Abstract
Context
Proteinuria can cause or exacerbate hypothyroidism, possibly due to urinary loss of protein-bound thyroid hormone. However, the precise relationship between proteinuria and hypothyroidism remains unclear.
Objective
This work aimed to determine the prevalence of hypothyroidism in patients with proteinuria and the relationship between hypothyroidism and degree of proteinuria.
Design
A retrospective cohort study was conducted from December 1979 to March 2015.
Setting
This study was conducted at a large academic hospital.
Patients
All paired samples of urine protein and serum thyrotropin (TSH), measured within 24 hours, were obtained from adults (age > 18 years) with at least one instance of urine protein greater than 0.2 g/day or mg/mg creatinine.
Main Outcome Measures
Samples were stratified by urine protein tertile. Mean TSH and risk of TSH elevation were compared among tertiles using analysis of covariance and generalized estimating equations controlled for age, sex, samples per patient, and levothyroxine treatment.
Results
A total of 2676 samples were identified from 2136 patients. Mean ± SE TSH (mIU/L) was increased in the highest tertile of urine protein (> 1.75g/day) compared to the lower 2 tertiles (2.09 ± 0.07 vs 1.59 ± 0.07, 1.59 ± 0.06, P < .001). The highest tertile had a greater prevalence of TSH greater than 5 mIU/L (17.2% vs 10.5%, 11.9%, P < .001) but a similar risk of TSH greater than 5 mIU/L (odds ratio [OR] 1.44; 95% CI, 0.67-3.09, P = .35). The highest tertile also had a higher prevalence (6.2% vs 3.4%, 2.6%, P = .003) and risk (OR 1.72; 95% CI, 1.05-2.84, P = .008) of TSH greater than 10 mIU/L. Similar results were observed when comparing samples with nephrotic-range proteinuria (> 3.5g/day) to those with lesser proteinuria.
Conclusion
Hypothyroidism is common among adults with proteinuria, and the risk of hypothyroidism is directly related to the severity of proteinuria.
Keywords: urine protein, nephrotic syndrome, TSH, thyroid function, hypothyroidism
Thyroid dysfunction has been long recognized in patients with nephrotic syndrome (NS). Children with congenital NS have massive proteinuria and may develop overt primary hypothyroidism that resolves after bilateral nephrectomy (1), which suggests a causative relationship between NS and hypothyroidism in these patients. Similarly, case reports have demonstrated an increased risk of subclinical hypothyroidism in children with steroid-resistant NS, which is typically milder than congenital NS (2, 3). The proposed cause of hypothyroidism in NS is urinary loss of thyroid hormone, of which more than 99% circulates bound to plasma proteins including thyroxine-binding globulin (TBG) and albumin. This hypothesis is supported by reports of elevated urinary concentrations of TBG and thyroxine (T4) in patients with NS that directly correlate to the severity of urinary protein loss (4-6).
Although proteinuria is more common in adults than in children, few data exist on the prevalence of thyroid dysfunction in adults with proteinuria. Several case reports have documented increased thyroid hormone requirements in patients with treated hypothyroidism who develop new or worsening proteinuria (7-10), and a small study has reported an association between proteinuria and thyrotropin (TSH) elevation in patients with NS (11), but data regarding the risk of hypothyroidism in adults with proteinuria—particularly nonnephrotic-range proteinuria—remain sparse (4, 5, 8, 12). To our knowledge, no large study has investigated the prevalence of hypothyroidism in adults with proteinuria or the relationship between hypothyroidism and the degree of proteinuria. Elucidating this relationship may inform practice regarding the appropriateness of and approach to screening for thyroid dysfunction in patients with proteinuria. We hypothesized that the prevalence of primary hypothyroidism is increased in patients with proteinuria, and that the prevalence and severity of hypothyroidism is directly related to the degree of proteinuria. To test this hypothesis, we analyzed the relationship between thyroid function and urinary protein excretion in a large cohort of adults with proteinuria in a large academic medical center.
