Abstract
Tomatoes are consumed worldwide as fresh vegetables because of their high contents of essential nutrients and antioxidant-rich phytochemicals. Tomatoes contain minerals, vitamins, proteins, essential amino acids (leucine, threonine, valine, histidine, lysine, arginine), monounsaturated fatty acids (linoleic and linolenic acids), carotenoids (lycopene and β-carotenoids) and phytosterols (β-sitosterol, campesterol and stigmasterol). Lycopene is the main dietary carotenoid in tomato and tomato-based food products and lycopene consumption by humans has been reported to protect against cancer, cardiovascular diseases, cognitive function and osteoporosis. Among the phenolic compounds present in tomato, quercetin, kaempferol, naringenin, caffeic acid and lutein are the most common. Many of these compounds have antioxidant activities and are effective in protecting the human body against various oxidative stress-related diseases. Dietary tomatoes increase the body’s level of antioxidants, trapping reactive oxygen species and reducing oxidative damage to important biomolecules such as membrane lipids, enzymatic proteins and DNA, thereby ameliorating oxidative stress. We reviewed the nutritional and phytochemical compositions of tomatoes. In addition, the impacts of the constituents on human health, particularly in ameliorating some degenerative diseases, are also discussed.
Keywords: nutrients, tomatoes, phytochemicals, antioxidants, human health, degenerative diseases
1. Introduction
Tomatoes (Solanum lycopersicum L.), which are frequently included in the Mediterranean diet and are widely consumed as vegetables, play an important role in nutrition because of their well-established health benefits [1]. Tomatoes are used in many processed food products such as sauces, salads, soups, and pastes [2]. Common nutrients reported to be present in tomatoes are vitamins, minerals, fiber, protein, essential amino acids, monounsaturated fatty acids, carotenoids and phytosterols [3,4,5,6]. These nutrients perform various body functions including constipation prevention, reduction in high blood pressure, stimulation of blood circulation, maintenance of lipid profile and body fluids, detoxification of body toxins and maintaining bone structure as well as strength [1,7,8]. Tomatoes are also an excellent source of nutrients and bioactive compounds, commonly known as secondary metabolites, the concentrations of which are correlated with the prevention of human chronic degenerative diseases, such as cardiovascular disease (CVD), cancer, and neurodegenerative diseases [9,10,11]. Due to the high concentrations of different natural antioxidant chemicals, such as carotenoids (β-carotenoids and lycopene), ascorbic acid (vitamin C), tocopherol (vitamin E) and bioactive phenolic compounds (quercetin, kaempferol, naringenin and lutein, as well as caffeic, ferulic and chlorogenic acids), tomatoes can help ameliorate many diseases, especially chronic diseases [12,13]. These compounds play beneficial roles in inhibiting reactive oxygen species (ROS) by scavenging free radicals, inhibiting cellular proliferation and damage, inhibiting apoptosis as well as metal chelation, modulation of enzymatic activities, cytokine expression and signal transduction pathways [12,14]. The main carotenoid in tomato is lycopene, which is responsible for its red color. The pharmacological activities of lycopene and other phenolic compounds include anticancer, anti-inflammatory, antidiabetic, anti-allergenic, anti-atherogenic, antithrombotic, antimicrobial, antioxidant, vasodilator and cardioprotective effects [15,16,17,18]. In addition to having good nutritive value and health promoting activities, the polyphenolic compounds and carotenoids also contribute to sensory activities including maintaining good aroma, taste, and texture [19]. Tomato is an important dietary source of both soluble and insoluble dietary fibers, namely cellulose, hemicelluloses and pectins [20]. In general, these fibers are resistant to intestinal digestion in the large intestine and are believed to ameliorate bowel disorders, cancer, diabetes, CVDs, and obesity [21,22]. Important proximate composition parameters for tomatoes include sugar content, pH, energy, acidity and reducing sugar contents [4]. The proximate compositions help in the characterization and identification of tomato nutrients. The combination of vitamins, minerals, amino acids, and fats all together contribute to making tomato part of a balanced diet. Phytosterols, which are involved in the prevention of colon cancer and heart disease, are present in tomatoes in lower amounts than that found in other fruits and vegetables [23]. Among the phytosterols, β-sitosterol, campesterol and stigmasterol are the main ones [5]. The antioxidant compounds predominately present in tomato consist of several different types of carotenoids, vitamin C, vitamin E, and phenolic compounds that confer their antioxidant activities by neutralizing reactive oxygen species (ROS) and protecting the cell membrane against lipid peroxidation [24,25].
Nutritional composition of tomato varies based on the tomato cultivar, extraction procedures, analysis methods and environmental conditions. During the processing of tomato products, up to 30% of their original weight are turned into waste, which may still contain some nutritive values [26]. For example, the seeds and the peel are the main waste product of tomato, which are rich in protein, dietary fibers, bioactive compounds and lycopene [27]. The by-products are used as food additives especially in the meat industries [28]. Nevertheless, although the waste products of tomato are a rich source of nutrients, proper research should be undertaken before their consumption. In spite of having health benefits, tomatoes demonstrate some undesired effects on the body when consumed in large amounts or in abnormal body conditions. The adverse effects of tomato intake are associated with renal problems, allergies, arthritis, heartburn, and migraine [1].
Although scattered data are available, there is a lack of updated compiled information on the nutrient composition and the health benefits of tomatoes. Therefore, in this review, we bring together information on all the nutrient compositions of tomato such as proximate composition, minerals, heavy metals, vitamins, fatty acids, amino acids, carotenoids, phytosterols, antioxidant activity and different types of bioactive compounds. Consequently, we discuss the associated health benefits of the bioactive compounds present in tomato in preventing chronic degenerative diseases, such as CVDs, diabetes, and cancer.
2. Methodology
A comprehensive literature search was performed by combining the appropriate keywords including “tomato”, “nutritional composition”, “proximate composition”, “phytochemicals”, “physiochemical properties”, “mineral”, “vitamin”, “fatty acid”, “amino acid”, “carotenoid”, “phytosterol”, “antioxidant properties”, “bioactive compounds”, “health benefits”, “human degenerative diseases”, “cardiovascular diseases”, “diabetes” and “cancer”. As for the search engine, Google Scholar, Scopus, Web of Science, ScienceDirect, and Pubmed were independently searched. Only English language published articles were considered. There was no year restriction, and the final search was conducted on 27 July 2020. The references were managed with EndNote software (version X7).
3. Nutritional Composition of Tomato
3.1. Proximate Composition
Proximate analysis is one of the first approaches for food characterization, particularly for the identification of nutrients in any food products. Generally, water, ash, protein, lipid, carbohydrate, sugar and reducing sugar contents, as well as pH, energy and acidity are the key proximate compositions of a food sample [29]. For instance, ash content is an important step in the analysis of nutritional element contents in food products. Ash refers to the inorganic residue (mineral content) that remains after the complete oxidation of organic matter and removal of water by heating (ashing) of a food sample in a furnace [30,31]. Next, moisture content (total solids) is important because it affects the chemical and physical aspects of food, which determine its freshness and storage stability [32,33]. Protein, lipids, and carbohydrates are principal components of foods and are the main elements in proximate composition analysis.
Proteins, which are macromolecules present in food, are important for cellular structure and biological functions. Protein analysis is crucial for nutritional labeling, as well as in describing the biological activities and functional properties of food products [33,34,35]. Lipids are another group of macromolecules that are generally insoluble in water but are soluble in organic solvents. In fact, precise and accurate analysis of lipid content in food is mandatory for the standard of quality and nutritional labeling and is important in ensuring manufacturing specification [36].
Carbohydrate analysis is also important as a major (more than 70%) energy source. Carbohydrate analysis yields nutritional information, standard of identity, water holding capacity, flavors, desirable textures, and stability of food products [37,38]. In addition, pH analysis of food samples is essential for food processing and storage. Dietary fiber is another important component of proximate analysis because it ensures a variety of health benefits, including protection against heart disease, colon cancer and diabetes [39]. In a more recent study based on previously published original research articles, an average tomato consists of ash 8.75%, water 94.17 (g/100 g), moisture 91.18 (g/100 g), total protein 17.71 (g/100 g), lipid 4.96 (g/100 g), carbohydrates 5.96 (g/100 g), total sugar 50.60 (g/100 g), pH 3.83, energy 34.67 kcal/100 g, acidity 0.48%, reducing sugar 35.84%, fructose 2.88%, glucose 2.45%, sucrose 0.02% and total fiber 11.44 (g/100 g) (Table 1).
Table 1.
Proximate composition of tomato.
| Parameters | Values | Range | References |
|---|---|---|---|
| Energy (kcal/100 g) | 34.67 ± 18.74 | 18.00–75.00 | [3,4,5,40,41,42,43,44,45,46,47,48,49,50,51,52] |
| Ash (%) | 8.75 ± 1.69 | 5.90–10.60 | |
| Moisture (g/100 g) | 91.18 ± 6.83 | 68.03–96.17 | |
| Total protein (g/100 g) | 17.71 ± 5.40 | 10.50–25.03 | |
| Lipid (g/100 g) | 4.96 ± 1.19 | 3.62–5.39 | |
| Carbohydrates (g/100 g) | 5.96 ± 1.37 | 3.92–8.00 | |
| Total sugar (g/100 g) | 50.60 ± 3.69 | 47.00–56.45 | |
| pH | 3.83 ± 0.21 | 3.61–4.08 | |
| Acidity (%) | 0.48 ± 0.07 | 0.39–0.55 | |
| Reducing sugar (%) | 35.84 ± 4.57 | 30.03–41.21 | |
| Fructose (%) | 2.88 ± 0.49 | 1.15–3.42 | |
| Glucose (%) | 2.45 ± 0.48 | 1.74–3.18 | |
| Sucrose (%) | 0.02 ± 0.05 | 0.01–0.02 | |
| Total fiber (g/100 g) | 11.44 ± 9.31 | 1.32–19.36 |
Values are expressed as mean ± standard deviation.
3.2. Mineral Content
Minerals are naturally occurring inorganic solid substances. They are essential for a variety of bodily functions, including the regulation of metabolic pathways, formation of vital organs, maintenance of bodily physiological functions, regulation of pH balance, fluid balance, blood pressure, nerve transmission, muscle contraction and energy production [53,54,55,56]. Some minerals, such as calcium (Ca), potassium (K), sodium (Na), phosphorus (P), magnesium (Mg), sulfur (S) and chlorine (Cl), are highly essential (average daily intake ˃ 50 mg) and are therefore known as major elements. Others include iron (Fe), iodine (I), zinc (Zn), fluorine (F), copper (Cu), selenium (Se), manganese (Mn), cobalt (Co), chromium (Cr), nickel (Ni), molybdenum (Mo) and selenium (Se), which are required in comparatively smaller amounts (< 50 mg/day) and are known as trace elements. Other elements, such as aluminum (Al), arsenic (As), boron (B), barium (Ba), bismuth (Bi), bromine (Br), lead (Pb), cadmium (Cd), cesium (Cs), germanium (Ge), lithium (Li), mercury (Hg), rubidium (Rb), silicon (Si), antimony (Sb), tin (Sn), samarium (Sm), strontium (Sr), tungsten (W), titanium (Ti) and thallium (Tl), which are needed in even smaller amounts, (1 µg/day) are known as ultratrace elements [57,58,59]. Pb, As, Hg, Cd, Cu, Cr, Ni, Zn and Mn are heavy metals that are toxic if present in low concentrations because they tend to accumulate in living cells [59,60].
From a nutritional perspective, tomato is a good source of minerals and other elements [4,44]. In this review, 23 types of minerals and their amounts present in tomato are compiled, including the major elements (calcium, potassium, sodium, phosphorus, magnesium, sulfur, chlorine) and trace elements (iron, iodine, zinc, fluorine, cupper, manganese, cobalt, chromium, nickel, aluminum, arsenic, boron, lead, cadmium, nitrate, chlorine, selenium, silicon) (Table 2).
Table 2.
Mineral contents in tomato.
| Elements | Units | Concentrations | Range | References |
|---|---|---|---|---|
| Sodium (Na) | mg/100 g | 70.38 ± 12.20 | 56.90–80.65 | [3,4,41,42,44,45,47,51,61,62,63] |
| Potassium (K) | mg/100 g | 403.02 ± 254.41 | 16.63–1097.00 | |
| Calcium (Ca) | mg/100 g | 105.21 ± 22.76 | 48.47–162.07 | |
| Magnesium (Mg) | mg/100 g | 172.58 ± 58.92 | 76.87–265.93 | |
| Phosphorus (P) | mg/100 g | 300.99 ± 32.12 | 173.00–379.31 | |
| Chlorine (Cl) | μg/100 g | 517.24 ± 0.00 | 517.24 | |
| Boron (B) | μg/g | 36.83 ± 3.27 | 25.84–48.59 | |
| Nickel (Ni) | mg/100 g | 0.66 ± 0.00 | 0.66 | |
| Nitrate (NO3-) | mg/100 g | 274.37 ± 156.75 | 86.21–459.00 | |
| Iron (Fe) | mg/100 g | 4.55 ± 2.18 | 1.50–6.45 | |
| Zinc (Zn) | mg/100 g | 2.48 ± 1.05 | 0.17–3.17 | |
| Cobalt (Co) | mg/100 g | 19.66 ± 9.66 | 10.00 -29.31 | |
| Copper (Cu) | mg/100 g | 0.67 ± 0.15 | 0.06–1.10 | |
| Manganese (Mn) | mg/100 g | 0.60 ± 0.12 | 0.11–1.88 | |
| Chromium (Cr) | μg/100 g | 193.80 ± 133.80 | 60.00–327.59 | |
| Iodine (I) | mg/100 g | 2.65 ± 1.44 | 0.18–3.97 | |
| Fluorine (F) | μg/100 g | 413.79 ± 0.00 | 413.79 | |
| Aluminum (Al) | μg/100 g | 1241.38 ± 0.00 | 1241.38 | |
| Silicon (Si) | μg/100 g | 46.55 ± 0.00 | 46.55 | |
| Selenium (Se) | μg/100 g | 13.45 ± 3.45 | 10.00–16.90 | |
| Lead (Pb) | μg/ g | 1.21 ± 0.06 | 1.15–1.27 | |
| Cadmium (Cd) | μg/ g | 0.17 ± 0.06 | 0.11–0.22 | |
| Arsenic (As) | μg/ g | 0.20 ± 0.005 | 0.19–0.20 |
Concentrations are expressed as mean ± standard deviation.
3.3. Vitamin Content
The accurate and precise analysis of vitamin content is important for a standard balanced diet because low or excessive amounts of vitamins can contribute to disease conditions by hampering normal cell growth [64]. Tomatoes are one of the most versatile and widely consumed vegetables in many countries and are a rich source of vitamins [65,66]. Vitamins C, B-complex, A, E and K are the main types of vitamins present in tomato, with vitamin C reported to be the highest (Table 3). Vitamins C and E (tocopherol) exhibit antioxidant activities making tomato a useful therapeutic agent for the prevention of various diseases, including CVDs and cancer [12,67,68,69,70]. Among the various types of vitamin B-complexes, the amount of folate is comparatively high in tomatoes. Nevertheless, excessive amounts of water-soluble vitamin B do not cause any toxicity because these vitamins can be easily excreted from the body. Vitamins perform various functions, such as maintaining the nervous system, producing red blood cells and enzymatic function [71,72].
Table 3.
