Abstract
Autophagy and apoptosis are powerful regulators of multiple facets of cellular metabolism and homeostasis. Here, we uncover that galanin, a pleiotropic peptide, regulates cardiac autophagy and deactivates apoptotic cell death through the Forkhead box protein O1 (FoxO1) pathway. In hypertrophied heart, galanin promotes autophagy and metabolic shift from fatty acid (FA) to glucose oxidation and preserves mitochondrial integrity. In cardiomyoblasts, galanin triggers autophagosome formation and alleviates hypertrophy, apoptotic cell death, and mitochondrial stress. Mechanistically, galanin dictates cell autophagic and anti-apoptotic phenotypes through FoxO1 pathway. Together, these findings uncover a previously unknown role for galanin in the regulation of cardiac autophagy and provide new insights into the molecular mechanisms supporting cell survival in the hypertrophic reprogramming of the heart.
Keywords: Galanin, Autophagy, Hypertrophy, Cardiac remodeling, Metabolism, Apoptosis
Graphical abstract
Highlights
-
•
Galanin dictates autophagic phenotype in cardiomyoblasts.
-
•
Galanin suppresses myocardial apoptosis and mitochondrial oxidative stress in hypertrophic remodeling.
-
•
Galanin promotes metabolic shift from fatty acid to glucose oxidation in the hypertrophied hearts.
-
•
Galanin regulates cardiac autophagy and apoptosis through FoxO1 pathway.
1. Introduction
Heart failure (HF) is a major cause of mortality and long-term disability after myocardial infarction (MI) [1]. Cardiac hypertrophy is a major predictor of progressive HF and an adverse prognosis [1]. Despite major therapeutic advances, the morbidity and mortality of HF remain unacceptably high and the current approach to tackle cardiac hypertrophy is still reductionist [2]. Therefore, novel insights into the biology and pathophysiology of hypertrophic remodeling of the heart are required to develop novel therapeutic intervention.
Complex cell machineries orchestrate the survival and death decisions in hypertrophied and failing heart. Initially, cardiac hypertrophy is an adaptive response to stress, which may turn maladaptive and fatal culminating in the loss of cardiac performance [3,4]. Considering that hypertrophy is associated with numerous biological processes including apoptosis, oxidative stress and mitochondrial metabolism [5], there is a great need for improved understanding of the mechanistic biology underlying cell growth. Impairment of autophagy, a cellular degradation pathway for the clearance of damaged proteins and organelles, has also been implicated in cardiac hypertrophy and HF phenotype [6]. The process of autophagy starts by sequestering cytosolic proteins or organelles into autophagosomes that then fuse with lysosomes to form autolysosomes for the degradation of sequestered contents by lysosomal hydrolases [7]. In cardiac cells, autophagy is constitutively active, maintaining an important housekeeping role in physiological and pathological situations. Autophagy may protect against myocardial injury by promoting cell survival or contribute to cardiac cell death [8,9]. Importantly, autophagy and apoptosis often occur in the same cell and loss or gain of either autophagy or apoptosis drives numerous pathological events [10].
Galanin is an endogenous peptide consisting of a chain of 29 amino acids (30 amino acids in humans) that is widely expressed in the central and peripheral nervous systems and endocrine axis [11]. The amino acid sequence of galanin is very conserved (almost 90% among species), indicating the physiopathological importance of the peptide in the body. Galanin orchestrates numerous physiological functions, including energy homeostasis, nociception, sleep regulation, cognition, neuroendocrine activities and central cardiovascular control [12]. Galanin is linked to a number of diseases including, epilepsy [13], depression [14], and eating disorders [15]. Galanin regulates cellular multi-responses by activating three distinct G-protein-coupled receptors, galanin receptor 1 (GalR1), GalR2, and GalR3, which differ in their pharmacology, signaling, and distribution [[16], [17], [18]]. Galaninergic system has also been associated with regulation of neurogenesis, stroke-related damage, inflammation, cancer, diabetes, and myocardial infarction [[19], [20], [21], [22], [23], [24]]. Despite the significance of galaninergic system in a wide variety of physiological and pathophysiological processes, there is no evidence to date that galanin plays a role in autophagy. Here, we describe a novel function for galanin in controlling of autophagy in the hypertrophied heart. We report that galanin promotes autophagosome formation and alleviates apoptotic priming, mitochondrial oxidative stress and injury in the failing myocardium. Furthermore, we dissect that galanin activates cardiac autophagic and anti-apoptotic responses through FoxO1 pathway.
2. Methods
2.1. Animal studies
The investigation conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85–23, revised 1985) and was performed in accordance with the recommendations of the French Accreditation of the Laboratory Animal Care (approved by the local Centre National de la Recherche Scientifique ethics committee). Two-month old wild-type male C57BL6/J mice purchased from Janvier Labs.
A mouse model of ischemia-reperfusion (I/R) injury was used as previously described [25]. In brief, the mice were incubated and placed under mechanical ventilation after undergoing general anesthesia, induced by i. p. injection of ketamine (35 mg·kg-1) and xylazine (5 mg kg−1). A left parasternotomy was performed to expose hearts, and a 7–0 silk suture (Softsilk; US Surgical, Norwalk, CT, USA) was placed around the left anterior descending coronary artery. A snare was placed on the suture, and regional myocardial ischemia was produced by tightening the snare. After 30 min of ischemia, the occlusive snare was released to initiate reperfusion up to 24 h. Sham-operated control mice underwent the same surgical procedures except that the snare was not tightened. The animals were randomly divided into four groups: (i) sham vehicle; (ii) I/R vehicle; (iii) sham galanin; and (iv) I/R galanin. Galanin (0,7 μg/kg, i. v.) or vehicle (PBS) was injected into the jugular vein at 15 min of reperfusion phase.
2.2. Determination of myocardial infarct size
Infarct size was determined by the triphenyltetrazolium chloride (TTC) staining technique as previously described [25]. In brief, the hearts at the end of the experimental protocol, Evans blue dye (1.5%, 1.0 ml) was injected into the left ventricular cavity. This turns viable tissue a dark red color, whereas non-viable, necrotic myocardium appears pale. The hearts were incubated for 10 min in TTC before staining in 10% formaldehyde solution to increase contrast between necrotic and viable myocardium. The areas of infarcted tissue, the risk zone, and the whole left ventricle were determined by computer morphometry. The area for each region was averaged from slices. Infarct size was expressed as a percentage of the ischemic risk area.
2.3. Histology
Ultrastructural studies of cardiac tissue by electron microscopy were done as before [26]. Briefly, cardiac tissue were fixed in cold 2.5% glutaraldehyde/1% paraformaldehyde, post-fixed in 2% osmium tetroxide, embedded in resin, and sectioned. Hematoxylin-eosin (H&E) and wheat germ agglutinin (WGA) staining were performed on 10 μm heart cryosections were done according to standard methods. The extent of cardiac fibrosis was quantified using ImageJ software.
2.4. Cell culture, transfection and treatments
The rat embryonic cardiomyoblastic cell line H9C2 was cultured in DMEM medium (Life Technologies) supplemented with 10% FBS and 1% penicillin-streptomycin in a 37 °C, 5% CO2 incubator. siRNA transfection was performed with LipofectamineRNAiMAX (Life Technologies) according to manufacturer's instructions. For hypoxic treatment, cells were pretreated for 30 min with galanin (10 nM) or vehicle (PBS) and then subjected to normoxia (5% CO2; 21% O2, balance N2) or hypoxia in a hypoxic chamber (5% CO2, 1% O2, balance N2) for 2 h (for mitochondrial oxidative stress) or 16 h (for apoptosis). To assess cell hypertrophy, the medium was replaced and cells were further incubated for 24 h in normoxic conditions (reoxygenation) in the continuous presence of galanin or PBS.
2.5. Reagents and antibodies
Antibodies used in this study are: anti-HSP60 (sc-13115), anti-HSP90 (sc-13119) and anti-RhoGDI (sc-373723) from Santa Cruz Biotechnology; anti-LC3B (3868 for immunofluorescence and 2775 for Western blot), anti-β-actin (3700) and anti-FoxO1 (2880) from Cell Signaling; anti-CD68 (MCA1957GA) from Bio-Rad (formerly AbDSerotec). Fluorescent Alexa-coupled secondary antibodies and DAPI were from Life Technologies and HRP-coupled secondary antibodies from Cell Signaling Technology. Galanin was purchased from GeneCust and was referred to as Gal throughout this study. Bafilomycin A1, a proton-ATPase inhibitor, from Sigma-Aldrich, was used to inhibit the autophagic flux. All other chemicals were from Sigma-Aldrich unless otherwise stated.
