Abstract
Acinetobacter non-baumannii species are becoming common etiologic agents of nosocomial infections. Furthermore, clinical isolates belonging to this group of bacteria are usually resistant to one or more antibiotics. The current information about antibiotic resistance genes in the different A. non-baumannii species has not yet been studied as a whole. Therefore, we did a comparative study of the resistomes of A. non-baumannii pathogens based on information available in published articles and genome sequences. We searched the available literature and sequences deposited in GenBank to identify the resistance gene content of A. calcoaceticus, A. lwoffii, A. junii, A. soli, A. ursingii, A. bereziniae, A. nosocomialis, A. portensis, A. guerrae, A. baylyi, A. calcoaceticus, A. disperses, A. johnsonii, A. junii, A. lwoffii, A. nosocomialis, A. oleivorans, A. oryzae, A. pittii, A. radioresistens, and A. venetianus. The most common genes were those coding for different β-lactamases, including the carbapenemase genes blaNDM-1 and blaOXA-58. A. pittii was the species with the most β-lactamase resistance genes reported. Other genes that were commonly found include those encoding some aminoglycoside modifying enzymes, the most common being aph(6)-Id, ant(3″)-IIa, and aph(3″)-Ib, and efflux pumps. All or part of the genes coding for the AdeABC, AdeFGH, and AdeIJK efflux pumps were the most commonly found. This article incorporates all the current information about A. non-baumannii resistance genes. The comparison of the different resistomes shows that there are similarities in the genes present, but there are also significant differences that could impact the efficiency of treatments depending on the etiologic agent. This article is a comprehensive resource about A. non-baumannii resistomes.
Keywords: Acinetobacter non-baumannii, antimicrobial resistance, resistome
1. Introduction
The genus Acinetobacter is a very diverse group of bacteria considered one of the most troublesome opportunistic nosocomial pathogens. It comprises 62 species with assigned names (http://www.bacterio.net/a/acinetobacter.html). A. baumannii is responsible for most nosocomial infections and has an intrinsic ability to acquire resistance to all available antibiotics. The problems caused by this bacterium led World Health Organization (WHO) experts and researchers from the Division of Infectious Diseases at the University of Tübingen (Germany) to include it in the critical priority group. Furthermore, the rise of carbapenem-resistant Acinetobacter led to its placement within a group of five pathogens considered as urgent threats to human health by the Center for Disease Control [1]. Although in the past decades the vast majority of Acinetobacter infections used to be caused by the species baumannii, in the past few years numerous other Acinetobacter species became commonly identified as causative agents of nosocomial infections. A. calcoaceticus, A. lwoffii, A. junii, A. soli, A. ursingii, A. bereziniae and A. nosocomialis are also becoming common culprits of hospital infections [2,3]. Despite their diversity, a common characteristic of these species is the presence of multiple antibiotic resistance genes. Likewise, multiple antibiotic resistance mechanisms have been documented in other Acinetobacter species (A. lwoffii, A. johnsonii, A. junnii, A. nosocomialis and A. pittii) mainly recovered from soil and wastewater, as well as vegetables and meats. These species are important environmental reservoirs of resistance genetic determinants, which could later give rise to clinically relevant strains [2,3,4,5,6,7].
In this work, we conduct a comprehensive analysis of reported antibiotic resistance genes in A. non-baumannii species. Other genes found in a lower number of isolates were those coding for resistance to trimethoprim (dfrA-like), macrolide (ereA2, ermB), colistin (mcr-1, mcr-4), fluoroquinolone (qnrD1, qnrS1), rifampicin (arr-2), chloramphenicol (cat-like), lincosamide (InuF, InuG) and tetracycline (tet-like).
