Abstract
Background
Controversy exists as to whether low-dose cabergoline is associated with clinically significant valvulopathy. Few studies examine hard cardiac endpoint data, most relying on echocardiographic findings.
Objectives
To determine the prevalence of valve surgery or heart failure in patients taking cabergoline for prolactinoma against a matched nonexposed population.
Design
Population-based cohort study based on North East London primary care records.
Methods
Data were drawn from ~1.5 million patients’ primary care records. We identified 646 patients taking cabergoline for >6 months for prolactinoma. These were matched to up to 5 control individuals matched for age, gender, ethnicity, location, diabetes, hypertension, ischemic heart disease, and smoking status. Cumulative doses/durations of treatment were calculated. Cardiac endpoints were defined as cardiac valve surgery or heart failure diagnosis (either diagnostic code or prescription code for associated medications).
Results
A total of 18 (2.8%) cabergoline-treated patients and 62 (2.33%) controls reached a cardiac endpoint. Median cumulative cabergoline dose was 56 mg (interquartile range [IQR] 27-123). Median treatment duration was 27 months (IQR 15-46). Median weekly dose was 2.1 mg. Neither univariate nor multivariate analysis demonstrated a significant association between cabergoline treatment at any cumulative dosage/duration and an increased incidence of cardiac endpoints. In a matched analysis, the relative risk for cardiac complications in the cabergoline-treated group was 0.78 (95% CI, 0.41-1.48; P = 0.446). Reanalysis of echocardiograms for 6/18 affected cabergoline-treated patients showed no evidence of ergot-derived drug valvulopathy.
Conclusions
The data did not support an association between clinically significant valvulopathy and low-dose cabergoline treatment and provide further evidence for a reduction in frequency of surveillance echocardiography.
Keywords: cabergoline, cardiac valvulopathy, dopamine agonist, prolactinoma, hyperprolactinemia, pituitary adenoma
Cabergoline, an ergot-derived dopamine agonist, is first-line therapy for patients with symptomatic hyperprolactinemia consequent upon a lactotrope pituitary adenoma. It normalizes prolactin levels, rectifies subfertility, abolishes galactorrhea, and decreases tumor size in the vast majority of patients. Since the first reports of an association between high dose (>3 mg/day) cabergoline for the treatment of Parkinson disease and fibrotic heart disease (1, 2), its potential to cause similar cardiac defects at “endocrine” doses (typically 0.5-1.0 mg weekly) for the treatment of hyperprolactinemic states has been extensively investigated. Regulatory authorities published stringent guidelines (3), which mandated an echocardiogram at the start of treatment, again after 3 to 6 months, followed by further scans every 6 to 12 months for the duration of treatment or sooner in the event of clinical suspicion of cardiac valvulopathy. The literature examining the consequences for cardiac function of cabergoline therapy for hyperprolactinemia is now extensive and includes case-control studies (4-16), meta-analyses (17-19), and evidence-assimilating professional society “position statements” (20). The vast majority of this literature is based on echocardiographic findings, with heterogeneous classification and, in one study, evidence of reporting bias (21). Most of this previous work is reassuring, with one worrying observation of a markedly increased incidence of clinically significant tricuspid valve disease observed in one early study (6) not replicated elsewhere, or indeed by the same group in a later study (22).
It is highly unlikely that further cross-sectional or case-control studies will advance the field and a large prospective study would be prohibitively expensive. Additionally, until now, studies have relied on echocardiogram findings for reporting the severity of valvular regurgitation. Here, we investigate the possible link between the use of cabergoline for the treatment of hyperprolactinemic states and cardiac valvulopathy using documented hard endpoints of cardiac failure or valve disease as outcome measures derived from a large primary care clinical database.
Methods
Formal approval for this study was received from the East of England, Cambridge, UK central committee of the National Health Service (NHS) Research Ethics Service (REC reference 20/EE/0013). Pseudonymized patient data were obtained from the Discovery Data Service (DDS) (https://discoverydataservice.org/Content/Home.htm). This service has a data sharing agreement with 305 primary care practices and 3 hospital trusts in 4 clinical commissioning groups (CCGs) in inner North East London, UK, an area also served by the authors’ (C.S., G.L., S.B., and W.D.) secondary/tertiary care institution, St Bartholomew’s Hospital. Primary care physicians use electronic patient records (EMIS and SystmOne systems) to generate diagnostic and drug prescription codes. The DDS draws upon the electronic patient records of ~1.5 million patients from participating primary care practices. A curated dataset was generated based upon our research questions and the inclusion/exclusion criteria described. Cases and controls were included/excluded based on the presence/absence of certain codes within their primary care record.
DDS enrollment of primary care practices started in 2016. All patients who were alive at of the start of a practice’s enrollment were included—with all their prior data available, irrespective of whether they died or left the practice between this time and the date of the data draw. Patients who died or left a participating practice prior to its DDS enrollment were not included in DDS at the time of the study. It can reasonably be assumed that an equal number of patients joined participating practices as left.
Inclusion criteria for the study were: age ≥18 years when cabergoline therapy was commenced, and use of cabergoline therapy for hyperprolactinemia caused by lactotrope pituitary adenoma.
Exclusion criteria for the study were: age <18 years; previous diagnosis of any sort of Parkinson disease, Parkinsonism, acromegaly/gigantism, or restless legs syndrome; and cabergoline therapy for <6 months (in order to avoid the inadvertent inclusion of women prescribed cabergoline to aid cessation of lactation).
