Abstract
Context
Bariatric surgery, particularly Roux-en-Y gastric bypass (RYGB), is associated with an increased risk of osteoporotic fractures. It is unknown whether RYGB or sleeve gastrectomy (SG) have different effects on bone health.
Objective
To compare changes in bone mineral density and markers of bone turnover 1 year after SG and RYGB.
Design, Setting, Patients, and Interventions
Randomized, triple-blind, single-center trial at a tertiary care center in Norway. The primary outcome was diabetes remission. Patients with severe obesity and type 2 diabetes were randomized and allocated (1:1) to SG or RYGB.
Main Outcome Measures
Changes in areal bone mineral density (aBMD) and bone turnover markers.
Results
Femoral neck, total hip, and lumbar spine aBMD, but not total body aBMD, decreased significantly more after RYGB (n = 44) than after SG (n = 48) (mean [95% confidence interval] between group differences -2.8% [-4.7 to -0.8], -3.0% [-5.0 to -0.9], -4.2% [-6.4 to -2.1], and -0.5% [-1.6 to 0.6], respectively). The increase in procollagen type 1 N-terminal propeptide (P1NP) and C-telopeptide of type I collagen (CTX-1) were approximately 100% higher after RYGB than after SG (between group difference at 1 year, both P < 0.001). The changes in femoral neck, total hip, and lumbar spine aBMDs and the changes in P1NP and CTX-1 were independently associated with the surgical procedure (all P < 0.05) and not weight change.
Conclusions
Roux-en-Y gastric bypass was associated with a greater reduction in aBMD and a greater increase in bone turnover markers compared with SG. This finding could suggest greater skeletal fragility after RYGB.
Keywords: gastric bypass, sleeve gastrectomy, bone mineral density, bone turnover, type 2 diabetes, morbid obesity
Bariatric surgery has increased dramatically over the last few decades, with more than 600 000 procedures performed worldwide in 2016 (1). Today, sleeve gastrectomy and gastric bypass are the most commonly performed procedures, accounting for more than 80% of operations (1). Several observational studies have reported an increased risk of fractures after bariatric surgery (2–8). A recent large French retrospective cohort study including more than 40 000 bariatric patients aged 40–65 years with a body mass index (BMI) ≥ 40 kg/m2 and matched controls showed that gastric bypass, but not sleeve gastrectomy, was associated with an increased risk of major osteoporotic fractures (5). The underlying mechanisms explaining these observations are unclear. However, reduced areal bone mineral density (aBMD) and increased metabolic activity of bone, both known risk factors for osteoporotic fractures (9, 10), may contribute to an increased fracture risk after bariatric surgery (11).
A few nonrandomized clinical trials have reported a greater reduction in aBMD at some skeletal regions and/or a greater increase in markers of bone turnover after gastric bypass than after sleeve gastrectomy (12–15). By contrast, other nonrandomized studies have reported no between-group differences (16–19). A substudy of patients included in the randomized controlled STAMPEDE trial reported no difference in bone mineral density (BMD) at 2 years between subjects with type 2 diabetes after sleeve gastrectomy (n = 19) and gastric bypass (n = 17) (20). However, at 5 years, markers of bone turnover were significantly higher in patients randomized to gastric bypass than in patients receiving sleeve gastrectomy (21). In addition, a greater reduction in lumbar spine aBMD and a greater increase in bone remodeling markers after metabolic (200 cm biliopancreatic limb and 100 cm alimentary limb) gastric bypass (n = 15) than after sleeve gastrectomy (n = 15) has been reported in a small randomized controlled trial (22).
Diet-induced weight-loss is associated with reduced aBMD and increased bone turnover (23), and it is therefore possible that metabolic changes in bone after bariatric surgery is a physiological adaption to a lower body weight. However, it is also possible that the anatomical rearrangement, especially after gastric bypass, induces potentially deleterious effects on bone health.
Previous trials addressing changes in aBMD and bone turnover after gastric bypass and sleeve gastrectomy report divergent results, and some studies have major flaws. Most studies were nonrandomized clinical trials (12–19), and the 2 randomized studies (20–22) were limited by small study samples. It is therefore still unclear whether gastric bypass results in a greater reduction in aBMD and higher bone turnover than sleeve gastrectomy, and whether these changes are related to weight loss or the procedures per se. The main objective of this randomized controlled study was to compare changes in aBMD and markers of bone turnover in subjects with severe obesity and type 2 diabetes 1 year after sleeve gastrectomy and gastric bypass. In addition, the independent effects of weight loss and the procedures on these parameters were explored. We hypothesized greater aBMD loss and higher bone turnover after gastric bypass than after sleeve gastrectomy, and that these potential differences would be mediated by both greater weight loss and by the procedure per se.
Materials and Methods
Trial design
The Oseberg study is a randomized, triple-blind, parallel-group, single-center trial taking place at a tertiary care center at Vestfold Hospital Trust in Tønsberg, Norway. Patients with severe obesity and type 2 diabetes were randomized and allocated (1:1) to either Roux-en-Y gastric bypass (RYGB) or sleeve gastrectomy. The study protocol was approved by the Regional Committees for Medical and Health Research Ethics in Norway (2012/1427/REK sør-øst B) and has been published previously (24). The primary outcome of the Oseberg study was diabetes remission at 1 year (25).The study is ongoing with annual visits at 2, 3, 4, and 5 years after the surgical procedure. The ClinicalTrials.gov identifier is NCT01778738.
Participants
Inclusion criteria were age ≥18 years, current BMI ≥ 33.0kg/m2 with previously verified BMI ≥ 35.0kg/m2, and type 2 diabetes (Hemoglobin A1c [HbA1c] ≥ 6.5% or use of antidiabetic medications with HbA1c ≥ 6.1%). Key exclusion criteria were previous major abdominal surgery, cancer, severe medical conditions associated with increased risk of complications, drug or alcohol addiction, pregnancy, severe gastroesophageal reflux disease, and long-term systemic corticosteroid use (3 months cumulative use in the last 12 months or treatment the past 2 months) (24). All patients provided written informed consent.
