Abstract
Context
Growth hormone (GH) and IGF-1 help regulate hepatic glucose and lipid metabolism, and reductions in these hormones may contribute to development of nonalcoholic fatty liver disease (NAFLD).
Objective
To assess relationships between hepatic expression of IGF1 and IGF-binding proteins (IGFBPs) and measures of glycemia and liver disease in adults with NAFLD. Secondarily to assess effects of GH-releasing hormone (GHRH) on circulating IGFBPs.
Design
Analysis of data from a randomized clinical trial of GHRH.
Setting
Two US academic medical centers.
Participants
Participants were 61 men and women 18 to 70 years of age with HIV-infection, ≥5% hepatic fat fraction, including 39 with RNA-Seq data from liver biopsy.
Main Outcome Measures
Hepatic steatosis, inflammation, and fibrosis by histopathology and measures of glucose homeostasis.
Results
Hepatic IGF1 mRNA was significantly lower in individuals with higher steatosis and NAFLD Activity Score (NAS) and was inversely related to glucose parameters, independent of circulating IGF-1. Among the IGFBPs, IGFBP2 and IGFBP4 were lower and IGFBP6 and IGFBP7 (also known as IGFBP-related protein 1) were higher with increasing steatosis. Hepatic IGFBP6 and IGFBP7 mRNA levels were positively associated with NAS. IGFBP7 mRNA increased with increasing fibrosis. Hepatic IGFBP1 mRNA was inversely associated with glycemia and insulin resistance, with opposite relationships present for IGFBP3 and IGFBP7. GHRH increased circulating IGFBP-1 and IGFBP-3, but decreased IGFBP-2 and IGFBP-6.
Conclusions
These data demonstrate novel relationships of IGF-1 and IGFBPs with NAFLD severity and glucose control, with divergent roles seen for different IGFBPs. Moreover, the data provide new information on the complex effects of GHRH on IGFBPs.
Keywords: Nonalcoholic fatty liver disease (NAFLD), nonalcoholic steatohepatitis (NASH), insulin-like growth factor-1, insulin-like growth factor–binding proteins, human immunodeficiency virus, glucose
Insulin-like growth factor-1 (IGF-1) and IGF-binding proteins (IGFBPs) are produced in multiple tissues, including the liver, primarily in response to growth hormone (GH) signaling. Hepatic synthesis is responsible for the bulk of systemic circulating IGF-1 and IGFBPs (1). IGF-1 has multiple paracrine and endocrine actions, including growth-promoting effects, hypoglycemic effects at the insulin receptor, and negative feedback at the level of the pituitary to suppress GH secretion. IGFBPs bind IGF-1 and IGF-2 to regulate their action but also have independent biological actions (1). IGFBPs have higher affinity for IGFs than does the IGF-1 receptor, such that the IGFBPs not only prolong the half-life of IGF-1 in circulation but also modulate availability of IGF-1 to exert biological actions in various tissues (2).
GH and IGF-1 are important regulators of hepatic glucose and lipid metabolism, and increasing evidence suggests that reductions in GH and IGF-1 increase the risk of developing nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH). Due to the tight coupling of GH and IGF-1 levels in normal physiologic states, however, the distinct metabolic actions of GH versus IGF-1 have been difficult to tease apart. Animal models suggest that both GH and IGF-1 have anti-inflammatory and anti-fibrotic effects (3-5), whereas GH itself appears critical to prevent hepatic lipid accumulation, in large part through inhibition of de novo lipogenesis (6-8). GH and IGF-1 have divergent effects on glycemia, with GH acting as a counterregulatory hormone and IGF-1 exerting hypoglycemic effects, achieved through a high degree of homology between IGF-1 and insulin, as well as the insulin receptor and the IGF receptors. In NAFLD, the hypoglycemic effects of IGF-1 are likely to be particularly relevant, as IGF-1 sensitivity remains in spite of hepatic insulin resistance (9).
IGFBPs are only recently being explored for possible mechanistic effects and/or as key markers in NAFLD, with potential effects independent of IGF-1. To date, however, little is known regarding these effects. Whereas a recent analysis suggests that circulating levels of all the IGFBPs rise in later stages of fibrosis (10), other reports suggest that NAFLD is associated with lower IGFBP-1 (11, 12), IGFBP-2 (13, 14), and IGFBP-3 (15). In addition to IGFBPs 1 through 6, which have high affinity for binding IGFs, a member of the lower-affinity group of IGFBP-related proteins, called IGFBPrP1 or IGFBP-7, has recently emerged as a marker of insulin resistance (16) and NAFLD (17).
The current analysis leverages data from a randomized clinical trial of the growth hormone–releasing hormone (GHRH) analogue tesamorelin in men and women living with human immunodeficiency virus (HIV) and NAFLD for whom liver biopsy and detailed metabolic phenotyping were performed (18), showing novel effects to reduce liver fat and prevent fibrosis in a population with high prevalence of NAFLD. Using hepatic RNA-Seq data on expression levels of IGF1 and IGFBP1-IGFBP7 in conjunction with histopathological examination of liver biopsy samples and serum measures of glucose homeostasis, we investigate the hypothesis that hepatic expression of IGF1 will be lower in individuals with more advanced features of NAFLD, and that higher IGF1 expression will be associated with lower glucose and higher hepatic insulin sensitivity. Furthermore, we explore the relationships between hepatic IGFBP1-IGFBP7 expression and measures of glycemia and fatty liver disease, finding divergent associations among different IGFBPs and critical indices of NAFLD and glucose homeostasis. Finally, using data from proteomics, we report the changes in circulating IGFBP-1, -2, -3, -6, and -7 with tesamorelin treatment, considering how those alterations may variably affect glucose homeostasis during treatment.
Methods
These data are from a randomized, double-blind, placebo-controlled trial in which individuals with HIV-infection and NAFLD were assigned to receive the GHRH analogue tesamorelin 2 mg daily or identical placebo for 12 months (18). Liver biopsy was performed before and after 12 months of treatment, and the present analysis includes data on hepatic mRNA expression of IGF1 and IGFBPs, and circulating levels of IGFBPs, not previously analyzed or reported. Throughout the manuscript, “IGF1” and “IGFBP1”to“IGFBP7” refer to hepatic mRNA levels, whereas “IGF-1” and “IGFBP-1”-“IGFBP-7” refer to protein levels in systemic circulation.
The trial included 61 men and women 18 to 70 years of age with HIV-infection and hepatic steatosis as evidenced by hepatic fat fraction ≥5% on 1H-magnetic resonance spectroscopy (1H-MRS). Eligibility criteria included stable antiretroviral regimen for ≥3 months, CD4+ T cell count >100 cells/mm3, HIV viral load <400 copies/mL, no excessive alcohol use (use <20 g daily for women or <30 g daily for men), no active hepatitis B, hepatitis C, cirrhosis, or other known hepatic disease, and no uncontrolled diabetes mellitus, defined by HbA1c ≥7% (18). Participants were excluded for use of insulin, thiazolidinediones, or systemic glucocorticoids, and use of other antidiabetic medications must have been stable for ≥6 months prior to study entry. Participants were enrolled at the Massachusetts General Hospital (MGH, Boston, MA) and the National Institutes of Health (NIH, Bethesda, MD) between August 20, 2015 and January 16, 2019. Written informed consent was obtained from all participants, and the study was approved by the institutional review boards at both institutions.
Study procedures
Study procedures conducted at baseline and after 12 months of treatment included the following, all of which were performed in the fasting state: hepatic 1H-MRS for measurement of hepatic fat fraction; abdominal magnetic resonance imaging for measurement of cross-sectional visceral adipose tissue and subcutaneous adipose tissue area at the level of the L4 vertebra; whole-body dual-energy x-ray absorptiometry (DXA) scan for regional quantification of lean and fat mass; ultrasound-guided percutaneous liver biopsy yielding 2 cores, the first of which was used for histopathologic review by a single expert pathologist blinded to treatment (D.E.K., National Institutes of Health), and the second of which was placed in an RNA stabilization reagent (RNAlater, Qiagen) and stored at −80 oC for gene expression analysis; laboratory evaluation including transaminases, glucose, insulin, lipids, IGF-1; and standard 75-gram oral glucose tolerance test with measurement of glucose 120 minutes following glucose load. Participants at MGH also underwent a 2-step euglycemic hyperinsulinemic clamp procedure as follows: after a 14-hour overnight fast, a low-dose clamp with insulin infusion of 20 mU/m2/min was performed for 120 minutes and then followed by a high-dose clamp with insulin infusion of 80 mU/m2/min for 120 minutes as previously described (19). The final 20-minute period of each clamp was used to calculate insulin-stimulated glucose disposal (M) using the DeFronzo method (20). Twenty-eight individuals at MGH had clamp data available. Clinical labs, including measures of glucose and insulin as well as transaminases, were measured at the National Institutes of Health Clinical Laboratory and, for MGH, at LabCorp and Quest.