Specific cardiac disease exclusion criteria included: any patient with congenital cardiac disease; radiation-induced cardiac disease; thyrotoxicosis-, rheumatic-, amyloid-, carcinoid-, sarcoid-, glycogen storage- or, kyphoscoliotic-related cardiac disease; or infectious heart disease at any time. Any patient with cardiac disease predating initiation of cabergoline was excluded.
We also excluded any patient who had ever taken bromocriptine, methysergide, dexfenfluramine, fenfluramine, ergotamine, or phentermine. Additionally, we excluded any patients with a prescription code for the following drugs: co-careldopa, co-beneldopa, levodopa, selegiline, rasagiline, amantadine, ropinirole, pramipexole, Sinemet, and mirapexin as these are suggestive of an uncoded diagnosis of Parkinson disease.
A full list of codes and conditions that were used as inclusion/exclusion criteria are available in Supplementary Appendix 1 (23).
The age (±2 years), gender, ethnicity, primary care location, smoking status, presence of hypertension status, and diabetes mellitus status for each patient were obtained from primary care records. Based on these criteria, we attempted to match each index patient with 5 cabergoline-nonexposed controls. These controls had no history of ischemic heart disease preceding the index patient’s cabergoline start date.
“Hard” indicators of the primary endpoint were considered to be any diagnosis of cardiac failure, cardiac valve disease, or the requirement for valve repair/replacement made after commencing cabergoline therapy (“cases” group). For the “controls,” the primary endpoint was reached if they received a diagnosis of cardiac failure/valve disease or underwent valve repair after the date that their “reference case” had started cabergoline.
“Soft” indicators of reaching the primary endpoint of cardiac failure were considered to be any primary care code for a patient taking any of the following medications—furosemide, torasemide, spironolactone, bumetanide, metolazone, or ethacrynic acid. Treatment with angiotensin converting enzyme inhibitors, angiotensin receptor antagonists or beta blockers were not used as exclusion criteria, as these are frequently used for the treatment of hypertension and heart rate control respectively but were considered less likely to be indicative of underlying heart failure.
A successful application to access confidential patient information without consent under section 251 of the NHS Act 2006 was also made to the Confidentiality Advisory Group (CAG reference 20/CAG/0079) in order to obtain patient identifiable information (UK NHS numbers) for any patient taking cabergoline who reached a primary endpoint. The NHS number was then used to interrogate the echocardiogram viewing application of the secondary/tertiary center (St Bartholomew’s Hospital). Where available, echocardiogram images were re-reported, independently but unblinded to the patient’s history of cabergoline exposure, by 2 very experienced consultant cardiologists (G.L. and S.B.) to assess whether the valves showed any of the signs consistent with valvulopathy caused by dopamine agonist therapy, namely, leaflet thickening, restricted movement, and calcification. Echocardiograms were scored using a system previously proposed for this purpose (20), derived from a scoring system for assessment of carcinoid heart disease (24) and using echocardiographic parameters set out in this latter paper. Once separate scores had been recorded, the examiners met to agree a consensus valve score.
Patients were not contacted individually about the study as the large sample size made this impractical. Instead, an electronic notice was placed on the NHS Trust’s website and posters were put up in the endocrine clinics, offering patients contact details for further information on the study and instructions on how to opt out. The data of patients participating in the national opt-out scheme concerning use of identifiable data for research purposes were excluded.
Statistical analysis
Patient demographics were summarized as medians and interquartile ranges (IQRs) for continuous variables or as percentages for categorical variables with comparisons made between those taking and those not taking cabergoline, using Wilcoxon rank-sum or Fisher exact tests, respectively. Unadjusted and adjusted relative risks (RRs) of cardiac complications in patients taking cabergoline compared with patients not taking cabergoline were calculated using Poisson regression with robust variances. For adjusted RRs, a stepwise selection procedure was used to determine which parameters of age, gender, ethnicity, CCG, diabetes, hypertension, ischemic heart disease, and smoking were associated with cardiac complications, keeping the use of cabergoline fixed in the model. Among patients taking cabergoline, the univariate and multivariate RRs of cardiac complications were similarly estimated according to cumulative dose and duration of use, treated as continuous variables, and in quartiles. A matched analysis was also performed to calculate the RR of cardiac complications in patients receiving cabergoline compared with nonexposed patients among matched groups where there were cardiac complications, using conditional fixed-effects Poisson regression. All analyses were performed using Stata version 15 (StataCorp, College Station, Texas).
After data were received from the DDS, and in order to verify the accuracy of the returned data, numbers of patients taking cabergoline and with heart failure diagnoses, heart failure drug prescriptions, or valve operations were compared to an anonymized EMIS search in the Tower Hamlets CCG—1 of the 4 contributing CCGs. This returned identical numbers to those returned by DDS in all categories, with the exception of 1 extra patient taking furosemide. It is likely that this patient started furosemide prior to cabergoline and so was excluded from our study and for this reason did not feature in the DDS cohort.
