Abstract
Nowadays, apart from having to know first-line Helicobacter pylori eradication regimens well, we must also be prepared to face treatment failures. The aim of this review is to summarize the role of rifabutin in the management of H. pylori infection. Bibliographical searches were performed in PubMed. Data on resistance and efficacy of rifabutin-containing regimens on H. pylori eradication were meta-analyzed. Mean H. pylori rifabutin resistance rate (39 studies, including 9721 patients) was 0.13%; when studies only including patients naïve to H. pylori eradication treatment were considered, this figure was even lower (0.07%). Mean H. pylori eradication rate (by intention-to-treat) with rifabutin-containing regimens (3052 patients) was 73%. Respective cure rates for second-, third-, fourth- and fifth-line therapies, were 79%, 69%, 69% and 72%. Most studies administered rifabutin 300 mg/day, which seemed to be more effective than 150 mg/day. The ideal length of treatment remains unclear, but 10–12-day regimens are generally recommended. Adverse events to rifabutin treatment in H. pylori studies were relatively infrequent (15%), and severe adverse events were exceptional (myelotoxicity was the most significant, although always reversible). In summary, rifabutin-containing therapy represents an encouraging strategy generally restricted, at present, to patients where previous (usually multiple) eradication regimens have failed.
Keywords: Helicobacter pylori, H. pylori, rifabutin, treatment, rescue
1. Introduction
Helicobacter pylori (H. pylori) is a worldwide infection that is the main cause not only of gastritis, but also of peptic ulcer disease and gastric cancer [1]. The most commonly used first-line therapies―combining a proton pump inhibitor (PPI) plus two antibiotics, generally including clarithromycin and either amoxicillin or metronidazole―currently fail in more than 20–30% of cases [2]. The major factor affecting our ability to eradicate H. pylori infection is antibiotic resistance, mainly to clarithromycin, which is increasing in many geographic areas [3].
At present, a rescue regimen including a quadruple combination of a PPI, bismuth, tetracycline, and metronidazole is generally used as the optimal second-line approach after initial H. pylori eradication failure [4,5,6]. However, this treatment fails to eradicate the infection in at least 20% of cases [7,8,9,10]. In addition, this regimen is associated with a high incidence of adverse events, is relatively complex, and many countries are currently experiencing a general unavailability of tetracycline and/or bismuth. On the other hand, quinolone-based rescue regimens, mainly including levofloxacin, achieve a mean eradication rate of only approximately 80%, probably due to the increasing H. pylori resistance to quinolones [7,8,9,11,12,13].
Consequently, in some cases, H. pylori infection persists even after two or more eradication treatments, and these patients constitute a therapeutic dilemma. Currently, a standard third/fourth-line therapy is lacking, and international guidelines recommend performing culture in these patients to select a rescue treatment according to microbial sensitivity to antibiotics, but this strategy is not practical [14]. Therefore, the evaluation of drugs without cross-resistance to macrolides, nitroimidazole or quinolones as components of retreatment combination therapies seems to be worthwhile [15,16].
Rifabutin is a rifamycin-S derivative, commonly used to treat Mycobacterium avium and Mycobacterium intracellulare, also referred to as Mycobacterium avium-intracellulare complex (MAC) in human immunodeficiency virus (HIV)-infected patients [17,18]. The in vitro sensitivity of H. pylori to this antibiotic is high, and it does not share resistance to clarithromycin, metronidazole or levofloxacin [15,16,19,20]. Furthermore, the selection of resistant H. pylori strains has been low in experimental conditions. Consequently, rifabutin-based rescue therapies represent a potential and attractive strategy for H. pylori eradication failures [21].
The aim of the present review is to summarize the role of rifabutin in the management of H. pylori infection. We will review the following topics: (1) rifabutin’s general antimicrobial activity and mechanism of action; (2) pharmacokinetics and pharmacodynamics of rifabutin; (3) prevalence of resistance of H. pylori to rifabutin; (4) efficacy of rifabutin for H. pylori eradication; (5) optimization strategies aimed to increase the efficacy of rifabutin-based therapies; (6) safety of rifabutin; and, finally, (7) limitations of rifabutin for the treatment of H. pylori infection.
2. Bibliographic Search and Statistical Analysis
A systematic bibliographic search was performed to identify studies evaluating the role of rifabutin in the management of H. pylori infection. An electronic search was conducted in PubMed up to October 2020 using the following algorithm (all fields): rifabutin AND (Helicobacter pylori OR H. pylori). In addition, the reference lists from the selected articles were reviewed to identify additional studies of potential interest. Articles published in any language were included.
For each study, the variables extracted evaluating the prevalence of rifabutin resistance were as follows: author, year of publication, country, pre- or post-treatment setting, number of patients, and resistance rate. On the other hand, the variables extracted evaluating the efficacy of rifabutin-containing treatments for each study were: author, publication year, country, therapy combination (drugs and doses), treatment duration, number of included patients, number of (previous) failed treatments, type of (previous) failed treatments, eradication rate, and adverse events rate.
The main outcome of interest was the successful eradication of H. pylori at least four weeks after completion of treatment, as assessed by at least one reliable diagnostic method (that is, histology, urea breath test or monoclonal stool antigen test); we excluded studies that used only serology testing for the detection of H. pylori. Analysis of H. pylori eradication efficacy was considered on an intention-to-treat basis (that is, including all eligible patients enrolled in the study regardless of compliance with the study protocol; patients with unevaluable data were assumed to have been unsuccessfully treated). Subanalyses of the data were performed by number of previous failed treatments and by duration of the rifabutin regimen.
Data of variables (including rifabutin resistance, efficacy, and safety of rifabutin-containing regimens on H. pylori eradication) were combined using the generic inverse variance method, which involves a weighted average of the effect estimates from the individual studies. The meta-analysis was performed using Review Manager (Cochrane Collaboration). If the results were homogeneous, a fixed effect model was used, and if the results were heterogeneous (I2 > 50%), a random effect model (DerSimonian and Laird) was applied.
3. Rifabutin General Antimicrobial Activity and Mechanism of Action
Rifabutin is structurally related to, and shares many of the properties of, rifampin (rifampicin) [22]. Rifabutin has a broad spectrum of antimicrobial activity, against mycobacteria, a variety of gram-positive and gram-negative bacteria, Toxoplasma gondii, and Chlamydia trachomatis. It is also active against Mycobacterium leprae, Mycobacterium tuberculosis, and atypical mycobacteria [22].
Rifabutin is generally more active in vitro than rifampicin against rifampicin-susceptible isolates of M. tuberculosis. It is also active against some rifampicin-resistant isolates, although there is substantial cross-resistance in vitro between the two drugs [23].
Rifabutin inhibits the β-subunit of H. pylori DNA-dependent RNA polymerase encoded by the rpoB gene [24]. Laboratory mutants of H. pylori, which are obtained after multiple serial passages in vitro and exhibit amino acid exchanges in codons 524 to 545 or in codon 585 of the rpoB gene, are resistant to rifabutin [24,25,26]. As an example, a low rate of resistance (0.24%) to rifabutin was noted in H. pylori strains isolated from 414 Japanese patients, and the only rifabutin resistant strain detected showed a point mutation in the rpoB gene and was isolated from a patient with a history of rifampin treatment for pulmonary tuberculosis [27].
It has been shown that past rifampicin usage is closely linked to high minimal inhibitory concentrations (MICs) of rifabutin and the prevalence of point mutations in rpoB gene, suggesting a cross-resistance between both antibiotics [28]. Therefore, although rifabutin is a potent candidate antibiotic for H. pylori eradication as a rescue option, careful consideration must be given to rifampicin treatment history prior to H. pylori eradication with rifabutin [25,28].
4. Pharmacokinetics and Pharmacodinamics of Rifabutin
Rifabutin is a highly lipid-soluble compound with a pKa of 6.9. It is well absorbed when given orally and exhibits high tissue-to-plasma ratios [22]. High concentrations of the drug are attained in all tissues, particularly those of the liver and lung [22].
Rifabutin is chemically stable at a wide pH range and its antibacterial activity is likely not to be hampered by the acidic environment of the stomach [22,24,29,30]. In one in vivo study performed in rats, the concentrations of rifabutin in gastric juice were 10 to 17 times higher than in blood, indicating extensive secretion into the stomach [31].
Mean absolute bioavailability is about 20% after a single dose [23]. When rifabutin is administered with food, its absorption is delayed but not decreased [23]. The apparent volume of distribution is about 8 to 9 L/kg, and the extent of binding to plasma proteins varies between 71% and 94% [23].
Rifabutin is extensively metabolized [23,32]. The mean plasma elimination half-life ranges from 32 to 67 h [23]. Although the pharmacokinetic properties of rifabutin are influenced by hepatic and renal impairment, dosage alteration is probably required only in patients with severe renal or hepatic dysfunction [23]. Rifabutin metabolism in liver microsomes may be inhibited by clarithromycin [33].
5. Resistance of H. pylori to Rifabutin
Antibiotic resistance to H. pylori is the main variable affecting the efficacy of current therapeutic regimens, and it is increasing worldwide [3]. Rifabutin shows good intracellular activity against H. pylori [34]. H. pylori has proven to be highly susceptible in vitro to rifabutin [23,24,29]. At present, the methodology to identify rifabutin resistance is mainly based on microbiological testing. The in vitro MIC of rifabutin against H. pylori [22,29,35] was remarkably lower than that found for amoxicillin, clarithromycin, and metronidazole [22,35].
Rifabutin is generally used to cure or prevent MAC disease in patients with advanced HIV infection, and for this reason, the secondary resistance of H. pylori to rifabutin is likely to be absent in the general healthy population [36]. Nevertheless, rifabutin resistance has generally been reported only after the administration of high doses for extended durations, such as those required for the treatment of mycobacterial infections. In this respect, the efficacy of rifabutin-containing regimens to treat MAC infection appear to be uninfluenced by prior prophylactic administration of the drug to patients with acquired immune deficiency syndrome (AIDS) [23].
The majority of rifampicin-resistant strains are isolated from patients after treatment failures, suggesting that previous, unsuccessful attempts of eradication seem to be an important risk factor for the development of rifabutin resistance and/or multiresistance [37,38]. In particular, some reports have documented the (exceptional) possibility of resistance to rifabutin, which was largely explained by previous exposure to this antibiotic [28].
An important concern regarding widespread use of rifabutin for treatment of H. pylori infection is the emergence of resistance in Mycobacterium tuberculosis. However, no correlation has been reported between short-term use of rifabutin for treatment of non-tuberculosis infections, such as those of H. pylori, and emergence of resistance [24,39]. Moreover, prior experience has shown that the risk of development of antimicrobial resistance with H. pylori is further reduced when it is used with another antimicrobial agent such as amoxicillin [40].
By analogy with clarithromycin resistance, it has been hypothesized that the presence of multiple strains of H. pylori, resistant and/or susceptible to the same antibiotic, in the same patient is possible [41]. Thus, several studies have reported the coexistence of the strains of H. pylori that determine the mixed pattern in the same patient [42]. In addition, the presence of H. pylori hetero-resistance between two biopsy sites (antrum and fundus) was recently shown in one-third of patients with secondary resistance, suggesting the necessity, in these cases, to perform an antimicrobial susceptibility test from both sites of the stomach [43]. Some authors have assessed the relationship between the presence of mixed infection of H. pylori and both antimicrobial susceptibility and virulence markers [44]. When different bacteria were isolated in the host, they showed a resistance to rifabutin (10%) that was higher than that of bacteria in single infection (4%) [44].
A recent systematic review of studies, published from 2006 to 2009, on primary H. pylori antibiotic resistance demonstrated that the prevalence rate of rifabutin resistance was very low (1.4%), but only two studies were included in the analysis [45]. The prevalence of primary antibiotic resistance of H. pylori in 2008 and 2009 in 18 European countries was assessed in another study, and the rate was 1.1% in adults [3]. More recently, in 2015, Ghotaslou et al. reviewed previous studies (published during the last six years) about the H. pylori antibiotic resistance, and the rifabutin resistance rate was relatively high (6.7%) [46]. However, in this review, rifampicin and rifabutin resistance rates were not clearly separated; while both antibiotics are structurally related and share many properties [22], and there is substantial cross-resistance in vitro between the two drugs [23], rifabutin is generally more active in vitro [23,38]. The highest (outlier) result was reported in one study where H. pylori eradication treatment was previously prescribed and only 16 patients were included [47].