Histopathological scoring of liver biopsies used the Nonalcoholic Steatohepatitis Clinical Research Network (NAS CRN) scoring system (21), in which scores for steatosis (grades 0-3), hepatocellular ballooning (grades 0-2), and lobular inflammation (grades 0-3), are added to calculate the NAS score. Fibrosis is independently staged as stages 0 to 4 (21). The presence or absence of NASH was also determined by histological review (D.E.K.).
Serum IGF-1 was measured centrally at Quest Laboratories using liquid chromatography/mass spectrometry. Circulating levels of IGFBP-1, IGFBP-2, IGFBP-3, IGFBP-6, and IGFBP-7 were measured through proteomics analysis. Analyses were performed using Proximity Extension Assay (PEA) technology (Olink Proteomics, Watertown, MA https://www.olink.com/resources-support/document-download-center/) (22, 23). Using oligonucleotide-labeled antibody probe pairs, PEA permits simultaneous assessment of multiple proteins maintaining the precision of dual-epitope ELISA without the loss of specificity of earlier generation multiplex assays. Signal generation requires both recognition of DNA-barcoding from sequence-specific oligonucleotides and dual recognition of correctly matched antibody pairs. The PEA assay reports fold change in log 2 (NPX) units.
From the initial study cohort of 61 participants, 39 participants (18 randomized to tesamorelin and 21 randomized to placebo) had adequate liver specimens for RNA-Seq and had high-quality, reliable data. These 39 individuals with IGF1 and IGFBP mRNA expression data are included in the cross-sectional analyses. Circulating levels of IGF-1 and (from proteomics analysis) the IGFBPs, were available for 61 individuals (30 placebo and 31 tesamorelin) at baseline and 46 individuals (25 placebo and 21 tesamorelin) at 12 months. This sample was used for the description of change in circulating IGFBPs following tesamorelin treatment compared with placebo.
cDNA library construction and RNA sequencing
Analyses were performed at the Broad Institute using their standardized methodology for RNA-Seq. Following extraction from liver tissue using RNeasy Plus Mini Kit (Qiagen), total RNA was quantified using the Quant-iT RiboGreen RNA Assay Kit and normalized to 5 ng/μl. Following plating, 2 μL of ERCC controls (using a 1:1000 dilution) and a k562 control were spiked into each sample. A 200-ng aliquot of each sample was taken for library preparation, using Illumina TruSeq Stranded mRNA Sample Preparation Kit. Oligo dT beads were used to select mRNA from the total RNA sample, followed by heat fragmentation and cDNA synthesis from the RNA template. The resultant 400-bp cDNA underwent dual-indexed library preparation: “A” base addition, adapter ligation using P7 adapters, and PCR enrichment using P5 adapters. After enrichment, the libraries were quantified using Quant-iT PicoGreen (1:200 dilution). Pooled libraries were normalized to 2 nM and denatured using 0.1 N NaOH prior to sequencing. Flowcell cluster amplification and sequencing were performed according to the manufacturer’s protocols using the NovaSeq S2 to produce 101-bp paired-end reads with 8-base index barcodes. Data were analyzed using the Broad Picard Pipeline, which includes de-multiplexing and data aggregation. All samples were analyzed using the bcbio-nextgen RNA-seq analysis pipeline (https://bcbio-nextgen.readthedocs.org/en/latest/). BAM files (converted back to fastq read files) were examined for quality issues using FastQC (http://www.bioinformatics.babraham.ac.uk/projects/fastqc/) to ensure library generation and sequencing were suitable for further analysis. Reads were aligned to University of California, Santa Cruz (UCSC) build hg38 of the human genome (Homo sapiens), augmented with transcript information from Ensembl release 94 using hisat2 (24). Alignments were analyzed for evenness of coverage, rRNA content, genomic context (for example, alignments in known transcripts and introns), complexity, and other quality checks using a combination of FastQC, Qualimap, (25) MultiQC (https://github.com/ewels/MultiQC), and custom tools. Counts of reads aligning to known genes were generated by featureCounts (26) and used as input for principal component analysis and hierarchical clustering to identify possible outliers.
Statistical analyses
Associations between continuous variables were assessed using Pearson correlation. Associations between categorical variables and continuous variables were assessed using the Student t test for 2-level categorical variables or analysis of variance (ANOVA) for categorical variables with >2 levels. For the latter, test for linear trend using one-way ANOVA was also performed for comparisons of steatosis grade and fibrosis stage. To adjust for covariates, analysis of covariance (ANCOVA) was applied. For analysis of the change in circulating IGFBPs in tesamorelin versus placebo after 12 months, all available data were used in random intercept mixed effects modeling for continuous repeated measures using restricted maximum likelihood to assess the effect estimate for the time × randomization interaction. All statistical testing was 2-tailed. SAS 9.4, JMP 15 (SAS Institute), and Graphpad Prism 8.4.3 were used for all analyses. As this is an exploratory analysis of correlated endpoints, we did not adjust for multiple comparisons so as not to increase the probability of type II error (27).
Results
Baseline clinical characteristics of the cohort for which hepatic gene expression is available (N = 39) as well as the entire cohort (N = 61), for which changes in circulating IGFBPs with tesamorelin are available, are shown in Table 1. The cohort was predominantly male, aged 53 ± 7 years at baseline, with long-standing and well-treated HIV-infection. A small percentage of individuals had mild type 2 diabetes controlled with diet and/or metformin.
Table 1.
Baseline Clinical Characteristics
| Hepatic gene expression (N = 39) | Circulating IGFBP analysis (N = 61) | |
|---|---|---|
| Age (years) | 53 ± 7 | 53 ± 7 |
| % Male | 77 | 79 |
| % Race | ||
| White | 59 | 66 |
| Black | 36 | 30 |
| Other | 5 | 4 |
| % Hispanic | 8 | 15 |
| Duration of HIV-infection (years) | 17 ± 9 | 17 ± 9 |
| CD4 count (cells/mm3) | 758 ± 268 | 765 ± 276 |
| Viral load < 20 copies, mL (%) | 92 | 90 |
| % Current antiretroviral use | ||
| NRTI | 92 | 92 |
| PI | 26 | 25 |
| NNRTI | 36 | 38 |
| Integrase inhibitor | 64 | 64 |
| % Type 2 diabetes | 8 | 13 |
| Body mass index (kg/m2) | 32 ± 6 | 31 ± 6 |
| Hepatic fat fraction (%) | 14 ± 9 | 14 ± 8 |
| Alanine aminotransferase (U/L) | 31 ± 32 | 32 ± 29 |
| Aspartate aminotransferase (U/L) | 31 ± 24 | 32 ± 22 |
| % NASH | 31 | 33 |
| % Fibrosis | 41 | 40 |
| Stage 1 | 15 | 11 |
| Stage 2 | 15 | 18 |
| Stage 3 | 10 | 11 |
| Fasting plasma glucose (mg/dL) | 92 ± 12 | 96 ± 18 |
| Fasting insulin (μU/mL) | 15 ± 17 | 15 ± 15 |
| Hemoglobin A1c (%) | 5.7 ± 0.5 | 5.7 ± 0.5 |
Continuous variables are presented as mean ± standard deviation. Abbreviations: IGFBP, insulin-like growth factor–binding protein; NASH, nonalcoholic steatohepatitis; NNRTI, non-nucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; PI, protease inhibitor.
