Abstract
Background
Medicine and public health are shifting away from a purely “personal responsibility” model of cardiovascular disease (CVD) prevention towards a societal view targeting social and environmental conditions and how these result in disease. Given the strong association between social conditions and CVD outcomes, we hypothesize that accelerated aging, measuring earlier health decline associated with chronological aging through a combination of biomarkers, may be a marker for the association between social conditions and CVD.
Methods
We used data from the Coronary Artery Risk Development in Young Adults study (CARDIA). Accelerated aging was defined as the difference between biological and chronological age. Biological age was derived as a combination of 7 biomarkers (total cholesterol, HDL, glucose, BMI, CRP, FEV1/h2, MAP), representing the physiological effect of “wear and tear” usually associated with chronological aging. We studied accelerated aging measured in 2005-06 as a mediator of the association between social factors measured in 2000-01 and 1) any incident CVD event; 2) stroke; and 3) all-cause mortality occurring from 2007 through 18.
Results
Among 2978 middle-aged participants, mean (SD) accelerated aging was 3.6 (11.6) years, i.e., the CARDIA cohort appeared to be, on average, 3 years older than its chronological age. Accelerated aging partially mediated the association between social factors and CVD (N=219), stroke (N=36), and mortality (N=59). Accelerated aging mediated 41% of the total effects of racial discrimination on stroke after adjustment for covariates. Accelerated aging also mediated other relationships but to lesser degrees.
Conclusion
We provide new evidence that accelerated aging based on easily measurable biomarkers may be a viable marker to partially explain how social factors can lead to cardiovascular outcomes and death.
Keywords: Accelerated aging, Psychosocial stress, Cardiovascular disease
Highlights
-
•
Accelerated aging is consistent with a mediator between psychosocial stress and CVD.
-
•
Accelerated aging mediates the association between racial discrimination and stroke.
-
•
Accelerated aging may be a candidate mechanism for studying health disparities.
1. Introduction
Cardiovascular disease (CVD), including myocardial infarction, stroke, hypertension, and heart failure, is an immense burden on the U.S. population. Currently, 35% of Americans over the age of twenty suffer from at least one CVD (Benjamin et al., 2019) with higher prevalence of 46% and 48% among Black men and women, respectively. CVD is also the underlying cause for about 31% of all deaths in the U.S. Although a declining trend in death from CVD has been observed, due in part to advances in prevention and treatment, CVD is still the leading cause of death at nearly 50% prevalence in adults over 20 years of age (Benjamin et al., 2019). The reasons for this include the increasing age of the population and the obesity epidemic and its related morbidities (e.g. hypertension, diabetes). Medicine and public health are shifting away from a purely “personal responsibility” model of the risk factors for CVD towards a more societal view that focuses on the changes in societal and environmental conditions, the social determinants of health (SDOH) (Das & O'Keefe, 2006; Gebreab et al., 2012; Lagraauw et al., 2015) The overwhelming evidence linking SDOH to CVD caused the American Heart Association to issue a scientific statement detailing the social determinants of risk and outcomes for CVD (Havranek et al., 2015).
Given the established association between SDOH and CVD outcomes, a better understanding of the mechanisms through which SDOH may affect cardiovascular health is warranted. Previous research has shown that social stressors such as job strain, marital separation and divorce, adverse childhood experiences, and stress associated with medical illness are strong predictors of coronary heart disease (CHD) (Everson-Rose & Lewis, 2005). Depression in particular has emerged as a potent predictor of cardiovascular diseases (Pedersen et al., 2017). In addition to symptoms of depression being a predictor for cardiovascular disease, studies have shown that among those with a history of mood disorders, racial discrimination is associated with an increased risk of cardiovascular disease (David H Chae et al., 2012). The relationship between depression and discrimination is found at all socioeconomic levels and is actually stronger among African Americans of higher education levels (Hudson et al., 2016) likely because African American persons of higher socioeconomic status (SES) occupy more “white spaces” increasing the likelihood of discrimination. In order for many African Americans to succeed to a place of high education and SES they have likely had to find ways to cope with various forms of racial discrimination, which can lead to depression (Hudson et al., 2016).
Various mechanisms through which these factors are associated with CVD including activation of the hypothalamic-pituitary-adrenal axis and autonomic nervous system, inflammation, blood pressure reactivity, and platelet activation have been proposed (Everson-Rose & Lewis, 2005; Thomas & Advani, 2006). One possible marker, accelerated aging, has emerged from the health disparities literature. The idea of accelerated aging leading to poor health outcomes was first presented through Geronimus’ weathering hypothesis. Weathering is the phenomenon of African Americans showing health declines related to aging earlier than Whites due, presumably, to excessive stress (Geronimus, 1992). Weathering can be measured indirectly by examining aging and disease rates to determine whether disease onset is consistent with the weathering hypothesis (Thorpe et al., 2016) or directly through measures of cumulative dysregulation such as allostatic load (Geronimus et al., 2006), telomere length (Geronimus et al., 2010), and biological age (Forrester et al., 2019). Biological age, or the true global aging-related state of an organism as it evolves with aging, is intended to quantify the aspects of aging that may result from an accelerated chronological aging timeline and may be due to stress or other factors. Biological age, regardless of the method used, has shown associations with SDOH and social factors (Forrester et al., 2019; Geronimus et al., 2010) as well as with cardiovascular outcomes and risk factors (Forrester et al., Under Revise and Resubmit; Fuster & Andres, 2006; Fyhrquist et al., 2013; Haycock et al., 2014; Huzen et al., 2010; Rehkopf et al., 2016).
1.1. Current study
Using data from the Coronary Artery Risk Development in Yong Adults study (CARDIA), we have previously shown that accelerated aging defined as biological age minus chronological age is associated with SDOH (socioeconomic status, social participation, depressive symptoms) (blinded for review) and that accelerated aging is associated with future cardiovascular outcomes (blinded for review). Here, we define “social factors” as social determinants of health plus depressive symptoms and hypothesize that accelerated aging may capture a combination of explanatory variables that mediate the well-documented association of social factors and CVD in the CARDIA cohort.
2. Methods
2.1. Cohort description
CARDIA is a multicenter longitudinal study of 5115 Black or White individuals aged 18 to 30 in 1985–86, described in detail elsewhere (Friedman et al., 1988). Briefly, following the baseline examination at year 0 (Y0) in 1985–86, follow-up examinations were conducted at Y2, Y5, Y7, Y10, Y15, Y20, Y25, and Y30 (2015–16). Interim phone or mail contacts to ascertain vital status and hospitalizations were conducted yearly. At baseline, CARDIA participants had to be free of long-term disease and disability and were selected by random sampling after stratification so that there would be approximately equal numbers of Black and White persons, men and women, ages 18–24 and 25–30 and ≤ 12 and >12 years of education at each of the four CARDIA field centers in Birmingham, AL; Chicago, IL; Minneapolis, MN; and Oakland, CA. All participants provided informed consent and institutional review board approval was obtained at each field center (University of Alabama at Birmingham, Northwestern University, University of Minnesota, and Kaiser Permanente of Northern California). We excluded 2136 participants who did not attend the Y20 exam (N=1740, of whom 175 had died) or attended but did not have complete biological data at Y20 (N=396) for a sample size of 2978. Among Black persons, 38% of those present at baseline were not present at Y20 compared to just 23% of Whites. Men were more likely to be present at baseline and missing by Y20 compared to women and likelihood of presence at baseline and missing at Y20 decreased as education increased. Among Black men with less than a high school education 59% of those present at baseline were missing by year 20, the highest of all race/sex/education groups. This pattern of missingness is likely to attenuate our results as this group is likely to have the most accelerated aging.