2. Results and Discussion
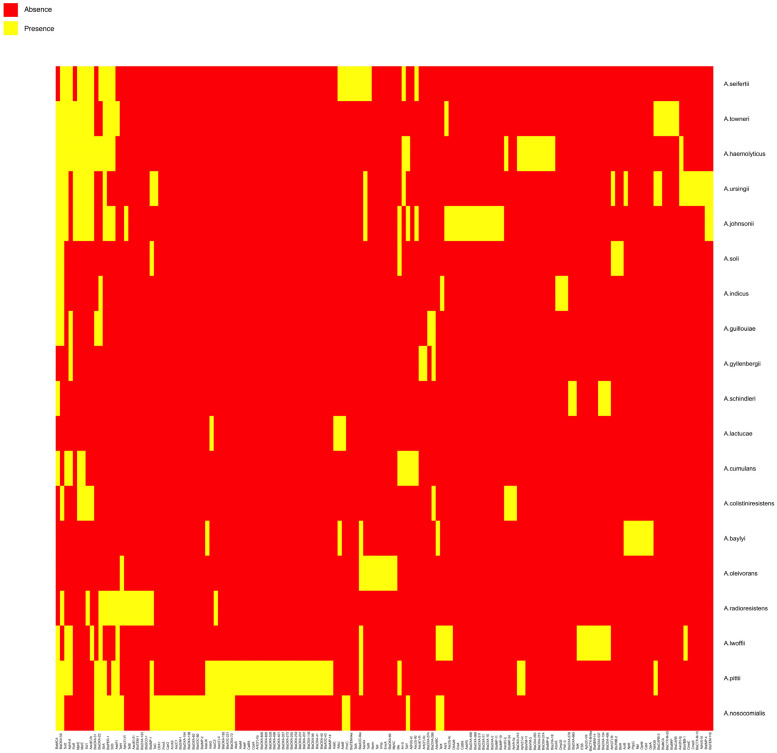
A summary of all A. non-baumannii species harboring potential antibiotic resistance genes (ARGs) reported in the literature is shown in Table 1. A detailed listing of the individual ARGs identified in each strain is shown in Table S1. The vast majority of ARGs code for β-lactamases of different classes (n = 153 for 31 isolates). The highest represented β-lactamase genes were blaNDM-1 and blaOXA-58 (14 and 12 strains, respectively) (Table S1). NDM-1 is a troublesome metallo-β-lactamase originally identified in a carbapenem-resistant Klebsiella pneumoniae isolate that, except for monobactams, confers resistance to all other β-lactam antibiotics [8,9,10]. The blaOXA-58 gene was first found in a multiresistant A. baumannii isolated in a hospital in France [11]. OXA-58 belongs to a group of enzymes with a reduced number of variants. A. baumannii strains carrying this enzyme showed different levels of resistance to carbapenems [10]. A. pittii was the species with the most β-lactamase resistance genes reported (n = 28), followed by A. nosocomialis (n = 15) and A. haemolyticus (n = 14) (Figure 1, Figure S1, and Table S1).
Table 1.
Number of antibiotic resistance genes (ARGs) in Acinetobacter non-baumannii isolates reported in the literature.
| Number of ARG in Acinetobacter non-baumannii Isolates Reported in the Literature | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β-lactamases | Aminoglycosides | Efflux Pump Genes | Sulfonamides | Tetracyclines | Macrolides | ABC-F | Rifampicin | Florfenicol | Bleomycin | Chloramphenicol | Carbapenem (CarO Specific) |
OMP | Fluoroquinolone | PBPs | Pmr | Trimhoprim |
| A. baylys (n = 1) | A. baylys (n = 1) | A. apis (n = 1) | A. colistiniresistens (n = 1) | A. indicus (n = 1) | A. colistiniresistens (n = 1) | A. colistiniresistens (n = 1) | A. cumulans (n = 1) | A. haemolyticus (n = 1) | A. cululans (n = 1) | A. baylyi (n = 1) | A. apis (n = 1) | A. apis (n = 1) | A. baylyi (n = 1) | A. baylyi (n = 1) | A. nosocomialis (n = 1) | A. nosocomialis (n = 1) |
| A. bereziniae (n = 1) | A. calcoaceticus (n = 1) | A. baylys (n = 6) | A. cumulans (n = 1) | A. lwoffii (n = 1) | A. cumulans (n = 1) | A. cumulans (n = 1) | A. haemolyticus (n = 1) | A. johnsonii (n = 1) | A. johnsonii (n = 1) | A. johnsonii (n = 1) | A. nosocomialis (n = 1) | A. indicus (n = 1) | A. ursingii (n = 1) | A. indicus (n = 1) | A. seifertii (n = 1) | A. johnsonii (n = 1) |
| A. beijerinckii (n = 1) | A. colistiniresistens (n = 3) | A. cumulans (n = 1) | A. gandensis (n = 1) | A. nosocomialis (n = 2) | A. haemolyticus (n = 1) | A. haemolyticus (n = 1) | A. johnsonii (n = 1) | A. seifertii (n = 1) | A. pittii (n = 1) | A. modestus (n = 1) | ||||||
| A. bohemicus (n = 1) | A. cumulans (n = 3) | A. haemolyticus (n = 1) | A. haemolyticus (n = 2) | A. oleivorans (n = 3) | A. indicus (n = 1) | A. johnsonii (n = 1) | A. lwoffii (n = 1) | A. towneri (n = 1) | A. soli (n = 2) | A. pittii (n = 1) | ||||||