Hepatic IGF1 expression and measures of liver disease
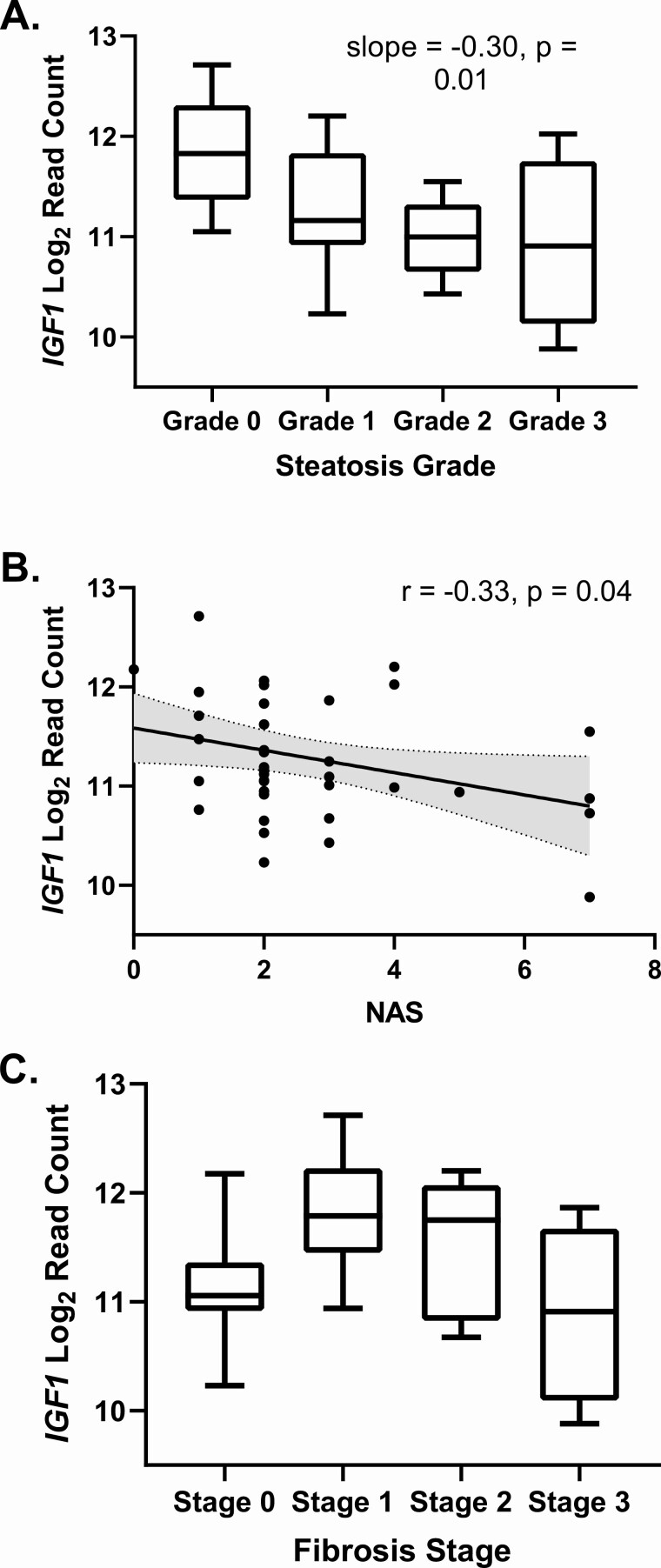
As shown in Table 2 and Fig. 1, hepatic IGF1 expression was significantly lower in individuals with higher grades of steatosis and higher NAS scores. There was a quadratic relationship between IGF1 expression and fibrosis stage, as shown in Fig. 1 C, with rising IGF1 expression at stages 1 and 2 and decreased expression at stage 3 (R2 for quadratic model 0.22, P = 0.01). These associations persisted with adjustment for sex and age. Although serum IGF-1 levels and hepatic IGF1 mRNA were positively correlated as expected (r = 0.35, P = 0.03), there were not significant relationships between serum IGF-1 and steatosis grade (P = 0.94), NAS (P = 0.86), or fibrosis stage (P = 0.67), and adjustment for serum IGF-1 did not attenuate the relationships between hepatic IGF1 mRNA and steatosis grade or NAS. As shown in Table 2, hepatic IGF1 expression was lower in individuals with higher body mass index (BMI) (r = −0.32, P = 0.05), whereas this was not true for serum IGF-1 level (r = 0.16, P = 0.33). Adjustment for BMI attenuated the relationship between hepatic IGF1 expression and steatosis grade (P = 0.06), NAS (P = 0.16), and the quadratic relationship with fibrosis stage.
Table 2.
Associations Between Hepatic IGF-1 and IGF-Binding Protein mRNA and Baseline Hepatic and Glycemic Measures and Body Composition
| IGF1 | IGFBP1 | IGFBP2 | IGFBP3 | IGFBP4 | IGFBP5 | IGFBP6 | IGFBP7 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| r | P | r | P | r | P | r | P | r | P | r | P | r | P | r | P | |
| Hepatic endpoints | ||||||||||||||||
| Steatosis grade | −0.30 | 0.01 | −0.47 | 0.08 | −0.49 | 0.01 | 0.09 | 0.07 | −0.12 | 0.01 | 0.04 | 0.59 | 0.25 | 0.004 | 0.35 | <0.0001 |
| NAFLD Activity Score | −0.33 | 0.04 | −0.16 | 0.33 | −0.23 | 0.16 | 0.14 | 0.39 | −0.25 | 0.12 | −0.05 | 0.76 | 0.47 | 0.003 | 0.83 | <0.0001 |
| Fibrosis stage | 0.04b | 0.66b | 0.28 | 0.19 | 0.23 | 0.19 | −0.04 | 0.30 | 0.04 | 0.27 | −0.03 | 0.61 | 0.15 | 0.03 | 0.25 | <0.0001 |
| ALT (U/L) | −0.38 | 0.02 | −0.13 | 0.45 | −0.12 | 0.46 | 0.12 | 0.46 | −0.15 | 0.35 | −0.13 | 0.42 | 0.34 | 0.03 | 0.60 | <0.0001 |
| AST (U/L) | −0.38 | 0.02 | −0.05 | 0.76 | −0.04 | 0.83 | 0.05 | 0.78 | −0.20 | 0.23 | −0.18 | 0.28 | 0.33 | 0.04 | 0.64 | <0.0001 |
| Glycemic endpoints | ||||||||||||||||
| Fasting Glucose (mg/dL) | −0.44 | 0.005 | −0.32 | 0.05 | −0.31 | 0.06 | 0.41 | 0.01 | −0.28 | 0.08 | 0.21 | 0.20 | 0.28 | 0.09 | 0.40 | 0.01 |
| Fasting Insulin (uIU/mL) | −0.46 | 0.005 | −0.16 | 0.35 | −0.20 | 0.24 | 0.28 | 0.10 | −0.22 | 0.19 | −0.02 | 0.89 | 0.27 | 0.11 | 0.64 | <0.0001 |
| Low-dose M (mg/kg/min)a | 0.34 | 0.08 | 0.46 | 0.02 | 0.36 | 0.07 | −0.37 | 0.06 | 0.50 | 0.007 | 0.09 | 0.65 | −0.19 | 0.34 | −0.10 | 0.63 |
| Two-hour glucose after 75g glucose load (mg/d) | −0.34 | 0.03 | −0.40 | 0.01 | −0.22 | 0.17 | 0.30 | 0.06 | −0.17 | 0.31 | 0.20 | 0.21 | 0.25 | 0.13 | 0.25 | 0.12 |
| High-dose M (mg/kg/min)* | 0.28 | 0.15 | 0.45 | 0.02 | 0.27 | 0.16 | −0.30 | 0.12 | 0.38 | 0.04 | 0.17 | 0.38 | −0.24 | 0.21 | −0.18 | 0.37 |
| Body composition | ||||||||||||||||
| Body mass index (kg/m2) | −0.32 | 0.05 | −0.51 | 0.001 | −0.59 | <0.0001 | 0.48 | 0.002 | −0.48 | 0.002 | −0.13 | 0.45 | 0.12 | 0.48 | 0.26 | 0.11 |
| Visceral adipose tissue area (cm2) | −0.19 | 0.25 | −0.25 | 0.13 | −0.06 | 0.72 | 0.03 | 0.85 | −0.16 | 0.33 | −0.00 | 0.99 | 0.02 | 0.90 | 0.37 | 0.02 |
| Subcutaneous adipose tissue area (cm2) | −0.20 | 0.24 | −0.37 | 0.02 | −0.46 | 0.004 | 0.34 | 0.04 | −0.32 | 0.05 | −0.22 | 0.19 | 0.08 | 0.63 | 0.06 | 0.72 |
For steatosis grade and fibrosis stage, r is slope in a 1-way ANOVA test for trend (from lowest to highest grade or stage) and P value is the P value for test for trend. For all other variables, r is Pearson’s correlation coefficient with corresponding P value.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; IGF1, insulin-like growth factor-1; IGFBP, insulin-like growth factor–binding protein; M, glucose disposal.
a Clamp data available for 28 subjects who participated at MGH.
b Please refer to text for quadratic relationship between IGF1 mRNA and fibrosis stage.
Figure 1.
Associations between hepatic IGF-1 expression and measures of NAFLD severity: A, steatosis grade; B, NAFLD Activity Score; and C, fibrosis stage. Slope from 1-way ANOVA test for linear trend, and r from Pearson correlation.
Expression of IGFBPs and measures of nonalcoholic fatty liver disease
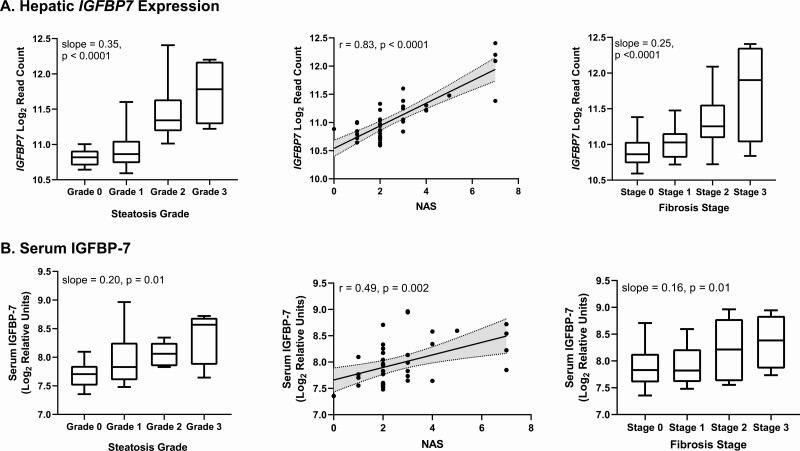
Relationships between hepatic expression of IGFBPs and measures of NAFLD are shown in Table 2. There were inverse relationships between IGFBP2 and IGFBP4 expression and steatosis grade. IGFBP1 to IGFBP5 were not otherwise strongly associated with any measures of NAFLD in our dataset. In contrast, expression of IGFBP6 and IGFBP7 were strongly positively associated with steatosis grade, NAS, fibrosis stage, and alanine aminotransferase (ALT). These associations remained significant after adjustment for BMI. Circulating levels of IGFBP-6 were not associated with hepatic expression of IGFBP6, nor were they associated with any liver endpoints. Circulating levels of IGFBP-7 were strongly associated with hepatic IGFBP7 expression (r = 0.52, P = 0.0007) as well as steatosis grade, NAS, and fibrosis stage (Fig. 2), although associations between circulating IGFBP-7 and liver measures were generally weaker than those with IGFBP7 expression. These associations also persisted after adjustment for BMI.
