Abstract
Background
Whereas biochemical response is often used as a primary study outcome, improvement in symptoms and health-related quality of life (HRQoL) is the relevant goal for patients to consider treatment successful. We performed a systematic review and meta-analysis to assess the effect of treatment on symptoms and HRQoL in acromegaly.
Methods
Seven electronic databases were searched for longitudinal studies assessing patient-reported symptoms or HRQoL in acromegaly. Meta-analyses were performed to assess differences during treatment for the Acromegaly Quality of Life Questionnaire (AcroQoL) and Patient-Assessed Acromegaly Symptom Questionnaire (PASQ), and standardized mean difference (SMD) for individual symptoms (interpretation: 0.2 small, 0.5 moderate, and 0.8 large effect). Treatment-naive and previously treated patients were assessed separately.
Results
Forty-six studies with 3301 patients were included; 24 contributed to quantitative analyses. Thirty-six studies used medication as main treatment, 1 transsphenoidal adenomectomy, and 9 various treatments. Symptoms and HRQoL both improved: AcroQoL increased 2.9 points (95% CI, 0.5 to 5.3 points), PASQ decreased –2.3 points (95% CI, –1.3 to –3.3 points), and individual symptom scores decreased for paresthesia –0.9 (95% CI, –0.6 to –1.2), hyperhidrosis –0.4 (95% CI, –0.1 to –0.6), fatigue –0.3 (95% CI, –0.1 to –0.6), arthralgia –0.3 (95% CI, –0.1 to –0.5), headache –0.3 (95% CI, 0.0 to –0.6), and soft-tissue swelling –0.2 (95% CI, 0.0 to –0.4).
Conclusion
Symptoms and HRQoL improved during acromegaly treatment. Consensus is needed on which symptoms should be included in a potential core outcome set, taking into account symptom frequency, severity, and sensitivity to change, which can be used in clinical practice and as outcome in trials.
Keywords: acromegaly, quality of life, symptoms, trials, cohort studies
Acromegaly results from an excess secretion of growth hormone (GH), which is usually produced by somatotroph cells in a GH-secreting pituitary adenoma (1). Hypersecretion of GH, and the resulting excess of insulin-like growth factor 1 (IGF-1), leads to multisystem morbidity, including diabetes mellitus (2), increased risk of cardiovascular diseases (3), and increased mortality risk (4).
Clinical symptoms of acromegaly include among others acral overgrowth, soft-tissue swelling, arthralgia, headache, fatigue, and symptoms due to local tumor effects (5). Patients with acromegaly report impaired health-related quality of life (HRQoL), which improves in a subset of patients when biochemical remission is achieved, although complete recovery is often not reached (6). To evaluate disease status during treatment, traditionally only biochemical parameters are evaluated (1), whereas improvement in symptoms and HRQoL is eventually the ultimate goal for all patients, and thus essential to consider a treatment as successful (7). Symptom status or HRQoL may respond congruently to biochemical status, but also incongruently (8). The necessity of using patient-reported outcomes is further accentuated by evidence that patients’ perception of their HRQoL might differ substantially from the perception of their physicians (9).
HRQoL can be described as a comprehensive outcome model, assessing outcomes at different levels from symptoms and bodily limitations to participation restrictions (10). The measurement of symptoms might be of extra interest, as they may be more directly responsive to acromegaly treatment, and often targetable treatment exists to relieve patients’ symptoms. To monitor symptom severity, the Patient-Assessed Acromegaly Symptom Questionnaire (PASQ) has been developed (11). The PASQ and variations of symptom lists based on the PASQ are frequently used in research, although these instruments have not been validated (12, 13). The PASQ consists of 5 acromegaly-related symptoms (soft-tissue swelling, arthralgia, headache, excessive perspiration, and fatigue), which are measured on a scale of 0 to 8 points (a higher score indicating more severe symptoms), and a cumulative score (scale 0 to 40 points) can be calculated by adding the individual components (11). To evaluate HRQoL, the disease-specific Acromegaly Quality of Life Questionnaire (AcroQoL) has been developed (14). The AcroQoL assesses physical and psychological aspects of quality of life in 22 questions, with a score ranging from 22 (worst) to 110 (best).
Study aim
The aim of the present study was to evaluate the impact of treatment for acromegaly on symptoms and HRQoL in longitudinal clinical studies in a systematic review and meta-analysis.
Materials and Methods
Eligibility criteria
Longitudinal clinical studies (ie, randomized or nonrandomized trials, and observational cohort studies) assessing patient-reported symptoms or HRQoL in adult patients with acromegaly were eligible for inclusion. Only comparative studies, measuring symptoms or HRQoL before and after study, were included. Studies assessing HRQoL were eligible for inclusion if HRQoL was assessed with a validated generic or disease-specific (for acromegaly, ie, AcroQoL) questionnaire. Studies assessing symptoms were eligible for inclusion if symptom questionnaires or symptom lists were used, provided that studies assessed symptoms in all included patients. There were no restrictions regarding treatment for acromegaly. At least 10 patients had to be included per study group to minimize risk of selection bias. Only articles written in the English language were considered. If multiple studies described (partially) overlapping populations, only the largest study was included. Three studies were not included because no full-text version of the article could be retrieved (15-17).
Search strategy
PubMed, Embase, Web of Science, Cochrane Library, EMCare, PsychINFO, and Academic Search Premier were systematically searched in May 2019 in cooperation with a specialized librarian to identify potentially relevant articles; see the data repository for the search strategy (18). PubMed was searched again in July 2020, but this did not result in additional articles that could contribute to quantitative analyses. The references of all included articles were searched for potentially eligible articles.
Data extraction
All identified references were entered in EndNote X9 (Thomson Reuters). First, the studies were screened by title and abstract only. Two independent reviewers reviewed potentially relevant articles in more detail.