Vitamin contents in tomato.
| Vitamins | Units | Concentrations | Range | References |
|---|---|---|---|---|
| Vitamin A | IU/100 g | 614.44 ± 248.18 | 267.33–833.00 | [4,46,61,62,65,69,73,74,75,76] |
| Vitamin E | μg/100 g | 15.08 ± 1.06 | 14.02–16.13 | |
| Vitamin K | μg/100 g | 98.28 ± 0.00 | 98.28 | |
| Vitamin C | mg/100 g | 36.16 ± 29.64 | 10.86–85.00 | |
| Thiamine | mg/100 g | 0.66 ± 0.44 | 0.04–0.98 | |
| Riboflavin | mg/100 g | 0.48 ± 0.34 | 0.02–0.81 | |
| Niacin | mg/100 g | 9.68 ± 0.00 | 9.68 | |
| Pantothenic Acid | mg/100 g | 4.93 ± 0.41 | 4.52–5.34 | |
| Vitamin B6 | mg/100 g | 1.51 ± 0.22 | 1.29–1.72 | |
| Biotin | μg/100 g | 68.97 ± 0.00 | 68.97 | |
| Folate | mg/100 g | 14.00 ± 1.00 | 13.00–15.00 |
Concentrations are expressed as mean ± standard deviation.
3.4. Fatty Acid Content
Tomato contains many different types of fatty acids (Table 4). Among them, linoleic and polyunsaturated fatty acids are the highest. Linoleic and linolenic acids are two essential fatty acids. Since the essential fatty acids cannot be synthesized by humans or animals, they must come from the dietary sources and tomato provides a good source of these acids. On the other hand, polyunsaturated fatty acids are also very important for the body since they are essential for the maintenance of plasma membrane integrity, cell growth and prevention of disease [77,78]. From this point of view, tomato is therefore a rich and highly nutritious food product.
Table 4.
Fatty acid contents in tomato.
| Fatty Acids | Concentrations (g/100 g) | Range | References |
|---|---|---|---|
| Myristic acid | 0.56 ± 0.22 | 0.32–0.93 | [5,41,47,49,62] |
| Palmitic acid | 18.07 ± 2.90 | 12.40–22.50 | |
| Stearic acid | 4.81 ± 1.50 | 2.80–6.84 | |
| Palmitoleic acid | 0.25 ± 0.10 | 0.03–0.32 | |
| Oleic acid | 14.24 ± 3.50 | 9.00–19.14 | |
| Linoleic acid | 49.40 ± 4.16 | 46.33–54.10 | |
| Linolenic acid | 10.17 ± 4.46 | 4.26–15.53 | |
| Caproic acid | 0.03 ± 0.02 | 0.01–0.05 | |
| Caprylic acid | 0.06 ± 0.04 | 0.02–0.10 | |
| Capric acid | 0.04 ± 0.03 | 0.01–0.07 | |
| Heptadecanoic acid | 0.26 ± 0.05 | 0.18–0.13 | |
| Lauric acid | 0.09 ± 0.05 | 0.04–0.15 | |
| Pentadecanoic acid | 0.12 ± 0.03 | 0.08–0.15 | |
| Arachidic acid | 0.88 ± 0.24 | 0.61–1.26 | |
| Eicosadienoic acid | 0.04 ± 0.02 | 0.02–0.06 | |
| Arachidonic acid | 0.04 ± 0.02 | 0.01–0.06 | |
| Eicosapentaenoic acid | 0.05 ± 0.01 | 0.03–0.06 | |
| Erucic acid | 0.02 ± 0.01 | 0.01–0.03 | |
| Docosadienoic acid | 0.07 ± 0.03 | 0.03–0.10 | |
| Behenic acid | 0.59 ± 0.19 | 0.31–0.82 | |
| Tricosanoic acid | 0.68 ± 0.54 | 0.16–1.52 | |
| Lignoceric acid | 0.74 ± 0.20 | 0.45–1.01 | |
| Saturated fatty acid | 27.40 ± 3.74 | 22.37–33.22 | |
| Monounsaturated fatty acid | 13.80 ± 2.42 | 11.00–17.66 | |
| Polyunsaturated fatty acid | 57.55 ± 23.51 | 55.78–58.63 | |
| Vaccenic acid | 0.53 ± 0.05 | 0.50–0.60 | |
| Eicosanoic acid | 0.10 ± 0.03 | 0.05–0.12 |
Concentrations are expressed as mean ± standard deviation.
3.5. Amino Acid Content
Amino acids are the building blocks of proteins that conduct important bodily functions, including the maintenance of cellular structure, transport and storage of nutrients, wound healing, and repair of damaged tissues [79]. A total of 17 amino acids have been identified in tomato (Table 5). It is estimated that essential amino acids constitute 39.75% of the total protein in tomato. Among these, the highest was glutamic acid (approximately 10.13 g/100 g protein). Among the various types of essential amino acids present in tomato, leucine is present in the highest concentration, while methionine is the lowest. Among the nonessential amino acids, glutamic acid is the most common, while cysteine is the least.
Table 5.
Amino acid contents of tomato.
| Amino Acids | Concentrations (g/100 g Protein) |
Range | References |
|---|---|---|---|
| Threonine * | 1.37 ± 0.97 | 0.40–2.34 | [3,41,62,80,81] |
| Valine * | 2.49 ± 2.09 | 0.40–2.49 | |
| Methionine * | 0.57 ± 0.45 | 0.12–1.02 | |
| Isoleucine * | 2.13 ± 1.73 | 0.40–3.86 | |
| Leucine * | 2.80 ± 2.28 | 0.52–5.07 | |
| Phenylalanine * | 1.77 ± 1.36 | 0.41–13.12 | |
| Histidine * | 1.93 ± 1.71 | 0.22–3.64 | |
| Lysine * | 2.45 ± 1.95 | 0.50–4.40 | |
| Arginine * | 2.33 ± 2.02 | 0.31–4.34 | |
| Aspartic Acid ** | 1.40 ± 0.70 | 0.70–2.09 | |
| Serine ** | 1.78 ± 1.30 | 0.48–3.08 | |
| Glutamic Acid ** | 10.13 ± 4.44 | 5.69–14.56 | |
| Proline ** | 1.53 ± 1.25 | 0.28–2.78 | |
| Glycine ** | 2.30 ± 1.99 | 0.31–4.29 | |
| Alanine ** | 2.74 ± 2.29 | 0.45–5.02 | |
| Cystine ** | 0.21 ± 0.19 | 0.02–0.39 | |
| Tyrosine ** | 1.82 ± 1.61 | 0.21–3.42 |
Concentrations are expressed as mean ± standard deviation. * denotes essential amino acids. ** denotes nonessential amino acids.
3.6. Carotenoid Content
Tomato contains various types of carotenoids and is rich in lycopene and β-carotenoids (Table 6). Carotenoids are plant pigments that play crucial roles in protecting plants from photo-oxidative processes. They are natural antioxidants useful for combating cellular oxidative damage [82]. Recent studies have suggested that carotenoids play important roles in improving vision [83], are effective for preventing CVDs [84], protect against sperm health [85] and can prevent various types of cancer [7,86,87]. On the other hand, carotenoids such as lutein and zeaxanthin improve skin health [88]. Lycopene is a type of carotenoid found in tomato that is helpful in the prevention of liver, lung, prostate, breast, and colon cancers [70,89].
Table 6.
Carotenoid contents in tomato.
| Units | Concentrations | Range | References | |
|---|---|---|---|---|
| β-carotene | μg/100 g | 9942.16 ± 264.74 | 3677.42–10,206.90 | [5,7,46,47,74,90,91,92,93,94] |
| α-carotene | μg/100 g | 101.00 | 101.00 | |
| Lycopene | μg/100 g | 8002.50 ± 243.54 | 5020.00–11,110.00 | |
| Lutein + zeaxanthin | μg/100 g | 60.67 ± 43.86 | 18.07–123.00 | |
| Phytoene | μg/100 g | 668.33 ± 361.95 | 430.00–1860.00 | |
| Phytofluene | μg/100 g | 500.00 ± 100.49 | 390.00–820.00 | |
| All trans-lutein | mg/kg | 5.00 ± 0.82 | 4.00–6.00 | |
| All trans-β carotene | mg/kg | 29.25 ± 27.26 | 4.00–75.00 | |
| 9-cis-β carotene | mg/kg | 6.50 ± 2.29 | 3.00–9.00 |
Concentrations are expressed as mean ± standard deviation.
3.7. Sterol Content
Sterols are mainly found in plants, animals, and microorganisms. Plant sterols, also known as phytosterols, commonly occur as a mixture of β-sitosterol, campesterol and stigmasterol. Phytosterols play important roles in human health. Phytosterols block cholesterol absorption sites in the human intestine and reduce cholesterol absorption, leading to a reduction in low-density lipoprotein cholesterol (LDL-C) and prevention of CVD [95]. Research has also suggested that phytosterols exhibit an anticancer effect by inhibiting cancer cell growth, carcinogens, angiogenesis, and invasion of metastasis by promoting the apoptosis of cancerous cells [96,97]. Phytosterols also act as antioxidants to prevent oxidative stress [98]. Additional important functions of phytosterols are stimulation of the immune system and anti-inflammatory activities [99,100]. In fact, tomato is an excellent source of phytosterols. Approximately 1283 mg of phytosterols are present per kg of tomato. Among them, β-sitosterol and stigmasterol are the main ones (Table 7).
Table 7.
Sterol contents in tomato.
| Concentrations (mg/kg) |
Range | References | |
|---|---|---|---|
| Campesterol | 147.50 ± 31.13 | 100.00–18.00 | [5,92] |
| Stigmasterol | 387.50 ± 88.71 | 260.00–510.00 | |
| Stigmastanol | 28.25 ± 10.92 | 10.00–38.00 | |
| β-sitosterol | 720.00 ± 175.64 | 520.00–1000.00 | |
| Δ5-Avenasterol | 62.30 ± 2.21 | 10.00–65.87 | |
| Cholestanol | 9.70 ± 1.80 | 2.10–11.54 | |
| Cholest-7-en-3-ol | 3.60 ± 0.13 | 0.42–4.40 | |
| Cholesterol | 41.90 ± 2.10 | 8.40–43.45 | |
| Lanost-8-en-3-β-ol | 52.40 ± 6.80 | 4.50–60.65 | |
| 24-Oxocholesterol | 67.50 ± 3.20 | 14.20–70.69 | |
| Total | 1283.25 ± 239.39 | 918.00–1570.00 |
Concentrations are expressed as mean ± standard deviation.
4. Antioxidant Properties and Bioactive Compounds in Tomato
Antioxidants are biomolecules that (1) prevent the oxidation of other molecules by inhibiting the initiation and elongation of the oxidizing chain reaction of ROS and (2) by inhibiting the proliferation of cells, free radical scavenging, the modulation of enzymatic activity via chelation of metallic ions and signal transduction pathways [101]. Antioxidants, which are bioactive reducing agents, prevent cellular damage caused by ROS, including superoxide anion radicals, hydroxyl radicals and hydrogen peroxide [102,103]. Antioxidants are considered as the first line of cellular defense used to minimize the harmful effects of free radicals by scavenging them while restoring the normal physiological system. Most medicinal plants are abundant sources of natural antioxidants, such as phenolic acid and flavonoids.
Tomatoes are consumed worldwide in various forms, either raw or processed and provide a significant amount of important antioxidants [104,105] including β-carotene, ascorbic acid, lycopene, tocopherol, phenolic acids, flavonoids, anthocyanins and other bioactive compounds (Table 8 and Table 9) [66,106,107,108]. The antioxidants present in tomato can delay, hinder, and prevent free radical oxidation, and thus forming stable radicals [109]. Generally, antioxidant compounds play important roles in the prevention of several human degenerative diseases, including CVDs, diabetes, cancer, neurological diseases, and aging, by minimizing oxidative stress caused by ROS [110,111].
Table 8.
Antioxidant constituents in tomato.
| Units | Concentrations | Range | References | |
|---|---|---|---|---|
| α-tocopherol | mg/100 g | 0.701 ± 0.110 | 0.59–0.88 | [3,5,49,50,61,65,90,113] |
| β-tocopherol | mg/100 g | 0.030 ± 0.004 | 0.02–0.03 | |
| γ-tocopherol | mg/100 g | 0.810 ± 0.720 | 0.40–2.24 | |
| δ-tocopherol | mg/100 g | 0.020 ± 0.010 | 0.01–0.02 | |
| Total tocopherol | mg/100 g | 1.200 ± 0.150 | 1.02–1.44 | |
| Vitamin C | mg/100 g | 36.160 ± 29.640 | 10.86–85.00 | |
| β-carotene | mg/100 g | 9.420 ± 2.640 | 3.67–10.21 | |
| Lycopene | mg/100 g | 7.960 ± 1.780 | 5.02–9.49 | |
| Phenolic acids | mg CIAE/g extract | 25.500 ± 3.590 | 21.34–31.23 | |
| Flavonoids | mg QE/g extract | 4.230 ± 1.280 | 3.06–6.36 | |
| Anthocyanins | mg ME/g extract | 0.870 ± 0.470 | 0.23–1.36 |
Concentrations are expressed as mean ± standard deviation. CIAE: chlorogenic acid equivalents. QE: quercetin equivalents. ME: malvidin 3-glucoside equivalents.
Table 9.