Materials and Methods
Participants and data collection
From the electronic medical record of Brigham & Women’s Hospital, we identified all patients older than 18 years who had at least one instance of elevated urinary protein excretion (proteinuria) documented between December 1979 and March 2015. Urinary protein excretion was defined either by 24-hour urine protein excretion (g/day) if available, or by urine protein:creatinine ratio (mg/mg) measured in a spot urine sample. Urine protein:creatinine ratio has been shown to correlate closely with daily urine protein excretion and is a reliable quantitative estimate of proteinuria (13); therefore, urinary protein excretion in this study is reported without units (representing either g/day or mg/mg). Urinary protein excretion of more than 0.2 was considered elevated. For each patient, we identified all instances in which urinary protein excretion and serum TSH had been measured within a 24-hour period. Each concurrent measurement of urinary protein excretion and TSH was considered a single sample for the purposes of analysis, and all analyses were conducted on a per-sample basis (for some patients, more than one sample was available). For each sample, we collected demographic and clinical data including patient age and sex, and whether the patient was taking thyroid-related medications (levothyroxine, methimazole, or propylthiouracil). Because excessive treatment with antithyroid medication is a cause of elevated TSH, samples obtained while on methimazole or propylthiouracil (n = 25) were excluded from analysis. Over the study period, a series of laboratory assays were used to measure TSH, urine protein, and urine creatinine concentrations. The reference range for TSH was 0.5 to 5.0 mIU/L prior to December 1, 2009, and 0.5 to 5.7 mIU/L after that date. The reference range for urine protein concentration was 0 to 15 mg/dL after March 26, 2001; there was no institutional reference range for this assay before that date, nor for urine creatinine concentration during the study period.
Statistical analysis
The association between TSH and urinary protein excretion as continuous variables was first assessed by linear regression. For further analysis we divided the samples into tertiles based on urinary protein excretion. Mean TSH concentration was compared among tertiles using analysis of covariance. Generalized estimating equations were used to compare the odds of having elevated TSH (> 5 or > 10 mU/L) among the proteinuria tertiles. In a secondary analysis, samples were compared between individuals with nephrotic-range proteinuria (> 3.5 g/day or mg/mg) (14) and those without. TSH concentration and urinary protein excretion were log-transformed for all analyses because of their nonnormal distribution. Where relevant, results of analyses were back-transformed to standard units for presentation. All primary analyses were controlled for age, sex, number of samples per patient, and levothyroxine treatment. In addition, to control further for the potential effect of levothyroxine treatment on TSH concentrations, we performed a sensitivity analysis by repeating all analyses after excluding samples obtained on levothyroxine treatment. Similarly, to account further for potential intraparticipant correlation, we performed sensitivity analyses that included only a single, randomly chosen sample for each individual participant. Analyses were performed using SPSS version 22 (SPSS IBM). Two-tailed P values of less than .05 were considered statistically significant. This research was approved by the Brigham & Women’s Hospital Office of Human Subjects Research.
Results
Among 2136 patients who had proteinuria documented during the study period, we identified 2676 samples consisting of concurrent measurements of serum TSH and urinary protein excretion (Table 1) that met inclusion criteria. The samples were obtained at a median (interquartile range) age of 56.4 years (range, 39.1-69.4 years), and most samples came from women. Nephrotic-range proteinuria was present in 18.7% of the samples. Elevated TSH of more than 5 mIU/L or more than 10 mIU/L was present in 13.2% and 4.0% of the samples, respectively. Among the 2136 participants, a maximum TSH greater than 5 mIU/L was observed in 296 (13.9%), and a maximum TSH greater than 10 mIU/L was observed in 91 (4.3%). Preliminary analysis by linear regression demonstrated an association between serum TSH and urinary protein excretion, with each 10% increase in urinary protein excretion associated with a 1.1 ± 0.2% increment in TSH. Although urinary protein excretion accounted for a small amount of the variance in TSH (R2 = 0.012), the relationship was highly significant (P < .001).
Table 1.