Interventions
The 2 intervention groups received identical pre- and postoperative treatment, including a low calorie diet (< 1200 kcal/day) during the 2 weeks preceding surgery. After surgery, patients were assessed at 5 weeks, 16 weeks, 34 weeks, and 1 year. Both groups were, when discharged from the hospital after surgery, prescribed identical vitamin and mineral supplementations, including daily oral supplementation with 2 multivitamin/mineral tablets, 1000 mg calcium carbonate, 800 IU vitamin D3, and 100 mg ferrous sulphate (in premenopausal women), and intramuscular injections of 1 mg vitamin B12 every third month. Vitamin and mineral supplementations were adjusted at each visit according to specific predefined algorithms aiming for serum levels of 25-OH-vitamin D between 50 and 125 nmol/l and calcium levels within the normal rage (2.10–2.60 mmol/l) (24).
Gastric bypass (25 ml pouch, 120 cm Roux-limb, and 60 cm biliopancreatic limb) and sleeve gastrectomy (35 Fr Bougie) were performed laparoscopically using identical skin incisions, as previously described (24).
Outcomes
The primary outcome results have been published (25). Areal BMD and body composition measured with dual-energy x-ray absorptiometry (DXA) at 1 year and procollagen type 1 N-terminal propeptide (P1NP), C-telopeptide of type I collagen (CTX-1), and bone alkaline phosphatase (BALP) in blood measured at 5 weeks, 16 weeks, 34 weeks, and 1 year are prespecified secondary outcomes in the Oseberg study.
Areal BMD of the total body with subregions, lumbar spine (L1–L4), and left proximal femur (total, neck, trochanter, and intertrochanter) was measured by DXA on a Hologic Delphi W instrument (Hologic, Inc, Bedford, Massachusetts) approximately 3 weeks prior to and 1 year after surgery. The total-body scan also provided measures of fat mass and lean mass. A trained team of 3 bioengineers performed all DXA scans. Each test day, the DXA machine was calibrated against a spine-phantom and a step-phantom supplied by the manufacturer. The presented Z-scores represent the number of standard deviations an actual aBMD deviates from the mean aBMD in the age- and gender-matched reference population (NHANES and Hologic studies given by the manufacturer). Areal BMD precision error reported by the manufacturer is < 1.0%.
Venous blood samples were obtained after an overnight fast at baseline, at 5 weeks, and at 1 year. At 16 and 34 weeks, nonfasting blood samples were collected. P1NP, CTX-1, and BALP were analyzed at the Hormone Laboratory, Oslo University Hospital, whereas vitamin D, parathyroid hormone (PTH), albumin, calcium, phosphate, and magnesium were analyzed at the Department of Laboratory Medicine, Vestfold Hospital Trust. Both laboratories are certified according to NO-EN ISO 15189. Serum CTX-1 and P1NP were measured using eletrochemicaliluminesence immunoassay (ECLIA; Roche Diagnostics, Mannheim, Germany). Serum BALP was analyzed using enzyme immunoassay (EIA; Metra Biosystems Inc, Mountain View, California) until February 2014 and by a competitive chemiluminescent assay (CLIA; Dia-Sorin, Saluggia, Italy) thereafter. The reported values until February 2014 were transformed to corresponding values using a documented regression algorithm [Y (new) = 0.38X (old) - 2.2, r = 0.95]. HbA1c was measured on a Tosoh high-performance liquid chromatography G8 analyzer (Tosoh Corporation, Tokyo, Japan). Vitamin D was analyzed using CLIA on the Architect i2000SR from Abbott Diagnostics (Abbott Laboratories, Abbott Park, Illinois) until November 2016, and from November 2016 until October 2017 using liquid chromatography tandem mass spectrometer, and thereafter by ECLIA on Roche Cobas 8000 (Roche Diagnostics, Basel, Switzerland). Method alignment during the study period was monitored in the laboratory. Plasma PTH was analyzed on the Architect i2000SR (CLIA) until October 2017 and on Roche Cobas 8000 (ECLIA) thereafter. The shift introduced a bias for PTH results corrected by a documented regression algorithm [Y (new) = 0.707X (old) + 0.483, r = 0.99]. Serum levels of albumin, calcium, phosphate, and magnesium were measured using dry slide technology on Vitros 5.1 (Ortho-Clinical Diagnostics, Raritan, New Jersey, USA) until October 2017 and by Roche Cobas 8000 thereafter. The regression algorithms for the analyses for which methods changed were obtained from comparison studies using patient samples performed according to standard operating procedures in the laboratory (data not shown). Analytical precision, method principles, sample matrix, and units used are given in Supplementary Table 1 located in a digital research materials repository (26).
Diabetes remission was defined as having an HbA1c ≤ 6.0 % without the use of diabetes medications.
Dietary intake was assessed through structured interviews performed by registered dieticians at baseline and at 1 year. Dietary intake was recorded using a validated food frequency questionnaire (27, 28). Questionnaire data was scanned using the Teleform program (10.0) (Datascan, Oslo, Norway). Calcium and vitamin D intakes (including supplementations) were calculated with an in-house (University of Oslo) data program (KBS version 7.3, 2017). The food database used (AE-14) is based on the official Norwegian Food Composition Table.
The SenseWear Armband (BodyMedia, Pittsburgh, Pennsylvania) was worn on the patient’s dominant upper arm for 4 days before and 1 year postsurgery to monitor physical activity (daily steps).
Sample size and randomization
The Oseberg study was powered (significance level to 5% and power to 80%) to detect differences in diabetes remission rates (n = 110) and changes in beta cell function (n = 100) (24).
The randomization procedure has previously been described in detail (24). Patients were randomized and allocated (1:1 ratio) to either gastric bypass or sleeve gastrectomy using a computerized random number generator with block sizes of 10. All study personnel, patients, and the primary outcome assessor were blinded to allocations. The surgeons did not participate in patient follow-up.
Statistical analysis
Due to missing DXA-scans (mostly due to body weight above the machine´s upper weight limit [150 kg]) data were analyzed according to per-protocol. The binary outcomes were analyzed using Fisher`s exact test. Continuous outcomes were analyzed using an independent samples t-test, the Mann-Whitney U test, a paired-samples t-test, linear mixed effects models for repeated measures, and analysis of covariance (including the baseline value of the dependent variable as a covariate). The age- and gender-adjusted effects of changes in body weight and body composition, daily steps and treatment group on changes in aBMD, and markers of bone turnover were assessed by multiple linear regression analysis. All tests were 2-sided and the significance level was set to 0.05. STATA software, version 15.0 was used to perform the linear mixed effects models. Other statistical analyses were performed using SPSS software, version 25.0.