2.2. Measurement
2.2.1. Biological age/accelerated aging
Accelerated Aging (AccA) was defined as the difference between biological age (BA) and chronological age (CA) (AccA = BA – CA) so that a positive value indicates that a person is biologically older than their chronological age and conversely a negative value indicates that a person is biologically younger than their CA. BA was calculated with the Klemera & Doubal Method (KDM)(Klemera & Doubal, 2006), as a linear combination of selected biomarkers associated with age and derived from regressions of chronological age on each biomarker. The KDM method uses the intercept, slope, and root mean squared error to solve the regression equation biomarker ~ chronological age in standardized units of the biomarker, and combines these estimated biological ages into BA, using CA, which shrinks BA toward CA. This results in BA being a linear combination of the biomarkers included in the calculation. In order to calculate biological age so that it represents the approximate chronological age at which a person in the general population would have the combination of biomarkers corresponding to the biological age, we used a reference dataset from NHANES to calculate the biological age parameters and applied the parameters to the CARDIA dataset (Levine, 2013). We used NHANES participants from 2007 through 2010 (the only consecutive years in which both c-reactive protein and lung function measures were available) that were non-pregnant and aged between 30 and 75 years. We ran the series of regressions required by KDM and calculated the parameters and then used the parameters along with the CARDIA participant’s chronological ages to create the final BA. We used 4-year weights provided by NHANES to ensure that our results are representative of the general population.
We selected 7 biomarkers based on general knowledge of their association with aging, availability in CARDIA, and significant association with CA in CARDIA: total and HDL cholesterol (mg/dL), glucose (mg/dL), body mass index (BMI), c-reactive protein (CRP) (mg/L), forced expiratory volume in 1 s (FEV1/h2) (liters), and mean arterial pressure (MAP; MAP = diastolic bp2 + systolic bp/3) (mmHg) see (Friedman et al., 1988) and (Lakoski et al., 2006) for more detail on biomarker measurement). We used biomarkers from the Y20 exam in 2005–2006.
2.2.2. Morbidity and mortality
Morbidity and mortality endpoints are determined through annual participant contacts, death certificates, and relevant medical and hospital records. Events are adjudicated by the CARDIA Endpoints Surveillance and Adjudication Committee (ESAS) according to established protocol (Bibbins-Domingo et al., 2009). In examining the temporal relationship between accelerated aging and morbidity and mortality, we used endpoints beginning in 2007 and ending in 2016 for morbidity and 2018 for mortality. Events before 2007 were not included in the analysis but the participants were not excluded. Thus, our endpoints are incident events, but do not necessarily represent incident CVD (although in 99% of cases they do). As an individual with CVD before 2007 might still have an incident event between 2007 and 2018, we performed a sensitivity analysis excluding participants who had undergone an event before 2007. Our CVD endpoint was defined as having at least one of the following endpoints between 2007 and 2018: myocardial infarction (MI), non-MI acute coronary syndrome (non-MI ACS), coronary revascularization, congestive heart failure (CHF), stroke, transient ischemic attack (TIA), coronary artery disease (CAD), peripheral artery disease (PAD), deep vein thrombosis (DVT), pulmonary embolism (PE), atrial fibrillation (AF), or death attributable to CVD.
2.2.3. Race
Race was self-reported at baseline (1985–1986) as Black/African American or White/Caucasian; ethnicity was not ascertained.
2.2.4. Depression symptoms (measured at Y15.)
The Center for Epidemiological Studies – Depression Scale (CES-D)(Radloff, 1977), a 20-question scale that asks about frequency of experiencing symptoms of depression (e.g. sleep changes, weight changes, “feeling blue”) measured depression symptoms. Responses range from 0 (none of the time) to 3 (most or all of the time) for a total score range of 0-60.
2.2.5. Socioeconomic status (measured at Y15)
Socioeconomic status (SES) included 7 categorical variables: education level (less than high school [<12 years], high school [9-12 years], undergraduate [13-16 years], graduate school [17-20 years]), difficulty paying for medical expenses (very hard to not very hard), difficulty paying for basics, income (<$5000 to >= $100,000 in $10,000 increments), assets (<=$500 to >=$500,000 in $10,000 increments), home status (owned or being bought to occupied without payment of money), and food insecurity (often don’t have enough food to have enough food and kinds of food). We conducted a factor analysis and used a factor score where a higher score indicated higher socioeconomic status. The score derived from the factor analysis is hereafter referred to as “SES score.”
2.2.6. Discrimination (measured at Y15.)
Racial and socioeconomic discrimination were measured via the Experiences of Discrimination Scale (Williams et al., 1997). The scale asks about discrimination in seven domains–at school, finding a job, work, housing, medical care, home, and in public. The Experiences of Discrimination Scale has a score range of 0 to 7 for each subscale. Each subscale asks if the respondent has been discriminated against or made to feel inferior due to a given factor (e.g., race, SES, gender, age). Racial discrimination and SES discrimination were separate subscales and therefore there was no overlap in attribution of discrimination. In keeping with previous CARDIA analyses (Borrell et al., 2013) we modeled discrimination categorically. Each discrimination variable (racial and SES) was defined as endorsing 3 or more domains of discrimination within the respective subscale, less than 3 domains, or none.
2.2.7. Social participation
(measured at Y15.) was ascertained from a questionnaire querying the types of groups that a participant may belong to (e.g. church groups, unions, community groups, etc.). Social participation was defined as a continuous score that represented the number of groups reported (0–6). Social participation score was used from the exam.
2.2.8. Covariates
Sex, study site, chronological age, smoking, and alcohol use were used as adjustment variables in analysis. Sex is well known to be associated with CVD outcomes while chronological age is associated with both AccA and CVD outcomes. There is known variation in health status by site in the CARDIA dataset. In this study CVD and mortality differed by site as did AccA. Sex was self-reported as male or female. Site indicated at which center the participant had their baseline interviews conducted. Smoking was defined as never/former smokers vs. current smokers at Y20. Alcohol use was defined as mL alcohol per day at Y20. Chronological age was defined as age at Y20.
2.3. Statistical analysis
All analyses used morbidity or mortality endpoints as outcomes. Most morbidity endpoints (above) had prevalence <1% between 2007 and 2018 so we created a composite CVD endpoint (above). CVD was modeled as absent or present where present indicated that at least one cardiovascular endpoint was adjudicated between 2007 and 2018. The cardiovascular endpoints included in the CVD variable are based on the American Heart Association’s definition of CVD (American Heart Association, 2017). Although hypertension and diabetes were more prevalent than CVD in the sample, we omitted them as individual outcomes because serum glucose and mean arterial pressure are included in the biological age equation. The analysis was completed with the following endpoints – CVD, stroke, and all-cause mortality. Although stroke is included in the CVD definition, we chose to analyze stroke as a separate endpoint because it is historically racially disparate and significantly different by race in this sample. Social factors were measured in 2000–2001 (Y15), accelerated aging was measured in 2005–2006 (Y20) and morbidity and mortality endpoints were measured between 2007 and 2018.
We measured the unadjusted relationship between each social determinant variable and each endpoint separately using logistic regression. Those social variables that were significantly associated with any of the endpoints were retained for mediation analyses. We tested for mediation using the Binary Mediation program in Stata 15, which standardizes the coefficients and then uses the product of coefficients approach to measure indirect effects, both with and without adjustment for covariates. Binary Mediation uses the Baron and Kenny (Baron & Kenny, 1986) approach to measuring mediation but can also handle both dichotomous and continuous variables. We used bootstrapping to obtain standard errors and 95% confidence intervals for the direct and indirect effects. Analyses were done with Stata 15 (StataCorp, 2017) and R 4.0 (R Core Team, 2017).
