗) is placed on the lateral aspect of the lateral femoral condyle retracting the patella and a 2-prong rake (∗∗) is used to retract the medial soft tissues exposing the lateral aspect of the intercondylar notch. (A) A marking pen is used to make a small mark at the 12-o'clock position on the chondral surface of each graft (X) to maintain the correct orientation. (B-C) Forceps (Y) are used to carefully align each graft with the aperture of the donor site (arrow) and digital pressure with the surgeon's thumb is used for insertion. This process is repeated for each allograft until all donor sites are back-filled.
Recipient Site Preparation and Donor Graft Implantation
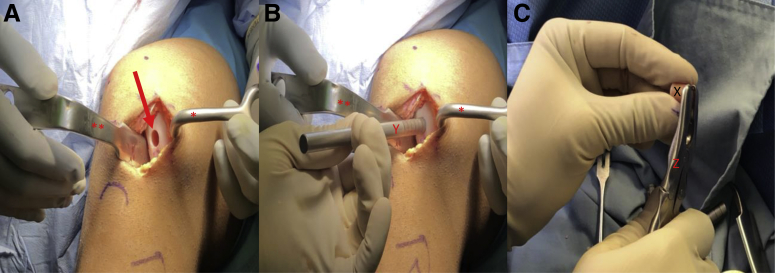
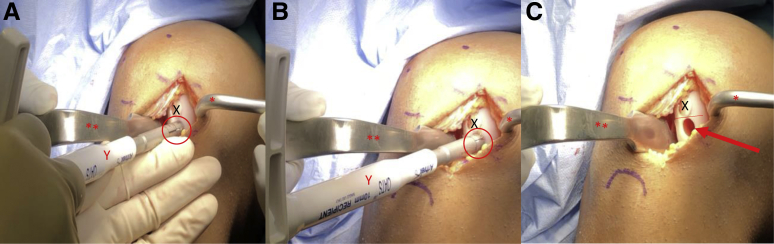
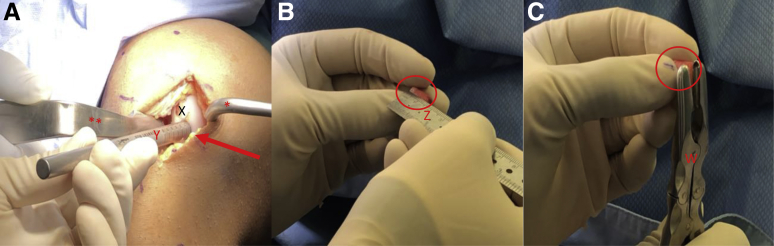
A ruler is used to confirm the length of each previously harvested autograft plug (Fig 9A). The authors prefer a minimum combined (cartilage + bone) length of 8 to 10 mm to ensure adequate graft fixation. A disposable commercially available single-use OAT system is used for recipient site preparation. Using this system, 6-, 8-, and 10-mm osteochondral plugs can be harvested. The harvester is placed perpendicular to the articular surface and aligned with the previously marked chondral lesion(s) on the affected condyle (Fig 9B). A mallet is then used to advance the harvester to the appropriate depth (Fig 9C). While applying an axial load, the harvester handle is twisted 90° clockwise and then 90° counterclockwise to disengage the graft from the underlying subchondral bone (Fig 10A). The extruder stylus is then reinserted into the harvester handle to release the recipient site osteochondral plug from the harvester. If a second autograft plug is needed, the authors prefer to proceed with preparation and implantation of the first donor graft prior to preparation of the second recipient site. A metal sizing tamp is then used to confirm the depth of the newly created recipient site (Fig 10B). A rongeur is used to remove any excess bone from the autograft plug to ensure that the cartilage surface of the graft sits flush with the cartilage surface of the surrounding condyle (Fig 10C). The prepared autograft plug is placed into a clear graft delivery tube with the bony surface of the plug toward the tapered end of the delivery tube (Fig 11A). The tapered end of the delivery tube is then inserted into the prepared recipient site. The metal sizing tamp is inserted into the clear delivery tube and gently malleted until the autograft plug is engaged in the recipient site (Fig 11B). The delivery tube is then removed and digital pressure via the surgeon's thumb is applied until the cartilage surface of the plug is flush or slightly recessed compared with the cartilage surface of the surrounding condyle (Fig 11C). If a second autograft plug is needed, the authors now prefer to proceed with the preparation of the second recipient site. The disposable harvester is aligned with the previously marked chondral lesion on the affected condyle and impacted to a depth of 8 to 10 mm (Fig 12, A and B). While applying an axial load, the harvester handle is twisted 90° clockwise and then 90° counterclockwise to disengage the graft from the underlying subchondral bone. Care is taken to maintain a 3- to 5-mm osteochondral bridge between the previously placed autograft plug and the newly created second graft recipient site to ensure adequate bony fixation (Fig 12C). A metal sizing tamp is used to confirm the depth of the second recipient site (Fig 13A). A ruler is used to confirm the length of the second autograft dowel (Fig 13B). The authors prefer