Results
Patient flow and recruitment
Between October 2012 and September 2017, 1305 consecutive patients scheduled for bariatric surgery were screened for study eligibility. Out of 319 patients with severe obesity and type 2 diabetes, 115 were ineligible and 95 declined participation. Consequently, 109 patients were randomly assigned to gastric bypass (n = 54) or sleeve gastrectomy (n = 55) and underwent a baseline examination between January 2013 and February 2018. Two patients were lost to follow-up, 11 patients did not perform the DXA at baseline due to a body weight above 150 kg, and 4 patients rejected the DXA scan at 1 year, leaving 48 patients in the sleeve gastrectomy group and 44 patients in the gastric bypass group to be included in the analyses.
Baseline
At baseline, the 92 patients (67% women) had a mean (standard deviation [SD]) age of 48.7 years (9.0), a mean BMI of 40.6 kg/m2 (4.1), a median duration of diabetes of 5 years (interquartile range 2–10), and median HbA1c of 7.7% (6.8–8.9) or 61 mmol/mol (51–74). Areal BMD in the femoral neck, total hip, spine, and total body and markers of bone turnover did not differ between the 2 groups (Table 1). Mean aBMD at the femoral neck, total hip, and lumbar spine were approximately 1 to 1.5 SDs above the mean aBMD in the age- and gender-matched reference populations (Z-scores), with no between-group differences. Thirty-six (39%) patients reported a total of 45 fractures in the following anatomical locations: foot (n = 6), tibia/fibula (n = 10), femur (n = 1), hand (n = 3), radius/ulna (n = 7), humerus (n = 2), clavicular (n = 2), scapula (n = 1), rib (n = 1), face (n = 2), and unspecified location (n = 10). Of these, 1 patient (in the sleeve gastrectomy group) reported a low-energy clavicle fracture. No patients used antiosteoporotic medications. With the exception of higher lean mass in the sleeve gastrectomy group than in the gastric bypass group, other patient demographics and characteristics did not differ significantly between groups (Table 1).
Table 1.
Patient demographics and characteristics at baseline
| Sleeve Gastrectomy | Gastric Bypass | P-value | |
|---|---|---|---|
| Number | 48 | 44 | |
| Age (years) | 48 (10) | 50 (8) | 0.414 |
| Female (yes) | 30 (63%) | 35 (80%) | 0.108 |
| Postmenopausal (yes) | 15 (50%) | 16 (46%) | 0.806 |
| White ethnicity (yes) | 47 (98%) | 43 (98%) | 1.00 |
| Current smoker (yes) | 3 (6%) | 4 (9%) | 0.759 |
| Alcohol units per week | 0 (0–2) | 1 (0–1) | 0.849 |
| Previous bone fracture (yes) | 21 (44%) | 15 (34%) | 0.396 |
| Daily steps | 5050 (1936) | 5485 (2235) | 0.320 |
| Body composition | |||
| Body weight (kg) | 121.6 (15.9) | 118.4 (17.8) | 0.361 |
| Height (m) | 1.72 (0.10) | 1.69 (0.08) | 0.089 |
| Body mass index (kg/m2) | 41.1 (4.1) | 41.5 (4.5) | 0.661 |
| Lean mass (kg) | 67.9 (12.3) | 62.9 (10.4) | 0.041 |
| Fat mass (kg) | 49.6 (8.3) | 50.8 (8.6) | 0.491 |
| Bone mineral content (kg) | 2.91 (0.51) | 2.73 (0.41) | 0.070 |
| Total mass (kg) | 120.4 (14.9) | 116.4 (16.5) | 0.234 |
| Diabetes status | |||
| HbA1c (%) | 7.7 (6.8–9.8) | 7.7 (6.8–8.5) | 0.407 |
| Duration of diabetes (years) | 5 (2–10) | 5 (2–10) | 0.946 |
| Medications | |||
| Diabetes medication (yes) | 43 (90%) | 38 (86%) | 0.752 |
| Insulin (yes) | 9 (19%) | 9 (21%) | 1.00 |
| Metformin (yes) | 36 (75%) | 34 (80%) | 0.629 |
| Dipeptidyl peptidase-4 inhibitors (yes) | 14 (29%) | 11 (25%) | 0.815 |
| Sodium-glucose co-transporter-2 inhibitors (yes) | 2 (4%) | 1 (2%) | 1.000 |
| Sulphonylureas (yes) | 11 (23%) | 4 (9%) | 0.093 |
| Glucagon-like peptide-1 receptor agonists (yes) | 7 (15%) | 11 (25%) | 0.293 |
| Pioglitazone (yes) | 0 (0%) | 1 (2%) | 0.478 |
| Proton pump inhibitor (yes) | 8 (17%) | 11 (25%) | 0.440 |
| Levothyroxine supplementation (yes) | 4 (8%) | 7 (16%) | 0.341 |
| Menopausal hormone therapy (yes) | 1 (3%) | 1 (3%) | 1.00 |
| Bone density | |||
| Femoral neck aBMD (g/cm2) | 0.904 (0.117) | 0.926 (0.155) | 0.449 |
| Femoral neck Z-score (SD) | 0.92 (0.93) | 1.30 (1.19) | 0.086 |
| Total hip aBMD (g/cm2) | 1.094 (0.117) | 1.096 (0.138) | 0.941 |
| Total hip Z-score (SD) | 1.22 (0.74) | 1.51 (1.01) | 0.117 |
| Lumbar spine L1-4 aBMD (g/cm2) | 1.107 (0.132) | 1.141 (0.154) | 0.258 |
| Lumbar spine Z-score (SD) | 0.97 (1.29) | 1.51 (1.65) | 0.080 |
| Total body aBMD (g/cm2) | 1.272 (0.117) | 1.249 (0.096) | 0.306 |
| Vitamin D status | |||
| P-PTH (pmol/l) | 7.0 (3.0) | 6.3 (2.1) | 0.223 |
| S-25-OH-vitamin D (nmol/l) | 60.4 (19.0) | 61.4 (25.0) | 0.837 |
Data are presented as mean (standard deviation), median (interquartile range), or number (percent). P-values were calculated using the independent samples t-test, the Mann-Whitney U test, or the Fisher`s exact test.
Abbreviations: aBMD, areal bone mineral density; HbA1c, hemoglobin A1c; SD, standard deviation.