2.6. Protein extraction and western blotting
Proteins from cardiac tissues and H9C2 cells were extracted using RIPA buffer and quantified using the Bio-Rad Protein Assay (Bio-Rad). Proteins were resolved by SDS-PAGE and western blotting. Immunoreactive bands were detected by chemiluminescence with the Clarity Western ECL Substrate (Bio-Rad) on a ChemiDoc MP Acquisition system (Bio-Rad).
2.7. RNA extraction and quantitative polymerase chain reaction (qRT-PCR)
The expression of genes was assessed using quantitative polymerase chain reaction. Total RNAs were isolated from rat embryonic cardiomyoblastic cell line H9C2 or mice heart using the RNeasy mini kit (Qiagen). Total RNAs (300 ng) were reverse transcribed using Superscript II reverse transcriptase (Invitrogen) in the presence of a random hexamers. Real-time quantitative PCR was performed as previously described [26]. The expression of target mRNA was normalized to GAPDH mRNA expression. Primers for qRT-PCR used in this study are as detailed in Supplemental Table 1.
2.8. Evaluation of apoptosis and mitochondrial oxidative stress
The apoptosis level was assessed using DeadEndFluorometric TUNEL system according to manufacturer's instructions (Promega, Madison, WI, USA). TUNEL is a general method to detect nuclear DNA fragmentation during apoptosis. TUNEL technique relies on the use of endogenous enzymes that allow the incorporation of labeled nucleotides into the 3′-hydroxyl (3′OH) recessed termini of DNA breaks. The added value in this approach resides in the possibility of evaluating both morphological and staining features in the same sample. Mitochondrial reactive oxygen species (mROS) production on cells and heart cryosections was measured using MitoSOX Red indicator (Life Technologies) as described [26].
2.9. Evaluation of autophagy
The autophagy was assed using Western blot and immunofluorescence techniques both in vitro and in vivo. In H9C2 cells, 50 nM of galanin was added into culture medium (various intervals) to evaluate the expression of microtubule-associated protein 1 light chain 3B (LC3B) showed as LC3-II/LC3-I ratio. Bafilomycin A1 was used to inhibit the autophagic flux at the final concentration of 100 nM. For the immunofluorescence, H9C2 cells were seeded on glass coverslips and then treated with galanin for 18 h. Both cells and heart cryosections were fixed in 4% paraformaldehyde and permeabilized with 0.2% Triton X-100 in PBS. Subsequently, samples were incubated with blocking solution: 1% BSA-PBS. After, the primary antibody directed against LC3B was incubated overnight at 4 °C. Samples were then incubated in Alexa 488, conjugated anti-rabbit secondary antibody. LC3B dots per cells in vitro and per field (3.6 × 105 μm2) in vivo were measured by ImageJ. In addition, an autophagy assay kit (MAK138) from Sigma-Aldrich was used in vitro according to the company datasheet. The bright blue dots per cells were measured by ImageJ. All images were acquired using WF Zeiss Cell Obs. HS inv. microscope.
2.10. Measurements of FA and glucose oxidation
The oxidation of FA was measured using [1–14C]-palmitate in heart tissue and isolated cardiomyocytes as previously described [26]. The heart tissue samples were incubated in modified Krebs–Henseleit buffer containing 1.5% FA-free bovine serum albumin, 5 mmol l–1 glucose, 1 mmol l–1 palmitate and 0.5 μCi ml–1 [14C]palmitate (PerkinElmer, Courtaboeuf, France) for 60 min. Isolated cardiomyocytes were incubated in the same modified Krebs–Henseleit buffer, but without glucose. After incubation, tissues were homogenized in 800 μl lysis buffer. Complete oxidation was determined by acidifying the incubation buffer with 1 ml of 1 mol l–1 H2SO4, and the 14CO2 was trapped by benzethonium hydroxide (Sigma) placed in a 0.5 ml microtube. After 120 min, the radioactivity was counted (Cytoscint; MP Biomedicals, Strasbourg, France). Similarly, glucose oxidation was assessed by measuring the [14C]-glucose oxidation in cardiac tissue as previously described. Sample incubation for 1 h was performed using a modified Krebs–Henseleit buffer containing 0.2% bovine serum albumin, 20 mM Hepes, 10 mM glucose, 0.8 μCi ml–1 [14C]glucose (PerkinElmer). Results were normalized for mg of proteins.
2.11. Analysis of mitochondrial respiration
Mitochondrial respiration was analyzed using a Seahorse XFe 24 workflow. Briefly, 100.000 cells per well were seeded. Twenty-four hours later, cells were treated or not with galanin (10 nM) for 30min. Medium was replaced by Seahorse medium without serum but with 25 mM glucose added. Oligomycin (1 μg/ml), FCCP (3 μM), and a mixture of rotenone (1 μM) plus antimycin A (1 μM) were then added sequentially to, respectively, inhibit the ATP synthase, uncouple oxidative phosphorylation, and gauge non-mitochondrial respiration.
2.12. Statistical analysis
Data are expressed as mean ± SEM. Comparison between two groups was performed by Student's two-tailed t-test while comparison of multiple groups was performed by one-way ANOVA followed by a Bonferroni's post hoc test, unless otherwise stated, using GraphPad Prism version 5.00 (GraphPad Software, Inc).
3. Results
3.1. Galanin promotes cardiomyoblast autophagosome formation and mitochondrial respiratory bioenergetics
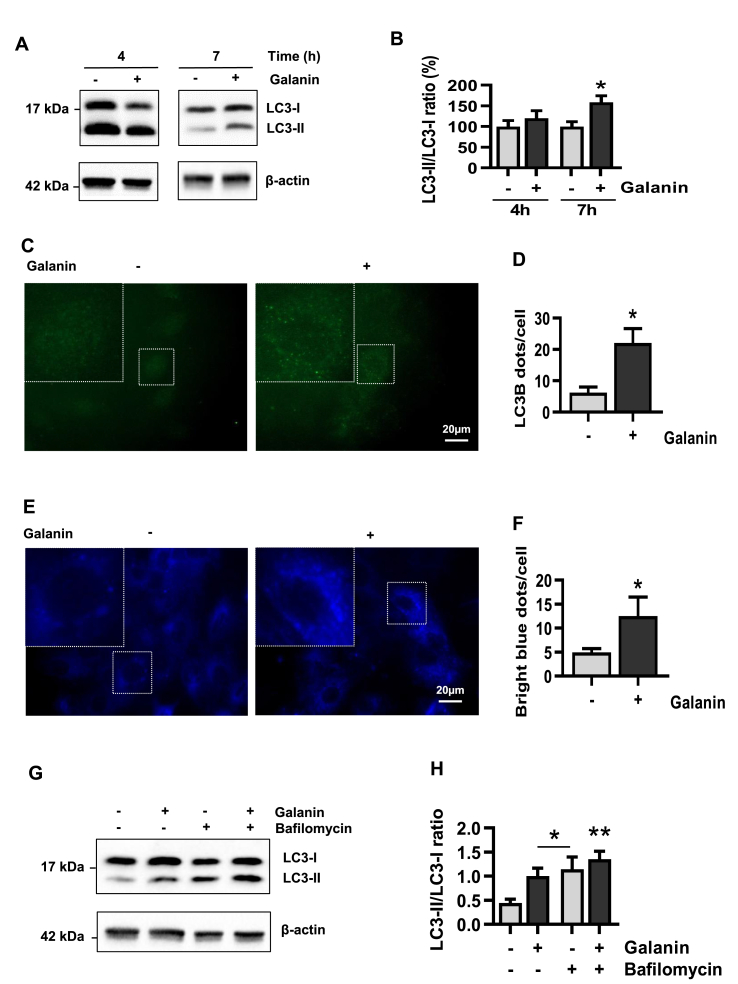
Autophagy is a major contributor to cellular metabolism and constitutes a quality control mechanism crucial in cell homeostasis [[27], [28], [29]]. To date, the role of galanin in autophagy is unknown, therefore, we first examined whether galanin affects autophagy in H9C2 cardiomyoblast cells. Autophagy was monitored by expression of LC3B using immunofluorescence and Western blot analysis. As shown in Fig. 1A and B, galanin induced cardiomyoblast LC3B conversion from LC3B–I to an autophagosome-associating LC3-II in a time-dependent manner. Induction of autophagy was confirmed by immunofluorescence that showed a galanin-mediated increase in LC3B punctate structure formation (Fig. 1C–F). Autophagosome accumulation can reflect an increased de novo autophagosome biosynthesis or an autophagy inhibition [30]. To explore whether galanin could alter autophagic flux, cardiomyoblasts were treated with galanin for 24 h in combination or not with the late-stage inhibitor Bafilomycin A1. As expected, Bafilomycin A1 treatment caused an increase of LC3B conversion from form I to II (Fig. 1G and H). This effect was more evident when cells were exposed to the combination of galanin and Bafilomycin A1.