The following data were extracted from all included studies: study design, number of patients, study treatment, previous treatment, treatment goal (improvement or no deterioration) in articles studying previously treated patients, age in years, sex (% male), tumor size (% macroadenoma), duration of follow-up in months, symptom questionnaires or symptom lists and HRQoL questionnaires used, outcomes at the start and end of the study period, and the interpretation of the outcomes according to the study. If data were presented only in a figure without numbers, we made an estimate from the figure (19-22).
If data were presented only according to patient categories (eg, sex), the data were combined into one outcome score using a fixed-effects meta-analysis. For articles presenting a score before a study and a difference between before and after the study, we calculated the score after the study, imputing the SD from before the study as the best estimate of the SD after the study.
Risk of bias assessment
Risk of bias was assessed for all included studies using a component approach (23). We included the following components, that could potentially bias a reported association between treatment for acromegaly and symptoms or HRQoL:
Loss to follow-up: Less than 5% was considered low risk of bias.
Missing outcome data: Missing data in less than 5% of patients was considered low risk of bias.
Inclusion of patients: Consecutive inclusion of all eligible patients or a random sample was considered low risk of bias.
Criteria for diagnosis of acromegaly: For low risk of bias, at least an oral glucose tolerance test had to be performed because of its high specificity for the diagnosis of acromegaly and to ensure comparability between patient groups in the included studies.
Assay for measurement of GH and/or IGF-1: Use of radioimmunoassay or immunoradiometric assay was considered low risk of bias because of their high sensitivity and specificity for detecting these hormones and to ensure comparability between hormone levels reported by the included studies.
We used the risk of bias assessment to explore potential heterogeneity. Because data were insufficient to estimate risk of confounding at the study level, baseline characteristics were summarized for all included articles.
Study end points
Symptom scores were analyzed separately for all symptoms reported by at least 2 studies: arthralgia, soft-tissue swelling, headache, hyperhidrosis, paresthesia, and fatigue. Furthermore, PASQ total scores were pooled for those studies using a scale of 0 to 40 points only, with higher scores representing more severe symptoms, because this is the original and most used way of calculating the PASQ total score. For this analysis 2 studies were excluded using a scale of 0 to 10 points (20, 24). HRQoL scores were pooled for AcroQoL total score, with a scale of 0 to 100 points, where a higher score represents a better HRQoL.
Stratified analyses were performed for studies with treatment-naive patients, patients who were treated previously and who were not in remission at the start of the study (categorized by us as “improvement as treatment goal”), and patients treated previously who were in remission at the start of the study (categorized by us as “no deterioration as treatment goal”). Mixed groups of treatment-naive and treated patients were coded as treated previously. For studies including a placebo group, results of placebo-treated patients after the study period were excluded from this meta-analysis.
Statistical analysis and reporting
Primary study outcome was the pooled standardized mean difference (SMD) before vs after study for symptoms for active treatment groups. Secondary study outcome was the pooled (nonstandardized) difference before vs after each study for AcroQoL and PASQ for active treatment groups. For AcroQoL and PASQ, a random-effects model was used if the questionnaire was used by at least 5 studies. A fixed-effects model was used for analyses including fewer than 5 studies, because in this case the between-study variance cannot be estimated reliably. For interpreting the SMD, as a rule of thumb an effect size of 0.2 represents a small effect, 0.5 a moderate effect, and 0.8 a large effect (25). A random-effects model was used for pooling individual symptom scores, as no fixed effect could be assumed because of heterogeneity in scale and the use of nonvalidated symptom lists.
Sensitivity analyses were performed for all studies using at least an oral glucose tolerance test to diagnose acromegaly, and for all studies using only a radioimmunoassay or immunoradiometric assay to measure GH and/or IGF-1. All outcomes were accompanied by a 95% CI. All analyses were performed in Stata 16.0 (Stata Corp). For reporting, the PRISMA (preferred reporting items for systematic reviews and meta-analyses) statement was used (26).
Results
Study selection
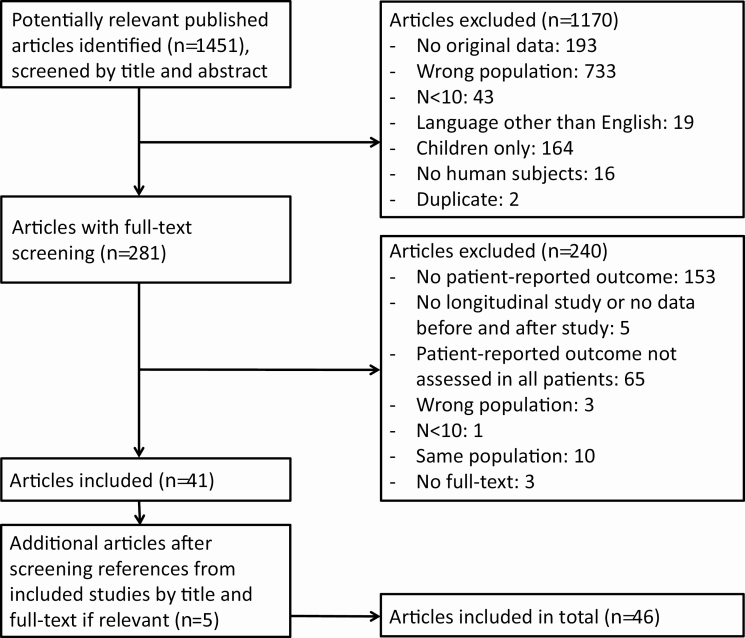
A total of 1451 unique articles were screened for title and abstract, of which 281 articles were screened in full text. After searching through the references of the included articles, another 5 articles could be included. In total, 46 articles were included for systematic review and meta-analysis, of which 24 reported on symptoms only, 10 on HRQoL only, and 12 reported on both symptoms and HRQoL. Reasons for exclusion are summarized in Fig. 1.