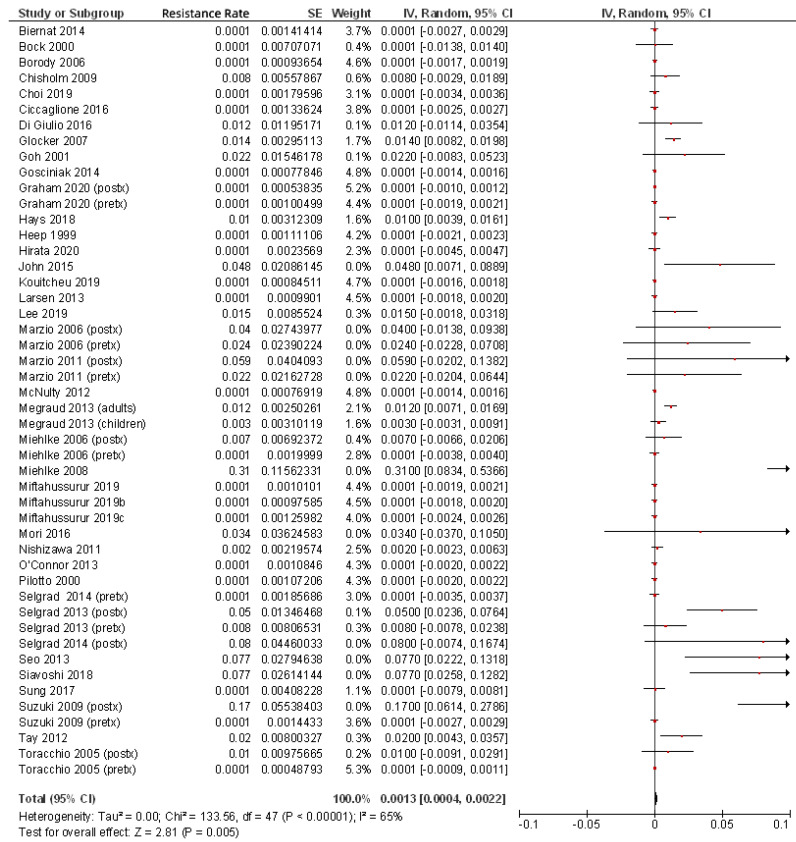
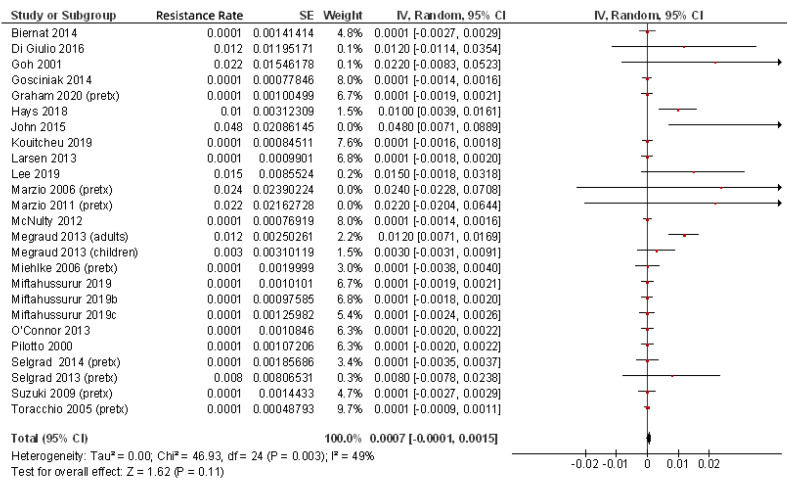
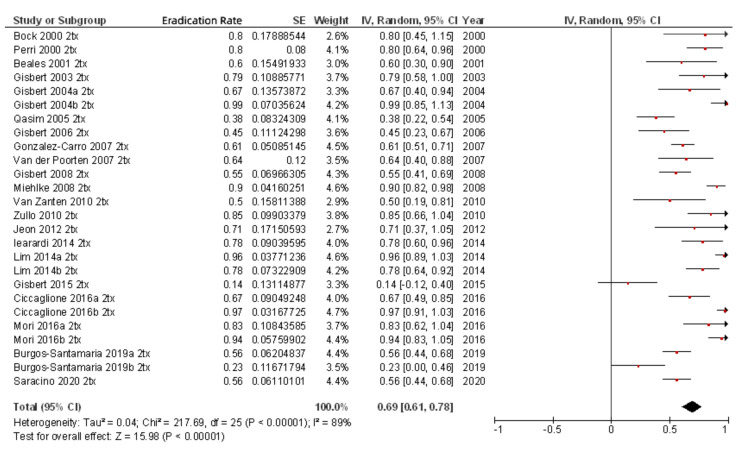
Finally, for the present review of the most up to date literature, we summarized in Table 1 all the studies that have evaluated H. pylori rifabutin resistance rates [3,24,25,27,28,38,41,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78]. From these 39 studies, including a total of 9721 patients, an overall rifabutin resistance rate of 0.13% was calculated (Figure 1); however, when only studies including pre-treatment patients (that is, naïve to H. pylori eradication treatment) were considered, this figure was even lower (0.07%), as is summarized in Figure 2. Continuous audit of the resistance figures in several populations is needed to keep rifabutin resistance information updated.
Table 1.
Resistance of H. pylori to rifabutin.
| Author | Year | Country | Pre-Treatment (Naïve) or Post-Treatment Setting | Number of Patients | Resistance Rate (%) |
|---|---|---|---|---|---|
| Heep [24] | 1999 | Germany | Post (mostly) | 81 | 0 |
| Bock [48] | 2000 | Germany | Post (R) | 2 | 0 |
| Pilotto [49] | 2000 | Italy | Pre | 87 | 0 |
| Toracchio [41] | 2005 | Italy | Pre | 420 | 0 |
| Toracchio [41] | 2005 | Italy | Post (C and M; no R) | 104 | 1 |
| Marzio [50] | 2006 | Italy | Pre | 41 | 2.4 |
| Marzio [50] | 2006 | Italy | Post | 51 | 4 |
| Miehlke [51] | 2006 | Germany | Pre | 145 | 0.7 |
| Miehlke [51] | 2006 | Germany | Post (C and M; no R) | 25 | 0 |
| Borody [52] | 2006 | Australia | Post | 114 | 0 |
| Suzuki [28] | 2009 | Japan | Pre | 48 | 0 |
| Suzuki [28] | 2009 | Japan | Post | 46 | 17 |
| Chisholm [53] | 2007 | UK | Post (70%) | 255 | 0.8 |
| Glocker [38] | 2007 | Germany | Post (R in some) | 1585 | 1.4 |
| Miehlke [47] | 2008 | Germany | Post (R) | 16 | 31 |
| Goh [54] | 2011 | Malaysia | Pre | 90 | 2.2 |
| Marzio [55] | 2011 | Italy | Pre | 46 | 2.2 |
| Marzio [55] | 2011 | Italy | Post | 34 | 5.9 |
| Nishizawa [27] | 2011 | Japan | Pre and post | 414 | 0.2 |
| McNulty [56] | 2012 | UK | Pre and post | 169 | 0 |
| Tay [57] | 2012 | Australia | Post | 306 | 2 |
| Megraud(adults) [3] | 2013 | Europe | Pre | 1893 | 1.2 |
| Megraud(children) [3] | 2013 | Europe | Pre | 311 | 0.3 |
| O’Connor [58] | 2013 | Ireland | Pre | 85 | 0 |
| Larsen [59] | 2013 | Norway | Pre | 102 | 0 |
| Selgrad [60] | 2013 | Germany | Pre | 122 | 0.8 |
| Selgrad [60] | 2013 | Germany | Post | 262 | 5 |
| Seo [61] | 2013 | Korea | - | 91 | 7.7 |
| Gosciniak [62] | 2014 | Poland | Pre | 165 | 0 |
| Biernat [63] | 2014 | Poland | Pre | 50 | 0 |
| Selgrad [64] | 2014 | Germany | Pre | 29 | 0 |
| Selgrad [64] | 2014 | Germany | Post | 37 | 8 |
| John [65] | 2015 | Qatar | Pre | 105 | 4.8 |
| Di Giulio [66] | 2016 | Italy | Pre | 83 | 1.2 |
| Mori [67] | 2016 | Japan | Post | 25 | 3.4 |
| Ciccaglione [68] | 2016 | Italy | Post | 56 | 0 |
| Sung [69] | 2017 | Korea | Post | 6 | 0 |
| Hays [25] | 2018 | France | Pre and post | 1015 | 1 |
| Siavoshi [70] | 2018 | Iran | - | 104 | 7.7 |
| Choi [71] | 2019 | Korea | Pre and post | 31 | 0 |
| Lee [72] | 2019 | Korea | Pre | 202 | 1.5 |
| Kouitcheu M. [73] | 2019 | Cameroon | Pre | 140 | 0 |
| Miftahussurur [74] | 2019 | Indonesia | Pre | 63 | 0 |
| Miftahussurur [75] | 2019 | Indonesia | Pre | 105 | 0 |
| Miftahussurur [76] | 2019 | Nepal & Bangladesh | Pre | 98 | 0 |
| Graham [77] | 2020 | USA | Pre | 345 | 0 |
| Hirata [78] | 2020 | Japan | Post | 18 | 0 |
| Graham [77] | 2020 | USA | Post | 99 | 0 |
C: clarithromycin; M: metronidazole; R: rifabutin; Pre: pre-treatment (naïve) setting (that is, before H. pylori eradication has been administered); Post: post-treatment setting (that is, when H. pylori eradication has been previously prescribed).
Figure 1.
Resistance of H. pylori to rifabutin including all patients (both pre- and post-treatment).
Figure 2.
Resistance of H. pylori to rifabutin including only patients naïve to H. pylori eradication treatment.
6. Efficacy of Rifabutin for H. pylori Eradication
In 2015, Liu et al. conducted a systematic review and meta-analysis of clinical trials for eradication of H. pylori that included a treatment arm with a PPI, rifabutin, and amoxicillin. Twenty-one studies were included, and the overall eradication rate was 70% (by intention-to-treat) [79]. More recently, Gingold-Belfer et al. performed a systematic review of prospective clinical trials (thus excluding retrospective studies) with a treatment arm consisting of PPI, amoxicillin, and rifabutin, and a meta-analysis of randomized controlled trials [80]. The pooled eradication success in the 33 studies selected for subjects treated with rifabutin triple therapy was 71.8%.
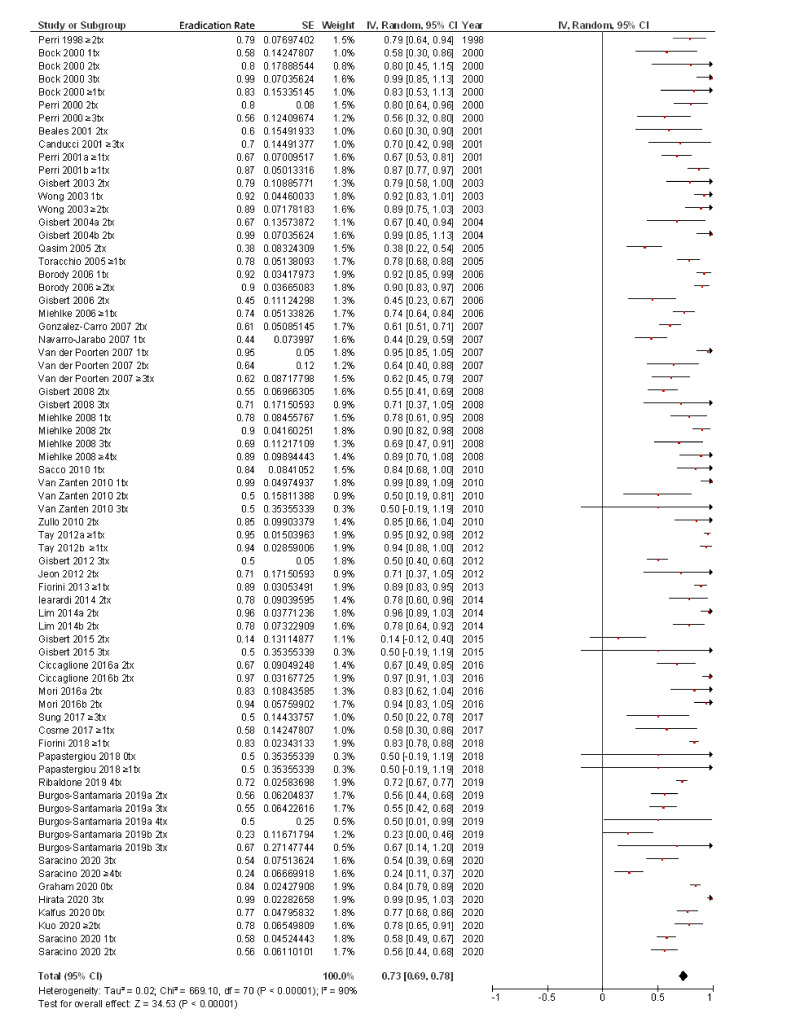
Finally, for the present review, to the best of our knowledge the most updated in the literature, all the studies that have evaluated the efficacy of rifabutin-containing regimens for the treatment of H. pylori infection are summarized in Table 2 [19,20,36,39,41,47,48,51,52,57,67,68,69,77,78,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108]. Overall, from the 3052 patients treated with rifabutin-containing regimens included in Table 2, a mean H. pylori eradication rate (by intention-to-treat analysis) of 73% (95% confidence interval (CI), 69–78%) was calculated (Figure 3). In most cases, rifabutin was administered at doses of 300 mg per day (either as 300 mg once a day or as 150 mg twice daily). Duration of treatment was 7 to 14 days in most of the studies. Almost all the protocols included patients with at least one previous H. pylori eradication attempt. Type of previous (failed) H. pylori eradication treatment varied markedly depending on the study, but in most of the cases included a first-line treatment with a PPI, clarithromycin, and either amoxicillin or nitroimidazole.
Table 2.
Rifabutin-containing therapies for the eradication of H. pylori.
