Abstract
Context
Many patients with unilateral primary aldosteronism (PA) have normal adrenal imaging, but little is known about their outcome following adrenalectomy.
Objective
To evaluate biochemical and clinical outcomes after adrenalectomy in patients with unilateral PA and normal-appearing adrenal imaging.
Design
Retrospective cohort study of patients seen between January 2006 and May 2018.
Setting
A Canadian tertiary care PA referral center.
Patients
Consecutive individuals with PA, normal cross-sectional adrenal imaging, and lateralizing adrenal vein sampling (AVS) who underwent adrenalectomy during the study period.
Primary Outcome
Biochemical response to adrenalectomy graded according to the Primary Aldosteronism Surgical Outcome criteria.
Results
A total of 40 patients were included. Biochemical outcomes were available for 33 people (mean age, 54.7 years; 91% male; median follow-up, 2.7 months), with 28 (85%) showing a complete or partial response and 5 (15%) with no response. Clinical outcomes were available for 36 people (mean age, 54.6 years; 86% male; median follow-up, 9.8 months), with 31 (86%) demonstrating a complete or partial response and 5 (14%) with no response.
Conclusions
The prognosis after adrenalectomy is highly favorable for patients with unilateral PA and normal-appearing adrenal imaging. Patients with lateralizing disease should be considered for surgery despite apparently normal adrenal imaging.
Keywords: normal imaging, adrenal vein sampling, adrenalectomy
Primary aldosteronism (PA) is the most common form of remediable hypertension, and it is associated with high rates of cardiovascular morbidity (1–4). Disease-targeted treatment is highly effective for lowering blood pressure and mitigating the risk of cardiovascular complications (5–8). A considerable proportion of patients with PA have unilateral disease that may be amenable to surgery (1, 9, 10). When possible, adrenalectomy is the guideline-recommended treatment of choice, as it is associated with high rates of cure (1, 4).
It is increasingly recognized that PA exists across a wide clinical spectrum (3). While the benefits of adrenalectomy are well established for patients with lateralizing disease that is associated with easily visualized aldosterone-producing adenomas (11, 12), the surgical outcome for patients who have no distinct adrenal nodularity on imaging remains unclear (13). Notably, individuals in the latter category have similar lateralization rates to those with distinct adrenal nodules (13, 14), yet they are more often male and tend to be older (13), factors which may potentially predispose to worse surgical outcomes (5, 15). Moreover, smaller nodules may also be associated with higher rates of persistent disease (5).
Little is known about the long-term outcomes in patients with unilateral PA and normal adrenal imaging who undergo surgery. Experience is limited, as these patients are often presumed to have adrenocortical hyperplasia and are therefore commonly overlooked for adrenalectomy (14, 16–20). Determining their prognosis, however, is crucial to understanding whether surgical recommendations can be safely generalized to this population. Addressing this, we systematically evaluated the biochemical and clinical outcomes of patients with unilateral PA and normal-appearing adrenal glands who underwent adrenalectomy.
Materials and Methods
Study design and data sources
This study was approved by the University of Calgary Conjoint Health Research Ethics Board and was reported according to the Strengthening the Reporting of Observational studies in Epidemiology statement.
We reviewed consecutive patients with PA and normal adrenal imaging who were evaluated with adrenal vein sampling (AVS) and referred for adrenalectomy for unilateral disease between January 2006 and May 2018. Specifically, we analyzed data from the regional AVS database from Calgary, Alberta, Canada. This database provides complete capture of all patients with PA from Southern Alberta and the interior of British Columbia, Canada, who underwent AVS during the study period, and it includes detailed demographic information, AVS results, and diagnostic imaging. These data are considered high-quality and have been used in numerous studies (13, 21–24). We augmented these data with manual review of clinical records, including those from charts, anatomical pathology, and laboratory reports to describe the characteristics of the affected individuals and to determine their rates of postoperative success.
Patients were referred for AVS based on an elevated aldosterone-to-renin ratio (ARR) and high-probability features of PA (eg, resistant hypertension, diuretic-induced hypokalemia, and/or spontaneous hypokalemia) (13, 24, 25). The ARR upper limit of normal was based on the specific units of measurement and local laboratory standards (>60pmol/L per mIU/L and >550pmol/L per ng/mL/hr) (25). Per our established protocols, the decision to perform confirmatory testing was at the discretion of the managing physician, although it was not needed for most cases (22, 26). Cross-sectional imaging of the abdomen was performed with computed tomography (CT), with at least 1 plane at or below 3 mm in thickness to assess the adrenal glands, and radiology reports were reviewed to determine imaging status. As previously described (13) and in accordance with prior studies (18, 19, 27), we restricted our primary analysis to patients who had no apparent adrenal nodules, which was defined as no discernible lesions greater than or equal to 1 cm. All subjects received AVS according to a standardized protocol (13, 23, 24) following the discontinuation of potentially interfering medications. Successful cannulation of the adrenal veins was based on a selectivity index (comparing adrenal vein to peripheral cortisol levels) of either ≥2:1 at baseline or ≥3:1 following cosyntropin stimulation. An unstimulated lateralization index cutoff of >3:1, comparing the adrenal vein aldosterone-to-cortisol ratios of the dominant to the nondominant side, was used to identify unilateral aldosterone hypersecretion (23, 24). Patients with unilateral disease who consented to surgery were then referred for adrenalectomy and were subsequently evaluated for postoperative outcomes.
Measures
Baseline characteristics, including age, sex, physical measurements (eg, weight, height, and blood pressure), blood pressure medications, and laboratory measures (eg, lowest serum potassium on record, plasma aldosterone, highest ARR collected without potentially interfering medications, and renal function at time of AVS) were retrieved. The abbreviated Modification of Diet in Renal Disease formula (28) was used to calculate estimated glomerular filtration rates (eGFR).
Blood pressure records reflected a variety of measurement techniques (eg, attended vs unattended) and devices (eg, automated vs manual). When available, out-of-office blood pressure measurements (eg, 24-hour ambulatory blood pressure monitor and home blood pressure measurements) were preferred (29). Otherwise, office readings were used. When multiple readings were measured at the same sitting, the average was recorded. Based on the Primary Aldosteronism Surgical Outcome (PASO) criteria and contemporaneous clinical practice guidelines, normal blood pressure was defined as <140/90 mm Hg for office-based blood pressure readings, <135/85 mm Hg for home blood pressure series, or <130/80 mm Hg for a 24-hour mean ambulatory blood pressure (5). All medications with blood pressure-lowering effects were recorded, even if they were not primarily prescribed for hypertension (eg, a beta blocker for atrial fibrillation). To facilitate comparisons between the intensity of antihypertensive drugs that were used before and after surgery, medications were quantified as defined daily doses (DDD) according to the World Health Organization Collaborating Centre for Drug Statistics Methodology ATC/DDD Index 2020 (https://www.whocc.no/atc_ddd_index/; accessed October 8, 2020) (5).
