Abstract
Whitebacked planthopper (WBPH) is a pest that causes serious damage to rice in Asian countries with a mild climate. WBPH causes severely rice yield losses and grain poor quality each year so needs biological control. Plants resist biotic and abiotic stress using expressing variety genes, such as kinase, phytohormones, transcription factors, and especially secondary metabolites. In this research, quantitative trait locus (QTL) mapping was performed by assigning the WBPH resistance score in the Cheongcheong/Nagdong doubled haploid (CNDH) line in 2018 and 2019. The RM280-RM6909 on chromosome 4 was detected as a duplicate in 2018, 2019, and derived from Cheongcheong. This region includes cell function, kinase, signaling, transcription factors, and secondary metabolites that protect plants from the stress of WBPH. The RM280-RM6909 on chromosome 4 contains candidate genes that are similar to the flavanone 3-hydroxylase (F3H) of rice. The F3H are homologous genes, which play an important role in biosynthesis defending against biotic stress in plants. After WBPH inoculation, the relative expression level of F3H was higher in resistant line than in a susceptible line. The newly identified WBPH resistance gene F3H by QTL mapping can be used for the breeding of rice cultivars that are resistant against WBPH.
Keywords: biotic stress, quantitative trait locus, rice, secondary metabolite, whitebacked planthopper
1. Introduction
Whitebacked planthoppers (WBPH, Sogatella furcifera) cause serious damage to rice from long-distance migratory pests that exist in rice cultivation areas around the world, including South Korea, and cause enormous economic losses due to reduced production. Rice is one of the world’s three largest crops and, including South Korea, is the most popular crop that two-thirds of the world’s population consumes in the main meal [1]. However, in the fields where rice is bred, many pests that damage rice is also distributed. In particular, WBPH acts as a vector for southern rice black-streaked dwarf virus (SRBSDV) and is the most destructive rice pest [2] that causes various damages such as wilting, blight, and lodging of rice [3,4]. WBPH is a flying pest that arrives in South Korea from late June to July every year in Southern China and Southeast Asia [5]. The amount of WBPH flying is more than 10 times higher than that of brown planthopper (BPH), and the largest WBPH time of occurrence in South Korea is from the end of July to the beginning of August, and the injury symptom is shown after the end of August [6]. Currently, in the case of South Korea, WBPH control depends on chemicals, but it is difficult to detect WBPH damage at an early stage. Since spraying of agrochemicals in a state where the degree of damage is clearly indicated already missed the optimal time for recovery of damage, it is difficult to control WBPH only with agrochemical control, so the development of WBPH resistant cultivar is required [7,8,9,10]. The incidence of WBPH has increased in China and Vietnam, and a new kind of SRBSDV has been discovered [11]. Previous attempts to understand WBPH resistance in rice confirmed many important genes such as Wbph1, Wbph2, Wbph3, wbph4, Wbph5 [12], Wbph6(t) [13,14], Wbph7(t), and Wbph8(t) [15].
In South Korea, Cheongcheong is resistant to WBPH, but it is an indica type cultivar. In order to develop a japonica-type resistant cultivar, it must be crossed with indica type rice. In general, WBPH resistance has been successfully introduced to indica cultivars, but reports of japonica cultivars breeding are very poor [16]. This is because the resistant genetic resources of WBPH are mostly derived from indica cultivars. In the process of introducing resistance genetic resources into japonica type rice through the remote cross, agriculturally inferior traits were introduced together, making it difficult to develop practical japonica type cultivars [17].
WBPH resistance is a quantitative trait associated with polygenes with diverse and complex biological properties. Molecular markers, along with high-density loci, have been widely used to find quantitative trait locus (QTL) for resistance to biological stress such as insects and pathogens in many plants [18]. There are insufficient reports of QTL related to WBPH resistance in rice [19]. Sogawa et al., 2003 [20] discovered a QTL associated with resistance and susceptibility based on the sucking response of WBPH in the ‘Zhaiyeqing8/Jingxi17’ doubled haploid (DH) lines. Yamasaki et al., 2003 [21] discovered antibiotics related QTLs based on WBPH oviposition reactions using recombinant inbreeding lines obtained from ‘Asominori/IR24’. QTL analysis can be used to identify loci associated with WBPH resistance and apply the Marker-assisted selection (MAS) system to select lines with WBPH resistance [22].
In this research, Cheongcheong (Indica) and Nagdong (Japonica) were crossed to breeding Cheongcheong/Nagdong doubled haploid (CNDH) rice in order to establish a MAS system for breeding WBPH resistant rice cultivar. The DH line was used to mapping and analyze key genes and QTLs for various agricultural traits [23,24,25]. The main purpose of this research is to identify WBPH resistance genes by analysis candidate genes that have the greatest effect on WBPH resistance in the CNDH line using QTL mapping. This WBPH resistance gene can improve resistance to unpredictable WBPH infection due to extreme climate change in the future and can be effectively used for breeding WBPH resistance rice cultivars.