| Author | Year | Country | Drugs and Doses | Duration of Treatment (Days) | Number of Patients | Number of Previous Failed Treatments | Type of Previous Treatments | Eradication Rate ¶ (%) | Adverse Events (%) |
|---|---|---|---|---|---|---|---|---|---|
| Perri [81] | 1998 | Italy | Rifabutin 300 mg/24 h Amoxicillin 1 g/12 h Pantoprazole 40 mg/12 h |
7 | 28 | ≥2 | PPI-containing tx | 79 | 7.1 |
| Bock [48] | 2000 | Germany | Rifabutin 150 mg/12 h Amoxicillin 1 g/12 h Lansoprazole 30 mg/12 h |
7 | 12 | 1 | PPI+A- and C-containing tx | 58 | 0 |
| Bock [48] | 2000 | Germany | Rifabutin 150 mg/12 h Amoxicillin 1 g/12 h Lansoprazole 30 mg/12 h |
7 | 5 | 2 | PPI+A- and C-containing tx | 80 | 0 |
| Bock [48] | 2000 | Germany | Rifabutin 150 mg/12 h Amoxicillin 1 g/12 h Lansoprazole 30 mg/12 h |
7 | 2 | 3 | PPI+A- and C-containing tx | 100 | 0 |
| Bock [48] | 2000 | Germany | Rifabutin 150 mg/12 h Amoxicillin 1 g/12 h Lansoprazole 30 mg/12 h |
7 | 6 | ≥1 | PPI+A- and C-containing tx | 83 | 0 |
| Perri [82] | 2000 | Italy | Rifabutin 300 mg/24 h Amoxicillin 1 g/12 h Pantoprazole 40 mg/12 h |
7 | 25 | 2 | (1) PPI+C+A (2) PPI+C+M, PPI+C+T |
80 | 3 |
| Perri [82] | 2000 | Italy | Rifabutin 300 mg/24 h Amoxicillin 1 g/12 h Pantoprazole 40 mg/12 h |
7 | 16 | ≥3 | (1) PPI+C+A (2) PPI+C+A, PPI+C+M, PPI+C+T 3/(4) PPI+A, PPI+C+M, PPI+A+T, PPI+C+T, RBC+A+T, RBC+C, Q |
56 | 3 |
| Beales [83] § | 2001 | UK | Rifabutin 300 mg/24 h Amoxicillin 1 g/12 h Omeprazole 20 mg/12 h |
14 | 10 | 2 | C- and M-containing tx | 60 | - |
| Canducci [84] | 2001 | Italy | Rifabutin 300 mg/24 h Amoxicillin 1 g/12 h Omeprazole 20 mg/12 h |
10 | 10 | ≥3 | - | 70 | 10 |
| Perri [36] | 2001 | Italy | Rifabutin 150 mg/24 h Amoxicillin 1 g/12 h Pantoprazole 40 mg/12 h |
10 | 45 | ≥1 | PPI+C+A, PPI+C+M, RBC+C | 67 | 9 |
| Perri [36] | 2001 | Italy | Rifabutin 300 mg/24 h Amoxicillin 1 g/12 h Pantoprazole 40 mg/12 h |
10 | 45 | ≥1 | PPI+C+A, PPI+C+M, RBC+C | 87 | 11 |
| Gisbert [85] | 2003 | Spain | Rifabutin 150 mg/12 h Amoxicillin 1 g/12 h Omeprazole 20 mg/12 h |
14 | 14 | 2 | (1) PPI+C+A (2) Q, RBC+T+M |
79 | 36 |
| Wong [86] | 2003 | China | Rifabutin 300 mg/24 h Levofloxacin 500 mg/24 h Rabeprazole 20 mg/12 h |
7 | 37 | 1 | PPI+C+A, PPI+C+M, PPI+A+M | 92 | 34 |
| Wong [86] | 2003 | China | Rifabutin 300 mg/24 h Levofloxacin 500 mg/24 h Rabeprazole 20 mg/12 h |
7 | 19 | ≥2 | C-containing tx (100%), M-containing tx (76%) | 89 | 34 |
| Gisbert [87] | 2004 | Spain | Rifabutin 150 mg/12 h Amoxicillin 1 g/12 h Omeprazole 20 mg/12 h |
14 | 12 | 2 | (1) PPI+C+A (2) RBC+T+M |
67 | - |
| Gisbert [87] | 2004 | Spain | Rifabutin 150 mg/12 h Amoxicillin 1 g/12 h Omeprazole 20 mg/12 h |
14 | 2 | 2 | (1) RBC+C+A (2) RBC+T+M |
100 | - |
| Qasim [88] | 2005 | Ireland | Rifabutin 300 mg/24 h Amoxicillin 1 g/12 h PPI/12 h |
10 | 34 | 2 | (1) PPI+C+A, PPI+C+M (2) PPI+C+A, PPI+C+M, Q |
38 | 2.9 |
| Toracchio [41] ¥ | 2005 | Italy | Rifabutin 150 mg/12 h Amoxicillin 1 g/12 h Pantoprazole 40 mg/12 h |
10 | 65 | ≥1 | - | 78 | 1.5 |
| Borody [52] | 2006 | Australia | Rifabutin 150 mg/24 h Amoxicillin 1–1.5 g/8 h Pantoprazole 80 mg/8 h |
12 | 63 | 1 | C-containing tx | 92 | 40 |
| Borody [52] | 2006 | Australia | Rifabutin 150 mg/24 h Amoxicillin 1–1.5 g/8 h Pantoprazole 80 mg/8 h |
12 | 67 | ≥2 | C-containing tx | 90 | 40 |
| Gisbert [89] | 2006 | Spain | Rifabutin 150 mg/12 h Amoxicillin 1 g/12 h Omeprazole 20 mg/12 h |
10 | 20 | 2 | (1) PPI+C+A (2) Q, RBC+T+M |
45 | 60 |
| Miehlke [51] § | 2006 | Germany | Rifabutin 150 mg/12 h Amoxicillin 1 g/12 h Esomeprazole 20 mg/12 h |
7 | 73 | ≥1 (24% 1 tx, 52% 2 tx, 24% 3 tx) | PPI+C+A, PPI+C+M, PPI+A+M, Q, PPI+A+L, others | 74 | 35 |
| Gonzalez Carro [90] | 2007 | Spain | Rifabutin 150 mg/12 h Amoxicillin 1 g/12 h Pantoprazole 40 mg/12 h |
10 | 92 | 2 | (1) PPI+C+A (2) Q |
61 | 2 |
| Navarro-Jarabo [91] | 2007 | Spain | Rifabutin 150 mg/12 h Amoxicillin 1 g/12 h Omeprazole 20 mg/12 h |
7 | 45 | 1 | PPI+C+A, PPI+C+M | 44 | 44 |
| Van der Poorten [39] | 2007 | Australia | Rifabutin 150 mg/12 h Amoxicillin 1 g/12 h PPI/12 h |
10 | 19 | 1 | C-containing tx | 95 | 10 |
| Van der Poorten [39] | 2007 | Australia | Rifabutin 150 mg/12 h Amoxicillin 1 g/12 h PPI/12 h |
10 | 16 | 2 | (1) C-containing tx (2) Q (42% of the cases) |
64 | 10 |
| Van der Poorten [39] | 2007 | Australia | Rifabutin 150 mg/12 h Amoxicillin 1 g/12 h PPI/12 h |
10 | 31 | ≥3 | (1) C-containing tx (2) Q (42% of the cases) (3) Other |
62 | 10 |
| Gisbert [92] | 2008 | Spain | Rifabutin 150 mg/12 h Amoxicillin 1 g/12 h Omeprazole 20 mg/12 h |
10-14 | 51 | 2 | (1) PPI+C+A (2) Q, RBC+T+M, PPI+A+L |
55 | 47 |
| Gisbert [92] | 2008 | Spain | Rifabutin 150 mg/12 h Amoxicillin 1 g/12 h Omeprazole 20 mg/12 h |
10-14 | 7 | 3 | (1) PPI+C+A (2) Q, RBC+T+M (3) PPI+A+L |
71 | 86 |
| Miehlke [47] ¥ | 2008 | Germany | Rifabutin 300 mg/24 h Moxifloxacin 400 mg/24 h Esomeprazole 40 mg/24 h |
7 | 24 | 1 | PPI+A, PPI+C, PPI+C+A, PPI+C+M, Q, PPI+A+L | 78 | 30 |
| Miehlke [47] ¥ | 2008 | Germany | Rifabutin 300 mg/24 h Moxifloxacin 400 mg/24 h Esomeprazole 40 mg/24 h |
7 | 52 | 2 | PPI+A, PPI+C, PPI+C+A, PPI+C+M, Q, PPI+A+L | 90 | 30 |
| Miehlke [47] ¥ | 2008 | Germany | Rifabutin 300 mg/24 h Moxifloxacin 400 mg/24 h Esomeprazole 40 mg/24 h |
7 | 17 | 3 | PPI+A, PPI+C, PPI+C+A, PPI+C+M, Q, PPI+A+L | 69 | 30 |
| Miehlke [47] ¥ | 2008 | Germany | Rifabutin 300 mg/24 h Moxifloxacin 400 mg/24 h Esomeprazole 40 mg/24 h |
7 | 10 | ≥4 | PPI+A, PPI+C, PPI+C+A, PPI+C+M, Q, PPI+A+L | 89 | 30 |
| Sacco [93] | 2009 | Italy | Rifabutin 150 mg/12 h Amoxicillin 1 g/12 h Esomeprazole 20 mg/12 h |
10 | 19 | 1 | PPI+A+Mx | 84 | 0 |
| Van Zanten [94] | 2010 | Canada | Rifabutin 300 mg/24 h Amoxicillin 1 g/12 h PPI/12 h |
7 | 4 | 1 | - | 100 | 0 |
| Van Zanten [94] | 2010 | Canada | Rifabutin 300 mg/24 h Amoxicillin 1 g/12 h PPI/12 h |
7 | 10 | 2 | (1) PPI+C+A, PPI+C+M, RBC+C (2) PPI+C+A, PPI+C+M, Q |
50 | 0 |
| Van Zanten [94] | 2010 | Canada | Rifabutin 300 mg/24 h Amoxicillin 1 g/12 h PPI/12 h |
7 | 2 | 3 | (1) PPI+C+A, Q (2) PPI+A, PPI+C+M (3) PPI+C+A |
50 | 0 |
| Zullo [95] | 2010 | Italy | Rifabutin 150 mg/12 h Amoxicillin 1 g/12 h Omeprazole 20 mg/12 h |
10 | 13 | 2 | (1) PPI+C+A, PPI+C+M, PPI+A+M (2) PPI+A+L |
85 | 15 |
| Tay [57] | 2012 | Australia | Rifabutin 150 mg/12 h Amoxicillin 1 g/8 h Ciprofloxacin 500 mg/12 h Rabeprazole 20 mg/8 h |
5-7 | 210 | ≥1 | (1) PPI+C+A +/- others (mostly PPI+C+M) | 95 | - |
| Tay [57] | 2012 | Australia | Rifabutin 150 mg/12 h Bismuth 240 mg/6 h Ciprofloxacin 500 mg/12 h Rabeprazole 20 mg/8 h |
7 | 69 | ≥1 | PPI+C+A +/- others (mostly PPI+C+M) | 94 | - |
| Gisbert [96] | 2012 | Spain | Rifabutin 150 mg/12 h Amoxicillin 1 g/12 h PPI/12 h |
10 | 100 | 3 | (1) PPI+C+A (2) Q (3) PPI+A+L |
50 | 30 |
| Jeong [97] | 2012 | Korea | Rifabutin 150 mg/12 h Amoxicillin 1 g/12 h PPI/12 h |
10 | 7 | 2 | (1) PPI+C+A (2) Q |
71 | - |
| Fiorini [99] | 2013 | Italy | Rifabutin 150 mg/24 h Amoxicillin 1 g/8 h Esomeprazole 40 mg/12 h |
12 | 105 | ≥1 | - | 89 | 15 |
| Ierardi [100] | 2014 | Italy | Rifabutin 150 mg/12 h Minocycline 100 mg/12 h Bismuth 120 mg/6 h Rabeprazole 20 mg/12 h |
10 | 21 | 2 | (1) PPI+C+A, sequential (2) PPI+A+L |
78 | 7.4 |
| Lim [98] | 2014 | Korea | Rifabutin 150 mg/12 h Amoxicillin 1 g/8 h Lansoprazole 60 mg/12 h |
7 | 27 | 2 | (1) PPI+C+A (2) Q |
96 | 11 |
| Lim [98] | 2014 | Korea | Rifabutin 150 mg/12 h Amoxicillin 1 g/8 h Lansoprazole 30 mg/12 h |
7 | 32 | 2 | (1) PPI+C+A (2) Q |
78 | 31 |
| Gisbert [101] # | 2015 | Spain | Rifabutin 150 mg/12 h Clarithromycin 500 mg/12 h Omeprazole 20 mg/12 h |
10 | 7 | 2 | (1) PPI+C+M (2) Q |
14 | 71 |
| Gisbert [101] # | 2015 | Spain | Rifabutin 150 mg/12 h Clarithromycin 500 mg/12 h Omeprazole 20 mg/12 h |
10 | 2 | 3 | (1) PPI+C+M (2) Q (3) PPI+C+L |
50 | 100 |
| Ciccaglione [68] | 2016 | Italy | Rifabutin 150 mg/12 h Amoxicillin 1 g/12 h Pantoprazole 20 mg/12 h |
10 | 27 | 2 | (1) PPI+C+A (2) PPI+A+M/L/Mx |
67 | 0 |
| Ciccaglione [68] | 2016 | Italy | Rifabutin 150 mg/12 h Amoxicillin 1 g/12 h Pantoprazole 20 mg/12 h Bismuth 240 mg/12 h |
10 | 29 | 2 | (1) PPI+C+A (2) PPI+A+M/L/Mx |
97 | 0 |
| Mori [67] | 2016 | Japan | Rifabutin 300 mg/24 h Amoxicillin 500 mg/8 h Esomeprazole 20 mg/8 h |
10 | 12 | 2 | (1) PPI+C+A (2) PPI+A+M |
83 | 75 |
| Mori [67] | 2016 | Japan | Rifabutin 300 mg/24 h Amoxicillin 500 mg/8 h Esomeprazole 20 mg/8 h |
10 | 17 | 2 | (1) PPI+C+A (2) PPI+A+M |
94 | 94 |
| Sung [69] | 2017 | Korea | Rifabutin 150 mg/12 h Amoxicillin 1 g/12 h PPI 20 mg/12 h |
7-14 | 12 | ≥3 | - | 50 | 18 |
| Cosme [102] | 2017 | Spain | Rifabutin 150 mg/12 h Amoxicillin 1 g/12 h Omeprazole 20 mg/12 h |
10 | 12 | ≥1 | - | 58 | 17 |
| Fiorini [20] | 2018 | Italy | Rifabutin 150 mg/24 h Amoxicillin 1 g/12 h Esomeprazole 40 mg/12 h |
12 | 257 | ≥1 | - | 83 | 18 |
| Papastergiou [103] | 2018 | Greece | Rifabutin 150 mg/12 h Amoxicillin 1 g/8 h Esomeprazole 40 mg/12 h |
7 | 2 | 0 (naïve) | - | 50 | 25 |
| Papastergiou [103] | 2018 | Greece | Rifabutin 150 mg/12 h Amoxicillin 1 g/8 h Esomeprazole 40 mg/12 h |
7 | 2 | ≥1 | - | 50 | 25 |
| Ribaldone [105] | 2019 | Italy | Rifabutin 150 mg/12 h Amoxicillin 1 g/12 h PPI/12 h |
14 | 302 | 4 | (1) PPI+C+A (2) PPI+C+A+M (3) Q (4) PPI+A+L |
72 | 7.3 |
| Burgos-Santamaría [106] | 2019 | Spain | Rifabutin 150 mg/12 h Amoxicillin 1 g/12 h Omeprazole 20 mg/12 h |
10-14 | 64 | 2 | (1) PPI+C+A (2) Q |
56 | 45 |
| Burgos-Santamaría [106] | 2019 | Spain | Rifabutin 150 mg/12 h Amoxicillin 1 g/12 h Omeprazole 20 mg/12 h |
10-14 | 60 | 3 | (1) PPI+C+A (2) Q (3) PPI+A+L (or vice versa) |
55 | 45 |
| Burgos-Santamaría [106] | 2019 | Spain | Rifabutin 150 mg/12 h Amoxicillin 1 g/12 h Omeprazole 20 mg/12 h |
10-14 | 4 | 4 | (1) PPI+C+A (2) Q (3) PPI+A+L (or vice versa) (4) Other |
50 | 45 |
| Burgos-Santamaría [106] # | 2019 | Spain | Rifabutin 150 mg/12 h Clarithromycin 500 mg/12 h Omeprazole 20 mg/12 h |
10-14 | 13 | 2 | (1) PPI+C+M (2) Q (or vice versa) |
23 | 75 |
| Burgos-Santamaría [106] # | 2019 | Spain | Rifabutin 150 mg/12 h Clarithromycin 500 mg/12 h Omeprazole 20 mg/12 h |
10-14 | 3 | 3 | (1) PPI+C+M (2) Q (or vice versa) (3) PPI+C+L |
67 | 75 |
| Saracino [108] | 2020 | Italy | Rifabutin 150 mg/24 h Amoxicillin 1 g/12 h Esomeprazole 40 mg/12 h |
12 | 119 | 1 | - | 58 | 46 |
| Saracino [108] | 2020 | Italy | Rifabutin 150 mg/24 h Amoxicillin 1 g/12 h Esomeprazole 40 mg/12 h |
12 | 66 | 2 | - | 56 | 46 |
| Saracino [108] | 2020 | Italy | Rifabutin 150 mg/24 h Amoxicillin 1 g/12 h Esomeprazole 40 mg/12 h |
12 | 44 | 3 | - | 54 | 46 |
| Saracino [108] | 2020 | Italy | Rifabutin 150 mg/24 h Amoxicillin 1 g/12 h Esomeprazole 40 mg/12 h |
12 | 41 | ≥4 | - | 24 | 46 |
| Graham [77] | 2020 | USA | Rifabutin 50 mg/8 h Amoxicillin 1 g/8 h Omeprazole 40 mg/12 h |
14 | 228 | 0 (naïve) | - | 84 | 36 |
| Hirata [78] | 2020 | Japan | Rifabutin 150 mg/12 h Amoxicillin 750 mg/12 h Vonoprazan 20 mg/12 h |
10 | 19 | 3 | (1) C-containing tx (2) M-containing tx (3) S-containing tx |
100 | 42 |
| Kalfus [107] | 2020 | USA | Rifabutin 150 mg/8 h Amoxicillin 1 g/8 h Omeprazole 40 mg/8 h (Talicia® single capsule) |
14 | 77 | 0 (naïve) | - | 77 | 51 |
| Kuo [19] | 2020 | China | Rifabutin 150 mg/12 h Amoxicillin 1 g/12 h Esomeprazole 40 mg/12 h |
10 | 40 | ≥2 | - | 78 | 23 |
PPI: proton pump inhibitor (standard dose); Type of previous treatments: (1) indicates 1st line treatment, (2) indicates 2nd line treatment, and (3) indicates 3rd line treatment; Tx: treatments; C: clarithromycin; A: amoxicillin; Q: bismuth quadruple therapy (PPI, bismuth, tetracycline, and nitroimidazole); RBC: ranitidine bismuth citrate; T: tetracycline; M: metronidazole; L: levofloxacin; Mx: moxifloxacin; S: sitafloxacin. ¶ Intention-to-treat analysis. § Patients infected with H. pylori resistant to both clarithromycin and metronidazole/tinidazole. ¥ Patients infected with H. pylori resistant to both clarithromycin and metronidazole/tinidazole, and susceptible to rifabutin. # Patients allergic to penicillin.