Figure 1.
Flowchart of study inclusion.
Study characteristics
We included 11 cohort studies (13, 21, 27-35), 24 nonrandomized trials (20, 22, 24, 36-56), and 11 randomized trials (11, 12, 19, 57-64), of which 2 were placebo controlled (11, 12). Studies were published between 1990 and 2019. In total, included studies described 3301 patients.
Baseline characteristics varied between included studies. Studies’ reported mean or median age ranged from 41.4 to 61.8 years, studies’ reported percentage male patients ranged from 20% to 70%, studies’ reported duration of follow-up ranged from 2 to 85 months, and studies’ reported percentage of pituitary macroadenomas ranged from 24% to 100%. Thirty-six studies used medication as the main treatment, 1 used transsphenoidal adenomectomy, and 9 used various treatments. A detailed overview of study characteristics of all included studies can be found in Supplementary Table 1 (18).
Risk of bias assessment
Loss to follow-up was reported by 24 studies (52%), with a range of 0% to 55% loss to follow-up. Eleven studies (24% of 46 studies) reported a loss to follow-up of less than 5%. There were 6 studies (13%) reporting missing outcome data of less than 5%. Seven articles (15%) explicitly reported recruiting consecutive patients. To establish the diagnosis acromegaly, 26 studies (57%) used at least an oral glucose tolerance test. To measure GH and/or IGF-1, 16 studies (35%) used only a radioimmunoassay or immunoradiometric assay. A detailed risk of bias assessment at the study level can be found in Supplementary Table 2 (18).
Study outcomes
In the 36 studies reporting on symptoms of acromegaly, symptoms improved in 30 (83%) and remained similar in 6 (17%) studies. In the 22 studies reporting HRQoL, HRQoL improved in 12 (55%) and remained similar in 10 (45%) studies. Of the 12 studies comparing symptoms and HRQoL both before and after a study, only one showed a different outcome for HRQoL (remained similar) and symptoms (improved). The 2 placebo-controlled trials showed an improvement in symptoms and HRQoL for the treatment groups and a deterioration for the placebo group during the study period. Detailed study outcomes can be found in Supplementary Table 3 (18) and an overview of abbreviated questionnaire names in Supplementary Table 4 (18).
Meta-analyses of symptoms and health-related quality of life in patients with acromegaly
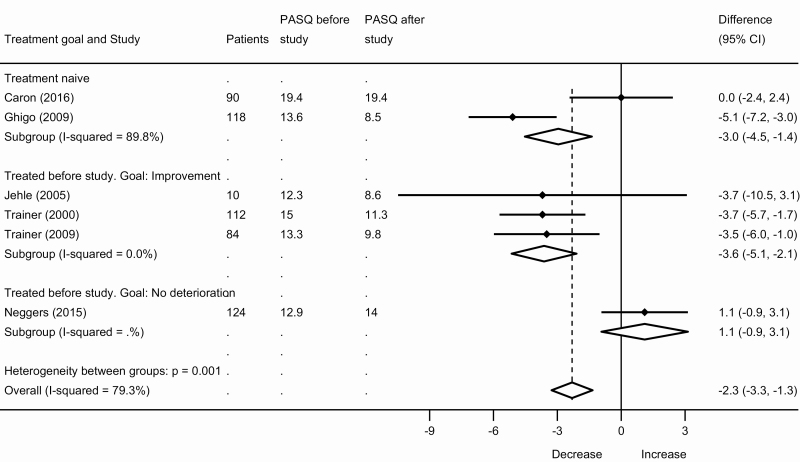
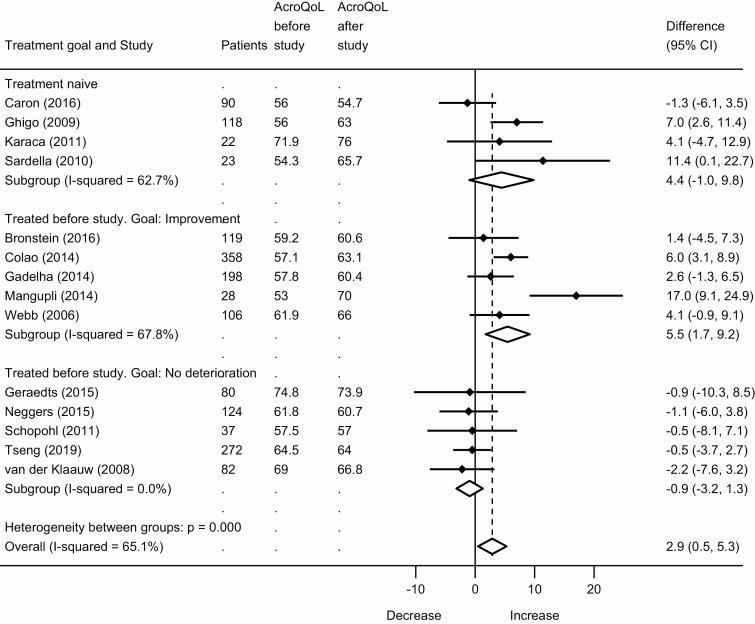
There were 24 papers that contributed data for quantitative analyses. Symptoms and HRQoL improved during treatment. The primary outcome, SMD for individual symptoms, showed a decrease in symptom scores during treatment: –0.9 (95% CI, –0.6 to –1.2) for paresthesia, –0.4 (95% CI, –0.1 to –0.6) for hyperhidrosis, –0.3 (95% CI, –0.1 to –0.6) for fatigue, –0.3 (95% CI, –0.1 to –0.5) for arthralgia, –0.3 (95% CI, 0.0 to –0.6) for headache, and –0.2 (95% CI, 0.0 to –0.4) for soft-tissue swelling; see Fig. 2. Total PASQ score decreased –2.3 points (95% CI, –1.3 to –3.3 points); see Fig. 3. AcroQoL increased: 2.9 points (95% CI, 0.5 to 5.3), see Fig. 4. For all outcomes, a larger effect size was seen in treatment-naive patients and in previously treated patients with improvement as the treatment goal, than in previously treated patients with a treatment goal of no further deterioration (eg, hyperhidrosis SMD –0.5 vs 0.1; AcroQoL 4.4 and 5.5 points vs –0.9 points); see Figs. 2-4. Sensitivity analyses for all studies using at least an oral glucose tolerance test to diagnose acromegaly, and for all studies using only a radioimmunoassay or immunoradiometric assay to measure GH and/or IGF-1, showed similar results to the main analyses; see the Supplementary Data (18).