2.3.1. Mediation analysis
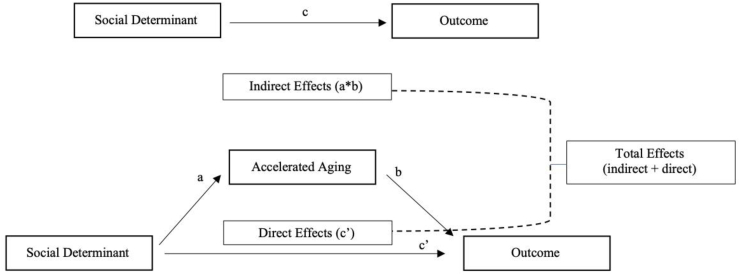
See Fig. 1 for a conceptual model and a visual representation of the following explanation of the binary meditation analysis. The binary mediation program runs a series of regression analyses: (1) the regression of the mediator (accelerated aging) on the predictor (social determinant variable) results in the “path a” coefficient; (2) the regression of the outcome (morbidity/mortality) on the predictor (social determinant variable) results in the “path c” coefficient”; and (3) the regression of the outcome (morbidity/mortality) on the mediator (accelerated aging and the predictor (social determinant variable) results in the “path b” coefficient (accelerated aging) and the “path c’ (c-prime)” coefficient (social determinant variable). For each regression that includes the dichotomous morbidity/mortality outcomes, the binary mediation program standardizes each estimate by the standard deviation of the predictor and the outcome and uses the product of the coefficients approach to calculate the indirect effect. See MacKinnon & Dwyer (Mackinnon & Dwyer, 1993) for the equations used in binary mediation to compute direct, indirect, and total effects. Briefly, the indirect effect is the result of multiplication of the path a and standardized path b coefficients. The direct effect is the standardized path c’ coefficient (the association between the predictor and outcome adjusted for the mediator). The total effect is calculated as path a*path b + path c’. Finally, the percentage of total effect mediated is the percentage of the total effect that is indirect and is calculated as indirect effect/total effect * 100.
Fig. 1.
Conceptual model of the hypothesized relationships between social determinants, accelerated aging, and cardiovascular outcomes. Where “c” is the relationship between stressors and the outcome not adjusted for accelerated aging; “a” is the relationship between stressors and accelerated aging; “b” is the relationship between accelerated aging and the outcome; and “c’ ” (c-prime) is the relationship between stressors and the outcome adjusted for the mediator, accelerated aging.
We first ran unadjusted binary mediation models for each outcome and the social determinant variables that were significantly associated with each outcome and used the bootstrap estimation approach with 500 samples to test the significance of the direct, indirect, and total effects. We next repeated each of the models in binary mediation adjusting for sex, chronological age, race, site, smoking status, and mL alcohol per week.
2.3.2. Sensitivity analyses
Our main analysis was a complete case analysis that included only those who had complete biological data at Y20. Among participants with a CVD outcome, 34% were missing biological data at Y20, and among the 234 participants who died between 2007 and 2018, 75% were missing biological data at Y20. To see whether these exclusions influenced our results, we completed a sensitivity analysis using multiple imputation, based on exams prior and subsequent to Y20 to include those who were not available at Y20. We used multiple imputation, using the multivariate normal distribution, to impute missing biological data for those who were lost by attrition. We created 10 sets of imputed data and pooled the results across them. We then used the imputed datasets in the KDM calculations and conducted the same analyses (unadjusted logistic regression, unadjusted binary mediation, adjusted binary mediation) as previously using the imputed dataset. We completed a second sensitivity analysis where excluding participants who had a cardiovascular outcome before 2007 (n = 25).
3. Results
3.1. Cohort description
Our sample consisted of 2978 Black or White participants with a mean chronological age of 45 years in 2005–2006 (Y20 -Table 1). In the overall sample, accelerated aging (AccA) was 3.62 (SD = 11.7). Black persons had higher average accelerated aging than Whites (9.93 years versus minus 1.43 years) and women had lower average accelerated aging than men (3.34 years versus 3.99). The parameters used to calculate biological age-BA (intercept, slope, root mean squared error and r2 taken from regressions of age on biomarkers) were calculated in the NHANES reference sample and applied to the CARDIA sample (see Supplemental Table 1 for parameters used to calculate BA). When BA parameters weren’t independent of the CARDIA sample (e.g. BA parameters calculated in the CARDIA sample and then applied to the CARDIA sample) they were less precise (higher SDs). There were 219 participants (7%) with CVD events after Y20 in the final sample and 59 all-cause deaths after Y20 (2%). The mean CES-D score at Y15 in the sample was about 8.7 (SD = 7.7) and about 21% reported racial discrimination in more than 3 domains with 19% of the total percentage reported among Black persons.
Table 1.
Summary Statistics for Accelerated aging, Demographics, and Biomarkers: CARDIA, 2000–2018.
| Total (n = 2978) m (sd) or n (%) | Black (n = 1324) m (sd) or n (%) | White (n = 1654) m (sd) or n (%) | |
|---|---|---|---|
| Chronological Age (Y20) | 45.3 (3.6) | 44.6 (3.8) | 45.8 (3.4) |
| Biological Age (Y20) | 48.9 (11.9) | 54.5 (10.0) | 44.4 (11.3) |
| Accelerated aging (Y20) | 3.62 (11.7) | 9.9 (9.3) | -1.4 (10.8) |
| Female (Y20) | 1706 (57%) | 821 (62%) | 885 (53%) |
| Current smoker(Y20) | 532 (18.0) | 313 (23.8) | 219 (13.4) |
| mL Alcohol/week (Y20) | 10.9 (21.8) | 8.6 (22.5) | 12.7 (21.2) |
| Exercise score (EU) (Y20) | 342.5 (275.0) | 297.5 (282.7) | 378.9 (263.0) |
| CES-D (Y15) | 8.7 (7.7) | 10.2 (8.3) | 7.6 (7.0) |
| SES Factor Score (Y15) | 4.6 (1.2) | 4.0 (1.2) | 5.0 (1.0) |
| Racial Discrimination (Y15) | 0.67 (0.8) | 1.2 (0.8) | 0.3 (0.5) |
| Social Participation (Y15) | 7.7 (1.4) | 7.7 (1.5) | 7.7 (1.4) |
| Total Cholesterol (mg/dL) (Y20) | 186.2 (34.8) | 184.2 (35.6) | 187.7 (34.1) |
| HDL Cholesterol (mg/dL) (Y20) | 54.3 (16.6) | 54.4 (16.2) | 54.2 (17.0) |
| Glucose (mg/dL) (Y20) | 97.5 (25.0) | 99.6 (30.0) | 95.8 (19.9) |
| Body Mass Index (Y20) | 29.4 (7.1) | 31.4 (7.4) | 27.8 (6.5) |
| C-reactive protein (mg/L) (Y20) | 2.7 (4.5) | 3.6 (5.3) | 2.0 (3.5) |
| FEV1/h2 (liters) (Y20) | 3.1 (0.8) | 2.7(0.7) | 3.3 (0.8) |
| Mean arterial pressure (mmHg) (Y20) | 87.3 (12.0) | 91.1 (12.4) | 84.3 (10.8) |
| Cardiovascular Disease (2007–2018) | 219 (7%) | 125 (9%) | 94 (6%) |
| Stroke (2007–2018) | 36 (1%) | 29 (2%) | 7 (0.42%) |
| Mortality (2007–2018) | 59 (2%) | 29 (2%) | 30 (2%) |
FEV1/h2 = Forced expiratory volume in 1 s/height2; EU = Exercise Units; CES-D = Center for Epidemiologic Studies Depression Scale.