a minimum combined (cartilage + bone) length of 8 to 10 mm for adequate graft fixation. A rongeur is used to remove any excess bone from the second autograft to ensure that the cartilage surface of the graft sits flush with the cartilage surface of the initial graft and surrounding condyle (Fig 13C). The prepared autograft plug is then placed into a clear graft delivery tube, cartilage surface up and bony surface of the plug toward the tapered end of the delivery tube (Fig 14A) The tapered end of the delivery tube is then inserted into the prepared second recipient site. The metal sizing tamp is inserted into the clear delivery tube and gently malleted until the second autograft plug is engaged in the recipient site (Fig 14B). The delivery tube is removed and digital pressure via the surgeon's thumb is applied until the cartilage surface of the plug is flush or slightly recessed compared to the cartilage surface of the first autograft plug and the surrounding condyle (Fig 14C). This process is repeated if additional autograft plugs are needed. If the fit of the grafts is adequate, no bloody fluid should be expressed between the graft–condyle interface when firm digital pressure is applied to each graft. If fixation is inadequate, headless compression screws or chondral darts may be used. For larger lesions with multiple plugs, there may be small areas between the plugs with abnormal cartilage (Fig 15 A and B). The authors prefer to fill these areas with micronized extracellular cartilage (BioCartilage; Arthrex, Naples, FL) mixed with BMAC or platelet-rich plasma, as it has shown good cartilage reconstitution and fill on postoperative magnetic resonance imaging (MRI) (Fig 16 A-F, Fig 17 A-D). The BioCartilage is mixed with BMAC or platelet-rich plasma until a paste-like slurry is formed. The slurry is then placed in the gaps between the donor plugs and impacted manually with the surgeon's fingers (Fig 15C). A fibrin glue sealant is used to seal the edges of the lesion if the surgeon feels it is necessary.
Fig 9.
View through a mini-medial parapatellar arthrotomy of the right knee in the supine position. (A) A Paulsen retractor (∗) is placed on the medial aspect of the medial femoral condyle retracting the soft tissues and a bent Hohman retractor (∗∗) is used to retract the patella laterally exposing the previously marked chondral lesions on the medial femoral condyle. (A) A ruler (Z) is used to confirm the length of each autograft dowel (X). The authors prefer a minimum combined (cartilage + bone) length of 8 to 10 mm for adequate graft fixation. (B) A single-use OAT recipient site-specific harvester (Y) is aligned with the previously marked chondral lesion on the medial femoral condyle (arrow). (C) The single-use OAT recipient site specific harvester is impacted to a depth of 8-10 mm (oval) and the handle is twisted 90°clockwise and then 90° counterclockwise to disengage the graft from the underlying subchondral bone. (OAT, osteochondral autograft transfer.)
Fig 10.
View through a mini-medial parapatellar arthrotomy of the right knee in the supine position. (A) A Paulsen retractor (∗) is placed on the medial aspect of the medial femoral condyle retracting the soft tissues and a bent Hohman retractor (∗∗) is used to retract the patella laterally exposing the medial femoral condyle with prepared superior lesion recipient site (arrow). (B) A metal sizing tamp (Y) is used to confirm the depth of the recipient site. (C) A rongeur (Z) is used to remove any excess bone from the autograft (X) to ensure that the cartilage surface of the graft sits flush with the cartilage surface of the surrounding condyle.