Characteristics of samples of concurrently measured urinary protein excretion and serum thyrotropin (TSH) from patients with at least one episode of proteinuria (> 0.2 g/d or mg/mg)
| Samples | 2676 |
|---|---|
| Female sex | 1690 (63.1%) |
| Age, y | 56.4 (18.4-97.4) |
| Samples per patient | 1 (1-18) |
| 1 | 1807 (67.5%) |
| 2 | 235 (8.8%) |
| ≥ 3 | 94 (3.5%) |
| Urinary protein excretion, g/d or mg/mg | 0.95 (0-144) |
| Nephrotic-range proteinuria (> 3.5) | 500 (18.7%) |
| TSH, mIU/L | 1.91 (< 0.005-169) |
| TSH > 5 | 353 (13.2%) |
| TSH > 10 | 108 (4.0%) |
| Thyroid-related medications | |
| Levothyroxine | 342 (12.8%) |
Data are presented as n (%) or median (range).
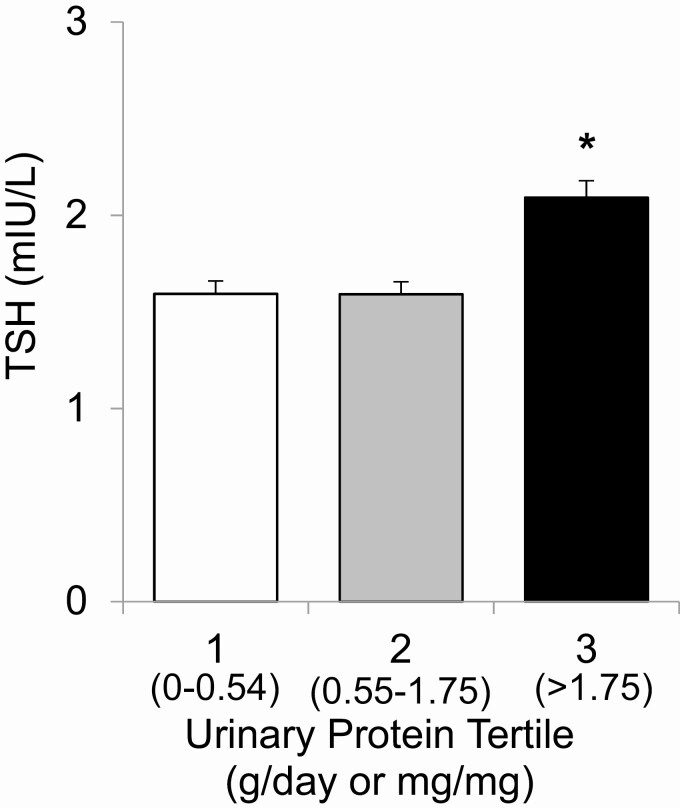
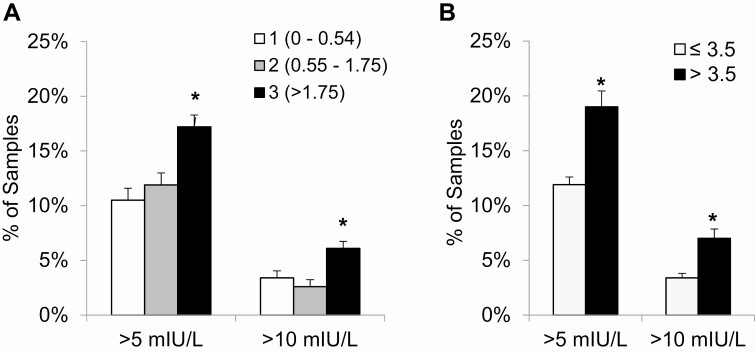
To gain further insight into this relationship, samples were divided into tertiles by urinary protein excretion (0-0.54, 0.55-1.75, and > 1.75). Of note, because some included patients contributed multiple samples and because proteinuria may vary over time, some included samples had normal urine protein (≤ 0.2). Mean ± SE TSH was significantly greater in the highest tertile of urinary protein excretion (2.09 ± 0.09 mIU/L, P < .001) than in the lowest and middle tertiles, which did not differ from each other (1.59 ± 0.07 vs 1.59 ± 0.06 mIU/L; Fig. 1). Likewise, the adjusted prevalence of elevated TSH values was significantly increased in samples with the highest urinary protein excretion (Fig. 2A). Mild TSH elevation (> 5 mIU/L) was observed in 10.5 ± 1.1% and 11.9 ± 1.1% of samples in the lowest and middle tertiles of urinary protein excretion, respectively, compared with 17.2 ± 1.1% of samples in the highest tertile (P < .001). TSH elevation greater than 10 mIU/L was present in 3.4 ± 0.6% and 2.6 ± 0.6% of samples in the lowest and middle tertiles, respectively, but in 6.1 ± 0.6% of samples in the highest tertile (P < .001).
