Abstract
Context
Questions regarding the superiority of free and bioavailable 25-hydroxyvitamin D [25(OH)D] in predicting health outcomes remain unresolved.
Objective
This study investigates the impact of vitamin D variables—total, bioavailable, or free 25(OH)D—on indices of bone and mineral metabolism, at baseline and in response to 2 vitamin D doses.
Design
Our objectives are implemented as exploratory analyses on data collected in a 1-year, double-blind, randomized controlled trial completed in July 2014.
Setting
Participants were recruited from 3 major hospitals in an ambulatory setting.
Participants
Participants were >65 years of age, overweight, and had a baseline serum 25(OH)D between 10 and 30 ng/mL. A total of 221 participants completed the study.
Intervention
Subjects were randomized to receive calcium and oral vitamin D3 (600 IU/day or 3750 IU/day) supplementation.
Results
Participants who received the higher vitamin D dose had levels that were 1.3- to 1.4-fold higher than those taking the lower dose, for all variables (P value < 0.001). Serum values of bioavailable and free 25(OH)D were associated with total 25(OH)D, with r values of 0.942 and 0.943, respectively (P value < 0.001). Parathyroid hormone (PTH) was negatively associated with all vitamin D variables, with correlation coefficients ranging from −0.22 to −0.25, while calcium and bone turnover markers (carboxy-terminal collagen crosslinks and osteocalcin) did not. Only total 25(OH)D had a positive relationship with % change bone mineral density (BMD) at the femoral neck at 12 months, while only free and bioavailable 25(OH) had a positive relationship with % change total body BMD at 12 months.
Conclusion
Calculated free and bioavailable 25(OH)D do not appear to be superior to total 25(OH)D in predicting indices of bone health in an elderly population.
Keywords: vitamin D, 25(OH)D, elderly, PTH, bone markers
The beneficial effect of vitamin D on bone metabolism, calcium homeostasis, and health is undisputed (1-3). Serum 25-hydroxyvitamin D [25(OH)D] is a fat-soluble hormone and is the precursor of the active metabolite 1,25-dihydroxyvitamin D [1,25 (OH)2D], otherwise known as calcitriol. Due to its long half-life, serum 25(OH)D best reflects vitamin D nutritional stores (4). Around 85% to 90% of vitamin D is tightly bound to the vitamin D binding protein (DBP), 10% to 15% is bound to albumin (a weaker carrier), and less than 1% is freely available (5). Although current guidelines recommend the measurement of total 25(OH)D to assess vitamin D status, there is increased interest in using other forms of 25(OH)D, such as free 25(OH)D and bioavailable 25(OH)D, which are defined as free and albumin bound 25(OH)D (6, 7). The free and bioavailable 25(OH)D can be calculated using the total 25(OH)D, albumin, and DBP, or can be directly measured (8, 9).
The described relationship between serum total 25(OH)D and various indices of mineral metabolism and outcomes, such as parathyroid hormone (PTH), bone mineral density (BMD), and fracture risk, has varied widely between studies due to variability in the populations studied (differing age categories, ethnicities, vitamin D nutritional status, assays used, and 25(OH)D forms assessed) (5, 10-14). It has been proposed that the free and bioavailable compounds might be more suitable predictors of vitamin D status. This is because they are more available for intracellular activation to the auto/paracrine metabolite, 1,25-dihydroxyvitamin D [1,25(OH)2D], or for binding to the vitamin D receptor, relative to 25(OH)D (which is largely bound to DBP) (15, 16). This paradigm applies to other steroid hormones such as thyroid hormones and sex steroids (17).
Powe et al refueled the debate by showing that the levels of bioavailable 25(OH)D were a better reflection of the similar PTH values and higher BMD seen in African Americans, despite lower total 25(OH)D compared to White Americans (18). Although the methods used have been challenged (19-22), and conclusions questioned since (23), the paper cast doubt on the reliability of total 25(OH)D, and whether bioavailable or free forms should be measured instead. This is especially important in conditions that affect DBP, such as pregnancy (24), liver disease (25, 26), and genetic variations in the DBP gene that might affect total 25(OH)D measurements (9, 27). To date, the question still remains whether serum free and bioavailable 25(OH)D might more consistently predict various skeletal (BMD and fractures) and extra-skeletal outcomes (cardiovascular disorders, cancers, and insulin resistance) (28-31), than total serum 25(OH)D. In a recent 1-year randomized vitamin D trial, our group showed comparable effects of low-dose and high-dose vitamin D on indices of insulin resistance (30) and on bone remodeling markers and percent change BMD (31) in elderly individuals, despite large differences in achieved serum 25(OH)D levels. This raises the intriguing possibility that free or bioavailable 25(OH)D, rather than total levels, are the main predictors of vitamin D effects on health outcomes. We hypothesize that the lack of a difference in BMD response previously demonstrated at 12 months between the 2 doses (31) may be explained by comparable levels of the bioavailable and free 25(OH)D.
The objective of this study is to capitalize on data from our randomized vitamin D trial to test the above hypothesis and to investigate which of the vitamin D variables is the best predictor of indices of bone and mineral metabolism at baseline and in response to 2 doses of vitamin D.
Methods
Study design
Our objectives are implemented as exploratory analyses on data collected in a 1-year, double-blind, randomized controlled trial (RCT) (NCT01315366) investigating the impact of 2 doses of vitamin D on indices of insulin resistance (30) and of bone and mineral metabolism (31) in elderly subjects. Participants were >65 years of age, overweight, ambulatory, and had a serum 25(OH)D between 10 and 30 ng/mL at screening. Individuals were excluded if they were diagnosed with diabetes mellitus, had impaired glucose tolerance and were on medication, had a chronic disease or major organ failure such as severe heart failure, liver or kidney failure (estimated glomerular filtration rate [eGFR] < 30 mL/min), had conditions or took medications known to affect bone metabolism, had a history of kidney stones, or had history of fragility fractures or an overall fracture risk >10% based on the FRAX Lebanon risk calculator. A total of 257 participants were enrolled in the trial, and 222 completed the 12-month follow-up period. Analyses were performed on the 221 individuals with data at 0, 6, and 12 months because 1 participant refused to share samples with other investigators. Recruitment, prescreening, and screening procedures were performed at 3 centers (American University of Beirut Medical Center [AUBMC], St Joseph University Hospital, and Rafic Hariri Governmental University Hospital) between January 2011 and July 2013, while enrollment and protocol implementation took place at AUBMC and the trial ended July 2014.