2. Results
2.1. Analysis of QTLs Associated with WBPH Resistance
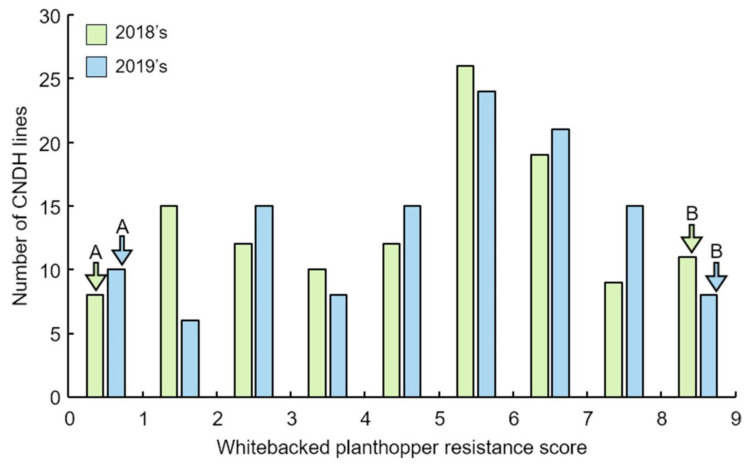
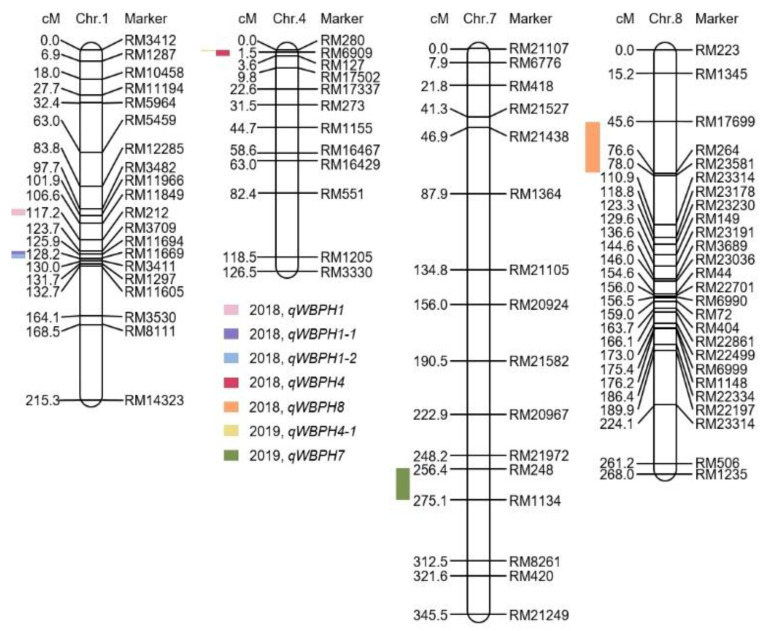
The phenotypes of CNDH lines were measured to screen for resistant lines. The genetic map with an average of 10.6 cM between markers was constructed from 120 CNDH lines, which included 222 SSR (Simple Sequence Repeats) markers. The frequency distribution was created by assigning a resistance score after WBPH inoculation to 120 CNDH lines in 2018 and 2019 (Figure 1). The WBPH resistance score in the CNDH lines represented normal distributions. Therefore, WBPH resistance was a quantitative trait associated with more than one gene. After inoculating WBPH to Cheongcheong, Nagdong, and CNDH lines based on the resistance score, WBPH resistance-related QTL mapping was performed (Supplementary Table S1). As a result, the LOD value was 3.0 or more in seven QTLs. QTL was detected on chromosomes 1, 4, 7, and 8. WBPH resistance-related QTL mapping was performed in 2018 and 2019. In 2018, QTL was detected on chromosomes 1, 4, and 8; in 2019, QTL was detected on chromosomes 4 and 7. In 2018, qWBPH1 was mapped at RM3482-RM11966 on chromosome 1 with an LOD value of 4.0 and a phenotypic change of 30%, qWBPH1-1 was mapped at RM3709-RM11694 on chromosome 1 with an LOD value of 3.5 and a phenotypic change of 30%, qWBPH1-2 was mapped at RM11694-RM11669 on chromosome 1 with an LOD value of 3.3 and a phenotypic change of 30%, qWBPH4 was mapped at RM280-RM127 on chromosome 4 with an LOD value of 3.2 and a phenotypic change of 30%, and qWBPH8 was mapped at RM17699-RM264 on chromosome 8 with an LOD value of 3.3 and a phenotypic change of 30%. In 2019, qWBPH4-1 was mapped at RM280-RM6909 on chromosome 4 with an LOD value of 3.5 with a phenotypic change of 30% and qWBPH7 was mapped at RM248-RM1134 on chromosome 7 with an LOD value of 3.0 and a phenotypic change of 30%. Of the WBPH resistance-related QTLs detected in 2018 and 2019, all were derived from Cheongcheong, except qWBPH1, which was derived from Nagdong. As a result of QTL mapping associated with WBPH resistance, RM280-RM6909 with an LOD value of 3.2 over on chromosome 4 was detected in duplicate for two years (Figure 2).
Figure 1.
Frequency distribution based on the Whitebacked planthopper (WBPH) resistance score in the CNDH line. The WBPH resistance score showed normal distribution in the Cheongcheong/Nagdong doubled haploid (CNDH) line. Therefore, the response to WBPH resistance involved more than one gene. A, Cheongcheong. B, Nagdong.
Figure 2.
Chromosomal region of quantitative trait locus (QTL) associated with the WBPH resistance gene in the CNDH lines. QTL mapping was analyzed by assigning the WBPH resistance score in the Cheongcheong/Nagdong doubled haploid (CNDH) line in 2018, and 2019. WBPH resistance-related QTL mapping results indicated that the WBPH resistance gene was located on chromosomes 1, 4, 7, and 8. On chromosome 4, the RM280-RM127 was detected in 2018, and the RM280-RM6909 was detected in 2019.
2.2. Candidate Gene Search Associated with WBPH Resistance Based on QTL Mapping
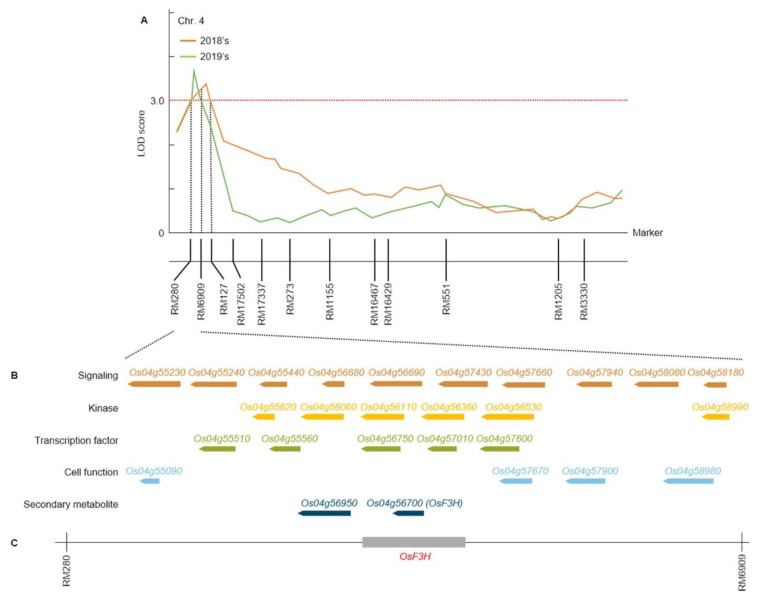
We investigated candidate genes associated with WBPH resistance by selecting RM280-RM6909 on chromosome 4, which was detected in duplicate as a result of QTL mapping associated with WBPH resistance in 2018 and 2019. Twenty-seven open reading frames (ORFs) associated with WBPH resistance were identified using RiceXPro and the RAP-DB (https://rapdb.dna.affrc.go.jp). RM280-RM6909 on chromosome 4 contained candidate genes that correspond to cell function, kinase, signaling, secondary metabolites, and transcription factor associated with WBPH resistance (Figure 3, Supplementary Table S2).
