Abstract
Context
Male infertility is defined as the inability to conceive following 1 year of regular unprotected intercourse. It is the causative factor in 50% of couples and a leading indication for assisted reproductive techniques (ART). Testicular failure is the most common cause of male infertility, yet the least studied to date.
Evidence Acquisition
The review is an evidence-based summary of male infertility due to testicular failure with a focus on etiology, clinical assessment, and current management approaches. PubMed-searched articles and relevant clinical guidelines were reviewed in detail.
Evidence Synthesis/Results
Spermatogenesis is under multiple levels of regulation and novel molecular diagnostic tests of sperm function (reactive oxidative species and DNA fragmentation) have since been developed, and albeit currently remain as research tools. Several genetic, environmental, and lifestyle factors provoking testicular failure have been elucidated during the last decade; nevertheless, 40% of cases are idiopathic, with novel monogenic genes linked in the etiopathogenesis. Microsurgical testicular sperm extraction (micro-TESE) and hormonal stimulation with gonadotropins, selective estrogen receptor modulators, and aromatase inhibitors are recently developed therapeutic approaches for men with the most severe form of testicular failure, nonobstructive azoospermia. However, high-quality clinical trials data is currently lacking.
Conclusions
Male infertility due to testicular failure has traditionally been viewed as unmodifiable. In the absence of effective pharmacological therapies, delivery of lifestyle advice is a potentially important treatment option. Future research efforts are needed to determine unidentified factors causative in “idiopathic” male infertility and long-term follow-up studies of babies conceived through ART.
Keywords: testes, male infertility, testosterone, semen, spermatogenesis, sperm quality
Infertility is defined as being unable to conceive after 12 months of regular (at least twice weekly) unprotected intercourse (1, 2). Male infertility is due to abnormal sperm parameters in the male partner and contributes to 50% of all cases of infertility (3, 4). A recent systematic review reported a fall in total sperm counts by 59.3% since the 1970s, in North America, Europe, and Australasia (5); however, other studies have failed to observe such a change (6, 7). Furthermore, male infertility is increasingly observed as a “canary in the coal mine” for future male health conditions (8), with an association with cardiovascular disease, testicular cancer, quality of life, and increased all-cause mortality (9). Male infertility may be broadly subdivided into 3 categories: (1) hypothalamo-pituitary disease causing secondary hypogonadism; (2) obstruction of seminal outflow (usually termed, obstructive azoospermia, OA); and testicular dysfunction (which may be associated with primary hypogonadism) (10–12).
Azoospermia is defined as the absence of sperm in ejaculate. It affects approximately 1% of all men and is the most severe manifestation of testicular failure (13). Secondary hypogonadism has been extensively summarized in the literature and has established therapies, gonadotropin-releasing hormone (GnRH), and gonadotropin replacement (14, 15). By contrast, OA and testicular dysfunction are less well-studied but represent rapidly evolving conditions with emerging endocrine and surgical therapies for affected patients. Our review, therefore, focuses on primary testicular dysfunction as a cause of male infertility.
Physiology of spermatogenesis
During spermatogenesis, male germ cells (spermatogonia) develop into mature spermatozoa through 3 distinct phases: spermatogonia divide by mitosis into primary spermatocytes, which in turn undergo meiosis (I and II) to form spermatids (16). Spermatids then develop by cytodifferentiation into elongated spermatozoa during spermiogenesis. The time taken for spermatogenesis is species-specific (17). Studies suggest that the entire spermatogenic process is between 42 and 76 days in men (18), which is an important consideration when assessing the therapeutic impact of any lifestyle/medical changes in subsequent semen analysis. In humans, spermatogenesis occurs in the recesses of the Sertoli cells located along the entire length of the seminiferous tubules of the testes in a helical arrangement, with several stages represented in a single cross-section (19). Sertoli cells provide structural and functional support to germ cells, and Leydig cells synthesize testosterone. These testicular functions are dependent on the hypothalamic-pituitary-testicular (HPT) axis (20). Pulsatile secretion of GnRH stimulates follicle-stimulating hormone (FSH) and luteinizing hormone (LH) secretion from the anterior pituitary. FSH stimulates Sertoli cell function and spermatogenesis (21). Sertoli and Leydig cells secrete inhibin B and testosterone, respectively. Testosterone and inhibin B have negative feedback effects at pituitary and hypothalamic levels. Optimal spermatogenesis requires the action of both testosterone and FSH. Derangements at any of these steps can lead to testicular dysfunction and male infertility (22). Multiple other hormones and their receptors (23) are observed in testes and spermatozoa of animals and humans such as AMH anti-Mullerian hormone (AMH) (24 ,25), insulin-like factor 3 (INSL-3) (26, 27), leptin (28–30), insulin (31), and kisspeptin (32), with postulated paracrine and endocrine roles in regulating testicular functions.
Sperm quality
Conventional semen analysis is the hallmark diagnostic test for male infertility (Table 1). It reflects the production of spermatozoa in the testes, the patency of the duct system and the glandular secretory activity (33). Lower reference ranges for semen parameters were generated based on data from fertile men whose partners had time to pregnancy of within 1 year (34). Reference ranges for semen parameters were also defined for unselected men (with unknown fertility status); these may provide a more appropriate reference population for screening the male population without regard to prior fertility (35). Men with sperm parameters below the World Health Organization (WHO) normal values are considered to have male factor infertility (36) and semen quality is used as a surrogate measure of male fecundity (35). The total number of spermatozoa per ejaculate and the sperm concentration are associated with fertility outcomes such as conception (37), time to pregnancy (38), and pregnancy rates (39). However, semen analysis is descriptive and not a direct marker of sperm function, and its predictive value for natural conception and fertility rates are low in most settings. In addition, WHO thresholds (34) are based on the 5th centile, so natural conception is entirely possible below this reference value. Furthermore, these threshold values are generated from data within a discrete reference group. which might not apply to the individual patient. The most significant sperm defects associated with infertility are summarized in Table 1.
Table 1.
WHO reference range for semen analysis with examples of main abnormalities related to semen analysis
| Semen Parameter | Reference Range | Abnormality | Description |
|---|---|---|---|
| Semen volume | ≥1.5 ml | ||
| pH | ≥7.2 | ||
| Sperm concentration | ≥15 million sperm/ml | Azoospermia | Absence of sperm in seminal plasma |
| Oligozoospermia | <15 million spermatozoa/ml | ||
| Cryptozoospermia | <1 million spermatozoa/ml | ||
| Total sperm count | ≥39 million sperm/ ejaculate | ||
| Total sperm motility | ≥40% motile sperm | Asthenozoospermia | <40% total motile spermatozoa or <32% progressive motile spermatozoa |
| Progressive sperm motility | ≥32% progressively motile sperm | Asthenozoospermia | <40% total motile spermatozoa or <32% progressive motile spermatozoa |
| Sperm morphology | ≥4% morphologically normal sperm | Teratozoospermia | <4% normal form/morphology |
| Oligoasthenoteratozoospermia (OAT syndrome) | Combination of <15 million spermatozoa/ml, <32% progressive motile spermatozoa, and <4% normal form |
Based on WHO reference range for semen analysis (Cooper et al 2010 (34)).
Abbreviations: pH, potential of hydrogen; WHO, World Health Organization.
Oxidative stress and sperm DNA fragmentation
Semen analysis parameters are subject to marked biological variation, with standard deviations comparable to mean levels (34). Considerable focus has therefore been placed on developing novel molecular diagnostic tests of sperm function. Semen reactive oxidative species (ROS) (40, 41) and sperm DNA fragmentation index (42) are novel tests of sperm function. Seminal ROS are released physiologically by leucocytes and as by-products of intracellular metabolic pathways and during adenosine triphosphate (ATP) production from the sperm mitochondria. Small amounts of semen ROS are essential for optimum sperm function and fertilization. However, there are a number of exogenous factors such as genitourinary infections, varicocele and obesity that may elevate semen ROS (43, 44). Methods used to measure ROS include chemiluminescence assay and electrochemical assay to measure oxidation reduction potential (sORP) (45). High ROS may lead to male infertility by adversely affecting sperm membrane lipid peroxidation, sperm motility, the acrosome reaction, chromatin maturation, and subsequent sperm DNA fragmentation (46, 47). Sperm DNA fragmentation is the percentage of spermatozoa with fragmented or damaged DNA. Assays for DNA fragmentation include sperm chromatin structure assay (SCSA), sperm chromatin dispersion (SCD), terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end-labeling and Comet assay (48). There is large variability in these assays, with a lack of consensus or external quality control for any method. Furthermore, there is insufficient evidence to support the routine use of ROS and sperm DNA fragmentation in male factor infertility, and these are not recommended by current clinical guidelines. It is suggested that use of antioxidants and lifestyle changes may reduce the risk of high ROS and sperm DNA fragmentation to improve male infertility; however, there is paucity in the data with a lack of good quality randomized controlled trials available (49, 50). Studies have reported worse outcomes of ART (51–53), with elevated levels of DNA fragmentation, including recurrent pregnancy loss (54).
Etiology of Male Infertility Due to Testicular Dysfunction
Causes of male infertility due to testicular dysfunction are summarised in Table 2.
Table 2.
Causes and risk factors of testicular failure and genital tract abnormalities
| Congenital |
|---|
| Klinefelter’s syndrome and variants |
| Male XX syndrome |
| Robertsonian translocation/inversions |
| Y chromosome microdeletions: partial and complete |
| Cystic Fibrosis |
| Novel monogenic mutations, eg, TEX11 |
| Immotile cilia/Kartagener’s syndrome |
| Congenital cryptorchidism |
| Acquired |
| Infections, eg, mumps orchitis, echovirus, gonorrhea, chlamydia |
| Infiltrative disease, eg, TB |
| Testicular torsion or trauma or malignancy |
| Chemotherapy, pelvic irradiation, or surgery |
| Large varicoceles |
| Medications |
| Idiopathic |
| Environmental factors/systemic disease |
| Obesity |
| Endocrine-disrupting chemicals |
| Lifestyle factors, eg, alcohol, smoking, recreational drug use |
| Genital tract abnormalities (defect in sperm transport) |
| Obstruction: congenital absence of vas deferens, infections, vasectomy |
| Others: ejaculatory dysfunction |
Abbreviation: TB, tuberculosis; TEX11, testis-expressed gene 11.
Genetic
Chromosomal abnormalities affect both sex and autosomal chromosomes and can cause numerical or structural aberrations (55) (Table 3).
Table 3.
Common congenital causes of testicular failure and obstructive azoospermia with genotype–phenotype correlations
| Genetic Aberration | Phenotype |
|---|---|
| Klinefelter syndrome | Azoospermia to severe oligozoospermia |
| Robertsonian translocation | |
| Y chromosome microdeletions | |
| AZFa deletion | Azoospermia |
| AZFb deletion | Azoospermia |
| AZFc deletion | Azoospermia to normozoospermia |
| 46 XX male syndrome | Azoospermia |
| CFTR | Obstructive azoospermia |
| INSL3-LGR8 | Cryptorchidism |
Abbreviations: AZF, azoospermia factor; CFTR, cystic fibrosis transmembrane conductance regulator; INSL3-LGR8, insulin-like factor 3–leucine-rich repeat-containing G protein-coupled receptor 8.
Klinefelter’s syndrome
Klinefelter’s syndrome (KS) is the most common genetic aneuploidy of testicular failure in oligozoospermic and azoospermic men (10–15%) (56, 57). It affects 1/1000 to 1/500 males (58). There are 2 main forms: nonmosaic 47XXY (80–90%) or mosaic 47XXY/46XY (5–10%). The classical phenotype is that of a tall male with small, firm testes and gynaecomastia (59), however the phenotype may vary from a fully virilized male to one with androgen deficiency. Consequently, 70% of men with KS remain undiagnosed until late adulthood (60). The endocrine profile reveals hypergonadotropic hypogonadism (low–normal testosterone, high FSH and LH levels, and undetectable inhibin B consistent with testicular failure). Semen analysis normally reveals azoospermia and may be the only phenotypical abnormality in men with KS. Testicular histology may include extensive fibrosis and hyalinization of seminiferous tubules with loss of spermatogonial stem cells (61), Sertoli cell degeneration, and hyperplasia of Leydig cells (62). However, some men may have single-residual foci of spermatogenesis within their testis (56). These foci of spermatogenesis in KS appear to arise from 46XY stem cells, and thus the vast majority of offspring have a normal karyotype. Therefore, testicular sperm retrieval techniques (TESE, see section on ‘Surgical sperm retrieval techniques’) offer therapeutic options for biological paternity. In a recent meta-analysis, a quarter of adult men with KS negative for mature spermatozoa on testicular biopsy were positive for spermatogonia, thus indicating they had sperm maturation arrest (63). Therefore, future research and advances in spermatogonial stem cell in vitro propagation, transplantation, and differentiation may offer new options for fertility preservation. These are still experimental methods, particularly cryopreservation of spermatogonial stem cells in the context of peripubertal or prepubertal boys with KS (64). Furthermore, patients with KS are found to have increased risks of cardiovascular disease, metabolic syndrome, diabetes mellitus, autoimmune diseases, and venous thromboembolism (65). This highlights the need for regular medical follow-up, especially for men on treatment with testosterone, which is the mainstay of treatment in hypogonadal nonfertility-seeking KS patients.
