Abstract
Patient: Female, 65-year-old
Final Diagnosis: Type 1 diabetes mellitus
Symptoms: Unconsciousness
Medication:—
Clinical Procedure: Insulin pump
Specialty: Endocrinology and Metabolic
Objective:
Unusual or unexpected effect of treatment
Background:
Hypoglycemia is a frequent complication observed in diabetic patients under treatment. This metabolic complication is associated with an increased mortality rate in diabetic patients. The use of sensor-augmented pump therapy with predictive low glucose management systems has improved blood glucose level control and reduced the incidence of hypoglycemic attacks. However, this therapy may be associated with adverse events.
Case Report:
A 65-year-old Japanese woman with type 1 diabetes mellitus underwent hemodialysis with end-stage renal failure due to diabetic nephropathy. The patient received sensor-augmented pump therapy with the predictive low glucose management system to prevent recurrent severe hypoglycemia. Hypoglycemia was infrequent when the sensor-augmented pump therapy with a predictive low-glucose management system was properly working. However, the patient suddenly died 3 months after starting the treatment. A record of continuous glucose monitoring showed that hypoglycemia occurred before the sudden death of the patient.
Conclusions:
The current case shows that sudden death associated with severe hypoglycemia may also occur during sensor-augmented pump therapy with a predictive low glucose management system. This case report underscores the need for close follow-up of diabetic patients receiving sensor-augmented pump therapy with the predictive low glucose management system and the critical importance of patient education on diabetes technology in high-risk patients.
MeSH Keywords: Death, Sudden; Diabetes Mellitus; Hemodialysis; Hypoglycemia; Kidney Falure, Chronic
Background
Previous studies reported hypoglycemia associated with an increased mortality rate in diabetic patients [1, 2]. Prevention of hypoglycemia is critical in the medical care of patients with diabetes mellitus treated with insulin [2]. In general, patients with type 1 diabetes mellitus under insulin therapy frequently develop insulinopenia that predisposes them to hypoglycemia [3]. Several studies have shown the benefit of continuous glucose monitoring sensors for reducing the incidence of hypoglycemia in patients at risk [4–6]. In recent years, the use of sensor-augmented pump (SAP) therapy with the predictive low glucose management (PLGM) system has improved the control of glycemia and reduced the incidence of hypoglycemic attacks [7]. However, reports show that this novel therapeutic approach does not entirely prevent bouts of hypoglycemia [8]. Here, we report a case of sudden death associated with hypoglycemia after an indication of SAP therapy with the PLGM function. The case was a patient with type 1 diabetic patient and multiple microvascular complications, including end-stage renal failure. Tanenberg et al. reported the first case of sudden death using a retrospective continuous glucose monitoring system (CGM) [9]. Here, we report the first case of sudden death during SAP therapy with the PLGM system. This case report shows the need for close follow-up of diabetic patients receiving SAP therapy with the PLGM system and the critical importance of patient education on diabetes technology in high-risk patients.
Case Report
The patient was a 65-year-old Japanese woman with type 1 diabetes mellitus. She was diagnosed with type 1 diabetes mellitus when she was 32 years old after complaining of thirst and frequent urination. She underwent bilateral vitrectomy and photocoagulation for diabetes retinopathy at the age of 43 years. She started hemodialysis at the age of 59 years due to end-stage renal disease caused by diabetic nephropathy. She had no family history of diabetes mellitus or renal diseases. She has been on medication for arterial hypertension since age 47 years and Hashimoto’s thyroiditis with hypothyroidism since age 58 years. She underwent C4–7 anterior cervical discectomy and fusion when she was 63 years old. She had no allergy to any food or drug and no habit of drinking or smoking. She was receiving treatment with multiple daily injections (MDI) of insulin. However, she had repeated hospital admissions because of frequent crises of hyperglycemia or hypoglycemia. At the age of 56 years, she started treatment with continuous subcutaneous insulin infusion (CSII) at Mie University Hospital (MiniMed Paradigm 722, Medtronic, Inc., Northridge, CA, USA). However, it was difficult for her to operate the device. Therefore, the attending physician re-indicated MDI therapy 4 months after starting CSII. The physician indicated intermittently scanned-continuous glucose monitoring (isCGM) 16 months before initiating SAP therapy. The result of the isCGM suggested the presence of repeated hypoglycemia. She was transferred to the hospital for severe hypoglycemic attacks 3 times in 6 months and was hospitalized twice before starting SAP therapy. Because appropriate treatment with MDI was not possible, we indicated SAP therapy with PLGM function after her admission to Mie University Hospital.