Fig 11.
View through a mini-medial parapatellar arthrotomy of the right knee in the supine position. (A) A Paulsen retractor (∗) is placed on the medial aspect of the medial femoral condyle retracting the soft tissues and a bent Hohman retractor (∗∗) is used to retract the patella laterally exposing the medial femoral condyle with prepared superior lesion recipient site (arrow). The prepared autograft plug (X) is placed into a clear graft delivery tube (oval) with the bony surface of the plug towards the tapered end of the delivery tube. (B) The tapered end of the delivery tube is then inserted into the prepared recipient site (arrow). The metal sizing tamp (Y) is inserted into the clear delivery tube and gently malleted until the autograft plug is engaged in the recipient site. (C) The delivery tube is removed and digital pressure via the surgeon's thumb is applied until the cartilage surface of the plug is flush or slightly recessed compared to the cartilage surface of the surrounding condyle.
Fig 12.
View through a mini-medial parapatellar arthrotomy of the right knee in the supine position. (A) A Paulsen retractor (∗) is placed on the medial aspect of the medial femoral condyle retracting the soft tissues and a bent Hohman retractor (∗∗) is used to retract the patella laterally exposing the previously marked inferior chondral lesion on the medial femoral condyle. (A-B) A single-use osteochondral autograft transfer recipient site specific harvester (Y) is aligned with the previously marked inferior chondral lesion on the medial femoral condyle (oval) and impacted to a depth of 8-10 mm. The handle is twisted 90° clockwise and then 90° counterclockwise to disengage the graft from the underlying subchondral bone. (C) Care is taken to maintain a 3- to 5-mm osteochondral bridge (line) between the previously placed superior autograft plug (X) and the newly created inferior graft recipient site (arrow) to ensure adequate bony fixation.
Fig 13.
View through a mini-medial parapatellar arthrotomy of the right knee in the supine position. (A) A Paulsen retractor (∗) is placed on the medial aspect of the medial femoral condyle retracting the soft tissues and a bent Hohman retractor (∗∗) is used to retract the patella laterally exposing the medial femoral condyle with inserted superior graft (X) and prepared inferior lesion recipient site (arrow). A metal sizing tamp (Y) is used to confirm the depth of the recipient site. (B) A ruler (Z) is used to confirm the length of the autograft dowel (oval). The authors prefer a minimum combined (cartilage + bone) length of 8-10 mm for adequate graft fixation. (C) A rongeur (W) is used to remove any excess bone from the autograft (oval) to ensure that the cartilage surface of the graft sits flush with the cartilage surface of the surrounding condyle.
Fig 14.
View through a mini-medial parapatellar arthrotomy of the right knee in the supine position. (A) A Paulsen retractor (∗) is placed on the medial aspect of the medial femoral condyle retracting the soft tissues and a bent Hohman retractor (∗∗) is used to retract the patella laterally exposing the medial femoral condyle with inserted superior graft (X) and prepared inferior lesion recipient site (arrow). The prepared autograft plug (Z) is placed into a clear graft delivery tube (oval) with the bony surface of the plug towards the tapered end of the delivery tube. (B) The tapered end of the delivery tube is then inserted into the prepared recipient site (arrow). The metal sizing tamp (Y) is inserted into the clear delivery tube and gently malleted until the autograft plug is engaged in the recipient site. (C) The delivery tube is removed and digital pressure via the surgeon's thumb is applied until the cartilage surface of the plug is flush or slightly recessed compared to the cartilage surface of the surrounding condyle. There is a 3- to 5-mm osteochondral bridge (line) between each graft.
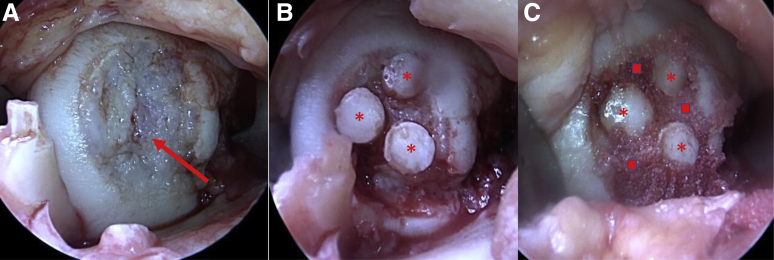
Fig 15.