Study variables of interest
The vitamin D variables were not specified amongst primary nor secondary outcomes in the original RCT protocol. We present exploratory analyses of the variables of interest collected in the trial. These include total, bioavailable, and free 25(OH)D as well as total 1,25(OH)2D; indices of bone mineral metabolism, including calcium, parathyroid hormone (PTH), and bone remodeling markers (osteocalcin and crosslaps [CTX]); and percentage change of BMD (lumbar spine, femoral neck, total hip, and total body), calculated as follows:
Intervention
All subjects received daily a total of 1000 mg elemental calcium and a total daily intake of vitamin D equivalent to 600 IU/day in the low-dose group and 3750 IU/d in the high-dose group. Compliance was >90%, for both calcium and vitamin D pills, for both treatment arms (31).
Measurements
Hormonal studies were performed at the Mayo Clinic Clinical Laboratories (Mayo Clinic, Rochester, Minnesota). Serum 25(OH)D was measured with the use of liquid chromatography–mass spectroscopy (LC-MS). PTH was measured by solid phase, 2-site immunoassay on the Siemens Immulite 2000 automated immunoassay system (Siemens Healthcare Diagnostics, Deerfield, IL). Routine chemistry tests including calcium, phosphorus, and creatinine were measured at the American University of Beirut (AUB) using the Roche Cobas 6000 auto-analyzer (Roche Diagnostics GmbH, Mannheim, Germany). DBP was measured using the radial immunodiffusion assay (32). Dual-energy x-ray absorptiometry measurements of the spine, hip, forearm, and total body BMD were made on a Hologic 4500A Horizon machine (Hologic, Bedford, MA, USA). Total body BMD was used as per recommendation of International Society of Clinical Densitometry (33). Precision estimates were based on duplicate scans obtained daily for each skeletal site. CTX and osteocalcin were measured by a 2-site immunoenzymatic sandwich assay on the Roche Cobas e411 (Roche Diagnostics, Indianapolis, IN 46250). Tests were done while participants were fasting. Intra-assay and inter-assay CVs were previously published (31).
Vitamin D calculation
Free and bioavailable 25(OH)D were calculated using the Bikle (5) and Vermeulen (34) equations obtained from Dr. Roger Bouillon’s laboratory (9). We proceeded with Bikle’s equation because the difference between these 2 equations was minimal (only in the second decimal point). Calculated free 25(OH)D using such equations yielded a good correlation with direct measurement in sera from elderly men and children (35). The equation does not account for genetic variation of DBP. However, the most common genotypes have the same affinity for 25(OH)D (8). The units for bioavailable and free 25(OH)D used are μg/L and ng/L, corresponding to ng/mL and pg/mL, respectively. We have reported total, bioavailable, and free 25(OH)D in ng/mL using a conversion factor of 2.49 from nmol/L.
Data analysis
We analyzed the data using descriptive statistics, independent t tests for continuous variables between arms and repeated ANOVA to compare means over time within treatments arm. Results are expressed as means ± standard deviation (SD) or standard error (SE). To increase the power of the study, the 3 values of measurements performed at baseline, 6 months, and 12 months for each subject were treated as separate entries, with an overall total number of over 650 data points for each of the variables of interest. We applied the general linear model (GLM) with generalized estimating equations to take into account the lack of independence for variables for each subject, and the repeated measures over time (0, 6, and 12 months) (36). The subject variable was the subject ID, and the within subject variable was time (0, 6, and 12 months). The independent correlation matrix was used because it provided the best goodness of fit as assessed by Quasi-likelihood Information Criteria (QIC). The predictors were serum total 25(OH)D, free 25(OH)D, or bioavailable 25(OH)D levels, the factor was time, and the outcomes were PTH and indices of bone metabolism. We derived the partial correlation coefficient R, from the GLM using the following formula:
Z is the maximum likelihood Wald statistic, and m is the number of participants. The values of total, bioavailable, and free 25(OH)D were also divided into quartiles, each quartile including an average of 164 subjects, and we performed the same analyses with 25(OH)D expressed in quartiles. We implemented pairwise comparisons for PTH levels, by serum 25(OH)D quartile with the addition of age as part of the model, with Bonferroni correction for multiplicity of testing, using the same general linear model. Patients receiving vitamin D achieve maximal serum values that plateau within 8 weeks after start of supplementation (31, 37). We therefore also evaluated the relationship between 12-month serum 25(OH)D values and bone density at various skeletal sites by Pearson correlations.
We used SPSS version 25.0 (SPSS, Armonk, NY), and a P value < 0.05 was considered statistically significant. Considering the exploratory nature of our analyses, we did not adjust for multiple t testing.
Results
Baseline characteristics of study subjects
Baseline characteristics of the participants are outlined in Table 1. A little over half (55%) of the participants were women. Briefly, subjects were 71.1 ± 4.7 years of age and had a mean body mass index (BMI) of 30.2 ± 4.5 kg/m2. There were no differences in age, BMI, calcium and vitamin D supplementation, dietary calcium intake, serum 25(OH)D, BMD, or any other biochemical, skeletal, and hormone levels across treatment arms in the overall group, and in the subgroups divided by gender (31). At baseline, 118 participants (53%) had total 25(OH)D values below 20 ng/mL. There was no gender difference in total 25(OH)D value distribution, as previously published (31).
Table 1.
Baseline Characteristics of Study Subjects
| Overall (N = 221) | Female (N = 122) | Male (N = 99) | |
|---|---|---|---|
| Age, years | 71 ± 4.7 | 69.9 ± 3.7 | 72.4 ± 5.5 |
| BMI, kg/m 2 | 30.2 ± 4.5 | 31.5 ± 4.9 | 28.7 ± 3.3 |
| Total 25(OH)D, ng/mL | 20.4 ± 7.4 | 21.1 ± 8.2 | 19.5 ± 6.3 |
| Vitamin D binding protein, mg/L | 284.6 ± 34.9 | 289.83 ± 36.4 | 278.1 ± 32.1 |
| Albumin (g/L) | 44.1 ± 2.9 | 44.2 ± 3.2 | 44.1 ± 2.6 |
| Bioavailable 25(OH)D (Bikle a ), μg/L | 2.1 ± 0.8 | 2.2 ± 0.9 | 2.1 ± 0.7 |
| Bioavailable 25(OH)D (Vermeulen a ), μg/L | 2.2 ± 0.8 | 2.2 ± 0.9 | 2.1 ± 0.7 |
| Free 25(OH)D (Bikle a ), ng/L | 5.4 ± 2.0 | 5.5 ± 2.2 | 5.2 ± 1.7 |
| Free 25(OH)D (Vermeulen a ), ng/L | 5.4 ± 2.0 | 5.5 ± 2.3 | 5.3 ± 1.7 |
| PTH, pg/mL | 68 ± 32.6 | 68.1 ± 31.1 | 67.8 ± 34.4 |
| Calcium, mg/dL | 9.5 ± 0.4 | 9.5 ± 0.4 | 9.4 ± 0.4 |
| Calcium or vitamin D supplementation, N (%) | 22 (10%) | 21 (17.2%) | 1 (1%) |
| Creatinine, mg/dL | 0.8 ± 0.2 | 0.7 ± 0.1 | 0.9 0.2 |
| eGFR b , mL/min/1.73m 2 | 81.34 ± 13.1 | 83.2 ± 12.8 | 79 ± 13.1 |
Abbreviations: 25(OH)D, 25-hydroxyvitamin D; BMI, body mass index; eGFR, estimated glomerular filtration rate; PTH, parathyroid hormone.