| A. calcoaceticus (n = 3) | A. gerneri (n = 2) | A. johnsonii (n = 1) | A. johnsonii (n = 2) | A. pitii (n = 1) | A. johnsonii (n = 2) | A. seifertii (n = 1) | A. seifertii (n = 1) | A. ursingii (n = 1) | ||||||||
| A. chinensis (n = 1) | A. guillouiae (n = 1) | A. lactucae (n = 4) | A. lwoffii (n = 1) | A. radioresistens (n = 2) | A. oleivorans (n = 1) | A. towneri (n = 1) | A. towneri (n = 1) | |||||||||
| A. colistiniresistens (n = 3) | A. gyllenbergii (n = 3) | A. lwoffii (n = 3) | A. pittii (n = 1) | A. seifertii (n = 2) | A. seifertii (n = 1) | A. ursingii (n = 1) | A. ursingii (n = 1) | |||||||||
| A. cumulans (n = 1) | A. haemolyticus (n = 7) | A. nosocomialis (n = 8) | A. radioresistens (n = 1) | A. towneri (n = 1) | A. towneri (n = 1) | |||||||||||
| A. dijkshoorniae (n = 3) | A. johnsonii (n = 9) | A. oleivorans (n = 3) | A. seifertii (n = 2) | A. ursingii (n = 1) | A. ursingii (n = 1) | |||||||||||
| A. disperusus (n = 1 | A. lwoffii (n = 5) | A. pittii (n = 5) | A. towneri (n = 2) | |||||||||||||
| A. gandensis (n = 2) | A. nosocomialis (n = 7) | A. seifertii (n = 2) | A. ursingii (n = 2) | |||||||||||||
| A. guillouiae (n = 6) | A. oleivorans (n = 1) | A. ursingii (n = 3) | ||||||||||||||
| A. gyllenbergii (n = 1) | A. parvus (n = 1) | |||||||||||||||
| A. haemolyticus (n = 14) | A. pittii (n = 9) | |||||||||||||||
| A. indicus (n = 3) | A. radioresistens (n = 6) | |||||||||||||||
| A. johnsonii (n = 13) | A. rudis (n = 1) | |||||||||||||||
| A. kyonggiensis (n = 1) | A. seifertii (n = 6) | |||||||||||||||
| A. lwoffii (n = 9) | A. soli (n = 1) | |||||||||||||||
| A. nosocomialis (n = 15) | A. ursingii (n = 5) | |||||||||||||||
| A. oleivorans (n = 2) | A. towneri (n = 5) | |||||||||||||||
| A. parvus (n = 1) | ||||||||||||||||
| A. pitti (n = 28) | ||||||||||||||||
| A. proteolyticus (n = 1) | ||||||||||||||||
| A. radioresistens (n = 7) | ||||||||||||||||
| A. schindleri (n = 6) | ||||||||||||||||
| A. seifertii (n = 5) | ||||||||||||||||
| A. soli (n = 4) | ||||||||||||||||
| A. tandoii (n = 1) | ||||||||||||||||
| A. towneri (n = 8) | ||||||||||||||||
| A. ursingii (n = 8) | ||||||||||||||||
| A. variabilis (n = 2) | ||||||||||||||||
| Total Number of ARGs | ||||||||||||||||
| N = 153 | N = 77 | N = 38 | N = 16 | N = 14 | N = 10 | N = 7 | N = 7 | N = 5 | N = 4 | N = 4 | N = 2 | N = 2 | N = 2 | N = 2 | N = 2 | N = 2 |
The antibiotic resistance genes were identified searching PubMed. The search was carried out inputting the full name of each species with the All Fields selection, followed by identification of the antibiotic resistance genes in each article. ABC-F ATP-binding cassette ribosomal protection protein gene resistance; OMP: Outer membrane porin drug resistance genes; PBPs: penicllin-binding proteins; Pmr: phosphoethanolamine transferase.