Robertsonian translocations and inversions
These involve chromosomal rearrangement and the “crossing over” of chromosomes (66). Individuals with Robertsonian translocation have 45 chromosomes. These are the most common structural autosomal abnormalities in infertile men, affecting 1/1000 men, with a higher prevalence in oligozoospermic men (1.6%) (64). These can be further classified as balanced or unbalanced structural aberrations (67). Balanced aberrations are characterized by a deviation from normal chromosome structure but without a net loss or gain of genetic material. These mainly result in spermatogenic failure (oligozoospermic or azoospermia) and male infertility, as well as in a risk of genetic imbalances among the offspring (68). The breakpoints of chromosomal rearrangements are critical in determining the resulting phenotype and implications for offspring; clinical genetics expertise is therefore required to interpret results. In contrast, unbalanced structural chromosomal abnormalities lead to a net gain or net loss of genetic material, and they are generally incompatible with life and cause a spectrum of disease (the exception to this rule being Y chromosome microdeletions, see section on ‘Y-chromosome microdeletions’). These structural aberrations can be either de novo or inherited. As with other genetic causes of male infertility, men with structural chromosomal abnormalities should be offered preconception genetic counselling and the option for preimplantation genetic diagnosis, prior to using their ejaculated or testicular sperm during ART (69).
Y-chromosome microdeletions
Y-chromosome microdeletions are genomic deletions of specific sections of the Y chromosome, containing genes needed for spermatogenesis (70). These observed in men with severe oligozoospermia (< 5 million/ml) (3–7%) and azoospermia (8–12%) (71). These microdeletions are termed “azoospermia factor,” namely AZFa, AFZb, AZFc, according to the genomic region deleted. AZFc deletions (65–70%) are the most common form of the long arm of the Y-chromosome microdeletions, with low levels of sperm in ejaculate or testicular biopsies (sperm retrieval rates of 50–75%) (72). In contrast, complete deletions of the AZFa and AZFb region result in complete spermatogenic failure, with severe testicular histology phenotypes including Sertoli cell only (SCO) or complete spermatogenic arrest such that no sperm is found at the time of TESE (73).
46 XX male syndrome
This is a disorder of sex development affecting approximately 1/9000 to 1/20 000 men (74). The most common suggested genetic mechanism leading to 46XX testicular disorder of sex development is translocation of part of the Y chromosome, including the SRY (Sex Determining Region Y) gene to the X chromosome during paternal meiosis. Males with 46XX SRY positive have completely differentiated male external and internal genitalia, with the majority remaining undiagnosed until adulthood when they present with infertility. Affected patients lack all of the AZF genes needed for spermatogenesis, so they cannot produce sperm (azoospermia) (75, 76).
Monogenic causes of male infertility
The cystic fibrosis transmembrane conductance regulator (CFTR) is required for formation of the ejaculatory duct, seminal vesicles, vas deferens, and distal two-thirds of the epididymis. Heterozygous loss of function CFTR variants are seen in approximately 2% to 3% of Caucasian men with obstructive azoospermia but are rare in Asians and other ethnicities. It is clinically characterized by congenital bilateral absence of vasa deferens (CBAVD), full epididymis, and low volume acidic ejaculate (77). Although patients with cystic fibrosis (CF) have mutations in both copies of the CFTR gene, majority of patients with CBAVD are carriers of mutations in only 1 copy of the gene. With more than 1500 known CFTR mutations (78), CBAVD is a heterogeneous genetic condition, with many cases associated with mild forms of CF and others having no relationship with CF (79). Female partners of men with CBAVD are recommended to also have CFTR carrier genetic testing to stratify the risk of having offspring with clinical CF. Several groups worldwide are searching for genes causing NOA. Testis-expressed gene 11 (TEX11) is an X-linked gene encoding a protein crucial for male germ cell meiotic DNA recombination and chromosomal synapsis. Inactivating mutations cause NOA and meiotic arrest in mice (80) and humans (81). Two other novel genes associated with NOA include NR5A1 and DMRT1 (82). Other rarer inherited causes of defective spermatozoa transport include primary ciliary dyskinesia (with or without Kartagener’s syndrome, which is inherited in an autosomal recessive pattern). Infertility is from spermatozoa immobility due to defective sperm-flagella movement (83). There is a higher prevalence of this condition in communities with consanguinity.
Developmental
Undescended testis (UDT) or cryptorchidism is associated with impaired spermatogenesis and testicular germ cell tumors (84). Treatment with orchidopexy is therefore recommended between 6 and 18 months of age to conserve spermatogenesis (85) and hormone production, and decrease the risk of testicular tumors (86). However, UDT are often damaged and treatment with orchidopexy risks causing obstructive azoospermia but more commonly testicular atrophy (87). Genetic analysis is not required, however, unless the patient is azoospermic or severely oligozoospermic, although novel mutations in insulin-like factor 3 (INSL3) and leucine-rich repeat-containing G protein-coupled receptor 8 (LGR8) genes are observed to be associated with cryptorchidism (88) (Table 3).
Acquired
Genitourinary infections
Infections of the male genitourinary tract from bacteria, viruses, or protozoa are associated with 10% to 15% of cases of male infertility (89). These are potentially curable causes of male infertility and present as urethritis, prostatitis, orchitis, or epididymitis (90). These infections are more common in developing countries and can be both acute or chronic, with Chlamydia trachomatis and Neisseria gonorrhea as the 2 most common associated pathogens. Chlamydia infection may cause urethritis, epididymitis(-orchitis), and prostatitis. Inflammation of the epididymis from the infection can induce infertility through sperm tract obstruction (91). Sperm damage may also occur from elevated ROS levels in the semen resulting from neutrophil activation. Furthermore, a recent study reported the detection of chlamydia-specific DNA and protein in testicular biopsies of infertile men, which may represent a further component of chlamydia-related male infertility (92). Other pathogens such as Mycoplasma gentitalium, tuberculosis (TB), Ureaplasma urealitycum, and mumps are also associated with male infertility (93). Tuberculosis epididymitis is typically chronic and occurs in high-risk groups such as men with immunodeficiency and high prevalence countries. There is increased incidence and risk of infertility among men with Hepatitis B and Hepatitis C infections compared with men without, but it is not known whether hepatitis infections directly impair male reproductive function. Many pathogens of the male genitourinary tract are asymptomatic, and it is often difficult to distinguish colonization from infection, which is detrimental to fertility (94). The underlying mechanisms are, however, unclear and hypothesized to include damage to the germinal epithelium, ischemia, or immune dysfunction, with cell damage from increased ROS (95). Spermatozoa may be affected at different levels of their development, maturation, and transport.
Infections are also associated with obstruction along the seminal tract, such as urethral strictures. Bacteriospermia is suspected if there are >1 million peroxidase-positive white blood cells per ml of ejaculate (leukocytospermia), with a semen culture or PCR to confirm the pathogen. Antibiotic treatment may improve sperm quality and prevent testicular damage and complications, but its effects on natural conception are not yet elucidated (96, 97). Furthermore, leucocytospermia is a sign of inflammation and may not be associated with a bacterial or viral process, hence its clinical significance in the ejaculate is controversial (98, 99). In addition, normal colonization of genitourinary tract with pathogens such as mycoplasma also hinders the assessment of the pathogenic effect. Furthermore, many of these pathogens may co-exist and potentiate the detrimental effect. Recently, viruses such as human papilloma virus (HPV) has been found in semen of men with male infertility (100); however, further good quality studies are needed to define its true clinical impact and association with sperm quality (101).
Cancer and its treatment
The 3 most common cancers associated with male infertility during reproductive years include: leukaemia, Hodgkin’s lymphoma, and testicular germ cell tumors. Nearly 5% to 8% of men with testicular germ cell tumors have azoospermia prior to any cancer treatment (102). The underlying mechanisms for direct testicular dysfunction include germinal epithelial hypoplasia, spermatogonial apoptosis, increased ROS, and sperm DNA damage (103, 104). Sperm damage is dependent on multiple variables, including tumor type, burden of disease, drug dose/combination therapy, and individual sensitivity—hence, the recovery time is unpredictable. Sperm cryopreservation is the only effective prevention for male infertility from testicular damage due to gonadotoxic treatment (105). There are clear guidelines to suggest that all patients should be provided with information on the impact of their cancer treatment on spermatogenesis (2, 106). Fertility-preserving procedures such as sperm cryopreservation and TESE should be offered from midpuberty onwards prior to gonadotoxic chemoradiotherapy or pelvic surgery.
Varicocele
This is defined as the abnormal dilatation of the pampiniform plexus of veins within the scrotum (107). Its incidence in infertile men ranges from 35% to 40%, although may occur in up to 15% of the normal male population. Most clinically detectable varicoceles are solely left sided; clinical varicoceles are only bilateral when severe (108). Large varicoceles (palpable and visible on standing) may impair spermatogenesis by mechanisms including compromised testicular cooling, hypoxia, increased intratesticular ROS, and sperm DNA damage (109). Varicocele repair is a debated therapy for male infertility; 2 recent meta-analyses support the surgical varicocelectomy for men with clinically significant varicoceles (110, 111). Varicocele repair is not recommended in subclinical varicoceles or in men with normal semen parameters (112). A subclinical varicocele is not palpable or visible during either rest or Valsalva maneuver, but can be shown by radiographic tests, eg, Doppler ultrasound studies.
Medications
Numerous medications have been reported to be associated with testicular failure (Table 4). The adverse effects can range from direct testicular spermatogenic impairment and antiandrogenic effects to ejaculatory/sexual dysfunction. Alkylating agents such as cyclophosphamide and immunosuppressants, particularly sirolimus, are toxic to germ cells (113); sperm cryopreservation is normally recommended beforehand, and contraception is advised during treatment due to potential teratogenicity. Observational studies have suggested that Selective serotonin reuptake inhibitors are associated with impaired semen quality (114) and damaged sperm DNA integrity in men (115). Radioiodine therapy for thyroid disease may cause testicular damage and abnormal spermatogenesis (116); however, these adverse effects are usually dose- and time-dependent, with improvements in sperm parameters after cessation of therapy. The risk is, however, increased with repeated higher cumulative doses. Alpha adrenergic blockers may cause retrograde ejaculation due to dysautonomia. Serotonin reuptake inhibitor therapy may cause anejaculation due to neurological effects (117).
Table 4.
Medications associated with testicular failure
| Impairment of Spermatogenesis |
|---|
| Chemotherapeutic agents |
| Calcium channel blockers |
| Colchicine |
| Sulphasalazine |
| Nitrofurantoin |
| Corticosteroids |
| Opioidsa |
| Radioiodine |
| Testosterone replacementa |
| Androgensa |
| Antiandrogenic |
| Cimetidine |
| Spironolactone |
| Flutamide |
| Ejaculatory failure |
| Alpha blockers |
| Serotonin reuptake inhibitors |
a Treatment suppresses spermatogenesis by negative feedback on the hypothalamo–pituitary axis (hypogonadotropic hypogonadism).
