Abstract
Context
Approximately 10% to 20% of prolactinomas are resistant to dopamine agonist therapy. The ErbB signaling pathway may drive aggressive prolactinoma behavior.
Objective
We evaluated lapatinib, an ErbB1-epidermal growth factor receptor (EGFR)/ErbB2 or human EGFR2 (HER2) tyrosine kinase inhibitor (TKI), in aggressive prolactinomas.
Design
A prospective, phase 2a multicenter trial was conducted.
Setting
This study took place at a tertiary referral pituitary center.
Patients
Study participants included adults with aggressive prolactinomas showing continued tumor growth despite maximally tolerated dopamine agonist therapy.
Intervention
Intervention included oral lapatinib 1250 mg/day for 6 months.
Main Outcome Measures
The primary end point was 40% reduction in any tumor dimension assessed by magnetic resonance imaging at study end; tumor response was assessed by Response Evaluation Criteria in Solid Tumors criteria. Secondary end points included prolactin (PRL) reduction, correlation of response with EGFR/HER2 expression, and safety.
Results
Owing to rigorous inclusion criteria, of 24 planned participants, only 7 consented and 4 were treated. None achieved the primary end point but 3 showed stable disease, including 2 with a 6% increase and 1 with a 16.8% decrease in tumor diameter. PRL response was not always concordant with tumor response, as 2 showed 28% and 59% increases in PRL. The fourth participant had a PRL-secreting carcinoma and withdrew after 3 months of lapatinib because of imaging and PRL progression. EGFR/HER2 expression did not correlate with treatment response. Lapatinib was well tolerated overall, with reversible grade 1 transaminitis in 2 patients, grade 2 rash in 2 patients, and grade 1 asymptomatic bradycardia in 2 patients.
Conclusions
An oral TKI such as lapatinib may be an effective option for a difficult-to-treat patient with an aggressive prolactinoma.
Keywords: prolactinoma, ErbB, epidermal growth factor receptor, HER2, tyrosine kinase inhibitor, lapatinib
Prolactinomas are benign pituitary adenomas that typically respond well to dopamine agonist (DA) therapy. However, approximately 10% to 20% are treatment resistant, and 5% to 10% behave aggressively and persist or recur despite standard doses of DA, particularly in men (1-3). Despite increased treatment dose, tumor size may not stabilize, and patients may experience increased risk of adverse effects, including cardiovascular toxicity from valvulopathy (4) and impulse control disorders (5). Surgery and radiotherapy may be considered for DA-resistant tumors, but potential adverse effects include new-onset hypopituitarism, cerebrospinal fluid leak, vascular injury, and visual field compromise; late recurrence after surgery is a concern (6), particularly in cases of large tumors, and radiation therapy may not address prolactin (PRL) levels (7, 8).
Temozolomide is a recommended treatment option for aggressive prolactinomas (9), and reports from retrospective studies and clinical practice surveys show good response rates (10, 11). However, the ability to achieve long-term, consistent, sustained efficacy remains a challenge (11, 12).
To address the unmet need for treatment of very aggressive pituitary tumors, over the past decade we have been studying therapeutic targeting of the ErbB pathway, particularly in rare, aggressive prolactinomas (13-15).
The 4 transmembrane receptors in the ErbB family, namely, ErbB1 or epidermal growth factor receptor (EGFR), ErbB2 or human EGFR2 (HER2), ErbB3, and ErbB4, and ligands are expressed in nontumoral lactotrophs (16) and induce PRL gene expression and PRL secretion (17, 18). In animal models, lactosomatotroph-derived tumors also express ErbB receptors and ligands, and targeting this system suppresses PRL gene expression, PRL secretion, and tumor size (19, 20). Human prolactinomas express nuclear EGFR and membranous HER2, ErbB3, and ErbB4, and their expression correlates with tumor invasion (13). Pituitary tumor cell lines transfected with EGFR and ErbB2 manifest downstream effects on PRL gene expression and PRL secretion, as well as on cell proliferation (14, 21); animal models implanted with these overexpressing transfectants developed larger tumors and PRL elevations compared with plasmid cloning DNA3–implanted controls (14).
In a proof-of-concept study, we assessed effects of the EGFR/HER2-targeting tyrosine kinase inhibitor (TKI) lapatinib in 2 patients with aggressive prolactinomas. After 6 months of treatment, one showed near normal levels of PRL and a 22% reduction in tumor size, while the other showed a 42% reduction in PRL and stable tumor size (13). Given these encouraging preliminary data, we conducted a phase 2a multicenter study of the ErbB2-targeting lapatinib in patients with aggressive prolactinomas and provide evidence for a new therapeutic target in these resistant tumors.
Materials and Methods
In a prospective, phase 2a, single-arm, open-label, multicenter trial, we treated adult patients with recurrent, treatment-resistant prolactinomas with 6 months of oral lapatinib 1250 mg/day (ClinicalTrials.gov identifier NCT00939523). The study was approved by the Cedars-Sinai Institutional Review Board, monitored by an external data safety monitoring board, and conducted according to Declaration of Helsinki principles. Written informed consent was received from participants prior to inclusion in the study.