Results
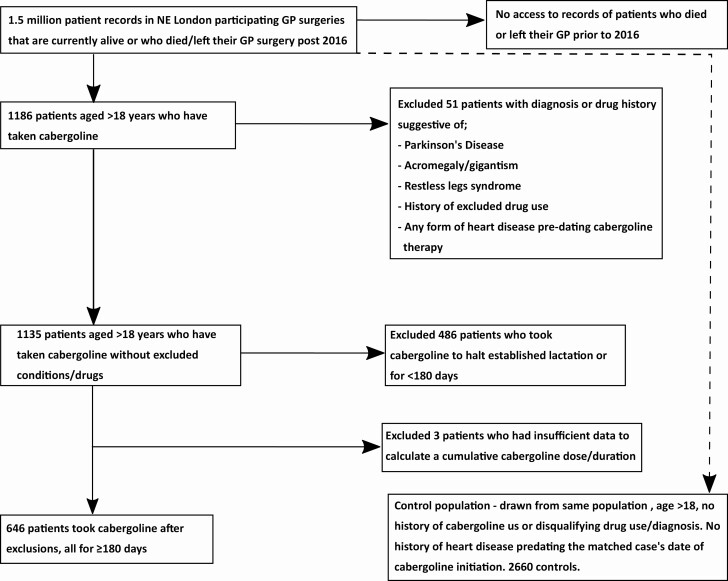
From a DDS population of ~1.5 million patients, a total of 646 patients who took cabergoline for hyperprolactinemia and were treated for >6 months were identified (Fig. 1). These were matched to 2660 cabergoline-nonexposed controls. Our matching criteria meant that we were unable to match all patients with 5 controls; 473 patients had 5 matched controls, 21 had 4, 37 had 3, 37 had 2, 26 had 1, and for 52 the database was unable to find even a single match. Our prevalence figure of 43 per 100 000 is strikingly similar to a previous UK primary care study (25), which found a prevalence of 44 per 100 000 and was considered to provide reassurance that the capture of patients taking cabergoline was robust.
Figure 1.
Breakdown of the number of patients at each inclusion/exclusion stage of selection.
As in previous studies, there was a strong female preponderance in our study group (F, 542 [84%] vs M, 104 [16%] Table 1). Matching between cabergoline-exposed and nonexposed patients resulted in a mean age of 41 vs 42 years (P = 0.004), respectively. The difference is likely to have been the result of a tolerance of ±2 years being applied in the matching criteria to maximize the number of matches. An even recruitment from across the CCGs was observed. There was a proportionally higher percentage of patients with diabetes mellitus and hypertension in the cabergoline-exposed group compared to the nonexposed population; conversely, there was a higher prevalence of smoking in the cabergoline-nonexposed group. This could be accounted for by differences in the number of matches between patients.
Table 1.
Patient Demographics. Figures are Median (interquartile range) or Number (percentage). CCG—clinical Commissioning Groups, Effectively Geographic Areas Containing Several Primary Care Practices
| Cabergoline | P value | ||
|---|---|---|---|
| Yes (n = 646) | No (n = 2660) | ||
| Age (years) | 41 (34-49) | 42 (35-51) | 0.004 |
| Gender | |||
| Female | 542 (84%) | 2246 (84%) | 0.763 |
| Male | 104 (16%) | 414 (16%) | |
| Ethnicity | |||
| Asian/Asian British | 126 (20%) | 510 (19%) | 0.048 |
| Black/Black British | 213 (33%) | 796 (30%) | |
| Mixed | 20 (3.1%) | 44 (1.7%) | |
| Unknown | 70 (11%) | 303 (11%) | |
| White | 217 (34%) | 1007 (38%) | |
| CCG | |||
| City and Hackney | 153 (24%) | 597 (22%) | 0.917 |
| Newham | 202 (31%) | 835 (31%) | |
| Tower Hamlets | 136 (21%) | 579 (22%) | |
| Waltham Forest | 155 (24%) | 649 (24%) | |
| Diabetes | 36 (5.6%) | 67 (2.5%) | <0.001 |
| Hypertension | 83 (13%) | 203 (7.6%) | <0.001 |
| IHD | 6 (0.9%) | 0 (0%) | <0.001 |
| Smoking status | |||
| Smoker | 169 (26%) | 1101 (41%) | <0.001 |
| Never smoked | 350 (54%) | 1492 (56%) | |
| Unknown | 127 (20%) | 67 (2.5%) | |
| Cumulative cabergoline dose (mg) | 56 (27-123) | - | - |
| Duration of cabergoline use (months) | 27 (15-46) | - | - |
| Cardiac complications | 18 (2.8%) | 52 (2.0%) | 0.221 |
Figures are median (interquartile range) or number (percentage).
Abbreviations: CCG, Clinical Commissioning Groups (effectively geographic areas containing several primary care practices); IHD, ischemic heart disease.
Within the cabergoline-treated patients, median cumulative cabergoline dose was 56 mg (IQR, 27-123), mean 136 mg (SD ± 370.2). Median duration of treatment was 27 months (IQR, 15-46) mean 40 months (SD ± 40.3). Median weekly dose was therefore 2.1 mg.
In total, 18 (2.8%) cabergoline-treated and 62 (2.3%) nonexposed patients met a cardiac complication endpoint of either a heart failure diagnosis, or commenced a heart failure–associated medication or had a heart valve replacement/repair (Table 2). Where applicable, median time to heart failure diagnosis/valve repair was 842 days (range, 221-5306; SD ± 1389) and median cumulative cabergoline dose to heart failure diagnosis/valve repair = 100 mg (mean 183, SD ± 85).
Table 2.