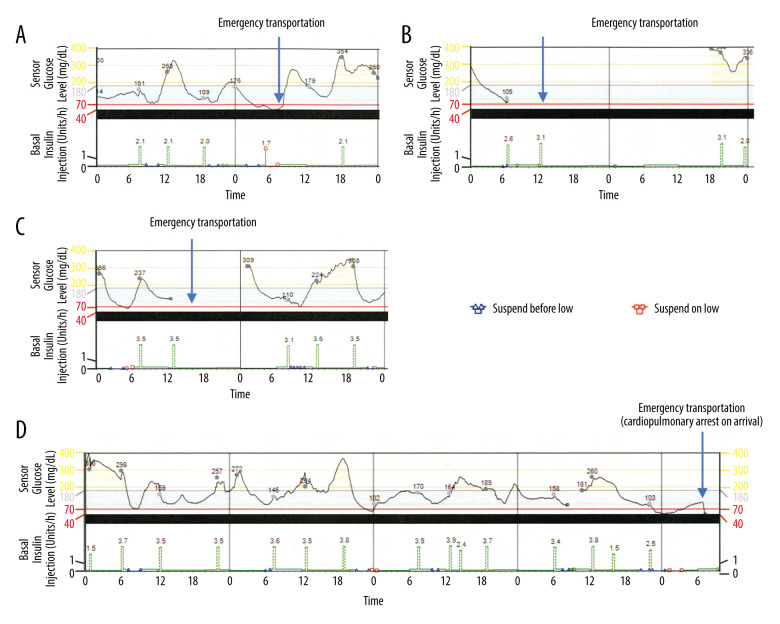
The treatment for diabetes that the patient was receiving before admission was insulin lispro 3 U before every meal and insulin degludec 5 U before breakfast. The clinical examination on admission showed loss of bilateral Achilles tendon reflex and decreased vibration sensation, suggesting diabetic neuropathy. Electrocardiography showed a left axis deviation and no arrhythmia. The coefficient of variation of R-R was 0.93%. We started SAP therapy with the PLGM function after admission (MiniMed™ 640G system, including MiniMed™ 640 insulin pump and Enlite sensor, Medtronic, Inc., Northridge, CA, USA). In patients with diabetic retinopathy-related visual impairment and poor fine-motor skills caused by diabetic neuropathy and cervical spondylosis, we consider use of the infusion set and Enlite sensor to be risky. However, the present patient learned to perform the procedures after receiving support from the medical staff. Infusion of insulin lispro through an insulin pump was performed based on the following setting conditions: basal pattern 0: 00–6: 00 0.125 U/hour, 6: 00–12: 00 0.2 U/hour, 12: 00–20: 00 0.1 U/hour, 20: 00–0: 00 0.125 U/hour (3.25 U/day), bolus 1.8 U/every meal, target blood glucose 70–180 mg/dL, with the limit glucose level of “suspend on low” and “suspend before low” of 70 mg/dL. We focused our attention on improving the patient’s ability to operate the device and preventing insulin infusion during hypoglycemia by the PLGM system. Therefore, we did no set up the insulin/correctional factor on the insulin pump. She learned to self-adjust her bolus insulin to 2.0–4.5 U depending on the preprandial blood glucose levels. The setting of basal insulin was reduced during follow-up at the outpatient clinic as follows: 0: 00–6: 00 0.075 U/hour, 6: 00–12: 00 0.2 U/hour, 12: 00–20: 00 0.1 U/hour, 20: 00–0: 00 0.125 U/hour, (2.95 U/day). The patient received instructions to check her blood glucose and take a 10-g solution of glucose orally when she had hypoglycemia symptoms or when the PLGM system was activated. She was referred to the hospital with severe hypoglycemia 3 times, once immediately after SAP therapy and twice 2 months after starting SAP therapy. Administrations of glucose at the Emergency Room promptly improved her hypoglycemia without the need for hospitalization during the 3 emergent adverse events. The first severe hypoglycemia occurred immediately following the bolus infusion after suspending the basal insulin by the PLGM function (Figure 1A). The second severe hypoglycemia event occurred when the patient mistakenly set the device on
Figure 1.