Figure 1.
Thyrotropin (TSH) levels by tertile of urinary protein excretion. Adjusted TSH is expressed as mean (SE). Analysis is controlled for age, sex, number of samples per patient, and levothyroxine treatment. *P less than .001.
Figure 2.
The relationship between prevalence of hypothyroidism and degree of proteinuria (g/day or mg/mg), defined as A, the tertile of urinary protein excretion or B, the clinical threshold of nephrotic-range proteinuria (> 3.5). Analyses are corrected for age, sex, number of samples per patient, and levothyroxine treatment. *P less than .001.
Compared to the lowest tertile of urinary protein excretion, the odds of TSH elevation greater than 10 mIU/L did not differ in the middle tertile (odds ratio [OR] 0.84; 95% CI, 0.49-1.44, P = .52) but were significantly greater in the highest tertile (OR 1.72; 95% CI, 1.05-2.84, P = .008). The odds of TSH elevation greater than 5 mIU/L were not significantly elevated in the middle tertile (OR 0.93; 95% CI, 0.49-1.79, P = .84) or the highest tertile (OR 1.44; 95% CI, 0.67-3.09, P = .35), compared to the lowest tertile.
In addition to the empirical threshold of 1.75 g/day or mg/mg derived from the observed tertiles of urinary protein excretion, we performed a secondary analysis of the same outcomes using the clinical definition of nephrotic-range proteinuria (> 3.5 g/day or mg/mg), with results similar to the primary analysis (Fig. 2B). Participants with nephrotic-range proteinuria had higher mean ± SE TSH than individuals with lesser proteinuria (2.40 ± 0.12 vs 1.62 ± 0.04 mIU/L, P < .001), as well as increased odds of both TSH elevation greater than 5 mIU/L (OR 1.86; 95% CI, 1.38-2.52, P < .001) and TSH greater than 10 mIU/L (OR 2.21; 95% CI, 1.33-3.69, P = .002).
Of the covariates controlled for in these analyses, older age, levothyroxine treatment, and having multiple samples measured were significantly associated with higher TSH. We conducted additional sensitivity analyses to control further for the association of TSH concentration with levothyroxine treatment and multiple samples. Mean ± SE TSH concentration was greater among samples obtained on levothyroxine treatment than among samples without levothyroxine (2.85 ± 0.15 vs 1.62 ± 0.04 mIU/L, P < .001), and the prevalence of TSH elevation was higher among samples obtained on levothyroxine for thresholds both of greater than 5 mIU/L (33.9 ± 2.6% vs 10.2 ± 0.6%, P < .001) and greater than 10 mIU/L (15.5 ± 2.0% vs 2.4 ± 0.3%, P < .001). After excluding samples obtained on levothyroxine treatment, results were similar to those of the primary analyses, except that a lower prevalence of TSH elevation was observed at all levels of urine protein excretion. The adjusted prevalence of TSH elevation greater than 5 mIU/L was 6.9 ± 1.1%, 9.5 ± 1.1% (P = .08), and 14.2 ± 1.1% (P < .001) in the lowest, middle, and highest tertiles of urinary protein excretion, respectively. The adjusted prevalence of TSH elevation greater than 10 mIU/L in each respective tertile was 1.6 ± 0.5%, 1.6 ± 0.5% (P = .96), and 3.9 ± 0.5% (P = .003). In addition, among samples obtained without levothyroxine, the odds of TSH elevation greater than 10 mIU/L in the highest tertile of urinary protein excretion (OR 2.77; 95% CI, 1.42-5.39, P = .003) and among subjects with nephrotic-range proteinuria (OR 2.66; 95% CI, 1.37-5.18, P = .004) were greater than in the overall cohort. In separate sensitivity analyses including only a single sample from each participant, results again were similar to those of the primary analyses, except that the risk of TSH elevation greater than 5 mIU/L was significantly increased in the highest tertile of urinary protein excretion (OR 1.58; 95% CI, 1.13-2.21, P = .008).