Figure 3.
Efficacy (intention-to-treat analysis) of rifabutin-containing therapies for the eradication of H. pylori.
Clinical experience with rifabutin for treatment of H. pylori infection has focused mainly on patients in whom one or more courses of anti-H pylori treatment previously failed [7,8,9,109,110,111]. However, three recent studies evaluated the role of rifabutin in H. pylori treatment naïve patients [77,103,107]. The meta-analysis of the efficacy (intention-to-treat) of these studies (including only 307 patients) showed a mean eradication rate of 82% (95% CI, 76–88%). Ultimately, the place of rifabutin as a first-line regimen in anti-H. pylori treatment will be determined by factors such as treatment success, adherence, cost, and availability. For the time being, rifabutin may be considered for first-line treatment in regions with high clarithromycin, metronidazole, and levofloxacin resistance (>15%) if bismuth is unavailable [8]. Obviously, head-to-head comparisons against reliable, well-established, and effective first-line regimens will expand efficacy and safety data and further define the role of rifabutin in the treatment of H. pylori infections.
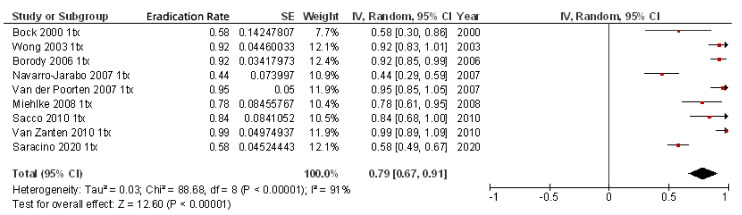
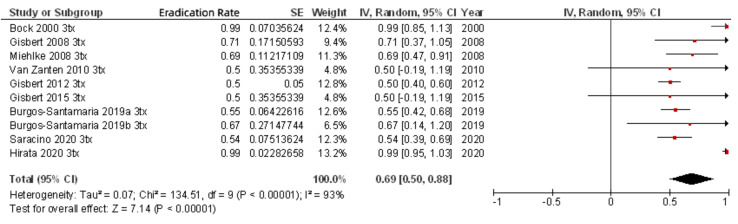
As previously noted, most of the studies included rescue regimens after at least one previous eradication failure (Table 2). When only second-line rifabutin-containing therapies administered to the 342 patients with one previous eradication failure were included, the mean eradication rate was 79% (95% CI, 67–91%) (Figure 4). When efficacy of third-line rifabutin-containing therapies for the eradication of H. pylori in patients with two previous eradication failures was calculated (including 678 patients), the cure rate was lower (69%; 95% CI, 61–78%) (Figure 5), and similar results were obtained in fourth-line treatment (256 patients; 69% cure rate; 95% CI, 50–88%). Finally, as a fifth-line rescue regimen (including 306 patients), the rifabutin-containing regimen was still quite effective (72%; 95% CI, 67–77%).
Figure 4.
Efficacy (intention-to-treat analysis) of second-line rifabutin-containing therapies for the eradication of H. pylori in patients with one previous eradication failure.
Figure 5.
Efficacy (intention-to-treat analysis) of third-line rifabutin-containing therapies for the eradication of H. pylori in patients with two previous eradication failures.
It has been suggested that rifabutin efficacy decreases with increasing number of previous (failed) therapies, perhaps due to patients who failed eradication therapy and possibly harbored H. pylori strains that were more refractory to eradication treatment [39,108]. Accordingly, in the meta-analysis by Gingold-Belfer et al. previously mentioned, treatment success with rifabutin triple therapy was 82% in treatment-naïve patients, 73% in patients receiving second-line treatment, and 64% when this regimen was prescribed as a third-line regimen [80]. However, as summarized in Figure 6, where studies assessing the efficacy of rifabutin in fourth-line regimens are included, quite favorable results may be obtained even after three previous eradication failures. Thus, the mean eradication rate in this scenario was approximately 70%. Accordingly, the efficacy of rifabutin treatment was not significantly influenced by the number of previous treatment failures in a recent study: eradication rates in patients with one, two, three, and four or more previous failures were 78.3%, 89.6%, 68.6%, and 88.9%, respectively (non-statistically significant differences) [47].
Figure 6.
Efficacy (intention-to-treat analysis) of fourth-line rifabutin-containing therapies for the eradication of H. pylori in patients with three previous eradication failures.
Just a few studies have directly compared—that is, in the same protocol of H. pylori eradication—rifabutin vs. other antibiotics [36,51,86,87,91,104]. Firstly, few studies have compared a PPI, amoxicillin, and rifabutin triple combination with the commonly used bismuth-containing quadruple regimen. Perri et al. [36] were the first to perform a randomized study where patients who failed eradication after standard triple therapy were treated for 10 days with a PPI, amoxicillin, and rifabutin (300 mg/24 h), or with bismuth quadruple therapy. On intention-to-treat analysis, eradication rates were 87% for rifabutin-containing therapy and 67% for bismuth quadruple therapy (these differences being statistically significant). More recent studies have shown better results with the rifabutin-triple [87] or bismuth-quadruple [91,104,108] regimen. Finally, the rifabutin-levofloxacin regimen has also been compared with the bismuth quadruple treatment for second-line treatment, reporting similar high eradication rates (91%) and similar good compliance (>95%) [86].
Miehlke et al., compared the rifabutin regimen with other treatments different to the bismuth-based quadruple regimen [51]. Patients infected with H. pylori strains resistant to both clarithromycin and metronidazole were randomized to receive esomeprazole, rifabutin, and amoxicillin for 7 days, or omeprazole 40 mg and amoxicillin 1000 mg, each given three times daily for 14 days. Eradication rates (intention-to-treat) were 74% and 70%, respectively. Therefore, they concluded that high-dose omeprazole/amoxicillin dual regimen and rifabutin triple therapy are comparable for rescue therapy.
Finally, other studies directly compared rifabutin to levofloxacin as third-line therapy for H. pylori. In a first study, a statistically significant superiority for the levofloxacin regimen was shown, although it should be noted that the eradication rate of 45% for rifabutin triple therapy in this study was surprisingly low [89]. In another study, on the contrary, higher cure rates were achieved with the rifabutin regimen (in this case, it was the eradication rate of the levofloxacin regimen, only 57%, which was unexpectedly low) [97].
Finally, it should be noted that rifabutin therapy was highly effective even when applied to H. pylori infection with primary resistance to clarithromycin or metronidazole (or both) [41,48,49,51,52,77,83,86], and even in patients with triple resistance to these two antibiotics plus quinolones [20], which is the usual scenario after several eradication therapy failures. In this respect, some authors evaluated, in the same study, different regimens after failure of two or more eradication treatments and achieved a final (overall) eradication rate of almost 100% [83,92,106,112,113,114,115,116,117]. This emphasizes the recommendation that in designing a treatment strategy we should prescribe two or more therapies which, if used consecutively, come as close to the 100% cure rate as possible [118]. In this respect, the aforementioned studies underline the fact that a wider perspective of the benefits of retreating H. pylori infection can be obtained if cumulative eradication rates, and not only absolute figures, with successive retreatments are taken into account.
7. How to Optimize Rifabutin-Based Treatments for H. pylori
In this section we will review the optimization strategies aimed to increase the efficacy of rifabutin-based H. pylori eradication therapies.
7.1. Rifabutin Dose and Frequency
Almost all studies administered rifabutin 300 mg/day for treating H. pylori infection. The H. pylori cure rate in some (few) studies prescribing rifabutin 150 mg/day was only approximately 40–70% [36,108], although other studies reported higher eradication rates [20,52,99]. A single randomized study directly compared both doses [36]: patients were treated for 10 days with pantoprazole, amoxicillin, and rifabutin 150 mg once daily or 300 mg once daily. Eradication rates were 67% in the rifabutin 150 mg group and significantly higher (87%) in the rifabutin 300 mg group. On the other hand, other authors showed that 12 days of half the dose of rifabutin (150 mg daily) in combination with increasing frequency of dosing with amoxicillin (1 g/8 h) and pantoprazole (80 mg/8 h) achieved an eradication rate as high as 91% [52]. Furthermore, by increasing the dosage of amoxicillin to 1.5 g/8 h for 12 days, an excellent overall eradication rate (97%) was achieved. These data suggest that frequent dosing of a high-dose amoxicillin and a double dose PPI in the presence of low-dose rifabutin (150 mg daily) are critical in increasing the efficacy of triple rifabutin-based therapy [52].
The incidence of adverse events is probably related to the rifabutin dose, with events reported more frequently in patients treated with doses of ≥450 mg daily than in those receiving 300 mg daily, and in those receiving 300 mg compared with 150 mg daily [23].
Finally, regarding the dose of amoxicillin, a meta-analysis by Gingold-Belfer et al. found that treatment was more likely to be successful when daily amoxicillin dose was ≥3000 mg [80].
7.2. Duration of Treatment
The ideal length of treatment for the rifabutin regimen remains unclear. In some reports, a 7-day course is equally efficacious compared to 10- to 14-day regimens, while others have found that the 7-day duration dramatically reduced the efficacy, with eradication rates at only 44% [91]. Although some studies have suggested that rifabutin treatment could be more likely to be successful when treatment duration is 14 days [80], many other studies have shown that therapy between 12 and 14 days has yielded results similar to the 10-day course and are likely to increase the incidence of adverse events [39]. Recently, a randomized controlled trial compared 10-day vs. 14-day eradication therapy with esomeprazole 20 mg/6 h, amoxicillin 500 mg/6 h, and rifabutin 300 mg/24 h [67]. Intention-to-treat eradication rates were 83% for the 10-day group and 94% for the 14-day group, respectively. Therapy was stopped due to adverse events in 8% and 29% of patients in the 10-day and 14-day groups, respectively. Therefore, the authors concluded that the 14-day therapy resulted in successful eradication in over 90% of patients, but the 10-day treatment may be enough to obtain a successful eradication rate, considering the tolerability of therapy [67]. Accordingly, from studies included in Table 2 and Figure 3, a mean H. pylori eradication rate of 79% for the 14-day regimen was calculated, while the corresponding figure for the 10-day regimen was similar (73%). In summary, current evidence suggests that 10 days may be more effective than 7 days, but no clear additional benefit has been shown with 14 days, which may increase the side effect burden [7,9,111].
7.3. Type and Dose of Antisecretory Drug
In a recent study, H. pylori-infected patients with two previous eradication failures were randomly assigned to receive either lansoprazole 30 mg/12 h or lansoprazole 60 mg/12 h, together with amoxicillin (1 g/8 h) and rifabutin (150 mg/12 h) for 7 days [98]. Eradication rates were higher in the high-dose PPI group (96% vs. 78%). Therefore, a key factor to successful rescue therapy with rifabutin-amoxicillin-PPI regimen may be to increase doses of PPI. Accordingly, in the meta-analysis by Gingold-Belfer et al., rifabutin treatment was more likely to be successful when daily PPI dose was ≥80 mg [80].
In this same line, studies on rifabutin-based regimens with a potassium-competitive acid blocker such as vonoprazan instead of PPIs may additionally improve the success of the rifamycin-containing eradication therapy. In a recent study, patients who failed H. pylori eradication by clarithromycin-based first-line, metronidazole-based second-line, and sitafloxacin-based third-line therapies were prescribed vonoprazan (20 mg), amoxicillin (750 mg), and rifabutin (150 mg) twice daily for 10 days, and H. pylori eradication was confirmed in all patients (19/19; 100%) [78].