Bone mineral density
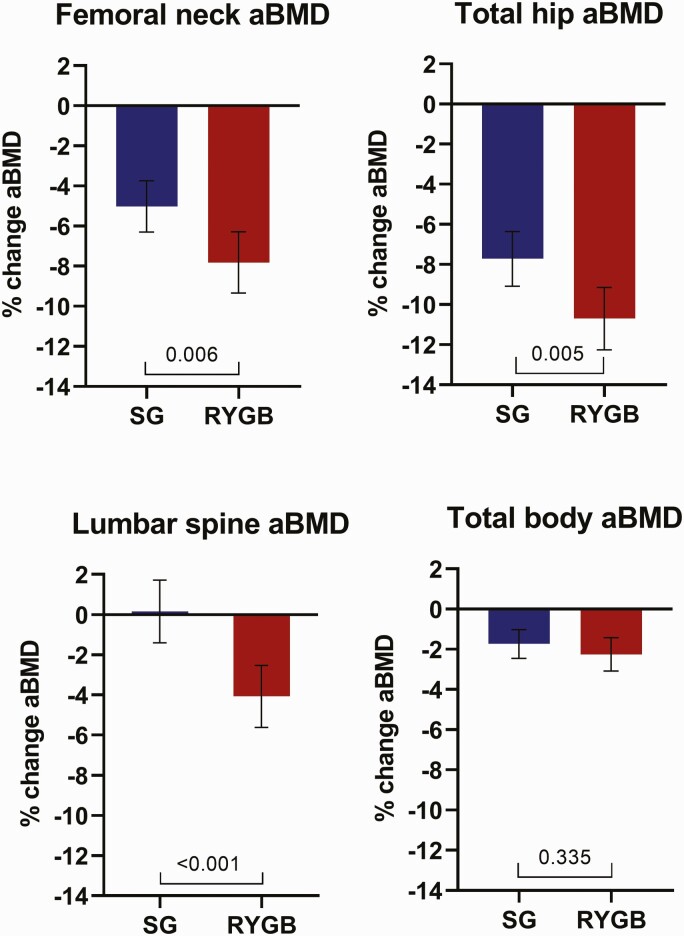
Areal BMD in the femoral neck, total hip, and lumbar spine decreased significantly more after gastric bypass than after sleeve gastrectomy during follow-up (mean [95% CI] between-group differences -2.8% [-4.7 to -0.8], -3.0% [-5.0 to -0.9], and -4.2% (-6.4 to -2.1), respectively) (Fig. 1). By contrast, the reduction in total body aBMD did not differ between the 2 groups (mean [95% CI] between-group differences -0.5% [-1.6 to 0.6]) (Fig. 1). Moreover, the reductions in aBMD at the trochanter and intertrochanter regions and at lumbar vertebrae 1–4 were significantly greater after gastric bypass than after sleeve gastrectomy (Data are available in Supplementary Table 2 located in a digital research materials repository (26)).
Figure 1.
Mean percent change in areal bone mineral density (aBMD) from baseline to 1 year after sleeve gastrectomy (SG) and Roux-en-Y gastric bypass (RYGB). Bars indicate 95% confidence intervals. P-values were calculated using independent samples t-test.
Z-scores at the femoral neck, total hip, and lumbar spine at 1 year were significantly lower in the gastric bypass group than in the sleeve gastrectomy group but still remained above zero in both groups (mean [95% CI] 0.55 SD [0.43–0.66] vs 0.74 SD [0.63–0.85], P = 0.020; 0.56 SD [0.47–0.65] vs 0.82 SD [0.73–0.91], P < 0.001; and 0.86 SD [0.71–1.0] vs 1.27 SD [1.12–1.42], P < 0.001, respectively).
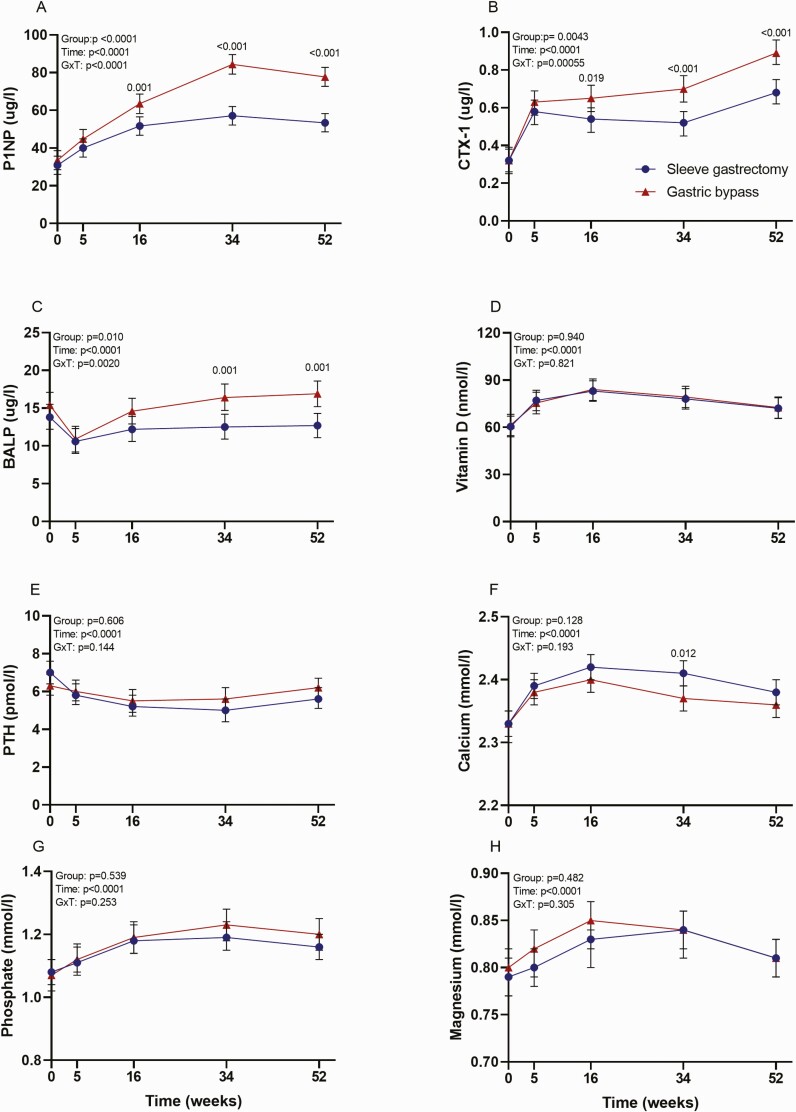
Serum levels of bone turnover markers
The increase in P1NP and CTX-1 was approximately 100% higher after gastric bypass than after sleeve gastrectomy (Fig. 2). BALP had a transient decrease in both groups 5 weeks after surgery (Fig. 2). At 1 year, BALP was significantly higher in the gastric bypass group than in the sleeve gastrectomy group. Vitamin D, PTH, calcium, phosphate, and magnesium changed significantly during follow-up, but there were no between-group differences (Fig. 2). Vitamin D, calcium, phosphate, and magnesium increased transiently, whereas PTH decreased transiently during follow-up, with peak and nadir levels at weeks 16 to 34, respectively.