Figure 2.
Meta-analysis of individual symptoms in patients with acromegaly.
Figure 3.
Meta-analysis of the Patient-Assessed Acromegaly Symptom Questionnaire (PASQ) in patients with acromegaly.
Figure 4.
Meta-analysis of the Acromegaly Quality of Life Questionnaire (AcroQoL) in patients with acromegaly.
Discussion
Our systematic review and meta-analysis indicates that acromegaly symptoms improve during longitudinal clinical studies in patients with acromegaly consistent with HRQoL outcomes. There is a larger effect for treatment-naive patients and previously treated patients with improvement as the treatment goal than for previously treated patients with a treatment goal of no further deterioration. Although there is no consensus yet on the method to evaluate symptoms, given the large variation described in the included studies, it is extremely valuable for patients that symptoms and HRQoL be evaluated as key treatment outcome parameters in clinical practice, in addition to biochemical outcomes.
Our results are in line with a previously performed systematic review on predictors of quality of life in patients with acromegaly (8). This study showed improved HRQoL after treatment with somatostatin receptor ligands, although there was insufficient evidence to state that biochemical control or treatment of acromegaly in general was associated with improved HRQoL, and other associated factors such as body mass index and depression were identified. Hence, the authors suggested focusing on treatment strategies to improve HRQoL in addition to normalizing biochemical markers, for example, by using alternative interventions such as psychosocial or weight-lowering interventions (8). Our findings are also in line with another systematic review on impairments in quality of life in patients with a pituitary adenoma (65). This study found an improvement in SF-36 health survey scores for patients with acromegaly after treatment compared with before treatment, but no normalization compared to a healthy control population. After patients with Cushing disease, patients with acromegaly showed the worst quality of life (65). We have performed a similar study for patients with Cushing disease, also showing an improvement of quality of life after treatment compared to before treatment (66). This suggests that our study results might be applicable to other chronic conditions, and highlights the importance of evaluating symptoms and HRQoL in all patients with chronic conditions, in clinical practice as well as in research settings.
Although HRQoL has been studied in systematic reviews before, there is no previous systematic review and meta-analysis on patient-reported symptoms, which also is an important outcome measure for the patient, and which is probably more responsive to acromegaly treatment. Therefore, a direct comparison between our study and a similar previous study was impossible to perform. Further strengths of our study are the inclusion of only longitudinal studies in our meta-analysis with measurement of symptoms or HRQoL both before and after the study period, and our separate analyses for treatment-naive and previously treated patients. The following study limitations need to be taken into account when interpreting our study results. Regarding the symptoms, most included symptom lists have not been validated, including the frequently used PASQ. However, because the PASQ is the most frequently used symptom questionnaire, and there is a need for consensus on which questionnaire to use, we suggest proceeding with the PASQ. Unfortunately, owing to a lack of individual patient data, no separate analyses could be performed per treatment method. Therefore, it remains unknown which treatment option should be preferred when aiming for the best possible HRQoL and the lowest symptom burden for patients with acromegaly. We see no clear difference in study outcomes over time that could correspond with a shift in treatment strategies, but this effect may be masked by the different years during which different centers shifted their treatment strategy, and sometimes the long periods during which patients were recruited for each study. Future studies comparing different treatment methods head to head are needed to provide a definitive answer to the question of what is the optimal treatment method.
Included studies showed risk of bias, mainly through loss to follow-up, and potential confounding through study heterogeneity (eg, percentage macroadenomas, treatment with surgery and radiotherapy). Adjustment of analyses was impossible because of a lack of individual patient data. Unfortunately, no separate sensitivity analyses with only low-risk-of-bias studies could be performed, because there were no studies with a low risk of bias on all items. Sensitivity analyses for all studies using at least an oral glucose tolerance test, and for all studies using only a radioimmunoassay or immunoradiometric assay showed results similar to the main analyses. Loss to follow-up could have resulted in a too large observed difference (ie, more improvement in symptoms and HRQoL in our meta-analyses than in reality) if mainly the worst patients were lost to follow-up. However, it is more likely that patients who perform worse find it most important to participate in quality-of-life research, leading to overparticipation of the worst patients. Therefore our results might be too pessimistic, and improvement in symptoms and HRQoL may actually be larger than observed. Risk of publication bias was minimized by searching for otherwise-unpublished meeting abstracts in Embase, Web of Science, and Cochrane Library, which did not result in additional included studies. Three studies were not found in full-text form and could not be included based on the abstract only (15-17). In total, these 3 studies included 136 patients, and they all describe improvement in symptoms without reporting individual symptoms in the abstract. Most likely, our results would have remained similar had we been able to include these 3 articles. Differences in total length of treatment are unlikely to have caused bias because one would expect a larger effect after a longer total duration of treatment for studies lasting less than 2 years, or studies offering patients multiple treatment options during the course of the study, because the treatment needs time to show effect and because if the treatment is ineffective, other treatments could be tried. The fact that our meta-analysis showed a larger effect for patients with improvement as the treatment goal than for patients with no further deterioration as the treatment goal suggests that differences in symptoms and HRQoL are due to the treatment itself and not the total length of treatment or the passing of more time.