(A) Arthroscopic view through a medial parapatellar arthrotomy of the medial femoral condyle of a right knee in the supine position showing a 20 × 25-mm osteochondritis dissecans lesion. (B) Same arthroscopic view showing placement of three 6-mm osteochondral autograft plugs labeled with asterisks. (C) Same arthroscopic view showing placement of BioCartilage in gaps between osteochondral plugs labeled with squares.
Fig 16.
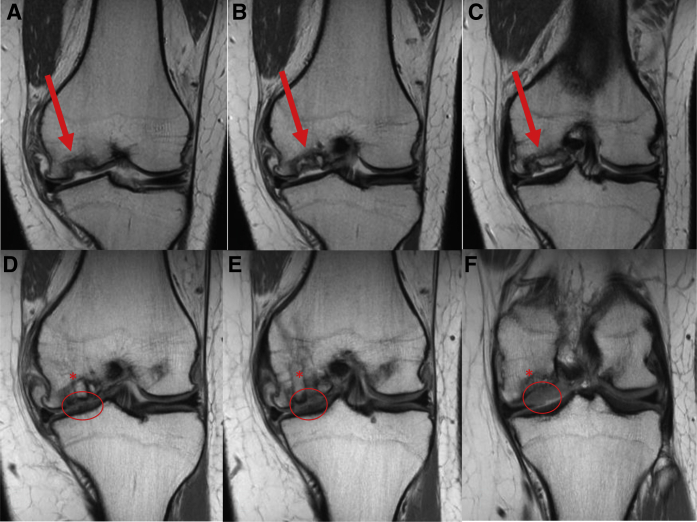
(A-C) Preoperative coronal T1 MRI from anterior to posterior demonstrating large osteochondritis dissecans lesion (arrow) of the medial femoral condyle in a 21-year-old female patient. (D-F) Three-month postoperative T1 MRI of corresponding coronal cuts from anterior to posterior showing early integration of osteochondral autograft plugs (∗) and significant cartilage fill (oval). (MRI, magnetic resonance imaging.)
Fig 17.
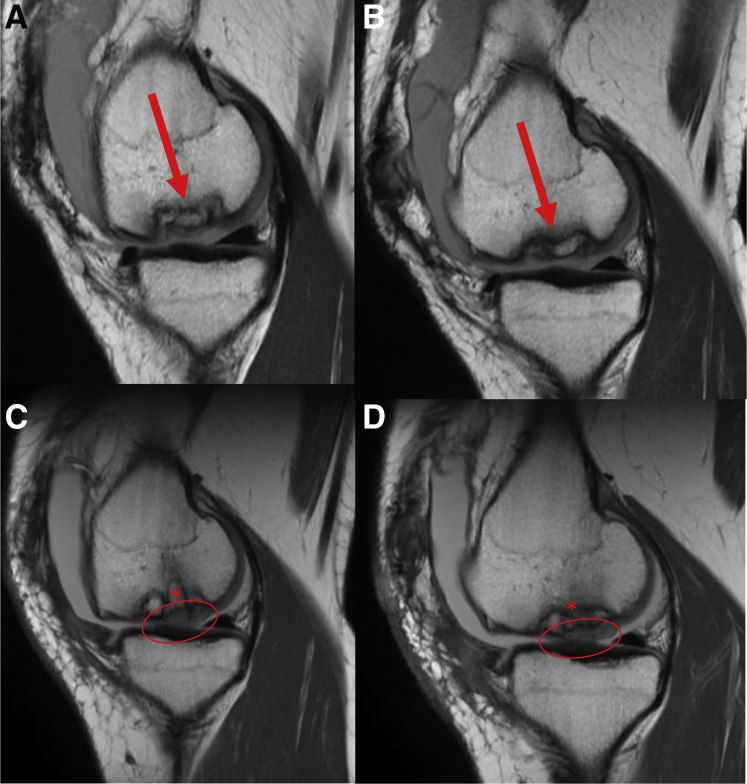
(A-B) Preoperative sagittal T1 MRI from medial to lateral demonstrating large osteochondritis dissecans lesion (arrow) of the medial femoral condyle in a 21-year-old female patient. (C-D) Three-month postoperative T1 MRI of corresponding sagittal cuts from medial to lateral showing early integration of osteochondral autograft plugs (∗) and significant cartilage fill (oval). (MRI, magnetic resonance imaging.)