Discussion
In this study, we show that the risk of hypothyroidism is directly related to the degree of urinary protein excretion in patients with proteinuria. Case reports have suggested a relationship between hypothyroidism and NS, and a small study of 60 patients has shown an association between TSH and degree of proteinuria (11). Another study reported a 6-fold increased prevalence of subclinical hypothyroidism in 159 patients with proteinuria compared with 900 controls (8). In contrast, smaller series have found no difference in serum TSH or free T4 levels between adults with NS and healthy controls (4, 12). These discrepancies may be due to sampling bias and to the small size of many studies, as well as the potentially dynamic clinical course of proteinuria, which might obscure a relationship if thyroid function and the degree of proteinuria are not measured concurrently. In the present study of a large cohort of patients with proteinuria, we confirm a high prevalence of hypothyroidism and demonstrate that the risk of hypothyroidism increases with the severity of proteinuria. In this cohort, the risk of significant TSH elevation (> 10 mIU/L) increased by 72% in participants in the highest tertile of urinary protein excretion, and the risk was increased 2-fold among individuals with nephrotic-range proteinuria. As might be expected, TSH concentrations were significantly higher in patients treated with levothyroxine, who presumably had a known diagnosis of hypothyroidism. Notably, the increase in risk of TSH elevation greater than 10 mIU/L associated with large proteinuria (highest tertile or nephrotic range) was significantly higher among individuals not treated with levothyroxine, which may reflect the fact that patients with known hypothyroidism may have their levothyroxine dose adjusted to address TSH elevation. The odds of milder TSH elevation greater than 5 mIU/L was also significantly increased in individuals with nephrotic-range proteinuria and, when the analysis was restricted to a single sample per participant, in the highest tertile of urinary protein excretion.
The prevalence of TSH elevation greater than 5 mIU/L in our cohort of participants with proteinuria (13.9%) is higher than some reported prevalence estimates of hypothyroidism in the United States. The National Health and Nutrition Examination Survey (NHANES III) study reported a 4.6% prevalence of hypothyroidism (TSH > 4.5 mIU/L) among 16 533 adults without known thyroid disease (15), whereas the Colorado Thyroid Prevalence Study reported a prevalence of hypothyroidism (TSH > 5 mIU/L) of 8.9% in a self-selected population attending a health fair (16). Although proteinuria may be a direct cause of hypothyroidism, our observational study cannot assess the causality of this association. In addition, the association of hypothyroidism with proteinuria observed in our cohort could be related to other confounding factors that we were not able to assess, such as the severity or etiology of underlying illness, comorbid conditions or autoimmune disease, or use of other medications.
Nevertheless, the association of hypothyroidism with significant proteinuria may have clinical consequences. Hypothyroidism may be associated with increased risk of mortality and adverse cardiovascular outcomes including coronary heart disease and congestive heart failure (17-19). Because patients with NS have dyslipidemia and are at significantly increased risk of myocardial infarction and coronary death (20), our findings raise the question of whether hypothyroidism may exacerbate—or even play a causative role in—these complications of NS. Although this issue remains to be clarified by future studies, awareness of the risk of hypothyroidism in patients with proteinuria may facilitate early detection and treatment that avoid exposing such patients to possible additional cardiovascular risk.
Several studies have suggested that hypothyroidism in patients with proteinuria may be caused by urinary loss of protein-bound thyroid hormone. One of the first studies supporting this hypothesis, published in 1979 by Afrasiabi et al, showed that individuals with proteinuria had increased urinary loss of T4, 3,5,3′-triiodothyronine, and TBG (4). A subsequent study by Chandurkar and colleagues showed that mean 24-hour urinary T4 excretion was 4.7 times higher in patients with proteinuria compared with controls, and that urinary T4 excretion correlated directly with urinary protein excretion; however, serum TSH concentrations were not measured in these patients (6). The development of NS has also been described as a cause of increasing levothyroxine requirement in hypothyroid patients previously controlled with stable doses of levothyroxine (10). Urinary loss of protein-bound thyroid hormone is therefore a plausible mechanism for an association between severity of proteinuria and risk of hypothyroidism, but there are other possible explanations as well, such as a potential common etiology of both proteinuria and hypothyroidism. Although our observational study cannot address the mechanism underlying the observed relationship between hypothyroidism and proteinuria, our findings should raise awareness of this clinically important association.