Breakdown of Cardiac Complication Endpoints Between Cabergoline-Exposed and Nonexposed Patients (some patients met 2 or more endpoint criteria)
| Endpoint | Cabergoline exposure | |
|---|---|---|
| Yes (n = 18) | No (n = 62) | |
| Valve repair/replacement | 1 | 3 |
| Heart failure diagnosis | 2 | 5 |
| Heart failure medication prescription | 17 | 57 |
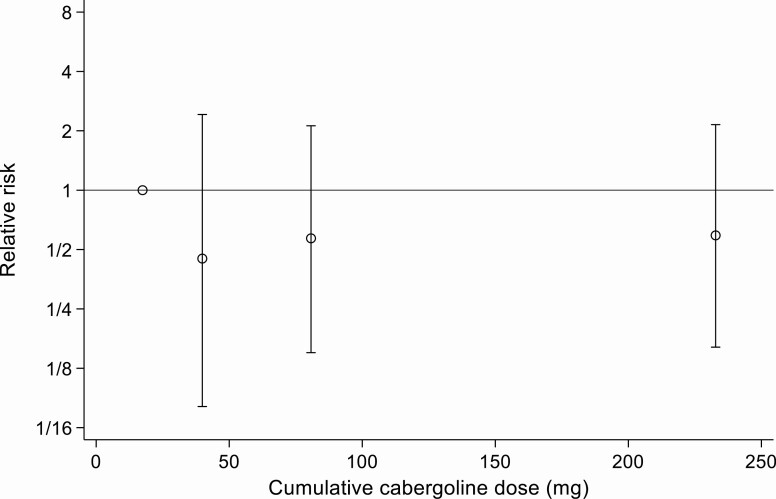
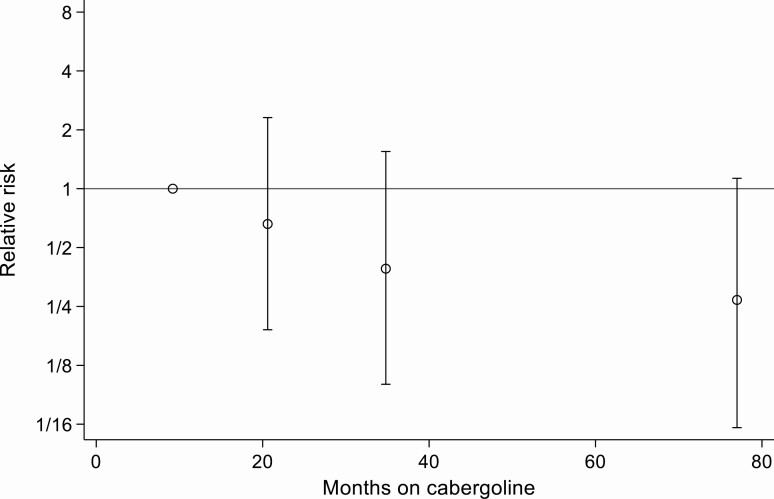
Neither univariate nor multivariate analysis demonstrated a significant association between cabergoline treatment at any cumulative dosage or duration and an increased incidence of valve replacement or heart failure (Table 3, Figs. 2 and 3). In a matched analysis the RR for cardiac complications in the cabergoline-treated group was 0.78 (95% CI, 0.41-1.48; P = 0.446).
Table 3.
Association Between Cabergoline and Cardiac Complications: Unmatched Analysis
| Unadjusted | Adjusted | |||
|---|---|---|---|---|
| RR (95% CI) | P value | RR (95% CI) | P value | |
| Cabergoline use | ||||
| No | 1 (ref) | - | 1 (ref) | - |
| Yes | 1.43 (0.84-2.42) | 0.189 | 0.98 (0.60-1.60)a | 0.922 |
| Cumulative cabergoline dose (per 100mg) | 1.02 (0.98-1.07) | 0.379 | 0.96 (0.81-1.14)b | 0.628 |
| Quartiles of cabergoline dose | ||||
| Quartile 1 (<27.0 mg) | 1 (ref) | - | 1 (ref) | - |
| Quartile 2 (27.1-56.1 mg) | 0.50 (0.09-2.71) | 0.424 | 0.45 (0.08-2.42)b | 0.350 |
| Quartile 3 (56.2-122.9 mg) | 1.25 (0.34-4.58) | 0.736 | 0.57 (0.15-2.12)b | 0.402 |
| Quartile 4 (>123.0 mg) | 1.76 (0.53-5.90) | 0.359 | 0.59 (0.16-2.15)b | 0.422 |
| Duration of cabergoline use (per month) | 1.00 (1.00-1.01) | 0.341 | 0.99 (0.98-1.00)b | 0.107 |
| Quartiles of duration of cabergoline use | ||||
| Quartile 1 (6-14.5 months) | 1 (ref) | - | 1 (ref) | - |
| Quartile 2 (14.6-26.6 months) | 0.80 (0.22-2.95) | 0.743 | 0.66 (0.19-2.31)b | 0.511 |
| Quartile 3 (26.7-46.4 months) | 0.60 (0.15-2.47) | 0.479 | 0.39 (0.10-1.55)b | 0.182 |
| Quartile 4 (>46.4) | 1.21 (0.38-3.88) | 0.752 | 0.27 (0.06-1.13)b | 0.072 |
a Adjusted for age, diabetes, Black or Black British ethnicity, gender, hypertension, Newham CCG
b Adjusted for age, diabetes, hypertension
Figure 2.
Relative risk of cardiac complications according to quartiles of cumulative cabergoline dose (points plotted at median cumulative dose within quartiles).
Figure 3.
Relative risk of cardiac complications according to quartiles of duration of cabergoline use (points plotted at median duration of cabergoline use within quartiles).
This matched analysis is based on a total of 12 endpoints in 57 (8.8%) cabergoline-treated patients and 52 endpoints in 234 (8.8%) cabergoline-nonexposed controls. 52 (8%) cabergoline users, accounting for 6 of the 52 had cardiac complications, were dropped from the analysis as they had no cabergoline-nonexposed matched controls. In the remainder, the absence of any cardiac endpoint for comparison meant that no analysis could be performed, and these subjects were therefore also excluded.