Postoperative Rehabilitation
The patient is placed in a hinged knee brace and allowed to toe-touch weight bear on the operative side for the first 1 to 2 weeks. By week 2, the patient is advanced to weight-bearing as tolerated with no range of motion restrictions and the brace may be removed. A continuous passive range of motion device may be used for the first 4 weeks to assist with regaining knee range of motion on a case-by-case basis. Early rehabilitation focuses on strengthening of the core and pelvic stabilizers. By the end of 6 weeks, the patient should have full knee range of motion, a normalized gait, and 5/5 proximal muscle strength (hip flexors, gluteal muscles, core, quadriceps and hamstrings). During weeks 6-18, the patient focuses on patellar mobility, stair climbing, progressive jogging, and plyometrics. Patients return to unrestricted physical activity by an average of 5 months postoperatively.
Discussion
OAT provides the most reliable and fastest return-to-sport of any cartilage restoration procedure making it the preferred technique for the young high-demand athlete. A study by Werner et al.9 showed that young, high-level athletes were able to return to sport by an average of 82.9 ± 25 days with a mean postoperative International Knee Documentation Committee score of 84.5 ± 9.5. Furthermore, OAT has shown a 72% success rate with good-to-excellent patient-reported outcome scores at an average long-term follow-up of 10.2 years.10 Disadvantages to the technique include the need to harvest tissue from the patient's ipsilateral knee and the subsequent limitation in terms of treatable lesion size (Table 2). Moreover, there is a learning curve to the OAT procedure, with a number of surgical technique–specific risks (Table 3). Back-filling of donor sites with precut osteochondral allograft plugs at the time of index surgery prevents the issues of patellar maltracking or fibrocartilage hypertrophy seen when donor sites are left alone or treated with isolated bone graft.5,6 Furthermore, BioCartilage augmentation fills in the gaps between osteochondral autograft plugs potentially allowing for better cartilage fill of larger lesions. This has been seen on 3-month postoperative MRI scans for multiple patients in the senior author's case series (Fig 16, A-F and Fig 17, A-D). The authors believe that the use of BioCartilage in conjunction with OAT increases the lesion size that can be treated by the OAT method. Despite a lack of clinical evidence for this application, the use of BioCartilage in conjunction with MFx for the treatment of grade 3 or 4 lesions of the femoral condyles has been shown to enhance T2 mapping properties of the repair tissue on postoperative MRI compared with MFx alone.11 Fortier et al.12 showed similar findings in an equine model. Further studies looking at the short-term clinical and radiographic outcomes of patients treated via this method are currently under investigation by the senior author. Overall, this technique allows the surgeon to treat focal cartilage lesions of the knee with autologous osteochondral plugs enhancing patient return to sport, recovery time, and long-term patient reported outcomes while minimizing donor-site morbidity. Future studies comparing this technique to traditional OAT and/or OCA are also needed.
Table 2.
Advantages and Disadvantages of OAT With BioCartilage Augmentation and Donor-Site Back-Fill
| Advantages | Disadvantages |
|---|---|
| Ability to treat larger lesions than with OAT alone | Technically challenging |
| Decreased donor-site morbidity | Precut cores with limited shelf-life |
| Single-stage autologous chondrocyte transfer | Limited treatable lesion size (2-5 cm2) |
| Precut allograft cores readily available | No long-term outcome data with this technique |
| Precut allograft cores easily sized using same instrumentation for OAT |
OAT, osteochondral autograft transfer.
Table 3.