3.2. Mediation analysis
In our initial, unadjusted analysis we tested depression symptoms, SES score, racial discrimination, SES discrimination, and social participation as predictors of each outcome based on a previous finding showing that these variables are associated with AccA in CARDIA (blinded for review) and we found significant relationships between depression symptoms and CVD, socioeconomic status and CVD, depression symptoms and stroke, racial discrimination and stroke, SES and stroke, depression symptoms and death, social participation and death, and SES and death. All social determinant variables were significantly associated with accelerated aging. We therefore proceeded to study the mediating effect of accelerated aging for eight of the statistically significant associations noted in Table 2.
Table 2.
Unadjusted odds ratios (95% confidence intervals) for morbidity/mortality endpoints in 2007–2018 on social factors in 2000–2001: CARDIA, 2000–2018a.
| CVD OR (95% CI) | Stroke OR (95% CI) | Death OR (95% CI) | |
|---|---|---|---|
| Depression Symptoms | 1.03 (1.01, 1.04) | 1.05 (1.02, 1.09) | 1.06 (1.03, 1.08) |
| SES Score | 0.79 (0.71, 0.89) | 0.76 (0.59, 0.96) | 0.63 (0.53, 0.76) |
| Racial Discrimination | 1.15 (0.97, 1.37) | 1.75 (1.17, 2.60) | 0.98 (0.70, 1.37) |
| SES Discrimination | 1.10 (0.90, 1.34) | 1.07 (0.68, 1.70) | 1.18 (0.84, 1.68) |
| Social Participation | 0.98 (0.88, 1.08) | 0.93 (0.73, 1.19) | 0.74 (0.60, 0.92) |
Units: 1-point increment in each social factor.
Bold type denotes significant association.
3.2.1. Cardiovascular disease
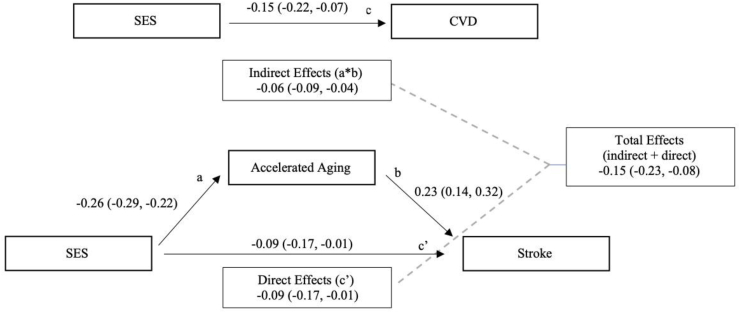
Our initial analysis of social factors and morbidity and mortality outcomes showed that depression symptoms and socioeconomic status score were significant predictors of cardiovascular disease (Table 2). Table 3 shows the results of the unadjusted and adjusted binary mediation analyses for depression and socioeconomic status. Both adjusted and unadjusted analyses of the relationship between depression and cardiovascular disease are consistent with accelerated aging as a partial mediator of this relationship. The results are consistent with partial mediation because when the mediator is included in the model the coefficient for the relationship between CVD and depression is attenuated but remains statistically significant (0.10, 95% CI: 0.03, 0.17). Approximately 8% of the association between depression and CVD is accounted for by accelerated aging and covariates. Similarly, the unadjusted relationship between socioeconomic status and CVD is consistent with partial mediation by accelerated aging (-0.09, 95% CI: -0.17, -0.02) (Fig. 2). After adjustment the model was consistent with AccA fully mediating the relationship between SES and CVD as the addition of AccA to the model rendered path between SES and CVD no longer statistically significant (-0.08, 95% CI: -0.16, 0.01).
Table 3.
Binary Mediation Coefficients for Accelerated aging at Y20 (2005–2006) Mediating the Relationship between Social Factors at Y15 (2000–2001) and Cardiovascular Disease after at Y20 (2007–2016): CARDIA, 2000–2016*.
| Depression Score (Points, Continuous) |
Socioeconomic Status Score (Points, Continuous) |
|||
|---|---|---|---|---|
| Binary Mediation Resultsa | Unadjusted (95% CI) | Adjustedb (95% CI) | Unadjusted (95% CI) | Adjusted (95% CI) |
| Path a | 0.12 (0.09, 0.16) | 0.03 (-0.001,0.07) | -0.26 (-0.30, -0.22) | -0.09 (-0.13, -0.06) |
| Path b | 0.25 (0.17, 0.33) | 0.23 (0.13, 0.33) | 0.23 (0.15, 0.32) | 0.23 (0.13, 0.34) |
| a*b (indirect effect of X on Y) | 0.03 (0.02, 0.04) | 0.01 (0.001, 0.02) | -0.06 (-0.08, -0.04) | -0.02 (-0.03, -0.01) |
| Total Effect of X on Y | 0.13 (0.06, 0.19) | 0.09 (0.03, 0.16) | -0.15 (-0.23, -0.08) | -0.10 (-0.17, -0.02) |
| Percent total effect mediated (% indirect of total effect) | 24.35% | 7.82% | 39.78% | 22.52% |
Note.
Path a = pathway from independent variable (depression score or SES score) to mediator (accelerated aging); Path b = pathway from mediator (accelerated aging) to outcome (cardiovascular disease). The coefficient for Path a corresponds to the association between the independent variable and the mediator; the coefficient for path b corresponds to the association between the mediator and the outcome; a*b corresponds to the effect of the mediation. 95% CI of a*b is bias-corrected and tests whether different than zero; The total effect of X on Y reports the indirect and direct effect of the independent variable on the outcome; percent total effect mediated is the percentage of the outcome that can be attributed to the mediator.
Adjusted for sex, chronological age, race, site, smoking status, and mL alcohol per week.
Fig. 2.
Unadjusted Mediation Model of SES, Accelerated aging, and CVD. a = pathway from SES to accelerated aging; b = pathway from accelerated aging to CVD; c = unadjusted pathway from SES to CVD; c’= pathway from SES to CVD adjusted for accelerated aging. Indirect effects = a*b; Direct effect = effects of SES on CVD in the presence of accelerated aging (c’); Total effects = indirect effect + direct effect. Numbers refer to standardized coefficient (bias-corrected 95% confidence interval) for each path.
3.2.2. Stroke
Table 4 shows the unadjusted and adjusted binary mediation results for the models with stroke as the outcome.
Table 4.
Binary Mediation Coefficients for Accelerated aging at Y20 (2005–2006) Mediating the Relationship between Social Factors at Y15 (2000–2001) and Stroke after at Y20 (2007–2016): CARDIA, 2000–2016.
| Depression Score (Points, Continuous) |
Racial Discrimination Score (Points, Continuous) |
Socioeconomic Status Score (Points, Continuous) |
||||
|---|---|---|---|---|---|---|
| Binary Mediation Resultsa | Unadjusted (95% CI) | Adjustedb (95% CI) | Unadjusted (95% CI) | Adjustedc (95% CI) | Unadjusted (95% CI) | Adjusted (95% CI) |
| Path a | 0.12 (0.09, 0.16) | 0.03 (-0.001, 0.07) | 0.30 (0.26, 0.34) | 0.28 (0.24, 0.31) | -0.26 (-0.30, 0.22) | -0.09 (-0.13, -0.06) |
| Path b | 0.38 (0.23, 0.53) | 0.33 (0.12, 0.54) | 0.37 (0.22, 0.52) | 0.37 (0.19, 0.56) | 0.40 (0.23, 0.56) | 0.37 (0.15, 0.60) |
| a*b (indirect effect of X on Y) | 0.05 (0.03, 0.07) | 0.01 (0.003, 0.02) | 0.11 (0.07, 0.15) | 0.10 (0.06, 0.15) | -0.10 (-0.14, -0.07) | -0.04 (-0.06, -0.02) |
| Total Effect of X on Y | 0.21 (0.10, 0.31) | 0.15 (-0.01, 0.27) | 0.25 (0.07, 0.41) | 0.25 (0.06, 0.42) | -0.16 (0.02, -0.31) | 0.01 (-0.21, 0.26) |
| Percent total effect mediated (% indirect of total effect) | 22.49% | 7.33% | 46.22% | 40.84% | 68.45% | -485.89%d |
The coefficient for Path a corresponds to the association between the independent variable and the mediator; the coefficient for path b corresponds to the association between the mediator and the outcome; a*b corresponds to the effect of the mediation. 95% CI of a*b is bias-corrected and tests whether different than zero; The total effect of X on Y reports the indirect and direct effect of the independent variable on the outcome; percent total effect mediated is the percentage of the outcome that can be attributed to the mediator.