Figure 3.
QTL mapping analysis based on WBPH resistance score in the CNDH lines. QTL mapping related to WBPH resistance was performed in 2018 and 2019. (A) RM280-RM6909 with an LOD value of 3.2 over on chromosome 4 was detected in duplicate for 2 years. (B) As a result of the physical map analysis of this region, candidate genes for signaling, kinase, transcription factor, cell function, and secondary metabolite involved in WBPH resistance was contained. (C) Among these candidate genes, F3H, a secondary metabolite, was identified.
2.3. Comparative Analysis of the Selection of Candidate Genes for WBPH Resistance
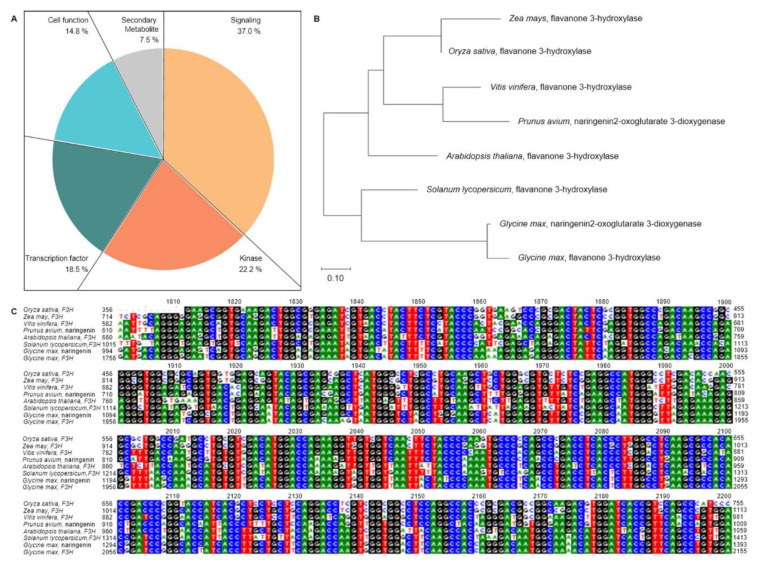
Of the 27 ORFs, 37.0% were signaling, 22.2% were kinases, 18.5% were transcription factors, 14.8% were cell function, and 7.5% were secondary metabolites (Figure 4A). Among these candidate genes, LOC_Os04g56700 has a similar function to flavanone 3-hydroxylase (F3H) in rice and acts as an important enzyme for flavonoid biosynthesis. Flavonoids are major secondary metabolites of plants and play important roles in defense mechanisms in plants. Because the F3H overexpression in rice showed WBPH resistance, the F3H gene was selected [26].
Figure 4.
Candidate gene distribution based on WBPH resistance-related QTL mapping phylogenetic tree of F3H. (A) Candidate genes associated with WBPH resistance included cell function, kinase, signaling, transcription factors, and secondary metabolites. Signaling was 37.0%, kinase was 22.2%, transcription factors were 18.5%, cell function was 14.8%, and secondary metabolites were 7.5%. (B) Comparison of homology between F3H of Oryza sativa (O. sativa) and F3H of A. thaliana, Z. mays, G. max, P. avium, S. lycopersicum, and V. vinifera. F3H of O. sativa was the most similar to F3H of Z. mays and showed a relatively high similarity to V. vinifera and P. avium. (C) Multiple sequence alignments of F3H. Comparison to the conserved nucleic acid sequences found in the F3H domain region of O. sativa, A. thaliana, Z. mays, G. max, P. avium, S. lycopersicum, and V. vinifera.
2.4. Phylogenetic Tree and Homology Sequence Analysis of Candidate Genes
BLAST analysis of the WBPH resistance gene F3H showed a similar sequence to naringenin. F3H was present not only in O. sativa but also in A. thaliana, Glycine max, and Zea mays as well as in Prunus avium, Solanum lycopersicum, and Vitis vinifera, which have high flavonoid contents. A phylogenetic tree was created to confirm the genetic similarity between the F3H and naringenin and the F3H present in rice (Figure 4B). The F3H of O. sativa was classified into the same group as F3H in Z. mays and had the highest homology. The F3H of V. vinifera and naringenin 2-oxoglutarate 3-dioxygenase of P. avium were also grouped and had a relatively high homology (Figure 4C). These F3H genes had homologous domain regions.