Outcomes
Biochemical
Our primary outcome of interest was the biochemical response to surgery (4, 5). Complete biochemical success was determined by the correction of hypokalemia (serum potassium <3.6 mmol/L, if previously present) after surgery and normalization of the ARR. Partial success was defined as correction of hypokalemia (if previously present) but a persistently elevated ARR along with a ≥50% decrease in the plasma aldosterone compared with baseline. Patients with persistent hypokalemia or with an elevated ARR and a <50% decrease in plasma aldosterone were considered to have absent biochemical success. Outcomes ascertainment was usually more than 6 to 12 months after surgery, as we reported the longest available follow-up for each person, but it was occasionally less than 6 months for biochemical outcomes (30).
Clinical
We used the PASO criteria to define clinical success as a secondary outcome (4, 5). Specifically, complete clinical success was characterized by the normalization of blood pressure without requiring any blood pressure-lowering medication. Partial success was defined as maintaining the same blood pressure with less medications, or as a reduction in blood pressure with either the same amount or less medication. An absent clinical response was assigned when blood pressure did not improve after surgery with either the same amount or more medication.
Histopathological
Surgical pathology reports were reviewed and specimens were categorized as follows: normal adrenal tissue, solitary adenoma, multifocal adenoma, or adrenal hyperplasia.
Statistical analysis
Descriptive statistics were reported for baseline and postoperative characteristics. Variables with a normal distribution were presented as means with standard deviations (SDs), and those with non-normal distributions were presented as medians with interquartile ranges (IQRs). We restricted our analysis to subjects where biochemical and/or clinical outcomes were measured for a complete case analysis using PASO criteria. Preoperative and postoperative values were compared, and absolute and relative changes were calculated for all biochemical and clinical measures of interest to determine outcome response. For each outcome of interest, the proportion of complete, partial, and absent success was calculated. Statistical testing for differences in characteristics between those with either a complete or partial response, and those without a response, for both biochemical and clinical outcome groups, was conducted with a 2-tailed test and a P-value < 0.05 considered as significant. Continuous variables with a normal distribution were compared via the t-test and those with a non-normal distribution were compared using the Mann-Whitney U test. Fisher’s exact test was used to compare proportions. Finally, sensitivity analysis was performed to account for possible misclassification based on adrenal imaging. The analysis was repeated on a cohort of patients who had a more stringent definition of “normal” adrenal imaging, defined by the absence of any adrenal gland abnormality on CT, including adrenal nodules <1 cm in size, or any description of “bulkiness” or “hyperplasia” on the radiology report. Analyses were performed using Microsoft Excel version 1908 (Microsoft Corporation, Redmond, Washington).
Results
Study participants
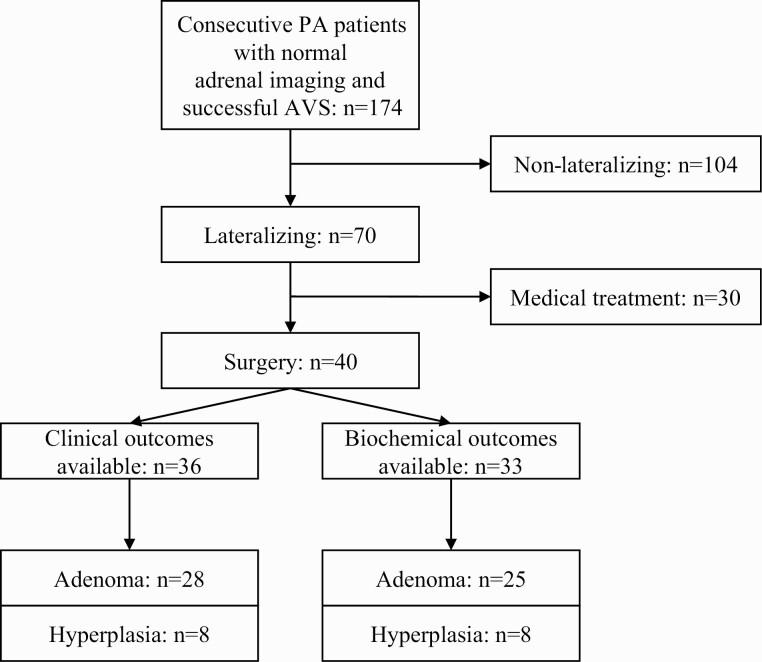
A total of 354 patients were referred for PA and underwent AVS during the study period. Of these, 179 (51%) had normal adrenal imaging. Five people were excluded because AVS was technically unsuccessful, leaving 174 patients. A total of 70 (40%) patients lateralized on AVS, of whom 40 consented to adrenalectomy for presumed unilateral PA. Of these individuals, biochemical outcomes were available for 33 and clinical outcomes for 36 (Fig. 1). Both biochemical and clinical outcomes were available for 32 of these people.
Figure 1.
Flow diagram of patients with primary aldosteronism (PA) and normal adrenal imaging.
Baseline characteristics
Preoperative characteristics of the study cohort are shown in Table 1. Among those who received adrenalectomy and for whom biochemical outcomes were available, the mean age was 54.7 years and the majority were male (91%). All subjects were at least 40 years of age. Patients were typically obese with a mean body mass index of 31.4 kg/m2 (SD, 4.5). Resistant hypertension was common with a mean baseline systolic blood pressure (BP) of 149 mm Hg (SD, 18) and a median medication intensity of 3.5 DDD (IQR, 3.5). The median baseline ARR was highly elevated at 467% of the assay-specific upper limit of normal (IQR, 945%). Hypokalemia was frequent, occurring in 31 (94%) individuals, and renal function was normal on average. Adrenal vein sampling was strongly consistent with lateralization, with a median lateralization index of 16.7 (IQR, 28.5). The median size of an adrenal nodule on CT imaging was 0.6 cm (IQR, 0.8). Those individuals who were evaluated for clinical outcomes were broadly similar in their baseline characteristics (Table 1).
Table 1.