*Values are expressed as mean ± SD
a Values were derived from the Bikle equation using a spreadsheet supplied by the laboratory of Dr. Roger Bouillon (9).
b eGFR was estimated using the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) equation. (GFR = 141 * min(Scr/κ,1)α * max(Scr/κ, 1)-1.209 * 0.993Age * 1.018 [if female] * 1.159 [if black])
Response to vitamin D supplementation
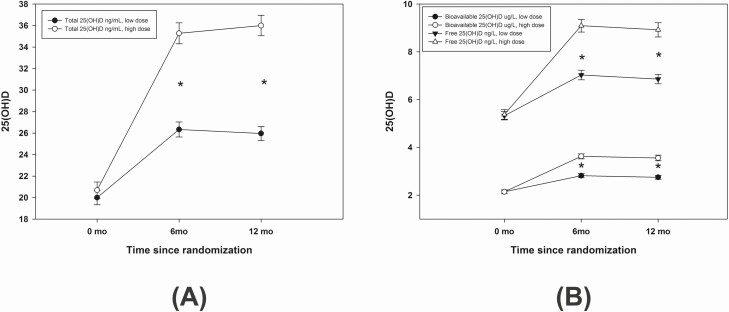
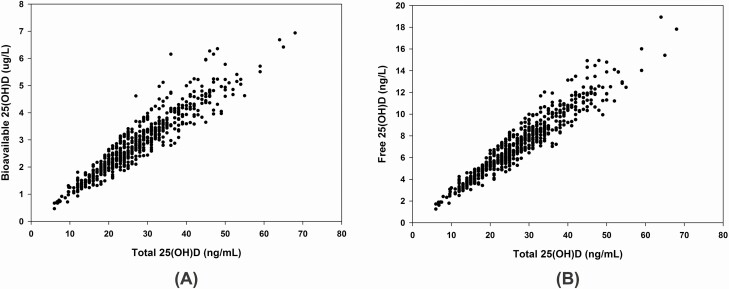
There was an increase in total, bioavailable, and free 25(OH)D levels at 12 months (P < 0.001) with both low-dose and high-dose supplementation. Subjects who received the higher vitamin D dose (3750 IU) had higher serum levels of all 3 serum 25(OH)D variables at 12 months compared with those who were supplemented with a low vitamin D dose (600 IU) (P < 0.001) (Fig. 1). Total 25(OH)D increased from 20.06 (6.92) ng/mL to 25.96 (6.88) ng/mL after low-dose supplementation, and from 20.65 (7.89) to 36 (9.73) ng/mL after high-dose supplementation. Bioavailable 25(OH)D increased from 2.14 (0.8) to 2.75 (0.88) μg/L after low-dose, and from 2.14(0.84) to 3.56 (1.25) μg/L, after high-dose supplementation. Free 25(OH)D increased from 5.33 (1.87) to 6.86 (2.07) ng/L after low-dose, and from 5.38 (2.14) to 8.92 (3.15) ng/L, after high-dose supplementation. The values of bioavailable and free 25(OH)D were associated with total 25(OH)D (P < 0.001), with an estimated r value of 0.942 and 0.943, respectively (Fig. 2).
Figure 1.
Total 25(OH)D (2-A) and bioavailable and free 25(OH)D (2-B) levels at 0, 6, and 12 months divided by vitamin D supplementation with high dose and low dose. Asterisk (*) placed between 6 months and 12 months values indicate significant difference between high-dose and low-dose supplementation. There was a significant increase of total (ng/mL), bioavailable (μg/L), and free 25(OH)D (ng/L) at 6 and 12 months compared to values at baseline using repeated-measures ANOVA within each treatment arm.
Figure 2.
Relationship between total 25(OH)D (ng/mL) with free (ng/L) or bioavailable 25(OH)D (μg/L). The values of bioavailable and free 25(OH)D were significantly associated with total 25(OH)D (P value < 0.001), with estimated r2 values of 0.887 and 0.889, respectively.
Relationship between vitamin D variables with indices of mineral and bone metabolism
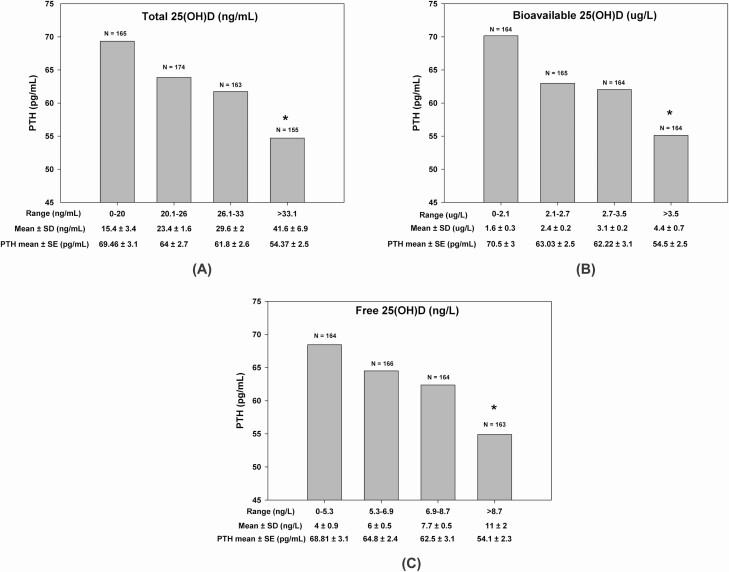
Serum total, bioavailable, and free 25(OH)D were all negatively correlated with PTH (P < 0.001). The correlation coefficients were very similar and ranged from −0.22 to −0.25. Similarly, this translated into a consistent trend for a negative relationship between mean PTH level and mean 25(OH)D level stratified by the quartiles of all 3 variables (Fig. 3). Mean PTH values were 1.27- to 1.29-fold higher in the first quartile, as compared with the fourth quartiles of each of the total, bioavailable, and free vitamin D variables (P < 0.001). Conversely, 1,25(OH)2D did not show any significant relationship with PTH, calcium, or bone remodeling markers (data not shown).