Figure 2.
Bone turnover markers, vitamin D, parathyroid hormone, and electrolytes after sleeve gastrectomy and gastric bypass during 1-year follow-up. P-values were derived from linear mixed effects models for repeated measures. Bars indicate 95% confidence intervals. Abbreviations: BALP, bone alkaline phosphatase; CTX-1, C-telopeptide of type I collagen; P1NP, procollagen type 1 N-terminal propeptide; PTH, parathyroid hormone.
Body weight and body composition
The reductions in body weight, fat mass, and bone mineral content from baseline to 1 year were significantly greater after gastric bypass than after sleeve gastrectomy (mean [95% CI] -34.5 kg [-37.3 to -31.7] vs -28.7 kg [-32.4 to -24.9], P = 0.014; -23.0 kg [-25.1 to -21.0] vs -18.2 kg [-20.9 to -15.5], P = 0.005; and -123 g [-153 to -93] vs -74 g [-99 to -49], P = 0.012, respectively). By contrast, changes in lean mass did not differ between the gastric bypass group and the sleeve gastrectomy group (-10.2 kg [-11.2 to -9.2] vs -9.8 kg [-10.9 to -8.8], P = 0.614).
Glycemic control
The 1-year reduction in HbA1c did not differ between the sleeve gastrectomy group and the gastric bypass group (mean [95% CI] -2.0% [-2.5 to -1.6] vs -2.0% [-2.4 to -1.5], P = 0.769). However, the number of patients using at least 1 diabetes medication at 1 year was lower, and the 1-year diabetes remission rate was higher after gastric bypass than after sleeve gastrectomy (6 [14%] vs 16 [33%], P = 0.031 and 33 [75%] vs 24 [50%], P = 0.018).
Physical activity
The increase in number of daily steps did not differ significantly between patients randomized to sleeve gastrectomy or gastric bypass (mean [95% CI] 1678 [951–2405] vs 653 [-293–1600], P = 0.085).
Areal BMD and markers of bone turnover: associations with weight loss, surgical procedures, and physical activity
Weight change from baseline to 1 year was, after adjustments for age and gender, significantly associated with the percent change in lumbar spine aBMD and with 1-year changes in P1NP and CTX-1 (Table 2). No significant associations were found between weight change and percent change in femoral neck aBMD, total hip aBMD, total body aBMD, or change in BALP (Table 2). Replacing weight change with fat and lean mass changes showed significant associations between fat mass change and changes in lumbar spine aBMD, P1NP, and CTX-1 (Data are available in Supplementary Table 3 located in a digital research materials repository (26)). Lean mass change was associated with neither aBMD changes nor changes in bone turnover markers (Data are available in Supplementary Table 3 located in a digital research materials repository (26)). Including both weight change and treatment group as independent variables in the regression analyses revealed that the percent change in aBMD at the femoral neck, total hip, and lumbar spine, and the change in P1NP and CTX-1 were all independently associated with the surgical procedure and not the weight change (Table 2). By contrast, the percent change in total body aBMD and change in BALP were associated with neither weight change nor treatment group (Table 2).
Table 2.
Independent effects of weight loss and treatment group on percent change in areal bone mineral density and absolute change in serum markers of bone turnover from baseline to one year
| Model 1 | Model 2 | |||||
|---|---|---|---|---|---|---|
| Weight change | Treatment group | Weight change | ||||
| Dependent variable | β | P-value | β | P-value | β | P-value |
| Femoral neck aBMD | 0.122 | 0.259 | -0.278 | 0.012 | 0.035 | 0.750 |
| Total hip aBMD | 0.104 | 0.333 | -0.252 | 0.022 | 0.025 | 0.823 |
| Lumbar spine aBMD | 0.267 | 0.012 | -0.300 | 0.005 | 0.173 | 0.103 |
| Total body aBMD | 0.152 | 0.163 | -0.088 | 0.443 | 0.126 | 0.268 |
| P1NP | -0.224 | 0.039 | 0.459 | <0.001 | -0.080 | 0.432 |
| CTX-1 | -0.279 | 0.010 | 0.377 | <0.001 | -0.161 | 0.125 |
| BALP | -0.044 | 0.687 | 0.184 | 0.105 | 0.014 | 0.904 |
Multiple linear regression: Model 1: weight change, age, and gender; Model 2: weight change, age, gender, and treatment group (sleeve gastrectomy = 1, gastric bypass = 2), β=standardized coefficients.
Abbreviations: aBMD, areal bone mineral density; BALP, bone alkaline phosphatase; CTX-1, C-telopeptide of type I collagen; P1NP, procollagen type 1 N-terminal propeptide.
The 1-year changes in daily steps were not associated with changes in aBMD or bone turnover markers (Data are available in Supplementary Table 3 located in a digital research materials repository (26)).
Vitamin and mineral supplementation and intake
Adherence to vitamin and mineral supplementation was high, with 85% to 100% of the patients in both groups taking calcium, vitamin D, and multivitamin supplementation as prescribed (Table 3). Estimated calcium and vitamin D intake increased significantly after both gastric bypass and sleeve gastrectomy during follow-up, but did not differ between the 2 groups at 1 year (Table 3).
Table 3.