Fig. 1.
Galanin induces autophagy in cardiomyoblasts.
A,B Immunoblot and quantification of the LC3-II/I ratio in H9C2 cells in presence or absence of galanin (50 nM) after 4 and 7 h. C,D Representative immunofluorescence images showing LC3B staining and quantification of the LC3B dots per cells subjected to 18 h of stimulation with galanin. E,F Representative images and quantification of bright blue dots per cells subjected to 18 h of stimulation with galanin and incubated with the Autophagosome Detection Reagent. G,H Immunoblot and quantification of the LC3-II/I ratio in H9C2 cells in presence or absence of galanin and bafilomycin (100 nM) after 24 h. Data represents the mean ± SEM. *P < 0.05; **P < 0.01 vs control. Statistical analysis was carried out by Student's two-tailed t-test. For H statistical analysis was carried out by one-way ANOVA. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
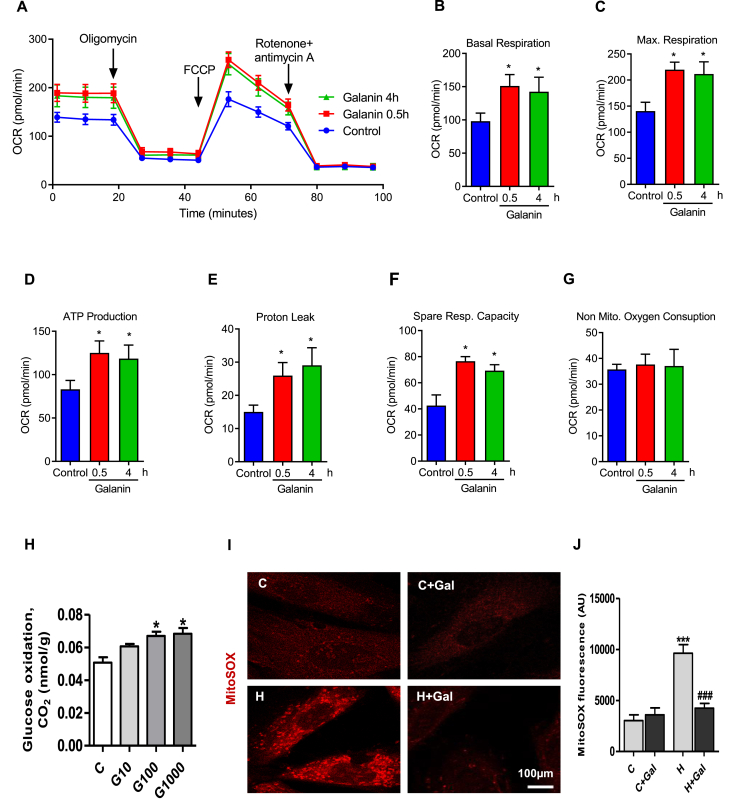
We further investigated the effect of galanin on mitochondrial metabolism of living cells through oxygen consumption rate (OCR) quantification using the Seahorse technology (Fig. 2A–G). Real time simultaneous measurements of OCR were monitored in a basal state and after the addition of oligomycin to block ATP synthesis, carbonilcyanide p-triflouromethoxyphenylhydrazone (FCCP) to uncouple ATP synthesis from the electron transport chain, and rotenone and antimycin A to block complex I and III of the electron transport chain, respectively (Fig. 2A). Seahorse analysis revealed that galanin significantly enhanced mitochondrial respiration basal and maximal mitochondria respiration in a time-dependent manner (Fig. 2B and C). As compared to control conditions galanin markedly increased ATP production, proton leak, spare respiratory capacity in cardiomyoblasts (Fig. 2D–F) without significant changes in non-mitochondrial oxygen consumption (Fig. 2G). As shown in Fig. 2H, galanin increased glucose oxidation in a dose-dependent manner in cardiomyoblasts. Galanin-mediated improvement of mitochondrial respiratory function was associated with reduced mROS production in H9C2 cells exposed to hypoxia as compared to normoxic conditions (Fig. 2I and J).
Fig. 2.
Galanin promotes mitochondrial biogenesis and suppresses the mROS production in vitro.
A Baseline oxygen consumption rate (OCR) following treatments with 10 nM galanin for 30 min (0.5 h) and 4 h in H9C2 cells. B Basal respiration, C maximal mitochondria respiration, D ATP production, E proton leak, F spare respiratory capacity, G non-mitochondrial oxygen consumption. H Glucose oxidation in H9C2 cells treated with galanin (10, 100, 1000nM). I, J Representative images and quantification of MitoSox Red staining in H9C2 cells subjected to 2 h of hypoxia (H) or normoxia (C) in presence or absence of 10 nM galanin. Data represents the mean ± SEM. *P < 0.05; ***P < 0.001 vs control (C). ###P < 0.001 vs H. Statistical analysis was carried out Student's two-tailed t-test. For J statistical analysis was carried out by one-way ANOVA. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3.2. Galanin inhibits cell apoptosis, mROS production and hypertrophy in cardiomyoblasts
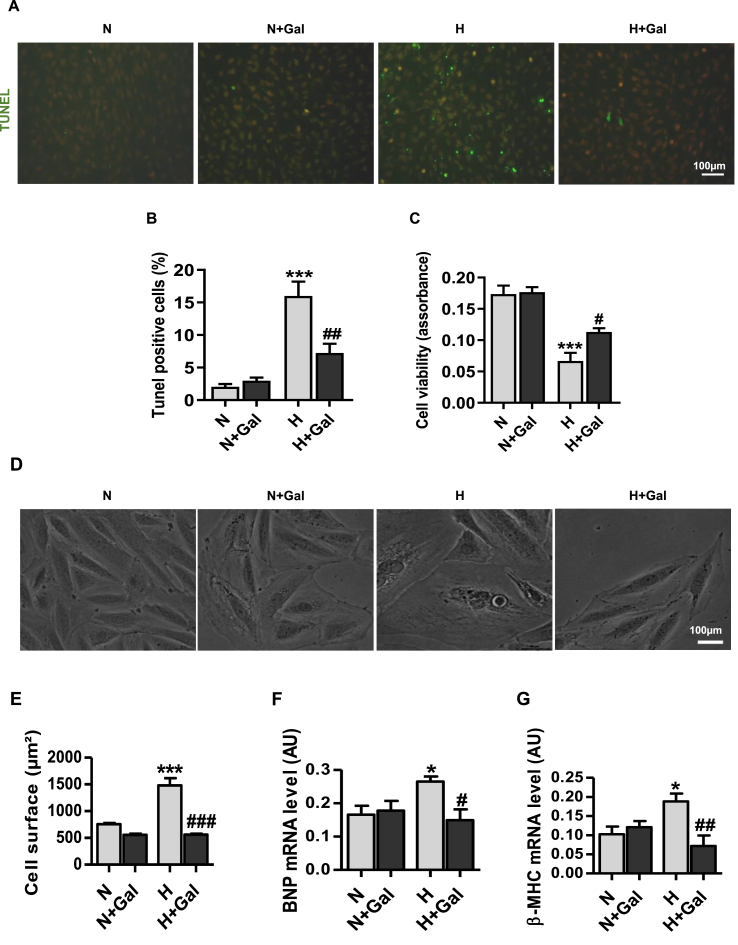
To explore cell survival and cell death decisions in relation to galanin-mediated autophagic and metabolic phenotypes, we next examined the effects of galanin on mitochondrial oxidative stress and apoptosis under hypoxia. As shown in Fig. 2I,J, treatment of cardiomyoblasts with galanin abolished hypoxia-induced mROS production as compared to normoxic conditions. In response to hypoxic stress galanin was able to reduce the number of TUNEL-positive apoptotic in H9C2 and increase cell viability (Fig. 3A-C). Furthermore, we found the anti-hypertrophic activity of galanin in hypoxia-stressed cardiomyoblasts as evidenced by decrease in cell size (Fig. 3D and E) and mRNA expression of hypertrophic factors including B-type natriuretic peptide (BNP) and beta-myosin heavy chain (β-MHC) (Fig. 3F and G).