Figure 3.
The relationship between serum PTH and 25 (OH)D expressed as A, total 25(OH)D; B, bioavailable 25(OH)D; and C, free 25(OH)D, each divided into their respective quartiles. Asterisk (*) indicates significantly lower PTH levels in fourth quartile of serum total, bioavailable, and free 25(OH)D compared with the first quartile.
Serum total, bioavailable, and free 25(OH)D levels were not correlated with serum calcium levels nor with indices of bone remodeling: CTX and osteocalcin. There was no correlation between serum total, bioavailable, and free 25(OH)D and BMD, when correlated with each other, both at study entry and at 12 months (at the end of the study). The only relationship between the different vitamin D variables at 12 months and percent change BMD (spine, hip, total body) was between serum total 25(OH)D, but not the free or bioavailable forms, and percentage change BMD at the femoral neck (R2 = 0.021, Y = 0.059X–1.28, P = 0.033). There was a significant relationship between bioavailable (R2 = 0.022, Y = 0.353X–0.729, P = 0.029) and free (R2 = 0.025, Y = 0.154X–0.834, P = 0.018), but not total 25(OH)D and total body BMD.
Discussion
In this RCT, vitamin D supplementation at a dose of 3750 IU/day resulted in serum levels of total, bioavailable, and free 25(OH)D, that were 1.28- to 1.38-fold higher than levels reached with 600 IU/day dose. This revokes the remote possibility that the comparable BMD reached at 12 months with the 2 doses (31) could be explained by a lack of difference in serum values of the free or bioavailable 25(OH)D. Serum values of bioavailable and free 25(OH)D were strongly associated with total 25(OH)D, and none of the variables was a better predictor of serum levels of PTH or bone remodeling markers. Only serum total 25(OH)D correlated with percent increments in femoral neck BMD at 12 months.
The increments in serum levels of free or bioavailable 25(OH)D noted in our trial are consistent with those from previous studies, although they may differ in absolute levels attained or fold increase compared with baseline values (10, 27, 35, 38, 39). Table 2 summarizes results from several randomized trials, examining the impact of vitamin D supplementation on indices of mineral metabolism, with an N > 200. Only 1 reports postsupplementation levels in free 25(OH)D. There was a substantial increase in free 25(OH)D from 2.9 and 3.0 at t = 0 to 10.3 ng/mL and 10.22 ng/mL (converted units) at 12 months on 4800 IU/day, in African American and Caucasian women, respectively (38). The 3-fold proportional increment may in part be explained by the lower baseline serum vitamin D levels and interestingly was identical in the 2 ethnic groups. An incremental increase from 1.3 to 7.1 pg/mL with measured free, and 1.9 to 2.8 ng/mL with bioavailable 25(OH)D variables, was reported with a dose of 2000 IU/day at 10 weeks postsupplementation (39).
Table 2.
Randomized Vitamin D Trials Outlining the Relation Between Vitamin D Metabolites and Bone Health Outcomes
| Article Intervention | N | Age Gender Race | Relation to total 25(OH)D | Relation to PTH | Relation to calcium + BTM + BMD | Assay | Formula for calculated 25(OH)D | Ranges of vitamin D level |
|---|---|---|---|---|---|---|---|---|
| Sollid et al, 2016 (27) | 472 | Age 62 years on average | Measured free 25(OH)D | Total 25(OH)D | Total 25(OH) D by LC-MS | Vermeulen’s equation | Total 25(OH)D ~ 63 ± 22 nmol/L, 25.2 ± 8.8 ng/mLCalculated free 25(OH) D 20 ± 7.4 pmol/L, 8 ± 2.96 ng/L | |
| Intervention: 20,000 IU/week | Male/Female 293/179 | r = 0.73, S | r = −0.21* | Free 25(OH) D by ELISA | Measured free 25(OH)D 13.4 ± 4.2 pmol/L, 5.36 ± 1.68 ng/L | |||
| OR 2857 IU/day for 12 mos | Calculated free 25(OH)D | Measured Free 25(OH)D | Calculated bioavailable 25(OH)D 8.5 ± 3.1 nmol/L, 3.4 ± 1.24 μg/L | |||||
| * no r provided | r = −0.17* | After supplementation: | ||||||
| Total 25(OH)D ~ 109 ± 26.2 nmol/L, 43.6 ± 10.48 ng/mL | ||||||||
| Calculated free 25(OH)D 36.9 ± 9.4 pmol/l, 14.7 ± 3.7 ng/L | ||||||||
| Measured free 25(OH)D 21.9 ± 5.2 pmol/L, 8.7 ± 2.08 ng/L | ||||||||
| Yao et al, 2017 (45) | 448 | Age 30 years on average | Baseline bioavailable 25(OH)D | Supplementation Total 25(OH)D and Bioavailable 25(OH)D*, r not provided | Bioavailable 25(OH) D and Calcium, r = 0.11* | Total 25(OH) D by LC-MS | Vermeulen’s equation | Total 25(OH)D 32.8 nmol/L, 13.2 ± 3.52 ng/ mL |
| Intervention: 2000 IU/day for 20 weeks | Male/ Female 141/307 | NS | Bioavailable 25(OH)D 2.7 ± 0.8 nmol/L, 1.08 ± 0.32 μg/L | |||||
| Supplementation Bioavailable 25(OH)D*, no r provided | After supplementation: | |||||||
| Total 25(OH)D 67.3 ± 23.1 nmol/L, 26.92 ± 9.24 ng/mL | ||||||||
| Bioavailable 25(OH)D 3.0 ± 3 nmol/L, 1.2 ± 1.2 μg/L | ||||||||
| Smith et al, 2019 (38) | 358 | Older women: | Baseline free 25(OH) D, S, r = 0.73 | Total and measured free 25(OH)D* | Total 25OHD by LC-MS | Total 25 (OH)D ~ 50.9 ± 13.2 nmol/L, 20.36 ± 5.28 ng/mL | ||
| Intervention: 400 to 4800 IU/day for 12 mos | Age 66 years on average | Supplementation free 25(OH)D r = 0.86* | Free 25(OH) D measured by ELISA | Free 25 (OH)D ~ 9.21 ± 2.88 nmol/L, 3.68 ± 1.15 ng/L | ||||
| Caucasian 140 (62%) | ||||||||
| African American 88 (38%) | ||||||||
| Younger women: | ||||||||
| Age 37 years on average | ||||||||
| Caucasian 91(70%) | ||||||||
| African American 39 (30%) | ||||||||
| Meryl LeBoff et al, 2020 (48) | 771 | Age 63 years on average |
pQCT
percent change, NS |
Total 25(OH) D by LC-MS | Total 25(OH)D 69.1 ± 22.7 nmol/L, 27.64 ± 9.08 ng/mL | |||
| Intervention: 2000 IU/ day for 2 years | Male/ Female: 411/360 | Free 25(OH) D by ELISA | Free 25(OH)D 14.6 ± 4.7 pmol/L, 5.9 ± 1.88 ng/L | |||||