Participants
The study was designed for a planned enrollment of 24 individuals to be recruited from tertiary referral pituitary centers at Cedars-Sinai Medical Center, Massachusetts General Hospital, Johns Hopkins Hospital, Barrow Neurological Institute, and Oregon Health & Science University. Adults age 18 to 80 years with diagnosed prolactinomas were eligible for enrollment if they showed continued tumor growth on maximally tolerated DA therapy, regardless of whether radiotherapy was previously administered. Pregnant women and patients with unstable visual fields were excluded, as were patients with left ventricular ejection fraction of less than 50%, moderate to severe hepatic impairment, active hepatitis, HIV positivity, concurrent cancers, and life expectancy of less than 1 year. Patients with stable but preexisting visual field deficits were eligible for enrollment with monthly monitoring.
End points
The primary end point was a 40% reduction in any tumor dimension assessed by magnetic resonance imaging (MRI) from baseline to study end. Response was determined according to Response Evaluation Criteria in Solid Tumors (RECIST) criteria (22). Specifically, complete response was defined as disappearance of the prolactinoma, partial response as a 30% or greater decrease in maximal tumor diameter, progressive disease as a 20% or greater increase in diameter, and stable disease as neither sufficient shrinkage to qualify for partial response nor increase for progressive disease.
Secondary end points were 50% PRL reduction, PRL normalization, percentage PRL reduction, percentage tumor volume reduction, and immunohistochemical correlation of clinical response with ErbB receptor expression. Tumor volume was calculated using the formula 1/2 (length × width × height).
Treatment
Lapatinib is approved by the US Food and Drug Administration at a dose of 1250 mg/day given orally once daily on days 1 to 21 in combination with capecitabine for patients with HER2-positive metastatic breast cancer. It is also approved at a dose of 1500 mg/day given orally once daily in combination with letrozole for use in patients with HER2-positive, estrogen receptor–positive advanced breast cancer.
After consultation with oncology on dose selection, we treated participants with the 1250 mg/day dose of lapatinib, given as a single oral dose of five 250-mg tablets once daily continuously for 6 months. A 30-day supply of tablets was dispensed at each monthly visit after confirming compliance.
Patients were maintained on their current DA therapy, and doses remained unchanged for the duration of the study.
Evaluations
All study visits were completed at Cedars-Sinai. Participants were tested at baseline and at monthly visits for all pituitary hormone levels, complete blood count, and complete metabolic panel. Echocardiogram and electrocardiogram assessments were performed at baseline, repeated every 2 months, and performed again at study end. Ophthalmic visual field examination testing was performed at baseline and every 3 months. Patients with stable preexisting visual field deficits were tested monthly for the first 3 months; if visual fields remained stable, testing was performed every 3 months for the remainder of the study. MRI was performed at baseline and every 3 months and evaluated by a designated neuroradiologist.
Treatment-emergent adverse events were graded according to National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTC) criteria. Based on the known side effects of lapatinib, participants were specifically monitored for decreased ejection fraction, interstitial lung disease/pneumonitis, anorexia, diarrhea, nausea, vomiting, hepatotoxicity, rash, and fatigue. Individuals with decreased ejection fraction, pulmonary toxicity, retinal toxicity, worsening of visual fields, tumor growth on MRI, or any toxicity grade greater than 2 on NCI-CTC were withdrawn and referred for follow-up treatment as appropriate.
Immunohistochemistry
After deparaffinization, stored resected tissue was pretreated for 36 minutes with On Board High pH buffer and for 8 minutes with On Board Protease 1 using Ventana Benchmark Ultra (Ventana Medical Systems) for HER2 and EGFR, respectively. Slides were incubated with prediluted primary antibody for 8 minutes for HER2 (Roche Diagnostics; catalog No. 790-2991) and for 20 minutes for EGFR (Roche Diagnostics, catalog No. 790–2988). Detection was performed with Ventana ultraView DAB. Breast cancer tissue was used as a positive control.
Statistical analysis
The study was designed for a planned enrollment of 24 participants. The null hypothesis was H0: no reduction P less than .20 vs the alternative hypothesis Ha: P greater than .40. The target sample size of 24 individuals was estimated to achieve power of greater than 80%, using the one-tailed hypothesis, α of .10, with an effect size of .20. Per protocol, analyses were planned for exact CI and significance test to be obtained from the binomial distribution and numerical variables to be summarized as median (range), with a P value less than or equal to .05 considered statistically significant.
Results
Owing to rigorous inclusion/exclusion criteria, only 7 individuals provided consent. Three were excluded before the first study dose was dispensed: 1 for personal reasons unrelated to the study, and 2 because of unstable visual fields and elevated transaminase levels at baseline. The remaining 4 participants were enrolled and treated during the reporting period.