Fig. 3.
Galanin inhibits apoptosis and hypertrophic response in vitro.
A,B Representative images and quantification of TUNEL-positive H9C2 cells exposed to hypoxia (H) or normoxia (N) for 16 h in presence or absence of galanin (10 nM). C Cell viability in normoxic or hypoxic H9C2 cells in presence or absence of 10 nM galanin measured by CellTiter-Glo assay. D,E Representative images and quantification of cross-sectional area, and F,G BNP and β-MHC mRNA expression levels in galanin-treated (10 nM) H9C2 cells exposed to 16 h of hypoxia (H) or normoxia (N). Data represents the mean ± SEM. *P < 0.05; ***P < 0.001 vs N. #P < 0.05; ##P < 0.01; ###P < 0.001 vs H. Data represents the mean ± SEM. Statistical analysis was carried out by one-way ANOVA.
3.3. Galanin prevents cardiac hypertrophy and damage in I/R-challenged hearts
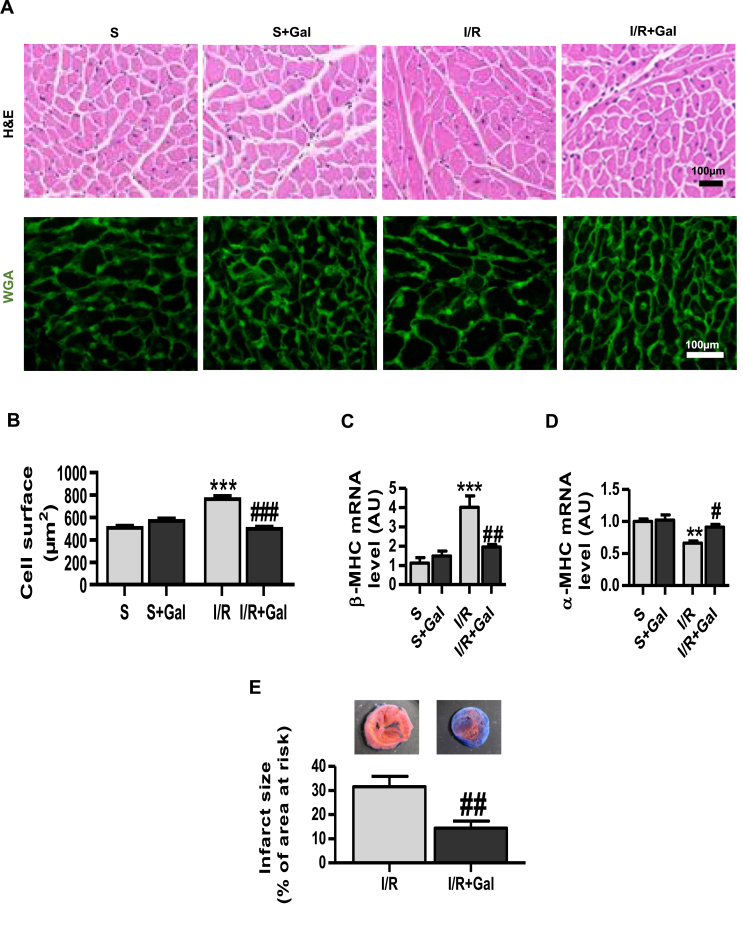
We then proceeded to explore whether galanin has an important role in modulating the hypertrophic responses in vivo. To investigate cardiac cell hypertrophic phenotype linked to myocardial damage, we employed a mouse model of I/R-induced injury. A single dose of galanin was administrated 15 min after the beginning of reperfusion in mice. Cardiac hypertrophy was evaluated by determining the cardiomyocyte size and the expression of cardiac hypertrophic markers. Histological analyses of cardiac sections revealed a significant decrease in myocyte cross-sectional area in galanin-treated mice as compared with vehicle-treated mice subjected to I/R injury (Fig. 4A and B). Galanin-dependent anti-hypertrophic action was further characterized by the downregulation of β-MHC with a concomitant upregulation of alpha-myosin heavy chain (ɑ-MHC) in cardiac tissue (Fig. 4C and D). Importantly, we found that galanin post-treatment significantly reduced the infarct size in post-infarct-remodelled heart (Fig. 4E).
Fig. 4.
Treatment with galanin prevents cardiac hypertrophy in I/R-remodelled hearts.
A Histological analysis of cardiac tissue stained with H&E (the first row) and WGA (the second row) from vehicle- or galanin (Gal)-treated mice subjected to sham surgery or myocardial ischemia for 30 min followed by reperfusion for 24 h. Galanin (0,7 μg/kg, i. v.) or vehicle (PBS) was injected at 15 min of reperfusion. B Comparison of the average cross-sectional area of cardiomyocytes from A, n = 6–8 mice per group. C,D Myocardial β-MHC and ɑ-MHC mRNA expression levels in vehicle or galanin-treated mice after 24 h of I/R, n = 4–5 mice per group. E Myocardium ischemic infarct size as percentage of the area at risk (AAR) in I/R and Gal-treated mice. Data represents the mean ± SEM. **P < 0.01; ***P < 0.001 vs Sham (S). #P < 0.05; ##P < 0.01; ###P < 0.001 vs I/R. Statistical analysis was carried out by one-way ANOVA. For E statistical analysis was carried out by Student's two-tailed t-test.
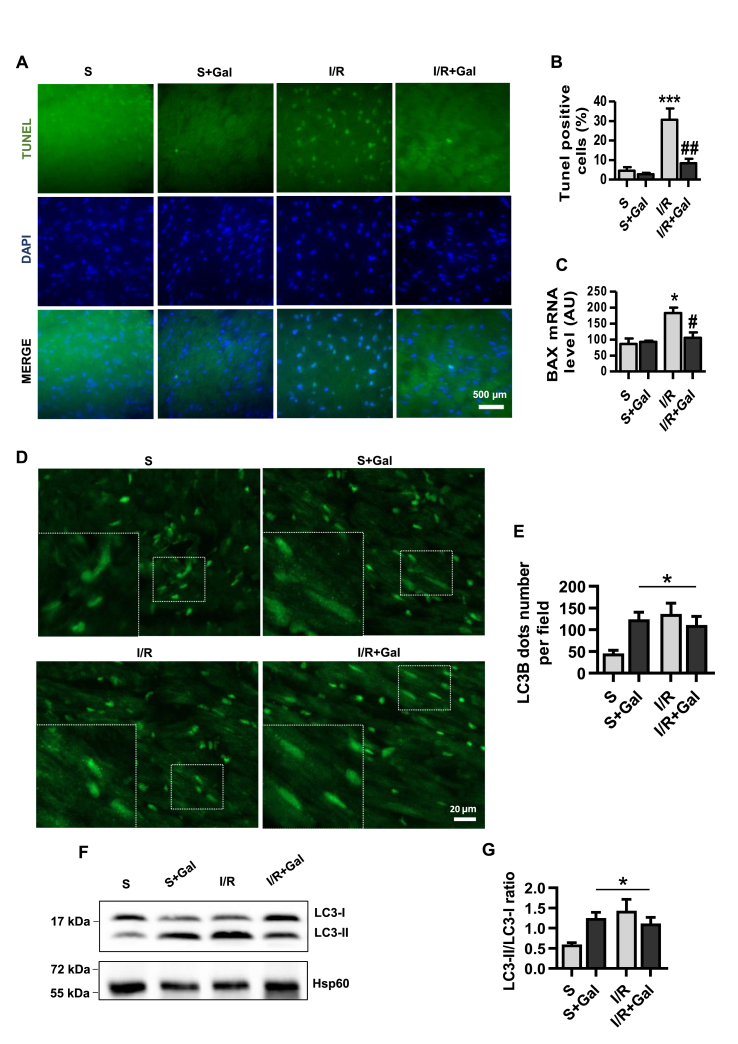
Both autophagy and apoptosis are pivotal processes for cellular metabolism in the failing heart [28]. We next examined the effects of galanin on I/R-induced apoptotic/autophagic status and metabolic remodeling in mice. As compared to vehicle-treated I/R group, the myocardial apoptosis and the expression of pro-apoptoc BCL2 associated X (BAX) gene (Fig. 5A–C), were significantly reduced in galanin-treated mice after 24 h of reperfusion. Autophagic phenotype monitoring revealed in both sham and I/R groups, galanin treatment enhanced cardiac LC3B punctate structure formation, an indicator of autophagosome accumulation (Fig. 5D and E). These observations were also strengthened by western blotting analysis to detect the conversion of LC3-I to LC3-II in I/R-induced myocardial remodeling. As expected, galanin promoted LC3B conversion from LC3 form I to an autophagosome-associating LC3 form II in mice subjected to 24 h of sham or I/R conditions (Fig. 5F and G).