Surgical Technique Risks
| Carefully examine preoperative magnetic resonance imaging scan to determine the depth of the lesion's bony involvement; if diseased bone is left behind, inserted grafts can subside. |
| If consecutive autograft plugs are taken from the same location, a minimum 3- to 5-mm bone bridge between sites must be maintained to avoid convergence of the donor sites and accidental truncation of the subchondral bone plugs |
| If the single-use osteochondral autograft transfer harvester is not inserted perpendicular to the articular surface of the donor site, the cartilage surface of the subsequent autograft plug will be oblique and therefore will be more difficult to match the contour of the cartilage surrounding the recipient site |
| When harvesting autograft donor plugs from the intercondylar notch, it is possible that the harvested plug does not disengage from the subchondral bone when the harvester is removed. If so, a small curved osteotome may be needed to dislodge the plug. This can result in accidental plug truncation and/or creating an uncontained donor site lesion no longer capable of being back-filled. |
| Inadequate donor site spacing (3-5 mm bone bridge between grafts) can lead to loss of fixation and graft failure of back-filled precut allograft plugs |
| Inadequate recipient site spacing (3-5 mm bone bridge between grafts) can lead to loss of fixation and graft failure of harvested autograft plugs |
| If the depth of the donor site or the length of the precut allograft plug is inadequately measured the implanted allograft plug may be proud resulting in increased contact pressures and chance of cartilage death/graft failure |
| If the depth of the recipient site or the length of the autograft plug is inadequately measured the implanted autograft plug may be proud resulting in increased contact pressures and chance of cartilage death/graft failure |
| Persistent pain due to failure of bony integration of the back-filled allograft plugs and/or donor autograft plugs |
| Persistent pain due to cartilage necrosis of the back-filled allograft plugs and/or donor autograft plugs |
Footnotes
The authors report the following potential conflicts of interest or sources of funding: R.J.W. and K.J.J. report other from Arthrex and JRF Ortho, outside the submitted work. Full ICMJE author disclosure forms are available for this article online, as supplementary material.
Supplementary Data
This video demonstrates the surgical technique for osteochondral autograft transfer for focal cartilage lesions of the medial femoral condyle of a right knee with donor-site back-fill using precut osteochondral allograft plugs. The patient is placed in the supine position and the right lower extremity is prepped and draped in the normal sterile fashion. A diagnostic arthroscopy using standard anteromedial and anterolateral portals is then performed to document the location, morphology, grade, and size of the chondral lesion and address any additional intra articular pathology. A small ∼6-cm medial vertical skin incision is made in-line with the previous arthroscopic portal. Metzenbaum scissors are used to release any superficial adhesions between the skin and underlying fascia in order to enhance skin mobility. A corresponding arthrotomy is performed and a combination of bent Hohmann and Paulsen retractor are used to retract the patella and soft tissues exposing the affected condyle. The end of a metal sizing tamp of the desired diameter is then colored with a marking pen and used as a stencil to mark out of the location of the future donor plugs. A 10-mm single-use osteochondral autograft transfer system harvester is then placed over the desired harvest site at the lateral aspect of the intercondylar notch. Care is taken to make sure that the harvester is placed perpendicular to the articular surface. The harvester is then impacted to the desired depth of approximately 10 mm. While applying an axial load, the harvester handle is twisted 90° clockwise and then 90° counterclockwise to disengage the graft from the underlying subchondral bone. The extruder stylus is then reinserted into the harvester handle to release the osteochondral plug from the harvester. These steps are repeated for the harvest of a second autograft plug. The metal sizing tamp is inserted into each recipient site and the arthroscope is used to help confirm the exact depth. A marking pen and the metal sizing tamp are then used to mark the appropriate depth of bone on each 10-mm × 12-mm osteochondral allograft plug. Bone marrow elements are then flushed from the graft using 1-2 minutes of pulsatile lavage. A rongeur is used to remove any excess bone. A forceps is then used to carefully insert the precut allograft plugs into the corresponding donor sites. Digital pressure is then applied and the grafts are found to be well seated with no evidence of articular step off. Next, a ruler is used to confirm to total length of the previously harvested autograft plugs before preparation of the recipient sites. A 10-mm single-use osteochondral autograft transfer system harvester is used for recipient site preparation. The harvester is placed perpendicular to the articular surface and malleted in to the appropriate depth. While applying an axial load, the harvester handle is twisted 90° clockwise and then 90° counterclockwise to disengage the graft from the underlying subchondral bone. These steps are repeated for each recipient site. Depth measurements for the articular surface are then read directly off of the metal sizing tamp at the 12-, 9-, 6-, and 3-o'clock positions. A ruler is then used to match the recipient site dimensions to the dimensions of the harvested autograft plug. Excess bone is removed with a rongeur and may be placed in the recipient site if needed. The autograft plug is then placed in a clear tube inserter. Care is taken to make sure that the articular surface of the plug matches the articular surface of the recipient site and that the plug is placed in the predetermined orientation. A mallet and metal tamp are then used to gently impact the autograft plug into the recipient site. This process is repeated for each osteochondral plug. It is important to leave a 3- to 5-mm bone bridge between recipient sites. These small bone bridges will help assure that the osteochondral plugs have adequate bony fixation. For larger lesions with multiple plugs there may be small areas between the plugs with abnormal cartilage. The authors prefer to fill these areas with micronized extracellular cartilage (BioCartilage) mixed with bone marrow aspirate concentrate or platelet-rich plasma, as it has shown good cartilage reconstitution and fill on postoperative magnetic resonance imaging.