Because statistical analysis of this small sample was not possible, we report here raw data results on patients 1 to 4. Brief case history summaries for these 4 individuals are included to provide additional context for our findings, identify potential predictors of response to TKI therapy, and define the potential role of TKI therapy in the treatment paradigm.
Clinical characteristics
Baseline characteristics are shown in Table 1. Three individuals were age 26 years or younger (range, 19-26 years) and the fourth was age 70 years; half of the participants were female. Patient 3 was recently diagnosed, with a disease duration of only 1 year. Patients 2 and 4 had a 5- to 6-year history of disease, whereas patient 1 had a 35-year history. All had undergone at least 1 prior surgery (range, 1-3) and patient 1 had also received radiotherapy and temozolomide. Weekly cabergoline dose ranged from 1 mg to 6 mg. Hormone deficiencies were common, although only patient 3 had panhypopituitarism; hypothyroidism was present in patient 1 and hypogonadism in all but patient 1.
Table 1.
Clinical characteristics
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | |
|---|---|---|---|---|
| Demographics | ||||
| Age, y | 70 | 26 | 20 | 19 |
| Sex | Female | Male | Male | Female |
| BMI, kg/m2 | 29.5 | 31.2 | 39.2 | 30.8 |
| Disease history | ||||
| Duration of disease, y | 35 | 5 | 1 | 6 |
| Tumor diameter at diagnosis, mm | NA | 40 | 60 | 16 |
| PRL at initial diagnosis, µ/L | NA | 14 000 | 1347 | 113 |
| Hormone deficiencies | ||||
| Hypopituitarism | Yes | Yes | Yes | Yes |
| Adrenal insufficiency | No | No | Yes | No |
| Hypothyroidism | Yes | No | Yes | No |
| Hypogonadism | No | Yes | Yes | Yes |
| Treatment history | ||||
| Surgeries, n | 3 | 1 | 2 | 2 |
| Radiotherapy | Yes | No | No | No |
| Temozolomide | Yes | No | No | No |
| Weekly cabergoline dose, mg | 1 | 6 | 6 | 3 |
Abbreviations: BMI, body mass index; NA, not available; PRL, prolactin.
Effect of lapatinib therapy on tumor size
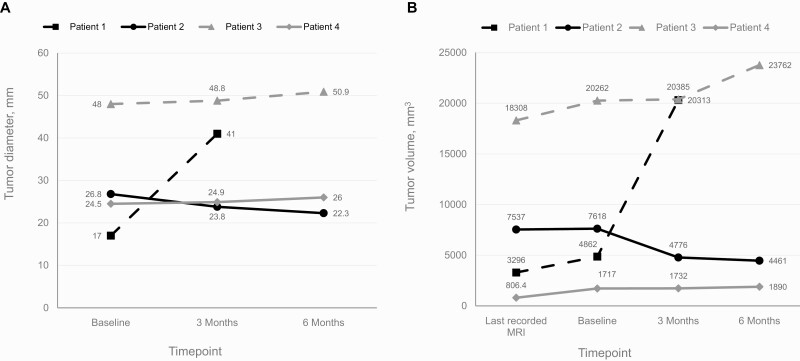
By study end, by the RECIST criteria, 3 participants exhibited stable disease, defined as a less than 30% decrease and a less than 20% increase in maximal tumor diameter (Table 2, Fig. 1A). Patient 1, who had a metastatic carcinoma at enrollment, showed progressive disease, with an increase in tumor diameter by 141% (17-41 mm) from baseline to month 3, after which she withdrew from the study. In patient 2, tumor diameter decreased by 16.8% (26.8-22.3 mm) from baseline to study end. Patient 3 showed a 6.0% increase from baseline (48-50.9 mm), and patient 4 showed a 6.1% increase (24.5-26 mm).
Table 2.
Response to lapatinib
| Patient 1a | Patient 2 | Patient 3 | Patient 4 | |
|---|---|---|---|---|
| Tumor diameter | ||||
| At baseline, mm | 17 | 26.8 | 48 | 24.5 |
| At study end, mm | 41 | 22.3 | 50.9 | 26 |
| Change from baseline, % | 141 | –16.8 | 6.0 | 6.1 |
| Tumor volume | ||||
| At baseline, mm3 | 4862 | 7618 | 20 262 | 1717 |
| At study end, mm3 | 20 313 | 4461 | 23 762 | 1890 |
| Change from baseline, % | 318 | –41.4 | 17.3 | 10.0 |
| PRL level | ||||
| At baseline, µ/L | 11748 | 238 | 1347 | 113.7 |
| Nadir, µ/L | 11753 | 123.1 | 1258.2 | 141.9 |
| Change from baseline to nadir, % | 0.04 | –48.2 | –6.6 | 24.8 |
| At study end, µ/L | 23 850 | 137.5 | 1725.5 | 181 |
| Change from baseline to study end, % | 103 | –42 | 28 | 59 |
| Safety | ||||
| Transaminitis | No | Yes (grade 1) | No | Yes (grade 1) |
| Rash | No | No | Yes (grade 2) | Yes (grade 2) |
| Bradycardia | No | No | Yes (grade 1) | Yes (grade 1) |
| Diarrhea | No | No | No | Yes (grade 1) |
Abbreviation: PRL, prolactin.
a Patient 1 withdrew after 3 months of treatment. Values given are from the final study visit.