Bioactive compounds in tomato.
| Name of Bioactive Compound | IUPAC Name | Molecular Formulas | Structure | Other Sources | References |
|---|---|---|---|---|---|
| Quercetin | 3,5,7,3′,4′-pentahydroxyflavone | C15H10O7 |
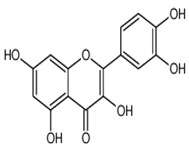
|
Onion family, chocolate, fortified wine, berry wine, grape wine, sparkling wine, cereals, herbs, fruit juices, tea infusions, spices, nuts, fruit | [3,5,13,44,92,104,114,115,116,117,118,119] |
| Kaempferol | 3,5,7,4′-tetrahydroxyflavone | C15H10O6 |
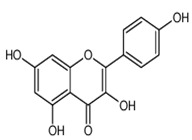
|
Pod vegetables, pulses, wine, fruit juices, spices, tea infusions, nuts, fruit | |
| Naringenin | 5,7-Dihydroxy-2-(4-hydroxyphenyl)chroman-4-one | C15H12O5 |
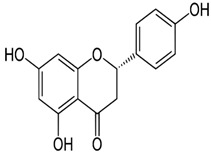
|
Herbs, fruits, vegetables, beans, drynaria | |
| Caffeic acid | 3,4-dihydroxycinnamic acid | C9H8O4 |
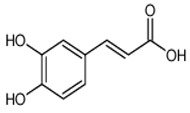
|
Grape wine, beer, fortified wine, cereal products, sparkling wine, cereals, tubers, berries, dried drupes, other dried fruits, citrus fruits, pomes, unknown coffee beverages, drupe juices, berry juices, pome juices, vegetable oils, herbs, other seasonings, tropical fruit juices, other types of seed oils, spices, fruit vegetables, cabbages, leafy vegetables, root vegetables | |
| Rutin | Rutoside; quercetin 3-rutinoside | C27H30O16 |
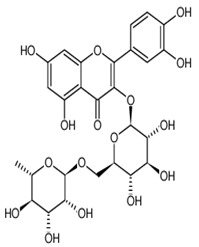
|
Invasive plant species (Carpobrotusedulis) | |
| Chlorogenic acid | 3-caffeoylquinic and neochlorogenic acid | C16H18O9 |
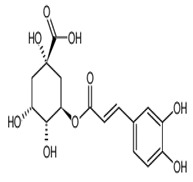
|
Grape wines, tea infusions, pomes, dried drupes, pome jams, tropical fruits, robusta coffee beverages, root vegetables, unknown coffee beverages, cabbages | |
| Ferulic acid | Transferulic acid; 4-hydroxy-3-methoxycinnamic acid | C10H10O4 |
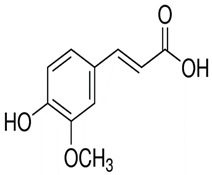
|
Cabbages, fruit vegetables, beer, fortified wine, sparkling wine, other dried fruits, cereals, soy based products, grape wine, cereal products, pome juices, chocolate, berries, citrus fruits, other fruits, pomes, berry juices, tropical fruit juices, fruit vegetable oils, other seed oils, herbs, nuts, beans and lentils | |
| P-coumaric acid | p-hydroxycinnamic acid | C9H8O3 |
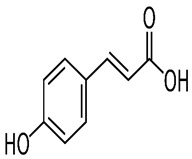
|
Sparkling wines, citrus fruits, beers, nut liquors, grape wines, fortified wines, dried drupes, other dried fruits, lentils, berries, other fruits, pomes, berry juices, pome juices, tropical fruit juices, fruit vegetable oils, other seed oils, herbs, other seasonings, spices, nuts, fruit vegetables | |
| Lycopene | lycopene, (7-cis,7’-cis 9-cis,9’-cis)-isomer | C40H56 |
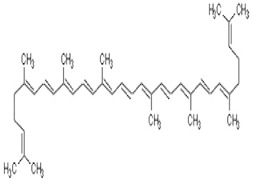
|
autumn olive, guac, guava, papaya, grapefruit, sea buckthorn, wolfberry, watermelon | |
| Resveratrol | 3,5,4′-trihydroxy-trans-stilbene | C14H12O3 |
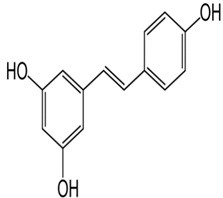
|
Grapes, wine, peanuts and soy | |
| Chrysin | C15H10O4 |
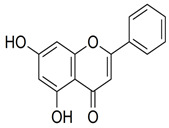
|
Fruit juices, especially citrus | ||
| Epicatechin | (2R,3R)-2-(3,4-dihydroxyphenyl)-3,4-dihydro-2H-chromene-3,5,7-triol | C15H14O6 |
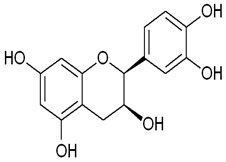
|
Tea, cocoa, grapes | |
| Catechin | (2R,3S)-2-(3,4-dihydroxyphenyl)-3,4-dihydro-2H-chromene-3,5,7-triol | C15H14O6 |
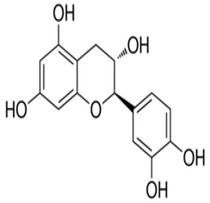
|
Tea, cocoa, grapes | |
| Luteolin | 2-(3,4-dihydroxyphenyl)- 5,7-dihydroxy-4-chromenone | C15H10O6 |
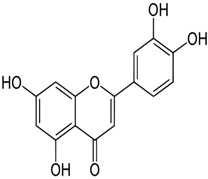
|
Honey, fruit, vegetable oils, nuts, herbs, shoot vegetables, fruit, vegetables, lentils, carrots, olive oil | |
| Cinnamic acid | 2-propenoic acid | C9H8O2 |
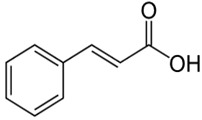
|
Propolis, fruit and vegetable oils, berries, citrus juices, fruit, vegetables, spices | |
| Phloretic acid | 3-(4-hydroxyphenyl) propanoic acid | C9H10O3 |
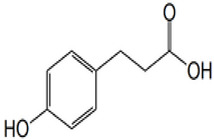
|
Dried grass | |
| Sinapic acid | 3-(4-hydroxy-3,5-dimethoxyphenyl)prop-2-enoic acid | C11H12O5 |
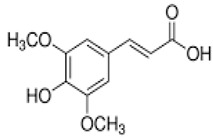
|
Black mustard seeds | |
| Vanillic acid | 4-hydroxy-3-methoxybenzoic acid | C8H8O4 |
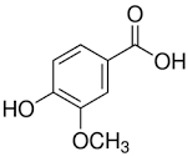
|
Rum, beer, brandy, wine, herbs, grapes, citrus fruit, whiskey |
Phenolic compounds present in tomato are considered as the primary antioxidant based on their ability to donate hydrogen atoms to reactive free radicals [104]. Carotenoid, ascorbic acid, lycopene, tocopherol, and anthocyanins are other important antioxidants in tomato. Carotenoids act as antioxidants by quenching singlet oxygen and peroxyl-radicals [112]. Normally, antioxidant activity is confirmed using 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay, thiobarbituric acid reactive substance (TBARS) inhibition, ferric reducing power (FRAP) and ferrous ion chelating activities.
5. Health Benefits of Tomato
The health benefits of tomatoes mainly associated with its rich supply of nutrients and secondary metabolites, including vitamins, minerals, essential fatty acids, carotenoids, antioxidants, and other bioactive compounds. In addition to its high amounts of vitamins A, C, E, K and B-complex [5,37], tomato is also a good source of important minerals as previously stated [3,47]. Additionally, tomato contains some dietary fiber, protein, essential amino acids, and a number of bioactive anti-oxidative organic compounds including lycopene, quercetin, kaempferol, naringenin, caffeic acid, rutin, resveratrol, catechin and luteolin, which contribute to the maintenance of good health [44]. Vitamins C and E are natural antioxidants that can prevent degenerative diseases caused by free radicals [120].
Lycopene is a natural antioxidant that can help combat different types of cancer, including prostate, breast, lung, stomach, colorectal, oral, esophageal, pancreatic, bladder, cervical and ovarian cancers [10,17]. Abundant amounts of minerals are responsible for maintenance of body’s physiological functions including blood pressure, blood clotting, nerve transmission, muscle contraction and energy production [56,121], while the vitamins help to maintain the health of the nervous system, facilitate blood cell production and enzymatic function [122].
The consumption of carotenoid-rich tomato has been reported to protect against vitamin A deficiency disorders and other chronic diseases including light-induced eye damage, the development of cataracts and age-related macular degeneration [123]. In fact, a high dietary intake of carotenoids (lutein and zeaxanthin) can prevent the risk of age-related macular degeneration, making tomato useful in ameliorating eye diseases [124]. Other important constituents present in tomato are phytosterols, which prevent intestinal cholesterol absorption by displacing it from the micelles, and thus stimulating its excretion, preventing CVDs, and ameliorating different types of cancer including colon, prostate, and breast cancers [1,125,126].
Oxidative stress is the main contributor to the development of chronic diseases in humans. ROS, including superoxide anion radicals, hydroxyl radicals and hydrogen peroxide, are highly reactive oxidant molecules that are endogenously induced by regular metabolic activity in the body, diet, and secondary lifestyle activities [127,128]. They react with cellular components (DNA, lipids, and proteins) to cause oxidative damage [129]. Antioxidants are super-protective agents that inactivate ROS and prevent oxidative damage [130]. Natural antioxidants such as vitamins C and E, different types of carotenoids and phenolic compounds including quercetin, kaempferol, caffeic acid, naringenin, chlorogenic acid, lutin, ferulic acid, lycopene, resveratrol, catechin and luteolin are present in tomato [6,92]. These bioactive compounds will protect endogenously produced reactive oxidant molecules and prevent oxidative damage. Therefore, they prevent the development of different types of cancer, diabetes and cardiovascular, eye, hypertension, inflammatory and neurodegenerative diseases [1,131,132,133,134].
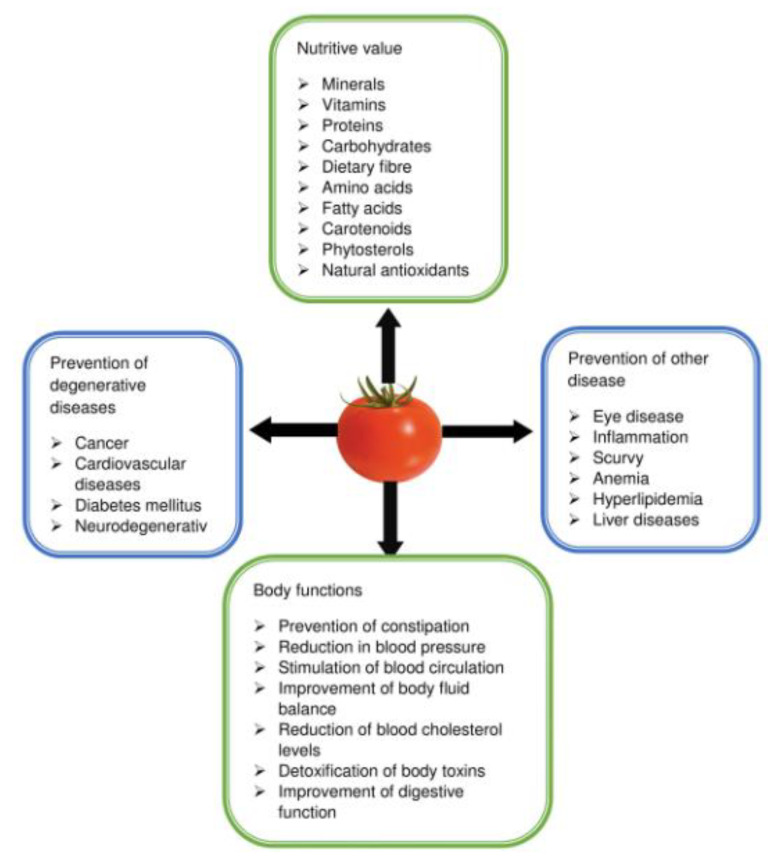
In summary, the health benefits of tomato are associated with its rich supply of nutrients to the body, such as minerals, vitamins, proteins, essential amino acids, fatty acids, and other antioxidants. The consumption of tomato is associated with the relief of cancer, diabetes, CVDs, eye disease and constipation, with blood pressure reduction, improved blood circulation, improved body fluid balance, cholesterol reduction, detoxification of toxins, reduction in inflammation, prevention of premature aging and improvement of digestive function (Figure 1).
Figure 1.
Summary of health benefits of tomato.
6. Effects of Bioactive Compounds of Tomato on Some Human Degenerative Diseases
6.1. Tomato in CVDs
Generally, CVD is a category of disease that affects the blood vessels and the heart [135]. Common predisposing factors include hypertension, gender, age, hypercholesterolemia, obesity, diabetes, and unhealthy lifestyle, such as minimal physical activity, smoking, the consumption of a high fat diet and excessive alcohol [136,137]. The bioactive compounds in tomato not only reduce the risk but also prevent or ameliorate CVDs [138] (Figure 2). The antiplatelet aggregation effects of tomato and tomato products support the prevention of CVD disorders [139]. For example, lycopene can improve endothelial function among patients who suffer from CVD [140]. Lycopene functions as a crucial hypolipidemic and antioxidant agent and inhibits factors that play important prothrombosis and pro-inflammatory roles, and thus improves CVDs [141]. Additionally, it is hypothesized that lycopene can increase LDL degradation and reduce cholesterol synthesis. It has been reported that both the thickness of the intima wall and myocardial infarction (MI) can be minimized by high lycopene intake [142]. As a radical scavenger, lycopene mops up singlet oxygen along with other active free radicals, and thus protects against vascular cell damage, which contributes to CVD [141]. Lycopene also shows antiplatelet and antithrombotic activities by hindering phospholipase C activation, which inhibits the breakdown of phosphoinositol and the formation of thromboxane B2. Subsequently, the mobilization of intracellular calcium, which is beneficial in CVDs, is impeded. In addition, the cyclic guanosine monophosphate (cGMP) /nitrate formation in the platelets activated by lycopene also constrains platelet aggregation [143].
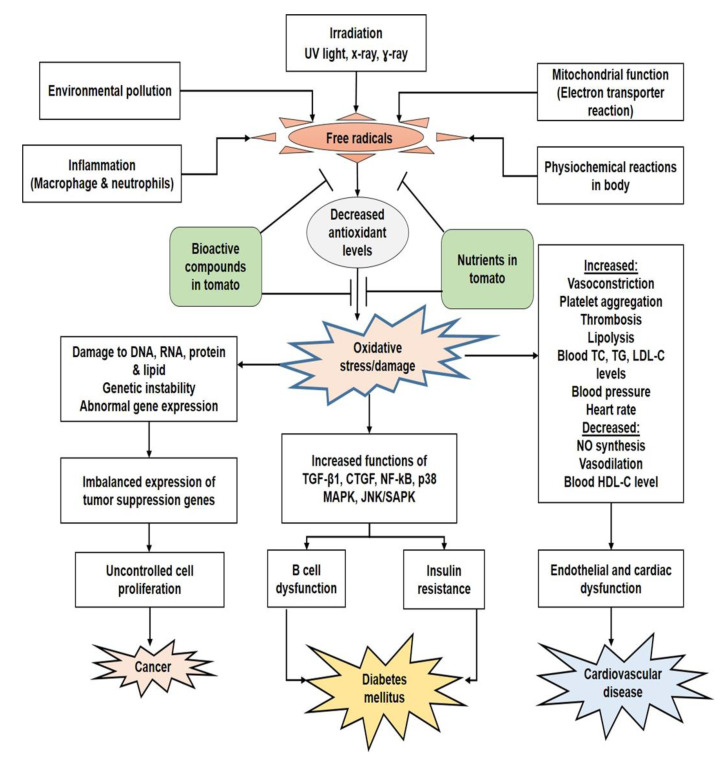
Figure 2.
Summary of the effects of nutrients and bioactive compounds in tomato on cancer, diabetes mellitus and cardiovascular disease (CVD). UV: ultraviolet; DNA: deoxyribonucleic acid; RNA: ribonucleic acid; TGF-β1: transforming growth factor beta 1; CTGF: connective tissue growth factor; NF-kB: nuclear factor kappa-light-chain enhancer of activated B cells; p38 MAPK: p38 mitogen-activated protein kinase; JNK/SAPK: c-Jun N-terminal kinases/stress-activated protein kinases; TC: total cholesterol; TG: triglyceride; LDL-C: low-density lipoprotein cholesterol; NO: nitric oxide; HDL-C: high-density lipoprotein cholesterol.
Quercetin is a potential chelator of metal ions, constraining xanthine oxidase while reducing lipid peroxidation and scavenging free radicals, which ultimately help to reduce the risk of CVD [144,145]. Furthermore, it reduces the levels of oxidized LDL in the plasma, since LDL oxidation is stimulated by macrophages or myeloperoxidase, inhibits the generation of hyperoxide and reduces systolic blood pressure in obese patients [146,147,148]. Quercetin also decreases the expression of endothelin-1 messenger ribonucleic acid (mRNA), which stimulates the dilation of coronary vessels and improves endothelial function [149]. Another study showed that quercetin regulates the expression of p47phox or neutrophil cytosol factor 1 and therefore reduces the levels of superoxide anion (O2−), as mediated by nicotinamide adenine dinucleotide phosphate oxidase (NADPH oxidase), ultimately preventing endothelial dysfunction in hypertension [150].