7.4. Addition of Bismuth
Bismuth has an additive effect with antibiotics, overcomes levofloxacin and clarithromycin resistance, and its efficacy is not affected by metronidazole resistance [119,120]. In addition, bismuth is one of the few antimicrobials to which resistance is not developed [121,122]. Bismuth exerts its antibacterial action mainly by preventing bacterial colonization and adherence to gastric epithelium and by binding toxins produced by H. pylori [123]. In addition, bismuth decreases mucin viscosity, reduces the bacterial load, and has a synergistic effect with antibiotics [119]. Therefore, combining bismuth and rifabutin in the same regimen is suggested as a promising option.
A combination of a triple therapy with PPI-amoxicillin-rifabutin but adding bismuth and thus converting this triple regimen into a quadruple one, has recently been evaluated, with encouraging results [57,68,100]. Ciccaglione et al. reported that the addition of bismuth to a triple therapy that included PPI, amoxicillin, and rifabutin in patients treated for the third time for H. pylori infection, resulted in 30% therapeutic gain compared to rifabutin-based triple therapy alone [68].
Finally, this bismuth-rifabutin regimen has recently been evaluated in the context of the “European Registry on H. pylori Management” (Hp-EuReg), an international multicenter prospective non-interventional registry starting in 2013 aimed to evaluate the decisions and outcomes in H. pylori management by European gastroenterologists [124]. Thirty European countries, with over 300 recruiters, are actively participating in this project, where patients are managed and registered according to their routine clinical practice [10]. To assess the effectiveness of empirical rescue therapies on third and subsequent lines in Europe, 1782 rescue treatments were evaluated in this registry. The effectiveness of the rifabutin-based quadruple regimen (that is, a PPI, rifabutin, amoxicillin, and bismuth) in these very refractory cases was 59% (by intention-to-treat) [125].
7.5. All-in-One Single Capsule Including Omeprazole, Amoxicillin, and Rifabutin
A recent development is the 2019 approval by the United States Food and Drug Administration (FDA) of a combination product (Talicia®; RedHill Biopharma, Raleigh, NC, USA) containing omeprazole, rifabutin, and amoxicillin. This is the first and only FDA-approved rifabutin-based H. pylori therapy. Potentially, it may improve patient compliance with treatment because of its relative simplicity. In the “ERADICATE Hp2” trial, this combination successfully eradicated H. pylori in 84% of patients compared to 58% of those who received the same doses of omeprazole and amoxicillin, but without rifabutin [77]. The recommended dose for this product is four capsules taken three times daily for 14 days; total daily doses are omeprazole 120 mg, rifabutin 150 mg, and amoxicillin 3 g.
A limitation of the aforementioned study is that it was only conducted in the United States and excluded persons of Asian descent. The higher effectiveness of H. pylori eradication therapy reported in some studies in Asian populations is possibly due to genetic factors. For example, CYP2C19 polymorphism associated with poor PPI metabolism is more common in Asian populations [80]. This phenotype leads to higher serum PPI levels and more potent gastric acid inhibition and may partially account for the superior eradication rate [126]. While this may lead to a higher bactericidal activity of amoxicillin, CYP2C19 polymorphism is unlikely to affect rifabutin efficacy, which is chemically stable at a wide pH range [80].
8. Tolerability of Rifabutin
The overall incidence rate for the occurrence of at least one adverse event (51%) was comparable to placebo (50%) in patients with AIDS treated prophylactically with rifabutin 300 mg daily [23]. Myalgia and taste perversion were, however, significantly more frequent with rifabutin than with placebo [23]. Other reported adverse effects include rash (3%), nausea/vomiting (0.4%), neutropenia (0.4%), anemia (0.4%) and, rarely, impairment of liver function [23,127]. In studies where rifabutin is administered to treat non-H. pylori infections, the rate of adverse events is reported as approximately 25–30% [79,80,128]. In our updated meta-analysis of all studies including rifabutin (Table 2), mean rate of adverse effects was 15% (95% CI, 14–17%) (Figure 7), which seems to be a reasonable figure when compared with other well-established eradication regimens, such as the bismuth and non-bismuth quadruple therapies [129]. Furthermore, severe adverse events with rifabutin are exceptional; in a recent review [80], only one severe adverse event was reported (diabetic ketoacidosis) [77].
Figure 7.
Adverse events of rifabutin-containing therapies.
Myelotoxicity is the most significant adverse event of rifabutin [130,131]. Overall, this complication is rare and is far more likely when high dose (600 mg/day) and prolonged duration therapy is used [130,131]. Although several cases of myelotoxicity have been reported during H. pylori therapy [39,41,82,84,86,88,89,96,99], this adverse event was not reported in most studies evaluating rifabutin for H. pylori infection. In the studies reporting this complication, myelotoxicity was observed in 1.5% to 3% of patients [39,41,86,88,96], although in some studies the incidence was higher [84,89]. In the meta-analysis by Gingold-Belfer et al., neutropenia was addressed in 27 studies, of which 19 studies reported no cases of neutropenia and eight studies reported at least one case; in total, only 17 patients developed neutropenia across all studies [80].
All patients from the literature recovered from myelotoxicity uneventfully in just a few days, although it took 15 days to normalize white cell count after rifabutin discontinuation in one case [84]. In some cases, the myelotoxicity was clinically apparent with fever [39,84]. However, there are no reports of infection or other adverse outcomes related to reduced white cell count, at least in the scenario of H. pylori treatment [39,41,82,84,86,88,89,96].
It has been suggested that during short-term rifabutin treatment blood cell count should be checked at any suspicious symptom. Moreover, some authors have recommended performing systematic blood controls in all patients receiving rifabutin, despite being asymptomatic [85,89]. However, others suggest that a practical approach may be to check the full blood count only if fever or other signs of systemic toxicity occur.
Only a few studies have directly compared—in the same protocol—the safety of rifabutin and other antibiotics in H. pylori eradication regimens. Firstly, some studies have compared a triple combination of rifabutin together with a PPI and amoxicillin vs. the widely used bismuth quadruple regimen [36,51,86,91]. As an example, in the study by Perri et al. [36], side effects were less frequent in rifabutin-treated patients than in those on bismuth quadruple therapy. Thus, approximately 15% of patients on quadruple therapy experienced moderate to severe side effects, and 6% had to discontinue treatment. In contrast, in the rifabutin-treated groups, no moderate or severe side effects were observed, and no patients discontinued treatment because of side effects.
Other studies compared rifabutin treatment with other regimens different to the bismuth quadruple therapy. Miehlke et al. [51] compared rifabutin-based triple therapy vs. high-dose dual therapy for rescue treatment of H. pylori. Patients were randomized to a PPI, rifabutin, and amoxicillin for 7 days, or to a PPI and amoxicillin 1000 mg/8 h for 14 days. Premature discontinuation of treatment occurred in 2% and 5% of patients, respectively. Finally, one study directly compared rifabutin to levofloxacin as third-line therapy for H. pylori, and adverse events were reported in 60% and 50% of cases, respectively [89].
9. Limitations of Rifabutin Treatment
Several concerns remain regarding rifabutin for H. pylori eradication treatment. First, this drug is quite expensive. Second, as previously reviewed, severe leucopenia has been reported in some (although exceptional) cases. Finally, although an argument raised consistently against the wider use of rifabutin is the concern regarding propagation of resistance, especially in mycobacterial species, it should be taken into account that the major use of rifabutin is for treatment of tuberculosis and other mycobacteria especially in the setting of immunodeficiency or HIV infection [39]. Acquired rifabutin resistance has been noted in these cohorts, but only when associated with CD4 counts <100 cells/mm3 and when intermittent dosing is used [132]. Even in the setting of prolonged tuberculosis treatment, continuous daily rifabutin has led to negligible rates of resistance [132,133]. Moreover, no reports have yet to definitively link rifabutin resistance with short course treatment of rifabutin for H. pylori or other non-mycobacterial indications [24,39].
10. Conclusions
Even with the current most effective treatment regimens, a relevant proportion of patients will fail to eradicate H. pylori infection. Nowadays, apart from having to know first-line eradication regimens well, we must also be prepared to face treatment failures. In this context, rifabutin has potential utility against H. pylori as this antibiotic shows good in vitro activity against this microorganism, and the prevalence rate of rifabutin resistance is very low (less than 1%).
Mean H. pylori eradication rate (overall results, by intention-to-treat analysis) with rifabutin-containing regimens was 73%. Respective cure rates for second-, third-, fourth-, and fifth-line rifabutin therapies, were 79%, 69%, 69%, and 72%, respectively. Almost all studies administered rifabutin 300 mg/day for treating H. pylori infection, which seems to be more effective than 150 mg/day. The ideal length of treatment for rifabutin regimen remains unclear, but 10- to 12-day regimens are usually recommended.
The mean rate of adverse effects to rifabutin treatment in H. pylori studies was relatively low (15%), and severe adverse events are exceptional. Myelotoxicity is the most significant adverse event of rifabutin during H. pylori therapy, although this complication was rare, always reversible, and without clinical consequences (such as infection) in the setting of H. pylori treatment.
Regarding the positioning of rifabutin regimens, clinical experience with rifabutin for treatment of H. pylori infection has focused mainly on patients in whom one or more courses of anti-H pylori treatment have previously failed. Since resistance to rifabutin is practically inexistent, and that rifabutin therapy is highly effective even in patients with primary resistance to clarithromycin, metronidazole, and levofloxacin, rifabutin use in an empirical manner as “rescue” therapy without culture in those patients in whom these antibiotics have failed, may be suggested. Recent studies have evaluated the role of rifabutin in H. pylori treatment naïve patients, with encouraging results. Nevertheless, the consideration of rifabutin as a novel first-line treatment option for H. pylori infection should be carefully weighed against concerns regarding microbial resistance, treatment cost (rifabutin is quite expensive), and the availability and effectiveness of alternative drugs.
At present, therefore, rifabutin is generally restricted to patients where previous (usually multiple) eradication regimens with key antibiotics such as amoxicillin, clarithromycin, metronidazole, tetracycline, and levofloxacin have failed. Thus, it may be suggested that the position of rifabutin in the algorithm of H. pylori treatment may be, usually, at a fourth-line rescue regimen. Nevertheless, rifabutin could be also recommended in an earlier scenario, for example, as second- or third-line treatment, if antibiotic resistance (for example to quinolones) is demonstrated or suspected; or even as a first-line treatment in regions with high clarithromycin, metronidazole, and levofloxacin resistance if bismuth is unavailable.
Abbreviations
| Helicobacter pylori | H. pylori |
| proton pump inhibitor | PPI |
Funding
This research received no external funding.