The CGM of the patient. (A–C) CGM data on the day of patient referral to our Emergency Room due to hypoglycemia after starting SAP therapy with the PLGM function. The values on the graph of the sensor glucose levels show the blood glucose level measured by self-monitoring blood glucose. The values on the graph of basal insulin infusion show the units of insulin used for bolus infusion. (D) CGM data during the last 5 days before the sudden death of the patient.
“Airplane Mode”, during which the pump is unable to send or receive wireless communications (Figure 1B). The third hypoglycemic event occurred when the PLGM function was unable to work due to contact failure of the Enlite sensor (Figure 1C).
The night before her sudden death, the patient underwent her regular hemodialysis session at the nearby hospital. After returning home, she infused her preprandial bolus of insulin. However, she did not eat her dinner (the food remained un-eaten when a neighbor found her lying on the floor). The patient was immediately transferred to the hospital. However, she had a cardiopulmonary arrest on arrival and died without responding to resuscitation procedures. There were no specific findings on the computed tomography scan to explain the cause of death. The record of her CGM collected subsequently showed that a 2.5-U bolus insulin was infused immediately after suspension of the basal insulin by the PLGM function. The record also revealed that the blood glucose level was low for about 5 hours after a bolus infusion of insulin, which then increased (Figure 1D). The daily average time below the range (TBR) of the blood glucose levels during 28 days before her death was 3%. Permission was not given to perform an autopsy.
Discussion
Hypoglycemia is a frequent complication of diabetes therapy [10]. Hypoglycemia is defined by the presence of a low plasma glucose level (<70 mg/dl in patients treated with insulin or insulin secretagogue), neurogenic and neuroglycopenic symptoms, and symptoms that ameliorate after carbohydrate administration. Patients with type 1 diabetes treated with hypoglycemic agents are at high risk of developing hypoglycemia [3]. Also, an increased incidence of hypoglycemia may contribute to increased mortality in diabetic patients [1]. Several studies have shown that isCGM and SAP therapy with or without the PLGM function can efficiently prevent hypoglycemia [7,8,11]. We indicated the use of SAP therapy with the PLGM system to our patient because she had repeated hypoglycemic attacks even with the simultaneous use of MDI and isCGM. After starting SAP therapy with the PLGM function, she experienced 3 events of severe hypoglycemia. However, except for the first event, the hypoglycemic attack occurred only when the PLGM function was improperly working. Despite the adverse events, the patient felt satisfied and better after starting the SAP therapy with the PLGM function.
The scenario preceding the sudden death of the patient suggests that she had severe hypoglycemia. The patient had infused her preprandial insulin before a neighbor found her lying on the floor, and the dinner food remained uneaten. Also, impaired gluconeogenesis caused by end-stage renal failure probably further worsened her hypoglycemic state by delaying recovery from hypoglycemia by the PGLM function [12]. According to the CGM data, the duration of hypoglycemia before her death was approximately 5 hours. Prolonged hypoglycemia can lead to encephalopathy, brainstem damage, cardio-respiratory arrest, and death [13,14]. Prolonged hypoglycemia can also cause severe arrhythmia and ischemic cardiovascular attacks [10]. However, the accuracy of CGM glucose levels during cardiopulmonary resuscitation setting is doubtful [15]. Therefore, whether hypoglycemia caused the patient’s sudden death remains unclear.