Acromegaly symptoms result mainly from an excess of GH and IGF-1 (1). Treatment of acromegaly is generally aimed at biochemical disease control (1). By controlling GH and IGF-1, symptom load will be reduced with immediate beneficial effect for the patient. However, a correlation between biochemical control and symptoms or HRQoL is not always seen in research or clinical practice (8). There are several reasons for this. First, symptoms may be only partly caused by GH overproduction or may be only partly reversible, for example, because of irreversible damage caused by prolonged GH and IGF-1 excess, such as on joints. Which symptoms are more sensitive to change after prolonged GH and IGF-1 excess is still unclear. Second, biochemical cutoff values in trials may not reflect the individual set point of “homeostasis,” and an IGF-I level within the normal reference ranges, even when adjusted for age and sex, may still be too high for the individual patient, resulting in ongoing symptoms. HRQoL is a complex model and only treating acromegaly, albeit beneficial, may not be detectable in HRQoL measures. So, all outcome measures have their intrinsic shortcomings, which is an extra reason to measure all of them. A reduction in symptom load (and potentially also GH and IGF-1) is likely to induce an improved HRQoL. Treatment-naive patients and patients treated previously with improvement as the treatment goal showed a larger effect, which is likely due to the worse situation at the start of the study, which gives more room for improvement. However, for some outcomes (paresthesia, arthralgia, headache) patients who were effectively treated before the start of the study showed improvement, meaning that a change in treatment might be beneficial for patients willing to participate in a clinical study despite previous treatment results. This also suggests that even in biochemically controlled patients, improvement is still possible regarding symptoms and HRQoL. A detailed comparison between biochemistry and patient-reported outcomes such as symptoms and HRQoL is needed to effectively implement these patient-reported outcomes in research and clinical practice.
In conclusion, symptoms and HRQoL improve during clinical studies both in treatment-naive and previously treated patients with acromegaly. The correlation between these patient-reported outcomes and biochemistry is a topic that requires further study, meaning clinicians should pay attention to symptoms and HRQoL even after biochemical disease control is established. Normalization in symptom scores and HRQoL compared to a healthy control population is also still unknown and should be investigated further in future studies to estimate the potential for further improvement. The optimal treatment method for improvement in symptoms and HRQoL should be investigated in head-to-head trials, and consists ideally of a multidisciplinary approach including treatment and follow-up by endocrinologists, neurosurgeons, and psychologists, as well as early diagnosis to minimize risk of irreversible organ damage. Ultimately, a core outcome set should be developed and validated, including symptoms in addition to traditional biochemical outcomes. Such a core outcome set could be used in clinical trials, ensuring standardized outcome measurement. In clinical practice this could be used to optimally monitor patients over time. For the time being, we suggest using the PASQ in addition to biochemistry because to date this is the most frequently used symptom questionnaire and therefore comparable between studies.
Acknowledgments
The authors are indebted to Drs J.W. Schoones for his help in conducting the literature search, and to M. Guijt for his help in checking the data extraction.
Financial Support: This work was supported by Chiasma, Inc.
Author Contributions: The study protocol was written by A.H.Z.N. and N.R.B. with input from Chiasma, Inc. The search strategy was designed by L.H.A.B. and A.H.Z.N.. Data extraction and analyses were performed by L.H.A.B. and A.H.Z.N. L.H.A.B. performed the analyses. Data interpretation was performed by L.H.A.B., A.H.Z.N., A.M.P., O.M.D., W.R.v.F., and N.R.B. L.H.A.B. wrote the first version of the manuscript, which was revised for intellectual content by L.H.A.B., A.H.Z.N., A.M.P., O.M.D., W.R.v.F., and N.R.B. L.H.A.B., A.H.Z.N., A.M.P., O.M.D., W.R.v.F., and N.R.B. approved the final version of the manuscript. Chiasma, Inc had no role in the study conduct, data collection, analyses, data interpretation, and the decision to submit the manuscript.
Glossary
Abbreviations
- AcroQoL
Acromegaly Quality of Life Questionnaire
- GH
growth hormone
- HRQoL
health-related quality of life
- IGF-1
insulin-like growth factor-1
- PASQ
Patient-Assessed Acromegaly Symptom Questionnaire
- SMD
standardized mean difference.
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability
All data generated or analyzed during this study are included in this published article or in the data repository listed in “References.”