Figure 2.
Associations between A, hepatic IGFBP7 expression and B, circulating IGFBP-7, and steatosis grade, NAFLD Activity Score, and fibrosis stage. Slopes from 1-way ANOVA test for linear trend, and r values from Pearson correlation.
With regard to grades for hepatocellular ballooning and lobular inflammation, the other 2 components of the NAS, hepatic expression of IGFBP6 was positively associated with lobular inflammation (r = 0.39, P = 0.01) and hepatocellular ballooning (r = 0.32, P = 0.047). Hepatic expression of IGFBP7 was also positively associated with lobular inflammation (r = 0.59, P < 0.0001) and hepatocellular ballooning (r = 0.79, P < 0.0001). Circulating IGFBP-7 was significantly positively associated with hepatocellular ballooning (r = 0.56, P = 0.0002) but not lobular inflammation (r = 0.23, P = 0.16).
Hepatic IGF1 expression and glucose homeostasis
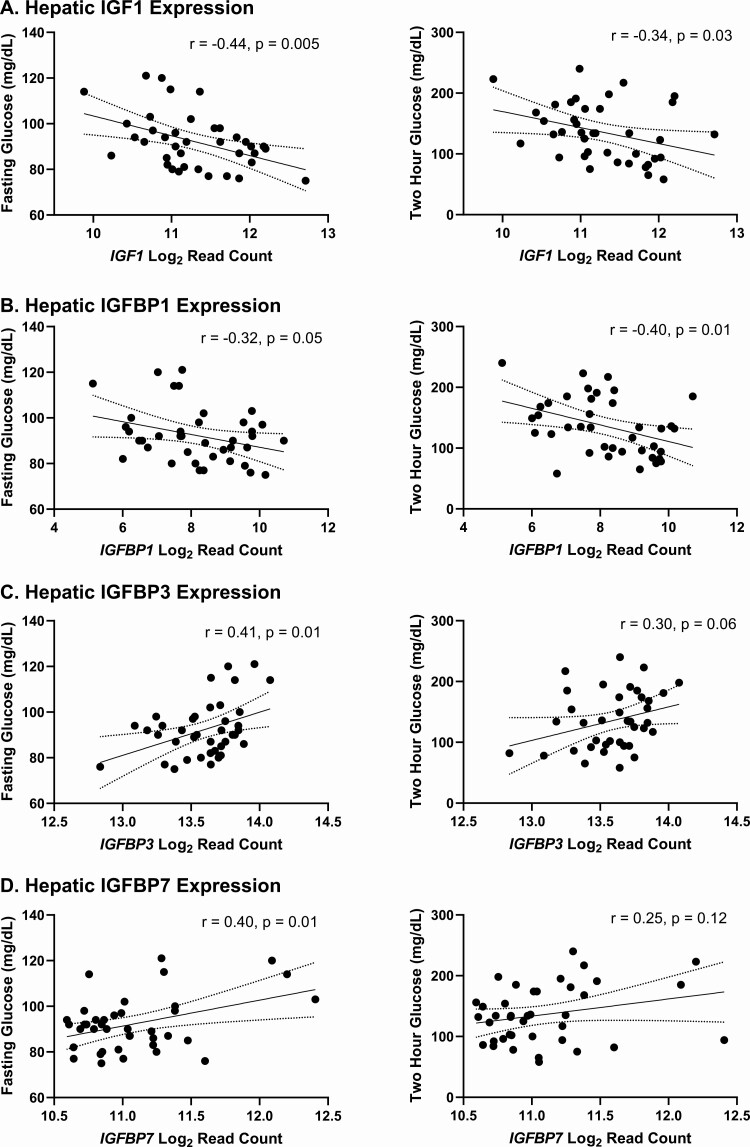
Higher hepatic IGF1 expression was strongly associated with lower levels of fasting glucose, fasting insulin, and 2-hour glucose following a 75-g oral glucose tolerance test, as shown in Table 2 and Fig. 3. By contrast, serum IGF-1 levels were not associated with fasting (r = 0.01, P = 0.93) or 2-hour glucose (r = −0.27, P = 0.10), fasting insulin (r = 0.06, P = 0.71), or insulin-stimulated glucose uptake during low-dose (r = 0.23, P = 0.24) or high-dose clamp (r = 0.07, P = 0.73). Adjustment for serum IGF-1 did not affect the relationships between hepatic IGF1 expression and fasting glucose (P = 0.002) or fasting insulin (P = 0.002), although it did attenuate the relationship between IGF1 expression and 2-hour glucose (P = 0.10). Similarly, adjustment for BMI did not substantially alter relationships between IGF1 expression and fasting glucose (P = 0.02) or fasting insulin (P = 0.02) but did attenuate the relationship between IGF1 expression and 2-hour glucose (P = 0.06).
Figure 3.
Associations between A, hepatic IGF1; B, IGFBP1; C, IGFBP3; and D, IGFBP7 expression and fasting glucose (left) and 2-hour glucose (right). r from Pearson correlation.
Expression of IGFBPs and glucose homeostasis
Relationships between hepatic IGFBP expression and measures of glucose homeostasis are shown in Table 2. Hepatic expression of IGFBP1 was negatively associated with fasting and 2-hour glucose (Fig. 3) and positively associated with insulin-stimulated glucose uptake during both low- and high-dose clamp. Following adjustment for BMI, the association between IGFBP1 mRNA and 2-hour glucose remained (P = 0.02), whereas associations with fasting glucose (P = 0.17) and insulin-stimulated glucose uptake during both low-dose (P = 0.33) and high-dose clamp (P = 0.14) were attenuated. Circulating IGFBP-1 was correlated with hepatic IGFBP1 mRNA (r = 0.31, P = 0.05) and was negatively associated with fasting glucose (r = −0.40, P = 0.01) and fasting insulin (r = −0.35, P = 0.04), with only the former association persisting after adjustment for BMI (P = 0.03).
By contrast, IGFBP3 expression was positively associated with fasting glucose (Fig. 3), persisting after adjustment for BMI (P = 0.04), and showed weaker associations with other measures of insulin resistance as well. Circulating IGFBP-3 was not correlated with hepatic IGFBP3 expression in our dataset, nor was it associated with any measures of glycemia. IGFBP7 expression was also positively associated with fasting glucose (Fig. 3) and measures of insulin resistance, although only the associations with fasting glucose (P = 0.03) and fasting insulin (P < 0.0001) persisted following adjustment for BMI. Circulating IGFBP-7 levels were also positively associated with fasting glucose (r = 0.43, P = 0.007), fasting insulin (r = 0.40, P = 0.02), and 2-hour glucose (r = 0.41, P = 0.009), all of which persisted after adjusting for BMI.
Expression of IGFBPs and adiposity
Hepatic expression of IGF1 and multiple IGFBPs were associated with BMI, as shown in Table 2. With the exception of IGFBP7, none were associated with visceral adipose tissue area, whereas many did show associations with abdominal subcutaneous adipose tissue area.
Change in circulating IGFBPs with tesamorelin
Changes in circulating levels of IGFBP-1, IGFBP-2, IGFBP-3, IGFBP-6, and IGFBP-7 following treatment with tesamorelin versus placebo are shown in Table 3. Circulating IGFBP-1 and IGFBP-3 increased with tesamorelin, whereas IGFBP-2 and IGFBP-6 were significantly reduced, and there was no effect on circulating IGFBP-7. As expected and previously reported, serum levels of IGF-1 increased significantly with tesamorelin treatment (18). There were associations between changes in serum IGF-1 and changes in plasma IGFBP-1 (r = 0.31, P = 0.04), IGFBP-2 (r = -0.35, P = 0.02), and IGFBP-3 (r = 0.35, P = 0.02). There were not significant associations between changes in serum IGF-1 and changes in plasma IGFBP-6 (r = −0.23, P = 0.13) or IGFBP-7 (r = −0.02, P = 0.92). Serum levels of acid labile subunit are not available; hepatic mRNA of acid labile subunit was increased by tesamorelin (0.3 ± 1.0 versus −0.4 ± 1.0 log2 fold change, tesamorelin versus placebo, 95% confidence interval [0.03, 1.3], P = 0.04).