Fig. 5.
Galanin counteracts apoptotic myocardial injury in I/R-challenged hearts and promotes autophagosome formation.
A Representative images of TUNEL-stained cardiac tissue from vehicle- or galanin (Gal)-treated mice subjected to sham surgery or myocardial ischemia for 30 min followed by reperfusion for 24 h. B Quantification of TUNEL-positive stained cells from A, and mRNA expression of pro-apoptotic Bax C in the indicated groups, n = 5–8 mice per group. D,E Representative immunofluorescence images showing LC3B staining and quantification of the LC3B dots per field in cardiac tissue from vehicle- or galanin-treated mice subjected to 24 h of I/R or sham, n = 6–7 mice per group. F,G Immunoblot and quantification of the LC3-II/I ratio in cardiac tissue from the indicated groups, n = 6–7 mice per group. Data are expressed as the mean ± SEM. *P < 0.05; ***P < 0.001 vs Sham (S). #P < 0.05; ##P < 0.01 vs I/R. Statistical analysis was carried out by one-way ANOVA. For E and G Newman-Keuls post hoc test was applied.
3.4. Galanin preserves mitochondrial integrity and promotes glucose metabolism in I/R-remodelled hearts
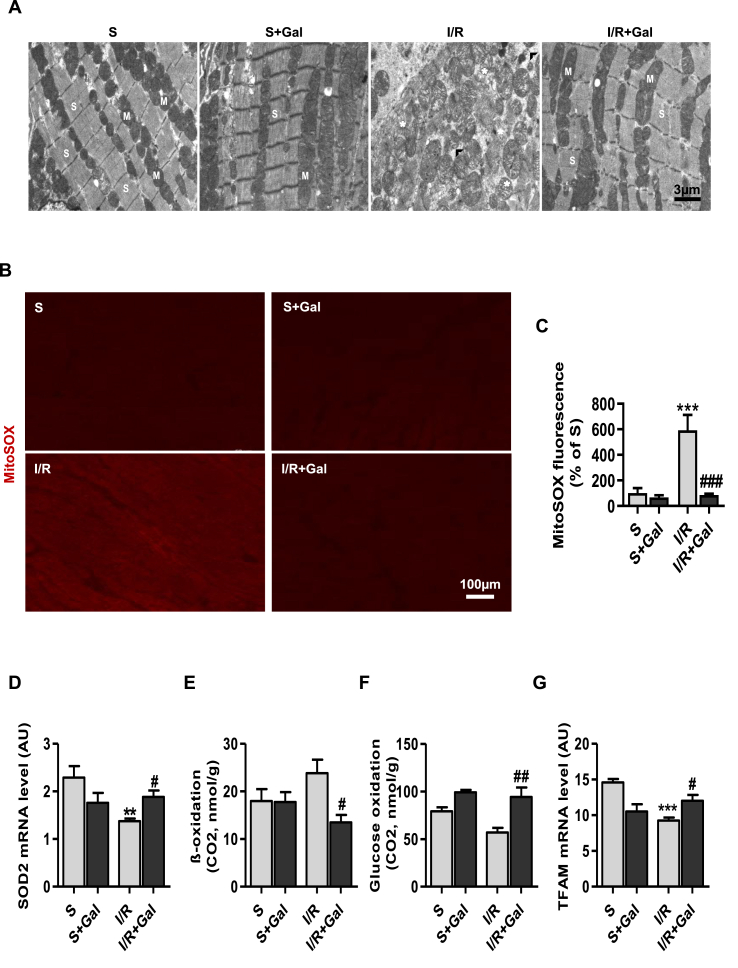
Myocardial energy metabolism and mitochondrial dysfunction have been recognized as the early events following MI [31]. We next evaluated whether galanin protects from I/R-triggered mitochondrial abnormalities and cardiometabolic defects in mice. Ultrastructual analysis demonstrated that control sham-operated mice showed normal myocardial ultrastucture with regular myofibrils interspersed with intact mitochondria (Fig. 6A). In contrast, I/R resulted in disruption of the myofibrils, mitochondrial swelling and disorganization of the mitochondrial cristae (Fig. 6A). As shown in Fig. 6A, treatment with galanin markedly prevented cardiomyocyte ultrastructural damage induced by IR injury as compared to vehicle-treated I/R group.
Fig. 6.
Galanin calibrates cardiometabolic reprogramming in I/R-remodelled hearts.
A Electron micrographs of hearts from vehicle- or galanin (Gal)-treated mice subjected to sham surgery or myocardial ischemia for 30 min followed by reperfusion for 24 h. Cardiac tissues were harvested for examination of myocardial ultrastructure by transmission electron microscopy (TEM). (M), mitochondria; (S), sarcomere, (*), damaged mitochondria, (∧), disrupted myofibrils. B,C Representative images of cardiac sections stained with MitoSox Red specific mROS indicator and quantification of MitoSox Red-positive stained tissue. D Myocardial SOD2 mRNA levels in vehicle- or galanin-treated mice subjected to 24 h of I/R or sham, n = 5–7 mice per group. E,F Palmitate oxidation and glucose oxidation in I/R-challenged hearts treated with vehicle or galanin. G Myocardial TFAM mRNA levels in vehicle- or galanin-treated mice subjected to 24 h of I/R or sham, n = 5–6 mice per group. Data are expressed as the mean ± SEM. **P < 0.01; ***P < 0.001 vs Sham (S). #P < 0.05; ##P < 0.01; ###P < 0.001 vs I/R. Statistical analysis was carried out by one-way ANOVA. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Abnormal mitochondria are a major source of ROS and metabolic dysregulation [32]. To detect the mitochondrial ROS generation in myocardial remodeling induced by I/R injury, MitoSOX Red dye was used in cardiac sections from sham and I/R animals. Analysis of cardiac tissue stained with MitoSOX Red mitochondrial superoxide indicator revealed reduced mROS accumulation in galanin-treated mice as compared to vehicle-treated mice (Fig. 6B and C). Consistently, mitochondrial superoxide dismutase 2 (SOD2) was upregulated in galanin-treated hearts after 24 h of reperfusion (Fig. 6D). Importantly, prevention of I/R-mediated mitochondrial damage and mROS production by galanin was associated with inhibition of FA oxidation (Fig. 6E) and promotion of glucose oxidation (Fig. 6F) in response to I/R stress. Moreover, we found that mRNA level of mitochondrial transcription factor A (TFAM), a key regulator of mitochondrial biogenesis, was increased in the group of galanin-treated I/R mice as compared to vehicle-treated I/R mice (Fig. 6G).
Interestingly, galanin-mediated metabolic shift from FA to glucose oxidation was associated with reduced inflammatory responses in cardiac tissue (Supplemetary Fig. 1). Inflammatory cell infiltration was found reduced on H&E staining in galanin-treated I/R group (Supplemetary Fig. 1A). As shown in Supplemetary Fig. 1B and C, galanin reduced macrophage marker expression of CD68 in cardiac tissue from mice subjected to I/R injury as compared with vehicle-treated mice.