Figure 1.
A. non-baumannii species which carry three or more ARGs as found in the literature. Heatmap showing species that harbor of three or more ARGs. Yellow, presence of the gene; red, absence of the gene in the corresponding species. A complete listing including A. non-baumannii species that carry any number of ARGs can be found in Table S1 and Figure S1.
The second most abundant resistance mechanism was that mediated by aminoglycoside modifying enzymes (n = 77 for 20 species, Table 1), aphA6 and strA being the most reported genes. Aminoglycosides are bactericidal antibiotics used to treat a wide range of bacterial infections, including those caused by Acinetobacter [12,13]. The most common mechanism of resistance to aminoglycosides is the enzymatic inactivation of the antibiotic molecule. Aminoglycoside modifying enzymes catalyze the transfer of acetyl, phosphate, or nucleotidyl groups to the –OH or –NH2 groups of the 2-deoxystreptamine or the sugar moieties of the molecule [14].
The third most abundant mechanism was the efflux pumps, i.e., cellular systems that can export compounds usually without specificity. This mechanism specifies resistance through detoxification [15,16]. A total of 38 antibiotic efflux pump genes were identified in 12 isolates (Figure 1, Figure S1, and Table S1). The resistance-nodulation-cell division (RND) family efflux systems AdeABC and AdeIJK were the most common efflux pumps present in A. non-baumannii species. Both efflux systems can expel a broad range of antibiotics [17,18,19]. In the case of A. baumannii, all isolates studied to date carry AdeIJK, and a majority, but not all, carry AdeABC [19]. A. pittii is the species with the highest variety ARGs (n = 46), followed by A. nosocomialis (n = 35), A. johnsonii (n = 33), A. and A. haemolyticus (n = 28). Thirty-four A. non-baumannii species did not carry any of the tested ARGs (Table S1).
Since the nucleotide sequence data banks include an excess of information when compared to that already published in scientific articles, another set of analyses was carried out using the Basic Local Alignment Search Tool (BLAST) software and the data from the databases CARD-RGI, ARG-ANNOT, and ResFinder. There were 1457 complete A. non-baumannii genomes in GenBank as of June 15, 2020. Each one of them was compared with the databases CARD-RGI, ARG-ANNOT and ResFinder using BLAST. Comparisons to information in the ARG-ANNOT database produced 9676 hits corresponding to efflux pumps genes in 1404 genomes. Genes related to AdeABC, AdeFGH and AdeIJK were the efflux pumps most commonly present, and AdeFGH and AdeIJK were those found more often in a complete version (Figure S2 and Table S2). The second group of predominant genes was β-lactamase resistance genes (n = 1574 for 963 genomes) (Figure S2 and Table S2). A total of 245 A. pittii, 187 A. sp., and 143 A. nosocomialis isolates harbored β-lactamase resistance genes. The blaOXA-421 and blaADC-4 genes were the highest represented β-lactamases. The species with the highest ARG content were A. pitti (n = 3841), A. sp. (n = 2915), and A. nosocomialis (n = 2277).