Conflicts of Interest
Gisbert has served as a speaker, a consultant, and advisory member for or has received research funding from Mayoly, Allergan, Diasorin, and Phathom.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References and Notes
- 1.Crowe S.E. Helicobacter pylori Infection. N. Engl. J. Med. 2019;380:1158–1165. doi: 10.1056/NEJMcp1710945. [DOI] [PubMed] [Google Scholar]
- 2.Gisbert J.P., Calvet X. Review article: The effectiveness of standard triple therapy for Helicobacter pylori has not changed over the last decade, but it is not good enough. Aliment. Pharmacol. Ther. 2011;34:1255–1268. doi: 10.1111/j.1365-2036.2011.04887.x. [DOI] [PubMed] [Google Scholar]
- 3.Megraud F., Coenen S., Versporten A., Kist M., Lopez-Brea M., Hirschl A.M., Andersen L.P., Goossens H., Glupczynski Y. Helicobacter pylori resistance to antibiotics in Europe and its relationship to antibiotic consumption. Gut. 2013;62:34–42. doi: 10.1136/gutjnl-2012-302254. [DOI] [PubMed] [Google Scholar]
- 4.Gisbert J.P., Pajares J.M. Review article: Helicobacter pylori “rescue” regimen when proton pump inhibitor-based triple therapies fail. Aliment Pharmacol. Ther. 2002;16:1047–1057. doi: 10.1046/j.1365-2036.2002.01276.x. [DOI] [PubMed] [Google Scholar]
- 5.Gisbert J.P. “Rescue” regimens after Helicobacter pylori treatment failure. World J. Gastroenterol. 2008;14:5385–5402. doi: 10.3748/wjg.14.5385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gisbert J.P., Molina-Infante J., Amador J., Bermejo F., Bujanda L., Calvet X. IV Spanish Consensus Conference on Helicobacter pylori infection treatment. Gastroenterol. Hepatol. 2016;39:697–721. doi: 10.1016/j.gastrohep.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 7.Fallone C.A., Chiba N., van Zanten S.V., Fischbach L., Gisbert J.P., Hunt R.H., Jones N.L., Render C., Leontiadis G.I., Moayyedi P., et al. The Toronto Consensus for the Treatment of Helicobacter pylori Infection in Adults. Gastroenterology. 2016;151:51–69. doi: 10.1053/j.gastro.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 8.Malfertheiner P., Megraud F., O’Morain C.A., Gisbert J.P., Kuipers E.J., Axon A.T., Bazzoli F., Gasbarrini A., Atherton J., Graham D.Y., et al. Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut. 2017;66:6–30. doi: 10.1136/gutjnl-2016-312288. [DOI] [PubMed] [Google Scholar]
- 9.Chey W.D., Leontiadis G.I., Howden C.W., Moss S.F. ACG Clinical Guideline: Treatment of Helicobacter pylori Infection. Am. J. Gastroenterol. 2017;112:212–239. doi: 10.1038/ajg.2016.563. [DOI] [PubMed] [Google Scholar]
- 10.Nyssen O.P., Bordin D., Tepes B., Perez-Aisa A., Vaira D., Caldas M., Bujanda L., Castro-Fernandez M., Lerang F., Leja M., et al. European Registry on Helicobacter pylori management (Hp-EuReg): Patterns and trends in first-line empirical eradication prescription and outcomes of 5 years and 21 533 patients. Gut. 2020;70:40–54. doi: 10.1136/gutjnl-2020-321372. [DOI] [PubMed] [Google Scholar]
- 11.Gisbert J.P., Morena F. Systematic review and meta-analysis: Levofloxacin-based rescue regimens after Helicobacter pylori treatment failure. Aliment. Pharmacol. Ther. 2006;23:35–44. doi: 10.1111/j.1365-2036.2006.02737.x. [DOI] [PubMed] [Google Scholar]
- 12.Gisbert J.P., Bermejo F., Castro-Fernandez M., Perez-Aisa A., Fernandez-Bermejo M., Tomas A., Barrio J., Bory F., Almela P., Sanchez-Pobre P., et al. Second-line rescue therapy with levofloxacin after H. pylori treatment failure: A Spanish multicenter study of 300 patients. Am. J. Gastroenterol. 2008;103:71–76. doi: 10.1111/j.1572-0241.2007.01500.x. [DOI] [PubMed] [Google Scholar]
- 13.Gisbert J.P., Castro-Fernandez M., Bermejo F., Perez-Aisa A., Ducons J., Fernandez-Bermejo M., Bory F., Cosme A., Benito L.M., Lopez-Rivas L., et al. Third-line rescue therapy with levofloxacin after two H. pylori treatment failures. Am. J. Gastroenterol. 2006;101:243–247. doi: 10.1111/j.1572-0241.2006.00457.x. [DOI] [PubMed] [Google Scholar]
- 14.Gisbert J.P. Empirical or susceptibility-guided treatment for Helicobacter pylori infection? A comprehensive review. Therap. Adv. Gastroenterol. 2020;13:1–16. doi: 10.1177/1756284820968736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Megraud F., Lamouliatte H. The treatment of refractory Helicobacter pylori infection. Aliment. Pharmacol. Ther. 2003;17:1333–1343. doi: 10.1046/j.1365-2036.2003.01592.x. Review article. [DOI] [PubMed] [Google Scholar]
- 16.Megraud F. Basis for the management of drug-resistant Helicobacter pylori infection. Drugs. 2004;64:1893–1904. doi: 10.2165/00003495-200464170-00003. [DOI] [PubMed] [Google Scholar]
- 17.Maddix D.S., Tallian K.B., Mead P.S. Rifabutin: A review with emphasis on its role in the prevention of disseminated Mycobacterium avium complex infection. Ann. Pharmacother. 1994;28:1250–1254. doi: 10.1177/106002809402801108. [DOI] [PubMed] [Google Scholar]
- 18.Rothstein D.M. Rifamycins, Alone and in Combination. Cold Spring Harb. Perspect. Med. 2016;6 doi: 10.1101/cshperspect.a027011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuo C.J., Lin C.Y., Le P.H., Chang P.Y., Lai C.H., Lin W.R., Chang M.L., Hsu J.T., Cheng H.T., Tseng C.N., et al. Rescue therapy with rifabutin regimen for refractory Helicobacter pylori infection with dual drug-resistant strains. BMC Gastroenterol. 2020;20:218. doi: 10.1186/s12876-020-01370-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fiorini G., Zullo A., Vakil N., Saracino I.M., Ricci C., Castelli V., Gatta L., Vaira D. Rifabutin Triple Therapy is Effective in Patients With Multidrug-resistant Strains of Helicobacter pylori. J. Clin. Gastroenterol. 2018;52:137–140. doi: 10.1097/MCG.0000000000000540. [DOI] [PubMed] [Google Scholar]
- 21.Gisbert J.P., Calvet X. Review article: Rifabutin in the treatment of refractory Helicobacter pylori infection. Aliment. Pharmacol. Ther. 2012;35:209–221. doi: 10.1111/j.1365-2036.2011.04937.x. [DOI] [PubMed] [Google Scholar]
- 22.Kunin C.M. Antimicrobial activity of rifabutin. Clin. Infect. Dis. 1996;22(Suppl. 1):S3–S13. doi: 10.1093/clinids/22.Supplement_1.S3. [DOI] [PubMed] [Google Scholar]
- 23.Brogden R.N., Fitton A. Rifabutin. A review of its antimicrobial activity, pharmacokinetic properties and therapeutic efficacy. Drugs. 1994;47:983–1009. doi: 10.2165/00003495-199447060-00008. [DOI] [PubMed] [Google Scholar]
- 24.Heep M., Beck D., Bayerdorffer E., Lehn N. Rifampin and rifabutin resistance mechanism in Helicobacter pylori. Antimicrob. Agents Chemother. 1999;43:1497–1499. doi: 10.1128/AAC.43.6.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hays C., Burucoa C., Lehours P., Tran C.T., Leleu A., Raymond J. Molecular characterization of Helicobacter pylori resistance to rifamycins. Helicobacter. 2018;23 doi: 10.1111/hel.12451. [DOI] [PubMed] [Google Scholar]
- 26.Heep M., Rieger U., Beck D., Lehn N. Mutations in the beginning of the rpoB gene can induce resistance to rifamycins in both Helicobacter pylori and Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2000;44:1075–1077. doi: 10.1128/AAC.44.4.1075-1077.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nishizawa T., Suzuki H., Matsuzaki J., Muraoka H., Tsugawa H., Hirata K., Hibi T. Helicobacter pylori resistance to rifabutin in the last 7 years. Antimicrob. Agents Chemother. 2011;55:5374–5375. doi: 10.1128/AAC.05437-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suzuki S., Suzuki H., Nishizawa T., Kaneko F., Ootani S., Muraoka H., Saito Y., Kobayashi I., Hibi T. Past rifampicin dosing determines rifabutin resistance of Helicobacter pylori. Digestion. 2009;79:1–4. doi: 10.1159/000191204. [DOI] [PubMed] [Google Scholar]
- 29.Akada J.K., Shirai M., Fujii K., Okita K., Nakazawa T. In vitro anti-Helicobacter pylori activities of new rifamycin derivatives, KRM-1648 and KRM-1657. Antimicrob. Agents Chemother. 1999;43:1072–1076. doi: 10.1128/AAC.43.5.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rossi G. An update on the antibiotic therapy of tuberculosis. Recenti. Prog. Med. 1999;90:241–243. [PubMed] [Google Scholar]
- 31.Koudriakova T., Iatsimirskaia E., Tulebaev S., Spetie D., Utkin I., Mullet D., Thompson T., Vouros P., Gerber N. In vivo disposition and metabolism by liver and enterocyte microsomes of the antitubercular drug rifabutin in rats. J. Pharmacol. Exp. Ther. 1996;279:1300–1309. [PubMed] [Google Scholar]
- 32.Blaschke T.F., Skinner M.H. The clinical pharmacokinetics of rifabutin. Clin. Infect. Dis. 1996;22:S15–S21. doi: 10.1093/clinids/22.Supplement_1.S15. [DOI] [PubMed] [Google Scholar]
- 33.Iatsimirskaia E., Tulebaev S., Storozhuk E., Utkin I., Smith D., Gerber N., Koudriakova T. Metabolism of rifabutin in human enterocyte and liver microsomes: Kinetic parameters, identification of enzyme systems, and drug interactions with macrolides and antifungal agents. Clin. Pharmacol. Ther. 1997;61:554–562. doi: 10.1016/S0009-9236(97)90135-1. [DOI] [PubMed] [Google Scholar]
- 34.Piccolomini R., Di Bonaventura G., Picciani C., Laterza F., Vecchiet J., Neri M. In vitro activity of clarithromycin against intracellular Helicobacter pylori. Antimicrob. Agents Chemother. 2001;45:1568–1571. doi: 10.1128/AAC.45.5.1568-1571.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rossi R., Jabes D., Della Bruna C. Volume 69 Prague, Czech Republic: 1994. In vitro Activity of Rifabutin, a Potential Antibiotic in the Therapy of Helicobacter Pylori, the 6th International Congress of Infectious Diseases (ICID) [Google Scholar]
- 36.Perri F., Festa V., Clemente R., Villani M.R., Quitadamo M., Caruso N., Bergoli M.L., Andriulli A. Randomized study of two “rescue” therapies for Helicobacter pylori-infected patients after failure of standard triple therapies. Am. J. Gastroenterol. 2001;96:58–62. doi: 10.1111/j.1572-0241.2001.03452.x. [DOI] [PubMed] [Google Scholar]
- 37.Heep M., Lehn N., Brandstatter B., Rieger U., Senzenberger S., Wehrl W. Detection of rifabutin resistance and association of rpoB mutations with resistance to four rifamycin derivatives in Helicobacter pylori. Eur. J. Clin. Microbiol. Infect. Dis. 2002;21:143–145. doi: 10.1007/s10096-001-0672-2. [DOI] [PubMed] [Google Scholar]
- 38.Glocker E., Bogdan C., Kist M. Characterization of rifampicin-resistant clinical Helicobacter pylori isolates from Germany. J. Antimicrob. Chemother. 2007;59:874–879. doi: 10.1093/jac/dkm039. [DOI] [PubMed] [Google Scholar]
- 39.Van der Poorten D., Katelaris P.H. The effectiveness of rifabutin triple therapy for patients with difficult-to-eradicate Helicobacter pylori in clinical practice. Aliment. Pharmacol. Ther. 2007;26:1537–1542. doi: 10.1111/j.1365-2036.2007.03531.x. [DOI] [PubMed] [Google Scholar]
- 40.Graham D.Y. Antibiotic resistance in Helicobacter pylori: Implications for therapy. Gastroenterology. 1998;115:1272–1277. doi: 10.1016/S0016-5085(98)70100-3. [DOI] [PubMed] [Google Scholar]
- 41.Toracchio S., Capodicasa S., Soraja D.B., Cellini L., Marzio L. Rifabutin based triple therapy for eradication of H. pylori primary and secondary resistant to tinidazole and clarithromycin. Dig. Liver Dis. 2005;37:33–38. doi: 10.1016/j.dld.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 42.Matsuoka M., Yoshida Y., Hayakawa K., Fukuchi S., Sugano K. Simultaneous colonisation of Helicobacter pylori with and without mutations in the 23S rRNA gene in patients with no history of clarithromycin exposure. Gut. 1999;45:503–507. doi: 10.1136/gut.45.4.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim J.J., Kim J.G., Kwon D.H. Mixed-infection of antibiotic susceptible and resistant Helicobacter pylori isolates in a single patient and underestimation of antimicrobial susceptibility testing. Helicobacter. 2003;8:202–206. doi: 10.1046/j.1523-5378.2003.00145.x. [DOI] [PubMed] [Google Scholar]
- 44.Cellini L., Grande R., Di Campli E., Di Bartolomeo S., Capodicasa S., Marzio L. Analysis of genetic variability, antimicrobial susceptibility and virulence markers in Helicobacter pylori identified in Central Italy. Scand. J. Gastroenterol. 2006;41:280–287. doi: 10.1080/00365520510024223. [DOI] [PubMed] [Google Scholar]
- 45.De Francesco V., Giorgio F., Hassan C., Manes G., Vannella L., Panella C., Ierardi E., Zullo A. Worldwide H. pylori antibiotic resistance: A systematic review. J. Gastrointestin. Liver Dis. 2010;19:409–414. [PubMed] [Google Scholar]
- 46.Ghotaslou R., Leylabadlo H.E., Asl Y.M. Prevalence of antibiotic resistance in Helicobacter pylori: A recent literature review. World J. Methodol. 2015;5:164–174. doi: 10.5662/wjm.v5.i3.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miehlke S., Schneider-Brachert W., Kirsch C., Morgner A., Madisch A., Kuhlisch E., Haferland C., Bastlein E., Jebens C., Zekorn C., et al. One-week once-daily triple therapy with esomeprazole, moxifloxacin, and rifabutin for eradication of persistent Helicobacter pylori resistant to both metronidazole and clarithromycin. Helicobacter. 2008;13:69–74. doi: 10.1111/j.1523-5378.2007.00588.x. [DOI] [PubMed] [Google Scholar]
- 48.Bock H., Koop H., Lehn N., Heep M. Rifabutin-based triple therapy after failure of Helicobacter pylori eradication treatment: Preliminary experience. J. Clin. Gastroenterol. 2000;31:222–225. doi: 10.1097/00004836-200010000-00007. [DOI] [PubMed] [Google Scholar]