Figure 1.
Effect of lapatinib therapy on tumor size. Changes in A, tumor diameter, and B, tumor volume based on magnetic resonance imaging (MRI) performed at baseline, 3 months, and 6 months. Patient 1 withdrew after 3 months of treatment.
Assessment of changes in tumor volume showed a similar pattern (Fig. 1B). Patient 1 showed a tumor volume increase of 318% (4862-20 313 mm3) before she withdrew at month 3. Patient 2 showed a 41.4% decrease in tumor volume (7618-4461 mm3), and patients 3 and 4 showed a 17.3% increase (20 262-23 762 mm3) and a 10.0% increase (1717-1890 mm3), respectively.
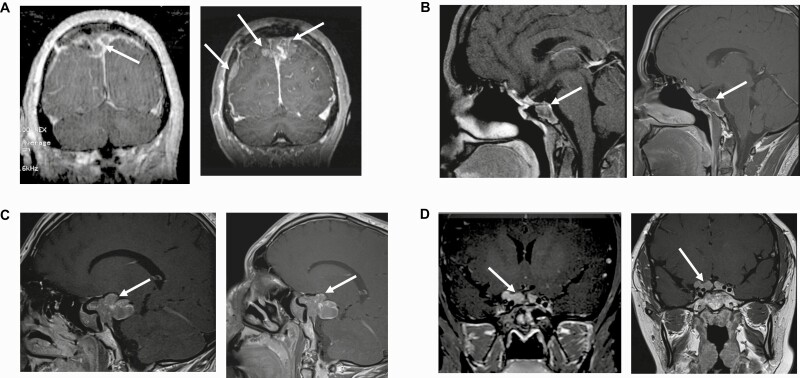
MRI showing changes from baseline to study end for all participants are depicted in Fig. 2.
Figure 2.
Magnetic resonance imaging (MRI) at baseline and study end. A, MRI of patient 1 at (left) baseline and (right) study end. Baseline MRI demonstrates a parasagittal mass on the right side. Lesion measures 17 × 22 × 26 mm. MRI after 3 months of lapatinib therapy shows a 141% increase in tumor diameter now extending to the left. Lesion measures 36.7 × 27 × 41 mm. In addition, there is a new dural metastasis. B, MRI of patient 2 at (left) baseline and (right) study end. Baseline MRI demonstrates a heterogeneous lesion with ill-defined margins and adjacent normal structures. Note extension into the left sphenoid sinus, parasellar structures, anterior clinoid, and superior clivus. The infundibulum is midline to slightly deviated toward the left side. Optic chiasm is normal. Lesion measures 21.8 × 26.8 × 26.1 mm. MRI after 6 months of lapatinib therapy shows a 16.8% decrease in tumor diameter. Lesion measures 16.6 × 22.3 × 24.1 mm. C, MRI of patient 3 at (left) baseline and (right) study end. Baseline MRI demonstrates a large pituitary mass that expands downward, ventrally, superiorly, and posteriorly, including into the interpeduncular cistern and the right temporal fossa, and compressing the right optic nerve. There are areas of hemorrhage within the mass. Lesion measures 48 × 24.4 × 34.6 mm. MRI after 6 months of lapatinib therapy shows a 6% increase in tumor diameter on the right-sided posterior component. Lesion measures 50.9 × 26.6 × 35.1 mm. D, MRI of patient 4 at (left) baseline and (right) study end. Baseline MRI demonstrates a lobulated mass associated with the undersurface of the optic chiasm and extending into the bilateral suprasellar cisterns. Lesion measures 16.3 × 8.6 × 24.5 mm. MRI after 6 months of lapatinib therapy shows a 6.1% increase in tumor diameter in each dimension. Lesion measures 15.8 × 9.2 × 26 mm.