Echocardiogram image reanalysis
Using the permission granted by the de-anonymization application, we were able to review the echocardiograms of 5 of the 18 patients who had taken cabergoline and reached an endpoint. Two of these patients had 2 echocardiograms (Table 4) separated in time. Neither of the examining cardiologists, both experts in the assessment of carcinoid heart disease, felt that any of the echocardiograms demonstrated any evidence of drug-induced valve disease. The highest (consensus) valve score was 9, out of a total possible score of 66. The scoring of each individual examiner can be seen in Supplementary Appendix 2 (23).
Table 4.
Valve Scores, Re-Reported Echocardiogram Comments, Associated Cabergoline Dose/Duration Parameters and Cardiac Endpoints for 6 Patients Reaching Cardiac Endpoints Where Echocardiogram Images were Available
| Patient | Consensus valve score | Cumulative cabergoline at echo (mg) | Duration of cabergoline treatment at echo (days) | Time since first exposure to cabergoline (days) | Approx. age at echo (years) | Time on cabergoline at endpoint (days) | Mean weekly dose (mg) | Endpoint | G.L. comments | S.B. comments |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0 | 27 | 241 | 241 | 48 | 1123 | 1.28 | HF drug | Poor quality scan for right side heart valves | |
| 1 | 2 | 160 | 1123 | 1253 | 51 | 1123 | 1 | HF drug | ||
| 2 | 2 | 67 | 322 | 2766 | 35 | 322 | 0.69 | HF drug | ||
| 2 | 2 | 67 | 322 | 3708 | 37 | 322 | 0.69 | HF drug | ||
| 3 | 0 | 51.75 | 777 | 309 | 77 | 1019 | 2.14 | HF drug | Poor quality scan for right side heart valves | |
| 4 | 6 | 1490 | 3787 | 6871 | 63 | 3787 | 0.36 | Valve repair | TAVI/prosthesis present. PPM lead through TV | Possible PPM related TR |
| 5 | 9 | 266 | 5306 | 5782 | 66 | 5306 | 2.85 | GP Diagnosis of HF | Aortic valve does not look degenerative | Possible LV dysfunction and secondary MR |
Scores: Normal = 0, Mild = 1, Moderate = 2 & Severe = 3. Total possible score 66.
Abbreviations: HF drug, prescription for heart failure drug; MV, mitral valve regurgitation; PPM, permanent pacemaker; TAVI, transcatheter aortic valve implantation; TR, tricuspid valve regurgitation; TV, tricuspid valve.
Discussion
To our knowledge, this is the first study to use hard clinical endpoints of heart failure or valve repair/replacement to examine the association between low-dose cabergoline for hyperprolactinemia and cardiac valvulopathy. Our previous work in this area (26-28), using echocardiographic data from patients taking cabergoline for hyperprolactinemia, found no clinically significant association between its use and cardiac valvulopathy. Furthermore, a previous prospective study examining the use of cabergoline in patients with acromegaly, in which patients are predisposed to cardiac dysfunction, found no significant effect (29). In this current study, no difference in the rate of heart failure diagnosis or valve repair was found between the cabergoline-exposed and nonexposed groups, even after adjustment for age, diabetes mellitus, and hypertension. There was no correlation between either the cumulative dose of cabergoline or the length of time over which it was taken and the occurrence of prescription or diagnostic indicators of heart failure or need for valve repair (Table 2, Figs. 2 and 3).
Up to September 2020, 13 case-control studies (4-16) examining the association between fibrotic valvulopathy and low-dose cabergoline had been published, together with 3 meta-analyses (17, 18, 30). This body of evidence has sufficiently reassured professional bodies that 2 professional bodies (the British Society of Echocardiography and the UK Society for Endocrinology) issued a joint position statement that diverges substantially from the original (and unchanged) Medicines and Healthcare products Regulatory Agency (MHRA) guidelines (20). It has been estimated (31) that strict adherence to these guidelines would result in an extra 94 000 echocardiograms/year in the UK, at a cost of £5.45 million [based on NHS national tariffs https://improvement.nhs.uk/resources/national-tariff/]. The findings reported here, of no increased prevalence of heart failure (as judged by relevant prescription/diagnostic codes) or valve surgery above a matched background population at these doses of cabergoline over the stated time period, provide further and complementary evidence that the regulatory authority’s recommended screening program should be rationalized. The study cannot exclude the possibility of a small effect of cabergoline on cardiac valves over a longer period than that reported here.
Previous studies have used echocardiography to explore the clinical question of low-dose cabergoline and cardiac valvulopathy and, although there are case reports of low-dose cabergoline induced valvulopathy in the literature (32-36), very few (if any) cases of valvulopathy causing clinical symptoms are reported within case-control series (17-19). While this echocardiographic study approach has been useful in exploring any potential association between the use of low-dose cabergoline and cardiac valvulopathy, it also has major limitations. Many of these studies omit an analysis of the valve morphology (valve thickness, leaflet restriction, or calcification), which shows characteristic changes in dopamine agonist–induced valvulopathy and so the etiology of any valve defect, even if not clinically significant, may be wrongly ascribed to dopamine agonist use. Intra-operator variability also makes judgment about the severity of valvular regurgitation subjective. Furthermore, the potential for significant ascertainment bias has been highlighted; prior knowledge that a patient has taken dopamine agonist therapy may influence the opinion of even experienced sonographers as to whether a valve is thickened and/or regurgitant (21). Additionally, the use of nonstandard methods to estimate the severity of tricuspid regurgitation may lead to overdiagnosis of clinically significant valvulopathy (6). With this in mind, we attempted to reexamine the echocardiograms of those cabergoline-treated patients who had reached a study endpoint, to assess the likelihood that the underlying etiology was dopamine agonist treatment. Although only 5/18 of these patients had scans that were accessible to us (7 studies in all), the fact that neither of the highly experienced cardiologists in our group felt that any of the echocardiograms showed any significant evidence of drug-induced valve disease and no patient had any enlargement of the right ventricle or any derangement in right ventricular function, underscores the potential for erroneous association between abnormal echocardiographic flow and the use of cabergoline. The highest possible valve score on an extensively used carcinoid heart disease scoring system was 66; none of our 5 patients had a consensus score greater than 9. Individual scoring produced differences of up to 4 between examiners, highlighting intra-operator variability even in very experienced hands, but the total score from an individual examiner was never greater than 10. The advantage of the population statistics based around clinical endpoints, as opposed to echocardiographic findings, is that by highlighting findings significant enough to impact on clinical care they avoid the inherent biases and lack of pathological specificity associated with the imaging data.