This case highlights the need for rigorous criteria to indicate diabetes technology and ensure its application is safe and effective. Patient education is an essential part of the criteria [16]. For example, all 4 severe hypoglycemic episodes our patient had after the SAP initiation occurred after the insulin bolus. The first hypoglycemic episode (induced by bolus infusion after basal insulin suspension) and the fourth hypoglycemic episode (related to lack of food intake after insulin bolus) particularly underscore the patient’s inability to self-management insulin use and the importance of patient education before SAP indication [16]. A safe device setting with glucose level alarms that efficiently alert during hypoglycemia is also of critical importance [17,18]. In the present case, the alarm was triggered when the glucose concentration was less than 70 mg/dl. Whether a stricter criterion is necessary for patients unable to master the technology entirely is a question to address in future investigations. It is worth noting that increasing evidence has shown that CGM underestimates the degree of hypoglycemia, suggesting that CGM may worsen the severity and delay the treatment of hypoglycemia [19].
Recent studies suggested using CGM metrics, including TBR, as a therapeutic goal in patients with diabetes mellitus [20,21]. The International Consensus recommends a target for TBR (<70 mg/dL) of <1% in the elderly/high-risk population [20]. The recommended therapeutic goal for TBR was not achieved in the present case. This observation underscores the critical importance of reaching the therapeutic target for TBR of <1% in high-risk diabetic patients.
In summary, we learned from this case that we should be very careful when indicating the use of SAP therapy with the PLGM system in diabetic patients with multiple complications. The present patient had severe hypoglycemia following a prepran-dial bolus infusion of insulin immediately after suspension of the basal insulin by the PLGM function. Therefore, careful monitoring of the insulin bolus infusion after the PLGM function and patient education on diabetes technology are critical for achieving therapeutic goals and improving clinical outcomes in high-risk diabetic patients.
Conclusions
This report is the first description of sudden death associated with severe hypoglycemia in a patient with type 1 diabetes mellitus receiving SAP therapy with the PLGM system. SAP therapy with the PLGM function may be an appropriate approach for treating patients with frequent and severe hypoglycemic attacks. However, this case shows that sudden death associated with severe hypoglycemia can also occur during SAP therapy with the PLGM system. This case report underscores the need for close follow-up of diabetic patients receiving SAP therapy with the PLGM system and the critical importance of patient education on diabetes technology in high-risk patients.
References:
- 1.Lu CL, Shen HN, Hu SC, et al. A population-based study of all-cause mortality and cardiovascular disease in association with prior history of hypoglycemia among patients with type 1 diabetes. Diabetes Care. 2016;39:1571–78. doi: 10.2337/dc15-2418. [DOI] [PubMed] [Google Scholar]
- 2.Zoungas S, Patel A, Chalmers J, et al. Severe hypoglycemia and risks of vascular events and death. N Engl J Med. 2010;363:1410–18. doi: 10.1056/NEJMoa1003795. [DOI] [PubMed] [Google Scholar]
- 3.Iwase M, Komorita Y, Fujii H, et al. Incidence of severe hypoglycemia and its association with serum adiponectin in Japanese patients with type 1 and insulin-treated type 2 diabetes: The Fukuoka Diabetes Registry. J Diabetes Investig. 2020;11(5):1258–64. doi: 10.1111/jdi.13253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beck RW, Riddlesworth T, Ruedy K, et al. Effect of continuous glucose monitoring on glycemic control in adults with type 1 diabetes using insulin injections: The DIAMOND Randomized Clinical Trial. JAMA. 2017;317:371–78. doi: 10.1001/jama.2016.19975. [DOI] [PubMed] [Google Scholar]
- 5.Heinemann L, Freckmann G, Ehrmann D, et al. Real-time continuous glucose monitoring in adults with type 1 diabetes and impaired hypoglycaemia awareness or severe hypoglycaemia treated with multiple daily insulin injections (HypoDE): A multicentre, randomised controlled trial. Lancet. 2018;391:1367–77. doi: 10.1016/S0140-6736(18)30297-6. [DOI] [PubMed] [Google Scholar]