| White 630, Black 67, Hispanic 26, Asian 15 | After supplementation: | |||||||
| Native American or Alaskan native 5 | Total 25(OH)D 98.6 nmol/L, 39.44 ng/mL | |||||||
| Free 25(OH)D 22.3 pmol/L, 8.92 ng/L | ||||||||
| Aspray et al, 2019 (47) | 379 | Age 74 years on average | Total and measured free 25(OH)D, S, no r | Change in BMD, NS | Total 25 (OH)D by LC-MS | Mathematical model | Total 25(OH)D 41.6 ± 19.9 nmol/L, 16.64 ± 7.96 ngl/mL | |
| Intervention: 12,000, 24,000, 48,000 IU/ mo for 12 mos, | Male/ Female: 260/112 | Free 25(OH)D 8.7 ± 4.2 pmol/L, 3.48 ± 1.68 ng/L | ||||||
| Equivalent to 400 IU/ day, 800 IU/day, 1600 IU/day | ||||||||
| After supplementation: | ||||||||
| Total 25(OH)D [55.0-79] nmol/L, [22-31.6] ng/mL | ||||||||
| Free 25(OH)D [11.7-16.8] pmol/L, [4.68-6.72] ng/L |
Values for 25(OH) D levels in italics provide values using units similar to ones in our study for ease of comparability, values of vitamin D metabolites correspond to values at baseline unless specified otherwise.
* indicates significance
Abbreviations: 25(OH)D, 25-hydroxyvitamin D; BMD, bone mineral density; BTM, bone turnover marker; LC-MS, liquid chromatography–mass spectrometry; NA, not available; NS, not significant; pQCT, peripheral quantitative computed tomography.
In our study, bioavailable and free 25(OH)D were significantly associated with total 25(OH)D with an r value of 0.942 and 0.943, respectively, findings comparable to others. In children, Simpson et al recently showed that total 25(OH)D correlated strongly with calculated free 25(OH)D, r = 0.89, while the association with directly measured free 25(OH)D was slightly weaker = 0.69 (35). Smith et al showed a correlation of r = 0.73 between measured free 25(OH)D and total 25(OH)D (38).
PTH is a key marker of bone metabolism, which swiftly increases in response to low vitamin D stores, best assessed through serum total 25(OH)D level (4, 12, 40, 41). We report a consistent inverse relationship between total, bioavailable, and free 25(OH)D with PTH levels, with correlation coefficients from −0.22 to −0.25 for the 3 variables. Although negative correlations were similarly reported in other trials, the association was not examined in any of them for all 3 variables concomitantly, and its strength was only documented in 1 trial, Table 2). The relationship does not seem to be dependent on age, with a reported r between calculated free 25 (OH) D and PTH of −0.17 in subjects 60 years of age (27) with a similar correlation in individuals with a mean age of 35 (42).
We found no relationship between any of the variables with calcium, and many studies support the same conclusion (39, 43, 44). However, in one randomized double-blind vitamin D3 trial conducted in older adults, Schwartz et al showed a weak positive correlation between calcium and total and free measured 25(OH)D, and negligible r2 (r2 = 0.08 and 0.07, respectively) (10). Similarly, we did not find any significant relationship between total, bioavailable, or free 25(OH)D and osteocalcin and crosslaps. Yao et al showed a positive relationship between bioavailable 25(OH)D and calcium (r = 0.22, P < 0.001) (45). Shwetz et al also showed that total 25(OH)D did not correlate with levels of bone-specific alkaline phosphatase, osteocalcin, CTX, or procollagen type 1 N-terminal pro-peptide (P1NP), either at baseline or at follow-up (46). Conversely, a positive correlation was shown between both total and free 25(OH)D and serum C-terminal telopeptides of type I collagen, but not P1NP, in an 8-week vitamin D RCT (39). The limited number of studies examining the relationship between total, bioavailable, and free 25(OH)D, with bone turnover markers, do not indicate any difference between the 3 variables. They are, however, not sufficient to deduce any definitive conclusions.
In this trial, we did not show any difference between the 2 vitamin D doses in BMD at 12 months at the spine or hip, but subtotal body BMD was higher with the high dose (31). Similarly, an RCT investigating the effect of vitamin D supplementation on BMD in older individuals showed no difference between doses of 400, 800, or 1600 IU/day (47). On additional analyses, we demonstrate a weak but significant relationship between 25(OH)D and percent change BMD at femoral neck only (R2 = 0.021, P = 0.033), and only mild significant correlation between the free and bioavailable 25(OH)D and the total body BMD at 12 months. The correlation between total, but not free and bioavailable 25(OH)D, with percent change BMD at the femoral neck was modest at best and may reflect the amount of variance of the relevant variables. The same applies for the observed correlations between the free and bioavailable 25(OH)D and not total 25(OH)D with total body (but not spine and hip). The VITamin D and OmegA-3 TriaL (VITAL) RCT showed no relationship between total 25(OH)D and percent change of peripheral quantitative computed tomography (48) after supplementation with vitamin D doses ranging from 400 to 4800 IU/day. While few observational studies reported a positive association between bioavailable and/or free 25(OH)D and BMD (49), including a study including young Lebanese women (50), we are unaware of any RCT that assessed the relationship between bioavailable or free vitamin D and percent change in BMD, after vitamin D supplementation. Furthermore, the relevance of the correlations between the free and bioavailable 25(OH) D forms and total body BMD (which is not routinely measured) to clinical applications, is unclear at this point.