2.5. Relative Expression Levels with Plant Defense Genes
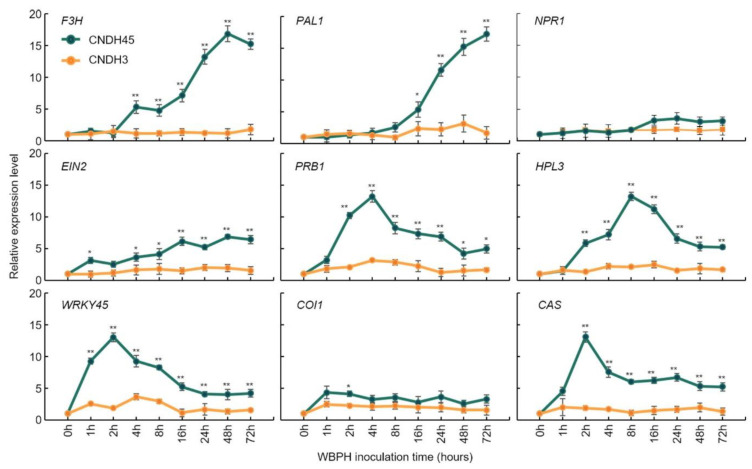
After inoculating WBPH to CNDH45 (WBPH-resistant line) and CNDH3 (WBPH-susceptible line), the relative expression level of F3H [27] was measured on certain time points. Moreover, plant defense genes of O. sativa and Arabidopsis thaliana, such as phenylalanine ammonia-lyase (PAL1) [28], non-expressor of pathogenesis-related genes 1 (NPR1 ) [29], ethylene insensitive 2 (EIN2) [30], pathogenesis-related protein (PRB1) [31], hydroperoxide lyase3 (HPL3) [32], WRKY45 [33], coronatine insensitive 1 (COI1) [34], and beta-caryophyllene synthase (CAS) [35], were compared in relative expression levels. These genes are resistant to biotic stress, such as insect wounding. The expression level of F3H in rice had a significant difference between the resistant and susceptible lines 4 h after WBPH inoculation (p = 0.01; Figure 5). The expression level continued to increase from 4 to 48 h after WBPH inoculation and decreased after 48 h. PAL1 also showed a similar tendency as F3H. PAL1 had a significant difference between the resistant and susceptible lines 16 h after WBPH inoculation (p = 0.05). The expression levels of EIN2, PRB1, HPL3, WRKY45, COI1, and CAS also had a difference between the resistant and susceptible lines. EIN2 and WRKY45 showed a significant difference 1 h after WBPH inoculation (p = 0.05 and 0.01, respectively). PRB1, HPL3, COI1, and CAS showed significant differences 2 h after WBPH inoculation (p = 0.01, 0.01, 0.05, and 0.01, respectively). However, COI1 showed a significant difference only 2 h after WBPH inoculation (p = 0.05), and there was no significant difference after that.
Figure 5.
Comparison of the relative expression levels of F3H depending on the WBPH inoculation time in WBPH-resistant and WBPH-susceptible lines. F3H revealed by WBPH resistance-related QTL mapping was compared to the relative expression levels of WBPH resistance genes in O. sativa and A. thaliana. All genes were highly expressed in the WBPH-resistant line than in the WBPH-susceptible line. F3H showed a significant difference between the resistant and susceptible lines 4 h after WBPH inoculation (p = 0.01) and showed higher gene expression levels over time. CNDH45, WBPH-resistant line; CNDH3, WBPH-susceptible line. * indicates a significant difference at p < 0.05; ** indicates a significant difference at p < 0.01.
3. Discussions
WBPH is a significant cause of biotic stress that seriously damage rice growth in Asia. In modern agriculture, a synthetic pest control agent is used to reduce pest damage. However, synthetic pest control agents cause environmental pollution and WBPH becomes resistant to these agents, resulting in ecosystem disruption. An eco-friendly alternative is to breed rice cultivars that confer WBPH resistance gene in rice. In this research, CNDH lines, which were developed through cross of Cheongcheong (WBPH-resistant cultivar) and Nagdong (WBPH-susceptible cultivar), were subjected to QTL mapping based on the WBPH resistance score in 2018 and 2019. The WBPH resistance score in the CNDH lines represented normal distributions. Therefore, WBPH resistance is a quantitative trait associated with more than one gene. As a result, seven QTLs on chromosomes 1, 4, 5, 7, and 8 were detected. Among them, RM280-RM6909 on chromosome 4 was detected in duplicate for 2 years with an LOD value of 3.2 over. The LOD values for 2018 and 2019 are 3.2 and 3.5 with a phenotypic change of 30%. Twenty-seven ORFs were identified in the RM280-RM6909 region of chromosome 4, where 37.0% were for signaling, 22.2% for kinases, 18.5% for transcription factors, 14.8% for cell function, and 7.5% for secondary metabolites. Plants have a mechanism for synthesizing defense substances in response to invading pathogens. Recent research on plant-pathogen systems has accelerated. In particular, research on the mechanism for synthesizing plant secondary metabolites in response to biotic stress, such as pathogen inoculation, has emerged in plant biology [36]. Therefore, secondary metabolites produced in response to pathogen inoculation were selected as an available substance for an eco-friendly pest control agent [37]. Plant secondary metabolites mainly act on other species and influence ecological interactions with the plant and the environment [38]. Of the secondary metabolites, flavonoids occur widely in plants and can be divided into subgroups, including anthocyanidins, flavonols, flavones, flavanones, chalcones, dihydrochalcones, and dehydroflavonols. Thus, they are biologically significant and chemically diverse. Flavonoids not only induce the activity of medicinal plants and have a pharmacological effect [39,40,41,42] but also are synthesized through physiologically active compounds of the plants themselves, stress protectors, attractants, or appetite suppressants. Pathogens of various types play a significant role in plant resistance [36]. Many researches have already reported that the accumulation of flavonoids in plants makes them resistant to a variety of abiotic and biotic stresses [43,44]. Specifically, Brunetti et al., 2013 reported that flavonoids as effective in removing reactive oxygen species. Therefore, flavonoids are involved in the plant defense system through various actions. Moreover, Jan et al., 2020 [26] reported that the flavonoid series Quercetin, Delphinidin, Kaempferol, and Cyanidin increased in concentration by a significant difference at the 1% level in overexpression of F3H compared to control. Plants with increased flavonoids had increased resistance to WBPH compared to control. Therefore, an increase in flavonoids can confer resistance to biotic stress, including WBPH, and reduce damage.