Baseline characteristics of the study cohort stratified by biochemical and clinical outcome
| Characteristicsa | Stratified by Biochemical Outcome | Stratified by Clinical Outcome | ||||
|---|---|---|---|---|---|---|
| Complete (n = 26) | Partial (n = 2) | Absent (n = 5) | Complete (n = 7) | Partial (n = 24) | Absent (n = 5) | |
| Age, mean (SD), years [n = 33; n = 36] | 54.9 (9.6) | 55.0 (13.6) | 53.6 (7.2) | 54.3 (9.2) | 54.4 (8.7) | 55.6 (13.4) |
| Age strata, no. (%) [n = 33; n = 36] | ||||||
| <40 years | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 40–49 years | 9 (35) | 1 (50) | 1 (20) | 3 (43) | 7 (29) | 2 (40) |
| 50–59 years | 10 (38) | 0 (0) | 3 (60) | 2 (29) | 10 (42) | 2 (40) |
| <60 years | 19 (73) | 1 (50) | 4 (80) | 5 (71) | 17 (71) | 4 (80) |
| ≥60 years | 7 (27) | 1 (50) | 1 (20) | 2 (29) | 7 (29) | 1 (20) |
| Male sex, no. (%) [n = 33; n = 36] | 24 (92) | 2 (100) | 4 (80) | 5 (71) | 21 (88) | 5 (100) |
| BMI, mean (SD), kg/m2 [n = 17; n = 18] | 32.1 (4.4) | ND | 29.1 (4.7) | 27.2 (2.9) | 32.1 (4.2) | 27.2 (5.5) |
| SBP, mean (SD), mm Hg [n = 33; n = 36] | 150 (20) | 139 (13) | 147 (12) | 149 (24) | 151 (17) | 140 (12) |
| DBP, mean (SD), mm Hg [n = 33; n = 36] | 92 (12) | 81 (6) | 89 (4) | 94 (13) | 92 (12) | 86 (8) |
| Medications, median (IQR), DDD [n = 33; n = 36] | 3.8 (3.7) | ND | 3.0 (5.9) | 4.0 (4.5) | 5.1 (3.3) | 1.5 (1.6) |
| PAC, median (IQR), pmol/L [n = 30; n = 32] | 648 (587) | ND | 572 (781) | 647 (619) | 589 (575) | 610 (1044) |
| ARR, median (IQR), % ULN [n = 32; n = 35] | 452 (1081) | ND | 635 (613) | 590 (1232) | 413 (1001) | 452 (540) |
| Serum potassium, mean (SD), mmol/L [n = 33; n = 36] | 3.0 (0.5) | 2.8 (0.1) | 3.0 (0.2) | 3.1 (0.6) | 3.0 (0.4) | 3.1 (0.2) |
| Hypokalemia (<3.6mmol/L), no. (%) [n = 33; n = 36] | 24 (92) | 2 (100) | 5 (100) | 6 (86) | 22 (92) | 5 (100) |
| Serum creatinine, mean (SD), µmol/L [n = 31; n = 34] | 90.3 (22.0) | ND | 97.5 (31.6) | 87.7 (24.8) | 91.0 (24.3) | 75.6 (14.8) |
| eGFR, mean (SD), mL/min/1.73 m2 [n = 31; n = 34] | 79.1 (20.4) | ND | 69.8 (16.6) | 77.0 (19.2) | 77.6 (19.7) | 96.9 (23.4) |
| AVS lateralization index, median (IQR) [n = 33; n = 36] | 16.1 (39.8) | ND | 20.9 (27.2) | 23.8 (68.4) | 12.5 (45.1) | 17.3 (9.5) |
Number of patients with missing data (no.; % of clinical outcomes group) included: BMI (18; 50%), PAC (4; 11%), ARR (1; 3%), creatinine (2; 6%), and eGFR (2; 6%). Number of patients with missing data (no.; % of biochemical outcomes group) included: BMI (16; 49%), PAC (3; 9%), ARR (1; 3%), creatinine (2; 6%), and eGFR (2; 6%).
Abbreviations: ARR, aldosterone-to-renin ratio; AVS, adrenal vein sampling; BMI, body mass index; DBP, diastolic blood pressure; DDD, defined daily dose; eGFR, estimated glomerular filtration rate; IQR, interquartile range; ND, no data; PAC, plasma aldosterone concentration; SBP, systolic blood pressure; SD, standard deviation; ULN, upper limit of normal.
a n shown for biochemical and clinical outcomes, respectively.
Biochemical outcomes
Biochemical outcomes were not measured for all patients, largely due to differences in practice over time. Among the 33 patients with biochemical outcomes, 26 (79%) exhibited a complete response, 2 (6%) had a partial response, and 5 (15%) had no response during a median follow-up interval of 2.7 months (IQR, 1.2–7.0; maximum, 46.5) (Table 2). Postoperatively, there was an average reduction in systolic BP by 16 mm Hg (SD, 20), diastolic BP by 8 mm Hg (SD, 11), and medications by 2.0 DDD (IQR, 2.6). Following pathological examination, adrenocortical adenomas were found to be present in 25 (76%) people with a median adenoma size of 0.9 cm (IQR, 0.4).
Table 2.