Aminoglycoside modifying enzyme coding genes were the third most abundant group of resistance determinants; 1084 genes being found in 599 genomes in the database. The species with the most aminoglycoside resistance genes were A. pitti, A. sp, A. indicus and A. haemolyticus. The most identified genes were aph(6)-Id, ant(3″)-IIa and aph(3″)-Ib (Figure S2).
No ARGs were identified in A. nectaris, A. marinus, A. equi, A. larvae, A. qingfengensis and A. boissieri by BLAST comparisons or the literature search. In the cases of A. albensis, A. bouvetii, A. brisouii, A. celticus, A. harbinensis, A. junii, A. kookii, A. pragensis and A. pseudolwoffii, no ARGs were identified in the literature. Still, at least one resistance-nodulation-cell division (RND) antibiotic efflux pump gene was identified by the BLAST comparisons. All adeIJK efflux pump genes were identified in A. defluvii, A. junii, A. venetianus, A.halotolerans, A. tjernbergiae, and A. wuhouensis. All adeFGH efflux pump genes were found in A. courvalinii. Besides the efflux pump, other ARGs genes were detected in A. bouvetii, A. courvalinii, A. defluvii, A. junni, A. pseudolwoffii, A. venetianus and A. wuhouensis).
The BLAST comparison permitted us to detect genes and the number of allelic variants (n) that are not yet reported in A. non-baumannii publications (Figures S1 and S2, Tables S1 and S2). The β-lactamase coding genes found are blaADC-like (n = 22), blaOXA-like (n = 46), blaKPC-1, blaCTX-M-65, blaNDM-3, blaPER-2, blaCARB-like (n = 4), blaTEM-4, blaVIM-4, blaVEB-7, blaGES-7. The aminoglycoside modifying enzyme coding genes identified were AAC(6′)-like (n = 12), AAC(3)-like (n = 3), aadA-like (n = 5), ANT(2″)-Ia, ANT(3″)-IIb, APH(4)-Ia, APH(3′)-like (n = 3). In addition, we identified genes that code for resistance to trimethoprim (dfrA-like (n = 7)), macrolide (ereA2, ermB), colistin (mcr-1, mcr-4), fluoroquinolone (qnrD1, qnrS1), rifampicin (arr-2), chloramphenicol (cat-like (n = 7)), lincosamide (InuF, InuG) and tetracycline (tet-like (n = 4)). Among the efflux pump related genes, we identified adeH, adeL, adeN, adeR, and adeS.
We also found ARGs described in A. non-baumannii publications that were not identified by the BLAST comparisons (Tables S1 and S2). The β-lactamase coding genes found are blaADC-221, blaIMP-like (n = 4), blaVIM-like (n = 2), blaCTX-M-15, blaNDM-14, blaCARB-2, blaSCO-1, blaOXA-like (n = 29), blaTEM-like (n = 3). The aminoglycoside modifying enzyme coding genes detected were aphA-like (n = 4), nptII, strA, cpaA, aacC2, aacC. Other genes identified were those coding for resistance to trimethoprim (dfrA-like (n = 2)), chloramphenicol (cat-like (n = 3)), macrolide (ereA, mphC, mphE, pld1-3), fluoroquinolone (acrB), tetracycline (tet-like (n = 4)). Among the efflux pump related genes, we identified AcrAB, adeE, dprA, cmeB, cmeC, craA, tolC, norm, emrA, pmrA, ump and mpl.
3. Materials and Methods
3.1. Literature Search
All known Acinetobacter species, as listed in the List of Prokaryotic names with Standing in Nomenclature [20], were used in a PubMed literature search. Two species, (A. portensis and A. guerrae), recently discovered by Caravalheira et. al. [21] were also included. The search was conducted by inputting the full name of each species with the All Fields selection in the PubMed database and then identifying the antibiotic resistance genes described in each article. CARD-RGI software [22] was used to verify the antibiotic resistance nature of genes that were not well defined in the publications.