Caffeic acid, which can increase the plasma level of vitamin E, scavenge free radicals, and reduce oxidative stress, demonstrates its effectiveness in MI [151,152]. It is also reported to be a potential antihypertensive agent as well [153]. Kaempferol effectively reduces oxidative stress, which is effective in CVD. On the other hand, naringenin, which is efficacious against interstitial fibrosis and cardiac hypertrophy, can improve the function of the left ventricle in mice with induced pressure overload. It also downregulates the activation of the c-Jun N-terminal kinases (JNK), extracellular signal–regulated kinases (ERK) and phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K)/protein kinase B (AKt) signaling pathways, which may offer some cardioprotective effects [154].
Lutein reduces carotid artery intima-media thickness, especially in patients with subclinical atherosclerosis [155]. Moreover, the use of dietary lutein has been reported to reduce atherosclerotic lesions, especially in combination with polyunsaturated fatty acids (PUFAs) [156]. Luteolin confers cardioprotective mechanisms against ischemia/reperfusion (I/R) injury by increasing pro-apoptotic molecules, Bcl2-associated agonist of cell death (BAD) phosphorylation and manganese superoxide dismutase (MnSOD) activity, which helps to inhibit the mitochondrial permeability transition pore (mPTP), stimulate the PI3K/AKt as well as myocardial endothelial nitric oxide synthase (eNOS) pathways, upregulate leukemic inhibitory factor (LIF) and anti-apoptotic proteins such as fibroblast growth factor receptor 2 (FGFR2) expression and decrease the ratio of Bax to Bcl-2, particularly in diabetes [157,158]. Moreover, luteolin inhibits thrombin activity, fibrin polymer formation and thrombosis, which is induced by oxidative stress and binds with thromboxane A2 receptor, which can hinder platelet aggregation, and thus confers some antithrombotic effect [159,160].
Vanillic acid reduces hypertension and infarct size in I/R, improves ventricular function and shows antioxidant mechanisms that can confer some cardioprotective effect [161,162,163]. Chlorogenic acid demonstrates antihypertensive, antiplatelet, antithrombotic and antioxidant activities involving A2A receptor and NF-κB and the adenylate cyclase/cyclic adenosine monophosphate/protein kinase A (adenylatecyclase/cAMP/PKA) pathways, which can improve CVDs [164,165,166]. On the other hand, ferulic acid improves the structure as well as the function of blood vessels and the heart [167]. Additionally, p-coumaric acid exhibits a preventive role in cardiac hypertrophy and MI by stimulating anti-hypertrophic and radical scavenging activities that constrain lysosomal dysfunction and ameliorate the levels of lysosomal enzymes [168,169].
On the other hand, chrysin has an anti-atherogenic activity and can ameliorate MI by activating peroxisome proliferator-activated receptor gamma (PPAR-ɣ) and inhibiting oxidative stress as well as inflammation, mediated by the advance glycation end product [170,171]. Catechin regulates lipid and blood lipid metabolism, reduces, and regulates blood pressure, protects vascular endothelial cells, reduces the cell proliferation of vascular smooth muscle, suppresses platelet adhesion, inhibits thrombogenesis and increases vascular integrity, thereby reducing the risk for CVDs [172,173]. Epicatechin can decrease platelet-induced endothelial activation along with catechin [174]. Cinnamic acid ameliorates myocardial ischemia due to its anti-inflammatory and anti-oxidative properties [175]. On the other hand, sinapic acid has membrane-stabilizing and free radical scavenging properties by which it can inhibit fibrosis and lysosomal dysfunction in heart diseases [176,177]. The protective effects of resveratrol occur by the upregulation of AMP-activated protein kinase (AMPK) and sirtuin (SITR1) and the activation of endogenous antioxidant enzymes against cardiovascular complications. It also confers some lipid-reducing, antiplatelet and anti-inflammatory properties that are beneficial in CVDs [178].
6.2. Tomato in Diabetes
Some bioactive compounds present in tomato are effective in diabetes (Figure 2). For instance, lycopene has been reported to exert hypoglycemic effects by increasing serum insulin levels and lowering glucose levels in diabetic animals as induced by streptozotocin (STZ) [179,180]. Lycopene reduces angiotensin converting enzyme (ACE) activity, the level of which can indicate diabetes or complications related to diabetes [181]. In addition, it has been reported to improve renal function and exhibit a defensive effect against diabetic nephropathy by regulating connecting tissue growth factor and p-Akt, reducing malondialdehyde levels and enhancing antioxidant activities [182]. It has also been reported that oocyte maturation, follicular growth and protection of ovaries are promoted by lycopene in diabetic conditions [183].
Quercetin stimulates glucose uptake by regulating mitogen-activated protein kinase (MAPK) insulin-dependent mechanisms, improving renal function, inhibiting the overexpression of transforming growth factor beta 1 (TGF-β1) and connective tissue growth factor (CTGF), hindering polyol accumulation as well as blocking aldose reductase, and thus reducing joint pain, irritation, and numbness, which are common symptoms observed in diabetes [184]. Quercetin elevates transmembrane potential and membrane fluidity and confers anti-inflammatory activity on immune and endothelial cells, which is beneficial especially in the late stage of diabetes [185]. Due to its antiplatelet activity, quercetin delays thrombus formation and plays a crucial role as an antioxidant by decreasing the generation of lipid hydroperoxides and increasing the activity of glutathione peroxidase in diabetes [186,187]. It also regulates NF-κB signaling and the mitochondrial pathway, significantly preventing the death of β cells [188].
On the other hand, kaempferol minimizes α-glucosidase activity, increases antioxidant activity, reduces lipid peroxidation level, protects β cell function and improves insulin sensitivity of the periphery, therefore exhibiting antidiabetic properties [189,190,191]. Kaempferol also downregulates I kappa B kinase (IKK), and thus inhibits the NF-κB pathway, which reduces inflammatory lesions in hepatic cells and improves insulin signaling [192]. Kaempferol also restores membrane-bound ATPase, which is normally affected in diabetes [193].
Naringenin, which has the potential to diminish nephropathy and improve endothelial complications along with lipid and glucose metabolisms in diabetes, shows antifibrotic, anti-oxidative and anti-inflammatory activities [194,195]. Interestingly, naringenin also inhibits increased cholinesterase (ChE) activity, which contributes to memory dysfunction [196]. Therefore, it is another important component present in tomato.
Caffeic acid has been reported to confer some anti-inflammatory and antiglycemic activities in diabetic kidney disease [197]. Chlorogenic acid reduces plasma glucose levels in fasting conditions and during late diabetes, where it lowers glycosylated hemoglobin (HbA1c). Additionally, by modulating the signaling pathway of the adiponectin receptor, chlorogenic acid improves diabetic kidney fibrosis [198]. It also prevents neurological complications and retinopathy accelerated by diabetes by improving memory, inhibiting TBARS production, reducing anxiety and reducing vascular hyperpermeability [199,200].
In pregnant women affected by gestational diabetes, lutein intake has been reported to reduce neonatal oxidative stress during birth [201]. Moreover, lutein, which is another important component present in tomato, prevents cataract progression and preserves the composition of free fatty acids, which is abnormal in diabetic conditions [202,203]. Luteolin can inhibit α-glucosidase action and improve insulin resistance [204,205], which is important in both diabetic encephalopathy and neuropathy [206,207].
Vanillic acid reduces blood pressure, plasma glucose and insulin by activating antioxidants, thereby reducing oxidative stress [208]. Ferulic acid reduces oxidative stress, and thus prevents testicular damage and downregulates both apoptosis and pro-inflammatory cytokine expression in diabetes [209,210]. Another important substance is p-coumaric acid, which exerts glucose-lowering activity by activating pancreatic glucose transporter 2 (GLUT 2), regulating lipid and glucose metabolisms and exhibiting some antidiabetic effects [211]. Moreover, p-coumaric acid shows anti-apoptotic, anti-inflammatory and antioxidant activities that counter hippocampal neurodegeneration in diabetes [212].
Cinnamic acid and some of its derivatives have the potential to be applied in the treatment of diabetes because of their many useful properties including (1) increased glucose uptake, adiponectin secretion, insulin secretion and hepatic glycolysis; (2) improved functionality of pancreatic β cells; (3) reduced adipogenesis; (4) improved hepatic gluconeogenesis, protein glycation, insulin fibrillation and intestinal glucose absorption and (5) decreased activity of certain enzymes, including α-glucosidase, dipeptidyl peptidase-4, pancreatic α-amylase and protein tyrosine phosphatase 1B [213].
Sinapic acid improves hyperglycemia and maximizes glucose utilization by regulating the signals of phospholipase C (PLC) and protein kinase C (PKC) in diabetes [214]. Catechin activates endothelial phosphoinositide (PI3K) signaling and consequently activates eNOS, resulting in the generation of nitrous oxide against vascular endothelial dysfunction (VED) in diabetes. In cases of vascular endothelial abnormalities (VEA), catechin confers a protective effect by reducing high glucose, lipid peroxidation and oxidative stress [215]. In addition, catechin protects against diabetic nephropathy by constraining the formation of advanced glycated end products and blocking inflammatory signaling pathways since catechin can trap the metabolite methylglyoxal [216].
Epicatechin, another important constituent of tomato, reduces insulin resistance, increases insulin sensitivity, and reduces oxidative stress [217]. It also improves pancreatic insulitis and islet mass as well as muscle function [218,219]. Chrysin hinders the activity of α-glucosidase, reduces oxidative stress, and generates moderate amounts of nitric oxide to prevent diabetes-related complications [220,221]. In addition to its antioxidant properties, chrysin has anti-inflammatory properties and the ability to regulate the apoptotic cascade by which it improves diabetic-associated cognitive deficits (DACD) and diabetic nephropathy [222,223]. Resveratrol is another important component present in tomato. In addition to having a significant effect on different signaling and metabolic pathways that can improve diabetes, it enhances mitochondrial biogenesis and reduces mitochondrial damage, oxidative damage, inflammation, lipid accumulation, liver steatosis and improves the action of insulin [224].
6.3. Tomato against Cancer
Many recent studies have suggested that regular intake of fruits and vegetables that are high in antioxidants prevents the progression of cancer cells [14,225]. There are many important bioactive phytochemicals present in fruits and vegetables that are responsible for cancer prevention. For example, bioactive phytochemicals can prevent cancer via various mechanisms including (1) inhibition of cancer cell proliferation [226], (2) prevention of oxidative stress via antioxidant effects [227], (3) alteration of cancer cell signaling pathways [228,229], (4) modification of enzymatic activity [230], (5) inhibition of oxidative DNA damage [231], (6) increasing the expression of phase II enzymes [232], and (7) inducing apoptosis of cancer cells [233,234] (Figure 2).
Many epidemiological studies have suggested that dietary intake of tomato can ameliorate cancer [13,70]. In fact, the excessive consumption of tomato is reported to confer some preventive effects against gastrointestinal tract cancer when compared to a control [235]. A research conducted by Colditz et al. confirmed that the intake of tomato and other types of vegetables is important for cancer prevention. In their research, from the 1271 elderly individuals investigated, 42 cancer patients died. However, those with higher intakes of tomato had a better prognosis [236]. Studies from different countries have reported that colon cancer is inversely correlated with a high tomato consumption [237,238]. Moreover, a case-control study from China and Italy indicated that approximately 60% of colon and rectal cancer patients showed some improvements following the intake of high amounts of tomato [235,239,240].
Lycopene, a major carotenoid present in tomato, has antioxidant properties and can prevent prostate cancer by blocking ROS generation, scavenging free radicals, and protecting both cell membrane and DNA from oxidative damage [10,17,241]. In another study, approximately 83% of prostate cancer was ameliorated in patients who received the highest amount of lycopene (0.40 µm/L) in comparison with lower amounts (0.18 µm/L) [242]. Siler et al. investigated the protective effects of lycopene and vitamin E (two important components present in tomatoes) on the growth of prostate tumors in a Dunning MAT-LyLu rat model [243], where rapidly growing tumor cells were injected into the ventral prostate of the experimental rats. Both lycopene- and vitamin E-treated experimental rats showed a significant reduction in tumor size and necrosis when compared to untreated rats. Moreover, molecular analysis of the tumor tissues indicated that vitamin E reduces androgen signaling, while lycopene downregulates the expression of 5-α reductase 1, interleukin 6 and insulin-like growth factor-1.
Quercetin confers both antioxidant and anticancer properties that prevent the proliferation of prostate cancer cells by increasing the levels of p21, Bax and caspase-3, and thus preventing the expression of the cell cycle regulator cdc2/cdk-1, cyclin B1, phosphorylated pRB and apoptosis markers Bcl-2 and Bcl-XL [244]. In another study, quercetin showed anticancer properties by inhibiting the P13k-Akt/PKB pathway [245]. Quercetin also protects against colon cancer by inhibiting β-catenin/Tcf signaling in SW480 colon cancer cell lines and reducing β-catenin/Tcf transcription activity [246]. Luteolin, which is also present in tomato, is an important polyphenol that exhibits anticancer properties by inhibiting rat aortic vascular smooth muscle cell proliferation and DNA synthesis as induced by platelet-derived growth factor-BB (PDGF-BB) via blockage of the phosphorylation of PDGF-BB receptor [247]. Apigenin, another important flavonoid in tomato, also has some anticancer properties since it inhibits pancreatic cancer cell proliferation by arresting the G2/M cell cycle, and thus decreases the concentration of cyclin A, cyclin B and the phosphorylated forms of cdc2 as well as cdc25 [248].
7. Limitations and Future Prospects
The nutritional composition of tomato depends on the maturation of fruits, ripening time, geographical location, tomato variety, freshness and whether they are tomato-based food products. The nutritional composition will also differ depending on the conditions of sample preparation, collection, and tomato parts. Nevertheless, to date, there are limited data on the nutritional contents of tomato peel and pulp for a good comparison to be made. Therefore, in this review, only the nutritional composition of fresh matured tomatoes is discussed. Further study needs to investigate the bioactive compounds present and investigate their impact on human health.
Additionally, although some research works have been published on tomato processing by-products containing many bioactive nutrients including lycopene, beta-carotene, amino acids, proteins, lipids, and dietary fibers [249,250], the bioavailability, safety, and pharmacological toxicity have not been investigated. Another study Salehi et al. demonstrated that tomato consumption, in addition to giving some beneficial effects, is also associated with some health risks including renal problems, irritable bowel syndrome (IBS), migration, allergy, body aches, arthritis, and urinary problems [1]. Therefore, research should also focus on the possible health risks that may arise due to tomato consumption and the amount consumed that leads to detrimental effects. Finally, there is a need to conduct randomized clinical trials that can assess the effects of long-term tomato consumption on its various health benefits.
8. Conclusions
Tomatoes are vegetables/fruits that contain significant amounts of dietary nutrients, including dietary fiber, reducing sugars, vitamins, minerals, protein, essential fatty acids, phytosterols and carotenoids. The nutritive elements play an important role in bodily function and are beneficial in ameliorating chronic diseases. Tomatoes are also rich with health promoting bioactive phytochemicals, such as phenolic compounds including lycopene, quercetin, kaempferol, naringenin, caffeic acid and lutein. The bioactive constituents show antioxidant, antiproliferative, antidiabetic, anti-inflammatory, and other health-promoting activities, indicating the vast potential of tomato in preventing and/or ameliorating several chronic degenerative diseases. Lycopene and β-carotene are two main active ingredients in tomato that have strong antioxidant properties, which are linked with many health benefits, including cancer and heart diseases. They also participate in preventing the development of cataracts. Additionally, the water content and dietary fibers help the body in terms of hydration, bowel movements, reducing constipation, improving obesity through weight loss, and preventing colon cancer. All immune stimulating activities of tomatoes make them active ingredients for the development of functional foods. Thus, tomato is an excellent source of dietary nutrients and is useful in disease prevention.