Careful scrutiny of the methodology of previous studies might explain some, but not all, of the differing results. Most studies started with comparable vitamin D and PTH levels, used similar assays to detect free, bioavailable, and total 25(OH)D and PTH, and used similar equations for calculated 25(OH)D values (Bikle’s or Vermeulen’s equation). However, even similar assays can lead to differing values that should be interpreted with caution if no calibration to international standards is ensured (4). In addition, values of directly measured free vitamin D are lower than obtained with calculations (23, 24, 51), while maintaining a strong correlation with each other (r = 0.80-0.83 for free and bioavailable 25(OH)D forms) (23). The direct measurement of free 25(OH)D is also not without limitations (7). It can be affected by ethnicity, DBP genotypes (23), and the type of antibody used (monoclonal versus polyclonal (52)). Furthermore, variability in several predictors of baseline levels of 25(OH)D forms, their relationship with mineral indices, and response to supplementation, may explain differences between studies. These include ethnic, genetic, demographic, and lifestyle factors, as well as type and duration of supplementation (10, 27, 42, 53-56). Directly measured free 25(OH)D correlated more strongly with PTH compared to calculated free 25(OH)D (24, 51, 57).
Our study may have some limitations, such as not having directly measured free 25(OH)D. However, as mentioned earlier, even the measured free 25(OH)D has its own limitation with its lack of standardization. To increase the power of our analyses, and increase the range of vitamin D values obtained, we used 3 observations from each subject, before and at 6 and 12 months postsupplementation. We, however, accounted for repeated measures and lack of independence in the specific general linear model chosen (36). Therefore, correlations obtained herein are not exactly comparable to those obtained in other studies but nevertheless are very comparable in direction and strength of association. The calculations at different time points are dependent on the vitamin D binding protein and albumin that were only measured at baseline. However, concentrations of DBP and albumin were previously shown to appear to be stable over 12 months (51). Our study included overweight individuals, with a mean age of 71 years, known to have lower total and free 25(OH)D (58) as well as higher PTH values compared with nonobese controls (59). PTH levels also increase by approximately 5% for each 10-year increase in age (60). However, although our findings may not be generalizable across age ranges, our cohort is typical of the population of interest for adverse musculoskeletal outcomes.
Indeed, our study has several other strengths. It concomitantly investigates relationships between the different 25(OH)D forms, under baseline conditions, in a population with a mean 25(OH)D of 20.4 ng/mL (Table 1), which is comparable to many other elderly cohorts. Over half (53%) of study subjects, had serum 25(OH) below the desirable level of 20 ng/mL (50 nmol/L) (13, 14). We systematically investigate the relationship between these different 25(OH)D forms in response to vitamin D supplementation, with doses of vitamin D that are comparable to the Institute of Medicine (IOM) recommended doses for the low dose, and the upper tolerable level for the high dose (14). It is also unique, as it investigates multiple indices of bone and mineral metabolism and BMD.
In conclusion, our study provides evidence for the lack of any advantage from measuring free or bioavailable over total 25(OH)D in assessing indices of bone health in an elderly population free of end-stage kidney or liver disease. It confirms findings from other large trials. Studies are needed to establish whether there is an additional value in measuring bioavailable and free 25(OH)D in populations where DBP and albumin are at risk of alteration, particularly in pregnant women, patients with liver failure, and possibly children (24, 61). However, the most recent study in children also revealed no added benefit of measuring free or bioavailable 25(OH)D (35). Finally, additional studies are needed to determine the accuracy of calculated and measured free and bioavailable 25(OH)D, as well as establish their normal range of reference, using international calibration standards.
Acknowledgments
The authors are grateful to study subjects for their participation and thank study coordinators and hospital personnel, administrators at the Lebanese Ministry of Social Affairs dispensaries, local dispensaries, for their time and dedication and making the study possible. The authors greatly appreciate the time and input of members of the data safety monitoring board, Heike Bischoff-Ferrari MD, DrPH (University of Zurich, Switzerland), Christopher Gallagher, MD (Creighton University, USA), and Reinhold Vieth PhD, FCACB (Mt Sinai Hospital, Montreal, Canada). The authors thank Euro-Pharm Canada for providing the vitamin D/identical placebo tablets and calcium citrate supplements. We thank Erik Van Herck who performed all DBP analyses.
Financial Support: This trial was supported by grants from the American University of Beirut (AUB), St Joseph University, and the Lebanese Council for National Scientific Research. Assays performed outside AUB were made in part possible thanks to institutional grants from the Mayo Clinic, Rochester, Minnesota, USA, and from Catholic University, Leuven, Belgium. Research reported in this publication was in part supported by the Fogarty International Center and Office of Dietary Supplements of the National Institutes of Health under Award Number D43 TW009118; PI Ghada El-Hajj Fuleihan. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Clinical Trial Information: ClinicalTrials.gov ID no. NCT01315366
Glossary
Abbreviations
- 1,25(OH)2D
1,25-dihydroxyvitamin D
- 25(OH)D
25-hydroxyvitamin D
- BMD
bone mineral density
- BMI
body mass index
- CTX
C-terminal telopeptide, crosslaps
- DBP
vitamin D binding protein
- PTH
parathyroid hormone
- RCT
randomized controlled trial
Additional Information
Disclosure Summary: Authors have no conflict of interest to disclose
Data Availability
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266-281. [DOI] [PubMed] [Google Scholar]
- 2. Lips P. Worldwide status of vitamin D nutrition. J Steroid Biochem Mol Biol. 2010;121(1-2):297-300. [DOI] [PubMed] [Google Scholar]