Vitamin and mineral supplementation and intake at baseline and at one year
| n | Sleeve Gastrectomy | n | Gastric Bypass | Estimated Between-group Difference (95% CI) | P-value | |
|---|---|---|---|---|---|---|
| Calcium supplementation | ||||||
| Baseline | 48 | 2 (4%) | 44 | 1 (2%) | 1.000 | |
| 1 year | 48 | 41 (85%) | 44 | 40 (91%) | – | 0.527 |
| Vitamin D supplementation | ||||||
| Baseline | 48 | 13 (27%) | 44 | 19 (43%) | 0.128 | |
| 1 year | 48 | 43 (90%) | 44 | 41 (93%) | – | 0.716 |
| Multivitamin supplementation | ||||||
| Baseline | 48 | 7 (15%) | 44 | 4 (9%) | 0.527 | |
| 1 year | 48 | 48 (100%) | 43 | 43 (100%) | – | NA |
| Vitamin D intake (μg) | ||||||
| Baseline | 46 | 15.9 (18.2) | 42 | 14.5 (11.2) | 0.675 | |
| 1 year | 44 | 32.0 (15.8)a | 42 | 35.9 (17.5)a | -3.8 (-11.2–3.5) | 0.304 |
| Calcium intake (g) | ||||||
| Baseline | 46 | 1296 (791) | 42 | 1193 (593) | 0.419 | |
| 1 year | 44 | 1830 (735)a | 42 | 1965 (799)a | -166 (-499–166) | 0.323 |
Data are presented as mean (standard deviation) or number (percentage). P-values were calculated using the Fisher`s exact test, an independent samples t-test, an analysis of covariance with adjustment for baseline value, and a paired samples t-test.
Abbreviation: NA, not applicable.
a P < 0.001 compared with baseline value, within-group differences.
Fractures during follow-up
One patient in the sleeve gastrectomy group reported a rib fracture after a bicycle accident.
Discussion
In this randomized controlled study (Oseberg), primarily designed to assess diabetes remission in patients with obesity and type 2 diabetes, patients who underwent gastric bypass had a greater reduction in total hip, femoral neck, and lumbar spine aBMD than patients who underwent sleeve gastrectomy. In addition, gastric bypass was associated with a greater increase in markers of both bone formation and reabsorption. The changes in aBMD and bone turnover seemed to be mediated by the surgical procedures per se and not by differences in weight loss.
The mechanisms underlying greater bone loss and greater bone turnover after gastric bypass than after sleeve gastrectomy may have several explanations. First, mechanical unloading of the skeleton due to weight loss induces bone loss and increases bone turnover (29). However, weight loss was not, as hypothesized, associated with reduced bone density or increased bone turnover independent of surgical procedure. Moreover, changes in physical activity had no impact on bone loss or bone turnover. Second, several nutritional factors may be involved. Gastric bypass induces malabsorption by bypassing the first part of the small intestine (30), which may result in vitamin and mineral deficiencies (31). Interestingly, similarly reduced calcium absorption in premenopausal women 1 and 2 years after gastric bypass and sleeve gastrectomy indicates reduced absorption also after sleeve gastrectomy (12). In keeping with this notion, vitamin D, calcium, and protein supplementation and physical activity decelerated bone loss and bone turnover in premenopausal women after gastric bypass and sleeve gastrectomy (32), underscoring the importance of adequate nutrition after bariatric surgery. In the present study, all patients were prescribed calcium and vitamin D supplements postsurgery, and the prescriptions were adjusted according to prespecified algorithms to secure calcium and vitamin D levels within the recommended range. This procedure resulted in increased intakes of both calcium and vitamin D in both groups, and serum calcium, vitamin D, and PTH levels were comparable between groups during the study. These observations weaken the potential role of insufficient calcium and vitamin D intake in explaining the differences in bone outcomes between the surgical procedures in our study. Finally, gut hormones are altered after bariatric surgery (33) and may alter bone health (34). Interestingly, peptide YY increase after gastric bypass was associated with both attenuated increase in P1NP and greater decrease in spine BMD estimated by quantitative computed tomography (35). The incretine hormones, gastric inhibitory polypeptide, and glucagon-like peptide-1, which are known to increase after gastric bypass and sleeve gastrectomy (33), have, in animal studies, been shown to promote bone formation and suppress bone resorption (34), and are therefore unlikely to explain the reduction in bone mass observed after these procedures.
Our results are in accordance with the randomized study by Guerrero- Pérez at al and a few small observational studies with a follow-up from 1 to 4 years, showing a greater aBMD reduction at the total hip (13, 15) and the femoral neck (12, 15, 22) after gastric bypass than after sleeve gastrectomy. By contrast, in most studies, including the subgroup analysis of the randomized STAMPEDE trial, the reduction in total hip and femoral neck aBMD did not differ significantly between subjects after the 2 procedures (13, 16–20). Moreover, to the best of our knowledge, our study is the first to report a significantly greater reduction in lumbar spine aBMD, providing a point of contrast from the above-mentioned studies (12, 13, 15–20, 22). Finally, our results support 2 studies reporting similar changes in total body aBMD after the 2 procedures (13, 16). In contrast, Carrasco et al found a significantly greater reduction in total body aBMD after gastric bypass than after sleeve gastrectomy during a 2-year period (12).
Our findings of higher 1-year levels of markers of bone formation (P1NP and BALP) and reabsorption (CTX-1) after gastric bypass than after sleeve gastrectomy are in keeping with most previous publications (12, 14, 15, 21). By contrast, the study by Muscitz et al including solely premenopausal women receiving neither calcium nor vitamin D supplementations is, to our knowledge, the only study reporting similar increases in bone turnover markers after gastric bypass and sleeve gastrectomy (16). Moreover, no difference between calcium, phosphate, magnesium, PTH, and vitamin D levels after gastric bypass and sleeve gastrectomy, as observed in our study, corresponds with previous publications (12, 13, 15–17, 21, 22).
Weight change has been shown to correlate with changes in femoral neck, total hip, lumbar spine, total body aBMD (13, 15, 16, 20), and with changes in P1NP and CTX-1 (14, 21) after gastric bypass and sleeve gastrectomy. This is partly in line with our result showing significant associations (adjusted for age and gender) between weight change and changes in lumbar spine aBMD and changes in P1NP and CTX-1. Furthermore, our results indicate that fat mass loss rather than reduced lean mass explains these associations. Interestingly, and in agreement with our results, Ivaska et al found gastric bypass to predict a greater increase in CTX-1 and P1NP even after adjusting for weight change (14). Moreover, we confirm the result from the randomized trial by Guerrero-Pérez at al (22) showing that gastric bypass, and not weight loss, is associated with a greater reduction in femoral neck and lumbar spine aBMD. These findings indicate that gastric bypass per se elicits bone turnover and induces bone loss, and does not support the idea that reduced aBMD after gastric bypass is solely a physiological adaption to a lower body weight.