Postoperative characteristics of the study cohort stratified by biochemical and clinical outcome
| Characteristicsa | Stratified by Biochemical Outcome | Stratified by Clinical Outcome | ||||
|---|---|---|---|---|---|---|
| Complete (n = 26) | Partial (n = 2) | Absent (n = 5) | Complete (n = 7) | Partial (n = 24) | Absent (n = 5) | |
| Pathology, no. (%) [n = 33; n = 36] | ||||||
| Adenoma | 20 (77) | 2 (100) | 3 (60) | 7 (100) | 19 (79) | 2 (40) |
| Hyperplasia | 6 (23) | 0 (0) | 2 (40) | 0 (0) | 5 (21) | 3 (60) |
| SBP (postoperative), mean (SD), mm Hg [n = 32; n = 36] | 133 (11) | 143 (5) | 130 (8) | 125 (10) | 134 (11) | 140 (10) |
| Reduction in SBPb, mean (SD), mm Hg [n = 32; n = 36] | 17 (21) | -4 (18) | 17 (12) | 24 (22) | 17 (17) | 0 (13) |
| Reduction in SBPb, mean (SD), % [n = 32; n = 36] | 10.1 (13.2) | -3.1 (13) | 10.9 (7.2) | 14.6 (14.7) | 10.4 (11.3) | -0.5 (11.0) |
| DBP (postoperative), mean (SD), mm Hg [n = 32; n = 36] | 84 (7) | 80 (0) | 82 (7) | 82 (3) | 84 (8) | 87 (8) |
| Reduction in DBPb, mean (SD), mm Hg [n = 32; n = 36] | 9 (12) | 1 (7) | 7 (5) | 12 (16) | 8 (10) | -1 (5) |
| Reduction in DBPb, mean (SD), % [n = 32; n = 36] | 7.9 (13.5) | 0.9 (8.7) | 7.7 (6.0) | 11 (16.9) | 7.8 (10.8) | -1.3 (6.1) |
| Medications (postoperative), median (IQR), DDD [n = 32; n = 36] | 1 (2.9) | ND | 3.3 (3.3) | 0 (0) | 2.2 (3) | 1.5 (2.4) |
| Reduction in medicationsb, median (IQR), DDD [n = 32; n = 36] | 2.3 (2.7) | ND | 1.7 (4.7) | 4.0 (4.5) | 2.2 (2) | 0 (2) |
| Reduction in medicationsb, median (IQR), % [n = 32; n = 36] | 71.4 (62.8) | ND | 33.3 (77.9) | 100 (0) | 56.8 (41.4) | 0 (79.2) |
| PAC (postoperative), median (IQR), pmol/L [n = 32; n = 33] | 164 (167) | ND | 470 (668) | 204 (166) | 165 (177) | 395 (736) |
| ARR (postoperative), median (IQR), % ULN [n = 32; n = 33] | 25 (26) | ND | 488 (354) | 20 (41) | 30 (74) | 54 (536) |
| Serum potassium (postoperative), mean (SD), mmol/L [n = 32; n = 34] | 4.6 (0.6) | 4.2 (0.3) | 4.2 (0.5) | 4.5 (0.4) | 4.6 (0.6) | 4.0 (0.5) |
| Serum creatinine (postoperative), mean (SD), µmol/L [n = 30; n = 31] | 111.4 (24.9) | 88.5 (14.8) | 101.3 (35.1) | 105.2 (20.3) | 111.7 (27.6) | 80.7 (7.5) |
| eGFR (postoperative), mean (SD), mL/min/1.73 m2 [n = 30; n = 31] | 61.2 (14.4) | 79.3 (19.0) | 63.4 (16.1) | 63.0 (13.5) | 60.7 (14.7) | 84.9 (6.2) |
| Reduction in eGFRb, mean (SD), mL/min/1.73 m2 [n = 28; n = 29] | 17.7 (18.3) | ND | -2.7 (0.4) | 15.3 (17.5) | 17.0 (19.4) | 24.2 (25.8) |
| Follow-up time, median (IQR), months [n = 33; n = 36] | 2.2 (3.9) | ND | 9.0 (26.0) | 3.1 (6.1) | 22.6 (56.2) | 2.5 (23.8) |
Number of patients with missing data (no.; % of clinical outcomes group) included: PAC (4; 11%), ARR (4; 11%), potassium (2; 6%), creatinine (5; 14%), and eGFR (5; 14%). Number of patients with missing data (no.; % of biochemical outcomes group) included: SBP, DBP, and medications (1; 3%), potassium (1; 3%), creatinine (3; 9%), and eGFR (3; 9%).
Abbreviations: ARR, aldosterone-to-renin ratio; DBP, diastolic blood pressure; DDD, defined daily dose; eGFR, estimated glomerular filtration rate; IQR, interquartile range; ND, no data; PAC, plasma aldosterone concentration; SBP, systolic blood pressure; SD, standard deviation; ULN, upper limit of normal.
an shown for biochemical and clinical outcomes, respectively.
b Change is postoperative value subtracted from preoperative value; a negative change indicates an increase in value.
Compared to individuals with an absent biochemical response, those with a complete or partial response had a larger median adenoma size on pathology (1.0 vs 0.5 cm; P < 0.01). There was also a greater relative decrease in the number of medications used (68.4% vs 33.3% reduction; P = 0.04). Plasma aldosterone (P < 0.01) and ARR (P < 0.01) were both lower postoperatively in those with a biochemical response. There were no significant differences in postoperative BP. Following surgery, those with a positive response exhibited a greater decrease in eGFR compared with those without a biochemical response (mean reduction, 19.1 vs -2.7 mL/min/1.73 m2; P < 0.01).
A detailed review of the 7 patients with partial or absent biochemical response revealed that of those without a response, 3 had evidence of hyperplasia on pathology and the remaining 2 with adrenocortical adenomas were clinically suspected of having bilateral aldosterone-producing adenomas; and of those with a partial response, both had adrenocortical adenomas (Table 3). In addition, plasma aldosterone and ARR decreased by at least 50% in 2 people, but the ARR still remained higher than the reference limit because of a persistently suppressed renin. In 5 people, absent or partial biochemical responses were accompanied by partial clinical success. In 1 of these individuals, the raised postoperative ARR (with suppressed renin levels) may have been related to early biochemical outcome ascertainment (at 2.3 months) prior to recovery of the renin-angiotensin-aldosterone system. In the other 4, whose biochemical outcomes were measured more distally, the persistently suppressed renin levels were likely related to the presence of bilateral disease.
Table 3.