3.2. Genome Sequences Collection and Antibiotic Resistance Genes Prediction
All complete A. non-baumannii genome nucleotide sequences were downloaded from GenBank NCBI. Predictive identification of antibiotic resistance genes (ARGs) was performed using the BLASTp, the CARD-RGI [22], ARG-ANNOT [23] and ResFinder [24] software databases. For ARGs prediction, 70% coverage, 95% amino acid identity and <10−6 e-value were used as the BLASTp parameters. Data prediction using the three databases were integrated for clustering analysis. Clustering analysis was carried out using the hclust and dist R package [25]. The Euclidean method was used as distance method. The agglomerative method was used as Hierarchical clustering method. Within the agglomerative methods, the complete-linkage algorithm was used for hierarchical clustering analysis. Agglomerative methods were reported as the most adequate to analyze the presence and absence of genes over genomes [26].
4. Conclusions
The importance of A. non-baumannii as pathogens, and the volume of information about their resistance to antimicrobials, is rapidly increasing. This article describes an initial study of the resistome of A. non-baumannii pathogens. The impact of this group of bacteria as causative agents of nosocomial infections is growing. As a consequence, while Acinetobacter infections are still mainly caused by A. baumannii [1,27], it is quite probable that other species will become as important in the future. It is already evident that multiple A. non-baumannii clinical isolates are resistant to one or more antibiotics [2,3]. The present and future efforts to design effective therapies against these pathogens require understanding their resistance profiles and genes. The comparison of existing data (literature and GenBank) about resistance genetic determinants showed similarities and significant differences that could impact the efficiency of treatments depending on the species that originate each infection.
Supplementary Materials
The following are available online at https://www.mdpi.com/2079-6382/10/1/16/s1, Figure S1. Heatmap showing absence and presence of ARGs in A. non-baumanii reported in the literature. Red color indicates the absence of genes and blue color indicates the presence of gene. The clustering analysis was done using hclust R package. The heatmap was done using to ggplot2 R package. Figure S2. Heatmap showing absence and presence of ARGs in A. non-baumannii genomes available in the GenBank database. Red color indicates the absence of a gene and blue color indicates the presence of a gene. The GenBank Assembly Accession of the genomes used is indicating in Table S3. The clustering analysis was done using the hclust R package. The heatmap was done using the ggplot2 R package. Table S1. List of ARGs in Acinetobacter, excluding A. baumannii, reported in the literature. The presence of ARGs is indicated with color cell and the absence of ARGs is indicated with white cell. Table S2. List of ARGs in Acinetobacter, excluding A. baumannii, predicted in available genomes in GenBank Database. The presence of ARGs is indicated with 1 and the absence of ARGs is indicated with 0. Table S3. List of Code used in Figure S2.
Author Contributions
Conceptualization, A.B., M.E.T., M.S.R.; methodology, A.B., G.M.T., M.E.T., M.S.R.; software, A.B., G.M.T.; formal analysis, A.B., G.M.T., S.M., M.E.T., M.S.R.; investigation, A.B., G.M.T., M.S.R.; resources, M.S.R.; data curation, A.B., G.M.T., S.M., M.E.T., M.S.R.; writing–original draft preparation, S.M., M.E.T., M.S.R.; writing–review & editing, A.B., S.M., M.E.T., M.S.R.; supervision, M.S.R.; project A.B. ministration, M.E.T., M.S.R.; funding acquisition, M.E.T. and M.S.R. All authors have read and agreed to the published version of the manuscript.
Funding
The authors’ work was supported by NIH SC3GM125556 to MSR and 2R15AI047115 to MET. AB was supported by grant MHRT 2T37MD001368 from the National Institute on Minority Health and Health Disparities, National Institute of Health.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations All authors have read and agreed to the published version of the manuscript..