The observed differences between our results and previous publications discussed above may have several explanations. First, there is a high risk of selection bias due to the lack of randomization in all but 2 small studies (20, 22). Second, there might also be a lack of statistical power due to the small sample size, with 6 studies having less than 50 participants (12, 14, 15, 19, 20, 22). Third, type 2 diabetes is, despite having an increased risk of fragility fractures, associated with a higher BMD and lower bone turnover markers (36, 37). Improved glycemic control postsurgery in our study population of patients solely with type 2 diabetes could therefore explain greater changes in aBMD and bone turnover markers than in some of the studies including few or no patients with diabetes (12, 13, 15–19). Fourth, there is a high degree of between-study heterogeneity. Ethnicity, age, gender distribution, menopausal status, and preoperative BMI differ considerably and may have affected the results.
Strengths and limitations
Our study has some limitations. First, the generalizability of the results is limited by the single-center design. Second, there was only a 1-year follow-up. Third, changes in aBMD and bone turnover markers were secondary endpoints, and the study was not powered to detect differences in these outcome measures. Fourth, most patients in our study are white, and the results can therefore not be generalizable to populations of other ethnicities. Fifth, multiple testing increases the risk of type 1 errors. We therefore recommend that results with P-values between 0.01 and 0.05 should be interpreted with caution. Finally, the study only includes surrogate outcomes for fractures. However, aBMD changes are strongly associated with fracture risks and are therefore important and clinically relevant surrogate outcomes (38). The major strengths of this study are the triple-blind randomized design and an unbiased sample.
Conclusion
In conclusion, we have shown both a greater reduction in bone mineral density and a greater increase in bone turnover 1 year after gastric bypass than after sleeve gastrectomy. This finding could suggest greater skeletal fragility after gastric bypass. However, the clinical implications of reduced bone mineral density and increased levels of bone turnover markers after bariatric surgery must be confirmed in larger long-term studies.
Acknowledgments
We thank the participants, the patient representative, and the study personnel. We particularly thank Linda Mathisen, Karina Bautz, Berit Mossing Bjørkås, Andreas Aarvik, Heidi Omre Fon, Hanna Lakso, and Astrid Hillestad (Morbid Obesity Center, Vestfold Hospital Trust, Tønsberg, Norway) for their continuous efforts, enthusiasm, and patient care, thereby ensuring the collection of high-quality data in the Oseberg study. We also thank Matthew McGee (Morbid Obesity Center, Vestfold Hospital Trust) for proofreading the manuscript and Anne Karine Skredegård and Richard Kirkevold for performing the DXA-scans.
Financial Support: The study is funded by the Morbid Obesity Center, Vestfold Hospital Trust. F.F. has received an educational grant (PhD) from South-Eastern Norway Regional Health Authority.
Clinical Trial Information: ClinicalTrials.gov: NCT01778738.
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability
All data generated or analyzed during this study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Angrisani L, Santonicola A, Iovino P, et al. IFSO worldwide survey 2016: primary, endoluminal, and revisional procedures. Obes Surg. 2018;28(12):3783–3794. [DOI] [PubMed] [Google Scholar]
- 2. Nakamura KM, Haglind EG, Clowes JA, et al. Fracture risk following bariatric surgery: a population-based study. Osteoporos Int. 2014;25(1):151–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lu CW, Chang YK, Chang HH, et al. Fracture risk after bariatric surgery: a 12-year nationwide cohort study. Medicine (Baltimore). 2015;94(48):e2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Axelsson KF, Werling M, Eliasson B, et al. Fracture risk after gastric bypass surgery: a retrospective cohort study. J Bone Miner Res. 2018;33(12):2122–2131. [DOI] [PubMed] [Google Scholar]
- 5. Paccou J, Martignène N, Lespessailles E, et al. Gastric bypass but not sleeve gastrectomy increases risk of major osteoporotic fracture: french population-based cohort study. J Bone Miner Res. 2020;35(8):1415–1423. [DOI] [PubMed] [Google Scholar]
- 6. Ahlin S, Peltonen M, Sjöholm K, et al. Fracture risk after three bariatric surgery procedures in Swedish obese subjects: up to 26 years follow-up of a controlled intervention study. J Intern Med. 2020;287(5):546–557. [DOI] [PubMed] [Google Scholar]
- 7. Fashandi AZ, Mehaffey JH, Hawkins RB, Schirmer B, Hallowell PT. Bariatric surgery increases risk of bone fracture. Surg Endosc. 2018;32(6):2650–2655. [DOI] [PubMed] [Google Scholar]
- 8. Rousseau C, Jean S, Gamache P, et al. Change in fracture risk and fracture pattern after bariatric surgery: nested case-control study. BMJ. 2016;354:i3794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Garnero P, Hausherr E, Chapuy MC, et al. Markers of bone resorption predict hip fracture in elderly women: the EPIDOS Prospective Study. J Bone Miner Res. 1996;11(10):1531–1538. [DOI] [PubMed] [Google Scholar]
- 10. Marshall D, Johnell O, Wedel H. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ. 1996;312(7041):1254–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gagnon C, Schafer AL. Bone health after bariatric surgery. JBMR Plus. 2018;2(3):121–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Carrasco F, Basfi-Fer K, Rojas P, et al. Calcium absorption may be affected after either sleeve gastrectomy or Roux-en-Y gastric bypass in premenopausal women: a 2-y prospective study. Am J Clin Nutr. 2018;108(1):24–32. [DOI] [PubMed] [Google Scholar]
- 13. Cadart O, Degrandi O, Barnetche T, et al. Long-term effects of Roux-en-Y gastric bypass and sleeve gastrectomy on bone mineral density: a 4-year longitudinal study. Obes Surg. 2020;30(9):3317–3325. [DOI] [PubMed] [Google Scholar]
- 14. Ivaska KK, Huovinen V, Soinio M, et al. Changes in bone metabolism after bariatric surgery by gastric bypass or sleeve gastrectomy. Bone. 2017;95:47–54. [DOI] [PubMed] [Google Scholar]