Strengths of the present study include the fact that we studied a common clinical problem in a real-world clinical setting, using a large number of participants and samples from a single health network. To reduce bias due to short-term changes in proteinuria and thyroid function, we included only paired urine and blood samples obtained within 24 hours of each other. Finally, multivariable analysis was used to control for potential confounding variables for which data were available, and sensitivity analyses confirmed the robustness of the observed associations.
This study also has several limitations. Data were not available to control for a number of potential confounders, including treatment with glucocorticoids or other medications that may affect TSH levels, or the presence of other risk factors for hypothyroidism such as thyroid autoantibodies, iodine status, or other autoimmune disease (including whether proteinuria itself was due to autoimmunity or to another etiology, such as type 2 diabetes). Glucocorticoids, in particular, can reduce TSH levels, so the lack of data on glucocorticoid treatment is, if anything, likely to underestimate the observed association between proteinuria and hypothyroidism. Nevertheless, these factors should be evaluated in future studies to improve our understanding of which patients with proteinuria are at greatest risk of developing hypothyroidism. Finally, over the long study period, there were institutional changes in laboratory assays that may affect results, which is a limitation of this retrospective analysis.
In conclusion, this study demonstrates that patients with severe proteinuria have a significant risk of hypothyroidism. Although this finding is not sufficient to recommend routine thyroid function testing in patients with proteinuria, clinicians should be aware of this risk and should consider thyroid function testing in this high-risk group, particularly if symptoms suggestive of hypothyroidism are present. Further prospective studies are necessary to determine whether improved detection and treatment of hypothyroidism in patients with proteinuria will improve clinical outcomes.
Acknowledgments
Financial Support: This work was supported by the National Institutes of Health (grant No. T32 DK007529).
Glossary
Abbreviations
- NS
nephrotic syndrome
- T4
thyroxine
- TBG
thyroxine-binding globulin
- TSH
thyrotropin
Contributor Information
Norra Kwong, Thyroid Section, Division of Endocrinology, Hypertension and Diabetes, Brigham & Women’s Hospital, Boston, Massachusetts, USA; Endocrinology, Diabetes and Metabolism Division, Palo Alto Medical Foundation, Palo Alto, California, USA.
Marco Medici, Department of Internal Medicine, Radboud University Medical Center, GA Nijmegen, the Netherlands; Department of Internal Medicine, Epidemiology and Academic Center for Thyroid Diseases, Erasmus University Medical Center, GD Rotterdam, the Netherlands.
Ellen Marqusee, Thyroid Section, Division of Endocrinology, Hypertension and Diabetes, Brigham & Women’s Hospital, Boston, Massachusetts, USA.
Ari J Wassner, Division of Endocrinology, Boston Children’s Hospital, Boston, Massachusetts, USA.