Table 3.
Changes in Circulating IGFBP With Tesamorelin Treatment
| Protein | Effect size (log2 fold change) | 95% CI of effect size | P value |
|---|---|---|---|
| IGFBP-1 | 0.64 | (0.18, 1.11) | 0.01 |
| IGFBP-2 | −0.46 | (−0.71, −0.21) | 0.0007 |
| IGFBP-3 | 0.24 | (0.01, 0.47) | 0.05 |
| IGFBP-6 | −0.23 | (−0.44, −0.02) | 0.04 |
| IGFBP-7 | 0.00 | (−0.23, 0.24) | 0.98 |
Effect size and P value for the time × randomization interaction in mixed effects modeling for continuous repeated measures in the entire study cohort (N = 61).
Among the entire cohort over 12 months, reductions in circulating IGFBP-2 seen with tesamorelin were associated with reductions in NAS score (r = 0.35, P = 0.02) and hepatocellular ballooning grade (r = 0.37, P = 0.02) but not changes in steatosis grade (r = 0.17, P = 0.28) or lobular inflammation grade (r = 0.17, P = 0.28). Over the course of the study, among the entire cohort, reductions in NAS score (r = 0.34, P = 0.03) and steatosis grade (r = 0.43, P = 0.005) were associated with changes in circulating IGFBP-7, consistent with baseline relationships. These longitudinal associations between circulating IGFBP-7 and liver indices were independent of tesamorelin treatment. There were not significant associations between changes in circulating IGFBP-1, IGFBP-3, or IGFBP-6 and changes in NAS or steatosis grade.
Discussion
Our data support and further elucidate roles of IGF-1 and certain IGFBPs in the regulation of glucose homeostasis and the pathophysiology of NAFLD. Most notably, hepatic IGF1 mRNA is strongly negatively associated with both features of NAFLD and measures of insulin resistance, whereas IGFBP7 (IGFBPrP1) is strongly positively associated with these measures. These findings extend the body of literature suggesting that IGF-1 has a protective role against NAFLD, and that IGFBP-7 is a circulating marker of NAFLD severity that may be pathogenic and of clinical utility as a noninvasive tool to assess disease severity.
Abundant literature demonstrates that perturbations in the GH/IGF-1 axis increase the risk for NAFLD and NASH. GH suppresses hepatic de novo lipogenesis (28-33), stimulates lipolysis (34, 35), and increases lipid beta-oxidation (36-38), all of which reduce hepatic triglyceride. Mice with liver-specific GH receptor knock-out demonstrate severe hepatic steatosis along with upregulation of inflammatory cytokines and increases in ALT and aspartate aminotransferase (AST), signaling hepatocellular damage (5, 8). In this model, rescue of GH signaling resolves hepatic steatosis, whereas IGF-1 infusion does not rescue the steatotic phenotype (8). The spontaneous dwarf rat, which harbors a mutation in the GH gene, also demonstrates severe steatosis, inflammation, and fibrosis (4). In this model, however, treatment with either GH or IGF-1 was sufficient to reverse the phenotype, with IGF-1 infusion having a particularly beneficial effect on fibrosis compared with GH infusion (4). Multiple additional studies in various animal models of NAFLD or pharmacologically induced liver damage demonstrate benefit of IGF-1, independent of GH, to reduce hepatic inflammation and fibrosis (3, 39-42). Clinical experience also supports a role of the GH/IGF-1 axis in NAFLD pathogenesis, as patients with GH deficiency have a higher prevalence of NAFLD/NASH, and GH replacement improves features of NAFLD (43-46). In the general population, adults with higher levels of serum IGF-1 and higher ratio of serum IGF-1/IGFBP-3 have lower risk of NAFLD (47). In adults with obesity and/or NAFLD, serum IGF-1 levels were lower in those with higher grades of inflammation and higher fibrosis stage (48, 49), and serum IGF-1:IGFBP-3 ratio decreases with increasing fibrosis (50). Furthermore, Hribal and colleagues have reported that hepatic IGF1 mRNA levels are inversely associated with fibrosis stage (49). Our findings extend these previous reports by showing that hepatic IGF1 mRNA is lower in those with higher grades of steatosis and higher NAS scores. In contrast to the previous report by Hribal and colleagues of decreasing IGF1 mRNA in livers with stage 1 or 2 fibrosis compared with no fibrosis, and further decrease in livers with stage 3 or 4 fibrosis, we found a quadratic relationship between IGF1 expression, with higher expression at fibrosis stage 1 and 2, and decreasing in stage 3. This discrepancy may reflect a difference in the population studied or be a result of relatively small sample size. The associations between IGF1 expression and measures of NAFLD in our data were attenuated with adjustment for BMI, which suggests either that BMI is a common cause of NAFLD and lower IGF1 expression, or that lower IGF1 expression is one mediator through which higher BMI is associated with higher risk of NAFLD. Given animal data showing direct effects of IGF-1 infusion to ameliorate NAFLD, the hypothesis of IGF-1 as a mediator is quite plausible: obesity is associated with relative reductions in GH (51-53), resulting in decreased hepatic GH signaling and decreased hepatic IGF1 expression, both of which may enable development of NAFLD.
More recent literature also suggests that some IGFBPs may be markers or etiologic agents in liver disease. In this regard, IGFBP-7, also known as IGFBPrP1 or mac25, has emerged as a potential contributor to hepatic inflammation and fibrosis. In a recent cross-sectional analysis of patients with chronic hepatitis C, with fibrosis stage estimated by FibroScan or FibroTest, there was an apparent increase of all IGFBPs (IGFBP-1 through IGFBP-7) at fibrosis stage 4, with IGFBP-7 being the only protein that distinguished fibrosis stages 1 and 2 from fibrosis stage 3 (10). Upregulation of IGFBP7 has been identified in an analysis of differentially expressed genes in a rodent model of NAFLD (54), and Liu and colleagues have reported mechanisms whereby IGFBP7, induced by transforming growth factor beta 1 (TGFβ1), promotes fibrogenesis by activating hepatic stellate cells (55-57). In our data, at baseline, both hepatic IGFBP7 mRNA and circulating levels of IGFBP-7 were strongly positively associated with steatosis grade, NAS, and fibrosis stage. Moreover, changes in NAS and steatosis grade longitudinally over the course of the study were highly related to changes in circulating IGFBP-7, independent of tesamorelin treatment. These observations, made for the first time in a human NAFLD population, support and extend previous reports. Of note, IGFBP7 is also reported to function as a tumor suppressor in multiple different types of cancer (58-60), and one group has reported that IGFBP7 overexpression inhibits growth of hepatocellular carcinoma (HCC), whereas IGFBP7 deletion accelerates growth of HCC (61). More research is needed to elucidate the distinct mechanistic roles of IGFBP-7 in NAFLD and HCC, and our data support a strong association that warrants further study. Moreover, effective serum markers for liver disease are extremely limited, and these data suggest a novel potential dynamic marker for NAFLD/NASH severity, circulating IGFBP-7, that might be clinically useful.
Hepatic expression of IGFBP6 was also significantly positively associated with steatosis, NAS, and fibrosis in our data. To our knowledge, this is the first report of the association between hepatic expression of IGFBP6 and features of NAFLD. Of note, circulating levels of IGFBP-6 were not associated with these measures. IGFBP2 was negatively associated with hepatic lipid content in our data, but also strongly negatively associated with BMI. In a recent report of transcriptome-wide differential gene expression analysis in a murine model of NAFLD, IGFBP2 emerged as a key nodal molecule, with significant downregulation of hepatic IGFBP2 expression in severe fatty liver as well as lower circulating levels of IGFBP-2 in adults with NAFLD (13). By contrast, hepatic IGFBP2 expression in patients with HCC was higher than in adjacent tissue (62). Similar to findings with IGFBP-7, this raises the possibility of distinct roles of certain IGFBPs in steatohepatitis progression and neoplastic growth, which requires further study.
With regard to glucose homeostasis, our data are consistent with the known insulin-like effects of IGF-1 and extend understanding of the roles of certain IGFBPs. In animals and humans, IGF-1 suppresses glucagon and hepatic gluconeogenesis, increases peripheral glucose utilization, and suppresses lipolysis and protein breakdown (63-65). These effects are thought to be largely mediated through IGF-1 action on the insulin receptor but also have been shown to occur in part through signaling at the IGF-1 receptor (66). Debate exists regarding whether the glucose-lowering effects of IGF-1 are primarily through effects of IGF-1 itself or IGF-1–mediated suppression of GH secretion (67). Our data suggest the possibility that hepatic IGF-1 may have a direct effect on glucose metabolism, as adjustment for serum IGF-1 did not change the association between hepatic IGF1 expression and fasting glucose or insulin. It should be noted, however, that mature hepatocytes are not thought to express IGF-1 receptors (68), such that an insulin-like effect of IGF-1 on hepatic glucose would likely require signaling through the insulin receptor, which has a much lower affinity for IGF-1 than does the IGF-1 receptor. Adjustment for serum IGF-1 did abrogate the association of hepatic IGF1 expression with two-hour glucose, supporting the hypothesis that suppression of GH may play a larger role in mediating IGF-1 effect on systemic insulin resistance (69). Of note, the effects of IGF-1 on systemic glucose homeostasis may have particular physiologic relevance in patients with NAFLD, who also have a high degree of insulin resistance. Petersen and colleagues have demonstrated that effects of IGF-1 to suppress endogenous glucose production and stimulate muscle glycogen synthesis are preserved in cirrhotic rats in spite of significant resistance to insulin in regulating these processes (9).