Obesity
Obesity is associated with a low serum SHBG and total testosterone, with normal serum LH and FSH (pseudohypogonadism). However, the pathogenesis of obesity on direct testicular dysfunction and spermatogenesis remains less clear (118, 119). The rising prevalence of obesity is a major factor postulated to contribute to oligozoospermia (120). A large meta-analysis of 21 studies including 13 077 men performed by Sermondade et al confirmed a positive relationship between increasing body mass index (BMI) and risk of oligozoospermia and azoospermia (118). Furthermore, a systematic review of 30 studies comprising 115 158 participants reported that obese men had a higher percentage of sperm with DNA fragmentation, abnormal morphology, and low mitochondrial membrane potential, and they were more likely to be infertile when compared with men with normal BMI (121). Previous observational studies have investigated the effects of dietary intervention on semen parameters during weight loss. Combined diet and exercise was associated with increases in sperm count, semen volume, and testosterone when compared with baseline in those men losing the most weight (17.2–25.4% of body weight) (119). Furthermore, voluntary weight loss among male partners was independently associated with an increased live birth rate following in vitro fertilisation (IVF) therapy (122). Obesity is associated with increased semen ROS levels and sperm DNA fragmentation. Increased aromatization of testosterone to estrogen may have direct negative testicular impact, as estrogen receptors are present in most cell types of the human testes, including Leydig and Sertoli cells (123). In addition, decreased testosterone levels are associated with leptin and insulin resistance of obesity by modulating the HPT axis at various levels (28, 124).
Emerging evidence in the burgeoning field of genetics and epigenetics has demonstrated that paternal obesity can affect offspring metabolic and reproductive phenotypes by means of epigenetic reprogramming of spermatogonial stem cells. Donkin et al (125) demonstrated that the expression level of specific mitochondrial RNAs, and small nuclear RNA (snRNA) fragments, was altered in the spermatozoa of men with obesity. They postulated that this altered expression modulated the expression of genes involved in behavior and food intake and could participate in predisposing the offspring to obesity. This highlights the benefit of paternal preconception advise in relation to obesity.
Endocrine disrupting chemicals
Endocrine disrupting chemicals (EDC) may exert estrogenic and/or antiandrogenic effects, or directly induce testicular toxicity by impairing Sertoli or Leydig cell function, increased oxidative stress, sperm DNA damage, or sperm epigenetic changes (126). Epidemiological and experimental studies have suggested that in utero exposure to EDC, including bisphenol A, phthalates, pesticides, and other environmental chemicals may disrupt gonadal development during fetal life and lead to cryptorchidism, hypospadias, poor semen quality, and a predisposition to testicular germ-cell cancers (known as testicular dysgenesis syndrome) (127, 128). Several animal and in vitro studies have demonstrated that exposure to EDCs during fetal, neonatal, and adult life has dose-dependent direct adverse effects on spermatogenesis and steroidogenesis (129). However, clinical evidence remains limited (130). Inconsistencies in observations may be due to differential effects of EDCs depending on the developmental stage of exposure, degree of exposure, difference in study populations, synergistic effects from exposure to multiple EDCs, and residual confounding (eg, lifestyle factors).
Lifestyle factors
Cross-sectional studies demonstrate that adverse health behaviors such as excessive alcohol intake (131, 132), smoking (133), and recreational drugs (134, 135) are associated with reduced fertility in men (136, 137). In contrast, a large birth cohort of young men observed that recreational drugs were not associated with any semen variables (138). Much of the current evidence comes from men presenting to infertility clinics and may not represent the effect of lifestyle factors on male fertility in the general population (139). In addition, almost all the studies focus on specific effects of 1 or at most 2 lifestyle factors under evaluation. In reality, exposure to these risk factors does not occur individually but simultaneously (140, 141). Therefore, we may be underestimating the consequences of each adverse lifestyle exposure to male infertility in the general population.
Smoking
A meta-analysis including 5865 patients showed a negative association between smoking and semen parameters (142), with a pronounced negative effect on moderate and heavy smokers. Smoking is associated with lower sperm motility, increased sperm morphological defects, and lower sperm concentration and fertility index (FI) in heavy smokers compared with mild or nonsmokers (143). Smoking is also associated with poorer clinical outcomes from ART (144). However, definitions of smoking vary between studies, and underlying mechanisms of male infertility are not elucidated.
Alcohol
Mice model studies have shown an association between the amount and duration of ethanol exposure with sperm quality and fertilization ability (145). Meta-analysis of 16 395 men from 15 cross-sectional studies showed that alcohol intake was negatively associated with semen volume and morphology, with a marked difference in daily versus occasional drinkers (146). A study of 1200 Danish male military recruits aged 18–28 years observed that modest habitual alcohol consumption of more than 5 units per week was associated with adverse effects on semen quality (147). Furthermore, ethanol has been proposed to be a Leydig cell toxin (148).
Recreational drug use
Recreational drug use, such as cannabis, androgens, and opioid abuse is correlated with reduced sperm parameters (135), high DNA fragmentation in sperm, and reduced male fertility (149). Cannabinoid receptors are found in testicular cells and spermatozoa (150). Androgens may cause hypogonadotropic hypogonadism due to negative feedback on the HPT axis (151). These negative effects of androgen abuse on the HPT axis are fully but slowly reversible (apart from testicular volume and SHBG) 6 to 18 months after ceasing androgen intake in the majority of men (152). Opioids are known to cause secondary hypogonadism by inhibiting kisspeptin-neurokinin B-dynorphin (KNDy) neuronal activity (153), but may also have direct testicular effects due to presence of endogenous opioid receptors through the testis (154).
Caffeine
Evidence on caffeine intake on testicular failure remains inconsistent and inconclusive. In in vitro cultured human Sertoli cells, high caffeine consumption was observed to reduce antioxidant capacity of Sertoli cells causing oxidative damage (155). However, a recent systematic review of observational studies in 19 967 men suggested that caffeine consumption did not adversely affect semen parameters but was associated with increased sperm aneuploidy and DNA breaks (156).
Physical activity
Men who are physically active have reported improvements in semen quality compared with sedentary controls (157). Therefore, supervised physical activity may improve reproductive function, especially if there are concomitant comorbidities such as diabetes and obesity. There is also some evidence that continuous high intensity strenuous physical activity may impair semen parameters compared with moderate intensity exercise (158). However, there are multiple potential confounding risk factors to this, including low body weight, fatigue leading to low libido, and abnormal calorie intake. Furthermore, published data for moderate-intensity exercise training are inconsistent. Other than the intensity of physical activity, the specific sport undertaken may also be important. Cycling represents a unique sport in regards to fertility. Prolonged or competitive cycling is associated with reductions in total motile sperm count and sperm concentration, and elevated sperm DNA fragmentation; this is due to increased scrotal temperature, which is known to damage sperm (159).
Assessment of Male Infertility Due to Testicular Failure
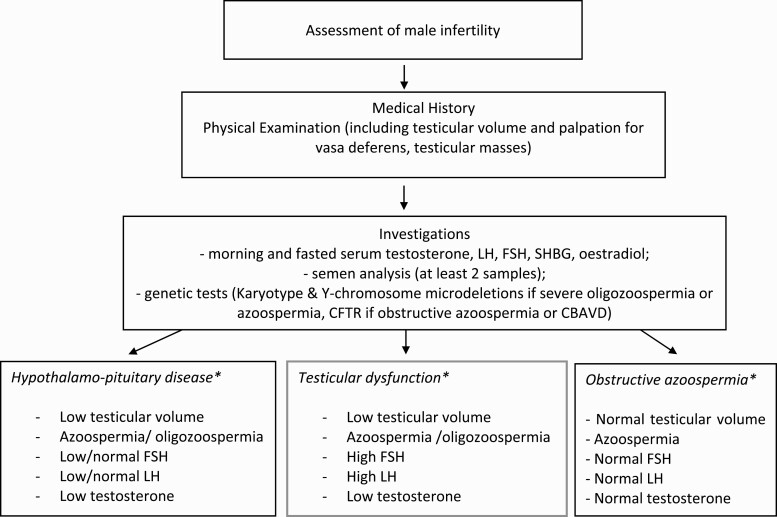
Testicular failure is often diagnosed incidentally during fertility investigations. A thorough history and physical examination, including the testes (the most accessible internal organ of the body) are the most important components for assessing and managing male infertility (Fig. 1). A detailed clinical investigation may shed light on the underlying pathology of testicular failure and identify potentially reversible factors of therapeutic value to the couple (Table 5 and Fig. 1).
Figure 1.
Flow diagram illustrating the assessment of an infertile male. The choice of investigations undertaken should be tailored to the clinical presentation and consider available health resources. *Typical diagnostic characteristics are provided, but some patients may have atypical characteristics.
Abbreviations: CBAVD, congenital bilateral absence of vasa deferens; FSH, follicle stimulating hormone; LH, luteinizing hormone; NOA, nonobstructive azoospermia; SHBG, sex hormone binding globulin.
Table 5.
Assessment of male infertility
| Typical Components of Fertility History |
| Duration of infertility |
| Frequency of intercourse |
| Symptoms of androgen deficiency, eg, low libido, decreased morning erections |
| Puberty |
| Past paternity |
| Undescended testes |
| Genitourinary infections |
| Gonadotoxic medications |
| Pelvic, bladder, or retroperitoneal surgery |
| Testicular trauma, torsion |
| Systemic illness, eg, obesity. diabetes, cancer, cardiovascular disease |
| Lifestyle factors, eg, alcohol, smoking, recreational drugs |
| Typical Components of Physical Examination |
| Secondary sexual characteristics |
| Features of Klinefelter’s syndrome, eg, tall stature |
| Location of urethral meatus |
| Testicular volume |
| Presence/absence of vasa deferens |
| Testicular mass, eg, varicocele |
| Undescended testes |
| Typical Investigations |
| Endocrine profile (early morning LH, FSH, testosterone, SHBG) |
| Semen analysis (at least 2 samples) |
| +/- US testis |
| Genetic counselling |
| Karyotype, Y-chromosome microdeletions (if severe oligozoospermia or azoospermia) |
| CFTR if obstructive azoospermia or CBAVD |
Abbreviations: CBAVD, congenital bilateral absence of vasa deferens; CFTR, cystic fibrosis transmembrane regulator; FSH, follicle stimulating hormone; LH, luteinizing hormone; SHBG, sex hormone binding globulin; US, Ultrasound.
History
Determine the frequency of intercourse and duration of infertility. There may be symptoms of hypogonadism, including decreased libido and morning erections. Clarify the details of puberty, past paternity, and any history of undescended testes or recent genitourinary infections. Determine medication history for gonadotoxic agents and past medical/surgical history for scrotal or pelvic irradiation, surgery or trauma (112). This is also a good opportunity to screen for comorbid systemic illnesses such as obesity and reversible causes such as alcohol excess, smoking, and recreational drug use (2).
Physical examination
Screen for secondary sexual characteristics and features of KS such as tall stature, gynaecomastia, and behavioral or educational problems. Location of urethral meatus should be confirmed, because it reflects prenatal androgen insufficiency. Testicular volume should be assessed using a Prader orchidometer. Reduced testicular volume (below 15 ml) by orchidometry is consistent with hypogonadism or testicular failure (160). Men may have untreated cryptorchidism or may have undergone orchidopexy as a child or adult; a small, previously undescended testis suggests testicular failure. Men with UDT also have a greater risk of germ cell tumours. A full or dilated epididymis may indicate an obstructive etiology. Varicoceles are best viewed with the patient standing and performing valsalva. Absence of the vasa deferentia may indicate CBVAD.
Investigations
These include endocrine hormone assessments (morning, fasted serum testosterone, LH, FSH, estradiol, SHBG), semen analysis, and/or ultrasound (US) of the testes, and appropriate genetic tests (161) (Fig. 1). Testosterone levels demonstrate a diurnal variation with maximum levels in the morning; overnight fasting increases morning serum testosterone (162) whilst food intake slightly suppresses these levels (163). Furthermore, various factors, including acute/subacute illness and several medications, may also affect the levels (164). A typical feature of OA is an entirely normal endocrine profile (and testicular volume). By contrast, serum FSH is often elevated in testicular failure (165), but LH and testosterone secretion may be preserved. However, the discriminatory power of FSH is limited around the upper limit of the normal reference group, with an overlap of FSH levels in obstructive azoospermia and NOA (166). The upper reference limit for serum FSH may often be higher in commercial assay kits due to inclusion of unselected older men or those with unrecognized reproductive illness (167). Semen analysis should be performed on at least 2 different occasions with 2 to 7 days of abstinence in accordance with established WHO standards (34). Testing for karyotype and Y chromosome microdeletions are recommend for all infertile men with suspected NOA or severe oligozoospermia (112). CFTR mutation screening should be performed in men with suspected OA.