- 49.Pilotto A., Franceschi M., Rassu M., Furlan F., Scagnelli M. In vitro activity of rifabutin against strains of Helicobacter pylori resistant to metronidazole and clarithromycin. Am. J. Gastroenterol. 2000;95:833–834. doi: 10.1111/j.1572-0241.2000.01900.x. [DOI] [PubMed] [Google Scholar]
- 50.Marzio L., Coraggio D., Capodicasa S., Grossi L., Cappello G. Role of the preliminary susceptibility testing for initial and after failed therapy of Helicobacter pylori infection with levofloxacin, amoxicillin, and esomeprazole. Helicobacter. 2006;11:237–242. doi: 10.1111/j.1523-5378.2006.00407.x. [DOI] [PubMed] [Google Scholar]
- 51.Miehlke S., Hansky K., Schneider-Brachert W., Kirsch C., Morgner A., Madisch A., Kuhlisch E., Bastlein E., Jacobs E., Bayerdorffer E., et al. Randomized trial of rifabutin-based triple therapy and high-dose dual therapy for rescue treatment of Helicobacter pylori resistant to both metronidazole and clarithromycin. Aliment. Pharmacol. Ther. 2006;24:395–403. doi: 10.1111/j.1365-2036.2006.02993.x. [DOI] [PubMed] [Google Scholar]
- 52.Borody T.J., Pang G., Wettstein A.R., Clancy R., Herdman K., Surace R., Llorente R., Ng C. Efficacy and safety of rifabutin-containing ‘rescue therapy’ for resistant Helicobacter pylori infection. Aliment. Pharmacol. Ther. 2006;23:481–488. doi: 10.1111/j.1365-2036.2006.02793.x. [DOI] [PubMed] [Google Scholar]
- 53.Chisholm S.A., Owen R.J. Frequency and molecular characteristics of ciprofloxacin- and rifampicin-resistant Helicobacter pylori from gastric infections in the UK. J. Med. Microbiol. 2009;58:1322–1328. doi: 10.1099/jmm.0.011270-0. [DOI] [PubMed] [Google Scholar]
- 54.Goh K.L., Navaratnam P. High Helicobacter pylori resistance to metronidazole but zero or low resistance to clarithromycin, levofloxacin, and other antibiotics in Malaysia. Helicobacter. 2011;16:241–245. doi: 10.1111/j.1523-5378.2011.00841.x. [DOI] [PubMed] [Google Scholar]
- 55.Marzio L., Cellini L., Amitrano M., Grande R., Serio M., Cappello G., Grossi L. Helicobacter pylori isolates from proximal and distal stomach of patients never treated and already treated show genetic variability and discordant antibiotic resistance. Eur. J. Gastroenterol. Hepatol. 2011;23:467–472. doi: 10.1097/MEG.0b013e328345d40f. [DOI] [PubMed] [Google Scholar]
- 56.McNulty C.A., Lasseter G., Shaw I., Nichols T., D’Arcy S., Lawson A.J., Glocker E. Is Helicobacter pylori antibiotic resistance surveillance needed and how can it be delivered? Aliment. Pharmacol. Ther. 2012;35:1221–1230. doi: 10.1111/j.1365-2036.2012.05083.x. [DOI] [PubMed] [Google Scholar]
- 57.Tay C.Y., Windsor H.M., Thirriot F., Lu W., Conway C., Perkins T.T., Marshall B.J. Helicobacter pylori eradication in Western Australia using novel quadruple therapy combinations. Aliment. Pharmacol. Ther. 2012;36:1076–1083. doi: 10.1111/apt.12089. [DOI] [PubMed] [Google Scholar]
- 58.O’Connor A., Taneike I., Nami A., Fitzgerald N., Ryan B., Breslin N., O’Connor H., McNamara D., Murphy P., O’Morain C. Helicobacter pylori resistance rates for levofloxacin, tetracycline and rifabutin among Irish isolates at a reference centre. Ir. J. Med. Sci. 2013;182:693–695. doi: 10.1007/s11845-013-0957-3. [DOI] [PubMed] [Google Scholar]
- 59.Larsen A.L., Ragnhildstveit E., Moayeri B., Eliassen L., Melby K.K. Resistance rates of metronidazole and other antibacterials in Helicobacter pylori from previously untreated patients in Norway. APMIS. 2013;121:353–358. doi: 10.1111/apm.12009. [DOI] [PubMed] [Google Scholar]
- 60.Selgrad M., Meissle J., Bornschein J., Kandulski A., Langner C., Varbanova M., Wex T., Tammer I., Schluter D., Malfertheiner P. Antibiotic susceptibility of Helicobacter pylori in central Germany and its relationship with the number of eradication therapies. Eur. J. Gastroenterol. Hepatol. 2013;25:1257–1260. doi: 10.1097/MEG.0b013e3283643491. [DOI] [PubMed] [Google Scholar]
- 61.Seo J.H., Jun J.S., Yeom J.S., Park J.S., Youn H.S., Ko G.H., Baik S.C., Lee W.K., Cho M.J., Rhee K.H. Changing pattern of antibiotic resistance of Helicobacter pylori in children during 20 years in Jinju, South Korea. Pediatr. Int. 2013;55:332–336. doi: 10.1111/ped.12048. [DOI] [PubMed] [Google Scholar]
- 62.Gosciniak G., Biernat M., Grabinska J., Binkowska A., Poniewierka E., Iwanczak B. The antimicrobial susceptibility of Helicobacter pylori strains isolated from children and adults with primary infection in the Lower Silesia Region, Poland. Pol. J. Microbiol. 2014;63:57–61. doi: 10.33073/pjm-2014-008. [DOI] [PubMed] [Google Scholar]
- 63.Biernat M.M., Poniewierka E., Blaszczuk J., Czapla L., Kempinski R., Ksiadzyna D., Grabinska J., Binkowska A., Megraud F., Gosciniak G. Antimicrobial susceptibility of Helicobacter pylori isolates from Lower Silesia, Poland. Arch. Med. Sci. 2014;10:505–509. doi: 10.5114/aoms.2013.36917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Selgrad M., Tammer I., Langner C., Bornschein J., Meissle J., Kandulski A., Varbanova M., Wex T., Schluter D., Malfertheiner P. Different antibiotic susceptibility between antrum and corpus of the stomach, a possible reason for treatment failure of Helicobacter pylori infection. World J. Gastroenterol. 2014;20:16245–16251. doi: 10.3748/wjg.v20.i43.16245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.John A., Al Kaabi S., Doiphode S., Chandra P., Sharma M., Babu R., Yacoub R., Derbala M. Does emerging Clarithromycin resistance signal an obituary to empirical standard triple therapy for Helicobacter pylori infection? Indian J. Gastroenterol. 2015;34:404–407. doi: 10.1007/s12664-015-0604-1. [DOI] [PubMed] [Google Scholar]
- 66.Di Giulio M., Di Campli E., Di Bartolomeo S., Cataldi V., Marzio L., Grossi L., Ciccaglione A.F., Nostro A., Cellini L. In vitro antimicrobial susceptibility of Helicobacter pylori to nine antibiotics currently used in Central Italy. Scand. J. Gastroenterol. 2016;51:263–269. doi: 10.3109/00365521.2015.1092577. [DOI] [PubMed] [Google Scholar]
- 67.Mori H., Suzuki H., Matsuzaki J., Tsugawa H., Fukuhara S., Miyoshi S., Hirata K., Seino T., Matsushita M., Nishizawa T., et al. Rifabutin-based 10-day and 14-day triple therapy as a third-line and fourth-line regimen for Helicobacter pylori eradication: A pilot study. United Eur. Gastroenterol. J. 2016;4:380–387. doi: 10.1177/2050640615618043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ciccaglione A.F., Tavani R., Grossi L., Cellini L., Manzoli L., Marzio L. Rifabutin Containing Triple Therapy and Rifabutin with Bismuth Containing Quadruple Therapy for Third-Line Treatment of Helicobacter pylori Infection: Two Pilot Studies. Helicobacter. 2016;21:375–381. doi: 10.1111/hel.12296. [DOI] [PubMed] [Google Scholar]
- 69.Sung J., Kim N., Park Y.H., Hwang Y.J., Kwon S., Na G., Choi J.Y., Kang J.B., Kim H.R., Kim J.W., et al. Rifabutin-based Fourth and Fifth-line Rescue Therapy in Patients with for Helicobacter pylori Eradication Failure. Korean J. Gastroenterol. 2017;69:109–118. doi: 10.4166/kjg.2017.69.2.109. [DOI] [PubMed] [Google Scholar]
- 70.Siavoshi F., Saniee P., Malekzadeh R. Effective antimicrobial activity of rifabutin against multidrug-resistant Helicobacter pylori. Helicobacter. 2018;23:e12531. doi: 10.1111/hel.12531. [DOI] [PubMed] [Google Scholar]
- 71.Choi Y.I., Jeong S.H., Chung J.W., Park D.K., Kim K.O., Kwon K.A., Kim Y.J., So S., Lee J.H., Jeong J.Y., et al. Rifabutin and Furazolidone Could Be the Candidates of the Rescue Regimen for Antibiotic-Resistant H. pylori in Korea. Can. J. Infect. Dis. Med. Microbiol. 2019;2019:9351801. doi: 10.1155/2019/9351801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lee J.Y., Kim N., Nam R.H., In Choi S., Lee J.W., Lee D.H. Primary and secondary antibiotic resistance of Helicobacter pylori in Korea from 2003 to 2018. Helicobacter. 2019;24:e12660. doi: 10.1111/hel.12660. [DOI] [PubMed] [Google Scholar]
- 73.Kouitcheu Mabeku L.B., Eyoum Bille B., Tepap Zemnou C., Tali Nguefack L.D., Leundji H. Broad spectrum resistance in Helicobacter pylori isolated from gastric biopsies of patients with dyspepsia in Cameroon and efflux-mediated multiresistance detection in MDR isolates. BMC Infect. Dis. 2019;19:880. doi: 10.1186/s12879-019-4536-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Miftahussurur M., Cruz M., Doohan D., Subsomwong P., Abreu J.A.J., Hosking C., Waskito L.A., Yamaoka Y. Five alternative Helicobacter pylori antibiotics to counter high levofloxacin and metronidazole resistance in the Dominican Republic. PLoS ONE. 2019;14:e0213868. doi: 10.1371/journal.pone.0213868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Miftahussurur M., Waskito L.A., Syam A.F., Nusi I.A., Siregar G., Richardo M., Bakry A.F., Rezkitha Y.A.A., Wibawa I.D.N., Yamaoka Y. Alternative eradication regimens for Helicobacter pylori infection in Indonesian regions with high metronidazole and levofloxacin resistance. Infect. Drug Resist. 2019;12:345–358. doi: 10.2147/IDR.S187063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Miftahussurur M., Aftab H., Shrestha P.K., Sharma R.P., Subsomwong P., Waskito L.A., Doohan D., Fauzia K.A., Yamaoka Y. Effective therapeutic regimens in two South Asian countries with high resistance to major Helicobacter pylori antibiotics. Antimicrob Resist. Infect. Control. 2019;8:40. doi: 10.1186/s13756-019-0482-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Graham D.Y., Canaan Y., Maher J., Wiener G., Hulten K.G., Kalfus I.N. Rifabutin-Based Triple Therapy (RHB-105) for Helicobacter pylori Eradication: A Double-Blind, Randomized, Controlled Trial. Ann. Intern. Med. 2020;172:795–802. doi: 10.7326/M19-3734. [DOI] [PubMed] [Google Scholar]
- 78.Hirata Y., Yamada A., Niikura R., Shichijo S., Hayakawa Y., Koike K. Efficacy and safety of a new rifabutin-based triple therapy with vonoprazan for refractory Helicobacter pylori infection: A prospective single-arm study. Helicobacter. 2020;25:e12719. doi: 10.1111/hel.12719. [DOI] [PubMed] [Google Scholar]
- 79.Liu X., Wang H., Lv Z., Wang Y., Wang B., Xie Y., Zhou X., Lv N. Rescue Therapy with a Proton Pump Inhibitor Plus Amoxicillin and Rifabutin for Helicobacter pylori Infection: A Systematic Review and Meta-Analysis. Gastroenterol. Res. Pract. 2015;2015:415648. doi: 10.1155/2015/415648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gingold-Belfer R., Niv Y., Levi Z., Boltin D. Rifabutin triple therapy for first-line and rescue treatment of Helicobacter pylori infection: A systematic review and meta-analysis. J. Gastroenterol. Hepatol. 2020 doi: 10.1111/jgh.15294. [DOI] [PubMed] [Google Scholar]
- 81.Perri F., Festa V., Andriulli A. Treatment of antibiotic-resistant Helicobacter pylori. N. Engl. J. Med. 1998;339:53. doi: 10.1056/NEJM199807023390116. [DOI] [PubMed] [Google Scholar]
- 82.Perri F., Festa V., Clemente R., Quitadamo M., Andriulli A. Rifabutin-based ‘rescue therapy’ for Helicobacter pylori infected patients after failure of standard regimens. Aliment. Pharmacol. Ther. 2000;14:311–316. doi: 10.1046/j.1365-2036.2000.00719.x. [DOI] [PubMed] [Google Scholar]
- 83.Beales I.L. Efficacy of Helicobacter pylori eradication therapies: A single centre observational study. BMC Gastroenterol. 2001;1:7. doi: 10.1186/1471-230X-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Canducci F., Ojetti V., Pola P., Gasbarrini G., Gasbarrini A. Rifabutin-based Helicobacter pylori eradication ‘rescue therapy’. Aliment. Pharmacol. Ther. 2001;15:143. doi: 10.1046/j.1365-2036.2001.00909.x. [DOI] [PubMed] [Google Scholar]
- 85.Gisbert J.P., Calvet X., Bujanda L., Marcos S., Gisbert J.L., Pajares J.M. ‘Rescue’ therapy with rifabutin after multiple Helicobacter pylori treatment failures. Helicobacter. 2003;8:90–94. doi: 10.1046/j.1523-5378.2003.00128.x. [DOI] [PubMed] [Google Scholar]
- 86.Wong W.M., Gu Q., Lam S.K., Fung F.M., Lai K.C., Hu W.H., Yee Y.K., Chan C.K., Xia H.H., Yuen M.F., et al. Randomized controlled study of rabeprazole, levofloxacin and rifabutin triple therapy vs. quadruple therapy as second-line treatment for Helicobacter pylori infection. Aliment. Pharmacol. Ther. 2003;17:553–560. doi: 10.1046/j.1365-2036.2003.01459.x. [DOI] [PubMed] [Google Scholar]
- 87.Gisbert J.P., Gisbert J.L., Marcos S., Pajares J.M. Empirical Helicobacter pylori “rescue” therapy after failure of two eradication treatments. Dig. Liver Dis. 2004;36:7–12. doi: 10.1016/j.dld.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 88.Qasim A., Sebastian S., Thornton O., Dobson M., McLoughlin R., Buckley M., O′Connor H., O′Morain C. Rifabutin- and furazolidone-based Helicobacter pylori eradication therapies after failure of standard first- and second-line eradication attempts in dyspepsia patients. Aliment. Pharmacol. Ther. 2005;21:91–96. doi: 10.1111/j.1365-2036.2004.02210.x. [DOI] [PubMed] [Google Scholar]
- 89.Gisbert J.P., Gisbert J.L., Marcos S., Moreno-Otero R., Pajares J.M. Third-line rescue therapy with levofloxacin is more effective than rifabutin rescue regimen after two Helicobacter pylori treatment failures. Aliment. Pharmacol. Ther. 2006;24:1469–1474. doi: 10.1111/j.1365-2036.2006.03149.x. [DOI] [PubMed] [Google Scholar]