Our data show a negative association between IGFBP1 and measures of insulin resistance as well as positive associations between IGFBP3 and IGFBP7 and these measures. Expression of IGFBP1 is directly suppressed by insulin (70), a mechanism that is sustained even in hepatic insulin resistance (1), such that our findings of inverse associations between IGFBP1 expression and fasting glucose and insulin are not surprising and support previous reports (12). Our finding that IGFBP3 is positively associated with fasting glucose and trends toward association with other measures of insulin resistance is consistent with animal studies demonstrating a role for IGFBP-3 in mediating glucose homeostasis. Transgenic mice overexpressing IGFBP3 have hyperglycemia and insulin resistance (71), presumably at least in part due to decreased bioavailability of IGF-1. In transgenic mice overexpressing a mutant IGFBP3 unable to bind IGF-1, however, a degree of glucose intolerance is still present, suggesting potential independent effects of IGFBP-3 that require further elucidation (72). Recently, a mouse model with a mutation in pregnancy-associated plasma protein-A2 (PAPP-A2), an IGFBP protease, has also shown insulin resistance in association with decreased serum free IGF-1 and increased serum IGFBP-3 (73).
Both hepatic IGFBP7 expression and circulating levels of IGFBP-7 were strongly positively associated with hyperglycemia in our cohort. In a previous report in men with and without diabetes, circulating IGFBP-7 levels were inversely associated with insulin sensitivity as well as adiponectin levels, and positively associated with circulating markers of inflammation (16). IGFBP-7 has much lower affinity for IGF-1 binding than do IGFBPs 1 to 6, such that reduced bioavailability of IGF-1 is an unlikely mechanism of this effect. Rather, IGFBP-7 specifically binds insulin and affects its interactions with the insulin receptor, a possible direct mechanism by which IGFBP-7 may cause insulin resistance (74). A more recent report suggests that IGFBP7 is expressed by liver macrophages in mice, with significant upregulation during high-fat feeding, and directly binds to the insulin receptor to induce gluconeogenesis and lipogenesis (75). Furthermore, IGFBP7 silencing in liver macrophages improved glucose tolerance and reduced accumulation of hepatic triglyceride (75). These data along with ours suggest a possible direct role of IGFBP-7 in regulating hepatic glucose and lipid metabolism.
We have previously demonstrated that tesamorelin is neutral to glucose homeostasis over long-term use (18). We now demonstrate distinct changes in various circulating IGFBP levels with tesamorelin treatment, with increases in IGFBP-1 and IGFBP-3 and reductions in IGFBP-2 and IGFBP-6. These data augment our knowledge of the multi-level effects of GHRH analogue on the GH/IGF-1 axis. GHRH increases circulating systemic GH levels (76), expected to increase insulin resistance, but also alters IGF-1 and IGFBPs to a degree that may balance effects on insulin resistance. Augmentation of IGF-1 is expected to have an insulin-like effect, and, if associations between IGFBP-1 and glucose demonstrated herein represent a causal mechanism, raising circulating levels of IGFBP-1 may also improve insulin sensitivity. In contrast, the effects of tesamorelin to increase circulating IGFBP-3 and reduce IGFBP-2 may antagonize insulin sensitivity. Together these data suggest that modulating the GH/IGF-1 axis at the level of the hypothalamus, which preserves the pituitary-level feedback whereby IGF-1 suppresses excessive GH release, has distinct effects on various components of the GH/IGF-1 system, potentially explaining overall neutrality with respect to glucose homeostasis.
Our study has strengths, leveraging a well-phenotyped cohort with detailed transcriptomic data from liver tissue, simultaneous proteomic analyses, and a novel intervention, but it also has some weaknesses. Our sample size is relatively small and predominantly male, such that we did not have adequate power to look at sex differences, and it is unclear if our results are generalizable to women. Furthermore, data from this cohort of individuals with long-standing HIV-infection may not be generalizable to those without HIV-infection, but such patients are quite stable and represent an interesting population with increased NAFLD in which to examine such relationships. The novel relationships of IGFBPs to glycemia and NAFLD determined in this analysis require further study to fully assess causality. For example, further large-scale trials investigating strategies for NAFLD/NASH should investigate whether changes in IGFBP-7 may mark changes in liver fat, fibrosis, and inflammation. Importantly, this study leverages a rich data set of proteomic and transcriptomic information to differentiate, for the first time, paracrine vs endocrine effects of IGF and IGFBPs on the liver in a human study.
Our data extend the existing literature and support an important protective relationship of IGF-1 to NAFLD pathogenesis, as well as a possible pathogenic role of IGFBP-7. Other IGFBPs, including IGFBP-6, may also be markers or play mechanistic roles, and warrant further study in this regard. Furthermore, our data support a role of IGF-1 in glucose metabolism and suggest that IGFBP-3 and IGFBP-7 promote insulin resistance. These mechanisms are physiologically relevant in individuals with HIV-infection and NAFLD and may represent therapeutic targets for NAFLD and NASH in other populations as well.
Acknowledgments
We would like to thank the research volunteers who participated in this study as well as the Clinical Research Center staff at the Massachusetts General Hospital and the National Institutes of Health. We would also like to thank James T. Billingsley and Shannan J. Ho Sui from the T.H. Chan Harvard School of Public Health Bioinformatics Core for their assistance with the RNA-Seq.
Financial Support: Supported by National Institutes of Health grants U01AI115711 (C.M.H. and S.K.G.), P30DK040561 (S.K.G.), K23HD100266 (L.T.F.), 1KL2TR002542 (L.T.F.), R01DK114144 (T.L.S.), R01DK108370 (R.T.C.), R01AI136715 (R.T.C), U19 AI082630 (R.T.C.), UL1 TR002541, and MGH Research Scholars Program (R.T.C.) Supported in part by the National Institute of Allergy and Infectious Disease Intramural Research Program and in part by the Intramural Research Program of the National Institutes of Health, National Cancer Institute.
Clinical Trial Information: Clinicaltrials.gov registration no. NCT02196831.
Glossary
Abbreviations:
- 1H-MRS
1H-magnetic resonance spectroscopy
- ALT
alanine aminotransferase
- ANOVA
analysis of variance
- BMI
body mass index
- GH
growth hormone
- GHRH
growth hormone–releasing hormone
- HCC
hepatocellular carcinoma
- HIV
human immunodeficiency virus
- IGF-1
insulin-like growth factor-1
- IGFBP
insulin-like growth factor–binding protein
- MGH
Massachusetts General Hospital
- NAFLD
nonalcoholic fatty liver disease
- NAS
NAFLD Activity Score
- NASH
nonalcoholic steatohepatitis
- PEA
Proximity Extension Assay
Additional Information
Disclosure Summary: T.L.S. reports research funding from Novo Nordisk, Inc. L.T.F. has served as a consultant for Theratechnologies, Inc. K.E.C. reports research funding from Boehringer Ingelheim and has served as a consultant to Novo Nordisk., Inc, and Bristol Myers Squibb. R.T.C. reports research funding from Abbvie, Gilead, Merck, BMS, Boehringer Ingelheim, Janssen, and Roche. S.K.G. has served as a consultant (personal) and currently serves as a consultant through an Institutional Consulting Agreement with Theratechnologies. The Massachusetts General Hospital has a royalty and license agreement with Theratechnologies for tesamorelin. Dr. Grinspoon is the inventor of a U.S. patent 10,799,562 titled “GHRH or Analogues Thereof for Use in Treatment of Hepatic Disease.” M.N.F., I.Z., C.M.M., M.T., D.E.K., and C.M.H. report no conflicts of interest.