In this research, as a result of QTL mapping associated with WBPH resistance, ORFs associated with secondary metabolites on chromosome 4 were detected. LOC_Os04g56700 on chromosome 4 had a similar sequence to F3H. Some of the previously reported WBPH resistance genes compared to LOC_Os04g56700 were WBPH resistance genes identified through QTL mapping, which confirmed their effects on WBPH resistance. F3H of rice had a similar effect to PAL1, which is an enzyme involved in the biosynthesis of polyphenolic compounds such as flavonoids [45]. Expression levels of F3H showed a significant difference between the resistant and susceptible line 4 h after WBPH inoculation (p = 0.01). These results proved that F3H identified in QTL mapping using the WBPH resistance score is precisely resistant to WBPH. The expression levels of F3H and PAL1 increased significantly in the latter period of WBPH inoculation, and those of PRB1, HPL3, WRKY45, and CAS gradually decreased after the expression level increased in the early period after WBPH inoculation. F3H exists not only in O. sativa but also in A. thaliana, G. max, P. avium, S. lycopersicum, V. vinifera, and Z. mays. The homology of the F3H sequence was compared to F3H in other plants. As a result, F3H of O. sativa was classified into the same group as F3H of Z. mays, so it had the highest homology. F3H of V. vinifera and naringenin 2-oxoglutarate 3-dioxygenase of P. avium, which have high flavonoid content, were also grouped, and had a relatively high homology. They have F3H and homology domains of rice, and it is possible to predict that they have similar functions. F3H is an enzyme involved in the biosynthetic pathway of dihydroflavonols in flavanones [46]. Dihydroflavonol synthesized by F3H is synthesized into leucocyanidin, which performs radical scavenging activities through dihydroflavonol 4-reductase (DFR) [47]. Leucocyanidin is synthesized by anthocyanidin synthase (ANS) into anthocyanidin, which prevents oxidative stress [48]. Finally, anthocyanin, which protects against invasion of bacteria and insects [49] and has excellent antioxidant effects, was synthesized by UDP-glucose flavonoid 3-O-glucosyltransferase (UFGT) [50,51].
4. Materials and Methods
4.1. Plant Materials and Treatments
The Cheongcheong/Nagdong double haploid (CNDH) line was bred in the field at Kyungpook National University from 2010. F1 obtained through crossing of Cheongcheong (Indica) and Nagdong (Japonica) was cultured to double haploid, and CNDH 120 line was developed. The CNDH line has been making generational progress for over 10 years and is currently being used as a bridging parent. In addition, it has been sufficiently used as a transforming and gene expression verification group also verified the stable expression of the gene using the CNDH line. The seeds were treated with a seed disinfectant in the dark at 25 °C for 4 days. The germinated seeds were sown on April 27, 2018 and April 26, 2019 at the experimental field at Kyungpook National University and transplanted on May 25, 2018 and May 24, 2019, with a planting distance of 30 × 15 cm. The amount of fertilizer applied was N–P2O5–K2O = 9.0–4.5–5.7 kg/10a, and the rice was cultivated according to the Rural Development Administration standard rice cultivation method.
4.2. WBPH Rearing
The rearing cage of WBPH was maintained at a temperature at 28 °C, humidity of 60%, and light intensity illumination of 16 h/day. Rice sowing was done weekly for feeding of WBPH and fresh seedlings of Chucheong was supplied for WBPH feeding. WBPH were able to move themselves to fresh plants. All WBPH had been reared in the oviposition stage.
4.3. Evaluation of WBPH Resistance in the CNDH Line
The WBPH resistance gene was evaluated using QTL mapping in WBPH-resistant and WBPH-susceptible lines. For bioassay analysis, seeds were sown in plastic trays (14 × 20 × 4.5 cm) at 3.5 × 4 cm intervals and cultivated to the seedling stage. Rice of the seedling stage was inoculated with second to third WBPH instars. About 15 WBPH were inoculated per plant. Phenotype change was observed after WBPH inoculation, and resistance scores were assigned based on plant damage evaluation. The evaluation of plant damage was performed using the Standard Evaluation System for Rice scale [52,53]. Resistance score was assigned 0 points if the plant was not damaged, 1 point if there were some damage, 3 points if the leaves were slightly underdeveloped, 5 points if the leaves were underdeveloped in more than half of the leaves, 7 points if more than half of the plants have died, and 9 points when the plant ultimately died. When Nagdong, a WBPH-susceptible cultivar, died, each plant was evaluated, and a resistance score of 9 was assigned.
4.4. QTLs Analysis of WBPH Resistance
QTL mapping was performed using WinQTL-cart2.5 [54]. The chromosome map of CNDH lines was created using the 222 SSR markers associated with WBPH resistance. Composite Interval Mapping was used to analyze WBPH resistance, and an LOD value of 3.0 or more was used to improve the accuracy of QTL mapping. The R2 value was used to represent the percentage of phenotypic changes that could be explained by QTL.
4.5. Identification of Candidate Genes through QTL Mapping
To identify candidate genes through QTL mapping, RiceXPro (https://ricexpro.dna.affrc.go.jp/) and Rapdb (https://rapdb.dna.affrc.go.jp/) were used. Candidate genes existing between markers obtained by QTL mapping were classified by function, and genes associated with WBPH resistance were analyzed.
4.6. Analysis of Expression Levels of Candidate Genes Resistant to WBPH
CNDH45 (WBPH-resistant line) and CNDH3 (WBPH-susceptible line) were inoculated with WBPH at the seedling stage when 3–4 main leaves were observed after sowing. The leaves were sampled at 0, 1, 2, 4, 8, 16, 24, 48, and 72 h after WBPH inoculation. RNA was extracted from the leaves using the RNeasy Plant Mini kit (QIAGEN, Hilden, Germany). cDNA was synthesized 80 ng RNA as a template using and qPCRBIO cDNA Synthesis kit (PCR Biosystems, Wayne, PA, USA). qRT-PCR was performed on the Eco Real-Time PCR System using WBPH resistance gene-specific primers (Supplementary Table S3). The qRT-PCR reaction contain 10 μL of 2× Real-time PCR Master Mix (BioFACT, Daejeon, Korea), 2 μL cDNA, 1 μL forward primer (10 pmol/μL), 1 μL reverse primer (10 pmol/μL), and DNase-free water to a final volume of 20 μL. The OsActin gene was used for the control. Each reaction was repeated three times, and the mean and standard deviation were represented.