Details of patients with partial or absent biochemical or clinical responses
| No. | PASO Outcomes | Imaging Findings | Pathology Findings | Notes | |
|---|---|---|---|---|---|
| Bioch. | Clin. | ||||
| 1 | Complete | Partial | Negative | Hyperplasia | 70 yo male seen in follow-up at 136.7 months. Residual hypertension felt to be due to obesity, OSA, and CKD, and was on a beta blocker for heart failure and ventricular tachycardia. |
| 2 | Complete | Partial | Left-sided 0.8-cm nodule | Multiple left-sided adenomas with surrounding hyperplasia | 55 yo male seen in follow-up at 86.2 months. Residual hypertension felt to be due to obesity. |
| 3 | Absent | Partial | Bilateral bulkiness | Right-sided 0.5-cm adenoma | 59 yo male with clinical follow-up at 49.3 mo and biochemical follow-up at 46.5 mo. Residual hypertension felt to be due to obesity and bilateral aldosterone-producing adenomas. There was a reduction of the ARR from 3490 pmol/L per ng/mL/hr preoperatively to 2910 pmol/L per ng/mL/hr (ie, PAC 291 pmol/L and PRA 0.1 ng/mL/hr) after surgery. AVS SI was >3 bilaterally postcosyntropin and LI was 38.3. |
| 4 | Complete | Partial | Negative | Hyperplasia | 52 yo male seen in follow-up at 6.5 mo. Residual hypertension felt to be due to obesity and OSA. |
| 5 | Complete | Absent | Negative | Hyperplasia | 47 yo male seen in follow-up at 21.2 mo. Residual hypertension felt to be due to obesity and an anxiety disorder, and was on a calcium channel blocker for atrial fibrillation. AVS SI was >2 bilaterally at baseline and >3 bilaterally postcosyntropin and LI was 17.3. |
| 6 | Partial | Partial | Right-sided 0.9-cm nodule | Right-sided 1.2-cm adenoma | 65 yo male with a history of obesity and OSA, with clinical follow-up at 2.7 mo and biochemical follow-up at 2.3 mo. There was a reduction of the ARR from 447 pmol/L per mIU/L preoperatively to 71 pmol/L per mIU/L (ie, PAC 135 pmol/L and DRC 1.9 mIU/L) after surgery. Lost to further follow-up. |
| 7 | Complete | Partial | Right-sided 0.9-cm nodule | Right-sided 1.3-cm adenoma with surrounding hyperplasia | 42 yo female with a history of migraine (on metoprolol) and white coat syndrome, seen in follow-up at 1.9 mo. Lost to further follow-up. |
| 8 | Complete | Absent | Left-sided 0.6-cm nodule | Left-sided 1.1-cm adenoma | 77 yo male seen in follow-up at 2.5 mo. At that time, 24-hr ambulatory blood pressure monitor showed an average BP of 149/80 mm Hg off treatment. He declined further postoperative pharmacological treatment. AVS SI was >3 bilaterally postcosyntropin and LI was 8.4. |
| 9 | Complete | Partial | Left-sided 0.9-cm nodule | Left-sided 0.8-cm adenoma with surrounding hyperplasia | 67 yo male seen in follow-up at 60.3 mo. Residual hypertension felt to be due to cyclosporine use after kidney transplant, progressive CKD, and obesity. |
| 10 | Complete | Partial | Negative | Left-sided 1.5-cm adenoma with surrounding hyperplasia | 48 yo male with a history of obesity, OSA, and diuretic use for chronic edema, seen in follow-up at 1.8 mo. Lost to further follow-up. |
| 11 | Complete | Partial | Right-sided 0.7-cm nodule | Right-sided 0.7-cm adenoma | 49 yo male seen in follow-up at 58.3 mo. Residual hypertension felt to be due to CKD and obesity. |
| 12 | Complete | Partial | Left-sided 0.8-cm nodule | Left-sided hyperplasia | 45 yo male seen in follow-up at 12.1 mo. Residual hypertension felt to be due to obesity and an anxiety disorder. |
| 13 | Complete | Partial | Negative | Right-sided 1.3-cm adenoma | 54 yo male seen in follow-up at 23 mo. Residual hypertension felt to be due to obesity. |
| 14 | ND | Partial | Left-sided 0.9-cm nodule | Left-sided 1.0-cm adenoma | 42 yo female seen in follow-up at 43.9 mo. Residual hypertension felt to be due to essential hypertension, and was on a beta blocker for atrial fibrillation. |
| 15 | Complete | Partial | Negative | Right-sided 1.1-cm adenoma | 69 yo male seen in follow-up at 57.2 mo. Residual hypertension felt to be due to CKD, and was on a beta blocker for ischemic heart disease. |
| 16 | Complete | Partial | Negative | Left-sided 1.0-cm adenoma with surrounding hyperplasia | 65 yo male seen in follow-up at 51.6 mo. Residual hypertension felt to be due to obesity. |
| 17 | ND | Partial | Left-sided 0.8-cm nodule | Left-sided 1.8-cm adenoma | 50 yo male seen in follow-up at 94.9 months. Residual hypertension felt to be due to obesity and daily nonsteroidal anti-inflammatory use for osteoarthritis. |
| 18 | Complete | Partial | Right-sided 0.9-cm nodule | Right-sided 1.1-cm adenoma with surrounding hyperplasia | 52 yo male with a history of obesity and white coat hypertension seen in follow-up at 0.3 mo. Lost to further follow-up. |
| 19 | ND | Partial | Right-sided nodule <1.0 cm | Right-sided 1.7-cm adenoma | 62 yo male seen in follow-up at 114.1 mo. Residual hypertension felt to be due to CKD. |
| 20 | Complete | Partial | Left-sided nodule <1.0-cm | Left-sided hyperplasia | 56 yo male seen in follow-up at 72.1 mo. Residual hypertension felt to be due to obesity, OSA, and was on a calcium channel blocker for atrial fibrillation. |
| 21 | Absent | Partial | Left-sided 0.7-cm nodule | Left-sided 0.6-cm adenoma | 60 yo male with clinical follow-up at 13.1 mo and biochemical follow-up at 13.1 mo. Residual hypertension felt to be due to bilateral aldosterone-producing adenomas. There was an increase in the ARR from 104 pmol/L per mIU/L preoperatively to 293 pmol/L per mIU/L (ie, PAC 293 pmol/L and DRC 1.0 mIU/L) after surgery. AVS SI was >3 bilaterally postcosyntropin and LI was 3.8. |
| 22 | Complete | Partial | Negative | Left-sided 0.9-cm adenoma | 40 yo male seen in follow-up at 13.8 mo. Residual hypertension felt to be due to obesity. |
| 23 | Complete | Absent | Left-sided bulkiness | Right-sided 0.8-cm adenoma with surrounding hyperplasia | 59 yo male seen in follow-up at 28.4 mo. Residual hypertension felt to be due to obesity. AVS SI was >3 bilaterally postcosyntropin and LI was 16.8. |
| 24 | Absent | Absent | Negative | Hyperplasia | 42 yo male with clinical follow-up at 0.7 mo and biochemical follow-up at 6.8 mo. Residual hypertension felt to be due to bilateral adrenal hyperplasia or unilateral adrenal hyperplasia with concurrent obesity. There was an increase in the ARR from 757 pmol/L per ng/mL/hr (approximately equivalent to 53 pmol/L per ng/mL/hr) preoperatively to 215 pmol/L per mIU/L (ie, PAC 862 pmol/L and DRC 4.0 mIU/L) after surgery. Lost to further follow-up. AVS SI was >2 bilaterally at baseline and >3 bilaterally postcosyntropin and LI was 20.9. |
| 25 | Absent | Absent | Right-sided 0.9-cm nodule and left-sided bulkiness | Left-sided hyperplasia | 52 yo male seen in clinical follow-up at 1.2 mo and biochemical follow-up at 0.8 mo. Residual hypertension felt to be due to bilateral adrenal hyperplasia. There was a reduction of the ARR from 4589 pmol/L per ng/mL/hr preoperatively to 4194 pmol/L per ng/mL/hr (ie, PAC 1057 pmol/L and PRA 0.25 ng/mL/hr) after surgery. Lost to further follow-up. AVS SI was >3 bilaterally postcosyntropin and LI was 23.2. |