Author Contributions
M.Y.A., L.N., and A.K. performed a literature search, compiled data and analyzed the data and M.Y.A. and S.S.K. wrote the manuscript. A.A.I.S. and E.M.T. contributed to the revision of the manuscript. M.I.K. and S.H.G. provided the overall concept, revised the manuscript, and critically edited and approved the final version of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by NHMRC Investigator Grant APP1175047 for AAIS.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
We declare that there are no conflicts of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Salehi B., Sharifi-Rad R., Sharopov F., Namiesnik J., Roointan A., Kamle M., Kumar P., Martins N., Sharifi-Rad J. Beneficial effects and potential risks of tomato consumption for human health: An overview. Nutrition. 2019;62:201–208. doi: 10.1016/j.nut.2019.01.012. [DOI] [PubMed] [Google Scholar]
- 2.Lenucci M.S., Cadinu D., Taurino M., Piro G., Dalessandro G. Antioxidant composition in cherry and high-pigment tomato cultivars. J. Agric. Food Chem. 2006;54:2606–2613. doi: 10.1021/jf052920c. [DOI] [PubMed] [Google Scholar]
- 3.Elbadrawy E., Sello A. Evaluation of nutritional value and antioxidant activity of tomato peel extracts. Arab. J. Chem. 2016;9:S1010–S1018. doi: 10.1016/j.arabjc.2011.11.011. [DOI] [Google Scholar]
- 4.Abdullahi I.I., Abdullahi N., Abdu A.M., Ibrahim A.S. Proximate, Mineral and Vitamin Analysis of Fresh and Canned Tomato. Biosci. Biotechnol. Res. Asia. 2016;13:1163–1169. doi: 10.13005/bbra/2147. [DOI] [Google Scholar]
- 5.Ramos-Bueno R.P., Romero-Gonzalez R., Gonzalez-Fernandes M.J., Guil-Guerrero J.L. Phytochemical composition and in vitro anti-tumour activities of selected tomato varieties. J. Sci. Food Agric. 2017;97:488–496. doi: 10.1002/jsfa.7750. [DOI] [PubMed] [Google Scholar]
- 6.Chaudhary P., Sharma A., Singh B., Nagpal A.K. Bioactivities of phytochemicals present in tomato. J. Food Sci. Technol. 2018;55:2833–2849. doi: 10.1007/s13197-018-3221-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campestrini L.H., Melo P.S., Peres L.E.P., Calhelha R.C., Ferreira I.C., De Alencar S.M. A new variety of purple tomato as a rich source of bioactive carotenoids and its potential health benefits. Heliyon. 2019;5:e02831. doi: 10.1016/j.heliyon.2019.e02831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vats S., Bansal R., Rana N., Kumawat S., Bhatt V., Jadhav P., Kale V., Sathe A., Sonah H., Jugdaohsingh R., et al. Unexplored nutritive potential of tomato to combat global malnutrition. Crit. Rev. Food Sci. Nutr. 2020:1–32. doi: 10.1080/10408398.2020.1832954. [DOI] [PubMed] [Google Scholar]
- 9.Cheng H.M., Koutsidis G., Lodge J.K., Ashor A.W., Siervo M., Lara J. Lycopene and tomato and risk of cardiovascular diseases: A systematic review and meta-analysis of epidemiological evidence. Crit. Rev. Food Sci. Nutr. 2019;59:141–158. doi: 10.1080/10408398.2017.1362630. [DOI] [PubMed] [Google Scholar]
- 10.Li N., Wu X., Zhuang W., Xia L., Chen Y., Wu C., Rao Z., Du L., Zhao R., Yi M., et al. Tomato and lycopene and multiple health outcomes: Umbrella review. Food Chem. 2020:128396. doi: 10.1016/j.foodchem.2020.128396. [DOI] [PubMed] [Google Scholar]
- 11.Park H.-A., Hayden M.M., Bannerman S., Jansen J., Crowe-White K.M. Anti-apoptotic effects of carotenoids in neurodegeneration. Molecules. 2020;25:3453. doi: 10.3390/molecules25153453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Agarwal S., Rao A.V. Tomato lycopene and its role in human health and chronic diseases. Can. Med. Assoc. J. 2000;163:739–744. [PMC free article] [PubMed] [Google Scholar]
- 13.Navarro-González I., García-Alonso J., Periago M.J. Bioactive compounds of tomato: Cancer chemopreventive effects and influence on the transcriptome in hepatocytes. J. Funct. Foods. 2018;42:271–280. doi: 10.1016/j.jff.2018.01.003. [DOI] [Google Scholar]
- 14.Hossen M.S., Ali M.Y., Jahurul M.H.A., Abdel-Daim M.M., Gan A.H., Khalil M.I. Beneficial roles of honey polyphenols against some human degenerative diseases: A review. Pharmacol. Rep. 2017;69:1194–1205. doi: 10.1016/j.pharep.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 15.Zhu R., Chen B., Bai Y., Miao T., Rui L., Zhang H., Xia B., Li Y., Gao S., Wang X.-D., et al. Lycopene in protection against obesity and diabetes: A mechanistic review. Pharmacol. Res. 2020;159:104966. doi: 10.1016/j.phrs.2020.104966. [DOI] [PubMed] [Google Scholar]
- 16.Sakemi Y., Sato K., Hara K., Honda M., Shindo K. Biological Activities of Z-Lycopenes Contained in Food. J. Oleo Sci. 2020;69:1509–1516. doi: 10.5650/jos.ess20163. [DOI] [PubMed] [Google Scholar]
- 17.Saini R.K., Rengasamy K.R., Mahomoodally F.M., Keum Y.-S. Protective effects of lycopene in cancer, cardiovascular, and neurodegenerative diseases: An update on epidemiological and mechanistic perspectives. Pharmacol. Res. 2020;155:104730. doi: 10.1016/j.phrs.2020.104730. [DOI] [PubMed] [Google Scholar]
- 18.Imran M., Ghorat F., Ul-Haq I., Ur-Rehman H., Aslam F., Heydari M., Shariati M.A., Okuskhanova E., Yessimbekov Z., Thiruvengadam M., et al. Lycopene as a natural antioxidant used to prevent human health disorders. Antioxidants. 2020;9:706. doi: 10.3390/antiox9080706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tohge T., Fernie A.R. Metabolomics-inspired insight into developmental, environmental and genetic aspects of tomato fruit chemical composition and quality. Plant Cell Physiol. 2015;56:1681–1696. doi: 10.1093/pcp/pcv093. [DOI] [PubMed] [Google Scholar]
- 20.Claye S.S., Idouraine A., Weber C.W. Extraction and fractionation of insoluble fiber from five fiber sources. Food Chem. 1996;57:305–310. doi: 10.1016/0308-8146(95)00250-2. [DOI] [Google Scholar]
- 21.Delzenne N.M., Olivares M., Neyrinck A.M., Beaumont M., Larsen T.M., Benitez-Paez A., Romani-Perez M., Garcia-Campayo V., Bosscher D., Sanz Y., et al. Nutritional interest of dietary fiber and prebiotics in obesity: Lessons from the MyNewGut consortium. Clin. Nutr. 2020;39:414–424. doi: 10.1016/j.clnu.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 22.Merenkova S., Zinina O., Stuart M., Okuskhanova E., Androsova N. Effects of dietary fiber on human health: A review. Чeлoвeк. Cпopт. Meдицинa. 2020;20:106–113. doi: 10.14529/hsm200113. [DOI] [Google Scholar]
- 23.Uddin M.S., Ferdosh S., Akanda J.H., Ghafoor K., Rukshana A.H., Ali E., Kamaruzzaman B.Y., Fauzi M.B., Hadijah S., Shaarani S., et al. Techniques for the extraction of phytosterols and their benefits in human health: A review. Sep. Sci. Technol. 2018;53:2206–2223. doi: 10.1080/01496395.2018.1454472. [DOI] [Google Scholar]
- 24.Kelebek H., Selli S., Kadiroğlu P., Kola O., Kesen S., Uçar B., Çetiner B. Bioactive compounds and antioxidant potential in tomato pastes as affected by hot and cold break process. Food Chem. 2017;220:31–41. doi: 10.1016/j.foodchem.2016.09.190. [DOI] [PubMed] [Google Scholar]
- 25.Sofy A.R., Dawoud R.A., Sofy M.R., Mohamed H.I., Hmed A.A., El-Dougdoug N.K. Improving regulation of enzymatic and non-enzymatic antioxidants and stress-related gene stimulation in cucumber mosaic cucumovirus-infected cucumber plants treated with glycine betaine, chitosan and combination. Molecules. 2020;25:2341. doi: 10.3390/molecules25102341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paulino S.L.J., Adrián Á.-T.G., Gabriela E.-A.L., Maribel V.-M., Sergio M.-G. Nutraceutical potential of flours from tomato by-product and tomato field waste. J. Food Sci. Technol. 2020;57:3525–3531. doi: 10.1007/s13197-020-04585-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu Z., Wang J., Gao R., Ye F., Zhao G. Sustainable valorisation of tomato pomace: A comprehensive review. Trends Food Sci. Technol. 2019;86:172–187. doi: 10.1016/j.tifs.2019.02.020. [DOI] [Google Scholar]
- 28.Domínguez R., Gullón P., Pateiro M., Munekata P.E.S., Zhang W., Lorenzo J.M. Tomato as Potential Source of Natural Additives for Meat Industry. A Review. Antioxidants. 2020;9:73. doi: 10.3390/antiox9010073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramdath D.D., Lu Z.-H., Maharaj P.L., Winberg J., Brummer Y., Hawke A. Proximate Analysis and Nutritional Evaluation of Twenty Canadian Lentils by Principal Component and Cluster Analyses. Foods. 2020;9:175. doi: 10.3390/foods9020175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harris G.K., Marshall M.R. Food Analysis. Springer; Cham, Switzerland: 2017. Ash analysis; pp. 287–297. [Google Scholar]
- 31.Maestri D., Barrionuevo D., Bodoira R., Zafra A., Jiménez-López J.C., Alché J.D.D. Nutritional profile and nutraceutical components of olive (Olea europaea L.) seeds. J. Food Sci. Technol. 2019;56:4359–4370. doi: 10.1007/s13197-019-03904-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nielsen S.S. Food Analysis Laboratory Manual. Springer; Boston, MA, USA: 2010. Determination of moisture content; pp. 17–27. [Google Scholar]
- 33.Aurand L.W. Food Composition and Analysis. Springer; Dordrecht, The Netherlands: 2013. [Google Scholar]
- 34.Pomeranz Y. Food Analysis: Theory and Practice. Springer; Boston, MA, USA: 2013. [Google Scholar]
- 35.Phizicky E., Bastiaens P.I.H., Zhu H., Snyder M., Fields S. Protein analysis on a proteomic scale. Nature. 2003;422:208–215. doi: 10.1038/nature01512. [DOI] [PubMed] [Google Scholar]
- 36.Christie W.W. Advances in Lipid Methodology. Volume 4 Woodhead Publishing Ltd.; Cambridge, UK: 1007. [Google Scholar]
- 37.BeMiller J.N. Carbohydrate Chemistry for Food Scientists. Woodhead Publishing Ltd.; Cambridge, UK: 2019. [Google Scholar]
- 38.BeMiller J.N. Food Analysis. Springer; Boston, MA, USA: 2010. Carbohydrate Analysis; pp. 147–177. [Google Scholar]
- 39.Buttriss J., Stokes C.S. Dietary fibre and health: An overview. Nutr. Bull. 2008;33:186–200. doi: 10.1111/j.1467-3010.2008.00705.x. [DOI] [Google Scholar]
- 40.Favier J.C., Ireland-Ripert J., Toque C., Feinberg M. Repertoire general des aliments, table de composition. Mitt. Geb. Lebensm. Hyg. 1997;88:209. [Google Scholar]
- 41.Souci S., Fachmann W., Kraut H. Food Composition and Nutrition Tables. 5th ed. Medpharm GmbH, Scientific Publishers; Stuttgart, Germany: CRC Press; Boca Raton, FL, USA: 1994. [Google Scholar]
- 42.David H., Linda L., Pamela P. National Nutrient Database for Standard Reference. USDA; Beltsville, MD, USA: 2016. Release 24. [Google Scholar]
- 43.Hernández M.H., Rodríguez E.R., Romero C.D. Chemical composition of tomato (Lycopersicon esculentum) from Tenerife, the Canary Islands. Food Chem. 2008;106:1046–1056. doi: 10.1016/j.foodchem.2007.07.025. [DOI] [Google Scholar]
- 44.Navarro-González I., García-Valverde V., García-Alonso J., Periago-Castón M.J. Chemical profile, functional and antioxidant properties of tomato peel fiber. Food Res. Int. 2011;44:1528–1535. doi: 10.1016/j.foodres.2011.04.005. [DOI] [Google Scholar]
- 45.Oboulbiga E.B., Parkouda C., Sawadogo-Lingani H., Compaoré E.W.R., Sakira A.K., Traoré A.S. Nutritional Composition, Physical Characteristics and Sanitary Quality of the Tomato Variety Mongol F1 from Burkina Faso. Food Nutr. Sci. 2017;8:444–455. doi: 10.4236/fns.2017.84030. [DOI] [Google Scholar]
- 46.Frusciante L., Carli P., Ercolano M.R., Pernice R., Di Matteo A., Fogliano V., Pellegrini N. Antioxidant nutritional quality of tomato. Mol. Nutr. Food Res. 2007;51:609–617. doi: 10.1002/mnfr.200600158. [DOI] [PubMed] [Google Scholar]
- 47.Guil-Guerrero J., Rebolloso-Fuentes M. Nutrient composition and antioxidant activity of eight tomato (Lycopersicon esculentum) varieties. J. Food Compos. Anal. 2009;22:123–129. doi: 10.1016/j.jfca.2008.10.012. [DOI] [Google Scholar]
- 48.Opara U.L., Al-Ani M.R., Al-Rahbi N.M. Effect of fruit ripening stage on physico-chemical properties, nutritional composition and antioxidant components of tomato (Lycopersicum esculentum) cultivars. Food Bioprocess Technol. 2012;5:3236–3243. doi: 10.1007/s11947-011-0693-5. [DOI] [Google Scholar]
- 49.Pinela J., Barros L., Carvalho A.M., Ferreira I.C. Nutritional composition and antioxidant activity of four tomato (Lycopersicon esculentum L.) farmer’ varieties in Northeastern Portugal homegardens. Food Chem. Toxicol. 2012;50:829–834. doi: 10.1016/j.fct.2011.11.045. [DOI] [PubMed] [Google Scholar]
- 50.Raffo A., Leonardi C., Fogliano V., Ambrosino P., Salucci M., Gennaro L., Bugianesi R., Giuffrida F., Quaglia G. Nutritional value of cherry tomatoes (Lycopersicon esculentum Cv. Naomi F1) harvested at different ripening stages. J. Agric. Food Chem. 2002;50:6550–6556. doi: 10.1021/jf020315t. [DOI] [PubMed] [Google Scholar]
- 51.Srivastava S., Kulshreshtha K. Nutritional Content and Significance of Tomato Powder. Ann. Arid Zone. 2013;52:121–124. [Google Scholar]
- 52.Ahmed M.J., Iya I.R., Dogara M.F. Proximate, Mineral and Vitamin Content of Flesh, Blanched and Dried Tomatoes (Lycopersicon esculentum) Asian Food Sci. J. 2020;18:11–18. doi: 10.9734/afsj/2020/v18i430223. [DOI] [Google Scholar]