4.7. Statistical Analysis
Data were analyzed by the SPSS program (IMMSPSS Statistics, version 22, IBMSPSS Statistics, version 22, Redmond, WC, USA). The mean and standard deviation were calculated and statistically analyzed through three repeated experiments. Furthermore, the candidate gene associated with WBPH resistance was compared to the control OsActin gene in terms of relative expression levels to analyze significant differences.
5. Conclusions
WBPH is a significant pest that causes severe damage to rice in Asian countries with a mild climate. Plants become resistant to biotic stress, such as WBPH, by synthesizing secondary metabolites to protect themselves. Therefore, secondary metabolites are essential elements in plant defense. QTL mapping was performed by assigning the WBPH resistance score in the CNDH line in 2018 and 2019. In the QTL mapping result for two years, the same region was mapped on chromosome 4. F3H was detected at the RM280-RM6909 region in rice. The F3H was involved in the synthesis of secondary metabolites. Moreover, this region was derived from Cheongcheong. Sequences similar to F3H have been found not only in O. sativa but also in Z. mays and have an essential role in biosynthesis defense against biotic stress in plants. They have homologous sequences, and F3H of O. sativa had the highest homology with F3H of Z. mays, so it was predicted that they would function similarly. After WBPH inoculation, the relative expression level of F3H was higher in the resistant line than in a susceptible line. The newly identified WBPH resistance gene F3H can be used for the development of rice cultivars that are resistant against WBPH, which has a negative impact on rice.
Supplementary Materials
The following are available online at https://www.mdpi.com/2223-7747/10/1/81/s1. Table S1. QTLs associated with WBPH resistance in the CNDH lines. Table S2. Twenty-seven candidate genes identified between RM280 and RM6909 markers and their ORFs, which include various proteins associated with WBPH resistance. Table S3. WBPH resistance gene-specific primers for qRT-PCR.
Author Contributions
Conceptualization, E.-G.K. and S.Y.; methodology, E.-G.K. and S.Y.; formal analysis, E.-G.K.; investigation, E.-G.K., J.-R.P., and K.-M.K.; writing—original draft preparation, E.-G.K. and S.Y.; writing—review and editing, E.-G.K.; project administration, K.-M.K. All authors read and approved the final manuscript.
Funding
This work was supported by National Research Foundation of Korea, grant number NRF-2017R1D1A3B04028676.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare that they have no competing interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ray D.K., Mueller N.D., West P.C., Foley J.A. Yield trends are insufficient to double global crop production by 2050. PLoS ONE. 2013;8:e66428. doi: 10.1371/journal.pone.0066428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Park H.H., Lee J.H., Kim S.T. Occurrence and Population Dynamics of Spiders in Transplanting Rice Fields under Different Levels of Pest Management. Korean J. Ecol. 2005;28:287–293. doi: 10.5141/JEFB.2005.28.5.287. [DOI] [Google Scholar]
- 3.Zhou G.H., Wen J.J., Cai D.J., Li P., Xu D.L., Zhang S.G. Southern rice black-streaked dwarf virus: A new proposed Fiji virus species in the family Reoviridae. Chin. Sci. Bull. 2008;53:3677–3685. doi: 10.1007/s11434-008-0467-2. [DOI] [Google Scholar]
- 4.Chandrasekar K., Suresh S., Soundararajan R.P., Boopathi T. Evaluation of resistance in some rice genotypes against Whitebacked Planthopper (WBPH) Sogatella furcifera (Horvath) J. Entomol. Zool. 2017;5:1575–1577. [Google Scholar]
- 5.Pu L., Xie G., Ji C., Ling B., Zhang M., Xu D., Zhou G. Transmission characteristics of Southern rice black-streaked dwarf virus by rice planthoppers. Crop Prot. 2012;41:71–76. doi: 10.1016/j.cropro.2012.04.026. [DOI] [Google Scholar]
- 6.Shin M.S., Ko J.K., Ko J.C., Kim B.K., Kang H.J., Kim Y.D., Nam J.K., Ha K.Y., Kim K.Y., Baek M.G., et al. A Brown Planthopper Resistance and High Quality Rice Variety ‘Chinnong’. Korean J. Breed. Sci. 2012;44:373–378. [Google Scholar]
- 7.Suh J.P., Jeung J.U., Kim Y.G., Jena K.K., Cho Y.C., Lee J.H., Kim M.K., Hong H.C., Lee J.H., Kim J.J., et al. A Brown Planthopper Resistant and High Grain Quality Rice Variety ‘Anmi’ Developed by Molecular Breeding Method. Korean J. Breed. Sci. 2014;46:152–159. doi: 10.9787/KJBS.2014.46.2.152. [DOI] [Google Scholar]
- 8.Choi S.Y., Song Y.H., Lee J.O., Park J.S. Studies on the Varietal Resistance of Rice to the White-backed Planthopper, Sogatella furcifera Horvath (III) Korean J. Appl. Entomol. 1973;12:139–142. [Google Scholar]
- 9.Lee Y.T., Heu M.H. Linkage analysis of the resistance genes to whitebacked planthopper (Sogatella furcifera Horvath) in rice. Korean J. Crop Sci. 1984;29:136–151. [Google Scholar]
- 10.Kim K.M., Park Y.H. Studies of the Life Cycle and Rearing Methods of Whitebacked Planthopper (Sogatella furcifera Horváth) J. Life Sci. 2018;28:357–360. [Google Scholar]