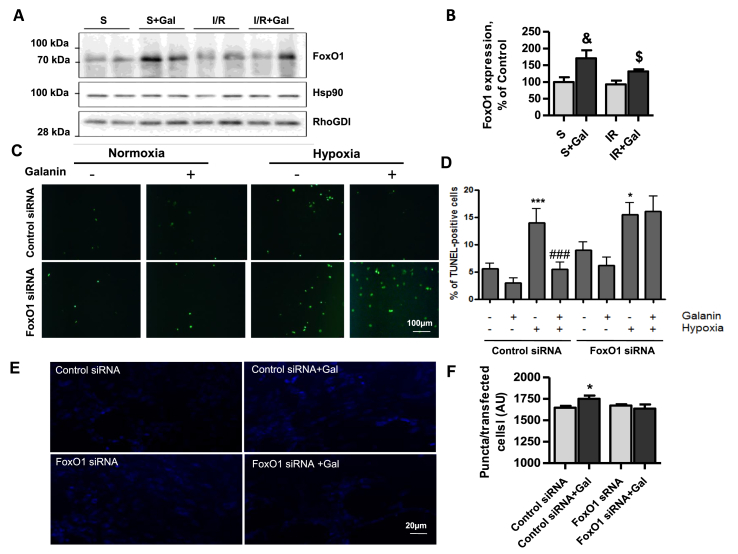
3.5. Galanin regulates cell apoptosis and autophagy through FoxO1 pathway
To explore the molecular mechanism by which galanin regulates apoptosis and autophagy, we examined the effects of galanin on myocardial expression of FoxO1, a key regulator of cell apoptosis, autophagy and metabolism. As shown in Fig. 7A–B, galanin treatment stimulated myocardial FoxO1 expression as compared with vehicle-treated mice subjected to I/R injury. At the cellular level, we then examined whether siRNA-mediated knockdown of FoxO1 could counteract galanin-mediated effects on cell death and autophagy in cardiomyoblasts. In cells exposed to hypoxia, galanin inhibited apoptotic cell death in control siRNA-transfected H9C2 cells, whereas these effects were abolished in the FoxO1 siRNA-transfected cells (Fig. 7C–D). As shown in Fig. 7E–F, FoxO1 knockdown also resulted in abolishment of galanin-mediated autophagic responses in cardiomyoblasts. Collectively, these data suggest that galanin regulates autophagic and apoptotic status in cardiomyoblaststs through FoxO1 pathway.
Fig. 7.
Galanin regulates autophagy and apoptotic cell death through FoxO1 pathway.
A,B Immunoblot and quantification of the FoxO1 protein expression in cardiac tissue from vehicle- or galanin-treated mice subjected to sham surgery or myocardial ischemia for 30 min followed by reperfusion for 24 h. C,D Representative images and quantification of TUNEL-stained H9C2 cells transfected with control siRNA or FoxO1 siRNA under hypoxia (H) or normoxia (N) in absence or presence of 10 nM galanin. E,F Representative images from H9C2 cells transfected with control siRNA or FoxO1 in absence or presence of 50 nM galanin and quantification of puncta for transfected cells and incubated with the Autophagosome Detection Reagent. Data represents the mean ± SEM from at least three independent experiments. *P < 0.05 vs control siRNA; ***P < 0.001 vs N + control siRNA; ###P < 0.001 vs H + control siRNA; & P < 0.05 vs contol sham-operated (S) mice; $ P < 0.05 vs I/R mice , n = 5–7 mice per group. Statistical analysis was carried out by one-way ANOVA.
4. Discussion
Autophagy and apoptosis orchestrate cell survival and death, and are implicated in the pathogenesis of cardiac hypertrophy and HF [6]. Here, we show that galanin dictates cardiac autophagic and anti-apoptotic responses through FoxO1 pathway. Our findings decode an unexpected activity of galanin that promotes cell autophagy in post-infarction hypertrophy. We found that galanin initiates autophagosome formation and metabolic shift from FA to glucose oxidation in hypertrophic remodeling of the heart. Galanin-mediated metabolism switches to glucose consumption is associated with preservation of mitochondrial integrity, reduced apoptosis and oxidative stress. These findings reveal a previously unknown role for galanin in the regulation of autophagy and offer a new alternative way to modulate cell autophagic status.
In response to stress, myocytes activate the autophagic and hypertrophic cell responses culminating in a complex series of metabolic, structural and functional events [33]. Under prolonged stress, loss of myocytes by apoptosis and myocardial energy deficit lead to decline in cardiac function and HF [34]. To prevent cardiac maladaptation, the surviving myocytes reactivate the expression of fetal genes that are normally silenced in the adult heart [35]. The return to the fetal gene program causes myocyte lengthening through the addition of new sarcomeres to counteract cardiac decompensation [36]. A shift from α-MHC to β-MHC isoform expression is often observed in failing human heart [37,38]. The present study provides evidence that galanin functions as a regulator of fetal gene program in hypertrophic remodeling in vivo. In response to early myocardial I/R injury, acute galanin post-treatment promotes α-MHC expression and inhibits β-MHC expression in cardiac tissues. In addition, an important pathological modification of cardiac muscle after infarction is a metabolic shifting in energy substrate metabolism [39]. Interestingly, we found that galanin treatment promotes metabolic shift from FA to glucose oxidation in the post-infarction hypertrophied heart. An important element of hypertrophic remodeling of the heart is a shift in energy substrate metabolism away from FA oxidation to an increased reliance on glucose, followed by an overall reduction in oxidative metabolism [39]. We show that galanin-mediated promotion in glucose metabolism is associated with reduced mROS generation suggesting an important role of galanin in metabolic reprogramming of the heart under stressful conditions. Furthermore, we demonstrated that galanin stimulates mitochondrial biogenesis and preserves mitochondrial integrity in response to stress. Since the adult heart has very limited regenerative capacity, galanin-dependent optimization of energy metabolism in surviving cells may limit the untoward effects of post-ischemic cardiac remodeling. Indeed, we found the reduced apoptotic cell death and infarct size in galanin-treated mice in I/R-challenged hearts. In agreement with previous studies [40,41], these results indicate that maintaining mitochondrial biogenesis against cardiac injury and mROS suppression may be promising mechanism in cardioprotection.
In the adult heart, cardiomyocytes are terminally differentiated cells and thus cannot proliferate. Long-lived, damaged, and dysfunctional cellular components are broken down for elimination or cellular renewal, providing building components for cellular recycling. Thereby, cytoplasmic components designated for autophagy are first sequestrated within de novo formed double-membraned vesicles that fuse with the lysosomes to form autolysosomes [30]. In our study, we provided the first evidence that galanin dictates autophagic phenotype in cardiac cells. Indeed, we shown that under basal conditions galanin promoted LC3B conversion from LC3–I to an autophagosome-associating LC3-II in cardiomyoblasts in a time-dependent manner. Furthermore, we found that a single injection of galanin induced cardiac autophagosome formation in both control sham-operated mice and mice submitted to I/R suggesting that galanin acts as a key regulator of autophagic status of cardiac cells in the basal state and in response to stress.
In mammals, FoxO transcription factors play a fundamental role in regulating cell autophagy, hypertrophy, cell survival, apoptosis, oxidative stress resistance and energy metabolism [42,43]. Among the FoxO subfamily, FoxO1 is the most important actor in governing cardiac equilibrium [42]. Conditional deletion of FoxO1 in the heart promotes cell death and reduces cardiac function in response to myocardial infarction, suggesting that FoxO1 promotes cardiomyocyte survival in response to oxidative stimuli [44]. Global loss of FoxO1 is fatal as it initiates embryonic cell death [43]. Interestingly, we found that galanin treatment increased protein expression of FoxO1 in both control sham-operated mice and mice submitted to I/R. In the recovery from I/R-induced acute cardiac injury, galanin suppressed mROS production and stimulated myocardial SOD2 expression in mice indicating that galanin can mediate protective effects against mitochondrial oxidative stress through regulation of antioxidant genes. Our in vitro results demonstrated that galanin attenuated mROS production, hypertrophy and apoptosis in cells, which were subjected to a mimic I/R injury induced by hypoxia. Importantly, we found that FoxO1 silencing suppressed galanin-mediated autophagosome formation and anti-apoptotic activity, suggesting that galanin controls autophagic and apoptotic status of cardiac cells through FoxO1 pathway. Demonstration of FoxO1-dependent activity of galanin in vitro and in vivo provides a mechanistic understanding of how galanin orchestrates cardiac cell functions under normal physiological and pathological conditions. FoxO1 has been found to be deregulated in a variety of human cardiovascular disorders [43,44]. A better understanding of the role of galaninergic system in the regulation of FoxO1 may contribute to the development of novel therapies for HF and associated FoxO-related diseases.
Activation of pro-inflammatory cell program is known to promote cardiac remodeling [45] and is a potentially important contributor to ventricular dysfunction [46]. Macrophage infiltration into the heart contributes to the excessive collagen deposition and ultimately cause HF [47,48]. Our data indicates that I/R induced inflammatory response in cardiac tissue, however, the number of inflammatory cells was reduced in galanin-treated mice in both early and late stages of myocardial remodeling suggesting anti-inflammatory profile of galanin.