- 53.Kumar V., Abbas A., Fausto N., Aster J. Robbins and Cotran Pathologic Basis of Disease. Elsevier; Philadelphia, PA, USA: 2005. [Google Scholar]
- 54.Baer L., Platman S.R., Fieve R.R. The role of electrolytes in affective disorders: Sodium, potassium, and lithium ions. Arch. Gen. Psychiatry. 1970;22:108–113. doi: 10.1001/archpsyc.1970.01740260012002. [DOI] [PubMed] [Google Scholar]
- 55.Paul S., Ali Y., Rumpa N.-E.-N., Tanvir E.M., Hossen S., Saha M., Bhoumik N.C., Gan S.H., Khalil I. Assessment of toxicity and beneficiary effects of garcinia pedunculata on the hematological, biochemical, and histological homeostasis in rats. Evid. Based Complementary Altern. Med. 2017;2017:4686104. doi: 10.1155/2017/4686104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gopalan C., Sastri B.R., Balasubramanian S. Nutrition Value of Indian Foods. National Institute of Nutrition; Hyderabad, India: 1980. [Google Scholar]
- 57.Ionete R.E., Dinca O.R., Geana E.I., Costinel D. Macro-and microelements as possible markers of quality and authenticity for fruits and derived products. Prog. Cryog. Isot. Sep. 2016;19:55. [Google Scholar]
- 58.Solayman M., Islam A., Paul S., Ali Y., Khalil I., Alam N., Gan S.H. Physicochemical properties, minerals, trace elements, and heavy metals in honey of different origins: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2016;15:219–233. doi: 10.1111/1541-4337.12182. [DOI] [PubMed] [Google Scholar]
- 59.Nielsen F.H. Ultratrace elements in nutrition. Annu. Rev. Nutr. 1984;4:21–41. doi: 10.1146/annurev.nu.04.070184.000321. [DOI] [PubMed] [Google Scholar]
- 60.Zugravu C.A., Parvu M., Patrascu D., Stoian A. Correlations between lead and cadmium pollution of honey and environmental heavy metal presence in two Romanian counties. Bull. UASVM Agric. 2009;66:230–233. [Google Scholar]
- 61.Campbell J.K., Canene-Adams K., Lindshield B.L., Boileau T.W.-M., Clinton S.K., Erdman J.J.W. Tomato phytochemicals and prostate cancer risk. J. Nutr. 2004;134:3486S–3492S. doi: 10.1093/jn/134.12.3486S. [DOI] [PubMed] [Google Scholar]
- 62.Favier J.C., Ireland-Ripert J., Toque C., Feinberg M. Répertoire Général des Alimentes: Table de Composition = Composition Tables. INRA; Paris, France: 1995. [Google Scholar]
- 63.Ramesh K.V., Paul V., Pandey R. Dynamics of mineral nutrients in tomato (Solanum lycopersicum L.) fruits during ripening: Part I—On the plant. Plant Physiol. Rep. 2020:1–15. doi: 10.1007/s40502-020-00546-0. [DOI] [Google Scholar]
- 64.Gerald F. The Vitamins Fundamental Aspects in Nutrition and Health. Elsevier; Oxford, UK: 2019. [Google Scholar]
- 65.Beecher G.R. Nutrient content of tomatoes and tomato products. Exp. Biol. Med. 1998;218:98–100. doi: 10.3181/00379727-218-44282a. [DOI] [PubMed] [Google Scholar]
- 66.Borguini R.G., Torres E.A.F.D.S. Tomatoes and tomato products as dietary sources of antioxidants. Food Rev. Int. 2009;25:313–325. doi: 10.1080/87559120903155859. [DOI] [Google Scholar]
- 67.Sies H., Stahl W., Sundquist A.R. Antioxidant functions of vitamins. Ann. N. York Acad. Sci. 1992;669:7–20. doi: 10.1111/j.1749-6632.1992.tb17085.x. [DOI] [PubMed] [Google Scholar]
- 68.Sies H., Stahl W. Vitamins E and C, beta-carotene, and other carotenoids as antioxidants. Am. J. Clin. Nutr. 1995;62:1315S–1321S. doi: 10.1093/ajcn/62.6.1315S. [DOI] [PubMed] [Google Scholar]
- 69.Canene-Adams K., Campbell J.K., Zaripheh S., Jeffery E.H., Erdman J.J.W. The tomato as a functional food. J. Nutr. 2005;135:1226–1230. doi: 10.1093/jn/135.5.1226. [DOI] [PubMed] [Google Scholar]
- 70.Giovannucci E. Tomatoes, tomato-based products, lycopene, and cancer: Review of the epidemiologic literature. J. Natl. Cancer Inst. 1999;91:317–331. doi: 10.1093/jnci/91.4.317. [DOI] [PubMed] [Google Scholar]
- 71.Sure B. The vitamins in health and disease. Nature. 1933;132:732. [Google Scholar]
- 72.Marcus R., Coulston A.M. Goodman & Gilman’s The Pharmacological Basis of Therapeutics. 10th ed. McGraw-Hill; New York, NY, USA: 1990. The vitamin B complex and ascorbic acid. [Google Scholar]
- 73.Souci S.W., Fachmann W., Kraut H. Food Composition and Nutrition Tables 1981/82. 2nd ed. Wissenschaftliche Verlagsgesellschaft mbH; Stuttgart, Germany: 1981. [Google Scholar]
- 74.USDA . National Nutrient Database for Standard Reference. US Department of Agriculture; Beltsville, MD, USA: 2008. Release 20. [Google Scholar]
- 75.Kadiri M., Ojewumi A., Olawale S. Minerals, vitamins and chlorophyll contents of fruits, stems and leaves of tomato and garden egg. Pak. J. Food Sci. 2015;25:150–154. [Google Scholar]
- 76.Erba D., Casiraghi M.C., Ribas-Agustí A., Cáceres R., Marfà O., Castellari M. Nutritional value of tomatoes (Solanum lycopersicum L.) grown in greenhouse by different agronomic techniques. J. Food Compos. Anal. 2013;31:245–251. doi: 10.1016/j.jfca.2013.05.014. [DOI] [Google Scholar]
- 77.Freitas H.R., Isaac A.R., Malcher-Lopes R., Diaz B.L., Trevenzoli I.H., Reis R.A.D.M. Polyunsaturated fatty acids and endocannabinoids in health and disease. Nutr. Neurosci. 2018;21:695–714. doi: 10.1080/1028415X.2017.1347373. [DOI] [PubMed] [Google Scholar]
- 78.Zárate R., El Jaber-Vazdekis N., Tejera N., Pérez J.A., Rodríguez C. Significance of long chain polyunsaturated fatty acids in human health. Clin. Transl. Med. 2017;6:1–9. doi: 10.1186/s40169-017-0153-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Elango R., Laviano A. Protein and amino acids: Key players in modulating health and disease. Curr. Opin. Clin. Nutr. Metab. Care. 2017;20:69–70. doi: 10.1097/MCO.0000000000000343. [DOI] [PubMed] [Google Scholar]
- 80.Tsatsaronis G.C., Boskou D.G. Amino acid and mineral salt content of tomato seed and skin waste. J. Sci. Food Agric. 1975;26:421–423. doi: 10.1002/jsfa.2740260406. [DOI] [PubMed] [Google Scholar]
- 81.Freeman J., Woodbridge C. Effect of maturation, ripening and truss position on the free amino acid content in tomato fruits. Proc. Am. Soc. Hortic. Sci. 1960;76:515–523. [Google Scholar]
- 82.Meléndez-Martínez A.J. An Overview of Carotenoids, Apocarotenoids, and Vitamin A in Agro-Food, Nutrition, Health, and Disease. Mol. Nutr. Food Res. 2019;63:e1801045. doi: 10.1002/mnfr.201801045. [DOI] [PubMed] [Google Scholar]
- 83.Perusek L., Maeda T. Vitamin A derivatives as treatment options for retinal degenerative diseases. Nutrients. 2013;5:2646–2666. doi: 10.3390/nu5072646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang Y., Chun O.K., Song W.O. Plasma and dietary antioxidant status as cardiovascular disease risk factors: A review of human studies. Nutrients. 2013;5:2969–3004. doi: 10.3390/nu5082969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mora-Esteves C., Shin D. Nutrient supplementation: Improving male fertility fourfold. Semin. Reprod. Med. 2013;31:293–300. doi: 10.1055/s-0033-1345277. [DOI] [PubMed] [Google Scholar]
- 86.Kuklev D.V., Domb A.J., Dembitsky V.M. Bioactive acetylenic metabolites. Phytomedicine. 2013;20:1145–1159. doi: 10.1016/j.phymed.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 87.Sharoni Y., Danilenko M., Walfisch S., Amir H., Nahum A., Ben-Dor A., Hirsch K., Khanin M., Steiner M., Agemy L., et al. Role of gene regulation in the anticancer activity of carotenoids. Pure Appl. Chem. 2002;74:1469–1477. doi: 10.1351/pac200274081469. [DOI] [Google Scholar]
- 88.Sayo T., Sugiyama Y., Inoue S. Lutein, a nonprovitamin A, activates the retinoic acid receptor to induce HAS3-dependent hyaluronan synthesis in keratinocytes. Biosci. Biotechnol. Biochem. 2013;77:1282–1286. doi: 10.1271/bbb.130124. [DOI] [PubMed] [Google Scholar]
- 89.Trejo-Solís C., Pedraza-Chaverrí J., Torres-Ramos M., Jiménez-Farfán D., Salgado A.C., Serrano-García N., Osorio-Rico L., Sotelo J. Multiple molecular and cellular mechanisms of action of lycopene in cancer inhibition. Evid.-Based Complementary Altern. Med. 2013;2013:1–17. doi: 10.1155/2013/705121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Knoblich M., Anderson B., Latshaw D. Analyses of tomato peel and seed byproducts and their use as a source of carotenoids. J. Sci. Food Agric. 2005;85:1166–1170. doi: 10.1002/jsfa.2091. [DOI] [Google Scholar]
- 91.Chang C.-H., Lin H.-Y., Chang C.-Y., Liu Y.-C. Comparisons on the antioxidant properties of fresh, freeze-dried and hot-air-dried tomatoes. J. Food Eng. 2006;77:478–485. doi: 10.1016/j.jfoodeng.2005.06.061. [DOI] [Google Scholar]
- 92.Kalogeropoulos N., Chiou A., Pyriochou V., Peristeraki A., Karathanos V.T. Bioactive phytochemicals in industrial tomatoes and their processing byproducts. LWT. 2012;49:213–216. doi: 10.1016/j.lwt.2011.12.036. [DOI] [Google Scholar]
- 93.Tonucci L.H., Holden J.M., Beecher G.R., Khachik F., Davis C.S., Mulokozi G. Carotenoid content of thermally processed tomato-based food products. J. Agric. Food Chem. 1995;43:579–586. doi: 10.1021/jf00051a005. [DOI] [Google Scholar]
- 94.Górecka D., Wawrzyniak A., Jędrusek-Golińska A., Dziedzic K., Hamułka J., Kowalczewski P.Ł., Walkowiak J. Lycopene in tomatoes and tomato products. Open Chem. 2020;18:752–756. doi: 10.1515/chem-2020-0050. [DOI] [Google Scholar]
- 95.Ostlund R.E., Racette S.B., Stenson W.F. Inhibition of cholesterol absorption by phytosterol-replete wheat germ compared with phytosterol-depleted wheat germ. Am. J. Clin. Nutr. 2003;77:1385–1389. doi: 10.1093/ajcn/77.6.1385. [DOI] [PubMed] [Google Scholar]
- 96.Bradford P.G., Awad A.B. Phytosterols as anticancer compounds. Mol. Nutr. Food Res. 2007;51:161–170. doi: 10.1002/mnfr.200600164. [DOI] [PubMed] [Google Scholar]
- 97.Woyengo T., Ramprasath V., Jones P. Anticancer effects of phytosterols. Eur. J. Clin. Nutr. 2009;63:813–820. doi: 10.1038/ejcn.2009.29. [DOI] [PubMed] [Google Scholar]
- 98.Yoshida Y., Niki E. Antioxidant effects of phytosterol and its components. J. Nutr. Sci. Vitaminol. 2003;49:277–280. doi: 10.3177/jnsv.49.277. [DOI] [PubMed] [Google Scholar]
- 99.Trautwein E.A., Demonty I. Phytosterols: Natural compounds with established and emerging health benefits. Oléagineux Corps Gras Lipides. 2007;14:259–266. doi: 10.1051/ocl.2007.0145. [DOI] [Google Scholar]
- 100.Bouic P.J. The role of phytosterols and phytosterolins in immune modulation: A review of the past 10 years. Curr. Opin. Clin. Nutr. Metab. Care. 2001;4:471–475. doi: 10.1097/00075197-200111000-00001. [DOI] [PubMed] [Google Scholar]
- 101.Sies H., Jones D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020;21:363–383. doi: 10.1038/s41580-020-0230-3. [DOI] [PubMed] [Google Scholar]
- 102.Ames B.N., Shigenaga M.K., Hagen T.M. Oxidants, antioxidants, and the degenerative diseases of aging. Proc. Natl. Acad. Sci. USA. 1993;90:7915–7922. doi: 10.1073/pnas.90.17.7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.García-Sánchez A., Miranda-Díaz A.G., Germán C.-M.E. The role of oxidative stress in physiopathology and pharmacological treatment with pro- and antioxidant properties in chronic diseases. Oxidative Med. Cell. Longev. 2020;2020:1–16. doi: 10.1155/2020/2082145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Martínez-Valverde I., Periago M.J., Provan G., Chesson A. Phenolic compounds, lycopene and antioxidant activity in commercial varieties of tomato (Lycopersicum esculentum) J. Sci. Food Agric. 2002;82:323–330. doi: 10.1002/jsfa.1035. [DOI] [Google Scholar]
- 105.Vlaisavljevic S., Martínez M.C., Stojanović A., Martínez-Huélamo M., Grung B., Raventós R.M.L. Characterisation of bioactive compounds and assessment of antioxidant activity of different traditional Lycopersicum esculentum L. varieties: Chemometric analysis. Int. J. Food Sci. Nutr. 2019;70:813–824. doi: 10.1080/09637486.2019.1587742. [DOI] [PubMed] [Google Scholar]
- 106.Clinton S.K. Lycopene: Chemistry, biology, and implications for human health and disease. Nutr. Rev. 1998;56:35–51. doi: 10.1111/j.1753-4887.1998.tb01691.x. [DOI] [PubMed] [Google Scholar]
- 107.Kotíková Z., Lachman J., Hejtmánková A., Hejtmánková K. Determination of antioxidant activity and antioxidant content in tomato varieties and evaluation of mutual interactions between antioxidants. LWT. 2011;44:1703–1710. doi: 10.1016/j.lwt.2011.03.015. [DOI] [Google Scholar]
- 108.Vallverdú-Queralt A., Medina-Remón A., Martínez-Huélamo M., Jáuregui O., Andres-Lacueva C., Lamuela-Raventos R.M. Phenolic profile and hydrophilic antioxidant capacity as chemotaxonomic markers of tomato varieties. J. Agric. Food Chem. 2011;59:3994–4001. doi: 10.1021/jf104400g. [DOI] [PubMed] [Google Scholar]