Path a = pathway from independent variable (depression score, racial discrimination, or SES score) to mediator (accelerated aging); Path b = pathway from mediator (accelerated aging) to outcome (stroke).
Adjusted for sex, chronological age, race, site, smoking status, and mL alcohol per week.
Model with racial discrimination as independent variable not adjusted for race.
Addition of covariates suppressed direct effect resulting in inconsistent mediation.
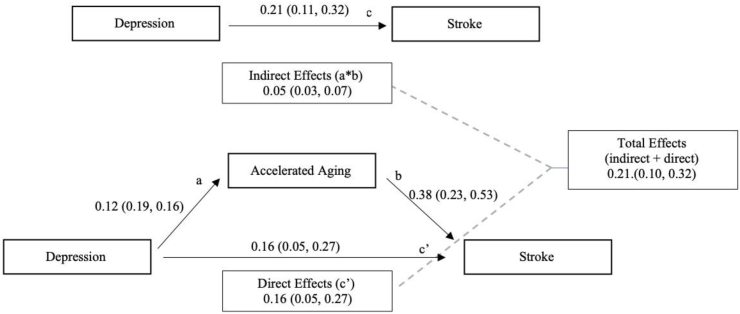
Fig. 3 illustrates the mediation analysis findings for accelerated aging as a mediator of the effect of depression symptoms on stroke. In unadjusted analyses the relationship between depression and stroke was significant as was the relationship between depression and accelerated aging. The direct effect remained significant in the presence of the mediator indicating partial mediation with 22% of the association between depression and stroke mediated by AccA. Adjusted analyses showed an attenuated association that rendered the direct effect not statistically significant consistent with full mediation (0.14, 95% CI: -0.01, 0.29).
Fig. 3.
Unadjusted Mediation Model of Depression, Accelerated aging, and Stroke. a = pathway from depression to accelerated aging; b = pathway from accelerated aging to stroke; c = unadjusted pathway from depression to stroke; c’= pathway from depression to stroke adjusted for accelerated aging. Indirect effects = a*b; Direct effect = effects of depression on stroke in the presence of accelerated aging (c’); Total effects = indirect effect + direct effect. Numbers refer to standardized coefficient (bias-corrected 95% confidence interval) for each path.
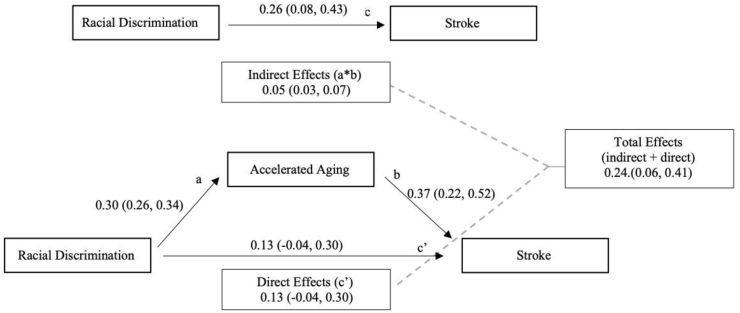
Unadjusted analyses with racial discrimination as the predictor showed a significant association between discrimination and stroke and discrimination and accelerated aging but a non-significant direct effect (0.13, 95% CI: -0.07, 0.31) indicating full mediation (Fig. 4). Adjusted analysis showed similar results (direct effect = 0.15, 95% CI: -0.06, 0.33). Consistent with the previous predictors, unadjusted analysis of SES and stroke indicated full mediation with insignificant direct effects (-0.06, 95% CI: -0.21, 0.16). In adjusted analysis, only the indirect effects remained significant (-0.04, 95% CI: -0.02, -0.06) while the direct effect was not significant and it flipped from a negative association to a positive association (0.04, 95% CI: -0.16, 0.24). The proportion mediated was -4.86 (486%) because in the presence of the mediator and the covariates, higher socioeconomic status was associated with increased likelihood of a stroke outcome (as opposed to decreased likelihood in the unadjusted model), though not significantly. This indicates inconsistent mediation and possible suppressor effects.
Fig. 4.
Unadjusted Mediation Model of Racial Discrimination, Accelerated aging, and Stroke. a = pathway from racial discrimination to accelerated aging; b = pathway from accelerated aging to stroke; c = unadjusted pathway from racial discrimination to stroke; c’= pathway from racial discrimination to stroke adjusted for accelerated aging. Indirect effects = a*b; Direct effect = effects of racial discrimination on stroke in the presence of accelerated aging (c’); Total effects = indirect effect + direct effect. Numbers refer to standardized coefficient (bias-corrected 95% confidence interval) for each path.
3.2.3. Mortality
Table 5 shows the results of the unadjusted and adjusted binary mediation analysis with all-cause mortality. Unadjusted relationships between depression, accelerated aging, and mortality were consistent with partial mediation as the direct effect remained significant (0.21, 95% CI: 0.09, 0.30) in the presence of the mediator. Adjustment for covariates resulted in slight attenuation of the effects but the association remained partially mediated.
Table 5.
Binary Mediation Coefficients for Accelerated aging at Y20 (2005–2006) Mediating the Relationship between Social Factors at Y15 (2000–2001) and All-cause Mortality (2007–2018): CARDIA, 2000–2018.
| Depression Score (Points, Continuous) (95% CI) |
Social Participation (Number of groups, Continuous) (95% CI) |
Socioeconomic Status Score (Points, Continuous) (95% CI) |
||||
|---|---|---|---|---|---|---|
| Binary Mediation Resultsa | Unadjusted | Adjustedb | Unadjusted | Adjusted | Unadjusted | Adjusted |
| Path a | 0.12 (0.09, 0.16) | 0.03 (-0.001, 0.07) | -0.04 (-0.08, -0.01) | -0.03 (-0.06, 0.003) | -0.26 (-0.30, -0.22) | -0.09 (-0.13, -0.06) |
| Path b | 0.23 (0.10, 0.37) | 0.27 (0.12, 0.42) | 0.24 (0.11, 0.37) | 0.27 (0.11, 0.42) | 0.19 (0.05, 0.33) | 0.24 (0.09, 0.40) |
| a*b (indirect effect of X on Y) | 0.02 (0.01, 0.05) | 0.01 (0.002, 0.03) | -0.01 (-0.02, -0.003) | -0.01 (-0.001, -0.02) | -0.04 (-0.09, 0.01) | -0.02 (-0.04, -0.01) |
| Total Effect of X on Y | 0.23 (0.12, 0.32) | 0.21 (0.07, 0.31) | -0.21 -0.07, -0.38) | -0.19 (-0.05, -0.36) | -0.29 (-0.38, -0.16) | -0.26 (-0.40, -0.11) |
| Percent total effect mediated (% indirect of total effect) | 12.45% | 4.39% | 5.10%% | 4.31% | 13.42% | 9.07% |
The coefficient for Path a corresponds to the association between the independent variable and the mediator; the coefficient for path b corresponds to the association between the mediator and the outcome; a*b corresponds to the effect of the mediation. 95% CI of a*b is bias-corrected and tests whether different than zero; The total effect of X on Y reports the indirect and direct effect of the independent variable on the outcome; percent total effect mediated is the percentage of the outcome that can be attributed to the mediator.