Data Availability
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Clemmons DR. Role of IGF-binding proteins in regulating IGF responses to changes in metabolism. J Mol Endocrinol. 2018;61(1):T139-T169. [DOI] [PubMed] [Google Scholar]
- 2. Hwa V, Oh Y, Rosenfeld RG. The insulin-like growth factor-binding protein (IGFBP) superfamily. Endocr Rev. 1999;20(6):761-787. [DOI] [PubMed] [Google Scholar]
- 3. Nishizawa H, Iguchi G, Fukuoka H, et al. IGF-I induces senescence of hepatic stellate cells and limits fibrosis in a p53-dependent manner. Sci Rep. 2016;6:34605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nishizawa H, Takahashi M, Fukuoka H, Iguchi G, Kitazawa R, Takahashi Y. GH-independent IGF-I action is essential to prevent the development of nonalcoholic steatohepatitis in a GH-deficient rat model. Biochem Biophys Res Commun. 2012;423(2):295-300. [DOI] [PubMed] [Google Scholar]
- 5. Liu Z, Cordoba-Chacon J, Kineman RD, et al. Growth hormone control of hepatic lipid metabolism. Diabetes. 2016;65(12):3598-3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cordoba-Chacon J, Majumdar N, List EO, et al. Growth hormone inhibits hepatic de novo lipogenesis in adult mice. Diabetes. 2015;64(9):3093-3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Villanueva-Ortega E, Méndez-García LA, Garibay-Nieto GN, et al. Growth hormone ameliorates high glucose-induced steatosis on in vitro cultured human HepG2 hepatocytes by inhibiting de novo lipogenesis via ChREBP and FAS suppression. Growth Horm IGF Res. 2020;53-54:101332. [DOI] [PubMed] [Google Scholar]
- 8. Fan Y, Menon RK, Cohen P, et al. Liver-specific deletion of the growth hormone receptor reveals essential role of growth hormone signaling in hepatic lipid metabolism. J Biol Chem. 2009;284(30):19937-19944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Petersen KF, Jacob R, West AB, Sherwin RS, Shulman GI. Effects of insulin-like growth factor I on glucose metabolism in rats with liver cirrhosis. Am J Physiol. 1997;273(6):E1189-E1193. [DOI] [PubMed] [Google Scholar]
- 10. Martínez-Castillo M, Rosique-Oramas D, Medina-Avila Z, et al. Differential production of insulin-like growth factor-binding proteins in liver fibrosis progression. Mol Cell Biochem. 2020;469(1-2):65-75. [DOI] [PubMed] [Google Scholar]
- 11. Petäjä EM, Zhou Y, Havana M, et al. Phosphorylated IGFBP-1 as a non-invasive predictor of liver fat in NAFLD. Sci Rep. 2016;6:24740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kotronen A, Lewitt M, Hall K, Brismar K, Yki-Järvinen H. Insulin-like growth factor binding protein 1 as a novel specific marker of hepatic insulin sensitivity. J Clin Endocrinol Metab. 2008;93(12):4867-4872. [DOI] [PubMed] [Google Scholar]
- 13. Fahlbusch P, Knebel B, Horbelt T, et al. Physiological disturbance in fatty liver energy metabolism converges on IGFBP2 abundance and regulation in mice and men. Int J Mol Sci. 2020;21(11):4144. doi:10.3390/ijms21114144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dali-Youcef N, Vix M, Costantino F, et al. Interleukin-32 contributes to human nonalcoholic fatty liver disease and insulin resistance. Hepatol Commun. 2019;3(9):1205-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Min HK, Maruyama H, Jang BK, et al. Suppression of IGF binding protein-3 by palmitate promotes hepatic inflammatory responses. Faseb J. 2016;30(12):4071-4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. López-Bermejo A, Khosravi J, Fernández-Real JM, et al. Insulin resistance is associated with increased serum concentration of IGF-binding protein-related protein 1 (IGFBP-rP1/MAC25). Diabetes. 2006;55(8):2333-2339. [DOI] [PubMed] [Google Scholar]
- 17. Yan H, Li T, Wang Y, Li H, Xu J, Lu X. Insulin-like growth factor binding protein 7 accelerates hepatic steatosis and insulin resistance in non-alcoholic fatty liver disease. Clin Exp Pharmacol Physiol. 2019;46(12):1101-1110. [DOI] [PubMed] [Google Scholar]
- 18. Stanley TL, Fourman LT, Feldpausch MN, et al. Effects of tesamorelin on non-alcoholic fatty liver disease in HIV: a randomised, double-blind, multicentre trial. Lancet HIV. 2019;6(12):e821-e830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Braun LR, Feldpausch MN, Czerwonka N, et al. Effects of pitavastatin on insulin sensitivity and liver fat: a randomized clinical trial. J Clin Endocrinol Metab. 2018;103(11):4176-4186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237(3):E214-E223. [DOI] [PubMed] [Google Scholar]
- 21. Kleiner DE, Brunt EM, Van Natta M, et al. ; Nonalcoholic Steatohepatitis Clinical Research Network . Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41(6):1313-1321. [DOI] [PubMed] [Google Scholar]
- 22. deFilippi C, Lo J, Christenson R, et al. Novel mediators of statin effects on plaque in HIV: a proteomics approach. Aids. 2018;32(7):867-876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Toribio M, Fitch KV, Stone L, et al. Assessing statin effects on cardiovascular pathways in HIV using a novel proteomics approach: analysis of data from INTREPID, a randomized controlled trial. Ebiomedicine. 2018;35:58-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kim D, Paggi JM, Park C, Bennett C, Salzberg SL. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat Biotechnol. 2019;37(8):907-915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. García-Alcalde F, Okonechnikov K, Carbonell J, et al. Qualimap: evaluating next-generation sequencing alignment data. Bioinformatics. 2012;28(20):2678-2679. [DOI] [PubMed] [Google Scholar]
- 26. Liao Y, Smyth GK, Shi W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics. 2014;30(7):923-930. [DOI] [PubMed] [Google Scholar]
- 27. Perneger TV. What’s wrong with Bonferroni adjustments. BMJ. 1998;316(7139):1236-1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Goodman HM. Effects of chronic growth hormone treatment on lipogenesis by rat adipose tissue. Endocrinology. 1963;72:95-99. [DOI] [PubMed] [Google Scholar]
- 29. Adamafio NA, Ng FM. Effects of growth hormone on lipogenesis and glucose oxidation in genetically GH-deficient mice. Mol Cell Endocrinol. 1984;37(2):241-244. [DOI] [PubMed] [Google Scholar]
- 30. Perry WF, Bowen HF. The effect of growth hormone on lipogenesis in intact and adrenalectomized rats. Endocrinology. 1955;56(5):579-583. [DOI] [PubMed] [Google Scholar]
- 31. Ng FM, Adamafio NA, Graystone JE. Effects of exogenous growth hormone on lipid metabolism in the isolated epididymal fat pad of the growth hormone-deficient little mouse. J Mol Endocrinol. 1990;4(1):43-49. [DOI] [PubMed] [Google Scholar]
- 32. Vernon RG. GH inhibition of lipogenesis and stimulation of lipolysis in sheep adipose tissue: involvement of protein serine phosphorylation and dephosphorylation and phospholipase C. J Endocrinol. 1996;150(1):129-140. [DOI] [PubMed] [Google Scholar]
- 33. Schwarz JM, Mulligan K, Lee J, et al. Effects of recombinant human growth hormone on hepatic lipid and carbohydrate metabolism in HIV-infected patients with fat accumulation. J Clin Endocrinol Metab. 2002;87(2):942. [DOI] [PubMed] [Google Scholar]
- 34. Keller U, Schnell H, Girard J, Stauffacher W. Effect of physiological elevation of plasma growth hormone levels on ketone body kinetics and lipolysis in normal and acutely insulin-deficient man. Diabetologia. 1984;26(2):103-108. [DOI] [PubMed] [Google Scholar]
- 35. Dietz J, Schwartz J. Growth hormone alters lipolysis and hormone-sensitive lipase activity in 3T3-F442A adipocytes. Metabolism. 1991;40(8):800-806. [DOI] [PubMed] [Google Scholar]
- 36. Fourman LT, Billingsley JM, Agyapong G, et al. Effects of tesamorelin on hepatic transcriptomic signatures in HIV-associated NAFLD. JCI Insight. 2020;5(16):e140134. doi:10.1172/jci.insight.140134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Leung KC, Ho KK. Stimulation of mitochondrial fatty acid oxidation by growth hormone in human fibroblasts. J Clin Endocrinol Metab. 1997;82(12):4208-4213. [DOI] [PubMed] [Google Scholar]
- 38. Møller N, Jørgensen JO. Effects of growth hormone on glucose, lipid, and protein metabolism in human subjects. Endocr Rev. 2009;30(2):152-177. [DOI] [PubMed] [Google Scholar]
- 39. Luo X, Jiang X, Li J, et al. Insulin-like growth factor-1 attenuates oxidative stress-induced hepatocyte premature senescence in liver fibrogenesis via regulating nuclear p53-progerin interaction. Cell Death Dis. 2019;10(6):451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sobrevals L, Rodriguez C, Romero-Trevejo JL, et al. Insulin-like growth factor I gene transfer to cirrhotic liver induces fibrolysis and reduces fibrogenesis leading to cirrhosis reversion in rats. Hepatology. 2010;51(3):912-921. [DOI] [PubMed] [Google Scholar]