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability
The data sets generated during and analyzed during the present study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Chadha V, Alon US. Bilateral nephrectomy reverses hypothyroidism in congenital nephrotic syndrome. Pediatr Nephrol. 1999;13(3):209-211. [DOI] [PubMed] [Google Scholar]
- 2. Dagan A, Cleper R, Krause I, Blumenthal D, Davidovits M. Hypothyroidism in children with steroid-resistant nephrotic syndrome. Nephrol Dial Transplant. 2012;27(6):2171-2175. [DOI] [PubMed] [Google Scholar]
- 3. Kapoor K, Saha A, Dubey NK, et al. Subclinical non-autoimmune hypothyroidism in children with steroid resistant nephrotic syndrome. Clin Exp Nephrol. 2014;18(1):113-117. [DOI] [PubMed] [Google Scholar]
- 4. Afrasiabi MA, Vaziri ND, Gwinup G, et al. Thyroid function studies in the nephrotic syndrome. Ann Intern Med. 1979;90(3):335-338. [DOI] [PubMed] [Google Scholar]
- 5. Fonseca V, Thomas M, Katrak A, Sweny P, Moorhead JF. Can urinary thyroid hormone loss cause hypothyroidism? Lancet. 1991;338(8765):475-476. [DOI] [PubMed] [Google Scholar]
- 6. Chandurkar V, Shik J, Randell E. Exacerbation of underlying hypothyroidism caused by proteinuria and induction of urinary thyroxine loss: case report and subsequent investigation. Endocr Pract. 2008;14(1):97-103. [DOI] [PubMed] [Google Scholar]
- 7. Junglee NA, Scanlon MF, Rees DA. Increasing thyroxine requirements in primary hypothyroidism: don’t forget the urinalysis! J Postgrad Med. 2006;52(3):201-203. [PubMed] [Google Scholar]
- 8. Gilles R, den Heijer M, Ross AH, Sweep FC, Hermus AR, Wetzels JF. Thyroid function in patients with proteinuria. Neth J Med. 2008;66(11):483-485. [PubMed] [Google Scholar]
- 9. Yeoh EC, Claude JR, Rajasoorya C. Paradox of rising thyroid stimulating hormone despite increasing thyroxine dose in hypothyroidism and the association with nephrotic syndrome. Nephrology (Carlton). 2013;18(9):647-648. [DOI] [PubMed] [Google Scholar]
- 10. Benvenga S, Vita R, Di Bari F, Fallahi P, Antonelli A. Do not forget nephrotic syndrome as a cause of increased requirement of levothyroxine replacement therapy. Eur Thyroid J. 2015;4(2):138-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jain D, Aggarwal HK, Pavan Kumar YM, Jain P. Evaluation of thyroid dysfunction in patients with nephrotic syndrome. Med Pharm Rep. 2019;92(2):139-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Feinstein EI, Kaptein EM, Nicoloff JT, Massry SG. Thyroid function in patients with nephrotic syndrome and normal renal function. Am J Nephrol. 1982;2(2):70-76. [DOI] [PubMed] [Google Scholar]
- 13. Ginsberg JM, Chang BS, Matarese RA, Garella S. Use of single voided urine samples to estimate quantitative proteinuria. N Engl J Med. 1983;309(25):1543-1546. [DOI] [PubMed] [Google Scholar]
- 14. Orth SR, Ritz E. The nephrotic syndrome. N Engl J Med. 1998;338(17):1202-1211. [DOI] [PubMed] [Google Scholar]
- 15. Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87(2):489-499. [DOI] [PubMed] [Google Scholar]
- 16. Canaris GJ, Manowitz NR, Mayor G, Ridgway EC. The Colorado Thyroid Disease Prevalence Study. Arch Intern Med. 2000;160(4):526-534. [DOI] [PubMed] [Google Scholar]
- 17. Hak AE, Pols HA, Visser TJ, Drexhage HA, Hofman A, Witteman JC. Subclinical hypothyroidism is an independent risk factor for atherosclerosis and myocardial infarction in elderly women: the Rotterdam Study. Ann Intern Med. 2000;132(4):270-278. [DOI] [PubMed] [Google Scholar]
- 18. Rodondi N, den Elzen WP, Bauer DC, et al. ; Thyroid Studies Collaboration . Subclinical hypothyroidism and the risk of coronary heart disease and mortality. JAMA. 2010;304(12):1365-1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chaker L, Baumgartner C, den Elzen WP, et al. ; Thyroid Studies Collaboration . Subclinical hypothyroidism and the risk of stroke events and fatal stroke: an individual participant data analysis. J Clin Endocrinol Metab. 2015;100(6):2181-2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ordoñez JD, Hiatt RA, Killebrew EJ, Fireman BH. The increased risk of coronary heart disease associated with nephrotic syndrome. Kidney Int. 1993;44(3):638-642. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets generated during and analyzed during the present study are not publicly available but are available from the corresponding author on reasonable request.