Adjusted for sex, chronological age, race, site, smoking status, and mL alcohol per week.
Path a = pathway from independent variable (depression score or racial discrimination) to mediator (accelerated aging); Path b = pathway from mediator (accelerated aging) to outcome (stroke).
The association between SES and mortality showed partial mediation by accelerated aging similar to mortality and depression. The association between SES and mortality was significant as was the relationship between SES and accelerated aging. The direct effect between SES and mortality remained significant when accelerated aging was included in the model (-0.24, 95% CI: -0.35, -0.11). Addition of covariates attenuated the relationship between SES and mortality thus reducing the indirect effect, but the direct effect remained similar (-0.26, 95% CI: -0.40, -0.11) and the effect remained consistent with partial mediation. Social participation showed a similar association consistent with partial mediation where the direct effect remained significant when the mediator was added to the model (-0.21, 95% CI: -0.37, -0.06). Adjustment for covariates attenuated the association but the direct effect (-0.19, 95% CI: -0.34, -0.03) and overall partial mediation remained the same.
3.3. Sensitivity analysis
For the first sensitivity analysis, imputing values not available at Y20, the main difference from the primary results was in the association between socioeconomic status and outcomes in the unadjusted logistic regression analysis. The primary analysis only showed a significant association between socioeconomic status and CVD whereas the analysis on the full imputed sample showed a significant association with all three primary outcomes. The binary mediation analyses were similar, though slightly attenuated, in the imputed sample versus the primary analysis (results not shown). The second sensitivity analyses excluding participants without cardiovascular outcomes before 2007 showed similar results as the primary analysis (results not shown).
4. Discussion
We found that accelerated aging, based on a linear combination of clinical biomarkers, could partially explain the association between social factors and CVD morbidity and mortality. Although previous studies have explored the association between other measures of accelerated aging (e.g. telomere length) and cardiovascular outcomes and mortality, we know of no studies examining accelerated aging as a function of biological and chronological age in this manner. Our findings are consistent with the hypothesis that accelerated aging may represent the mechanism relating social factors to cardiovascular outcomes.
4.1. Social factors and morbidity/mortality
An abundance of literature has confirmed the association of social factors and cardiovascular outcomes and death. The importance of this field of research is illustrated by the term “behavioral cardiology” (Rozanski, 2014). A common theme in this literature is the robust association of depression and cardiovascular outcomes. both predicts incident CVD and worse outcomes in those with CVD (Pedersen et al., 2017). In our fully adjusted models, we found that each one-point increase in CES-D score was associated with an increase in the odds of any CVD, stroke, and death, confirming the association. We also found that higher socioeconomic status was associated with a decreased risk of cardiovascular disease, stroke, and mortality.
4.2. Accelerated aging mediates the relationship between social factors and health outcomes
This hypothesis was designed to expand our finding from a previous report where we demonstrated that social factors could predict later accelerated aging (blinded for review). Our mediation analyses revealed that accelerated aging is a possible pathway for the associations between certain social factors (depression, racial discrimination, socioeconomic status, social participation) and cardiovascular outcomes. The strongest relationships that we found (based on proportion of effect mediated after adjustment) were between SES and CVD, and racial discrimination and stroke. This is particularly interesting in light of other research showing that higher SES tends to be better for mental and physical health but that among Black persons in particular, racial discrimination may cancel out the positive effects of higher SES (Hudson et al., 2012). This phenomenon could be due to the high effortful coping or “John Henryism” that is often required of Black persons who achieve high socioeconomic status (Hudson et al., 2016; McKetney & Ragland, 1996). Black persons are far less likely than White persons to be upwardly mobile (being born into a low SES and move up to a higher SES in adulthood) and when they do, they face many more obstacles including racial discrimination, the toll of which can lead to depression (Hertz, 2009, p. 165) and ultimately affect cardiovascular health. This research would benefit from a future analysis with greater power to test interactions between racial discrimination and depression among Black persons.
As previously discussed, depression has been linked to cardiovascular outcomes. Wolkowitz et al. have proposed a model in which major depressive disorder (MDD) causes accelerated aging through biochemical mediators associated with the HPA axis, oxidative stress, inflammation, and others, and accelerated aging results in disease (Wolkowitz et al., 2010, 2011). Although we didn’t measure diagnosed MDD, our findings support this model as our results were consistent with accelerated aging mediating the relationship between depression symptoms and stroke and depression symptoms and CVD overall. Others have also shown that MDD was associated with another measure of accelerated aging, shortened telomeres (Verhoeven et al., 2014). Our other major finding, that accelerated aging fully mediates the relationship between racial discrimination and stroke is less clear cut.
Although discrimination has been repeatedly linked to mental health outcomes, perceived discrimination as a risk factor for disease and poor health, has produced inconsistent results. Perceived discrimination (both overall and race-specific) has been associated with blood pressure (Krieger & Sidney, 1996), self-rated health (Colen et al., 2018), hypertensive status (Cozier et al., 2006; Dolezsar et al., 2014), risk of CVD (D. H. Chae et al., 2010), elevated diastolic blood pressure (Lewis et al., 2009), coronary artery calcification (Everage et al., 2012; Lewis et al., 2006), and poor physical and mental health (Borrell et al., 2006). The findings are mixed, however, because most of the relationships have qualifiers such as beliefs about Black people, chronological age, type of discrimination (lifetime versus chronic), nativity and childhood residence, and coping. Clark et al. propose a biopsychosocial model of racism as a stressor for African Americans (Clark et al., 1999). Our results seem to partially confirm this model; however, we were unable to stratify by race and hence cannot confirm that the model is specific to African Americans. Although African Americans by far reported the most racial discrimination (75% reported any racial discrimination, and 45% reported racial discrimination in three or more domains), 23% of Whites also reported some racial discrimination and a small proportion reported racial discrimination in three or more domains (3%). It is likely that the perception of racial discrimination is different by race and adjusting for race did significantly attenuate the relationship between racial discrimination and stroke (results not shown), indicating that racial discrimination experienced by Black persons likely drives the significant relationship.
Various biological mechanisms have been proposed for these associations including autonomic nervous system dysfunction, endothelial dysfunction, inflammation, prothrombotic state, and increased stress reactivity (Pedersen et al., 2017). Our findings may also encompass multiple previously delineated mechanisms. Inflammation for instance was part of our biological age measure so it is included in the definition of accelerated aging. The weathering hypothesis as originally stated was intended to show how chronic stress affected physical health so increased stress reactivity should, in theory, be captured by any measure of weathering. Presumably, our measure of accelerated aging is more comprehensive than inflammation or HPA axis dysfunction alone, and it is also easier to measure. The stark racial differences in accelerated aging also make it a candidate for a marker of disparities in health outcomes. Therefore, a logical next step would be to test accelerated aging as a marker in a bi-racial cohort with enough power to stratify by race.
4.3. Limitations and strengths
Our results should be understood in light of a few limitations. First, it is never ideal to “adjust out” race, however due to the low prevalence of outcomes in our sample we were underpowered to stratify or include race interactions. Next, our sample was overall relatively healthy thus limiting the generalizability to unhealthy populations. Further, most of our individual outcomes had a low prevalence in our study sample therefore limiting our power to detect associations. We adjusted for smoking and alcohol use rather than modeling them as mediators. Although substance use is likely on the pathway from stress to accelerated aging, we are unable to determine if smoking and alcohol use are a cause or consequence of the stressors that disadvantage minorities and thus, we didn’t expressly model these relationships. Finally, we did not include measures of diet and exercise which are known to affect certain CVD outcomes and could affect our results. The strengths of our study include longitudinal data allowing for temporal relationships between variables and that we used a validated method of biological age computation including calculations of biological age parameters in a reference dataset.