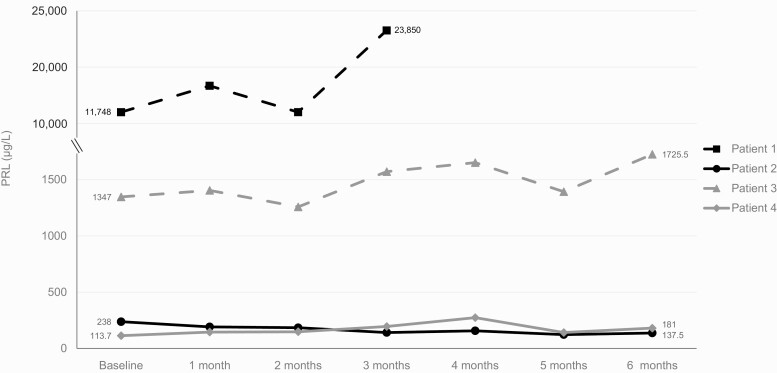
Effect of lapatinib therapy on prolactin
PRL response to lapatinib over the course of the study (Fig. 3) did not always align with tumor mass response. PRL increased by 103% (11 748-23 850 µ/L) in patient 1 and decreased by 42% (238-137.5 µ/L) in patient 2, concordant with changes in tumor size. By contrast, patients 3 and 4 experienced increased PRL with lapatinib treatment despite stable tumor response. In patient 3, PRL increased by 28% from baseline to study end (1347-1725.5 µ/L), whereas in patient 4, PRL increased by 59% from baseline to study end (113.7 to 181 µ/L).
Figure 3.
Effect of lapatinib therapy on prolactin (PRL). Values shown are from baseline and monthly visits. Patient 1 withdrew after 3 months of treatment.
Safety
Overall, lapatinib was well tolerated (Table 2). No participant discontinued treatment because of adverse effects and no grade 3/4 or serious adverse events were reported. Patients 2 and 4 developed transient grade 1 transaminitis, and liver function tests normalized while on continued lapatinib treatment. Grade 2 rash in patients 3 and 4 as well as grade 1 diarrhea in patient 4 were controlled with supportive measures. No decrease in ejection fraction was noted, although patients 3 and 4 developed grade 1 asymptomatic bradycardia. Patient 2 reported some nausea and fatigue during the first month on therapy that resolved uneventfully.
Immunohistochemistry
Tumor tissue derived from patients 2, 3, and 4 was immunonegative for EGFR and HER2 expression, and expression did not correlate with PRL or tumor response (data not shown). Testing was not performed on tumor tissue from patient 1, who withdrew after 3 months of treatment.
Case history brief summaries
Patient 1 was a 70-year-old woman with a 35-year history of widely metastatic recurrent prolactinoma. She was initially treated with and responded well to bromocriptine therapy, which was discontinued after 10 years. In 2006, her PRL was 160 µ/L and a recurrent mass was evident on MRI. She was restarted on bromocriptine then underwent transsphenoidal resection of the tumor and fractionated radiotherapy followed by craniotomy for persistent tumor growth; Ki-67 was not reported. She was then switched to cabergoline 1 mg/week and showed tumor stabilization for 3 years.
In 2013, the patient presented with cauda equina syndrome and a PRL of 6025 µ/L with radiologic confirmation of metastases to the spine, brain, and lungs. Despite 2 courses of radiotherapy to the spine/sacrum and 1 year of temozolomide, MRI showed continued progression of disease in the spine and new brain metastases. She was referred for trial enrollment after she underwent stereotactic radiosurgery to the brain metastases.
After withdrawing from the study, patient 1 was treated off-label with the BCR-ABL TKI imatinib and showed no response; she declined rapidly and died in hospice 3 months later.
Patient 2 was a 26-year-old man with a 5-year history of resistant prolactinoma and persistent hypogonadism on testosterone replacement. At diagnosis, his PRL was 140 00 µ/L and tumor size was 50 × 41 × 39 mm; escalating doses of cabergoline resulted in initial reduction in tumor size, but a subsequent increase was observed. The patient underwent transsphenoidal surgical tumor resection in June 2015; Ki-67 was reported as rare positive. He resumed cabergoline at a dose of 6 mg/week, but his PRL increased to 447 µ/L. MRI also showed continued tumor growth and he was referred for trial enrollment.
On poststudy follow-up, patient 2 showed continued response to cabergoline therapy, with a PRL nadir of 47 µ/L, and tumor size remained stable.
Patient 3 was a 20-year-old male diagnosed in April 2016 with a 62 × 55 × 44-mm prolactinoma and an initial PRL of 3500 µ/L. He underwent subtotal tumor resection via craniotomy in 2016; Ki-67 was 15%. He was treated with cabergoline, but his PRL increased to 1900 µ/L and a concomitant increase in tumor size was noted. Despite a second subtotal resection via craniotomy in August 2016 and continued treatment with cabergoline 6 mg/week, his PRL increased to 1286 µ/L and tumor growth was noted. The patient was offered radiotherapy and opted instead for trial enrollment.
After completing the trial, patient 3 underwent repeat pituitary surgery followed by off-label treatment with the mTOR (mammalian target of rapamycin) inhibitor everolimus and proton beam radiotherapy. Tumor volume and PRL levels both remain stable.
Patient 4 was a 19-year-old female with a resistant macroprolactinoma. She was diagnosed at age 13 years with a 13 × 13 × 15-mm tumor and a PRL of 310 µ/L. She was treated with cabergoline then underwent transsphenoidal tumor resection for continued tumor growth. Ki-67 was 2% to 3%. She was restarted on cabergoline then underwent resection of the residual tumor via craniotomy in 2014. Sequential MRIs over the next 3 years showed continued tumor enlargement despite escalating doses of cabergoline up to 3 mg/week before she was referred for trial enrollment.