- 109.Pandey K.B., Rizvi S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxidative Med. Cell. Longev. 2009;2:270–278. doi: 10.4161/oxim.2.5.9498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Abdel-Daim M.M., El-Tawil O.S., Bungau S., Atanasov A.G. Applications of antioxidants in metabolic disorders and degenerative diseases: Mechanistic approach. Oxidative Med. Cell. Longev. 2019;2019:1–3. doi: 10.1155/2019/4179676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Liu Z., Ren Z., Zhang J., Chuang C.-C., Kandaswamy E., Zhou T., Zuo L. Role of ROS and nutritional antioxidants in human diseases. Front. Physiol. 2018;9:477. doi: 10.3389/fphys.2018.00477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Stahl W., Sies H. Antioxidant activity of carotenoids. Mol. Asp. Med. 2003;24:345–351. doi: 10.1016/S0098-2997(03)00030-X. [DOI] [PubMed] [Google Scholar]
- 113.Leonardi C., Ambrosino P., Esposito F., Fogliano V. Antioxidative activity and carotenoid and tomatine contents in different typologies of fresh consumption tomatoes. J. Agric. Food Chem. 2000;48:4723–4727. doi: 10.1021/jf000225t. [DOI] [PubMed] [Google Scholar]
- 114.Burns J., Yokota T., Ashihara H., Lean M.E.J., Crozier A. Plant foods and herbal sources of resveratrol. J. Agric. Food Chem. 2002;50:3337–3340. doi: 10.1021/jf0112973. [DOI] [PubMed] [Google Scholar]
- 115.López-Lázaro M. Distribution and biological activities of the flavonoid luteolin. Mini-Rev. Med. Chem. 2009;9:31–59. doi: 10.2174/138955709787001712. [DOI] [PubMed] [Google Scholar]
- 116.Cichon M.J., Riedl K.M., Schwartz S.J. A metabolomic evaluation of the phytochemical composition of tomato juices being used in human clinical trials. Food Chem. 2017;228:270–278. doi: 10.1016/j.foodchem.2017.01.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Periago M.J., Garcia-Alonso J., Jacob K., Olivares A.B., Bernal M.J., Iniesta M.D., Martinez C., Ros G. Bioactive compounds, folates and antioxidant properties of tomatoes (Lycopersicum esculentum) during vine ripening. Int. J. Food Sci. Nutr. 2009;60:694–708. doi: 10.3109/09637480701833457. [DOI] [PubMed] [Google Scholar]
- 118.Del Giudice R., Raiola A., Tenore G.C., Frusciante L., Barone A., Monti D.M., Rigano M.M. Antioxidant bioactive compounds in tomato fruits at different ripening stages and their effects on normal and cancer cells. J. Funct. Foods. 2015;18:83–94. doi: 10.1016/j.jff.2015.06.060. [DOI] [Google Scholar]
- 119.Erge H.S., Karadeniz F. Bioactive compounds and antioxidant activity of tomato cultivars. Int. J. Food Prop. 2011;14:968–977. doi: 10.1080/10942910903506210. [DOI] [Google Scholar]
- 120.Carocho M., Ferreira I.C. A review on antioxidants, prooxidants and related controversy: Natural and synthetic compounds, screening and analysis methodologies and future perspectives. Food Chem. Toxicol. 2013;51:15–25. doi: 10.1016/j.fct.2012.09.021. [DOI] [PubMed] [Google Scholar]
- 121.Kumar V., Abbas A.K., Aster J.C. Robbins and Cotran Pathologic Basis of Disease. 9th ed. Elsevier; Philadelphia, PA, USA: 2014. [Google Scholar]
- 122.Combs G.F., Jr., McClung J.P. The Vitamins: Fundamental Aspects in Nutrition and Health. Academic Press; Cambridge, MA, USA: 2016. [Google Scholar]
- 123.Rao A.V., Rao L.G. Carotenoids and human health. Pharmacol. Res. 2007;55:207–216. doi: 10.1016/j.phrs.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 124.Dutta D., Chaudhuri U.R., Chakraborty R. Structure, health benefits, antioxidant property and processing and storage of carotenoids. Afr. J. Biotechnol. 2005;4:1510–1520. doi: 10.4314/ajfand.v4i13.71773. [DOI] [Google Scholar]
- 125.Thompson G.R., Grundy S.M. History and development of plant sterol and stanol esters for cholesterol-lowering purposes. Am. J. Cardiol. 2005;96:3–9. doi: 10.1016/j.amjcard.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 126.Quilez J., Garcia-Lorda P., Salas-Salvado J. Potential uses and benefits of phytosterols in diet: Present situation and future directions. Clin. Nutr. 2003;22:343–351. doi: 10.1016/S0261-5614(03)00060-8. [DOI] [PubMed] [Google Scholar]
- 127.Choudhury F.K., Rivero R.M., Blumwald E., Mittler R. Reactive oxygen species, abiotic stress and stress combination. Plant J. 2017;90:856–867. doi: 10.1111/tpj.13299. [DOI] [PubMed] [Google Scholar]
- 128.Liguori I., Russo G., Curcio F., Bulli G., Aran L., Della-Morte D., Gargiulo G., Testa G., Cacciatore F., Bonaduce D., et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging. 2018;13:757–772. doi: 10.2147/CIA.S158513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Jakubczyk K., Dec K., Kałduńska J., Kawczuga D., Kochman J., Janda K. Reactive oxygen species—Sources, functions, oxidative damage. Pol. Merkur. Lek. Organ Pol. Tow. Lek. 2020;48:124–127. [PubMed] [Google Scholar]
- 130.Bjørklund G., Chirumbolo S. Role of oxidative stress and antioxidants in daily nutrition and human health. Nutrition. 2017;33:311–321. doi: 10.1016/j.nut.2016.07.018. [DOI] [PubMed] [Google Scholar]
- 131.Martí R., Roselló S., Cebolla-Cornejo J. Tomato as a source of carotenoids and polyphenols targeted to cancer prevention. Cancers. 2016;8:58. doi: 10.3390/cancers8060058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Coyle J.T., Puttfarcken P. Oxidative stress, glutamate, and neurodegenerative disorders. Science. 1993;262:689–695. doi: 10.1126/science.7901908. [DOI] [PubMed] [Google Scholar]
- 133.Maritim A.C., Sanders R.A., Watkins J.B., III Diabetes, oxidative stress, and antioxidants: A review. J. Biochem. Mol. Toxicol. 2003;17:24–38. doi: 10.1002/jbt.10058. [DOI] [PubMed] [Google Scholar]
- 134.Wolak T., Sharoni Y., Levy J., Linnewiel-Hermoni K., Stepensky D., Paran E. Effect of Tomato Nutrient Complex on Blood Pressure: A Double Blind, Randomized Dose-Response Study. Nutrients. 2019;11:950. doi: 10.3390/nu11050950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.World Health Organization . Prevention of Cardiovascular Disease: Pocket Guidelines for Assessment and Management of Cardiovascular Risk:(WHO/ISH Cardiovascular Risk Prediction Charts for the Europe Region) WHO; Geneva, Switzerland: 2008. [Google Scholar]
- 136.Bourgeois D., Inquimbert C., Ottolenghi L., Carrouel F. Periodontal Pathogens as Risk Factors of Cardiovascular Diseases, Diabetes, Rheumatoid Arthritis, Cancer, and Chronic Obstructive Pulmonary Disease—Is There Cause for Consideration? Microorganisms. 2019;7:424. doi: 10.3390/microorganisms7100424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mensah G.A., Roth G.A., Fuster V. The Global Burden of Cardiovascular Diseases and Risk Factors: 2020 and Beyond. American College of Cardiology Foundation; Washington, DC, USA: 2019. [DOI] [PubMed] [Google Scholar]
- 138.Michaličková D., Belović M., Ilić N., Kotur-Stevuljević J., Slanař O., Šobajić S. Comparison of polyphenol-enriched tomato juice and standard tomato juice for cardiovascular benefits in subjects with stage 1 hypertension: A randomized controlled study. Plant Foods Hum. Nutr. 2019;74:122–127. doi: 10.1007/s11130-019-0714-5. [DOI] [PubMed] [Google Scholar]
- 139.Cámara M., Fernández-Ruiz V., Sánchez-Mata M.-C., Díaz L.D., Kardinaal A., Van Lieshout M. Evidence of antiplatelet aggregation effects from the consumption of tomato products, according to EFSA health claim requirements. Crit. Rev. Food Sci. Nutr. 2020;60:1515–1522. doi: 10.1080/10408398.2019.1577215. [DOI] [PubMed] [Google Scholar]
- 140.Gajendragadkar P.R., Hubsch A., Mäki-Petäjä K.M., Serg M., Wilkinson I.B., Cheriyan J. Effects of oral lycopene supplementation on vascular function in patients with cardiovascular disease and healthy volunteers: A randomised controlled trial. PLoS ONE. 2014;9:e99070. doi: 10.1371/journal.pone.0099070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mordente A., Guantario B., Meucci E., Silvestrini A., Lombardi E., Martorana G.E., Giardina B., Bohm V. Lycopene and cardiovascular diseases: An update. Curr. Med. Chem. 2011;18:1146–1163. doi: 10.2174/092986711795029717. [DOI] [PubMed] [Google Scholar]
- 142.Arab L., Steck S. Lycopene and cardiovascular disease. Am. J. Clin. Nutr. 2000;71:1691S–1695S. doi: 10.1093/ajcn/71.6.1691S. [DOI] [PubMed] [Google Scholar]
- 143.Hsiao G., Wang Y., Tzu N.-H., Fong T.-H., Shen M.-Y., Lin K.-H., Chou D.-S., Sheu J.-R. Inhibitory effects of lycopene on in vitro platelet activation and in vivo prevention of thrombus formation. J. Lab. Clin. Med. 2005;146:216–226. doi: 10.1016/j.lab.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 144.Song X., Wang Y., Gao L. Mechanism of antioxidant properties of quercetin and quercetin-DNA complex. DNA. 2020;3:5. doi: 10.1007/s00894-020-04356-x. [DOI] [PubMed] [Google Scholar]
- 145.Wu X.-J., Zhou X.-B., Chen C., Mao W. Systematic investigation of quercetin for treating cardiovascular disease based on network pharmacology. Comb. Chem. High Throughput Screen. 2019;22:411–420. doi: 10.2174/1386207322666190717124507. [DOI] [PubMed] [Google Scholar]
- 146.Egert S., Bosy-Westphal A., Seiberl J., Kürbitz C., Settler U., Plachta-Danielzik S., Wagner A.E., Frank J., Schrezenmeir J., Rimbach G., et al. Quercetin reduces systolic blood pressure and plasma oxidised low-density lipoprotein concentrations in overweight subjects with a high-cardiovascular disease risk phenotype: A double-blinded, placebo-controlled cross-over study. Br. J. Nutr. 2009;102:1065–1074. doi: 10.1017/S0007114509359127. [DOI] [PubMed] [Google Scholar]
- 147.Kostyuk V.A., Potapovich A.I., Suhan T.O., De Luca C., Korkina L. Antioxidant and signal modulation properties of plant polyphenols in controlling vascular inflammation. Eur. J. Pharmacol. 2011;658:248–256. doi: 10.1016/j.ejphar.2011.02.022. [DOI] [PubMed] [Google Scholar]
- 148.Al-Awwadi N.A., Araiz C., Bornet A., Delbosc S., Cristol J.-P., Linck N., Azay J., Teissedre P.-L., Cros G. Extracts enriched in different polyphenolic families normalize increased cardiac NADPH oxidase expression while having differential effects on insulin resistance, hypertension, and cardiac hypertrophy in high-fructose-fed rats. J. Agric. Food Chem. 2005;53:151–157. doi: 10.1021/jf048919f. [DOI] [PubMed] [Google Scholar]
- 149.Romero M., Jiménez R., Sánchez M., López-Sepúlveda R., Zarzuelo M.J., O’Valle F., Zarzuelo A., Pérez-Vizcaíno F., Duarte J. Quercetin inhibits vascular superoxide production induced by endothelin-1: Role of NADPH oxidase, uncoupled eNOS and PKC. Atherosclerosis. 2009;202:58–67. doi: 10.1016/j.atherosclerosis.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 150.Sánchez M., Galisteo M., Vera R., Villar I.C., Zarzuelo A., Tamargo J., Pérez-Vizcaíno F., Duarte J. Quercetin downregulates NADPH oxidase, increases eNOS activity and prevents endothelial dysfunction in spontaneously hypertensive rats. J. Hypertens. 2006;24:75–84. doi: 10.1097/01.hjh.0000198029.22472.d9. [DOI] [PubMed] [Google Scholar]
- 151.Lafay S., Gueux E., Rayssiguier Y., Mazur A., Remesy C., Scalbert A. Caffeic acid inhibits oxidative stress and reduces hypercholesterolemia induced by iron overload in rats. Int. J. Vitam. Nutr. Res. 2005;75:119–125. doi: 10.1024/0300-9831.75.2.119. [DOI] [PubMed] [Google Scholar]
- 152.Ozguner F., Altinbas A., Ozaydin M., Dogan A., Vural H., Kisioglu A.N., Cesur G., Yildirim N.G. Mobile phone-induced myocardial oxidative stress: Protection by a novel antioxidant agent caffeic acid phenethyl ester. Toxicol. Ind. Health. 2005;21:223–230. doi: 10.1191/0748233705th228oa. [DOI] [PubMed] [Google Scholar]
- 153.Bhullar K.S., Lassalle-Claux G., Touaibia M., Rupasinghe H.P.V. Antihypertensive effect of caffeic acid and its analogs through dual renin-angiotensin-aldosterone system inhibition. Eur. J. Pharmacol. 2014;730:125–132. doi: 10.1016/j.ejphar.2014.02.038. [DOI] [PubMed] [Google Scholar]
- 154.Zhang N., Yang Z., Yuan Y., Li F., Liu Y., Ma Z., Liao H., Bian Z., Zhang Y., Zhou H., et al. Naringenin attenuates pressure overload-induced cardiac hypertrophy. Exp. Ther. Med. 2015;10:2206–2212. doi: 10.3892/etm.2015.2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zou Z.-Y., Xu X.-R., Lin X.-M., Zhang H.-B., Xiao X., Ouyang L., Huang Y.-M., Wang X., Liu Y.-Q. Effects of lutein and lycopene on carotid intima-media thickness in Chinese subjects with subclinical atherosclerosis: A randomised, double-blind, placebo-controlled trial. Br. J. Nutr. 2014;111:474–480. doi: 10.1017/S0007114513002730. [DOI] [PubMed] [Google Scholar]
- 156.Shanmugasundaram R., Selvaraj R.K. Dietary lutein and fish oil interact to alter atherosclerotic lesions in a Japanese quail model of atherosclerosis. J. Anim. Physiol. Anim. Nutr. 2011;95:762–770. doi: 10.1111/j.1439-0396.2010.01106.x. [DOI] [PubMed] [Google Scholar]
- 157.Yang J.-T., Qian L.-B., Zhang F.-J., Wang J., Ai H., Tang L.-H., Wang H.-P. Cardioprotective effects of luteolin on ischemia/reperfusion injury in diabetic rats are modulated by eNOS and the mitochondrial permeability transition pathway. J. Cardiovasc. Pharmacol. 2015;65:349–356. doi: 10.1097/FJC.0000000000000202. [DOI] [PubMed] [Google Scholar]