References
- 1.Flanigan D.C., Harris J.D., Trinh T.Q., Siston R.A., Brophy R.H. Prevalence of chondral defects in athletes’ knees: A systematic review. Med Sci Sports Exerc. 2010;42:1795–1801. doi: 10.1249/MSS.0b013e3181d9eea0. [DOI] [PubMed] [Google Scholar]
- 2.Krych A.J., Pareek A., King A.H., Johnson N.R., Stuart M.J., Williams R.J. Return to sport after the surgical management of articular cartilage lesions in the knee: A meta-analysis. Knee Surg Sport Traumatol Arthrosc. 2016;25:3186–3196. doi: 10.1007/s00167-016-4262-3. [DOI] [PubMed] [Google Scholar]
- 3.Krych A.J., Harnly H.W., Rodeo S.A., Williams R.J. Activity levels are higher after osteochondral autograft transfer mosaicplasty than after microfracture for articular cartilage defects of the knee: A retrospective comparative study. J Bone Joint Surg A. 2012;94:971–978. doi: 10.2106/JBJS.K.00815. [DOI] [PubMed] [Google Scholar]
- 4.Solheim E., Hegna J., Strand T., Harlem T., Inderhaug E. Randomized study of long-term (15-17 years) outcome after microfracture versus mosaicplasty in knee articular cartilage defects. Am J Sports Med. 2018;46:826–831. doi: 10.1177/0363546517745281. [DOI] [PubMed] [Google Scholar]
- 5.Anil U., Strauss E.J. Donor-site-related mechanical symptoms following osteochondral autograft transfer: A case report. JBJS Case Connect. 2018;8:e84. doi: 10.2106/JBJS.CC.18.00060. [DOI] [PubMed] [Google Scholar]
- 6.LaPrade R.F., Botker J.C. Donor-site morbidity after osteochondral autograft transfer procedures. Arthroscopy. 2004;20:e69–e73. doi: 10.1016/j.arthro.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 7.Rowland R., Colello M., Wyland D.J. Osteochondral autograft transfer procedure: Arthroscopic technique and technical pearls. Arthrosc Tech. 2019;8:e713–e719. doi: 10.1016/j.eats.2019.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones K.J., Mosich G.M., Williams R.J. Fresh precut osteochondral allograft core transplantation for the treatment of femoral cartilage defects. Arthrosc Tech. 2018;7:e791–e795. doi: 10.1016/j.eats.2018.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Werner B.C., Cosgrove C.T., Gilmore C.J. Accelerated return to sport after osteochondral autograft plug transfer. Orthop J Sport Med. 2017;5 doi: 10.1177/2325967117702418. 2325967117702418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pareek A., Reardon P.J., Maak T.G., Levy B.A., Stuart M.J., Krych A.J. Long-term outcomes after osteochondral autograft transfer: A systematic review at mean follow-up of 10.2 years. Arthroscopy. 2016;32:1174–1184. doi: 10.1016/j.arthro.2015.11.037. [DOI] [PubMed] [Google Scholar]
- 11.Carter A.H., Guttierez N., Subhawong T.K. MR imaging of BioCartilage augmented microfracture surgery utilizing 2D MOCART and KOOS scores. J Clin Orthop Trauma. 2018;9:146–152. doi: 10.1016/j.jcot.2017.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fortier L.A., Chapman H.S., Pownder S.L. BioCartilage improves cartilage repair compared with microfracture alone in an equine model of full-thickness cartilage loss. Am J Sports Med. 2016;44:2366–2374. doi: 10.1177/0363546516648644. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This video demonstrates the surgical technique for osteochondral autograft transfer for focal cartilage lesions of the medial femoral condyle of a right knee with donor-site back-fill using precut osteochondral allograft plugs. The patient is placed in the supine position and the right lower extremity is prepped and draped in the normal sterile fashion. A diagnostic arthroscopy using standard anteromedial and anterolateral portals is then performed to document the location, morphology, grade, and size of the chondral lesion and address any additional intra articular