Testicular histology
Testicular biopsy is almost always performed in association with surgical sperm retrieval (see section on ‘Surgical sperm retrieval techniques’). Spermatogenesis is classified histologically into 4 main patterns (168): complete spermatogenesis is seen in normal testes and obstructive azoospermia; hypospermatogenesis (all cell types present in correct ratio but reduced numbers); maturation arrest (failure of spermatogenesis beyond a certain stage); and SCO syndrome, ie, no germ cells present. The most severe of these histological patterns is SCO with complete absence of germ cells.
Principles of Management
There are no approved pharmacological treatments to stimulate spermatogenesis in primary testicular failure. As a result, treatment of severe male factor infertility, including azoospermia or oligozoospermia encompasses ART such as IVF or intra-cytoplasmic sperm injection, which although effective, are unaffordable for some couples and healthcare systems (169) and have potential complications for the female partner, such as ovarian hyperstimulation syndrome (170). Utilization of ART is dependent on factors such as the availability of reproductive technology, the health system of the country, and funding from state or insurers. Principles of management are discussed below.
Optimization of chances for a spontaneous pregnancy
Factors that influence spontaneous conception include the duration of infertility, the age of the female partner, and the underlying disease causing infertility (171). The frequency of sexual intercourse should be 2 to 3 times weekly in order to optimize the likelihood of conception (172). Eighty percent of pregnancies will occur in the first 6 cycles with regular unprotected intercourse, while a further 10% of couples will conceive within 12 months, in line with the international definition of infertility. Furthermore, nearly 50% of couples who did not conceive in the first 12 months will conceive by 36 months (173). Around 30% of couples in whom the male partner has a sperm concentration of 1 to 5 million/ml will conceive spontaneously over 24 to 36 months (174). Therefore, the finding of a low sperm concentration at 1 million/ml does not preclude natural fertility; however, the probability declines over time as sperm defects become more severe, together with the compounding effects of female age or co-existing disease. Five percent of couples remain infertile after 48 months of regular unprotected intercourse, with a poor prognosis for spontaneous pregnancy (175).
Lifestyle changes
In the absence of any effective pharmacological interventions, delivery of lifestyle advice represents potentially important treatment for couples with male infertility. The benefits of lifestyle measures on general health and wellbeing are uncontroversial. Recent evidence from mice models (176, 177) and human studies (178) suggests that amelioration of adverse lifestyle factors such as alcohol consumption and smoking cessation may improve markers of male fertility and quality of life (179). Despite observed improvements in semen parameters, the effects on pregnancy and live birth outcomes are limited (122, 180). Increased BMI is negatively correlated to male fertility (118); however, there is inconclusive evidence from interventional studies to suggest that weight loss is an effective therapy for male infertility. Bariatric surgery is the most effective weight loss therapy for obesity. However, a longitudinal study by Legro et al (181) observed a paradoxical reduction in sperm concentration during the first 6 months following bariatric surgery. A recent meta-analysis concluded that bariatric surgery has no overall effect on sperm function in men with obesity up to 24 months postsurgery, despite normalization of the reproductive hormone profile (182). Recent observational studies have suggested that milder weight loss is associated with improved sperm function in obese men with infertility (122). Hakonsen et al conducted an uncontrolled study in 43 men, with obesity reporting that men losing the most weight (17.2–25.4% of body weight) had an increase in sperm count, semen volume, and testosterone when compared with baseline (119). Mir et al also showed that weight loss leads to a significant reduction in the DNA fragmentation index and, therefore, improved sperm morphology (183). However, data on ART outcomes are lacking. A recent meta-analysis showed that moderate intensity exercise can improve sperm concentration and progressive motility parameters (184). In conclusion, paternal preconception health advise may improve lifestyle factors, with a positive effect on sperm parameters.
Putative Hormonal Therapies for Testicular Failure
Gonadotropin replacement therapy and antiestrogens such as selective estrogen receptor modulators (SERMs) and aromatase inhibitors are empirically administered to men with azoospermia prior to surgical sperm retrieval, but robust evidence of their efficacies is currently lacking (185).
Gonadotropins
Gonadotropin therapy is highly effective for gonadotropin deficiency (14, 15) but not necessarily for men with idiopathic oligozoospermia. A Cochrane review of 6 RCTs with 456 participants observed a cumulative improvement in the live birth rate and spontaneous pregnancy rate in couples where male partners received FSH when compared with no treatment or placebo (186). However, the number of RCTs and participants included were small, with insufficient evidence to allow firm conclusions. Another prospective study of men with idiopathic oligozoospermia and normal FSH levels reported significantly improved sperm retrieval rates, fertilization, and pregnancy rates post-TESE-ICSI in men treated with FSH versus untreated controls (187). A recent meta-analysis of 15 controlled studies (9 RCTs, 6 nonrandomized studies) with 614 men treated with FSH and 661 men untreated or treated with placebo showed an improvement in pregnancy rate after FSH administration both spontaneously and after ART in men with idiopathic infertility (188). There was no significant difference in the mean basal FSH levels between the treated and nontreated groups of men included in this meta-analysis.
Antiestrogens
Selective estrogen receptor modulators reduce estradiol feedback and so disinhibit GnRH and pituitary gonadotropin secretion and increase testicular stimulation. Aromatase is an enzyme that converts testosterone and androstenedione to estradiol and estrone, respectively. Similar to SERMs, aromatase inhibitors decrease the negative estradiol feedback on the HPT axis (189, 189). A meta-analysis of 11 RCTs reported significant improvement in sperm concentration, motility, and spontaneous pregnancy rate in men with idiopathic infertility and OAT on antiestrogen therapy compared with controls (190). Another latest meta-analysis of idiopathic normogonadotrophic oligozoospermic men included 16 (controlled and noncontrolled) studies with improvement in sperm concentration, total sperm count, LH, FSH, and total testosterone levels following treatment with SERMs in men with idiopathic oligozoospermia (191). Similarly, one small randomized controlled study of 46 patients with NOA and cryptozoospermia (22 men received letrozole vs 22 men received placebo for 6 months) observed a significant increase in sperm concentration, motility, FSH, LH, and testosterone, with a reduction in estradiol in the men who received Letrozole versus placebo (192). However, the current data is controversial and heterogenous to draw any firm conclusion. Furthermore, there is lack of standardization on the duration of treatment, dose/type of antiestrogen therapy, and baseline patient characteristics.
The use of any of these pharmacological therapies for testicular failure pre-ICSI or TESE is controversial (193), and their usage is not supported by current guidelines (2, 112). The safety profiles of these off-label medications have not been established in men. Furthermore, there is no current consensus on the optimal medication and considerable ambiguity exists as to the perceived effects on fertility. Furthermore, antiestrogen therapies have associated risks such as increased thromboembolic disease, gynaecomastia with detrimental effects on male sexual function (sexual desire and erectile function), (194, 195) and long-term adverse effects on bone mineral density (196).
Nevertheless, many clinical centers consider these empirical medical therapies in specific cases, for example, in cases without elevated FSH (primary spermatogenic failure) and idiopathic oligozoospermia (197, 198), with the rationale being to increase FSH levels to stimulate spermatogenesis and optimize intratesticular testosterone, pre- ART. Future good quality multicenter RCTs are needed to better define the efficacy of these pharmacological agents.
Surgical sperm retrieval techniques
Sperm retrieval techniques
Men with obstructive azoospermia can be treated with epididymal or testicular sperm extraction, although epididymal extraction is typically less invasive (199). Epididymal sperm extraction can be done by microsurgical epididymal sperm aspiration (MESA), percutaneous epididymal sperm aspiration (PESA), reconstructive surgery. Men with NOA require testicular sperm extraction to search for areas of focal spermatogenesis for use during ICSI; this is performed by TESA (fine-needle transcutaneous aspiration, minimally invasive) or TESE/micro-TESE (scrotal incision with open testicular biopsy with or without a microscope to identify the seminiferous tubules) (200) (Fig. 2). A meta-analysis of 21 404 men with NOA observed that TESE/micro-TESE results in a testicular sperm retrieval rate of up to 50% in men with nonobstructive azoospermia, independent of age and hormonal profile (201). Currently, there are no clinical factors or biochemical tests that can reliably predict sperm retrieval in men with NOA prior to surgery. However, genetic disorders such as AZFa/AZFb microdeletions or XX male syndrome are contraindications to TESE due to their incompatibility with spermatogenesis. A meta-analysis of men with NOA due to KS suggested successful spermatozoa retrieval in up to 50% of cases, with nearly 50% pregnancy and live birth rates after TESE-ICSI, independent of male age, testes volume, and reproductive endocrine profile (202). Several groups have reported healthy offspring born after TESE-ICSI, with sperm extracted from men with KS (203), with the risk of chromosomal abnormalities being similar to those reported in men without KS. It is interesting to consider whether cryopreservation negatively impacts the reproductive potential of sperm. Cryopreservation increases the proportion of sperm in a sample which are immotile and/or nonviable (204). However, there does not appear to be evidence suggesting that cryopreserved testicular sperm negatively influences the pregnancy and live birth outcomes after TESE-ICSI (205). TESE complications include intratesticular hematoma and fibrosis. A recent meta-analysis reported that mean levels of total serum testosterone were reduced in men with NOA 6 months after TESE, with a recovery time ranging between 18 and 26 months (206). Therefore, endocrine surveillance for hypogonadism should be considered in men with NOA post-TESE.
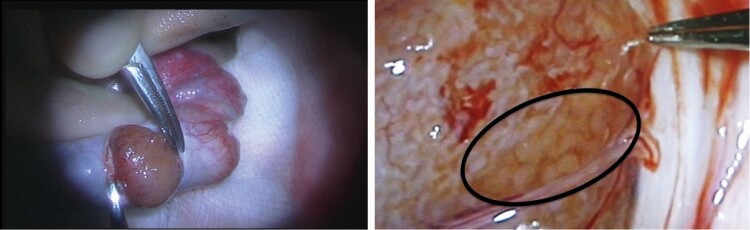
Figure 2.
Micro-TESE (microdissection testicular sperm extraction). Intraoperative photographs of a micro-TESE in a patient with nonobstructive azoospermia (NOA). The testis is transversely bivalved and dilated under optical magnification. Opaque tubules (circled in black) are retrieved, as these are more likely to contain seminiferous tubules with complete spermatogenesis.
Effects on offspring following ART for male infertility
It is important to consider whether the offspring of affected couples have increased risks of congenital defects potentially transferred through genetic and epigenetic mechanisms (207, 208). Multiple studies have suggested that the risk of sex and autosome chromosomal abnormalities is higher in offspring born following ICSI (commonly indicated in men with poor sperm quality) versus the general population (209, 210). In contrast, neonatal health, including birth parameters, major anomalies, and chromosomal abnormalities in a large cohort of children born with nonejaculated (epididymal and testicular) sperm, was similar compared with the outcome of children born with ejaculated sperm (211). Other studies have supported these findings (212, 213). However, there are multiple limitations to these studies, with limited sample size, and that are often performed without control groups. Further elucidation of the heritable factors causing male infertility will enable more robust screening and counselling of couples undergoing ART.
Conclusion
Underlying genetic predisposition, exposure to environmental factors, and adverse lifestyle behaviors contribute to the etiopathogenesis of testicular dysfunction. However, 40% of cases of testicular failure remain classified as idiopathic. Future research is needed to determine unidentified factors causative in idiopathic male factor infertility. This would lead to novel individualized and targeted pharmacological therapies to complement ART, which is unaffordable to many couples worldwide.
Acknowledgments
Financial Support: The Section of Endocrinology and Investigative Medicine is funded by grants from the MRC and NIHR and is supported by the NIHR Biomedical Research Centre Funding Scheme. The following authors have grant funding as follows: A.S., Imperial College Healthcare Charity Fellowship; C.N.J., NIHR Post-Doctoral Fellowship; W.S.D., NIHR Research Professorship.
Additional Information
Disclosure Summary: The views expressed are those of the authors and not necessarily those of the above-mentioned funders, the NHS, the NIHR, or the Department of Health. The authors have no conflicts of interest to disclose.
Data Availability
Data sharing is not applicable to this article, as no datasets were generated or analyzed during the current study.