| 26 | Partial | Partial | Left-sided 0.8-cm nodule with surrounding bulkiness | Left-sided 0.9-cm adenoma | 45 yo male seen in clinical follow-up at 0.7 mo and biochemical follow-up at 11.9 mo. Residual hypertension felt to be due to obesity. There was a reduction of the ARR from 10680 pmol/L per ng/mL/hr preoperatively to 690 pmol/L per ng/mL/hr (ie, PAC 283 pmol/L and PRA 0.41 ng/mL/hr) after surgery. Lost to further follow-up. |
| 27 | Complete | Partial | Negative | Left-sided 0.9-cm adenoma | 50 yo male seen in follow-up at 6.3 mo. Unknown cause of residual hypertension. |
| 28 | Absent | Partial | Left-sided bulkiness | Right-sided 0.5-cm adenoma with surrounding hyperplasia | 54 yo male seen in clinical follow-up at 22.2 mo and biochemical follow-up at 9.0 mo. Residual hypertension felt to be due to obesity, OSA, and CKD, and was on a diuretic for chronic edema. There was a reduction of the ARR from 422 pmol/L per mIU/L preoperatively to 134 pmol/L per mIU/L (ie, PAC 470 pmol/L and DRC 3.5 mIU/L) after surgery. AVS SI was >2 bilaterally at baseline and >3 bilaterally postcosyntropin, and LI was 3.3. |
| 29 | Complete | Partial | Left-sided bulkiness | Hyperplasia | 54 yo male with a history of obesity and type 2 diabetes mellitus seen in follow-up at 0.2 mo. Lost to further follow-up. |
Abbreviations: AVS, adrenal vein sampling; Bioch., Biochemical; BP, blood pressure; CKD, chronic kidney disease; Clin., Clinical; DRC, direct renin concentration; hr, hours; LI, lateralization index; mo, months; ND, no data; SI, selectivity index; OSA, obstructive sleep apnea; PAC, plasma aldosterone concentration; PRA, plasma renin activity; yo, year-old.
Clinical outcomes
For the 36 people with clinical outcomes, 7 (19%) demonstrated a complete response, 24 (67%) had a partial response, and 5 (14%) had no clinical improvement over a median follow-up of 9.8 months (IQR, 1.9–55.8; maximum, 136.7) (Table 2). Following adrenalectomy, patients had an average reduction in systolic BP by 16 mm Hg (SD, 19) and diastolic BP by 8 mm Hg (SD, 11), along with a decrease in the number of medications that were needed (median reduction of 2.0 DDD [IQR, 2.9]). Adrenocortical adenomas were found at histopathologic examination in 28 (78%) cases with a median size of 1.0 cm (IQR, 0.4) in the largest dimension.
Among patients with a complete or partial clinical response, there was a large reduction in systolic BP (mean reduction, 19 mm Hg [SD, 19]), which was accompanied by a decrease in the number of medications taken postoperatively (median reduction, 2.3 DDD [IQR, 2.7]). A low postoperative plasma aldosterone was associated with favorable treatment response (median 167 vs 395 pmol/L for those with complete or partial response vs absent response, respectively; P = 0.03). Those with a clinical response had a comparatively lower eGFR after surgery (mean 61.2 vs 84.9 mL/min/1.73m2; P < 0.01), although the mean reduction in eGFR was not significantly different.
Detailed review of the 5 people who had no evidence of clinical response showed that 4 had hyperplasia on pathology and the remaining 1 individual who had a distinct adrenocortical nodule had a persistently elevated 24-hour mean ambulatory BP of 149/80 mm Hg but without treatment (Table 3).
Sensitivity analyses
We conservatively restricted the analysis to 105 patients who had no evidence of any nodularity, bulkiness, or hyperplasia on imaging, and we found that 32 (31%) lateralized on AVS and 17 (16%) subsequently underwent adrenalectomy. Clinical outcomes data were available for 11 of these subjects, of whom 9 (82%) had a complete or partial clinical response. Biochemical outcomes were obtainable for 11 patients, among whom 10 (91%) had a complete biochemical response. The prognosis was similar to that observed in the main analysis.
Finally, we evaluated clinical outcomes among the subset of patients who had a complete biochemical response. Among these individuals, clinical outcomes were available for 25 people. Overall, 6 (24%) had a complete clinical response and 16 (64%) had a partial clinical response for a total of 22 (88%) patients with a positive clinical response.
Discussion
In this study, we evaluated surgical outcomes in patients with PA and normal-appearing adrenal imaging who lateralized on AVS. Patients generally had excellent surgical responses. Using PASO criteria, complete biochemical remission was seen in 79% of people and complete cure or partial clinical improvement in 86%, rates that are consistent with global reports for patients surgically treated for aldosterone-producing adenomas (5, 12). Notably, even though our study subjects were frequently older (with more than two-thirds aged 50 years or older) and predominantly male (representing 8 of 10 people), their overall response to surgery was not worse. There were no obvious clinical characteristics that were predictive of postoperative outcomes in this population. These findings support the argument that imaging alone is inadequate as a subtyping modality in PA, as it would lead to many patients missing a potentially curative procedure.
Our findings are consistent with and extend those of previous reports. Wachtel et al (31) evaluated 61 patients with PA with nonlocalizing imaging (potentially inclusive of people with bilateral masses as well as those with no adrenal masses) who lateralized on AVS (defined using an lateralization index [LI] of ≥4:1; median LI, 12.4 [IQR, 6.5–24.2]) and subsequently underwent adrenalectomy. They found that 39 (64%) of these patients had complete or partial clinical improvement after 6 to 12 months of follow-up. Their relatively low rates of clinical response may be in part due to a subset of patients who had asymmetrical nodular hyperplasia. As in other studies, we confirmed that most patients with apparently normal adrenal imaging actually have microadenomas that are commonly below the detection threshold for CT (31, 32). Importantly, our biochemical and clinical response rates are comparable to those observed in larger cohorts of patients with distinct adenomas (5, 12). In an international study of 12 centers, Williams et al (5) reported that adrenalectomy for unilateral PA was associated with clinical improvement in 84% of people (intercenter range, 68–100%) and biochemical remission of aldosteronism in 94% (range, 83–100%). Notably, even though patients in our study had a high frequency of poor prognostic factors, including the fact that many individuals were typically older (by an average of 5 years), obese, a larger proportion were male, and most had small subcentimetric adrenocortical adenomas in comparison to other studies (5, 12), our rates of clinical cure and biochemical response were not appreciably different than expected.