pathology. A small ∼6-cm medial vertical skin incision is made in-line with the previous arthroscopic portal. Metzenbaum scissors are used to release any superficial adhesions between the skin and underlying fascia in order to enhance skin mobility. A corresponding arthrotomy is performed and a combination of bent Hohmann and Paulsen retractor are used to retract the patella and soft tissues exposing the affected condyle. The end of a metal sizing tamp of the desired diameter is then colored with a marking pen and used as a stencil to mark out of the location of the future donor plugs. A 10-mm single-use osteochondral autograft transfer system harvester is then placed over the desired harvest site at the lateral aspect of the intercondylar notch. Care is taken to make sure that the harvester is placed perpendicular to the articular surface. The harvester is then impacted to the desired depth of approximately 10 mm. While applying an axial load, the harvester handle is twisted 90° clockwise and then 90° counterclockwise to disengage the graft from the underlying subchondral bone. The extruder stylus is then reinserted into the harvester handle to release the osteochondral plug from the harvester. These steps are repeated for the harvest of a second autograft plug. The metal sizing tamp is inserted into each recipient site and the arthroscope is used to help confirm the exact depth. A marking pen and the metal sizing tamp are then used to mark the appropriate depth of bone on each 10-mm × 12-mm osteochondral allograft plug. Bone marrow elements are then flushed from the graft using 1-2 minutes of pulsatile lavage. A rongeur is used to remove any excess bone. A forceps is then used to carefully insert the precut allograft plugs into the corresponding donor sites. Digital pressure is then applied and the grafts are found to be well seated with no evidence of articular step off. Next, a ruler is used to confirm to total length of the previously harvested autograft plugs before preparation of the recipient sites. A 10-mm single-use osteochondral autograft transfer system harvester is used for recipient site preparation. The harvester is placed perpendicular to the articular surface and malleted in to the appropriate depth. While applying an axial load, the harvester handle is twisted 90° clockwise and then 90° counterclockwise to disengage the graft from the underlying subchondral bone. These steps are repeated for each recipient site. Depth measurements for the articular surface are then read directly off of the metal sizing tamp at the 12-, 9-, 6-, and 3-o'clock positions. A ruler is then used to match the recipient site dimensions to the dimensions of the harvested autograft plug. Excess bone is removed with a rongeur and may be placed in the recipient site if needed. The autograft plug is then placed in a clear tube inserter. Care is taken to make sure that the articular surface of the plug matches the articular surface of the recipient site and that the plug is placed in the predetermined orientation. A mallet and metal tamp are then used to gently impact the autograft plug into the recipient site. This process is repeated for each osteochondral plug. It is important to leave a 3- to 5-mm bone bridge between recipient sites. These small bone bridges will help assure that the osteochondral plugs have adequate bony fixation. For larger lesions with multiple plugs there may be small areas between the plugs with abnormal cartilage. The authors prefer to fill these areas with micronized extracellular cartilage (BioCartilage) mixed with bone marrow aspirate concentrate or platelet-rich plasma, as it has shown good cartilage reconstitution and fill on postoperative magnetic resonance imaging.