References
- 1. Human Fertilisation and Embryology Authority. Fertility treatment in 2013: trends and figures. Hum Fertil Embryol Author. 2013; 1–46. [Google Scholar]
- 2. National Institute for Health and Care Excellence, (NICE). Fertility problems: assessment and treatment. ProMED-mail website. http://guidance.nice.org.uk/CG156. Accessed June 25, 2020. [PubMed]
- 3. Sharlip ID, Jarow JP, Belker AM, et al. . Best practice policies for male infertility. Fertil Steril. 2002;77(5):873–882. [DOI] [PubMed] [Google Scholar]
- 4. Practice Committee of the American Society for Reproductive Medicine. Diagnostic evaluation of the infertile male: a committee opinion. Fertil Steril. 2015;103(3):e18–e25. [DOI] [PubMed] [Google Scholar]
- 5. Levine H, Jørgensen N, Martino-Andrade A, et al. . Temporal trends in sperm count: a systematic review and meta-regression analysis. Hum Reprod Update. 2017;23(6):646–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rasmussen PE, Erb K, Westergaard LG, Laursen SB. No evidence for decreasing semen quality in four birth cohorts of 1055 Danish men born between 1950 and 1970. Fertil Steril. 1997;68(6):1059–1064. [DOI] [PubMed] [Google Scholar]
- 7. Jørgensen N, Joensen UN, Jensen TK, et al. . Human semen quality in the new millennium: a prospective cross-sectional population-based study of 4867 men. BMJ Open. 2012;2(4):e000990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Choy JT, Eisenberg ML. Male infertility as a window to health. Fertil Steril. 2018;110(5):810–814. [DOI] [PubMed] [Google Scholar]
- 9. Jensen TK, Jacobsen R, Christensen K, Nielsen NC, Bostofte E. Good semen quality and life expectancy: a cohort study of 43 277 men. Am J Epidemiol. 2009;170(5):559–565. [DOI] [PubMed] [Google Scholar]
- 10. Karavolos S, Stewart J, Evbuomwan I, McEleny K, Aird I. Assessment of the infertile male. Obstet Gynaecologist. 2013;15(1):1–9. [Google Scholar]
- 11. Stahl PJ, Stember DS, Goldstein M. Contemporary management of male infertility. Annu Rev Med. 2012;63:525–540. [DOI] [PubMed] [Google Scholar]
- 12. Krausz C. Male infertility: pathogenesis and clinical diagnosis. Best Pract Res.: Clin Endocrinol Metab. 2010;25(2):271–285. [DOI] [PubMed] [Google Scholar]
- 13. Jarow JP, Espeland MA, Lipshultz LI. Evaluation of the azoospermic patient. J Urol. 1989;142(1):62–65. [DOI] [PubMed] [Google Scholar]
- 14. Rastrelli G, Corona G, Mannucci E, Maggi M. Factors affecting spermatogenesis upon gonadotropin-replacement therapy: a meta-analytic study. Andrology. 2014;2(6):794–808. [DOI] [PubMed] [Google Scholar]
- 15. Dwyer AA, Raivio T, Pitteloud N. Gonadotrophin replacement for induction of fertility in hypogonadal men. Best Pract Res Clin Endocrinol Metab. 2015;29(1):91–103. [DOI] [PubMed] [Google Scholar]
- 16. Nishimura H, L’Hernault SW. Spermatogenesis. Curr Biol. 2017;27(18):R988–R994. [DOI] [PubMed] [Google Scholar]
- 17. Heller CG, Clermont Y. Spermatogenesis in man: an estimate of its duration. Science. 1963;140(3563):184–186. [DOI] [PubMed] [Google Scholar]
- 18. Misell LM, Holochwost D, Boban D, et al. . A stable isotope-mass spectrometric method for measuring human spermatogenesis kinetics in vivo. J Urol. 2006;175(1):242–6; discussion 246. [DOI] [PubMed] [Google Scholar]
- 19. Griswold MD. Spermatogenesis: the commitment to meiosis. Physiol Rev. 2016;96(1):1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Matsumoto AM, Bremner WJ. Endocrinology of the hypothalamic-pituitary-testicular axis with particular reference to the hormonal control of spermatogenesis. Baillieres Clin Endocrinol Metab. 1987;1(1):71–87. [DOI] [PubMed] [Google Scholar]
- 21. Kathrins M, Niederberger C. Diagnosis and treatment of infertility-related male hormonal dysfunction. Nat Rev Urol. 2016;13(6):309–323. [DOI] [PubMed] [Google Scholar]
- 22. Clavijo RI, Hsiao W. Update on male reproductive endocrinology. Transl Androl Urol. 2018;7(Suppl 3):S367–S372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Foresta C, Zuccarello D, Garolla A, Ferlin A. Role of hormones, genes, and environment in human cryptorchidism. Endocr Rev. 2008;29(5):560–580. [DOI] [PubMed] [Google Scholar]
- 24. Xu H, Zhang H, Xiao Z, Qiao J, Li R. Regulation of anti-Müllerian hormone (AMH) in males and the associations of serum AMH with the disorders of male fertility. Asian J Androl. 2019;21(2):109–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Matuszczak E, Hermanowicz A, Komarowska M, Debek W. Serum AMH in physiology and pathology of male gonads. Int J Endocrinol. 2013;2013:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ferlin A, Arredi B, Zuccarello D, Garolla A, Selice R, Foresta C. Paracrine and endocrine roles of insulin-like factor 3. J Endocrinol Invest. 2006;29(7):657–664. [DOI] [PubMed] [Google Scholar]
- 27. Cannarella R, Condorelli RA, La Vignera S, Calogero AE. Effects of the insulin-like growth factor system on testicular differentiation and function: a review of the literature. Andrology. 2018;6(1):3–9. [DOI] [PubMed] [Google Scholar]
- 28. Tena-Sempere M, Barreiro ML. Leptin in male reproduction: the testis paradigm. Mol Cell Endocrinol. 2002;188(1–2):9–13. [DOI] [PubMed] [Google Scholar]
- 29. Soyupek S, Armağan A, Serel TA, et al. . Leptin expression in the testicular tissue of fertile and infertile men. Arch Androl. 2005;51(3):239–246. [DOI] [PubMed] [Google Scholar]
- 30. Malik IA, Durairajanayagam D, Singh HJ. Leptin and its actions on reproduction in males. Asian J Androl. 2019;21(3):296–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schoeller EL, Schon S, Moley KH. The effects of type 1 diabetes on the hypothalamic, pituitary and testes axis. Cell Tissue Res. 2012;349(3):839–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sharma A, Thaventhiran T, Minhas S, Dhillo WS, Jayasena CN. Kisspeptin and testicular function-is it necessary? Int J Mol Sci. 2020;21(8):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Baskaran S, Finelli R, Agarwal A, Henkel R. Diagnostic value of routine semen analysis in clinical andrology. Andrologia (Berlin, West). 2020; e13614. Doi: 10.1111/and.13614. [DOI] [PubMed] [Google Scholar]
- 34. Cooper TG. WHO Laboratory Manual for the Examination and Processing of Human Semen. 5 ed. Geneva: WHO Press; 2010. [Google Scholar]
- 35. Cooper TG, Noonan E, von Eckardstein S, et al. . World Health Organization reference values for human semen characteristics. Hum Reprod Update. 2010;16(3):231–245. [DOI] [PubMed] [Google Scholar]
- 36. Plachot M, Belaisch-Allart J, Mayenga JM, Chouraqui A, Tesquier L, Serkine AM. Outcome of conventional IVF and ICSI on sibling oocytes in mild male factor infertility. Hum Reprod. 2002;17(2):362–369. [DOI] [PubMed] [Google Scholar]
- 37. Bonde JPE, Ernst E, Jensen TK, et al. . Relation between semen quality and fertility: a population-based study of 430 first-pregnancy planners. Lancet. 1998;352(9135): 1172–1177. [DOI] [PubMed] [Google Scholar]
- 38. Slama R, Eustache F, Ducot B, et al. . Time to pregnancy and semen parameters: a cross-sectional study among fertile couples from four European cities. Hum Reprod. 2002;17(2):503–515. [DOI] [PubMed] [Google Scholar]
- 39. Zinaman MJ, Brown CC, Selevan SG, Clegg ED. Semen quality and human fertility: a prospective study with healthy couples. J Androl. 2000;21(1):145–153. [PubMed] [Google Scholar]
- 40. Tremellen K. Oxidative stress and male infertility–a clinical perspective. Hum Reprod Update. 2008;14(3):243–258. [DOI] [PubMed] [Google Scholar]
- 41. Bisht S, Faiq M, Tolahunase M, Dada R. Oxidative stress and male infertility. Nat Rev Urol. 2017;14(8):470–485. [DOI] [PubMed] [Google Scholar]
- 42. Zeqiraj A, Beadini S, Beadini N, et al. . Male infertility and sperm DNA fragmentation. Open Access Maced J Med Sci. 2018;6(8):1342–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dutta S, Majzoub A, Agarwal A. Oxidative stress and sperm function: a systematic review on evaluation and management. Arab J Urol. 2019;17(2):87–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pourmasumi S, Sabeti P, Rahiminia T, Mangoli E, Tabibnejad N, Talebi AR. The etiologies of DNA abnormalities in male infertility: an assessment and review. Int J Reprod Biomed. 2017;15(6):331–344. [PMC free article] [PubMed] [Google Scholar]
- 45. Agarwal A, Roychoudhury S, Bjugstad KB, Cho C.. Oxidation-Reduction Potential of Semen: What Is Its Role in the Treatment of Male Infertility? London, England: SAGE Publications; 2016;1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Muratori M, Tamburrino L, Marchiani S, et al. . Investigation on the origin of sperm DNA fragmentation: role of apoptosis, immaturity and oxidative stress. Mol Med. 2015;21(1):109–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Agarwal A, Parekh N, Panner Selvam MK, et al. . Male oxidative stress infertility (MOSI): proposed terminology and clinical practice guidelines for management of idiopathic male infertility. World J Men’s Health. 2019;37(3):296–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Agarwal A, Cho C, Esteves SC, Majzoub A. Reactive oxygen species and sperm DNA fragmentation. Transl Androl Urol. 2017;6(Suppl 4):S695–S696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Showell MG, Brown J, Yazdani A, Stankiewicz MT, Hart RJ. Antioxidants for male subfertility. Cochrane Database Syst Rev. 2011; ( 1):CD007411. [DOI] [PubMed] [Google Scholar]
- 50. Smits RM, Mackenzie-Proctor R, Yazdani A, Stankiewicz MT, Jordan V, Showell MG. Antioxidants for male subfertility. Cochrane Database Syst Rev. 2019;3(3):CD007411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Simon L, Emery B, Carrell DT. Sperm DNA fragmentation: consequences for reproduction. Adv Exp Med Biol. 2019;1166:87–105. [DOI] [PubMed] [Google Scholar]
- 52. Nicopoullos J, Vicens-Morton A, Lewis SEM, et al. . Novel use of COMET parameters of sperm DNA damage may increase its utility to diagnose male infertility and predict live births following both IVF and ICSI. Hum Reprod. 2019;34(10):1915–1923. [DOI] [PubMed] [Google Scholar]
- 53. Bungum M, Humaidan P, Spano M, Jepson K, Bungum L, Giwercman A. The predictive value of sperm chromatin structure assay (SCSA) parameters for the outcome of intrauterine insemination, IVF and ICSI. Hum Reprod. 2004;19(6):1401–1408. [DOI] [PubMed] [Google Scholar]
- 54. Tan J, Taskin O, Albert A, Bedaiwy MA. Association between sperm DNA fragmentation and idiopathic recurrent pregnancy loss: a systematic review and meta-analysis. Reprod Biomed Online. 2019;38(6):951–960. [DOI] [PubMed] [Google Scholar]
- 55. McLachlan RI, O’Bryan MK. State of the art for genetic testing of infertile men. J Clin Endocrinol Metab 2010;95(3):1013–1024. [DOI] [PubMed] [Google Scholar]
- 56. Hofherr SE, Wiktor AE, Kipp BR, Dawson DB, Van Dyke DL. Clinical diagnostic testing for the cytogenetic and molecular causes of male infertility: the Mayo Clinic experience. J Assist Reprod Genet. 2011;28(11):1091–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Foresta C, Garolla A, Bartoloni L, Bettella A, Ferlin A. Genetic abnormalities among severely oligospermic men who are candidates for intracytoplasmic sperm injection. J Clin Endocrinol Metab. 2005;90(1):152–156. [DOI] [PubMed] [Google Scholar]