A complete understanding of the actions of galanin in the heart requires identification and functional characterization of galanin receptor subtypes. The galanin receptors are coupled to a variety of signal transduction pathways. GalR1 and GalR3 induce Gi-coupled inhibitory signaling, whereas GalR2 induces Gq-coupled stimulatory signaling [[16], [17], [18]]. GalR1 can influence inwardly rectifying K+ channels (GIRK). Activation of GalR1 can also stimulate MAPK activity through a PKC-independent mechanism. GalR2 is able to activate the stimulatory pathway of Gαq/11 class of G proteins and MAPK through a PKC- and Gαo-dependent mechanism. Both GalR1 and GalR2 activation can inhibit cAMP response element binding protein (CREB). The signaling properties of GalR3 are not yet fully defined. Activation of GalR3 receptors results in an inward K+ current that is characteristic of Gαi-coupled receptors. While peptide hormone-based drugs benefit from being selective for their specific receptors, the usage of native peptides as therapeutics is challenging due to the short half-life of the biologically active peptide form(s) on a scale of a few minutes. The short half-life of galanin in plasma of less than 10 min [49]. Thus, the development of metabolically stable galanin analogs that exert longer-lasting and more potent effects than the endogenous peptide represent promising candidates for the treatment of cardiovascular disorders.
In summary, these studies identify galanin as a critical regulator of cardiac autophagic and apoptotic responses in post-infarction hypertrophied hearts. We describe a novel FoxO1-dependent mechanism whereby galanin controls myocardial apoptosis and autophagy. These findings reveal a previously unknown role for galanin in the regulation of cardiac autophagy and provide a new strategy to modulate autophagic processes in a therapeutic arena.
Declaration of competing interest
The authors state that they have no conflict of interest to disclose.
Acknowledgments
This work was supported by the INSERM, Région Midi-Pyrénées and ERASMUS MUNDUS MEDEA project.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2021.101866.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Galanin reduces inflammation in post-infarct-remodelled heart.
A Representative image of H&E-stained cardiac sections from vehicle- or galanin-treated mice subjected to 24h of I/R or sham. B,C Representative images of CD68-stained cardiac sections and quantification in A, n = 5–7 mice per group. Data represents the mean ± SEM. ***P < 0.001 vs Sham (S). ###P < 0.001 vs I/R. Statistical analysis was carried out by one-way ANOVA.
References
- 1.Cahill T.J., Kharbanda R.K. Heart failure after myocardial infarction in the era of primary percutaneous coronary intervention: mechanisms, incidence and identification of patients at risk. World J. Cardiol. 2017;9(5):407–415. doi: 10.4330/wjc.v9.i5.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chioncel O., Collins S.P., Greene S.J., Pang P.S., Ambrosy A.P., Antohi E.L., Vaduganathan M., Butler J., Gheorghiade M. Predictors of post‐discharge mortality among patients hospitalized for acute heart failure. Card. Fail. Rev. 2017;3(2):122–129. doi: 10.15420/cfr.2017:12:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Segura A.M., Frazier O.H., Buja L.M. Fibrosis and heart failure. Heart Fail. Rev. 2014;19(2):173–185. doi: 10.1007/s10741-012-9365-4. [DOI] [PubMed] [Google Scholar]
- 4.Orogo A.M., Gustafsson Å.B. Therapeutic targeting of autophagy: potential and concerns in treating cardiovascular disease. Circ. Res. 2015;116(3):489–503. doi: 10.1161/CIRCRESAHA.116.303791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cao D.J., Gillette T.G., Hill J.A. Cardiomyocyte autophagy: remodeling, repairing, and reconstructing the heart. Curr. Hypertens. Rep. 2009;11(6):406–411. doi: 10.1007/s11906-009-0070-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levine B., Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132(1):27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jia G., Sowers J.R. Autophagy: a housekeeper in cardiorenal metabolic health and disease. Biochim. Biophys. Acta. 2015;1852(2):219–224. doi: 10.1016/j.bbadis.2014.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chun Y., Kim J. Autophagy: an essential degradation program for cellular homeostasis and life. Cells. 2018;7(12):278. doi: 10.3390/cells7120278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roca-Agujetas V., de Dios C., Lestón L., Marí M., Morales A., Colell A. Recent insights into the mitochondrial role in autophagy and its regulation by oxidative stress. Oxid. Med. Cell. Longev. 2019;2019:3809308. doi: 10.1155/2019/3809308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mariño G., Niso-Santano M., Baehrecke E.H., Kroemer G. Self-consumption: the interplay of autophagy and apoptosis. Nat. Rev. Mol. Cell Biol. 2014;15(2):81–94. doi: 10.1038/nrm3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tatemoto K., Rökaeus Å., Jörnvall H., McDonald T.J., Mutt V. Galanin - a novel biologically active peptide from porcine intestine. FEBS Lett. 1983;164(1):124–128. doi: 10.1016/0014-5793(83)80033-7. [DOI] [PubMed] [Google Scholar]
- 12.Lang R., Gundlach A.L., Kofler B. The galanin peptide family: receptor pharmacology, pleiotropic biological actions, and implications in health and disease. Pharmacol. Ther. 2007;115(2):177–207. doi: 10.1016/j.pharmthera.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 13.Lerner J.T., Sankar R., Mazarati A.M. Galanin and epilepsy. EXS. 2010;102:183–194. doi: 10.1007/978-3-0346-0228-0_13. [DOI] [PubMed] [Google Scholar]
- 14.Zhao X., Seese R.R., Yun K., Peng T., Wang Z. The role of galanin system in modulating depression, anxiety, and addiction-like behaviors after chronic restraint stress. Neuroscience. 2013;246:82–93. doi: 10.1016/j.neuroscience.2013.04.046. [DOI] [PubMed] [Google Scholar]
- 15.Baranowska B., Wolinska-Witort E., Wasilewska-Dziubinska E., Roguski K., Martynska L., Chmielowska M. The role of neuropeptides in the disturbed control of appetite and hormone secretion in eating disorders. Neuroendocrinol. Lett. 2003;24(6):431–434. [PubMed] [Google Scholar]
- 16.Lang R., Gundlach A.L., Holmes F.E., Hobson S.A., Wynick D., Hokfelt T., Kofler B. Physiology, signaling, and pharmacology of galanin peptides and receptors: three decades of emerging diversity. Pharmacol. Rev. 2015;67(1):118–175. doi: 10.1124/pr.112.006536. [DOI] [PubMed] [Google Scholar]
- 17.Howard A.D., Tan C., Shiao L.L., Palyha O.C., McKee K.K., Weinberg D.H., Feighner S.D., Cascieri M.A., Smith R.G., Van Der Ploeg L.H., Sullivan K.A. Molecular cloning and characterization of a new receptor for galanin. FEBS Lett. 1997;405(3):285–290. doi: 10.1016/s0014-5793(97)00196-8. [DOI] [PubMed] [Google Scholar]
- 18.Barreda-Gómez G., Giralt M.T., Pazos A., Rodríguez-Puertas R. Galanin activated Gi/o-proteins in human and rat central nervous systems. Neuropeptides. 2014;48(5):295–304. doi: 10.1016/j.npep.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 19.Zaben M.J., Gray W.P. Neuropeptides and hippocampal neurogenesis. Neuropeptides. 2013;47(6):431–438. doi: 10.1016/j.npep.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 20.Wynick D., Bacon A. Targeted disruption of galanin: new insights from knock-out studies. Neuropeptides. 2002;36(2–3):132–144. doi: 10.1054/npep.2002.0888. [DOI] [PubMed] [Google Scholar]
- 21.Lang R., Kofler B. The galanin peptide family in inflammation. Neuropeptides. 2011;45(1):1–8. doi: 10.1016/j.npep.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 22.Fang P., Shi M., Zhu Y., Bo P., Zhang Z. Type 2 diabetes mellitus as a disorder of galanin resistance. Exp. Gerontol. 2016;73:72–77. doi: 10.1016/j.exger.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 23.Legakis I., Mantzouridis T., Mountokalakis T. Positive correlation of galanin with glucose in type 2 diabetes. Diabetes Care. 2005;28(3):759–760. doi: 10.2337/diacare.28.3.759. [DOI] [PubMed] [Google Scholar]