References
- 1.CDC . Antibiotic Resistance Threats in the United States. Centers for Disease Control; Atlanta, GA, USA: 2019. [Google Scholar]
- 2.Wong D., Nielsen T.B., Bonomo R.A., Pantapalangkoor P., Luna B., Spellberg B. Clinical and pathophysiological overview of Acinetobacter infections: A century of challenges. Clin. Microbiol. Rev. 2017;30:409–447. doi: 10.1128/CMR.00058-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tavares L.C.B., Cunha M.P.V., Vasconcellos F.M., Bertani A.M.J., Barcellos T.A.F., Bueno M.S., Santos C.A., Sant’Ana D.A., Ferreira A.M., Mondelli A.L., et al. Genomic and clinical characterization of IMP-1-producing multidrug-resistant Acinetobacter bereziniae isolates from bloodstream infections in a brazilian tertiary hospital. Microb. Drug Resist. 2020 doi: 10.1089/mdr.2019.0210. [DOI] [PubMed] [Google Scholar]
- 4.Al Atrouni A., Joly-Guillou M.L., Hamze M., Kempf M. Reservoirs of non-baumannii Acinetobacter species. Front. Microbiol. 2016;7:49. doi: 10.3389/fmicb.2016.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zong Z., Zhang X. blaNDM-1-carrying Acinetobacter johnsonii detected in hospital sewage. J. Antimicrob. Chemother. 2013;68:1007–1010. doi: 10.1093/jac/dks505. [DOI] [PubMed] [Google Scholar]
- 6.Montana S., Palombarani S., Carulla M., Kunst A., Rodriguez C.H., Nastro M., Vay C., Ramirez M.S., Almuzara M. First case of bacteraemia due to Acinetobacter schindleri harbouring blaNDM-1 in an immunocompromised patient. New Microbes New Infect. 2018;21:28–30. doi: 10.1016/j.nmni.2017.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kiyasu Y., Hitomi S., Funayama Y., Saito K., Ishikawa H. Characteristics of invasive Acinetobacter infection: A multicenter investigation with molecular identification of causative organisms. J. Infect. Chemother. 2020;26:475–482. doi: 10.1016/j.jiac.2019.12.010. [DOI] [PubMed] [Google Scholar]
- 8.Yong D., Toleman M.A., Giske C.G., Cho H.S., Sundman K., Lee K., Walsh T.R. Characterization of a new metallo-beta-lactamase gene, bla(NDM-1), and a novel erythromycin esterase gene carried on a unique genetic structure in Klebsiella pneumoniae sequence type 14 from India. Antimicrob. Agents Chemother. 2009;53:5046–5054. doi: 10.1128/AAC.00774-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu W., Feng Y., Tang G., Qiao F., McNally A., Zong Z. NDM metallo-beta-lactamases and their bacterial producers in health care settings. Clin. Microbiol. Rev. 2019;32 doi: 10.1128/CMR.00115-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramirez M.S., Bonomo R.A., Tolmasky M.E. Carbapenemases: Transforming Acinetobacter baumannii into a yet more dangerous menace. Biomolecules. 2020;10:720. doi: 10.3390/biom10050720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Poirel L., Marque S., Heritier C., Segonds C., Chabanon G., Nordmann P. OXA-58, a novel class D {beta}-lactamase involved in resistance to carbapenems in Acinetobacter baumannii. Antimicrob. Agents Chemother. 2005;49:202–208. doi: 10.1128/AAC.49.1.202-208.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dozzo P., Moser H.E. New aminoglycoside antibiotics. Expert Opin. Ther. Pat. 2010;20:1321–1341. doi: 10.1517/13543776.2010.506189. [DOI] [PubMed] [Google Scholar]
- 13.Patel G., Perez F., Bonomo R.A. Carbapenem-resistant Enterobacteriaceae and Acinetobacter baumannii: Assessing their impact on organ transplantation. Curr. Opin. Organ. Transplant. 2010;15:676–682. doi: 10.1097/MOT.0b013e3283404373. [DOI] [PubMed] [Google Scholar]