- 3. Bikle DD. Vitamin D metabolism, mechanism of action, and clinical applications. Chem Biol. 2014;21(3):319-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fuleihan Gel-H, Bouillon R, Clarke B, et al. . Serum 25-hydroxyvitamin D levels: variability, knowledge gaps, and the concept of a desirable range. J Bone Miner Res. 2015;30(7):1119-1133. [DOI] [PubMed] [Google Scholar]
- 5. Bikle DD, Gee E, Halloran B, Kowalski MA, Ryzen E, Haddad JG. Assessment of the free fraction of 25-hydroxyvitamin D in serum and its regulation by albumin and the vitamin D-binding protein. J Clin Endocrinol Metab. 1986;63(4):954-959. [DOI] [PubMed] [Google Scholar]
- 6. Bouillon R, Van Assche FA, Van Baelen H, Heyns W, De Moor P. Influence of the vitamin D-binding protein on the serum concentration of 1,25-dihydroxyvitamin D3. Significance of the free 1,25-dihydroxyvitamin D3 concentration. J Clin Invest. 1981;67(3):589-596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tsuprykov O, Chen X, Hocher CF, Skoblo R, Yin Lianghong, Hocher B. Why should we measure free 25(OH) vitamin D? J Steroid Biochem Mol Biol. 2018;180:87-104. [DOI] [PubMed] [Google Scholar]
- 8. Bikle D, Bouillon R, Thadhani R, Schoenmakers I. Vitamin D metabolites in captivity? Should we measure free or total 25(OH)D to assess vitamin D status? J Steroid Biochem Mol Biol. 2017;173:105-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bouillon R, Schuit F, Antonio L, Rastinejad F. Vitamin D binding protein: a historic overview. Front Endocrinol (Lausanne). 2019;10:910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schwartz JB, Kane L, Bikle D. Response of vitamin D concentration to vitamin D3 administration in older adults without sun exposure: a randomized double-blind trial. J Am Geriatr Soc. 2016;64(1):65-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Herrmann M, Farrell CL, Pusceddu I, Fabregat-Cabello N, Cavalier E. Assessment of vitamin D status - a changing landscape. Clin Chem Lab Med. 2017;55(1):3-26. [DOI] [PubMed] [Google Scholar]
- 12. Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. ; Endocrine Society . Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911-1930. [DOI] [PubMed] [Google Scholar]
- 13. Lips P, Cashman KD, Lamberg-Allardt C, et al. . Current vitamin D status in European and Middle East countries and strategies to prevent vitamin D deficiency: a position statement of the European Calcified Tissue Society. Eur J Endocrinol. 2019;180(4):P23-P54. [DOI] [PubMed] [Google Scholar]
- 14. Ross AC, Manson JE, Abrams SA, et al. . The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96(1):53-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mendel CM. The free hormone hypothesis: a physiologically based mathematical model. Endocr Rev. 1989;10(3):232-274. [DOI] [PubMed] [Google Scholar]
- 16. Chun RF, Peercy BE, Orwoll ES, Nielson CM, Adams JS, Hewison M. Vitamin D and DBP: the free hormone hypothesis revisited. J Steroid Biochem Mol Biol. 2014;144(Pt A):132-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vermeulen A, Stoïca T, Verdonck L. The apparent free testosterone concentration, an index of androgenicity. J Clin Endocrinol Metab. 1971;33(5):759-767. [DOI] [PubMed] [Google Scholar]
- 18. Powe CE, Evans MK, Wenger J, et al. . Vitamin D-binding protein and vitamin D status of black Americans and white Americans. N Engl J Med. 2013;369(21):1991-2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hollis BW, Bikle DD. Vitamin D-binding protein and vitamin D in blacks and whites. N Engl J Med. 2014;370(9):879-880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bouillon R, Jones K, Schoenmakers I. Vitamin D-binding protein and vitamin D in blacks and whites. N Engl J Med. 2014;370(9):879. [DOI] [PubMed] [Google Scholar]
- 21. Chen Y. Vitamin D-binding protein and vitamin D in blacks and whites. N Engl J Med. 2014;370(9):878. [DOI] [PubMed] [Google Scholar]
- 22. Weintraub SJ. Vitamin D-binding protein and vitamin D in blacks and whites. N Engl J Med. 2014;370(9):878. [DOI] [PubMed] [Google Scholar]
- 23. Nielson CM, Jones KS, Chun RF, et al. ; Osteoporotic Fractures in Men (MrOS) Research Group . Free 25-hydroxyvitamin D: impact of vitamin D binding protein assays on racial-genotypic associations. J Clin Endocrinol Metab. 2016;101(5): 2226-2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schwartz JB, Lai J, Lizaola B, et al. . A comparison of measured and calculated free 25(OH) vitamin D levels in clinical populations. J Clin Endocrinol Metab. 2014;99(5):1631-1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bikle DD, Halloran BP, Gee E, Ryzen E, Haddad JG. Free 25-hydroxyvitamin D levels are normal in subjects with liver disease and reduced total 25-hydroxyvitamin D levels. J Clin Invest. 1986;78(3):748-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bikle DD, Schwartz J. Vitamin D binding protein, total and free vitamin D Levels in different physiological and pathophysiological conditions. Front Endocrinol (Lausanne). 2019;10:317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sollid ST, Hutchinson MY, Berg V, et al. . Effects of vitamin D binding protein phenotypes and vitamin D supplementation on serum total 25(OH)D and directly measured free 25(OH)D. Eur J Endocrinol. 2016;174(4):445-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bouillon R. Vitamin D and cardiovascular disorders. Osteoporos Int. 2019;30(11):2167-2181. [DOI] [PubMed] [Google Scholar]
- 29. Wactawski-Wende J, Kotchen JM, Anderson GL, et al. ; Women’s Health Initiative Investigators . Calcium plus vitamin D supplementation and the risk of colorectal cancer. N Engl J Med. 2006;354(7):684-696. [DOI] [PubMed] [Google Scholar]
- 30. El-Hajj Fuleihan G, Baddoura R, Habib RH, et al. . Effect of vitamin D replacement on indexes of insulin resistance in overweight elderly individuals: a randomized controlled trial. Am J Clin Nutr. 2016;104(2):315-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rahme M, Sharara SL, Baddoura R, et al. . Impact of calcium and two doses of vitamin D on bone metabolism in the elderly: a randomized controlled trial. J Bone Miner Res. 2017;32(7):1486-1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bouillon R, van Baelen H, de Moor P. The measurement of the vitamin D-binding protein in human serum. J Clin Endocrinol Metab. 1977;45(2):225-231. [DOI] [PubMed] [Google Scholar]
- 33. Petak S, Barbu CG, Yu EW, et al. . The Official Positions of the International Society for Clinical Densitometry: body composition analysis reporting. J Clin Densitom. 2013;16(4):508-519. [DOI] [PubMed] [Google Scholar]