Detailed evaluation of 2 patient echocardiograms separated in time was undertaken. The first patient had an echocardiogram after 241 and 1123 cumulative days of cabergoline treatment (corresponding to 241 and 1253 total days since first exposure and 27 and 160 mg of cabergoline, respectively). One cardiologist judged the earlier scan to show evidence of mild (ie, clinically nonsignificant) tricuspid regurgitation. On the subsequent scan, both cardiologists concurred that there was evidence of mild right-sided valve pathology. The second patient had 2 echocardiograms, both performed a considerable time after cessation of cabergoline therapy (2444 and 3386 days), a mean weekly dose of 0.7 mg over 322 days. The consensus valve score in this second patient for mild right-sided valve changes did not worsen between the 2 echocardiograms. Patient 3 had a consensus valve score of 0. Patient 4 took cabergoline for ~10 years (cumulative dose 1490 mg, mean weekly dose 0.36 mg) and had an echocardiogram ~8.5 years after cessation of cabergoline. By this point, there was a permanent cardiac pacemaker in situ, with a lead running through the tricuspid valve orifice, which may explain the observed moderate tricuspid regurgitation. The tricuspid valve was also felt to have mildly reduced mobility and moderate changes in morphology. Patient 5 had a consensus valve score of 9, largely due to left-sided valve changes, with moderate aortic and mitral thickening and moderately reduced mitral mobility. Any right-sided valve changes were graded as mild.
Our findings are similar to those of a 2012 Danish registry case-matched study examining valvulopathy in patients with a diagnosis of hyperprolactinemia (37). In this study, 19/2381 patients with hyperprolactinemia were diagnosed with valvular heart disease (vs 75/23 810 controls), although only 10/19 had received cabergoline. Within this group of 10, all had left-sided valve lesions and only 3 were symptomatic, 2 of whom had aortic stenosis and were aged 59 and 75 years. In keeping with the established literature, the majority of cases were reported not because of symptoms but through surveillance protocols, increasing the likelihood that the observed increase in valvulopathy was created by ascertainment bias. A further subgroup analysis of 2387 hyperprolactinemic patients in this same paper (37) showed no cardiac valve surgery in the cabergoline-treated patients, though the absolute numbers of cabergoline-treated patients in this study is not stated. Similarly, our study contained only 1 cabergoline-treated patient who underwent aortic valve surgery at the age of ~73.
Our study has obvious limitations. This was a retrospective analysis of a primary care database and is dependent on the accuracy with which the participating family physicians have entered the patient diagnoses and drug information. The cabergoline-exposed patients included in this study have a shorter cabergoline exposure and lower cumulative dose than those studied in previous case-control series (Table 1) (20). This may be because many of those studies were based on patients being treated in endocrine units whose clinicians, in the early aftermath of the published data on Parkinson disease, are likely to have selected patients within their clinical services who had been on cabergoline for long periods of time. Furthermore, academic endocrine units are likely to have a slightly misrepresentative proportion of patients with lactotrope macroadenomas within their cohorts (requiring more prolonged therapy) and of patients with tumors partially resistant to dopamine agonist (requiring higher doses). We calculated the cumulative doses of cabergoline taken by the patients using prescription data, which included a combination of start date, number of tablets, total number of tablets dispensed, dose, but not an end date. The end date (and therefore the treatment duration) was therefore derived from the above parameters. We cannot be certain that patients filled these prescriptions or indeed took the drugs as prescribed; however, a study comparing primary care prescription data with patient medication diaries demonstrated good correlation (38).
The mean age difference of 1 year between cabergoline-exposed and nonexposed patients, is highly unlikely to cause a significant difference in valvulopathy incidence. The incidence of clinically significant valvulopathy for any reason at the age of 41/42 years is very low (39). Moreover, this difference, along with those in the prevalence rates of diabetes mellitus and hypertension were accounted for in the multivariate analysis (Table 2).
In conclusion, this large cohort study of patients taking cabergoline for hyperprolactinemia does not support an increased prevalence of the cardiac endpoints of heart failure or valve repair above a matched nonexposed control population. These data support our previous proposals for a reduction in the frequency of echocardiographic surveillance in this patient group.
Acknowledgments
Financial Support: The UK National Institute of Health Research to Craig Edward Stiles and that the work was supported by a grant from the Clinical Endocrinology Trust
Glossary
Abbreviations
- CCG
clinical commissioning group
- DDS
Discovery Data Service
- IQR
interquartile range
- NHS
UK National Health Service
- RR
relative risk.
Additional Information
Disclosures: The authors have nothing to disclose.
Data Availability
Restrictions apply to the availability of some or all data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.