The finding that patients with PA and unilateral aldosterone hypersecretion have excellent postoperative responses, even when initial imaging appears normal, is important because it informs treatment decisions and may affect downstream outcomes. Over the long term, there is growing evidence that adrenalectomy is more effective than empiric medical treatment for patients with PA in lowering the risk of cardiovascular events, incident atrial fibrillation, progressive kidney disease, diabetes mellitus, and mortality (6–8, 10, 33). As patients with small and radiographically indiscernible nodules may potentially reflect those with a milder spectrum of disease, early intervention may help to mitigate late complications.
This study had many strengths. This is the only study to our knowledge reporting on surgical outcomes specifically for patients with “normal” adrenal imaging. Standardized criteria for evaluating clinical outcomes were applied in order to facilitate comparison with other studies (5, 12, 31). This is in contrast to most other adrenalectomy studies where reporting was inconsistent or incomplete (34). Additionally, we are the first to describe biochemical outcomes in these patients, which alongside clinical outcomes, provide a global assessment of postoperative success (4, 5). Long-term follow-up of clinical outcomes (up to 11 years) was obtained in many patients, indicating that clinical responses were durable.
There were some limitations to this study. First, because adrenal imaging was ordered as part of routine care, imaging protocols changed over the study period. However, all CT scans performed within our region had sufficient resolution and were comparable to those of contemporary publications (18, 19, 27), with at least 1 plane at or below 3 mm in thickness. In our sensitivity analysis, where we excluded patients with subtle radiographic features of adrenal bulkiness or thickening, we found that outcomes were essentially identical to our primary findings. Second, our study cohort did not have any patients under the age of 40 years, thus limiting generalizability. Although we could not evaluate if surgery was similarly beneficial in younger patients, we would expect their outcomes to be favorable, as advanced age is generally associated with worse outcomes (5, 11, 15, 35). Third, patients with lateralization on AVS were presumed to have unilateral disease, as based on our previous experience (23), but we could not exclude the possibility of misclassification because of asymmetric bilateral nodular disease, which could have been present in some cases where no biochemical response was seen after surgery (36). Fourth, we cannot exclude the possibility of selection bias as patients who consented to proceed with surgery may have been systematically different than those who declined. The generally high lateralization indices in those who received adrenalectomy may have been associated with the high rates of biochemical and clinical cure that we observed. Finally, long-term cardiovascular outcomes were not examined in this study, although these have independently been shown to improve after adrenalectomy for patients with unilateral PA (6, 8, 10).
Conclusions
Individuals with AVS-confirmed unilateral PA and normal-appearing adrenal imaging have high rates of biochemical and clinical success following adrenalectomy. Thus, eligible patients who are interested in surgery should be considered for potentially curative adrenalectomy, rather than treated empirically with medications for bilateral disease. The prognosis following surgery remains highly favorable, even among patients who are obese, older, and male. Future studies are needed to determine if other clinical factors are predictive of treatment response.
Acknowledgments
Financial Support: A.A.L. is supported by the Hypertension Canada New Investigator Award and the Canadian Institutes of Health Research Project Grant (159533). The funders had no role in study design, data collection, analysis, reporting, or the decision to submit for publication.
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability
Restrictions apply to the availability of data generated or analyzed during this study to preserve patient confidentiality. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.
References
- 1. Funder JW, Carey RM, Mantero F, et al. . The management of primary aldosteronism: case detection, diagnosis, and treatment: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2016;101(5):1889–1916. [DOI] [PubMed] [Google Scholar]
- 2. Monticone S, D’Ascenzo F, Moretti C, et al. . Cardiovascular events and target organ damage in primary aldosteronism compared with essential hypertension: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2018;6(1):41–50. [DOI] [PubMed] [Google Scholar]
- 3. Brown JM, Siddiqui M, Calhoun DA, et al. . The unrecognized prevalence of primary aldosteronism: a cross-sectional study. Ann Intern Med. 2020;173(1):10–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rossi GP, Bisogni V, Bacca AV, et al. . The 2020 Italian Society of Arterial Hypertension (SIIA) practical guidelines for the management of primary aldosteronism. Int J Cardiol Hypertens. 2020;5(April):100029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Williams TA, Lenders JWM, Mulatero P, et al. ; Primary Aldosteronism Surgery Outcome (PASO) investigators . Outcomes after adrenalectomy for unilateral primary aldosteronism: an international consensus on outcome measures and analysis of remission rates in an international cohort. Lancet Diabetes Endocrinol. 2017;5(9):689–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hundemer GL, Curhan GC, Yozamp N, Wang M, Vaidya A. Incidence of atrial fibrillation and mineralocorticoid receptor activity in patients with medically and surgically treated primary aldosteronism. JAMA Cardiol. 2018;3(8):768–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hundemer GL, Curhan GC, Yozamp N, Wang M, Vaidya A. Renal outcomes in medically and surgically treated primary aldosteronism. Hypertension. 2018;72(3):658–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hundemer GL, Curhan GC, Yozamp N, Wang M, Vaidya A. Cardiometabolic outcomes and mortality in medically treated primary aldosteronism: a retrospective cohort study. Lancet Diabetes Endocrinol. 2018;6(1):51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kline GA, Pasieka JL, Harvey A, So B, Dias VC. Medical or surgical therapy for primary aldosteronism: post-treatment follow-up as a surrogate measure of comparative outcomes. Ann Surg Oncol. 2013;20(7):2274–2278. [DOI] [PubMed] [Google Scholar]