- 58. Bojesen A, Juul S, Gravholt CH. Prenatal and postnatal prevalence of Klinefelter syndrome: a national registry study. J Clin Endocrinol Metab. 2003;88(2):622–626. [DOI] [PubMed] [Google Scholar]
- 59. Groth KA, Skakkebæk A, Høst C, Gravholt CH, Bojesen A. Klinefelter syndrome – a clinical update. J Clin Endocrinol Metab. 2013;98(1):20–30. [DOI] [PubMed] [Google Scholar]
- 60. Bonomi M, Rochira V, Pasquali D, Balercia G, Jannini EA, Ferlin A; Klinefelter ItaliaN Group (KING) . Klinefelter syndrome (KS): genetics, clinical phenotype and hypogonadism. J Endocrinol Invest. 2017;40(2):123–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wikström AM, Dunkel L. Klinefelter syndrome. Best Pract Res: Clin Endocrinol Metab. 2010;25(2):239–250. [DOI] [PubMed] [Google Scholar]
- 62. Aksglaede L, Wikström AM, Rajpert-De Meyts E, Dunkel L, Skakkebaek NE, Juul A. Natural history of seminiferous tubule degeneration in Klinefelter syndrome. Hum Reprod Update. 2006;12(1):39–48. [DOI] [PubMed] [Google Scholar]
- 63. Deebel NA, Galdon G, Zarandi NP, et al. . Age-related presence of spermatogonia in patients with Klinefelter syndrome: a systematic review and meta-analysis. Hum Reprod Update. 2020;26(1):58–72. [DOI] [PubMed] [Google Scholar]
- 64. Gies I, De Schepper J, Goossens E, Van Saen D, Pennings G, Tournaye H. Spermatogonial stem cell preservation in boys with Klinefelter syndrome: to bank or not to bank, that’s the question. Fertil Steril. 2012;98(2):284–289. [DOI] [PubMed] [Google Scholar]
- 65. Kanakis GA, Nieschlag E. Klinefelter syndrome: more than hypogonadism. Metabolism. 2018;86:135–144. [DOI] [PubMed] [Google Scholar]
- 66. Gardner RJM, Sutherland GR, Shaffer LG.. Chromosome Abnormalities and Genetic Counseling. 4 ed. Oxford; New York [u.a.]: Oxford University Press; 2012:161–182. [Google Scholar]
- 67. Morin SJ, Eccles MD, Jennifer MS, et al. . Translocations, inversions and other chromosome rearrangements. Fertil Steril. 2016;107(1):19–26. [DOI] [PubMed] [Google Scholar]
- 68. Spinner N, Conlin L, Mulchandani S, Emanuel B. Deletions and Other Structural Abnormalities of the Autosomes. Emery and Rimoin’s Principles and Practice of Medical Genetics. Academic Press; 2013:1–37. [Google Scholar]
- 69. Siffroi JP, Benzacken B, Straub B, et al. . Assisted reproductive technology and complex chromosomal rearrangements: the limits of ICSI. Mol Hum Reprod. 1997;3(10):847–851. [DOI] [PubMed] [Google Scholar]
- 70. Foresta C, Moro E, Ferlin A. Y chromosome microdeletions and alterations of spermatogenesis. Endocr Rev. 2001;22(2):226–239. [DOI] [PubMed] [Google Scholar]
- 71. Colaco S, Modi D. Genetics of the human Y chromosome and its association with male infertility. Reprod Biol Endocrinol. 2018;16(1):14–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Abur U, Gunes S, Ascı R, et al. . Chromosomal and Y-chromosome microdeletion analysis in 13,00 infertile males and the fertility outcome of patients with AZFc microdeletions. Andrologia. 2019;51(11):1–8. [DOI] [PubMed] [Google Scholar]
- 73. Hopps CV, Mielnik A, Goldstein M, Palermo GD, Rosenwaks Z, Schlegel PN. Detection of sperm in men with Y chromosome microdeletions of the AZFa, AZFb and AZFc regions. Hum Reprod. 2003;18(8):1660–1665. [DOI] [PubMed] [Google Scholar]
- 74. Lee PA, Nordenström A, Houk CP, et al. . Global disorders of sex development update since 2006: perceptions, approach and care. Horm Res Paediatr. 2016;85(3):158–180. [DOI] [PubMed] [Google Scholar]
- 75. Chakraborty P, Chakraborty P, Bhattacharjee R, et al. . Male factor infertility: clues to diagnose 46, XX male. J Obstet Gynecol India. 2016;66(S2): 662–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Delachapelle A, Hortling H, Niemi M, Wennstroem J. XX sex chromosomes in a human male. first case. Acta Med Scand. 1964;175(Suppl 412):25–38. [DOI] [PubMed] [Google Scholar]
- 77. Bieth E, Hamdi SM, Mieusset R. Genetics of the congenital absence of the vas deferens. Hum Genet. 2020; Doi: 10.1007/s00439-020-02122-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Chillón M, Casals T, Mercier B, et al. . Mutations in the cystic fibrosis gene in patients with congenital absence of the vas deferens. N Engl J Med. 1995;332(22):1475–1480. [DOI] [PubMed] [Google Scholar]
- 79. De Braekeleer M, Férec C. Mutations in the cystic fibrosis gene in men with congenital bilateral absence of the vas deferens. Mol Hum Reprod. 1996;2(9):669–677. [DOI] [PubMed] [Google Scholar]
- 80. Yang F, Gell K, van der Heijden GW, et al. . Meiotic failure in male mice lacking an X-linked factor. Genes Dev. 2008;22(5):682–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Yatsenko AN, Georgiadis AP, Röpke A, et al. . X-linked TEX11 mutations, meiotic arrest, and azoospermia in infertile men. N Engl J Med. 2015;372(22):2097–2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Cerván-Martín M, Castilla JA, Palomino-Morales RJ, Carmona FD. Genetic landscape of nonobstructive azoospermia and new perspectives for the clinic. J Clin Med. 2020;9(2):300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Sironen A, Shoemark A, Patel M, Loebinger MR, Mitchison HM. Sperm defects in primary ciliary dyskinesia and related causes of male infertility. Cell Mol Life Sci. 2020;77(11):2029–2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Niedzielski JK, Oszukowska E, Słowikowska-Hilczer J. Undescended testis – current trends and guidelines: a review of the literature. Arch Med Sci. 2016;12(3):667–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Wilkerson ML, Bartone FF, Fox L, Hadziselimovic F. Fertility potential: a comparison of intra-abdominal and intracanalicular testes by age groups in children. Horm Res Paediatr. 2001;55(1):18–20. [DOI] [PubMed] [Google Scholar]
- 86. Hadziselimovic F, Hocht B, Herzog B, Buser MW. Infertility in cryptorchidism is linked to the stage of germ cell development at orchidopexy. Horm Res. 2007;68(1):46–52. [DOI] [PubMed] [Google Scholar]
- 87. Chung E, Brock GB. Cryptorchidism and its impact on male fertility: a state of art review of current literature. Can Urol Assoc J. 2011;5(3):210–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Bogatcheva NV, Agoulnik AI. INSL3/LGR8 role in testicular descent and cryptorchidism. Reprod Biomed Online. 2005;10(1):49–54. [DOI] [PubMed] [Google Scholar]
- 89. Pellati D, Mylonakis I, Bertoloni G, et al. . Genital tract infections and infertility. Eur J Obstet Gynecol Reprod Biol. 2008;140(1):3–11. [DOI] [PubMed] [Google Scholar]
- 90. Rowe PJ, Comhaire FH, Hargreave TB, Mahmoud AMA, World Health Organization . WHO manual for the Standardized Investigation, Diagnosis and Management of the Infertile Male/Patrick J. Rowe ... [et al.]. Geneva: World Health Organization; 2000. [Google Scholar]
- 91. Stojanov M, Baud D, Greub G, Vulliemoz N. Male infertility: the intracellular bacterial hypothesis. New Microbes New Infect. 2018;26:37–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Bryan ER, McLachlan RI, Rombauts L, et al. . Detection of chlamydia infection within human testicular biopsies. Hum Reprod. 2019;34(10):1891–1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Taylor-Robinson D. Infections due to species of mycoplasma and ureaplasma: an update. Clin Infect Dis. 1996;23(4):671–682. [DOI] [PubMed] [Google Scholar]
- 94. Gimenes F, Souza RP, Bento JC, et al. . Male infertility: a public health issue caused by sexually transmitted pathogens. Nat Rev Urol. 2014;11(12):672–687. [DOI] [PubMed] [Google Scholar]
- 95. Adamopoulos DA, Lawrence DM, Vassilopoulos P, Contoyiannis PA, Swyer GI. Pituitary-testicular interrelationships in mumps orchitis and other viral infections. Br Med J. 1978;1(6121):1177–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Weidner W, Ludwig M, Miller J. Therapy in male accessory gland infection – what is fact, what is fiction? Andrologia. 1998;30(Suppl 1):87–90. [DOI] [PubMed] [Google Scholar]
- 97. Comhaire FH, Rowe PJ, Farley TM. The effect of doxycycline in infertile couples with male accessory gland infection: a double blind prospective study. Int J Androl. 1986;9(2):91–98. [DOI] [PubMed] [Google Scholar]
- 98. Trum JW, Mol BW, Pannekoek Y, et al. . Value of detecting leukocytospermia in the diagnosis of genital tract infection in subfertile men. Fertil Steril. 1998;70(2):315–319. [DOI] [PubMed] [Google Scholar]
- 99. Aitken RJ, Baker HW. Seminal leukocytes: passengers, terrorists or good samaritans? Hum Reprod. 1995;10(7):1736–1739. [DOI] [PubMed] [Google Scholar]
- 100. Lyu Z, Feng X, Li N, et al. . Human papillomavirus in semen and the risk for male infertility: a systematic review and meta-analysis. BMC Infect Dis. 2017;17(1):714–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Depuydt CE, Donders GGG, Verstraete L, et al. . Infectious human papillomavirus virions in semen reduce clinical pregnancy rates in women undergoing intrauterine insemination. Fertil Steril. 2019;111(6):1135–1144. [DOI] [PubMed] [Google Scholar]
- 102. Petersen PM, Skakkebæk NE, Vistisen K, Rørth M, Giwercman A.. Semen quality and reproductive hormones before orchiectomy in men with testicular cancer. J Clin Oncol. 1999;17(3):941–947. [DOI] [PubMed] [Google Scholar]
- 103. Meirow D, Schenker JG. Cancer and male infertility. Hum Reprod. 1995;10(8):2017–2022. [DOI] [PubMed] [Google Scholar]
- 104. Paoli D, Pallotti F, Lenzi A, Lombardo F. Fatherhood and sperm DNA damage in testicular cancer patients. Front Endocrinol (Lausanne). 2018;9:506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Shankara-Narayana N, Di Pierro I, Fennell C, et al. . Sperm cryopreservation prior to gonadotoxic treatment: experience of a single academic centre over 4 decades. Hum Reprod. 2019;34(5):795–803. [DOI] [PubMed] [Google Scholar]
- 106. Fertility preservation and reproduction in patients facing gonadotoxic therapies: an Ethics Committee opinion. Fertil Steril. 2018;110(3):380–386. [DOI] [PubMed] [Google Scholar]
- 107. Will MA, Swain J, Fode M, Sonksen J, Christman GM, Ohl D. The great debate: varicocele treatment and impact on fertility. Fertil Steril. 2011;95(3):841–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Macleod R, Biyani CS, Cartledge J, Eardley I. Varicocele. Clin Evid. 2015; 2015(07):1806–1828. [PMC free article] [PubMed] [Google Scholar]
- 109. Kim HH, Goldstein M. Adult varicocele. Curr Opin Urol. 2008;18(6):608–612. [DOI] [PubMed] [Google Scholar]
- 110. Esteves SC, Miyaoka R, Roque M, Agarwal A. Outcome of varicocele repair in men with nonobstructive azoospermia: systematic review and meta-analysis. Asian J Androl. 2016;18(2):246–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Kirby EW, Wiener LE, Rajanahally S, Crowell K, Coward RM. Undergoing varicocele repair before assisted reproduction improves pregnancy rate and live birth rate in azoospermic and oligospermic men with a varicocele: a systematic review and meta-analysis. Fertil Steril. 2016;106(6):1338–1343. [DOI] [PubMed] [Google Scholar]
- 112. Jungwirth A, Giwercman A, Tournaye H, et al. ; European Association of Urology Working Group on Male Infertility . European Association of Urology Guidelines on Male Infertility: the 2012 update. Eur Urol. 2012;62(2):324–332. [DOI] [PubMed] [Google Scholar]