- 14.Ramirez M.S., Tolmasky M.E. Aminoglycoside modifying enzymes. Drug Resist. Updates. 2010;13:151–171. doi: 10.1016/j.drup.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Delmar J.A., Su C.C., Yu E.W. Bacterial multidrug efflux transporters. Ann. Rev. Biophys. 2014;43:93–117. doi: 10.1146/annurev-biophys-051013-022855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tolmasky M.E. Strategies to prolong the useful life of existing antibiotics and help overcoming the antibiotic resistance crisis. In: Atta-ur-Rhaman, editor. Frontiers in Clinical Drug Research-Anti Infectives. Volume 1. Bentham Books; Sharjah, UAE: 2017. pp. 1–27. [Google Scholar]
- 17.Coyne S., Courvalin P., Perichon B. Efflux-mediated antibiotic resistance in Acinetobacter spp. Antimicrob. Agents Chemother. 2011;55:947–953. doi: 10.1128/AAC.01388-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Damier-Piolle L., Magnet S., Bremont S., Lambert T., Courvalin P. AdeIJK, a resistance-nodulation-cell division pump effluxing multiple antibiotics in Acinetobacter baumannii. Antimicrob. Agents Chemother. 2008;52:557–562. doi: 10.1128/AAC.00732-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sugawara E., Nikaido H. Properties of AdeABC and AdeIJK efflux systems of Acinetobacter baumannii compared with those of the AcrAB-TolC system of Escherichia coli. Antimicrob. Agents Chemother. 2014;58:7250–7257. doi: 10.1128/AAC.03728-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parte A.C., Sarda Carbasse J., Meier-Kolthoff J.P., Reimer L.C., Goker M. List of prokaryotic names with standing in nomenclature (LPSN) moves to the DSMZ. Int. J. Syst. Evol. Microbiol. 2020 doi: 10.1099/ijsem.0.004332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carvalheira A., Gonzales-Siles L., Salva-Serra F., Lindgren A., Svensson-Stadler L., Thorell K., Pineiro-Iglesias B., Karlsson R., Silva J., Teixeira P., et al. Acinetobacter portensis sp. nov. and Acinetobacter guerrae sp. nov., isolated from raw meat. Int. J. Syst. Evol. Microbiol. 2020;70:4544–4554. doi: 10.1099/ijsem.0.004311. [DOI] [PubMed] [Google Scholar]
- 22.Alcock B.P., Raphenya A.R., Lau T.T.Y., Tsang K.K., Bouchard M., Edalatmand A., Huynh W., Nguyen A.V., Cheng A.A., Liu S., et al. CARD 2020: Antibiotic resistome surveillance with the comprehensive antibiotic resistance database. Nucleic Acids Res. 2020;48:D517–D525. doi: 10.1093/nar/gkz935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gupta S.K., Padmanabhan B.R., Diene S.M., Lopez-Rojas R., Kempf M., Landraud L., Rolain J.M. ARG-ANNOT, a new bioinformatic tool to discover antibiotic resistance genes in bacterial genomes. Antimicrob. Agents Chemother. 2014;58:212–220. doi: 10.1128/AAC.01310-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bortolaia V., Kaas R.S., Ruppe E., Roberts M.C., Schwarz S., Cattoir V., Philippon A., Allesoe R.L., Rebelo A.R., Florensa A.F., et al. ResFinder 4.0 for predictions of phenotypes from genotypes. J. Antimicrob. Chemother. 2020 doi: 10.1093/jac/dkaa345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murtagh F., Legendre P. Ward’s hierarchical agglomerative clustering method: Which algorithms implement Ward’s criterion? J. Classif. 2014;31:274–295. doi: 10.1007/s00357-014-9161-z. [DOI] [Google Scholar]
- 26.Pedersen T.L. Hierarchical sets: Analyzing pangenome structure through scalable set visualizations. Bioinformatics. 2017;33:1604–1612. doi: 10.1093/bioinformatics/btx034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boucher H.W., Talbot G.H., Bradley J.S., Edwards J.E., Gilbert D., Rice L.B., Scheld M., Spellberg B., Bartlett J. Bad bugs, no drugs: No ESKAPE! An update from the Infectious Diseases Society of America. Clin. Infect. Dis. 2009;48:1–12. doi: 10.1086/595011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data sharing not applicable.