- 10. Rossi GP, Maiolino G, Flego A, et al. ; PAPY Study Investigators . Adrenalectomy lowers incident atrial fibrillation in primary aldosteronism patients at long term. Hypertension. 2018;71(4):585–591. [DOI] [PubMed] [Google Scholar]
- 11. Lumachi F, Ermani M, Basso SM, Armanini D, Iacobone M, Favia G. Long-term results of adrenalectomy in patients with aldosterone-producing adenomas: multivariate analysis of factors affecting unresolved hypertension and review of the literature. Am Surg. 2005;71(10):864–869. [PubMed] [Google Scholar]
- 12. Vorselaars WMCM, Nell S, Postma EL, et al. ; International CONNsortium study group . Clinical outcomes after unilateral adrenalectomy for primary aldosteronism. JAMA Surg. 2019;154(4):e185842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sam D, Kline GA, So B, Przybojewski SJ, Leung AA. Unilateral disease is common in patients with primary aldosteronism without adrenal nodules. Can J Cardiol. 2020. Doi: 10.1016/j.cjca.2020.05.013 [DOI] [PubMed] [Google Scholar]
- 14. Mulatero P, Stowasser M, Loh KC, et al. . Increased diagnosis of primary aldosteronism, including surgically correctable forms, in centers from five continents. J Clin Endocrinol Metab. 2004;89(3):1045–1050. [DOI] [PubMed] [Google Scholar]
- 15. Takeda M, Yamamoto K, Akasaka H, et al. ; JPAS Study Group . Clinical characteristics and postoperative outcomes of primary aldosteronism in the elderly. J Clin Endocrinol Metab. 2018;103(10):3620–3629. [DOI] [PubMed] [Google Scholar]
- 16. Mulatero P, Bertello C, Rossato D, et al. . Roles of clinical criteria, computed tomography scan, and adrenal vein sampling in differential diagnosis of primary aldosteronism subtypes. J Clin Endocrinol Metab. 2008;93(4):1366–1371. [DOI] [PubMed] [Google Scholar]
- 17. Dekkers T, Prejbisz A, Kool LJS, et al. ; SPARTACUS Investigators . Adrenal vein sampling versus CT scan to determine treatment in primary aldosteronism: an outcome-based randomised diagnostic trial. Lancet Diabetes Endocrinol. 2016;4(9):739–746. [DOI] [PubMed] [Google Scholar]
- 18. Kamemura K, Wada N, Ichijo T, et al. . Significance of adrenal computed tomography in predicting laterality and indicating adrenal vein sampling in primary aldosteronism. J Hum Hypertens. 2017;31(3):195–199. [DOI] [PubMed] [Google Scholar]
- 19. Umakoshi H, Tsuiki M, Takeda Y, et al. ; JPAS Study Group . Significance of computed tomography and serum potassium in predicting subtype diagnosis of primary aldosteronism. J Clin Endocrinol Metab. 2018;103(3):900–908. [DOI] [PubMed] [Google Scholar]
- 20. Williams TA, Burrello J, Sechi LA, et al. . Computed tomography and adrenal venous sampling in the diagnosis of unilateral primary aldosteronism. Hypertension. 2018;72(3):641–649. [DOI] [PubMed] [Google Scholar]
- 21. Rossi GP, Barisa M, Allolio B, et al. . The Adrenal Vein Sampling International Study (AVIS) for identifying the major subtypes of primary aldosteronism. J Clin Endocrinol Metab. 2012;97(5):1606–1614. [DOI] [PubMed] [Google Scholar]
- 22. Kline GA, Pasieka JL, Harvey A, So B, Dias VC. High-probability features of primary aldosteronism may obviate the need for confirmatory testing without increasing false-positive diagnoses. J Clin Hypertens (Greenwich). 2014;16(7):488–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kline G, Leung A, So B, Chin A, Harvey A, Pasieka JL. Application of strict criteria in adrenal venous sampling increases the proportion of missed patients with unilateral disease who benefit from surgery for primary aldosteronism. J Hypertens. 2018;36(6):1407–1413. [DOI] [PubMed] [Google Scholar]
- 24. Sam D, Kline GA, So B, Leung AA. Discordance between imaging and adrenal vein sampling in primary aldosteronism irrespective of interpretation criteria. J Clin Endocrinol Metab. 2019;104(6):1900–1906. [DOI] [PubMed] [Google Scholar]
- 25. Leung AA, Orton DJ, Chin A, Sadrzadeh H, Kline GA. Novel approach to establishing an aldosterone: renin ratio cutoff for primary aldosteronism. Hypertension. 2017;69(3):450–456. [DOI] [PubMed] [Google Scholar]
- 26. Wang K, Hu J, Yang J, et al. . Development and validation of criteria for sparing confirmatory tests in diagnosing primary aldosteronism. J Clin Endocrinol Metab. 2020;105(7):1–8. [DOI] [PubMed] [Google Scholar]
- 27. Umakoshi H, Ogasawara T, Takeda Y, et al. . Accuracy of adrenal computed tomography in predicting the unilateral subtype in young patients with hypokalaemia and elevation of aldosterone in primary aldosteronism. Clin Endocrinol (Oxf). 2018;88(5):645–651. [DOI] [PubMed] [Google Scholar]
- 28. Coresh J, Selvin E, Stevens LA, et al. . Prevalence of chronic kidney disease in the United States. JAMA. 2007;298(17):2038–2047. [DOI] [PubMed] [Google Scholar]
- 29. Aung K, Htay T. Relationship between outpatient clinic and ambulatory blood pressure measurements and mortality. Curr Cardiol Rep. 2019;21(5):28. [DOI] [PubMed] [Google Scholar]
- 30. Takamatsu K, Takeda T, Hattori S, et al. . Appropriate timing for a biochemical evaluation after adrenalectomy for unilateral aldosterone-producing adenoma. Clin Endocrinol (Oxf). 2020;92(6):503–508. [DOI] [PubMed] [Google Scholar]
- 31. Wachtel H, Bhandari S, Roses RE, Cohen DL, Trerotola SO, Fraker DL. Primary aldosteronism with nonlocalizing imaging. Surgery. 2019;165(1):211–218. [DOI] [PubMed] [Google Scholar]
- 32. Letavernier E, Peyrard S, Amar L, Zinzindohoué F, Fiquet B, Plouin PF. Blood pressure outcome of adrenalectomy in patients with primary hyperaldosteronism with or without unilateral adenoma. J Hypertens. 2008;26(9):1816–1823. [DOI] [PubMed] [Google Scholar]
- 33. Wu VC, Chueh SJ, Chen L, et al. ; TAIPAI Study Group . Risk of new-onset diabetes mellitus in primary aldosteronism: a population study over 5 years. J Hypertens. 2017;35(8):1698–1708. [DOI] [PubMed] [Google Scholar]
- 34. Lenders JWM, Deinum J, Passauer J, Januszewicz A, Chan OYA, Prejbisz A. Low quality of reports on blood pressure in patients adrenalectomized for unilateral primary aldosteronism. J Clin Endocrinol Metab. 2020;105(6):1–7. [DOI] [PubMed] [Google Scholar]
- 35. Zarnegar R, Young WF Jr, Lee J, et al. . The aldosteronoma resolution score: predicting complete resolution of hypertension after adrenalectomy for aldosteronoma. Ann Surg. 2008;247(3):511–518. [DOI] [PubMed] [Google Scholar]
- 36. De Sousa K, Boulkroun S, Baron S, et al. . Genetic, cellular, and molecular heterogeneity in adrenals with aldosterone-producing adenoma. Hypertension. 2020;75(4):1034–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Restrictions apply to the availability of data generated or analyzed during this study to preserve patient confidentiality. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.