- 113. Leroy C, Rigot JM, Leroy M, et al. . Immunosuppressive drugs and fertility. Orphanet J Rare Dis. 2015;10(1):136–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Koyuncu H, Serefoglu EC, Yencilek E, Atalay H, Akbas NB, Sarıca K. Escitalopram treatment for premature ejaculation has a negative effect on semen parameters. Int J Impot Res. 2011;23(6):257–261. [DOI] [PubMed] [Google Scholar]
- 115. Tanrikut C, Feldman AS, Altemus M, Paduch DA, Schlegel PN. Adverse effect of paroxetine on sperm. Fertil Steril. 2010;94(3):1021–1026. [DOI] [PubMed] [Google Scholar]
- 116. Sawka AM, Lea J, Alshehri B, et al. . A systematic review of the gonadal effects of therapeutic radioactive iodine in male thyroid cancer survivors. Clin Endocrinol (Oxf). 2008;68(4):610–617. [DOI] [PubMed] [Google Scholar]
- 117. Hendry WF. Disorders of ejaculation: congenital, acquired and functional. Br J Urol. 1998;82(3):331–341. [DOI] [PubMed] [Google Scholar]
- 118. Sermondade N, Faure C, Fezeu L, et al. . BMI in relation to sperm count: an updated systematic review and collaborative meta-analysis. Hum Reprod Update. 2013;19(3):221–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Håkonsen LB, Thulstrup AM, Aggerholm AS, et al. . Does weight loss improve semen quality and reproductive hormones? Results from a cohort of severely obese men. Reprod Health. 2011;8(1):24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Sermondade N, Faure C, Fezeu L, Lévy R, Czernichow S, Obesity-Fertility Collaborative Group . Obesity and increased risk for oligozoospermia and azoospermia. Arch Intern Med. 2012;172(5):440–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Campbell JM, Lane M, Owens JA, Bakos HW. Paternal obesity negatively affects male fertility and assisted reproduction outcomes: a systematic review and meta-analysis. Reprod BioMed Online. 2015;31(5):593–604. [DOI] [PubMed] [Google Scholar]
- 122. Belan M, Duval K, Jean-Denis F, et al. . Anthropometric and lifestyle changes in male partners of infertile couples in which the women is obese are associated with pregnancy – preliminary results. Can J Diabetes. 2015;39(6):530. [Google Scholar]
- 123. Cavaco JE, Laurentino SS, Barros A, Sousa M, Socorro S. Estrogen receptors alpha and beta in human testis: both isoforms are expressed. Syst Biol Reprod Med. 2009;55(4):137–144. [DOI] [PubMed] [Google Scholar]
- 124. Tsai EC, Matsumoto AM, Fujimoto WY, Boyko EJ. Association of bioavailable, free, and total testosterone with insulin resistance: influence of sex hormone-binding globulin and body fat. Diabetes Care. 2004;27(4):861–868. [DOI] [PubMed] [Google Scholar]
- 125. Donkin I, Versteyhe S, Ingerslev LR, et al. . Obesity and bariatric surgery drive epigenetic variation of spermatozoa in humans. Cell Metab. 2016;23(2):369–378. [DOI] [PubMed] [Google Scholar]
- 126. Sidorkiewicz I, Zaręba K, Wołczyński S, Czerniecki J.. Endocrine-disrupting chemicals—Mechanisms of action on male reproductive system. Toxicol Ind Health. 2017;33(7):601–609. [DOI] [PubMed] [Google Scholar]
- 127. Skakkebaek NE, Rajpert-De Meyts E, Main KM. Testicular dysgenesis syndrome: an increasingly common developmental disorder with environmental aspects. Hum Reprod. 2001;16(5):972–978. [DOI] [PubMed] [Google Scholar]
- 128. Skakkebaek NE, Rajpert-De Meyts E, Buck Louis GM, et al. . Male reproductive disorders and fertility trends: influences of environment and genetic susceptibility. Physiol Rev. 2016;96(1):55–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Sharma A, Mollier J, Brocklesby RWK, Caves C, Jayasena CN, Minhas S. Endocrine-disrupting chemicals and male reproductive health. Reprod Med Biol. 2020;19(3):243–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Bonde JP, Flachs EM, Rimborg S, et al. . The epidemiologic evidence linking prenatal and postnatal exposure to endocrine disrupting chemicals with male reproductive disorders: a systematic review and meta-analysis. Hum Reprod Update. 2016;23(1):104–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Anderson RA, Willis BR, Oswald C, Zaneveld LJD. Male reproductive tract sensitivity to ethanol: a critical overview. Pharmacol Biochem Behav. 1983;18:305–310. [DOI] [PubMed] [Google Scholar]
- 132. Jayasena C, Sharma A, Abbara A, et al. . Burdens and awareness of adverse self‐reported lifestyle factors in men with sub‐fertility: A cross‐sectional study in 1149 men. Clin Endocrinol (Oxf). 2020;93(3):312–321. [DOI] [PubMed] [Google Scholar]
- 133. Evans HJ, Fletcher J, Torrance M, Hargreave TB. Sperm abnormalities and cigarette smoking. Lancet. 1981;1(8221):627–629. [DOI] [PubMed] [Google Scholar]
- 134. Close CE, Roberts PL, Berger RE. Cigarettes, alcohol and marijuana are related to pyospermia in infertile men. J Urol. 1990;144(4):900–903. [DOI] [PubMed] [Google Scholar]
- 135. Bracken MB, Eskenazi B, Sachse K, McSharry JE, Hellenbrand K, Leo-Summers L. Association of cocaine use with sperm concentration, motility, and morphology. Fertil Steril. 1990;53(2):315–322. [DOI] [PubMed] [Google Scholar]
- 136. Braga DPdAF, Halpern G, Figueira RdCS, Setti AS, Iaconelli A, Borges E. Food intake and social habits in male patients and its relationship to intracytoplasmic sperm injection outcomes. Fertil Steril. 2012;97(1):53–59. [DOI] [PubMed] [Google Scholar]
- 137. Li Y, Lin H, Li Y, Cao J. Association between socio-psycho-behavioral factors and male semen quality: systematic review and meta-analyses. Fertil Steril. 2011;95(1):116–123. [DOI] [PubMed] [Google Scholar]
- 138. Hart RJ, Doherty DA, McLachlan RI, et al. . Testicular function in a birth cohort of young men. Hum Reprod. 2015;30(12):2713–2724. [DOI] [PubMed] [Google Scholar]
- 139. Jayasena CN, Sharma A, Abbara A, et al. . Burdens and awareness of adverse self-reported lifestyle factors in men with sub-fertility: a cross-sectional study in 1149 men. Clin Endocrinol. 2020; Doi: 10.1111/cen.14213. [DOI] [PubMed] [Google Scholar]
- 140. Boeri L, Capogrosso P, Ventimiglia E, et al. . Heavy cigarette smoking and alcohol consumption are associated with impaired sperm parameters in primary infertile men. Asian J Androl. 2019;21(5):478–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Gaur DS, Talekar MS, Pathak VP. Alcohol intake and cigarette smoking: impact of two major lifestyle factors on male fertility. Indian J Pathol Microbiol. 2010;53(1):35–40. [DOI] [PubMed] [Google Scholar]
- 142. Sharma R, Harlev A, Agarwal A, Esteves SC. Cigarette smoking and semen quality: a new meta-analysis examining the effect of the 2010 World Health Organization laboratory methods for the examination of human semen. Eur Urol. 2016;70(4):635–645. [DOI] [PubMed] [Google Scholar]
- 143. Mitra A, Chakraborty B, Mukhopadhay D, et al. . Effect of smoking on semen quality, FSH, testosterone level, and CAG repeat length in androgen receptor gene of infertile men in an Indian city. Syst Biol Reprod Med. 2012;58(5):255–262. [DOI] [PubMed] [Google Scholar]
- 144. Waylen AL, Metwally M, Jones GL, Wilkinson AJ, Ledger WL. Effects of cigarette smoking upon clinical outcomes of assisted reproduction: a meta-analysis. Hum Reprod Update. 2009;15(1):31–44. [DOI] [PubMed] [Google Scholar]
- 145. Anderson RA Jr, Willis BR, Oswald C, Zaneveld LJ. Ethanol-induced male infertility: impairment of spermatozoa. J Pharmacol Exp Ther. 1983;225(2):479–486. [PubMed] [Google Scholar]
- 146. Ricci E, Al Beitawi S, Cipriani S, et al. . Semen quality and alcohol intake: a systematic review and meta-analysis. Reprod Biomed Online. 2017;34(1):38–47. [DOI] [PubMed] [Google Scholar]
- 147. Jensen TK, Gottschau M, Madsen JO, et al. . Habitual alcohol consumption associated with reduced semen quality and changes in reproductive hormones; a cross-sectional study among 1221 young Danish men. BMJ Open. 2014;4(9):e005462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Muthusami KR, Chinnaswamy P. Effect of chronic alcoholism on male fertility hormones and semen quality. Fertil Steril. 2005;84(4):919–924. [DOI] [PubMed] [Google Scholar]
- 149. Safarinejad MR, Asgari SA, Farshi A, et al. . The effects of opiate consumption on serum reproductive hormone levels, sperm parameters, seminal plasma antioxidant capacity and sperm DNA integrity. Reprod Toxicol. 2013;36:18–23. [DOI] [PubMed] [Google Scholar]
- 150. du Plessis SS, Agarwal A, Syriac A. Marijuana, phytocannabinoids, the endocannabinoid system, and male fertility. J Assist Reprod Genet. 2015;32(11):1575–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Dohle GR, Smit M, Weber RF. Androgens and male fertility. World J Urol. 2003;21(5):341–345. [DOI] [PubMed] [Google Scholar]
- 152. Shankara-Narayana N, Yu C, Savkovic S, et al. . Rate and extent of recovery from reproductive and cardiac dysfunction due to androgen abuse in men. J Clin Endocrinol Metab. 2020;105(6):1827–1839. [DOI] [PubMed] [Google Scholar]
- 153. Skorupskaite K, George JT, Anderson RA. The kisspeptin-GnRH pathway in human reproductive health and disease. Hum Reprod Update. 2014;20(4):485–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Subirán N, Casis L, Irazusta J. Regulation of male fertility by the opioid system. Mol Med. 2011;17(7–8): 846–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Dias TR, Alves MG, Bernardino RL, et al. . Dose-dependent effects of caffeine in human Sertoli cells metabolism and oxidative profile: relevance for male fertility. Toxicology. 2015;328(1):12–20. [DOI] [PubMed] [Google Scholar]
- 156. Ricci E, Viganò P, Cipriani S, et al. . Coffee and caffeine intake and male infertility: a systematic review. Nutr J. 2017;16(1):37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Vaamonde D, Da Silva-Grigoletto ME, García-Manso JM, Barrera N, Vaamonde-Lemos R. Physically active men show better semen parameters and hormone values than sedentary men. Eur J Appl Physiol. 2012;112(9):3267–3273. [DOI] [PubMed] [Google Scholar]
- 158. Jóźków P, Rossato M. The impact of intense exercise on semen quality. Am J Men’s Health. 2016;11(3):654–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Jung A, Strauss P, Lindner HJ, Schuppe HC. Influence of moderate cycling on scrotal temperature. Int J Androl. 2008;31(4):403–407. [DOI] [PubMed] [Google Scholar]
- 160. Lotti F, Maggi M. Ultrasound of the male genital tract in relation to male reproductive health. Hum Reprod Update. 2015;21(1):56–83. [DOI] [PubMed] [Google Scholar]
- 161. Fleseriu M, Hashim IA, Karavitaki N, et al. . Hormonal replacement in hypopituitarism in adults: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2016;101(11):3888–3921. [DOI] [PubMed] [Google Scholar]
- 162. Sartorius G, Spasevska S, Idan A, et al. . Serum testosterone, dihydrotestosterone and estradiol concentrations in older men self-reporting very good health: the healthy man study. Clin Endocrinol (Oxf). 2012;77(5):755–763. [DOI] [PubMed] [Google Scholar]
- 163. Lehtihet M, Arver S, Bartuseviciene I, Pousette A. S-testosterone decrease after a mixed meal in healthy men independent of SHBG and gonadotrophin levels. Andrologia. 2012;44(6):405–410. [DOI] [PubMed] [Google Scholar]