References
- 1. Katznelson L, Laws ER Jr, Melmed S, et al. ; Endocrine Society Acromegaly: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2014;99(11):3933-3951. [DOI] [PubMed] [Google Scholar]
- 2. Fieffe S, Morange I, Petrossians P, et al. ; French Acromegaly Registry Diabetes in acromegaly, prevalence, risk factors, and evolution: data from the French Acromegaly Registry. Eur J Endocrinol. 2011;164(6):877-884. [DOI] [PubMed] [Google Scholar]
- 3. Berg C, Petersenn S, Lahner H, et al. ; Investigative Group of the Heinz Nixdorf Recall Study and the German Pegvisomant Observational Study Board and Investigators Cardiovascular risk factors in patients with uncontrolled and long-term acromegaly: comparison with matched data from the general population and the effect of disease control. J Clin Endocrinol Metab. 2010;95(8):3648-3656. [DOI] [PubMed] [Google Scholar]
- 4. Sherlock M, Ayuk J, Tomlinson JW, et al. Mortality in patients with pituitary disease. Endocr Rev. 2010;31(3):301-342. [DOI] [PubMed] [Google Scholar]
- 5. Melmed S. Medical progress: acromegaly. N Engl J Med. 2006;355(24):2558-2573. [DOI] [PubMed] [Google Scholar]
- 6. Crespo I, Valassi E, Webb SM. Update on quality of life in patients with acromegaly. Pituitary. 2017;20(1):185-188. [DOI] [PubMed] [Google Scholar]
- 7. Biermasz NR. The burden of disease for pituitary patients. Best Pract Res Clin Endocrinol Metab. 2019;33(2):101309. [DOI] [PubMed] [Google Scholar]
- 8. Geraedts VJ, Andela CD, Stalla GK, et al. Predictors of quality of life in acromegaly: no consensus on biochemical parameters. Front Endocrinol (Lausanne). 2017;8:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Guyatt GH, Feeny DH, Patrick DL. Measuring health-related quality of life. Ann Intern Med. 1993;118(8):622-629. [DOI] [PubMed] [Google Scholar]
- 10. World Health Organization. International Classification of Functioning, Disability and Health: ICF. Geneva: World Health Organization; 2001. [Google Scholar]
- 11. Trainer PJ, Drake WM, Katznelson L, et al. Treatment of acromegaly with the growth hormone-receptor antagonist pegvisomant. N Engl J Med. 2000;342(16):1171-1177. [DOI] [PubMed] [Google Scholar]
- 12. Neggers SJ, van Aken MO, de Herder WW, et al. Quality of life in acromegalic patients during long-term somatostatin analog treatment with and without pegvisomant. J Clin Endocrinol Metab. 2008;93(10):3853-3859. [DOI] [PubMed] [Google Scholar]
- 13. Schreiber I, Buchfelder M, Droste M, et al. ; German Pegvisomant Investigators Treatment of acromegaly with the GH receptor antagonist pegvisomant in clinical practice: safety and efficacy evaluation from the German Pegvisomant Observational Study. Eur J Endocrinol. 2007;156(1):75-82. [DOI] [PubMed] [Google Scholar]
- 14. Webb SM, Prieto L, Badia X, et al. Acromegaly Quality of Life Questionnaire (ACROQOL) a new health-related quality of life questionnaire for patients with acromegaly: development and psychometric properties. Clin Endocrinol (Oxf). 2002;57(2):251-258. [DOI] [PubMed] [Google Scholar]
- 15. Lundin L, Ljunghall S, Wide L, Boström H. Bromocriptine therapy in eleven patients with acromegaly. Acta Endocrinol Suppl (Copenh). 1978;216:207-216. [PubMed] [Google Scholar]
- 16. Marek J, Hána V, Krsek M, Justová V, Catus F, Thomas F. Long-term treatment of acromegaly with the slow-release somatostatin analogue lanreotide. Eur J Endocrinol. 1994;131(1):20-26. [DOI] [PubMed] [Google Scholar]
- 17. Trainer PJ. Lessons from 6 years of GH receptor antagonist therapy for acromegaly. J Endocrinol Invest. 2003;26(10 Suppl):44-52. [PubMed] [Google Scholar]
- 18. Broersen LHA, Zamanipoor Najafabadi AH, Pereira AM, Dekkers OM, van Furth WR, Biermasz NR. Supplemental data for “Improvement in symptoms and health-related quality of life in acromegaly patients: a systematic review and meta-analysis.” figshare. 2020. Deposited August 19, 2020. https://figshare.com/articles/online_resource/Supplemental_Data/12826826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Colao A, Cappabianca P, Caron P, et al. Octreotide LAR vs. surgery in newly diagnosed patients with acromegaly: a randomized, open-label, multicentre study. Clin Endocrinol (Oxf). 2009;70(5):757-768. [DOI] [PubMed] [Google Scholar]
- 20. Ezzat S, Gaspo R, Serri O, Ur E, Chik CL. A Canadian multi-centre, open-label long-term study of Pegvisomant treatment in refractory acromegaly. Clin Invest Med. 2009;32(6):E265. [DOI] [PubMed] [Google Scholar]
- 21. Geraedts VJ, Dimopoulou C, Auer M, Schopohl J, Stalla GK, Sievers C. Health outcomes in acromegaly: depression and anxiety are promising targets for improving reduced quality of life. Front Endocrinol (Lausanne). 2014;5:229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mangupli R, Lisette A, Ivett C, Paul C, de los Ríos Victoria C, Luis CJ. Improvement of acromegaly after octreotide LAR treatment. Pituitary. 2003;6(1):29-34. [DOI] [PubMed] [Google Scholar]
- 23. Dekkers OM, Vandenbroucke JP, Cevallos M, Renehan AG, Altman DG, Egger M. COSMOS-E: guidance on conducting systematic reviews and meta-analyses of observational studies of etiology. PLoS Med. 2019;16(2):e1002742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shimatsu A, Nagashima M, Hashigaki S, Ohki N, Chihara K. Efficacy and safety of monotherapy by pegvisomant, a growth hormone receptor antagonist, in Japanese patients with acromegaly. Endocr J. 2016;63(4):337-347. [DOI] [PubMed] [Google Scholar]