After completing the trial, patient 4 showed slight tumor growth despite treatment with cabergoline (5 mg/week).
Discussion
We report on the use of EGFR/HER2 TKI targeted therapy in aggressive prolactinomas in a clinical trial setting. Of the 4 individuals treated, 3 achieved tumor stabilization, and the remaining patient with metastatic disease at onset exhibited progressive disease.
Although our rigorous inclusion criteria that limited enrollment to patients with actively growing tumors undermined our ability to enroll sufficient patients to achieve adequate power, review of the 4 case histories suggests how lapatinib therapy might be integrated into the current treatment paradigm.
For example, patient 1 had been resistant to temozolomide, and proved resistant to lapatinib. Others have similarly reported that patients with aggressive pituitary tumors who failed temozolomide and were treated with lapatinib showed continued progression (11). These results suggest that TKI therapy may not be beneficial for temozolomide-resistant prolactinomas. At the same time, none of the other 3 patients had been previously treated with radiotherapy, yet all showed stable disease while on TKI despite evidence of progressive disease at enrollment. Larger sample sizes and a careful review of patient history will be important to elucidate the impact of prior therapy on TKI response, and to help determine whether TKI therapy administered at an earlier stage and/or concurrent with chemotherapy might be warranted for patients with aggressive prolactinomas.
Whether proliferation index can be used to identify likely responders also needs to be considered. Patient 3 had a Ki-67 level of 15%, whereas patients 2 and 4, as well as the 2 responders in our proof-of-concept trial (13) had low Ki-67 levels. Larger, longer-term clinical trials of lapatinib in a broader patient population could provide an opportunity to explore the potential role of tumor characteristics, such as Ki-67 levels, in defining subpopulations who might benefit most from this therapy, while balancing the risk of adverse effects from TKIs.
Because participants were maintained on cabergoline during lapatinib therapy, an important consideration is whether interaction between the 2 agents confounded our ability to assess PRL levels as an end point. Patients were kept on the same dose as at enrollment, and, per protocol, all had shown persistent disease progression despite cabergoline treatment. It therefore seems unlikely that continued use of cabergoline in our participants was a significant confounder. Nevertheless, a potential synergistic effect remains unknown. Patient 4 was temporarily off cabergoline for 3 days prior to the 3-month visit and for 2 weeks prior to the 4-month visit because of an interruption in insurance coverage. It is possible that the slight increase in PRL at the 3-month and 4-month visits shown in Fig. 3 resulted from rebound hyperprolactinemia, because her PRL level dropped again at the 5-month visit once cabergoline resumed. If true, this would suggest that maintaining the TKI while she was off cabergoline prevented rebound tumor growth, and that the added suppressive effect was lost after the lapatinib was discontinued. However, as PRL increased again by the 6-month visit despite the use of both cabergoline and lapatinib, it is also possible that there is little to no clinical effect of cabergoline withdrawal on PRL secretion in patients with DA resistance.
To our knowledge, evidence for interactions between the 2 signaling pathways is sparse. We identified 2 reports on this topic (23, 24) and their clinical significance remains unknown.
A secondary outcome of this study was the correlation between EGFR/HER2 expression and therapeutic response. Our previous studies with in vitro and in vivo models demonstrated the effects of the EGFR-targeting gefitinib and the EGFR/HER2-targeting lapatinib in prolactinomas. Specifically, we found EGF and heregulin ligands led to induction of PRL in rat lactosomatotroph tumor cells, and treatment with TKIs prevented ErbB2/ErbB3 heterodimerization and PRL secretion (21). In athymic nude mice implanted with rat growth hormone–secreting tumor (GH3) cells subcutaneously, those treated with gefitinib achieved a 50% reduction in tumor size, whereas vehicle-treated mice experienced a 26-fold tumor volume increase over baseline, and gefitinib-treated mice showed PRL levels reduced to 75% of controls (15). GH3 cell lines stably transfected with a constitutively active form of the receptor showed a 250-fold PRL induction in the absence of ligand, whereas treatment with lapatinib led to 40% suppression of PRL secretion and decreased phosphorylation of HER2 and its downstream signal (14). To recapitulate an endogenous prolactinoma model, Fischer rats were implanted with estrogen pellets and developed pituitary tumors with hyperprolactinemia and positive HER2 expression. After treatment with lapatinib, PRL levels and pituitary tumor size were suppressed by 35% compared to controls (14). Similarly, EGFR and HER2 transgenic female mice showed enlarged pituitaries or pituitary adenomas as well as increased circulating PRL levels at rates that were 4- to 5-fold those seen in wild-type mice. Treatment with lapatinib reduced PRL by 60% in EGFR transgenic mice and by 40% in HER2 transgenic mice, and PRL expression as well as extracellularly regulated kinase and protein kinase B phosphorylation were suppressed (25). In primary culture of human prolactinomas, expression of ErbB receptors and inhibitory effects of TKIs on PRL secretion and cell proliferation were confirmed. Treatment with lapatinib dose-dependently decreased PRL messenger RNA expression and PRL protein secretion, more so than did gefitinib, further emphasizing the role of HER2/ErbB2 in prolactinomas (14, 21).