- 34. Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84(10):3666-3672. [DOI] [PubMed] [Google Scholar]
- 35. Simpson CA, Zhang JH, Vanderschueren D, et al. . Relationship of total and free 25-hydroxyvitamin D to biomarkers and metabolic indices in healthy children. J Clin Endocrinol Metab 2020;105(4):e1631-e1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lipsitz SR, Leong T, Ibrahim J, Lipshultz S. A partial correlation coefficient and coefficient of determination for multivariate normal repeated measures data. J R Stat Soc. 2001;50(1):87-95 [Google Scholar]
- 37. Ish-Shalom S, Segal E, Salganik T, Raz B, Bromberg IL, Vieth R. Comparison of daily, weekly, and monthly vitamin D3 in ethanol dosing protocols for two months in elderly hip fracture patients. J Clin Endocrinol Metab. 2008;93(9):3430-3435. [DOI] [PubMed] [Google Scholar]
- 38. Smith LM, Gallagher JC. Effect of vitamin D supplementation on total and free 25 hydroxyvitamin D and parathyroid hormone. An analysis of two randomized controlled trials. J Intern Med. 2019;286(6):651-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Aloia J, Dhaliwal R, Mikhail M, et al. . Free 25(OH)D and calcium absorption, PTH, and markers of bone turnover. J Clin Endocrinol Metab. 2015;100(11):4140-4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Khundmiri SJ, Murray RD, Lederer E. PTH and Vitamin D. Compr Physiol. 2016;6(2):561-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dietary Reference Intakes for Calcium and Vitamin D. Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium Ross AC, Taylor CL, Yaktine AL, Valle HBD, eds. Washington, DC: National Academies Press (US); 2011. [PubMed] [Google Scholar]
- 42. Gallagher JC. Vitamin D and aging. Endocrinol Metab Clin North Am. 2013;42(2):319-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Johnsen MS, Grimnes G, Figenschau Y, Torjesen PA, Almås B, Jorde R. Serum free and bio-available 25-hydroxyvitamin D correlate better with bone density than serum total 25-hydroxyvitamin D. Scand J Clin Lab Invest. 2014;74(3):177-183. [DOI] [PubMed] [Google Scholar]
- 44. Li C, Chen P, Duan X, et al. . Bioavailable 25(OH)D but not total 25(OH)D is an independent determinant for bone mineral density in Chinese postmenopausal women. Ebiomedicine. 2017;15:184-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yao P, Sun L, Lu L, et al. . Effects of genetic and nongenetic factors on total and bioavailable 25(OH)D responses to vitamin D supplementation. J Clin Endocrinol Metab. 2017;102(1): 100-110. [DOI] [PubMed] [Google Scholar]
- 46. Schwetz V, Trummer C, Pandis M, et al. . Effects of vitamin D supplementation on bone turnover markers: a randomized controlled trial. Nutrients 2017;9(5):432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Aspray TJ, Chadwick T, Francis RM, et al. . Randomized controlled trial of vitamin D supplementation in older people to optimize bone health. Am J Clin Nutr. 2019;109(1):207-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. LeBoff MS, Chou SH, Murata EM, et al. . Effects of supplemental vitamin D on bone health outcomes in women and men in the VITamin D and OmegA-3 TriaL (VITAL). J Bone Miner Res. 2020;35(5):883-893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Allison RJ, Farooq A, Cherif A, Hamilton B, Close GL, Wilson MG. Why don’t serum vitamin D concentrations associate with BMD by DXA? A case of being ‘bound’ to the wrong assay? Implications for vitamin D screening. Br J Sports Med. 2018;52(8):522-526. [DOI] [PubMed] [Google Scholar]
- 50. Alwan A, Rizkallah M, Maalouf G, et al. . Positive correlations between free vitamin D and bone variables in a group of young lebanese women. J Clin Densitom. 2018;21(3):446-452. [DOI] [PubMed] [Google Scholar]
- 51. Oleröd G, Hultén LM, Hammarsten O, Klingberg E. The variation in free 25-hydroxy vitamin D and vitamin D-binding protein with season and vitamin D status. Endocr Connect. 2017;6(2):111-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Malmstroem S, Rejnmark L, Imboden JB, Shoback DM, Bikle DD. Current Assays to Determine Free 25-Hydroxyvitamin D in Serum. J AOAC Int. 2017;100(5):1323-1327. [DOI] [PubMed] [Google Scholar]
- 53. Saad RK, Akiki VC, Rahme M, Ajjour S, Assad M, El-Hajj Fuleihan GA. Time trends and predictors of hypovitaminosis D across the life course: 2009–2016. Metabolism 2020:154138. doi: 10.1016/j.metabol.2020.154138. [DOI] [PubMed] [Google Scholar]
- 54. Shieh A, Ma C, Chun RF, et al. . Effects of Cholecalciferol vs Calcifediol on total and free 25-hydroxyvitamin D and parathyroid hormone. J Clin Endocrinol Metab. 2017;102(4):1133-1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Shieh A, Ma C, Chun RF, et al. . associations between change in total and free 25-hydroxyvitamin D with 24,25-dihydroxyvitamin D and parathyroid hormone. J Clin Endocrinol Metab. 2018;103(9):3368-3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Binkley N, Borchardt G, Siglinsky E, Krueger D. Does vitamin D metabolite measurement help predict 25(OH)D change following vitamin D supplementation? Endocr Pract. 2017;23(4):432-441. [DOI] [PubMed] [Google Scholar]
- 57. Lopez-Molina M, Santillan C, Murillo M, et al. . Measured free 25-hydroxyvitamin D in healthy children and relationship to total 25-hydroxyvitamin D, calculated free 25-hydroxyvitamin D and vitamin D binding protein. Clin Biochem. 2018;61:23-27. [DOI] [PubMed] [Google Scholar]
- 58. Walsh JS, Evans AL, Bowles S, et al. . Free 25-hydroxyvitamin D is low in obesity, but there are no adverse associations with bone health. Am J Clin Nutr. 2016;103(6):1465-1471. [DOI] [PubMed] [Google Scholar]
- 59. Saarnio E, Pekkinen M, Itkonen ST, et al. . Low free 25-hydroxyvitamin D and high vitamin D binding protein and parathyroid hormone in obese Caucasians. A complex association with bone? PLoS One. 2018;13(2):e0192596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Carrivick SJ, Walsh JP, Brown SJ, Wardrop R, Hadlow NC. Brief report: Does PTH increase with age, independent of 25-hydroxyvitamin D, phosphate, renal function, and ionized calcium? J Clin Endocrinol Metab. 2015;100(5):2131-2134. [DOI] [PubMed] [Google Scholar]
- 61. Bikle DD, Malmstroem S, Schwartz J. Current controversies: are free vitamin metabolite levels a more accurate assessment of vitamin D status than total levels? Endocrinol Metab Clin North Am. 2017;46(4):901-918. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.