- 15. Bredella MA, Greenblatt LB, Eajazi A, Torriani M, Yu EW. Effects of Roux-en-Y gastric bypass and sleeve gastrectomy on bone mineral density and marrow adipose tissue. Bone. 2017;95:85–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Muschitz C, Kocijan R, Marterer C, et al. Sclerostin levels and changes in bone metabolism after bariatric surgery. J Clin Endocrinol Metab. 2015;100(3):891–901. [DOI] [PubMed] [Google Scholar]
- 17. Vilarrasa N, de Gordejuela AG, Gómez-Vaquero C, et al. Effect of bariatric surgery on bone mineral density: comparison of gastric bypass and sleeve gastrectomy. Obes Surg. 2013;23(12):2086–2091. [DOI] [PubMed] [Google Scholar]
- 18. Hsin MC, Huang CK, Tai CM, Yeh LR, Kuo HC, Garg A. A case-matched study of the differences in bone mineral density 1 year after 3 different bariatric procedures. Surg Obes Relat Dis. 2015;11(1):181–185. [DOI] [PubMed] [Google Scholar]
- 19. Tan HC, Tan MZ-W, Tham KW, et al. One year changes in QCT and DXA bone densities following bariatric surgery in a multiethnic Asian cohort. Osteoporos Sarcopenia. 2015;1(2):115–120. [Google Scholar]
- 20. Maghrabi AH, Wolski K, Abood B, et al. Two-year outcomes on bone density and fracture incidence in patients with T2DM randomized to bariatric surgery versus intensive medical therapy. Obesity (Silver Spring). 2015;23(12):2344–2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Crawford MR, Pham N, Khan L, Bena JF, Schauer PR, Kashyap SR. Increased bone turnover in type 2 diabetes patients randomized to bariatric surgery versus medical therapy at 5 years. Endocr Pract. 2018;24(3):256–264. [DOI] [PubMed] [Google Scholar]
- 22. Guerrero-Pérez F, Casajoana A, Gómez-Vaquero C, et al. Changes in bone mineral density in patients with type 2 diabetes after different bariatric surgery procedures and the role of gastrointestinal hormones. Obes Surg. 2020;30(1):180-188. [DOI] [PubMed] [Google Scholar]
- 23. Zibellini J, Seimon RV, Lee CM, et al. Does diet-induced weight loss lead to bone loss in overweight or obese adults? a systematic review and meta-analysis of clinical trials. J Bone Miner Res. 2015;30(12):2168–2178. [DOI] [PubMed] [Google Scholar]
- 24. Borgeraas H, Hjelmesæth J, Birkeland KI, et al. Single-centre, triple-blinded, randomised, 1-year, parallel-group, superiority study to compare the effects of Roux-en-Y gastric bypass and sleeve gastrectomy on remission of type 2 diabetes and β-cell function in subjects with morbid obesity: a protocol for the Obesity surgery in Tønsberg (Oseberg) study. BMJ Open. 2019;9(6):e024573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hofsø D, Fatima F, Borgeraas H, et al. Gastric bypass versus sleeve gastrectomy in patients with type 2 diabetes (Oseberg): a single-centre, triple-blind, randomised controlled trial. Lancet Diabetes Endocrinol. 2019;7(12):912–924. [DOI] [PubMed] [Google Scholar]
- 26. Supplementary tables. Hofsø et al. Bone mineral density and bone turnover after sleeve gastrectomy and gastric bypass, a randomized controlled trial. 2020 [cited 28.09.20]. ProMED-mail website. https://figshare.com/articles/dataset/Supplementary_tables_Hofs_et_al_Bone_mineral_density_and_bone_turnover_after_sleeve_gastrectomy_and_gastric_bypass_a_randomized_controlled_trial/13013828. Accessed September 28, 2020.
- 27. Andersen LF, Tomten H, Haggarty P, Løvø A, Hustvedt BE. Validation of energy intake estimated from a food frequency questionnaire: a doubly labelled water study. Eur J Clin Nutr. 2003;57(2):279–284. [DOI] [PubMed] [Google Scholar]
- 28. Andersen LF, Solvoll K, Johansson LR, Salminen I, Aro A, Drevon CA. Evaluation of a food frequency questionnaire with weighed records, fatty acids, and alpha-tocopherol in adipose tissue and serum. Am J Epidemiol. 1999;150(1):75–87. [DOI] [PubMed] [Google Scholar]
- 29. Zerwekh JE, Ruml LA, Gottschalk F, Pak CY. The effects of twelve weeks of bed rest on bone histology, biochemical markers of bone turnover, and calcium homeostasis in eleven normal subjects. J Bone Miner Res. 1998;13(10):1594–1601. [DOI] [PubMed] [Google Scholar]
- 30. Schafer AL, Weaver CM, Black DM, et al. Intestinal calcium absorption decreases dramatically after gastric bypass surgery despite optimization of vitamin D status. J Bone Miner Res. 2015;30(8):1377–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lupoli R, Lembo E, Saldalamacchia G, Avola CK, Angrisani L, Capaldo B. Bariatric surgery and long-term nutritional issues. World J Diabetes. 2017;8(11):464–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Muschitz C, Kocijan R, Haschka J, et al. The impact of vitamin D, calcium, protein supplementation, and physical exercise on bone metabolism after bariatric surgery: the BABS study. J Bone Miner Res. 2016;31(3):672–682. [DOI] [PubMed] [Google Scholar]
- 33. Malin SK, Kashyap SR. Differences in weight loss and gut hormones: Rouen-Y gastric bypass and sleeve gastrectomy surgery. Curr Obes Rep. 2015;4(2):279–286. [DOI] [PubMed] [Google Scholar]
- 34. Nauck MA, Meier JJ. Incretin hormones: their role in health and disease. Diabetes Obes Metab. 2018;20(Suppl 1):5–21. [DOI] [PubMed] [Google Scholar]
- 35. Kim TY, Shoback DM, Black DM, et al. Increases in PYY and uncoupling of bone turnover are associated with loss of bone mass after gastric bypass surgery. Bone. 2020;131:115115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Eller-Vainicher C, Cairoli E, Grassi G, et al. Pathophysiology and management of type 2 diabetes mellitus bone fragility. J Diabetes Res. 2020;2020:7608964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ma L, Oei L, Jiang L, et al. Association between bone mineral density and type 2 diabetes mellitus: a meta-analysis of observational studies. Eur J Epidemiol. 2012;27(5):319–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Black DM, Bauer DC, Vittinghoff E, et al. ; Foundation for the National Institutes of Health Bone Quality Project . Treatment-related changes in bone mineral density as a surrogate biomarker for fracture risk reduction: meta-regression analyses of individual patient data from multiple randomised controlled trials. Lancet Diabetes Endocrinol. 2020;8(8):672–682. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are not publicly available but are available from the corresponding author on reasonable request.