- 41. Muguerza B, Castilla-Cortázar I, García M, Quiroga J, Santidrián S, Prieto J. Antifibrogenic effect in vivo of low doses of insulin-like growth factor-I in cirrhotic rats. Biochim Biophys Acta. 2001;1536(2-3):185-195. [DOI] [PubMed] [Google Scholar]
- 42. Sanz S, Pucilowska JB, Liu S, et al. Expression of insulin-like growth factor I by activated hepatic stellate cells reduces fibrogenesis and enhances regeneration after liver injury. Gut. 2005;54(1):134-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Adams LA, Feldstein A, Lindor KD, Angulo P. Nonalcoholic fatty liver disease among patients with hypothalamic and pituitary dysfunction. Hepatology. 2004;39(4):909-914. [DOI] [PubMed] [Google Scholar]
- 44. Ichikawa T, Hamasaki K, Ishikawa H, Ejima E, Eguchi K, Nakao K. Non-alcoholic steatohepatitis and hepatic steatosis in patients with adult onset growth hormone deficiency. Gut. 2003;52(6):914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nishizawa H, Iguchi G, Murawaki A, et al. Nonalcoholic fatty liver disease in adult hypopituitary patients with GH deficiency and the impact of GH replacement therapy. Eur J Endocrinol. 2012;167(1):67-74. [DOI] [PubMed] [Google Scholar]
- 46. Sato T, Katabami T, Furukawa K, et al. Intracellular lipid content of liver and skeletal muscle in patients with adult growth hormone deficiency without diabetes mellitus. Obes Res Clin Pract. 2012;6(4):e263-e346. [DOI] [PubMed] [Google Scholar]
- 47. Runchey SS, Boyko EJ, Ioannou GN, Utzschneider KM. Relationship between serum circulating insulin-like growth factor-1 and liver fat in the United States. J Gastroenterol Hepatol. 2014;29(3):589-596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Dichtel LE, Corey KE, Misdraji J, et al. The Association Between IGF-1 Levels and the Histologic Severity of Nonalcoholic Fatty Liver Disease. Clin Transl Gastroenterol. 2017;8(1):e217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hribal ML, Procopio T, Petta S, et al. Insulin-like growth factor-I, inflammatory proteins, and fibrosis in subjects with nonalcoholic fatty liver disease. J Clin Endocrinol Metab. 2013;98(2):E304-E308. [DOI] [PubMed] [Google Scholar]
- 50. Polyzos SA, Perakakis N, Boutari C, et al. Targeted analysis of three hormonal systems identifies molecules associated with the presence and severity of NAFLD. J Clin Endocrinol Metab. 2020;105(3):e390-e400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Makimura H, Stanley T, Mun D, You SM, Grinspoon S. The effects of central adiposity on growth hormone (GH) response to GH-releasing hormone-arginine stimulation testing in men. J Clin Endocrinol Metab. 2008;93(11):4254-4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Miller KK, Biller BM, Lipman JG, Bradwin G, Rifai N, Klibanski A. Truncal adiposity, relative growth hormone deficiency, and cardiovascular risk. J Clin Endocrinol Metab. 2005;90(2):768-774. [DOI] [PubMed] [Google Scholar]
- 53. Iranmanesh A, Lizarralde G, Veldhuis JD. Age and relative adiposity are specific negative determinants of the frequency and amplitude of growth hormone (GH) secretory bursts and the half-life of endogenous GH in healthy men. J Clin Endocrinol Metab. 1991;73(5):1081-1088. [DOI] [PubMed] [Google Scholar]
- 54. Li T, Yan H, Geng Y, et al. Target genes associated with lipid and glucose metabolism in non-alcoholic fatty liver disease. Lipids Health Dis. 2019;18(1):211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Huang TJ, Ren JJ, Zhang QQ, et al. IGFBPrP1 accelerates autophagy and activation of hepatic stellate cells via mutual regulation between H19 and PI3K/AKT/mTOR pathway. Biomed Pharmacother. 2019;116:109034. [DOI] [PubMed] [Google Scholar]
- 56. Ren JJ, Huang TJ, Zhang QQ, et al. Insulin-like growth factor binding protein related protein 1 knockdown attenuates hepatic fibrosis via the regulation of MMPs/TIMPs in mice. Hepatobiliary Pancreat Dis Int. 2019;18(1):38-47. [DOI] [PubMed] [Google Scholar]
- 57. Liu LX, Huang S, Zhang QQ, et al. Insulin-like growth factor binding protein-7 induces activation and transdifferentiation of hepatic stellate cells in vitro. World J Gastroenterol. 2009;15(26):3246-3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hwa V, Tomasini-Sprenger C, Bermejo AL, Rosenfeld RG, Plymate SR. Characterization of insulin-like growth factor-binding protein-related protein-1 in prostate cells. J Clin Endocrinol Metab. 1998;83(12):4355-4362. [DOI] [PubMed] [Google Scholar]
- 59. Landberg G, Ostlund H, Nielsen NH, et al. Downregulation of the potential suppressor gene IGFBP-rP1 in human breast cancer is associated with inactivation of the retinoblastoma protein, cyclin E overexpression and increased proliferation in estrogen receptor negative tumors. Oncogene. 2001;20(27):3497-3505. [DOI] [PubMed] [Google Scholar]
- 60. Chen Y, Pacyna-Gengelbach M, Ye F, et al. Insulin-like growth factor binding protein-related protein 1 (IGFBP-rP1) has potential tumour-suppressive activity in human lung cancer. J Pathol. 2007;211(4):431-438. [DOI] [PubMed] [Google Scholar]
- 61. Akiel M, Guo C, Li X, et al. IGFBP7 deletion promotes hepatocellular carcinoma. Cancer Res. 2017;77(15):4014-4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Zhou Q, Mao YQ, Jiang WD, et al. Development of IGF signaling antibody arrays for the identification of hepatocellular carcinoma biomarkers. Plos One. 2012;7(10):e46851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Boulware SD, Tamborlane WV, Rennert NJ, Gesundheit N, Sherwin RS. Comparison of the metabolic effects of recombinant human insulin-like growth factor-I and insulin. Dose-response relationships in healthy young and middle-aged adults. J Clin Invest. 1994;93(3):1131-1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Englisch R, Wurzinger R, Fürnsinn C, et al. Effects of insulin-like growth factor I on basal and stimulated glucose fluxes in rat liver. Biochem J. 2000;351(Pt 1):39-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Boulware SD, Tamborlane WV, Matthews LS, Sherwin RS. Diverse effects of insulin-like growth factor I on glucose, lipid, and amino acid metabolism. Am J Physiol. 1992;262(1 Pt 1):E130-E133. [DOI] [PubMed] [Google Scholar]
- 66. Di Cola G, Cool MH, Accili D. Hypoglycemic effect of insulin-like growth factor-1 in mice lacking insulin receptors. J Clin Invest. 1997;99(10):2538-2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ohlsson C, Mohan S, Sjögren K, et al. The role of liver-derived insulin-like growth factor-I. Endocr Rev. 2009;30(5): 494-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Waraky A, Aleem E, Larsson O. Downregulation of IGF-1 receptor occurs after hepatic linage commitment during hepatocyte differentiation from human embryonic stem cells. Biochem Biophys Res Commun. 2016;478(4):1575-1581. [DOI] [PubMed] [Google Scholar]
- 69. Yakar S, Setser J, Zhao H, et al. Inhibition of growth hormone action improves insulin sensitivity in liver IGF-1-deficient mice. J Clin Invest. 2004;113(1):96-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Powell DR, Suwanichkul A, Cubbage ML, DePaolis LA, Snuggs MB, Lee PD. Insulin inhibits transcription of the human gene for insulin-like growth factor-binding protein-1. J Biol Chem. 1991;266(28):18868-18876. [PubMed] [Google Scholar]
- 71. Silha JV, Gui Y, Murphy LJ. Impaired glucose homeostasis in insulin-like growth factor-binding protein-3-transgenic mice. Am J Physiol Endocrinol Metab. 2002;283(5):E937-E945. [DOI] [PubMed] [Google Scholar]
- 72. Nguyen KH, Yao XH, Erickson AG, Mishra S, Nyomba BL. Glucose intolerance in aging male IGFBP-3 transgenic mice: differential effects of human IGFBP-3 and its mutant IGFBP-3 devoid of IGF binding ability. Endocrinology. 2015;156(2):462-474. [DOI] [PubMed] [Google Scholar]
- 73. Fujimoto M, Andrew M, Liao L, et al. Low IGF-I Bioavailability Impairs Growth and Glucose Metabolism in a Mouse Model of Human PAPPA2 p.Ala1033Val Mutation. Endocrinology. 2019;160(6):1363-1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Yamanaka Y, Wilson EM, Rosenfeld RG, Oh Y. Inhibition of insulin receptor activation by insulin-like growth factor binding proteins. J Biol Chem. 1997;272(49):30729-30734. [DOI] [PubMed] [Google Scholar]
- 75. Morgantini C, Jager J, Li X, et al. Liver macrophages regulate systemic metabolism through non-inflammatory factors. Nat Metab. 2019;1(4):445-459. [DOI] [PubMed] [Google Scholar]
- 76. Stanley TL, Chen CY, Branch KL, Makimura H, Grinspoon SK. Effects of a growth hormone-releasing hormone analog on endogenous GH pulsatility and insulin sensitivity in healthy men. J Clin Endocrinol Metab. 2011;96(1): 150-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.