- 24.Berger A., Santic R., Hauser-Kronberger C., Schilling F.H., Kogner P., Ratschek M., Gamper A., Jones N., Sperl W., Kofler B. Galanin and galanin receptors in human cancers. Neuropeptides. 2005;39(3):353–359. doi: 10.1016/j.npep.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 25.Pchejetski D., Foussal C., Alfarano C., Lairez O., Calise D., Guilbeau-Frugier C., Schaak S., Seguelas M.H., Wanecq E., Valet P., Parini A., Kunduzova O. Apelin prevents cardiac fibroblast activation and collagen production through inhibition of sphingosine kinase 1. Eur. Heart J. 2012;33(18):2360–2369. doi: 10.1093/eurheartj/ehr389. [DOI] [PubMed] [Google Scholar]
- 26.Alfarano C., Foussal C., Lairez O., Calise D., Attané C., Anesia R., Daviaud D., Wanecq E., Parini A., Valet P., Kunduzova O. Transition from metabolic adaptation to maladaptation of the heart in obesity: role of apelin. Int. J. Obes. 2014;39(2):312–320. doi: 10.1038/ijo.2014.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang K.Y., Wang J.N., Zhou Y.Y., Wu S.Z., Tao L.Y., Peng Y.P., Que J.Q., Xue Y.J., Ji K.T. Antithrombin III alleviates myocardial ischemia/reperfusion injury by inhibiting excessive autophagy in a phosphoinositide 3-kinase/akt-dependent manner. Front. Pharmacol. 2019;10:516. doi: 10.3389/fphar.2019.00516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nishida K., Yamaguchi O., Otsu K. Crosstalk between autophagy and apoptosis in heart disease. Circ. Res. 2008;103(4):343–351. doi: 10.1161/CIRCRESAHA.108.175448. [DOI] [PubMed] [Google Scholar]
- 29.Macchia A., Levantesi G., Marfisi R.M., Franzosi M.G., Maggioni A.P., Nicolosi G.L., Schweiger C., Tavazzi L., Tognoni G., Valagussa F., Marchioli R. Determinants of late-onset heart failure in myocardial infarction survivors: GISSI prevenzione trial results. Rev. Esp. Cardiol. 2005;58(11):1266–1272. doi: 10.1157/13080953. [DOI] [PubMed] [Google Scholar]
- 30.Klionsky D.J., Abdelmohsen K., Abe A., Abedin M.J., Abeliovich H., Arozena A.A., Adachi H., Adams C.M., Adams P.D., Adeli K., Adhihetty P.J., Adler S.G., Agam G., Agarwal R., Aghi M.K., Agnello M., Agostinis P., Aguilar P.V., Aguirre-Ghiso J., Airoldi E.M. Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy. 2016;12(1):1–222. doi: 10.1080/15548627.2015.1100356. 3rd edition. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakada Y., Canseco D.C., Thet S., Abdisalaam S., Asaithamby A., Santos C.X., Shah A.M., Zhang H., Faber J.E., Kinter M.T., Szweda L.I., Xing C., Hu Z., Deberardinis R.J., Schiattarella G., Hill J.A., Oz O., Lu Z., Zhang C.C., Kimura W., Sadek H.A. Hypoxia induces heart regeneration in adult mice. Nature. 2017;541(7636):222–227. doi: 10.1038/nature20173. [DOI] [PubMed] [Google Scholar]
- 32.Mangge H., Becker K., Fuchs D., Gostner J.M. Antioxidants, inflammation and cardiovascular disease. World J. Cardiol. 2014;6(6):462–477. doi: 10.4330/wjc.v6.i6.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Masoud W.G.T., Clanachan A.S., Lopaschuk G.D. The failing heart: is it an inefficient engine or an engine out of fuel? Cardiac Remodeling. 2013:65–84. [Google Scholar]
- 34.Bernardo B.C., Weeks K.L., Pretorius L., McMullen J.R. Molecular distinction between physiological and pathological cardiac hypertrophy: experimental findings and therapeutic strategies. Pharmacol. Ther. 2010;128(1):191–227. doi: 10.1016/j.pharmthera.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 35.Dirkx E., da Costa Martins P.A., De Windt L.J. Regulation of fetal gene expression in heart failure. Biochim. Biophys. Acta. 2013;1832(12):2414–2424. doi: 10.1016/j.bbadis.2013.07.023. [DOI] [PubMed] [Google Scholar]
- 36.Rajabi M., Kassiotis C., Razeghi P., Taegtmeyer H. Return to the fetal gene program protects the stressed heart: a strong hypothesis. Heart Fail. Rev. 2007;12(3–4):331–343. doi: 10.1007/s10741-007-9034-1. [DOI] [PubMed] [Google Scholar]
- 37.Reiser P.J., Portman M.A., Ning X.H., Moravec C.S. Human cardiac myosin heavy chain isoforms in fetal and failing adult atria and ventricles. Am. J. Physiol. Cell Physiol. 2001;280(4) doi: 10.1152/ajpheart.2001.280.4.H1814. H1814–1820. [DOI] [PubMed] [Google Scholar]
- 38.Barry S.P., Davidson S.M., Townsend P.A. Molecular regulation of cardiac hypertrophy. Int. J. Biochem. Cell Biol. 2008;40(10):2023–2039. doi: 10.1016/j.biocel.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 39.Ritterhoff J., Tian R. Metabolism in cardiomyopathy: every substrate matters. Cardiovasc. Res. 2017;113(4):411–421. doi: 10.1093/cvr/cvx017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Timotin A., Pisarenko O., Sidorova M., Studneva I., Shulzhenko V., Palkeeva M., Serebryakova L., Molokoedov A., Veselova O., Cinato M., Tronchere H., Boal F., Kunduzova O. Myocardial protection from ischemia/reperfusion injury by exogenous galanin fragment. Oncotarget. 2017;8(13):21241–21252. doi: 10.18632/oncotarget.15071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pisarenko O., Timotin A., Sidorova M., Studneva I., Shulzhenko V., Palkeeva M., Serebryakova L., Molokoedov A., Veselova O., Cinato M., Boal F., Tronchere H., Kunduzova O. Cardioprotective properties of N-terminal galanin fragment (2-15) in experimental ischemia/reperfusion injury. Oncotarget. 2017;8(60):101659–101671. doi: 10.18632/oncotarget.21503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Puthanveetil P., Wan A., Rodrigues B. FoxO1 is crucial for sustaining cardiomyocyte metabolism and cell survival. Cardiovasc. Res. 2013;97(3):393–403. doi: 10.1093/cvr/cvs426. [DOI] [PubMed] [Google Scholar]
- 43.Hosaka T., Biggs W.H., Tieu D., Boyer A.D., Varki N.M., Cavenee W.K., Arden K.C. Disruption of forkhead transcription factor (FOXO) family members in mice reveals their functional diversification. Proc. Natl. Acad. Sci. U.S.A. 2004;101(9):2975–2980. doi: 10.1073/pnas.0400093101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sengupta A., Molkentin J.D., Paik J.H., DePinho R.A., Yutzey K.E. FoxO transcription factors promote cardiomyocyte survival upon induction of oxidative stress. J. Biol. Chem. 2011;286(9):7468–7478. doi: 10.1074/jbc.M110.179242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hulsmans M., Sam F., Nahrendorf M. Monocyte and macrophage contributions to cardiac remodeling. J. Mol. Cell. Cardiol. 2016;93:149–155. doi: 10.1016/j.yjmcc.2015.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hulsmans M., Sager H.B., Roh J.D., Valero-Muñoz M., Houstis N.E., Iwamoto Y., Sun Y., Wilson R.M., Wojtkiewicz G., Tricot B., Osborne M.T., Hung J., Vinegoni C., Naxerova K., Sosnovik D.E., Zile M.R., Bradshaw A.D., Liao R., Tawakol A., Weissleder R., Rosenzweig A., Swirski F.K., Sam F., Nahrendorf M. Cardiac macrophages promote diastolic dysfunction. J. Exp. Med. 2018;215(2):423–440. doi: 10.1084/jem.20171274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nahrendorf M., Swirski F.K. Regulating repair: regulatory T cells in myocardial infarction. Circ. Res. 2014;115(1):7–9. doi: 10.1161/CIRCRESAHA.114.304295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Van Amerongen M.J., Harmsen M.C., Van Rooijen N., Petersen A.H., Van Luyn M.J.A. Macrophage depletion impairs wound healing and increases left ventricular remodeling after myocardial injury in mice. Am. J. Pathol. 2007;170(3):818–829. doi: 10.2353/ajpath.2007.060547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Harling H., Holst J.J. Circulating galanin: origin, metabolism, and pharmacokinetics in anesthetized pigs. Am. J. Physiol. 1992;262(1):E52–E57. doi: 10.1152/ajpendo.1992.262.1.E52. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Galanin reduces inflammation in post-infarct-remodelled heart.
A Representative image of H&E-stained cardiac sections from vehicle- or galanin-treated mice subjected to 24h of I/R or sham. B,C Representative images of CD68-stained cardiac sections and quantification in A, n = 5–7 mice per group. Data represents the mean ± SEM. ***P < 0.001 vs Sham (S). ###P < 0.001 vs I/R. Statistical analysis was carried out by one-way ANOVA.