- 90.Gonzalez Carro P., Perez Roldan F., De Pedro Esteban A., Legaz Huidobro M.L., Soto Fernandez S., Roncero Garcia Escribano O., Esteban Lopez-Jamar J.M., Pedraza Martin C., Ruiz Carrillo F. Efficacy of rifabutin-based triple therapy in Helicobacter pylori infected patients after two standard treatments. J. Gastroenterol. Hepatol. 2007;22:60–63. doi: 10.1111/j.1440-1746.2006.04375.x. [DOI] [PubMed] [Google Scholar]
- 91.Navarro-Jarabo J.M., Fernandez N., Sousa F.L., Cabrera E., Castro M., Ramirez L.M., Rivera R., Ubina E., Vera F., Mendez I., et al. Efficacy of rifabutin-based triple therapy as second-line treatment to eradicate helicobacter pylori infection. BMC Gastroenterol. 2007;7:31. doi: 10.1186/1471-230X-7-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gisbert J.P., Gisbert J.L., Marcos S., Jimenez-Alonso I., Moreno-Otero R., Pajares J.M. Empirical rescue therapy after Helicobacter pylori treatment failure: A 10-year single-centre study of 500 patients. Aliment. Pharmacol. Ther. 2008;27:346–354. doi: 10.1111/j.1365-2036.2007.03573.x. [DOI] [PubMed] [Google Scholar]
- 93.Sacco F., Spezzaferro M., Amitrano M., Grossi L., Manzoli L., Marzio L. Efficacy of four different moxifloxacin-based triple therapies for first-line H. pylori treatment. Dig. Liver Dis. 2010;42:110–114. doi: 10.1016/j.dld.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 94.Van Zanten S.V., Desai S., Best L., Cooper-Lesins G., Malatjalian D., Haldane D., Peltekian K. Rescue therapy using a rifabutin-based regimen is effective for cure of Helicobacter pylori infection. Can. J. Gastroenterol. 2010;24:303–306. doi: 10.1155/2010/637908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zullo A., De Francesco V., Manes G., Scaccianoce G., Cristofari F., Hassan C. Second-line and rescue therapies for Helicobacter pylori eradication in clinical practice. J. Gastrointestin. Liver Dis. 2010;19:131–134. [PubMed] [Google Scholar]
- 96.Gisbert J.P., Castro-Fernandez M., Perez-Aisa A., Cosme A., Molina-Infante J., Rodrigo L., Modolell I., Cabriada J.L., Gisbert J.L., Lamas E., et al. Fourth-line rescue therapy with rifabutin in patients with three Helicobacter pylori eradication failures. Aliment. Pharmacol. Ther. 2012;35:941–947. doi: 10.1111/j.1365-2036.2012.05053.x. [DOI] [PubMed] [Google Scholar]
- 97.Jeong M.H., Chung J.W., Lee S.J., Ha M., Jeong S.H., Na S., Na B.S., Park S.K., Kim Y.J., Kwon K.A., et al. Comparison of rifabutin- and levofloxacin-based third-line rescue therapies for Helicobacter pylori. Korean J. Gastroenterol. 2012;59:401–406. doi: 10.4166/kjg.2012.59.6.401. [DOI] [PubMed] [Google Scholar]
- 98.Lim H.C., Lee Y.J., An B., Lee S.W., Lee Y.C., Moon B.S. Rifabutin-based high-dose proton-pump inhibitor and amoxicillin triple regimen as the rescue treatment for Helicobacter pylori. Helicobacter. 2014;19:455–461. doi: 10.1111/hel.12147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fiorini G., Vakil N., Zullo A., Saracino I.M., Castelli V., Ricci C., Zaccaro C., Gatta L., Vaira D. Culture-based selection therapy for patients who did not respond to previous treatment for Helicobacter pylori infection. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2013;11:507–510. doi: 10.1016/j.cgh.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 100.Ierardi E., Giangaspero A., Losurdo G., Giorgio F., Amoruso A., De Francesco V., Di Leo A., Principi M. Quadruple rescue therapy after first and second line failure for Helicobacter pylori treatment: Comparison between two tetracycline-based regimens. J. Gastrointestin. Liver Dis. 2014;23:367–370. doi: 10.15403/jgld.2014.1121.234.qrth. [DOI] [PubMed] [Google Scholar]
- 101.Gisbert J.P., Barrio J., Modolell I., Molina-Infante J., Aisa A.P., Castro-Fernandez M., Rodrigo L., Cosme A., Gisbert J.L., Fernandez-Bermejo M., et al. Helicobacter pylori first-line and rescue treatments in the presence of penicillin allergy. Dig. Dis. Sci. 2015;60:458–464. doi: 10.1007/s10620-014-3365-2. [DOI] [PubMed] [Google Scholar]
- 102.Cosme A., Montes M., Ibarra B., Tamayo E., Alonso H., Mendarte U., Lizasoan J., Herreros-Villanueva M., Bujanda L. Antimicrobial susceptibility testing before first-line treatment for Helicobacter pylori infection in patients with dual or triple antibiotic resistance. World J. Gastroenterol. 2017;23:3367–3373. doi: 10.3748/wjg.v23.i18.3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Papastergiou V., Mathou N., Licousi S., Evgenidi A., Paraskeva K.D., Giannakopoulos A., Stavrou P.Z., Platsouka E., Karagiannis J.A. Seven-day genotypic resistance-guided triple Helicobacter pylori eradication therapy can be highly effective. Ann. Gastroenterol. 2018;31:198–204. doi: 10.20524/aog.2017.0219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cosme A., Torrente Iranzo S., Montes Ros M., Fernandez-Reyes Silvestre M., Alonso Galan H., Lizasoain J., Bujanda L. Helicobacter pylori antimicrobial resistance during a 5-year period (2013-2017) in northern Spain and its relationship with the eradication therapies. Helicobacter. 2019;24:e12557. doi: 10.1111/hel.12557. [DOI] [PubMed] [Google Scholar]
- 105.Ribaldone D.G., Fagoonee S., Astegiano M., Durazzo M., Morgando A., Sprujevnik T., Giordanino C., Baronio M., De Angelis C., Saracco G.M., et al. Rifabutin-Based Rescue Therapy for Helicobacter pylori Eradication: A Long-Term Prospective Study in a Large Cohort of Difficult-to-Treat Patients. J. Clin. Med. 2019;8:199. doi: 10.3390/jcm8020199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Burgos-Santamaría D., McNicholl A., Gisbert J.P. Empirical Helicobacter pylori rescue therapy: An 18-year singlecentre study of 1200 patients. GastroHep. 2019;1:311–324. doi: 10.1002/ygh2.372. [DOI] [Google Scholar]
- 107.Kalfus I.N., Graham D.Y., Riff D.S., Panas R.M. Rifabutin-Containing Triple Therapy (RHB-105) for Eradication of Helicobacter pylori: Randomized ERADICATE Hp Trial. Antibiotics. 2020;9 doi: 10.3390/antibiotics9100685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Saracino I.M., Pavoni M., Zullo A., Fiorini G., Saccomanno L., Lazzarotto T., Antonelli G., Cavallo R., Borghi C., Vaira D. Rifabutin-Based Triple Therapy Or Bismuth-Based Quadruple Regimen As Rescue Therapies For Helicobacter pylori Infection. Eur. J. Intern. Med. 2020 doi: 10.1016/j.ejim.2020.06.029. [DOI] [PubMed] [Google Scholar]
- 109.Song M., Ang T.L. Second and third line treatment options for Helicobacter pylori eradication. World J. Gastroenterol. 2014;20:1517–1528. doi: 10.3748/wjg.v20.i6.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Boyanova L., Markovska R., Hadzhiyski P., Kandilarov N., Mitov I. Rifamycin use for treatment of Helicobacter pylori infection: A review of recent data. Future Microbiol. 2020;15:1185–1196. doi: 10.2217/fmb-2020-0084. [DOI] [PubMed] [Google Scholar]
- 111.Fallone C.A., Moss S.F., Malfertheiner P. Reconciliation of Recent Helicobacter pylori Treatment Guidelines in a Time of Increasing Resistance to Antibiotics. Gastroenterology. 2019;157:44–53. doi: 10.1053/j.gastro.2019.04.011. [DOI] [PubMed] [Google Scholar]
- 112.Gasbarrini A., Ojetti V., Armuzzi A., Branca G., Canducci F., Torre E.S., Candelli M., Pastorelli A., Anti M., Fedeli G., et al. Efficacy of a multistep strategy for Helicobacter pylori eradication. Aliment. Pharmacol. Ther. 2000;14:79–83. doi: 10.1046/j.1365-2036.2000.00685.x. [DOI] [PubMed] [Google Scholar]
- 113.Seppala K., Kosunen T.U., Nuutinen H., Sipponen P., Rautelin H., Sarna S., Hyvarinen H., Farkkila M., Miettinen T.A. Cure of Helicobacter pylori infection after failed primary treatment: One-center results from 120 patients. Scand. J. Gastroenterol. 2000;35:929–934. doi: 10.1080/003655200750022977. [DOI] [PubMed] [Google Scholar]
- 114.Chan F.K., Sung J.J., Suen R., Wu J.C., Ling T.K., Chung S.C. Salvage therapies after failure of Helicobacter pylori eradication with ranitidine bismuth citrate-based therapies. Aliment. Pharmacol. Ther. 2000;14:91–95. doi: 10.1046/j.1365-2036.2000.00674.x. [DOI] [PubMed] [Google Scholar]
- 115.Zullo A., Hassan C., Campo S.M., Lorenzetti R., Febbraro I., De Matthaeis M., Porto D., Morini S. A triple therapy regimen after failed Helicobacter pylori treatments. Aliment. Pharmacol. Ther. 2001;15:1193–1197. doi: 10.1046/j.1365-2036.2001.01028.x. [DOI] [PubMed] [Google Scholar]
- 116.Gomollon F., Sicilia B., Ducons J.A., Sierra E., Revillo M.J., Ferrero M. Third line treatment for Helicobacter pylori: A prospective, culture-guided study in peptic ulcer patients. Aliment. Pharmacol. Ther. 2000;14:1335–1338. doi: 10.1046/j.1365-2036.2000.00833.x. [DOI] [PubMed] [Google Scholar]
- 117.Rokkas T., Sechopoulos P., Robotis I., Margantinis G., Pistiolas D., Cumulative H. pylori eradication rates in clinical practice by adopting first and second-line regimens proposed by the Maastricht III consensus and a third-line empirical regimen. Am. J. Gastroenterol. 2009;104:21–25. doi: 10.1038/ajg.2008.87. [DOI] [PubMed] [Google Scholar]
- 118.De Boer W.A., Tytgat G.N. Regular review: Treatment of Helicobacter pylori infection. BMJ. 2000;320:31–34. doi: 10.1136/bmj.320.7226.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Malfertheiner P. Infection: Bismuth improves PPI-based triple therapy for H. pylori eradication. Nat. Rev. Gastroenterol. Hepatol. 2010;7:538–539. doi: 10.1038/nrgastro.2010.131. [DOI] [PubMed] [Google Scholar]
- 120.Liao J., Zheng Q., Liang X., Zhang W., Sun Q., Liu W., Xiao S., Graham D.Y., Lu H. Effect of fluoroquinolone resistance on 14-day levofloxacin triple and triple plus bismuth quadruple therapy. Helicobacter. 2013;18:373–377. doi: 10.1111/hel.12052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Goodwin C.S., Marshall B.J., Blincow E.D., Wilson D.H., Blackbourn S., Phillips M. Prevention of nitroimidazole resistance in Campylobacter pylori by coadministration of colloidal bismuth subcitrate: Clinical and in vitro studies. J. Clin. Pathol. 1988;41:207–210. doi: 10.1136/jcp.41.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Megraud F. The challenge of Helicobacter pylori resistance to antibiotics: The comeback of bismuth-based quadruple therapy. Therap. Adv. Gastroenterol. 2012;5:103–109. doi: 10.1177/1756283X11432492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wagstaff A.J., Benfield P., Monk J.P. Colloidal bismuth subcitrate. A review of its pharmacodynamic and pharmacokinetic properties, and its therapeutic use in peptic ulcer disease. Drugs. 1988;36:132–157. doi: 10.2165/00003495-198836020-00002. [DOI] [PubMed] [Google Scholar]
- 124.McNicholl A.G., O’Morain C.A., Megraud F., Gisbert J.P., As Scientific Committee of the Hp-Eureg on Behalf of the National Coordinators Protocol of the European Registry on the management of Helicobacter pylori infection (Hp-EuReg) Helicobacter. 2019;24:e12630. doi: 10.1111/hel.12630. [DOI] [PubMed] [Google Scholar]
- 125.Burgos-Santamaría D., Nyssen O.P., Vaira D., Niv Y., Tepes B., Fiorini G., Perez-Aisa A., Rodrigo-Sáez L., Castro-Fernández M., Pellicano R., et al. European registry on H. pylori management (Hp-EuReg): Analysis of 1,782 empirical rescue therapies on third and subsequent lines. Helicobacter. 2020;25(Suppl. 1):41. [Google Scholar]
- 126.Gisbert J.P., McNicholl A.G. Optimization strategies aimed to increase the efficacy of H. pylori eradication therapies. Helicobacter. 2017;22:e12392. doi: 10.1111/hel.12392. [DOI] [PubMed] [Google Scholar]
- 127.Brughera M., Scampini G., Newman A.J., Castellino S., Sammartini U., Mazue G. Overview of toxicological data on rifabutin. Exp. Toxicol. Pathol. 1995;47:1–9. doi: 10.1016/S0940-2993(11)80273-3. [DOI] [PubMed] [Google Scholar]
- 128.Dautzenberg B. The use of rifabutin in Europe for the treatment of mycobacterial infection in AIDS patients. Infection. 1997;25:63–66. doi: 10.1007/BF02113518. [DOI] [PubMed] [Google Scholar]
- 129.Gisbert J.P., Calvet X. Review article: Non-bismuth quadruple (concomitant) therapy for eradication of Helicobater pylori. Aliment. Pharmacol. Ther. 2011;34:604–617. doi: 10.1111/j.1365-2036.2011.04770.x. [DOI] [PubMed] [Google Scholar]
- 130.Griffith D.E., Brown B.A., Girard W.M., Wallace R.J., Jr. Adverse events associated with high-dose rifabutin in macrolide-containing regimens for the treatment of Mycobacterium avium complex lung disease. Clin. Infect. Dis. 1995;21:594–598. doi: 10.1093/clinids/21.3.594. [DOI] [PubMed] [Google Scholar]
- 131.Apseloff G., Fluids G., LaBoy-Goral L., Kraut E., Vincent J. Severe neutropenia caused by recommended prophylactic doses of rifabutin. Lancet. 1996;348:685. doi: 10.1016/S0140-6736(05)65109-4. [DOI] [PubMed] [Google Scholar]
- 132.Burman W., Benator D., Vernon A., Khan A., Jones B., Silva C., Lahart C., Weis S., King B., Mangura B., et al. Acquired rifamycin resistance with twice-weekly treatment of HIV-related tuberculosis. Am. J. Respir. Crit. Care Med. 2006;173:350–356. doi: 10.1164/rccm.200503-417OC. [DOI] [PubMed] [Google Scholar]
- 133.Li J., Munsiff S.S., Driver C.R., Sackoff J. Relapse and acquired rifampin resistance in HIV-infected patients with tuberculosis treated with rifampin- or rifabutin-based regimens in New York City, 1997–2000. Clin. Infect. Dis. 2005;41:83–91. doi: 10.1086/430377. [DOI] [PubMed] [Google Scholar]