- 25. Grading of Recommendations Assessment, Development and Evaluation (GRADE) Working Group. About re-expressing SMD. Published online July 19, 2012. https://pregnancy.cochrane.org/sites/pregnancy.cochrane.org/files/public/uploads/re-expressing%20SMD%20new.pdf. Accessed August 12, 2020. [Google Scholar]
- 26. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cheung NW, Taylor L, Boyages SC. An audit of long-term octreotide therapy for acromegaly. Aust N Z J Med. 1997;27(1):12-18. [DOI] [PubMed] [Google Scholar]
- 28. Claessen KM, Ramautar SR, Pereira AM, et al. Increased clinical symptoms of acromegalic arthropathy in patients with long-term disease control: a prospective follow-up study. Pituitary. 2014;17(1):44-52. [DOI] [PubMed] [Google Scholar]
- 29. Fujio S, Arimura H, Hirano H, et al. Changes in quality of life in patients with acromegaly after surgical remission—a prospective study using SF-36 questionnaire. Endocr J. 2017;64(1):27-38. [DOI] [PubMed] [Google Scholar]
- 30. Jehle S, Reyes CM, Sundeen RE, Freda PU. Alternate-day administration of pegvisomant maintains normal serum insulin-like growth factor-I levels in patients with acromegaly. J Clin Endocrinol Metab. 2005;90(3):1588-1593. [DOI] [PubMed] [Google Scholar]
- 31. Mangupli R, Camperos P, Webb SM. Biochemical and quality of life responses to octreotide-LAR in acromegaly. Pituitary. 2014;17(6):495-499. [DOI] [PubMed] [Google Scholar]
- 32. Suliman M, Jenkins R, Ross R, Powell T, Battersby R, Cullen DR. Long-term treatment of acromegaly with the somatostatin analogue SR-lanreotide. J Endocrinol Invest. 1999;22(6):409-418. [DOI] [PubMed] [Google Scholar]
- 33. Tseng FY, Huang TS, Lin JD, et al. A registry of acromegaly patients and one year following up in Taiwan. J Formos Med Assoc. 2019;118(10):1430-1437. [DOI] [PubMed] [Google Scholar]
- 34. van der Klaauw AA, Biermasz NR, Hoftijzer HC, Pereira AM, Romijn JA. Previous radiotherapy negatively influences quality of life during 4 years of follow-up in patients cured from acromegaly. Clin Endocrinol (Oxf). 2008;69(1):123-128. [DOI] [PubMed] [Google Scholar]
- 35. Webb SM, Badia X, Surinach NL; Spanish AcroQol Study Group Validity and clinical applicability of the Acromegaly Quality of Life Questionnaire, AcroQoL: a 6-month prospective study. Eur J Endocrinol. 2006;155(2):269-277. [DOI] [PubMed] [Google Scholar]
- 36. Alexopoulou O, Abrams P, Verhelst J, et al. Efficacy and tolerability of lanreotide Autogel therapy in acromegalic patients previously treated with octreotide LAR. Eur J Endocrinol. 2004;151(3):317-324. [DOI] [PubMed] [Google Scholar]
- 37. Ambrosio MR, Franceschetti P, Bondanelli M, et al. Efficacy and safety of the new 60-mg formulation of the long-acting somatostatin analog lanreotide in the treatment of acromegaly. Metabolism. 2002;51(3):387-393. [DOI] [PubMed] [Google Scholar]
- 38. Bevan JS, Atkin SL, Atkinson AB, et al. Primary medical therapy for acromegaly: an open, prospective, multicenter study of the effects of subcutaneous and intramuscular slow-release octreotide on growth hormone, insulin-like growth factor-I, and tumor size. J Clin Endocrinol Metab. 2002;87(10):4554-4563. [DOI] [PubMed] [Google Scholar]
- 39. Bronstein MD, Fleseriu M, Neggers S, et al. ; Pasireotide C2305 Study Group Switching patients with acromegaly from octreotide to pasireotide improves biochemical control: crossover extension to a randomized, double-blind, phase III study. BMC Endocr Disord. 2016;16:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Caron PJ, Bevan JS, Petersenn S, Houchard A, Sert C, Webb SM; PRIMARYS Investigators Group Effects of lanreotide Autogel primary therapy on symptoms and quality-of-life in acromegaly: data from the PRIMARYS study. Pituitary. 2016;19(2):149-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chin SO, Chung CH, Chung YS, et al. Change in quality of life in patients with acromegaly after treatment with octreotide LAR: first application of AcroQoL in Korea. BMJ Open. 2015;5(6):e006898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Colao A, Pivonello R, Auriemma RS, et al. Efficacy of 12-month treatment with the GH receptor antagonist pegvisomant in patients with acromegaly resistant to long-term, high-dose somatostatin analog treatment: effect on IGF-I levels, tumor mass, hypertension and glucose tolerance. Eur J Endocrinol. 2006;154(3):467-477. [DOI] [PubMed] [Google Scholar]
- 43. Colao A, Auriemma RS, Rebora A, et al. Significant tumour shrinkage after 12 months of lanreotide Autogel-120 mg treatment given first-line in acromegaly. Clin Endocrinol (Oxf). 2009;71(2):237-245. [DOI] [PubMed] [Google Scholar]
- 44. Giusti M, Ciccarelli E, Dallabonzana D, et al. Clinical results of long-term slow-release lanreotide treatment of acromegaly. Eur J Clin Invest. 1997;27(4):277-284. [DOI] [PubMed] [Google Scholar]
- 45. Karaca Z, Tanriverdi F, Elbuken G, et al. Comparison of primary octreotide-LAR and surgical treatment in newly diagnosed patients with acromegaly. Clin Endocrinol (Oxf). 2011;75(5):678-684. [DOI] [PubMed] [Google Scholar]
- 46. Lancranjan I, Atkinson AB. Results of a European multicentre study with Sandostatin LAR in acromegalic patients. Sandostatin LAR Group. Pituitary. 1999;1(2):105-114. [DOI] [PubMed] [Google Scholar]