- 164. Brambilla DJ, Matsumoto AM, Araujo AB, McKinlay JB. The effect of diurnal variation on clinical measurement of serum testosterone and other sex hormone levels in men. J Clin Endocrinol Metab. 2009;94(3):907–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Martin-du-Pan RC, Bischof P. Increased follicle stimulating hormone in infertile men. Is increased plasma FSH always due to damaged germinal epithelium? Hum Reprod. 1995;10(8):1940–1945. [DOI] [PubMed] [Google Scholar]
- 166. Schoor RA, Elhanbly S, Niederberger CS, Ross LS. The role of testicular biopsy in the modern management of male infertility. J Urol. 2002;167(1):197–200. [PubMed] [Google Scholar]
- 167. Sikaris K, McLachlan RI, Kazlauskas R, de Kretser D, Holden CA, Handelsman DJ. Reproductive hormone reference intervals for healthy fertile young men: evaluation of automated platform assays. J Clin Endocrinol Metab. 2005;90(11):5928–5936. [DOI] [PubMed] [Google Scholar]
- 168. McLachlan RI, Rajpert-De Meyts E, Hoei-Hansen CE, de Kretser DM, Skakkebaek NE. Histological evaluation of the human testis – approaches to optimizing the clinical value of the assessment: mini review. Hum Reprod. 2007;22(1):2–16. [DOI] [PubMed] [Google Scholar]
- 169. Tournaye H, Krausz C, Oates RD. Concepts in diagnosis and therapy for male reproductive impairment. Lancet Diabetes Endocrinol. 2016;5(7):554–564. [DOI] [PubMed] [Google Scholar]
- 170. Inhorn MC, Patrizio P. Infertility around the globe: new thinking on gender, reproductive technologies and global movements in the 21st century. Hum Reprod Update. 2015;21(4):411–426. [DOI] [PubMed] [Google Scholar]
- 171. Vander Borght M, Wyns C. Fertility and infertility: definition and epidemiology. Clin Biochem. 2018;62:2–10. [DOI] [PubMed] [Google Scholar]
- 172. Stanford JB, Dunson DB. Effects of sexual intercourse patterns in time to pregnancy studies. Am J Epidemiol. 2007;165(9):1088–1095. [DOI] [PubMed] [Google Scholar]
- 173. Evers JL. Female subfertility. Lancet. 2002;360(9327):151–159. [DOI] [PubMed] [Google Scholar]
- 174. Schoysman R, Gerris J. Twelve-year follow-up study of pregnancy rates in 1291 couples with idiopathically impathically impaired male fertility. Acta Eur Fertil. 1983;14(1):51–56. [PubMed] [Google Scholar]
- 175. Gnoth C, Godehardt E, Frank-Herrmann P, Friol K, Tigges J, Freundl G. Definition and prevalence of subfertility and infertility. Hum Reprod. 2005;20(5):1144–1147. [DOI] [PubMed] [Google Scholar]
- 176. Anderson RA Jr, Willis BR, Oswald C, Zaneveld LJ. Partial reversal of ethanol-induced male reproductive pathology following abstinence. Alcohol Alcohol. 1985;20(3):273–286. [PubMed] [Google Scholar]
- 177. Oyeyipo I, Raji Y, Bolarinwa A. Nicotine alters male reproductive hormones in male albino rats: the role of cessation. J Hum Reprod Sci. 2013;6(1):40–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Prentki Santos E, López-Costa S, Chenlo P, et al. . Impact of spontaneous smoking cessation on sperm quality: case report. Andrologia. 2011;43(6):431–435. [DOI] [PubMed] [Google Scholar]
- 179. Teskereci G, Oncel S. Effect of lifestyle on quality of life of couples receiving infertility treatment. J Sex Marital Ther. 2013;39(6):476–492. [DOI] [PubMed] [Google Scholar]
- 180. Vanegas JC, Chavarro JE, Williams PL, et al. . Discrete survival model analysis of a couple’s smoking pattern and outcomes of assisted reproduction. Fertil Res Pract. 2017;3(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Legro RS, Kunselman AR, Meadows JW, et al. . Time-related increase in urinary testosterone levels and stable semen analysis parameters after bariatric surgery in men. Reprod Biomed Online. 2015;30(2):150–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Wei Y, Chen Q, Qian W. Effect of bariatric surgery on semen parameters: a systematic review and meta-analysis. Med Sci Monit Basic Res. 2018;24:188–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Mir J, Franken D, Andrabi SW, Ashraf M, Rao K. Impact of weight loss on sperm DNA integrity in obese men. Andrologia. 2018;50(4):e12957. [DOI] [PubMed] [Google Scholar]
- 184. Ibañez-Perez J, Santos-Zorrozua B, Lopez-Lopez E, Matorras R, Garcia-Orad A. An update on the implication of physical activity on semen quality: a systematic review and meta-analysis. Arch Gynecol Obstet. 2019;299(4):901–921. [DOI] [PubMed] [Google Scholar]
- 185. Corona G, Ratrelli G, Maggi M. The pharmacotherapy of male hypogonadism besides androgens. Expert Opin Pharmacother. 2015;16(3):369–387. [DOI] [PubMed] [Google Scholar]
- 186. Attia AM, Abou-Setta AM, Al-Inany HG, Attia AM. Gonadotrophins for idiopathic male factor subfertility. Cochrane Database System Rev. 2013; 2013(8):1–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Cocci A, Cito G, Russo GI, et al. . Effectiveness of highly purified urofollitropin treatment in patients with idiopathic azoospermia before testicular sperm extraction. Urologia. 2018;85(1):19–21. [DOI] [PubMed] [Google Scholar]
- 188. Santi D, Granata AR, Simoni M. FSH treatment of male idiopathic infertility improves pregnancy rate: a meta-analysis. Endocr Connect. 2015;4(3):R46–R58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Del Giudice F, Busetto G, De Berardinis E, et al. . A systematic review and meta-analysis of clinical trials implementing aromatase inhibitors to treat male infertility. Asian J Androl. 2020;22(4):360–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Chua ME, Escusa KG, Luna S, Tapia LC, Dofitas B, Morales M. Revisiting oestrogen antagonists (clomiphene or tamoxifen) as medical empiric therapy for idiopathic male infertility: a meta-analysis. Andrology. 2013;1(5):749–757. [DOI] [PubMed] [Google Scholar]
- 191. Cannarella R, Condorelli RA, Mongioì LM, Barbagallo F, Calogero AE, La Vignera S. Effects of the selective estrogen receptor modulators for the treatment of male infertility: a systematic review and meta-analysis. Expert Opin Pharmacother. 2019;20(12):1517–1525. [DOI] [PubMed] [Google Scholar]
- 192. Cavallini G, Biagiotti G, Bolzon E. Multivariate analysis to predict letrozole efficacy in improving sperm count of non-obstructive azoospermic and cryptozoospermic patients: a pilot study. Asian J Androl. 2013;15(6):806–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Ko EY, Siddiqi K, Brannigan RE, Sabanegh ES. Empirical medical therapy for idiopathic male infertility: a survey of the American Urological Association. J Urol. 2012;187(3):973–978. [DOI] [PubMed] [Google Scholar]
- 194. Birzniece V. Gonadal steroids and body composition, strength, and sexual function in men. N Engl J Med. 2013;369(25):2455–2457. [DOI] [PubMed] [Google Scholar]
- 195. Sartorius GA, Ly LP, Handelsman DJ. Male sexual function can be maintained without aromatization: randomized placebo-controlled trial of dihydrotestosterone (DHT) in healthy, older men for 24 months. J Sex Med. 2014;11(10):2562–2570. [DOI] [PubMed] [Google Scholar]
- 196. Vanderschueren D, Laurent MR, Claessens F, et al. . Sex steroid actions in male bone. Endocr Rev. 2014;35(6):906–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Anawalt BD. Approach to male infertility and induction of spermatogenesis. J Clin Endocrinol Metab. 2013;98(9):3532–3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Hussein A, Ozgok Y, Ross L, Rao P, Niederberger C. Optimization of spermatogenesis-regulating hormones in patients with non-obstructive azoospermia and its impact on sperm retrieval: a multicentre study. BJU Int. 2013;111(3 Pt B):E110–E114. [DOI] [PubMed] [Google Scholar]
- 199. Coward RM, Mills JN. A step-by-step guide to office-based sperm retrieval for obstructive azoospermia. Transl Androl Urol. 2017;6(4):730–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Bernie AM, Mata DA, Ramasamy R, Schlegel PN. Comparison of microdissection testicular sperm extraction, conventional testicular sperm extraction, and testicular sperm aspiration for nonobstructive azoospermia: a systematic review and meta-analysis. Fertil Steril. 2015;104(5):1099–1103.e3. [DOI] [PubMed] [Google Scholar]
- 201. Corona G, Minhas S, Giwercman A, et al. . Sperm recovery and ICSI outcomes in men with non-obstructive azoospermia: a systematic review and meta-analysis. Hum Reprod Update. 2019;25(6):733–757. [DOI] [PubMed] [Google Scholar]
- 202. Corona G, Pizzocaro A, Lanfranco F, et al. ; Klinefelter ItaliaN Group (KING) . Sperm recovery and ICSI outcomes in Klinefelter syndrome: a systematic review and meta-analysis. Hum Reprod Update. 2017;23(3):265–275. [DOI] [PubMed] [Google Scholar]
- 203. Nodar F, De Vincentiis S, Olmedo SB, Papier S, Urrutia F, Acosta AA. Birth of twin males with normal karyotype after intracytoplasmic sperm injection with use of testicular spermatozoa from a nonmosaic patient with Klinefelter’s syndrome. Fertil Steril. 1999;71(6):1149–1152. [DOI] [PubMed] [Google Scholar]
- 204. Degl’Innocenti S, Filimberti E, Magini A, et al. . Semen cryopreservation for men banking for oligospermia, cancers, and other pathologies: prediction of post-thaw outcome using basal semen quality. Fertil Steril. 2013;100(6):1555–63.e1. [DOI] [PubMed] [Google Scholar]
- 205. Hessel M, Robben JC, D’Hauwers KW, Braat DD, Ramos L. The influence of sperm motility and cryopreservation on the treatment outcome after intracytoplasmic sperm injection following testicular sperm extraction. Acta Obstet Gynecol Scand. 2015;94(12):1313–1321. [DOI] [PubMed] [Google Scholar]
- 206. Eliveld J, van Wely M, Meißner A, Repping S, van der Veen F, van Pelt AMM. The risk of TESE-induced hypogonadism: a systematic review and meta-analysis. Hum Reprod Update. 2018;24(4):442–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Thompson JG, Kind KL, Roberts CT, Robertson SA, Robinson JS. Epigenetic risks related to assisted reproductive technologies: short- and long-term consequences for the health of children conceived through assisted reproduction technology: more reason for caution? Hum Reprod. 2002;17(11):2783–2786. [DOI] [PubMed] [Google Scholar]
- 208. Davies MJ, Moore VM, Willson KJ, et al. . Reproductive technologies and the risk of birth defects. N Engl J Med. 2012;366(19):1803–1813. [DOI] [PubMed] [Google Scholar]
- 209. Van Steirteghem A, Bonduelle M, Devroey P, Liebaers I. Follow-up of children born after ICSI. Hum Reprod Update. 2002;8(2):111–116. [DOI] [PubMed] [Google Scholar]
- 210. Bonduelle M, Legein J, Derde MP, et al. . Comparative follow-up study of 130 children born after intracytoplasmic sperm injection and 130 children born after in-vitro fertilization. Hum Reprod. 1995;10(12):3327–3331. [DOI] [PubMed] [Google Scholar]
- 211. Belva F, De Schrijver F, Tournaye H, et al. . Neonatal outcome of 724 children born after ICSI using non-ejaculated sperm. Hum Reprod. 2011;26(7):1752–1758. [DOI] [PubMed] [Google Scholar]
- 212. Fedder J, Loft A, Parner ET, Rasmussen S, Pinborg A. Neonatal outcome and congenital malformations in children born after ICSI with testicular or epididymal sperm: a controlled national cohort study. Hum Reprod. 2013;28(1):230–240. [DOI] [PubMed] [Google Scholar]
- 213. Jin L, Li Z, Gu L, Huang B. Neonatal outcome of children born after ICSI with epididymal or testicular sperm: a 10-year study in China. Sci Rep. 2020;10(1):5145–5152. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article, as no datasets were generated or analyzed during the current study.