4.4. Conclusions and future directions
We have demonstrated that a definition of accelerated aging that is based on easily measurable biomarkers partially mediates associations between social factors and cardiovascular outcomes, even after adjustment for demographics, smoking, and alcohol consumption. Conceptually, these findings contribute to our quest to understand how social conditions “get under the skin” (Taylor et al., 1997) to affect health outcomes. A measure of accelerated aging that shows promise as a mechanism for cardiovascular health disparities could serve as a modifiable target in health equity interventions.
Funding
This work was supported through an NIH T32 (5T32HL120823-04) and a CCTS Clinical Scholar Award.
Author statement
Sarah Forrester: Conceptualization, Methodology, Writing - original draft. Rachel Zmora: Conceptualization. Pamela Schreiner: Resources, Investigation, Writing - review & editing. David Jacobs Jr: Data curation, Investigation, Writing - review & editing. Veronique Roger: Resources, Data curation, Investigation, Writing - review & editing. Roland Thorpe Jr: Resources, Writing - review & editing. Catarina Kiefe: Conceptualization, Writing - original draft, Investigation.
Ethics approval
Study protocols at each site were approved by their local IRB. Ethical approval was not required for this analysis because it utilized de-identified secondary data analysis. The primary author and Dr. Kiefe did complete and sign a Data and Materials Distribution Agreement with CARDIA prior to receiving data.
Declaration of competing interest
No authors have any conflicts of interest to disclose
Acknowledgments
The Coronary Artery Risk Development in Young Adults Study (CARDIA) is conducted and supported by the National Heart, Lung, and Blood Institute (NHLBI) in collaboration with the University of Alabama at Birmingham (HHSN268201800005I & HHSN268201800007I), Northwestern University (HHSN268201800003I), University of Minnesota (HHSN268201800006I), and Kaiser Foundation Research Institute (HHSN268201800004I). This manuscript has been reviewed by CARDIA for scientific content.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ssmph.2021.100733.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- American Heart Association . 2017. What is cardiovascular disease? [Google Scholar]
- Baron R.M., Kenny D.A. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology. 1986;51 doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Benjamin E.J., Muntner P., Alonso A., Bittencourt M.S., Callaway C.W., Carson A.P. Heart disease and stroke statistics-2019 update: A report from the American heart association. Circulation. 2019;139:e56–e66. doi: 10.1161/CIR.0000000000000659. [DOI] [PubMed] [Google Scholar]
- Bibbins-Domingo K., Pletcher M.J., Lin F., Vittinghoff E., Gardin J.M., Arynchyn A. Racial differences in incident heart failure among young adults. New England Journal of Medicine. 2009;360:1179–1190. doi: 10.1056/NEJMoa0807265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrell L.N., Kiefe C.I., Diez-Roux A.V., Williams D.R., Gordon-Larsen P. Racial discrimination, racial/ethnic segregation, and health behaviors in the CARDIA study. Ethnicity and Health. 2013;18:227–243. doi: 10.1080/13557858.2012.713092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrell L.N., Kiefe C.I., Williams D.R., Diez-Roux A.V., Gordon-Larsen P. Self-reported health, perceived racial discrimination, and skin color in African Americans in the CARDIA study. Social Science & Medicine. 2006;63:1415–1427. doi: 10.1016/j.socscimed.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Chae D.H., Lincoln K.D., Adler N.E., Syme S.L. Do experiences of racial discrimination predict cardiovascular disease among African American men? The moderating role of internalized negative racial group attitudes. Social Science & Medicine. 2010;71:1182–1188. doi: 10.1016/j.socscimed.2010.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae D.H., Nuru-Jeter A.M., Lincoln K.D., Arriola K.R.J. Racial discrimination, mood disorders, and cardiovascular disease among black Americans. Annals of Epidemiology. 2012;22:104–111. doi: 10.1016/j.annepidem.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R., Anderson N.B., Clark V.R., Williams D.R. Racism as a stressor for African Americans. A biopsychosocial model. American Psychologist. 1999;54:805–816. doi: 10.1037//0003-066x.54.10.805. [DOI] [PubMed] [Google Scholar]
- Colen C.G., Ramey D.M., Cooksey E.C., Williams D.R. Racial disparities in health among nonpoor African Americans and Hispanics: The role of acute and chronic discrimination. Social Science & Medicine. 2018;199:167–180. doi: 10.1016/j.socscimed.2017.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozier Y., Palmer J.R., Horton N.J., Fredman L., Wise L.A., Rosenberg L. Racial discrimination and the incidence of hypertension in US black women. Annals of Epidemiology. 2006;16:681–687. doi: 10.1016/j.annepidem.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Das S., O'Keefe J.H. Behavioral cardiology: Recognizing and addressing the profound impact of psychosocial stress on cardiovascular health. Current Atherosclerosis Reports. 2006;8:111–118. doi: 10.1007/s11883-006-0048-2. [DOI] [PubMed] [Google Scholar]
- Dolezsar C.M., McGrath J.J., Herzig A.J., Miller S.B. Perceived racial discrimination and hypertension: A comprehensive systematic review. Health Psychology. 2014;33:20–34. doi: 10.1037/a0033718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everage N.J., Gjelsvik A., McGarvey S.T., Linkletter C.D., Loucks E.B. Inverse associations between perceived racism and coronary artery calcification. Annals of Epidemiology. 2012;22:183–190. doi: 10.1016/j.annepidem.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everson-Rose S.A., Lewis T.T. Psychosocial factors and cardiovascular diseases. Annual Review of Public Health. 2005;26:469–500. doi: 10.1146/annurev.publhealth.26.021304.144542. [DOI] [PubMed] [Google Scholar]
- Forrester S.N., Jacobs D.R., Zmora R., Schreiner P., Roger V.L., Kiefe C.I. Racial differences in weathering and its associations with psychosocial stress: The CARDIA study. SSM - Population Health. 2019;7 doi: 10.1016/j.ssmph.2018.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrester S.N., Zmora R., Schreiner P., Jacobs D.R., Jr., Roger V.L., Thorpe R.J., Jr. (Under Revise and Resubmit). Association of weathering with future cardiovascular events and all-cause mortality: The coronary artery risk development in young adults study. Annals of Epidemiology. 2007 - 2018 doi: 10.1080/13557858.2020.1839021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman G.D., Cutter G.R., Donahue R.P., Hughes G.H., Hulley S.B., Jacobs D.R., Jr. CARDIA: Study design, recruitment, and some characteristics of the examined subjects. Journal of Clinical Epidemiology. 1988;41:1105–1116. doi: 10.1016/0895-4356(88)90080-7. [DOI] [PubMed] [Google Scholar]
- Fuster J.J., Andres V. Telomere biology and cardiovascular disease. Circulation Research. 2006;99:1167–1180. doi: 10.1161/01.RES.0000251281.00845.18. [DOI] [PubMed] [Google Scholar]
- Fyhrquist F., Saijonmaa O., Strandberg T. The roles of senescence and telomere shortening in cardiovascular disease. Nature Reviews Cardiology. 2013;10:274–283. doi: 10.1038/nrcardio.2013.30. [DOI] [PubMed] [Google Scholar]
- Gebreab S.Y., Diez-Roux A.V., Hickson D.A., Boykin S., Sims M., Sarpong D.F. The contribution of stress to the social patterning of clinical and subclinical CVD risk factors in African Americans: The Jackson heart study. Social Science & Medicine. 2012;75:1697–1707. doi: 10.1016/j.socscimed.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geronimus A.T. The weathering hypothesis and the health of African-American women and infants: Evidence and speculations. Ethnicity & Disease. 1992;2:207–221. [PubMed] [Google Scholar]