In a retrospective analysis of 29 aggressive prolactinomas, we showed 82% were immunopositive for EGFR and 92% for HER2, with ErbB receptor expression correlating with tumoral invasion (13). It was therefore somewhat surprising that our present study showed no association between response to lapatinib therapy and EGFR/HER expression. We intentionally selected immunochemistry over immunofluorescence used in our previous report (13) because immunohistochemistry is commonly used clinically. However, immunofluorescence is more sensitive and detects lower levels of protein expression (26). Immunofluorescence may thus be superior in assessing EGFR/HER2 expression in pituitary tissue and be more useful for assessing the relationship between EGFR expression and lapatinib activity.
Future research efforts will also be important in elucidating whether targeting only EGFR and HER2 may be insufficient in aggressive prolactinomas. In our previous report, in addition to high rates of EGFR and HER2 expression, we observed that 71% of samples expressed ErbB4 and 25% expressed ErbB3, which was associated with higher rates of radiologic tumor invasion (13). Whether a pan-ErbB–targeted therapy may prove a more effective approach compared to a selective EGFR/HER2 TKI such as lapatinib warrants additional study.
Results of this small, phase 2a study suggest that short-term lapatinib therapy may be effective in controlling tumor growth and reducing PRL levels in select patients with aggressive prolactinomas. Larger studies more inclusive of patients with a wider variety of clinical histories will define populations most likely to benefit from novel treatments and elucidate how such agents might best fit in the current treatment paradigm.
Acknowledgments
The authors thank James Mirocha, MS, for assistance with statistical design and Shira Berman for assistance with manuscript preparation.
Financial Support: This work was supported by the National Institutes of Health (NIH grant No. R21DK105405 to O.C.) and the National Center for Advancing Translational Sciences (NCATS grant Nos. UL1TR000124 and UL1TR001881). The study drug was kindly provided by Novartis. The funding sources had no role in study design, data analysis, or decision to publish.
Clinical Trial Information: ClinicalTrials.gov identifier No. NCT00939523 (registered July 15, 2009).
Glossary
Abbreviations
- DA
dopamine agonist
- EGFR
epidermal growth factor receptor
- HER2
human epidermal growth factor receptor 2
- MRI
magnetic resonance imaging
- NCI-CTC
National Cancer Institute Common Terminology Criteria for Adverse Events
- PRL
prolactin
- RECIST
Response Evaluation Criteria in Solid Tumors
- TKI
tyrosine kinase inhibitor
Additional Information
Disclosure Summary: M.F. has received research support to the university from Novartis and has served as scientific consultant to Novartis. The remaining authors have nothing to disclose.
Data Availability
Restrictions apply to some or all the availability of data generated or analyzed during this study to preserve patient confidentiality. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.
References
- 1. Di Sarno A, Landi ML, Cappabianca P, et al. . Resistance to cabergoline as compared with bromocriptine in hyperprolactinemia: prevalence, clinical definition, and therapeutic strategy. J Clin Endocrinol Metab. 2001;86(11):5256-5261. [DOI] [PubMed] [Google Scholar]
- 2. Delgrange E, Daems T, Verhelst J, Abs R, Maiter D. Characterization of resistance to the prolactin-lowering effects of cabergoline in macroprolactinomas: a study in 122 patients. Eur J Endocrinol. 2009;160:747-752. [DOI] [PubMed] [Google Scholar]
- 3. Melmed S. Pituitary-tumor endocrinopathies. N Engl J Med. 2020;382:937-950. [DOI] [PubMed] [Google Scholar]
- 4. Melmed S, Casanueva FF, Hoffman AR, et al. ; Endocrine Society . Diagnosis and treatment of hyperprolactinemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(2):273-288. [DOI] [PubMed] [Google Scholar]
- 5. Ioachimescu AG, Fleseriu M, Hoffman AR, Vaughan TB 3rd, Katznelson L. Psychological effects of dopamine agonist treatment in patients with hyperprolactinemia and prolactin-secreting adenomas. Eur J Endocrinol. 2019;180(1):31-40. [DOI] [PubMed] [Google Scholar]
- 6. Liu W, Zahr RS, McCartney S, Cetas JS, Dogan A, Fleseriu M. Clinical outcomes in male patients with lactotroph adenomas who required pituitary surgery: a retrospective single center study. Pituitary. 2018;21(5):454-462. [DOI] [PubMed] [Google Scholar]
- 7. Buchfelder M, Zhao Y, Schlaffer SM. Surgery for prolactinomas to date. Neuroendocrinology. 2019;109(1):77-81. [DOI] [PubMed] [Google Scholar]