References
- 1. Schade R, Andersohn F, Suissa S, Haverkamp W, Garbe E. Dopamine agonists and the risk of cardiac-valve regurgitation. N Engl J Med. 2007;356(1):29-38. [DOI] [PubMed] [Google Scholar]
- 2. Zanettini R, Antonini A, Gatto G, Gentile R, Tesei S, Pezzoli G. Valvular heart disease and the use of dopamine agonists for Parkinson’s disease. N Engl J Med. 2007;356(1):39-46. [DOI] [PubMed] [Google Scholar]
- 3.Medicines and Healthcare products Regulatory Agency. Ergot-derived dopamine agonists: risk of fibrotic reactions - GOV.UK 2008.https://www.gov.uk/drug-safety-update/ergot-derived-dopamine-agonists-risk-of-fibrotic-reactions. Accessed January 9, 2018.
- 4. Bogazzi F, Buralli S, Manetti L, et al. Treatment with low doses of cabergoline is not associated with increased prevalence of cardiac valve regurgitation in patients with hyperprolactinaemia. Int J Clin Pract. 2008;62(12):1864-1869. [DOI] [PubMed] [Google Scholar]
- 5. Boguszewski CL, dos Santos CM, Sakamoto KS, Marini LC, de Souza AM, Azevedo M. A comparison of cabergoline and bromocriptine on the risk of valvular heart disease in patients with prolactinomas. Pituitary. 2012;15(1):44-49. [DOI] [PubMed] [Google Scholar]
- 6. Colao A, Galderisi M, Di Sarno A, et al. Increased prevalence of tricuspid regurgitation in patients with prolactinomas chronically treated with cabergoline. J Clin Endocrinol Metab. 2008;93(10):3777-3784. [DOI] [PubMed] [Google Scholar]
- 7. Cordoba-Soriano JG L-OC, Hidalgo-Olivares VM, Tercero-Martinez A, Barambio-Ruiz M, Salas-Nieto J. Valvular heart disease in hyperprolactinaemic patients treated with low doses of cabergoline. Rev Esp Cardiol. 2013;66(5):3. [DOI] [PubMed] [Google Scholar]
- 8. Elenkova A, Shabani R, Kalinov K, Zacharieva S. Increased prevalence of subclinical cardiac valve fibrosis in patients with prolactinomas on long-term bromocriptine and cabergoline treatment. Eur J Endocrinol. 2012;167(1):17-25. [DOI] [PubMed] [Google Scholar]
- 9. Halperin I, Aller J, Varela C, et al. No clinically significant valvular regurgitation in long-term cabergoline treatment for prolactinoma. Clin Endocrinol (Oxf). 2012;77(2): 275-280. [DOI] [PubMed] [Google Scholar]
- 10. Herring N, Szmigielski C, Becher H, Karavitaki N, Wass JA. Valvular heart disease and the use of cabergoline for the treatment of prolactinoma. Clin Endocrinol (Oxf). 2009;70(1):104-108. [DOI] [PubMed] [Google Scholar]
- 11. Kars M, Delgado V, Holman ER, et al. Aortic valve calcification and mild tricuspid regurgitation but no clinical heart disease after 8 years of dopamine agonist therapy for prolactinoma. J Clin Endocrinol Metab. 2008;93(9):3348-3356. [DOI] [PubMed] [Google Scholar]
- 12. Lancellotti P, Livadariu E, Markov M, et al. Cabergoline and the risk of valvular lesions in Endocrine disease. Eur J Endocrinol. 2008;159(1):1-5. [DOI] [PubMed] [Google Scholar]
- 13. Nachtigall LB, Valassi E, Lo J, et al. Gender effects on cardiac valvular function in hyperprolactinaemic patients receiving cabergoline: a retrospective study. Clin Endocrinol (Oxf). 2010;72(1):53-58. [DOI] [PubMed] [Google Scholar]
- 14. Tan T, Cabrita IZ, Hensman D, et al. Assessment of cardiac valve dysfunction in patients receiving cabergoline treatment for hyperprolactinaemia. Clin Endocrinol (Oxf). 2010;73(3):369-374. [DOI] [PubMed] [Google Scholar]
- 15. Vallette S, Serri K, Rivera J, et al. Long-term cabergoline therapy is not associated with valvular heart disease in patients with prolactinomas. Pituitary. 2009;12(3):153-157. [DOI] [PubMed] [Google Scholar]
- 16. Wakil A, Rigby AS, Clark AL, Kallvikbacka-Bennett A, Atkin SL. Low dose cabergoline for hyperprolactinaemia is not associated with clinically significant valvular heart disease. Eur J Endocrinol. 2008;159(4):R11-R14. [DOI] [PubMed] [Google Scholar]
- 17. Bogazzi F, Manetti L, Raffaelli V, Lombardi M, Rossi G, Martino E. Cabergoline therapy and the risk of cardiac valve regurgitation in patients with hyperprolactinemia: a meta-analysis from clinical studies. J Endocrinol Invest. 2008;31(12):1119-1123. [DOI] [PubMed] [Google Scholar]
- 18. De Vecchis R, Esposito C, Ariano C. Cabergoline use and risk of fibrosis and insufficiency of cardiac valves. Meta-analysis of observational studies. Herz. 2013;38(8):868-880. [DOI] [PubMed] [Google Scholar]
- 19. Stiles CE, Tetteh-Wayoe ET, Bestwick J, Steeds RP, Drake WM. A meta-analysis of the prevalence of cardiac valvulopathy in hyperprolactinemic patients treated with Cabergoline. J Clin Endocrinol Metab. 2019;104(2):15. [DOI] [PubMed] [Google Scholar]