- 11.Lee J.H., Yeo U.S., Cho J.H., Lee J.Y., Song C.S., Shin M.S., Kang H.W., Sohn J.K. Marker Assisted Selection of Brown Planthopper Resistance and Development of Multi-Resistance to Insect and Diseases in Rice (Oryza sativa L.) Korean J. Breed. Sci. 2011;43:413–421. [Google Scholar]
- 12.Khush G.S., Brar D.S. Genetics of resistance to insects in crop plants. Adv. Agron. 1991;45:223–274. [Google Scholar]
- 13.Ma J.F., Goto S., Tamai K., Ichii M. Role of root hairs and lateral roots in silicon uptake by rice. Plant Physiol. 2001;127:1773–1780. doi: 10.1104/pp.010271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li H.B., Wong C.C., Cheng K.W., Chen F. Antioxidant properties in vitro and total phenolic contents in methanol extracts from medicinal plants. LWT Food Sci. Technol. 2008;41:385–390. doi: 10.1016/j.lwt.2007.03.011. [DOI] [Google Scholar]
- 15.Tan M., Zhong W., Song D., Thornton S., Jiang X. E. coli-expressed recombinant norovirus capsid proteins maintain authentic antigenicity and receptor binding capability. J. Med. Virol. 2004;74:641–649. doi: 10.1002/jmv.20228. [DOI] [PubMed] [Google Scholar]
- 16.Lee J.G., Hong S.S., Kim J.Y., Park K.Y., Lim J.W., Lee J.H. Occurrence of stink bugs and pecky rice damage by stink bugs in paddy fields in Gyeonggi-do, Korea. Korean J. Appl. 2009;48:37–44. doi: 10.5656/KSAE.2009.48.1.037. [DOI] [Google Scholar]
- 17.Nemoto H., Ikeda R., Kaneda C. New genes for resistance to brown planthopper, Nilaparvata lugens Stal, in rice. Jpn. J. Breed. 1984;39:23–28. doi: 10.1270/jsbbs1951.39.23. [DOI] [Google Scholar]
- 18.Yencho G.C., Cohen M.B., Byrne P.F. Applications of tagging and mapping insect resistance loci in lants. Annu. Rev. Entomol. 2000;45:393–422. doi: 10.1146/annurev.ento.45.1.393. [DOI] [PubMed] [Google Scholar]
- 19.Geethanjali S., Kadirvel P., Gunathilagaraj K., Maheswaran M. Detection of quantitative trait loci (QTL) associated with resistance to whitebacked planthopper (Sogatella furcifera) in rice (Oryza sativa) Plant Breed. 2009;128:130–136. doi: 10.1111/j.1439-0523.2008.001565.x. [DOI] [Google Scholar]
- 20.Sogawa K., Liu G.J., Shen J.H. A review on the hyper-susceptibility of Chinese hybrid rice to insect pests. Chin. J. Rice Sci. 2003;17:23–30. [Google Scholar]
- 21.Yamasaki M., Yoshimura A., Yasui H. Genetic basis of ovicidal response to whitebacked planthopper (Sogatella furcifera Horvath) in rice (Oryza sativa L.) Mol. Breed. 2003;12:133–143. doi: 10.1023/A:1026018821472. [DOI] [Google Scholar]
- 22.Lande R., Thompson R. Efficiency of marker-assisted selection in the improvement of quantitative traits. Genetics. 1990;124:743–756. doi: 10.1093/genetics/124.3.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Forster B.P., Thomas W.T. Doubled haploids in genetics and plant breeding. Plant Breed. Rev. 2005;25:57–88. [Google Scholar]
- 24.Bao J.S., Wu Y.R., Hu B., Wu P., Cui H.R., Shu Q.Y. QTL for rice grain quality based on a DH population derived from parents with similar apparent amylose content. Euphytica. 2002;128:317–324. doi: 10.1023/A:1021262926145. [DOI] [Google Scholar]
- 25.Huang N., Parco A., Mew T., Magpantay G., McCouch S., Guiderdoni E., Khush G.S. RFLP mapping of isozymes, RAPD and QTLs for grain shape, brown planthopper resistance in a doubled haploid rice population. Mol. Breed. 1997;3:105–113. doi: 10.1023/A:1009683603862. [DOI] [Google Scholar]
- 26.Jan R., Khan M.A., Asaf S., Lee I.J., Kim K.M. Overexpression of OsF3H modulates WBPH stress by alteration of phenylpropanoid pathway at a transcriptomic and metabolomic level in Oryza sativa. Sci. Rep. 2020;10:1–16. doi: 10.1038/s41598-020-71661-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lucini L., Baccolo G., Rouphael Y., Colla G., Bavaresco L., Trevisan M. Chitosan treatment elicited defence mechanisms, pentacyclic triterpenoids and stilbene accumulation in grape (Vitis vinifera L.) bunches. Phytochemistry. 2018;156:1–8. doi: 10.1016/j.phytochem.2018.08.011. [DOI] [PubMed] [Google Scholar]
- 28.Lin S., Nie P., Ding S., Zheng L., Chen C., Feng R., Zhou S. Quantitative proteomic analysis provides insights into rice defense mechanisms against Magnaporthe oryzae. Int. J. Mol. Sci. 2018;19:1950. doi: 10.3390/ijms19071950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chern M.S., Fitzgerald H.A., Yadav R.C., Canlas P.E., Dong X., Ronald P.C. Evidence for a disease-resistance pathway in rice similar to the NPR1-mediated signaling pathway in Arabidopsis. Plant J. 2001;27:101–113. doi: 10.1046/j.1365-313x.2001.01070.x. [DOI] [PubMed] [Google Scholar]
- 30.Duan C., Yu J., Bai J., Zhu Z., Wang X. Induced defense responses in rice plants against small brown planthopper infestation. Crop. J. 2014;2:55–62. doi: 10.1016/j.cj.2013.12.001. [DOI] [Google Scholar]
- 31.Satoh M., Gomi K., Matsumura M., Takabayashi J., Sasaki K., Ohashi Y., Kanno H. Planthoppers: New Threats to the Sustainability of Intensive Rice Production Systems in Asia. IRRI, International Rice research Institute; Los Baños, Philippines: 2009. Whitebacked planthopper–induced disease resistance in rice; pp. 327–340. [Google Scholar]