- Geronimus A.T., Hicken M., Keene D., Bound J. "Weathering" and age patterns of allostatic load scores among blacks and whites in the United States. American Journal of Public Health. 2006;96:826–833. doi: 10.2105/AJPH.2004.060749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geronimus A.T., Hicken M.T., Pearson J.A., Seashols S.J., Brown K.L., Cruz T.D. Do US black women experience stress-related accelerated biological aging?: A Novel theory and first population-based test of black-white differences in telomere length. Human Nature. 2010;21:19–38. doi: 10.1007/s12110-010-9078-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havranek E.P., Mujahid M.S., Barr D.A., Blair I.V., Cohen M.S., Cruz-Flores S. Social determinants of risk and outcomes for cardiovascular disease: A scientific statement from the American heart association. Circulation. 2015;132:873–898. doi: 10.1161/CIR.0000000000000228. [DOI] [PubMed] [Google Scholar]
- Haycock P.C., Heydon E.E., Kaptoge S., Butterworth A.S., Thompson A., Willeit P. Leucocyte telomere length and risk of cardiovascular disease: Systematic review and meta-analysis. BMJ. 2014;349:g4227. doi: 10.1136/bmj.g4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz T. 2009. Rags, riches, and race. Unequal chances: Family background and economic success. [Google Scholar]
- Hudson D.L., Bullard K.M., Neighbors H.W., Geronimus A.T., Yang J., Jackson J.S. Are benefits conferred with greater socioeconomic position undermined by racial discrimination among African American men? Journal of Men's Health. 2012;9:127–136. doi: 10.1016/j.jomh.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson D.L., Neighbors H.W., Geronimus A.T., Jackson J.S. Racial discrimination, John Henryism, and depression among African Americans. Journal of Black Psychology. 2016;42:221–243. doi: 10.1177/0095798414567757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huzen J., de Boer R.A., van Veldhuisen D.J., van Gilst W.H., van der Harst P. The emerging role of telomere biology in cardiovascular disease. Frontiers in Bioscience. 2010;15:35–45. doi: 10.2741/3604. [DOI] [PubMed] [Google Scholar]
- Klemera P., Doubal S. A new approach to the concept and computation of biological age. Mechanism of Ageing and Development. 2006;127:240–248. doi: 10.1016/j.mad.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Krieger N., Sidney S. Racial discrimination and blood pressure: The CARDIA study of young black and white adults. American Journal of Public Health. 1996;86:1370–1378. doi: 10.2105/ajph.86.10.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagraauw H.M., Kuiper J., Bot I. Acute and chronic psychological stress as risk factors for cardiovascular disease: Insights gained from epidemiological, clinical and experimental studies. Brain, Behavior, and Immunity. 2015;50:18–30. doi: 10.1016/j.bbi.2015.08.007. [DOI] [PubMed] [Google Scholar]
- Lakoski S.G., Herrington D.M., Siscovick D.M., Hulley S.B. C-reactive protein concentration and incident hypertension in young adults: The CARDIA study. Archives of Internal Medicine. 2006;166:345–349. doi: 10.1001/archinte.166.3.345. [DOI] [PubMed] [Google Scholar]
- Levine M.E. Modeling the rate of senescence: Can estimated biological age predict mortality more accurately than chronological age? Journals of Gerontology Series A: Biological and Medical Sciences. 2013;68:667–674. doi: 10.1093/gerona/gls233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis T.T., Barnes L.L., Bienias J.L., Lackland D.T., Evans D.A., Mendes de Leon C.F. Perceived discrimination and blood pressure in older African American and white adults. Journals of Gerontology Series A: Biological and Medical Sciences. 2009;64:1002–1008. doi: 10.1093/gerona/glp062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis T.T., Everson-Rose S.A., Powell L.H., Matthews K.A., Brown C., Karavolos K. Chronic exposure to everyday discrimination and coronary artery calcification in African-American women: The SWAN heart study. Psychosomatic Medicine. 2006;68:362–368. doi: 10.1097/01.psy.0000221360.94700.16. [DOI] [PubMed] [Google Scholar]
- Mackinnon D., Dwyer J.H. Estimating mediated effects in prevention studies. Evaluation Review. 1993;17:144–158. [Google Scholar]
- McKetney E.C., Ragland D.R. John Henryism, education, and blood pressure in young adults the CARDIA study. American Journal of Epidemiology. 1996;143:787–791. doi: 10.1093/oxfordjournals.aje.a008816. [DOI] [PubMed] [Google Scholar]
- Pedersen S.S., von Kanel R., Tully P.J., Denollet J. Psychosocial perspectives in cardiovascular disease. European Journal of Preventive Cardiology. 2017;24:108–115. doi: 10.1177/2047487317703827. [DOI] [PubMed] [Google Scholar]
- R Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2017. R: A language and environment for statistical computing. [Google Scholar]
- Radloff L.S. Vol. 1. 1977. (The CES-D scale: A self-reprot depression scale for research in the general population applied psychological measurement). [Google Scholar]
- Rehkopf D.H., Needham B.L., Lin J., Blackburn E.H., Zota A.R., Wojcicki J.M. Leukocyte telomere length in relation to 17 biomarkers of cardiovascular disease risk: A cross-sectional study of US adults. PLoS Medicine. 2016;13 doi: 10.1371/journal.pmed.1002188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozanski A. Behavioral cardiology: Current advances and future directions. Journal of the American College of Cardiology. 2014;64:100–110. doi: 10.1016/j.jacc.2014.03.047. [DOI] [PubMed] [Google Scholar]
- StataCorp . StataCorp LLC; College Station, Tx: 2017. Stata statistical software: Release 15. [Google Scholar]
- Taylor S.E., Repetti R.L., Seeman T. Health psychology: What is an unhealthy environment and how does it get under the skin? Annual Review of Psychology. 1997;48:411–447. doi: 10.1146/annurev.psych.48.1.411. [DOI] [PubMed] [Google Scholar]
- Thomas T.H., Advani A. Inflammation in cardiovascular disease and regulation of the actin cytoskeleton in inflammatory cells: The actin cytoskeleton as a target. Cardiovascular and Hematological Agents in Medicinal Chemistry. 2006;4:165–182. doi: 10.2174/187152506776369926. [DOI] [PubMed] [Google Scholar]
- Thorpe R.J., Jr., Fesahazion R.G., Parker L., Wilder T., Rooks R.N., Bowie J.V. Accelerated health declines among African Americans in the USA. Journal of Urban Health. 2016;93:808–819. doi: 10.1007/s11524-016-0075-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhoeven J.E., Revesz D., Wolkowitz O.M., Penninx B.W. Cellular aging in depression: Permanent imprint or reversible process?: An overview of the current evidence, mechanistic pathways, and targets for interventions. BioEssays. 2014;36:968–978. doi: 10.1002/bies.201400068. [DOI] [PubMed] [Google Scholar]
- Williams D.R., Yan Y., Jackson J.S., Anderson N.B. Racial differences in physical and mental health: Socio-economic status, stress and discrimination. Journal of Health Psychology. 1997;2:335–351. doi: 10.1177/135910539700200305. [DOI] [PubMed] [Google Scholar]
- Wolkowitz O.M., Epel E.S., Reus V.I., Mellon S.H. Depression gets old fast: Do stress and depression accelerate cell aging? Depression and Anxiety. 2010;27:327–338. doi: 10.1002/da.20686. [DOI] [PubMed] [Google Scholar]
- Wolkowitz O.M., Mellon S.H., Epel E.S., Lin J., Dhabhar F.S., Su Y. Leukocyte telomere length in major depression: Correlations with chronicity, inflammation and oxidative stress--preliminary findings. PloS One. 2011;6 doi: 10.1371/journal.pone.0017837. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.