- 20. Steeds RP, Stiles CE, Sharma V, Chambers JB, Lloyd G, Drake W. Echocardiography and monitoring patients receiving dopamine agonist therapy for hyperprolactinaemia: a joint position statement of the British Society of Echocardiography, the British Heart Valve Society and the Society for Endocrinology. Echo Res Pract. 2019;6(1):G1-G8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gu H, Luck S, Carroll PV, Powrie J, Chambers J. Cardiac valve disease and low-dose dopamine agonist therapy: an artefact of reporting bias? Clin Endocrinol (Oxf). 2011;74(5):608-610. [DOI] [PubMed] [Google Scholar]
- 22. Auriemma RS, Pivonello R, Perone Y, et al. Safety of long-term treatment with cabergoline on cardiac valve disease in patients with prolactinomas. Eur J Endocrinol. 2013;169(3):359-366. [DOI] [PubMed] [Google Scholar]
- 23. Stiles C. Appendix 1 discovery paper.docx. figshare. 2020. Posted November 13, 2020. 10.6084/m9.figshare.13235444.v1 [DOI]
- 24. Bhattacharyya S, Toumpanakis C, Caplin ME, Davar J. Usefulness of N-terminal pro-brain natriuretic peptide as a biomarker of the presence of carcinoid heart disease. Am J Cardiol. 2008;102(7):938-942. [DOI] [PubMed] [Google Scholar]
- 25. Fernandez A, Karavitaki N, Wass JA. Prevalence of pituitary adenomas: a community-based, cross-sectional study in Banbury (Oxfordshire, UK). Clin Endocrinol (Oxf). 2010;72(3):377-382. [DOI] [PubMed] [Google Scholar]
- 26. Drake WM, Stiles CE, Bevan JS, et al. ; UK Cabergoline valvulopathy study group . A follow-up study of the prevalence of valvular heart abnormalities in hyperprolactinemic patients treated with cabergoline. J Clin Endocrinol Metab. 2016;101(11):4189-4194. [DOI] [PubMed] [Google Scholar]
- 27. Drake WM, Stiles CE, Howlett TA, Toogood AA, Bevan JS, Steeds RP; UK Dopamine Agonist Valvulopathy Group . A cross-sectional study of the prevalence of cardiac valvular abnormalities in hyperprolactinemic patients treated with ergot-derived dopamine agonists. J Clin Endocrinol Metab. 2014;99(1):90-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stiles CE, Tetteh-Wayoe ET, Bestwick J, Steeds RP, Drake WM. A meta-analysis of the prevalence of cardiac valvulopathy in hyperprolactinemic patients treated with Cabergoline. J Clin Endocrinol Metab. 2018;104(2):523-538. [DOI] [PubMed] [Google Scholar]
- 29. Maione L, Garcia C, Bouchachi A, et al. No evidence of a detrimental effect of cabergoline therapy on cardiac valves in patients with acromegaly. J Clin Endocrinol Metab. 2012;97(9):E1714-E1719. [DOI] [PubMed] [Google Scholar]
- 30. Stiles CE, Steeds RP, Drake WM. Response to letter to the editor: “a meta-analysis of the prevalence of cardiac valvulopathy in patients with hyperprolactinemia treated with cabergoline”. J Clin Endocrinol Metab. 2019;104(10):4321-4322. [DOI] [PubMed] [Google Scholar]
- 31. Sherlock M, Toogood AA, Steeds R. Dopamine agonist therapy for hyperprolactinaemia and cardiac valve dysfunction; a lot done but much more to do. Heart. 2009;95(7):522-523. [DOI] [PubMed] [Google Scholar]
- 32. Caputo C, Prior D, Inder WJ. The need for annual echocardiography to detect cabergoline-associated valvulopathy in patients with prolactinoma: a systematic review and additional clinical data. Lancet Diabetes Endocrinol. 2015;3(11):906-913. [DOI] [PubMed] [Google Scholar]
- 33. Cawood TJ, Bridgman P, Hunter L, Cole D. Low-dose cabergoline causing valvular heart disease in a patient treated for prolactinoma. Intern Med J. 2009;39(4):266-267. [DOI] [PubMed] [Google Scholar]
- 34. D’Aloia A, Piovanelli B, Rovetta R, et al. A case of iatrogenic severe mitral regurgitation. Monaldi Arch Chest Dis. 2013;80(3):133-136. [DOI] [PubMed] [Google Scholar]
- 35. Izgi C, Feray H, Cevik C, Saltan Y, Mansuroglu D, Nugent K. Severe tricuspid regurgitation in a patient receiving low-dose cabergoline for the treatment of acromegaly. J Heart Valve Dis. 2010;19(6):797-800. [PubMed] [Google Scholar]
- 36. Moore S, Nana M, Dixon A. Severe aortic regurgitation associated with low cumulative dose cabergoline in prolactinoma: a case report. Endocrine Abstracts. 2019;65:CC6. doi:10.1530/endoabs.65.CC6 [Google Scholar]
- 37. Steffensen C, Maegbaek ML, Laurberg P, et al. Heart valve disease among patients with hyperprolactinemia: a nationwide population-based cohort study. J Clin Endocrinol Metab. 2012;97(5):1629-1634. [DOI] [PubMed] [Google Scholar]
- 38. Joseph RM, van Staa TP, Lunt M, Abrahamowicz M, Dixon WG. Exposure measurement error when assessing current glucocorticoid use using UK primary care electronic prescription data. Pharmacoepidemiol Drug Saf. 2019;28(2):179-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Singh JP, Evans JC, Levy D, et al. Prevalence and clinical determinants of mitral, tricuspid, and aortic regurgitation (the Framingham Heart Study). Am J Cardiol. 1999;83(6): 897-902. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Restrictions apply to the availability of some or all data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.