- 8. Lasolle H, Ilie MD, Raverot G. Aggressive prolactinomas: how to manage? Pituitary. 2020;23(1):70-77. [DOI] [PubMed] [Google Scholar]
- 9. Raverot G, Burman P, McCormack A, et al. ; European Society of Endocrinology . European Society of Endocrinology clinical practice guidelines for the management of aggressive pituitary tumours and carcinomas. Eur J Endocrinol. 2018;178(1):G1-G24. [DOI] [PubMed] [Google Scholar]
- 10. Lasolle H, Cortet C, Castinetti F, et al. . Temozolomide treatment can improve overall survival in aggressive pituitary tumors and pituitary carcinomas. Eur J Endocrinol. 2017;176(6):769-777. [DOI] [PubMed] [Google Scholar]
- 11. McCormack A, Dekkers OM, Petersenn S, et al. ; ESE survey collaborators . Treatment of aggressive pituitary tumours and carcinomas: results of a European Society of Endocrinology (ESE) survey 2016. Eur J Endocrinol. 2018;178(3):265-276. [DOI] [PubMed] [Google Scholar]
- 12. Elbelt U, Schlaffer SM, Buchfelder M, et al. . Efficacy of temozolomide therapy in patients with aggressive pituitary adenomas and carcinomas—a German survey. J Clin Endocrinol Metab. 2020;105(3):dgz211. [DOI] [PubMed] [Google Scholar]
- 13. Cooper O, Mamelak A, Bannykh S, et al. . Prolactinoma ErbB receptor expression and targeted therapy for aggressive tumors. Endocrine. 2014;46(2):318-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fukuoka H, Cooper O, Mizutani J, et al. . HER2/ErbB2 receptor signaling in rat and human prolactinoma cells: strategy for targeted prolactinoma therapy. Mol Endocrinol. 2011;25(1):92-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vlotides G, Siegel E, Donangelo I, Gutman S, Ren SG, Melmed S. Rat prolactinoma cell growth regulation by epidermal growth factor receptor ligands. Cancer Res. 2008;68(15):6377-6386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ben-Jonathan N, Chen S, Dunckley JA, LaPensee C, Kansra S. Estrogen receptor-alpha mediates the epidermal growth factor–stimulated prolactin expression and release in lactotrophs. Endocrinology. 2009;150(2):795-802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hapgood J, Libermann TA, Lax I, et al. . Monoclonal antibodies against epidermal growth factor receptor induce prolactin synthesis in cultured rat pituitary cells (GH3). Proc Natl Acad Sci U S A. 1983;80(21):6451-6455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen S, Bangaru ML, Sneade L, Dunckley JA, Ben-Jonathan N, Kansra S. Epidermal growth factor receptor cross-talks with ligand-occupied estrogen receptor-alpha to modulate both lactotroph proliferation and prolactin gene expression. Am J Physiol Endocrinol Metab. 2009;297(2):E331-E339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cooper O, Vlotides G, Fukuoka H, Greene MI, Melmed S. Expression and function of ErbB receptors and ligands in the pituitary. Endocr Relat Cancer. 2011;18(6):R197-R211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ben-Shlomo A, Cooper O. Role of tyrosine kinase inhibitors in the treatment of pituitary tumours: from bench to bedside. Curr Opin Endocrinol Diabetes Obes. 2017;24(4):301-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vlotides G, Cooper O, Chen YH, Ren SG, Greenman Y, Melmed S. Heregulin regulates prolactinoma gene expression. Cancer Res. 2009;69(10):4209-4216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Eisenhauer EA, Therasse P, Bogaerts J, et al. . New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228-247. [DOI] [PubMed] [Google Scholar]
- 23. Missale C, Castelletti L, Boroni F, Memo M, Spano P. Epidermal growth factor induces the functional expression of dopamine receptors in the GH3 cell line. Endocrinology. 1991;128(1):13-20. [DOI] [PubMed] [Google Scholar]
- 24. Huang H, Wu K, Ma J, Du Y, Cao C, Nie Y. Dopamine D2 receptor suppresses gastric cancer cell invasion and migration via inhibition of EGFR/AKT/MMP-13 pathway. Int Immunopharmacol. 2016;39:113-120. [DOI] [PubMed] [Google Scholar]
- 25. Liu X, Kano M, Araki T, et al. . ErbB receptor-driven prolactinomas respond to targeted lapatinib treatment in female transgenic mice. Endocrinology. 2015;156(1):71-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kajimura J, Ito R, Manley NR, Hale LP. Optimization of single- and dual-color immunofluorescence protocols for formalin-fixed, paraffin-embedded archival tissues. J Histochem Cytochem. 2016;64(2):112-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Restrictions apply to some or all the availability of data generated or analyzed during this study to preserve patient confidentiality. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.