- 32.Wang Q., Xin Z., Li J., Hu L., Lou Y., Lu J. (E)-β-caryophyllene functions as a host location signal for the rice white-backed planthopper Sogatella furcifera. Physiol. Mol. Plant Pathol. 2015;91:106–112. doi: 10.1016/j.pmpp.2015.07.002. [DOI] [Google Scholar]
- 33.Takatsuji H. Development of disease-resistant rice using regulatory components of induced disease resistance. Front. Plant Sci. 2014;5:630. doi: 10.3389/fpls.2014.00630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kazan K., Manners J.M. The interplay between light and jasmonate signalling during defence and development. J. Exp. Bot. 2011;62:4087–4100. doi: 10.1093/jxb/err142. [DOI] [PubMed] [Google Scholar]
- 35.Wang B., Zhou G., Xin Z., Ji R., Lou Y. (Z)-3-Hexenal, one of the green leaf volatiles, increases susceptibility of Rice to the white-backed Planthopper Sogatella furcifera. Plant Mol. Biol. Rep. 2015;33:377–387. doi: 10.1007/s11105-014-0756-7. [DOI] [Google Scholar]
- 36.Treutter D. Significance of flavonoids in plant resistance: A review. Environ. Chem. Lett. 2006;4:147. doi: 10.1007/s10311-006-0068-8. [DOI] [Google Scholar]
- 37.Keen N.T. The molecular biology of disease resistance. Plant Mol. Biol. 1992;19:109–122. doi: 10.1007/BF00015609. [DOI] [PubMed] [Google Scholar]
- 38.Croteau R., Kutchan T.M., Lewis N.G. Natural products (secondary metabolites) Biochem. Mol. Biol. 2000;24:1250–1319. [Google Scholar]
- 39.De Bruyne T., Pieters L., Deelstra H., Vlietinck A. Condensed vegetable tannins: Biodiversity in structure and biological activities. Biochem. Syst. Ecol. 1999;27:445–459. doi: 10.1016/S0305-1978(98)00101-X. [DOI] [Google Scholar]
- 40.Kong J.M., Chia L.S., Goh N.K., Chia T.F., Brouillard R. Analysis and biological activities of anthocyanins. Phytochemistry. 2003;64:923–933. doi: 10.1016/S0031-9422(03)00438-2. [DOI] [PubMed] [Google Scholar]
- 41.Marles M.S., Ray H., Gruber M.Y. New perspectives on proanthocyanidin biochemistry and molecular regulation. Phytochemistry. 2003;64:367–383. doi: 10.1016/S0031-9422(03)00377-7. [DOI] [PubMed] [Google Scholar]
- 42.Yilmaz Y., Toledo R.T. Health aspects of functional grape seed constituents. Trends Food Sci. Technol. 2004;15:422–433. doi: 10.1016/j.tifs.2004.04.006. [DOI] [Google Scholar]
- 43.Brunetti C., Di Ferdinando M., Fini A., Pollastri S., Tattini M. Flavonoids as antioxidants and developmental regulators: Relative significance in plants and humans. Int. J. Mol. Sci. 2013;14:3540–3555. doi: 10.3390/ijms14023540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cetinkaya H., Kulak M., Karaman M., Karaman H.S., Kocer F. Flavonoids—From Biosynthesis to Human Health. IntechOpen; London, UK: 2017. Flavonoid accumulation behavior in response to the abiotic stress: Can a uniform mechanism be illustrated for all plants? pp. 151–165. [Google Scholar]
- 45.Lister C.E., Lancaster J.E., Walker J.R. Phenylalanine ammonia-lyase (PAL) activity and its relationship to anthocyanin and flavonoid levels in New Zealand-grown apple cultivars. J. Am. Soc. Hortic. Sci. 1996;121:281–285. doi: 10.21273/JASHS.121.2.281. [DOI] [Google Scholar]
- 46.Samyor D., Das A.B., Deka S.C. Pigmented rice a potential source of bioactive compounds: A review. Int. J. Food Sci. Technol. 2017;52:1073–1081. doi: 10.1111/ijfs.13378. [DOI] [Google Scholar]
- 47.Park S., Choi M.J., Lee J.Y., Kim J.K., Ha S.H., Lim S.H. Molecular and biochemical analysis of two rice flavonoid 3’-hydroxylase to evaluate their roles in flavonoid biosynthesis in rice grain. Int. J. Mol. Sci. 2016;17:1549. doi: 10.3390/ijms17091549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wilmouth R.C., Turnbull J.J., Welford R.W., Clifton I.J., Prescott A.G., Schofield C.J. Structure and mechanism of anthocyanidin synthase from Arabidopsis thaliana. Structure. 2002;10:93–103. doi: 10.1016/S0969-2126(01)00695-5. [DOI] [PubMed] [Google Scholar]
- 49.Lev-Yadun S., Gould K.S. Anthocyanins. Springer; New York, NY, USA: 2008. Role of anthocyanins in plant defence; pp. 21–48. [Google Scholar]
- 50.Kumar A., Singh B., Singh K. Functional characterization of flavanone 3-hydroxylase gene from Phyllanthus emblica (L.) J. Plant Biochem. Biotechnol. 2015;24:453–460. doi: 10.1007/s13562-014-0296-0. [DOI] [Google Scholar]
- 51.Asem I.D., Imotomba R.K., Mazumder P.B., Laishram J.M. Anthocyanin content in the black scented rice (Chakhao): Its impact on human health and plant defense. Symbiosis. 2015;66:147–154. doi: 10.1007/s13199-015-0329-z. [DOI] [Google Scholar]
- 52.IBPGR-IRRI Rice Advisory Committee . Descriptors for Rice (Oryza Sativa L.) International Rice Research Institute; Manilla, Philippines: 1980. International Board for Plant Genetic Resources. [Google Scholar]
- 53.De Datta S.K., Malabuyoc J.A., Aragon E.L. A field screening technique for evaluating rice germplasm for drought tolerance during the vegetative stage. Field Crops Res. 1988;19:123–134. doi: 10.1016/0378-4290(88)90050-0. [DOI] [Google Scholar]
- 54.Wang S., Basten C.J., Zeng Z.B. Windows QTL Cartographer 2.5. Department of Statistics, North Carolina State University; Raleigh, NC, USA: 2007. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.