- 47. Mercado M, Borges F, Bouterfa H, et al. ; SMS995B2401 Study Group A prospective, multicentre study to investigate the efficacy, safety and tolerability of octreotide LAR (long-acting repeatable octreotide) in the primary therapy of patients with acromegaly. Clin Endocrinol (Oxf). 2007;66(6):859-868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Neggers SJ, Pronin V, Balcere I, et al. ; LEAD Study Group Lanreotide Autogel 120 mg at extended dosing intervals in patients with acromegaly biochemically controlled with octreotide LAR: the LEAD study. Eur J Endocrinol. 2015;173(3):313-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Salvatori R, Nachtigall LB, Cook DM, et al. ; SALSA Study Group Effectiveness of self- or partner-administration of an extended-release aqueous-gel formulation of lanreotide in lanreotide-naïve patients with acromegaly. Pituitary. 2010;13(2):115-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sardella C, Lombardi M, Rossi G, et al. Short- and long-term changes of quality of life in patients with acromegaly: results from a prospective study. J Endocrinol Invest. 2010;33(1):20-25. [DOI] [PubMed] [Google Scholar]
- 51. Sassolas G, Harris AG, James-Deidier A. Long term effect of incremental doses of the somatostatin analog SMS 201-995 in 58 acromegalic patients. French SMS 201-995 approximately equal to Acromegaly Study Group. J Clin Endocrinol Metab. 1990;71(2):391-397. [DOI] [PubMed] [Google Scholar]
- 52. Schopohl J, Strasburger CJ, Caird D, et al. ; German Lanreotide Study Group Efficacy and acceptability of lanreotide Autogel 120 mg at different dose intervals in patients with acromegaly previously treated with octreotide LAR. Exp Clin Endocrinol Diabetes. 2011;119(3):156-162. [DOI] [PubMed] [Google Scholar]
- 53. Sonino N, Scarpa E, Paoletta A, Fallo F, Boscaro M. Slow-release lanreotide treatment in acromegaly: effects on quality of life. Psychother Psychosom. 1999;68(3):165-167. [DOI] [PubMed] [Google Scholar]
- 54. van der Lely AJ, Bernabeu I, Cap J, et al. Coadministration of lanreotide Autogel and pegvisomant normalizes IGF1 levels and is well tolerated in patients with acromegaly partially controlled by somatostatin analogs alone. Eur J Endocrinol. 2011;164(3):325-333. [DOI] [PubMed] [Google Scholar]
- 55. Verhelst JA, Pedroncelli AM, Abs R, et al. Slow-release lanreotide in the treatment of acromegaly: a study in 66 patients. Eur J Endocrinol. 2000;143(5):577-584. [DOI] [PubMed] [Google Scholar]
- 56. Yetkin DO, Boysan SN, Tiryakioglu O, Yalin AS, Kadioglu P. Forty month follow-up of persistent and difficultly controlled acromegalic patients treated with depot long acting somatostatin analog octreotide. Endocr J. 2007;54(3):459-464. [DOI] [PubMed] [Google Scholar]
- 57. Cannavò S, Squadrito S, Curtò L, Almoto B, Trimarchi F. Effectiveness of slow-release lanreotide in previously operated and untreated patients with GH-secreting pituitary macroadenoma. Horm Metab Res. 2001;33(10):618-624. [DOI] [PubMed] [Google Scholar]
- 58. Colao A, Bronstein MD, Freda P, et al. ; Pasireotide C2305 Study Group Pasireotide versus octreotide in acromegaly: a head-to-head superiority study. J Clin Endocrinol Metab. 2014;99(3):791-799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Gadelha MR, Bronstein MD, Brue T, et al. ; Pasireotide C2402 Study Group Pasireotide versus continued treatment with octreotide or lanreotide in patients with inadequately controlled acromegaly (PAOLA): a randomised, phase 3 trial. Lancet Diabetes Endocrinol. 2014;2(11):875-884. [DOI] [PubMed] [Google Scholar]
- 60. Ghigo E, Biller BM, Colao A, et al. Comparison of pegvisomant and long-acting octreotide in patients with acromegaly naïve to radiation and medical therapy. J Endocrinol Invest. 2009;32(11):924-933. [DOI] [PubMed] [Google Scholar]
- 61. Madsen M, Poulsen PL, Orskov H, Møller N, Jørgensen JO. Cotreatment with pegvisomant and a somatostatin analog (SA) in SA-responsive acromegalic patients. J Clin Endocrinol Metab. 2011;96(8):2405-2413. [DOI] [PubMed] [Google Scholar]
- 62. Petersenn S, Schopohl J, Barkan A, et al. ; Pasireotide Acromegaly Study Group Pasireotide (SOM230) demonstrates efficacy and safety in patients with acromegaly: a randomized, multicenter, phase II trial. J Clin Endocrinol Metab. 2010;95(6):2781-2789. [DOI] [PubMed] [Google Scholar]
- 63. Trainer PJ, Ezzat S, D’Souza GA, Layton G, Strasburger CJ. A randomized, controlled, multicentre trial comparing pegvisomant alone with combination therapy of pegvisomant and long-acting octreotide in patients with acromegaly. Clin Endocrinol (Oxf). 2009;71(4):549-557. [DOI] [PubMed] [Google Scholar]
- 64. Trainer PJ, Newell-Price JDC, Ayuk J, et al. A randomised, open-label, parallel group phase 2 study of antisense oligonucleotide therapy in acromegaly. Eur J Endocrinol. 2018;179(2):97-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Andela CD, Scharloo M, Pereira AM, Kaptein AA, Biermasz NR. Quality of life (QoL) impairments in patients with a pituitary adenoma: a systematic review of QoL studies. Pituitary. 2015;18(5):752-776. [DOI] [PubMed] [Google Scholar]
- 66. Broersen LHA, Andela CD, Dekkers OM, Pereira AM, Biermasz NR. Improvement but no normalization of quality of life and cognitive functioning after treatment of cushing syndrome. J Clin Endocrinol Metab. 2019;104(11):5325-5337. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article or in the data repository listed in “References.”