- 6.Pratley RE, Kanapka LG, Rickels MR, et al. Effect of continuous glucose monitoring on hypoglycemia in older adults with type 1 diabetes: A Randomized Clinical Trial. JAMA. 2020;323:2397–406. doi: 10.1001/jama.2020.6928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Katayama A, Tone A, Watanabe M, et al. The hypoglycemia-prevention effect of sensor-augmented pump therapy with predictive low glucose management in Japanese patients with type 1 diabetes mellitus: A short-term study. Diabetol Int. 2020;11:97–104. doi: 10.1007/s13340-019-00408-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Battelino T, Nimri R, Dovc K, et al. Prevention of hypoglycemia with predictive low glucose insulin suspension in children with type 1 diabetes: A Randomized Controlled Trial. Diabetes Care. 2017;40:764–70. doi: 10.2337/dc16-2584. [DOI] [PubMed] [Google Scholar]
- 9.Tanenberg RJ, Newton CA, Drake AJ. Confirmation of hypoglycemia in the “dead-in-bed” syndrome, as captured by a retrospective continuous glucose monitoring system. Endocr Pract. 2010;16:244–48. doi: 10.4158/EP09260.CR. [DOI] [PubMed] [Google Scholar]
- 10.Hanefeld M, Duetting E, Bramlage P. Cardiac implications of hypoglycaemia in patients with diabetes – a systematic review. Cardiovasc Diabetol. 2013;12:135. doi: 10.1186/1475-2840-12-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Charleer S, De Block C, Van Huffel L, et al. Quality of life and glucose control after 1 year of nationwide reimbursement of intermittently scanned continuous glucose monitoring in adults living with type 1 diabetes (FUTURE): A Prospective Observational Real-World Cohort Study. Diabetes Care. 2020;43:389–97. doi: 10.2337/dc19-1610. [DOI] [PubMed] [Google Scholar]
- 12.Alsahli M, Gerich JE. Hypoglycemia, chronic kidney disease, and diabetes mellitus. Mayo Clin Proc. 2014;89:1564–71. doi: 10.1016/j.mayocp.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 13.Ikeda T, Takahashi T, Sato A, et al. Predictors of outcome in hypoglycemic encephalopathy. Diabetes Res Clin Pract. 2013;101:159–63. doi: 10.1016/j.diabres.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 14.Jones TW, McCarthy G, Tamborlane WV, et al. Mild hypoglycemia and impairment of brain stem and cortical evoked potentials in healthy subjects. Diabetes. 1990;39:1550–55. doi: 10.2337/diab.39.12.1550. [DOI] [PubMed] [Google Scholar]
- 15.Umpierrez GE, Klonoff DC. Diabetes technology update: use of insulin pumps and continuous glucose monitoring in the hospital. Diabetes Care. 2018;41:1579–89. doi: 10.2337/dci18-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choudhary P, Amiel SA. Hypoglycaemia in type 1 diabetes: Technological treatments, their limitations and the place of psychology. Diabetologia. 2018;61:761–69. doi: 10.1007/s00125-018-4566-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin YK, Groat D, Chan O, et al. Associations between the time in hypoglycemia and hypoglycemia awareness status in type 1 diabetes patients using continuous glucose monitoring systems. Diabetes Technol Ther. 2020;22(11):787–93. doi: 10.1089/dia.2020.0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin YK, Groat D, Chan O, et al. Alarm settings of continuous glucose monitoring systems and associations to glucose outcomes in type 1 diabetes. J Endocr Soc. 2020;4:bvz005. doi: 10.1210/jendso/bvz005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Farrell CM, McNeilly AD, Hapca SM, et al. Real-time continuous glucose monitoring during a hyperinsulinemic-hypoglycemic clamp significantly underestimates the degree of hypoglycemia. Diabetes Care. 2020;43:e142–43. doi: 10.2337/dc20-0882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: Recommendations from the international consensus on time in range. Diabetes Care. 2019;42:1593–603. doi: 10.2337/dci19-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Danne T, Nimri R, Battelino T, et al. International consensus on use of continuous glucose monitoring. Diabetes Care. 2017;40:1631–40. doi: 10.2337/dc17-1600. [DOI] [PMC free article] [PubMed] [Google Scholar]