- 158.Sun D., Huang J., Zhang Z., Gao H., Li J., Shen M., Cao F., Wang H. Luteolin limits infarct size and improves cardiac function after myocardium ischemia/reperfusion injury in diabetic rats. PLoS ONE. 2012;7:e33491. doi: 10.1371/journal.pone.0033491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Choi J.-H., Kim Y.-S., Shin C.-H., Lee H.-J., Kim S. Antithrombotic activities of luteolin in vitro and in vivo. J. Biochem. Mol. Toxicol. 2015;29:552–558. doi: 10.1002/jbt.21726. [DOI] [PubMed] [Google Scholar]
- 160.Guerrero J.A., Lozano M.L., Castillo J., Benavente-Garcia O., Vicente V., Rivera J. Flavonoids inhibit platelet function through binding to the thromboxane A2 receptor. J. Thromb. Haemost. 2005;3:369–376. doi: 10.1111/j.1538-7836.2004.01099.x. [DOI] [PubMed] [Google Scholar]
- 161.Kumar S., Prahalathan P., Saravanakumar M., Raja B. Vanillic acid prevents the deregulation of lipid metabolism, endothelin 1 and up regulation of endothelial nitric oxide synthase in nitric oxide deficient hypertensive rats. Eur. J. Pharmacol. 2014;743:117–125. doi: 10.1016/j.ejphar.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 162.Dianat M., Hamzavi G.R., Badavi M., Samarbaf-Zadeh A. Effect of vanillic acid on ischemia-reperfusion of isolated rat heart: Hemodynamic parameters and infarct size assays. Indian J. Exp. Biol. 2015;53:641–646. [PubMed] [Google Scholar]
- 163.Prince P.S.M., Dhanasekar K., Rajakumar S. Vanillic acid prevents altered ion pumps, ions, inhibits Fas-receptor and caspase mediated apoptosis-signaling pathway and cardiomyocyte death in myocardial infarcted rats. Chem.-Biol. Interact. 2015;232:68–76. doi: 10.1016/j.cbi.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 164.Zhao Y., Wang J., Ballevre O., Luo H., Zhang W. Antihypertensive effects and mechanisms of chlorogenic acids. Hypertens. Res. 2012;35:370–374. doi: 10.1038/hr.2011.195. [DOI] [PubMed] [Google Scholar]
- 165.Fuentes E., Caballero J., Alarcón M., Rojas A., Palomo I. Chlorogenic acid inhibits human platelet activation and thrombus formation. PLoS ONE. 2014;9:e90699. doi: 10.1371/journal.pone.0090699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Li Y., Shen D., Tang X., Li X., Wo D., Yan H., Song R., Feng J., Li P., Zhang J., et al. Chlorogenic acid prevents isoproterenol-induced hypertrophy in neonatal rat myocytes. Toxicol. Lett. 2014;226:257–263. doi: 10.1016/j.toxlet.2014.02.016. [DOI] [PubMed] [Google Scholar]
- 167.Alam M.A., Sernia C., Brown L. Ferulic acid improves cardiovascular and kidney structure and function in hypertensive rats. J. Cardiovasc. Pharmacol. 2013;61:240–249. doi: 10.1097/FJC.0b013e31827cb600. [DOI] [PubMed] [Google Scholar]
- 168.Roy A.J., Prince P.S.M. Preventive effects of p-coumaric acid on cardiac hypertrophy and alterations in electrocardiogram, lipids, and lipoproteins in experimentally induced myocardial infarcted rats. Food Chem. Toxicol. 2013;60:348–354. doi: 10.1016/j.fct.2013.04.052. [DOI] [PubMed] [Google Scholar]
- 169.Roy A.J., Prince P.S.M. Preventive effects of p-coumaric acid on lysosomal dysfunction and myocardial infarct size in experimentally induced myocardial infarction. Eur. J. Pharmacol. 2013;699:33–39. doi: 10.1016/j.ejphar.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 170.Anandhi R., Thomas P.A., Geraldine P. Evaluation of the anti-atherogenic potential of chrysin in Wistar rats. Mol. Cell. Biochem. 2013;385:103–113. doi: 10.1007/s11010-013-1819-z. [DOI] [PubMed] [Google Scholar]
- 171.Rani N., Bharti S., Bhatia J., Nag T., Ray R., Arya D.S. Chrysin, a PPAR-γ agonist improves myocardial injury in diabetic rats through inhibiting AGE-RAGE mediated oxidative stress and inflammation. Chem.-Biol. Interact. 2016;250:59–67. doi: 10.1016/j.cbi.2016.03.015. [DOI] [PubMed] [Google Scholar]
- 172.Chen X.Q., Hu T., Han Y., Huang W., Yuan H., Zhang Y.-T., Du Y., Jiang Y.-W. Preventive effects of catechins on cardiovascular disease. Molecules. 2016;21:1759. doi: 10.3390/molecules21121759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Bhardwaj P., Khanna D. Green tea catechins: Defensive role in cardiovascular disorders. Chin. J. Nat. Med. 2013;11:345–353. doi: 10.1016/S1875-5364(13)60051-5. [DOI] [PubMed] [Google Scholar]
- 174.Carnevale R., Loffredo L., Nocella C., Bartimoccia S., Bucci T., De Falco E., Peruzzi M., Chimenti I., Biondi-Zoccai G., Pignatelli P., et al. Epicatechin and catechin modulate endothelial activation induced by platelets of patients with peripheral artery disease. Oxidative Med. Cell. Longev. 2014;2014:1–9. doi: 10.1155/2014/691015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Song F., Li H., Sun J., Wang S. Protective effects of cinnamic acid and cinnamic aldehyde on isoproterenol-induced acute myocardial ischemia in rats. J. Ethnopharmacol. 2013;150:125–130. doi: 10.1016/j.jep.2013.08.019. [DOI] [PubMed] [Google Scholar]
- 176.Roy S.J., Prince P.S.M. Protective effects of sinapic acid on lysosomal dysfunction in isoproterenol induced myocardial infarcted rats. Food Chem. Toxicol. 2012;50:3984–3989. doi: 10.1016/j.fct.2012.08.017. [DOI] [PubMed] [Google Scholar]
- 177.Silambarasan T., Manivannan J., Priya M.K., Suganya N., Chatterjee S., Raja B. Sinapic acid prevents hypertension and cardiovascular remodeling in pharmacological model of nitric oxide inhibited rats. PLoS ONE. 2014;9:e115682. doi: 10.1371/journal.pone.0115682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Zordoky B.N., Robertson I.M., Dyck J.R. Preclinical and clinical evidence for the role of resveratrol in the treatment of cardiovascular diseases. Biochim. Biophys. Acta BBA—Mol. Basis Dis. 2015;1852:1155–1177. doi: 10.1016/j.bbadis.2014.10.016. [DOI] [PubMed] [Google Scholar]
- 179.Ozmen O., Topsakal S., Haligur M., Aydogan A., Dincoglu D. Effects of caffeine and lycopene in experimentally induced diabetes mellitus. Pancreas. 2016;45:579–583. doi: 10.1097/MPA.0000000000000489. [DOI] [PubMed] [Google Scholar]
- 180.Bayramoglu A., Bayramoglu G., Senturk H. Lycopene partially reverses symptoms of diabetes in rats with streptozotocin-induced diabetes. J. Med. Food. 2013;16:128–132. doi: 10.1089/jmf.2012.2277. [DOI] [PubMed] [Google Scholar]
- 181.Ozmutlu S., DeDe S., Ceylan E. The effect of lycopene treatment on ACE activity in rats with experimental diabetes. J. Renin-Angiotensin-Aldosterone Syst. 2012;13:328–333. doi: 10.1177/1470320311426024. [DOI] [PubMed] [Google Scholar]
- 182.Li W., Wang G., Lu X., Jiang Y.-X., Xu L., Zhao X. Lycopene ameliorates renal function in rats with streptozotocin-induced diabetes. Int. J. Clin. Exp. Pathol. 2014;7:5008–5015. [PMC free article] [PubMed] [Google Scholar]
- 183.Yildiz M., Sandikci M. Changes in rat ovary with experimentally induced diabetes and the effects of lycopene on those changes. Rom. J. Morphol. Embryol. 2016;57:703–713. [PubMed] [Google Scholar]
- 184.Chen S., Jiang H., Wu X., Fang J. Therapeutic effects of quercetin on inflammation, obesity, and type 2 diabetes. Mediat. Inflamm. 2016;2016:1–5. doi: 10.1155/2016/9340637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Margină D., Gradinaru D., Manda G., Neagoe I., Ilie M. Membranar effects exerted in vitro by polyphenols—quercetin, epigallocatechin gallate and curcumin—on HUVEC and Jurkat cells, relevant for diabetes mellitus. Food Chem. Toxicol. 2013;61:86–93. doi: 10.1016/j.fct.2013.02.046. [DOI] [PubMed] [Google Scholar]
- 186.Mosawy S., Jackson D.E., Woodman O.L., Linden M.D. The flavonols quercetin and 3′,4′-dihydroxyflavonol reduce platelet function and delay thrombus formation in a model of type 1 diabetes. Diabetes Vasc. Dis. Res. 2014;11:174–181. doi: 10.1177/1479164114524234. [DOI] [PubMed] [Google Scholar]
- 187.Braga C.P., Momentti A.C., Peixoto F.B., Baptista R.D.F.F., Dos Santos F.A., Fava F.H., Fernandes A.A.H. Influence of treatment with quercetin on lipid parameters and oxidative stress of pregnant diabetic rats. Can. J. Physiol. Pharmacol. 2013;91:171–177. doi: 10.1139/cjpp-2012-0173. [DOI] [PubMed] [Google Scholar]
- 188.Dai X., Ding Y., Zhang Z., Cai X., Li Y. Quercetin and quercitrin protect against cytokine-induced injuries in RINm5F β-cells via the mitochondrial pathway and NF-κB signaling. Int. J. Mol. Med. 2013;31:265–271. doi: 10.3892/ijmm.2012.1177. [DOI] [PubMed] [Google Scholar]
- 189.Peng X., Zhang G., Liao Y., Gong D. Inhibitory kinetics and mechanism of kaempferol on α-glucosidase. Food Chem. 2016;190:207–215. doi: 10.1016/j.foodchem.2015.05.088. [DOI] [PubMed] [Google Scholar]
- 190.Alkhalidy H., Moore W., Zhang Y., McMillan R., Wang A., Ali M., Suh K.-S., Zhen W., Cheng Z., Jia Z., et al. Small molecule kaempferol promotes insulin sensitivity and preserved pancreatic β-cell mass in middle-aged obese diabetic mice. J. Diabetes Res. 2015;2015:532984. doi: 10.1155/2015/532984. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 191.Al-Numair K.S., Chandramohan G., Veeramani C., AlSaif M.A. Ameliorative effect of kaempferol, a flavonoid, on oxidative stress in streptozotocin-induced diabetic rats. Redox Rep. 2015;20:198–209. doi: 10.1179/1351000214Y.0000000117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Luo C., Yang H., Tang C., Yao G., Kong L., He H., Zhou Y. Kaempferol alleviates insulin resistance via hepatic IKK/NF-κB signal in type 2 diabetic rats. Int. Immunopharmacol. 2015;28:744–750. doi: 10.1016/j.intimp.2015.07.018. [DOI] [PubMed] [Google Scholar]
- 193.Al-Numair K.S., Veeramani C., AlSaif M.A., Chandramohan G. Influence of kaempferol, a flavonoid compound, on membrane-bound ATPases in streptozotocin-induced diabetic rats. Pharm. Biol. 2015;53:1372–1378. doi: 10.3109/13880209.2014.982301. [DOI] [PubMed] [Google Scholar]
- 194.Tsai S.-J., Chuang C.-H., Mong M.-C., Kam W.-Y., Huang H.-Y., Yin M.-C. Anti-inflammatory and antifibrotic effects of naringenin in diabetic mice. J. Agric. Food Chem. 2011;60:514–521. doi: 10.1021/jf203259h. [DOI] [PubMed] [Google Scholar]
- 195.Ren B., Qin W., Wu F., Wang S., Pan C., Wang L., Zeng B., Ma S., Liang J. Apigenin and naringenin regulate glucose and lipid metabolism, and ameliorate vascular dysfunction in type 2 diabetic rats. Eur. J. Pharmacol. 2016;773:13–23. doi: 10.1016/j.ejphar.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 196.Rahigude A., Bhutada P., Kaulaskar S., Aswar M., Otari K. Participation of antioxidant and cholinergic system in protective effect of naringenin against type-2 diabetes-induced memory dysfunction in rats. Neuroscience. 2012;226:62–72. doi: 10.1016/j.neuroscience.2012.09.026. [DOI] [PubMed] [Google Scholar]
- 197.Chao C.-Y., Mong M.-C., Chan K.-C., Yin M.-C. Anti-glycative and anti-inflammatory effects of caffeic acid and ellagic acid in kidney of diabetic mice. Mol. Nutr. Food Res. 2010;54:388–395. doi: 10.1002/mnfr.200900087. [DOI] [PubMed] [Google Scholar]
- 198.Jin S., Chang C., Zhang L., Liu Y., Huang X., Chen Z. Chlorogenic acid improves late diabetes through adiponectin receptor signaling pathways in db/db mice. PLoS ONE. 2015;10:e0120842. doi: 10.1371/journal.pone.0120842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Stefanello N., Schmatz R., Pereira L.B., Rubin M.A., Rocha J.B.T., Facco G., Pereira M.E., Mazzanti C.M.D.A., Passamonti S., Rodrigues M.V., et al. Effects of chlorogenic acid, caffeine, and coffee on behavioral and biochemical parameters of diabetic rats. Mol. Cell. Biochem. 2014;388:277–286. doi: 10.1007/s11010-013-1919-9. [DOI] [PubMed] [Google Scholar]
- 200.Shin J.Y., Sohn J., Park K.H. Chlorogenic acid decreases retinal vascular hyperpermeability in diabetic rat model. J. Korean Med. Sci. 2013;28:608–613. doi: 10.3346/jkms.2013.28.4.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Lorenzoni F., Giampietri M., Ferri G., Lunardi S., Madrigali V., Battini L., Boldrini A., Ghirri P. Lutein administration to pregnant women with gestational diabetes mellitus is associated to a decrease of oxidative stress in newborns. Gynecol. Endocrinol. 2013;29:901–903. doi: 10.3109/09513590.2013.808329. [DOI] [PubMed] [Google Scholar]
- 202.Arnal E., Miranda M., Almansa I., Muriach M., Barcia J.M., Romero F.J., Diaz-Llopis M., Bosch-Morell F. Lutein prevents cataract development and progression in diabetic rats. Graefe’s Arch. Clin. Exp. Ophthalmol. 2009;247:115–120. doi: 10.1007/s00417-008-0935-z. [DOI] [PubMed] [Google Scholar]
- 203.Al-Malki A.L., Moselhy S.S. Free fatty acids profiling in response to carnitine synergize with lutein in diabetic rats. Afr. J. Tradit. Complementary Altern. Med. 2016;13:149–154. doi: 10.21010/ajtcam.v13i6.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Yan J., Zhang G., Pan J., Wang Y. α-Glucosidase inhibition by luteolin: Kinetics, interaction and molecular docking. Int. J. Biol. Macromol. 2014;64:213–223. doi: 10.1016/j.ijbiomac.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 205.Xu N., Zhang L., Dong J., Zhang X., Chen Y.-G., Bao B., Liu J. Low-dose diet supplement of a natural flavonoid, luteolin, ameliorates diet-induced obesity and insulin resistance in mice. Mol. Nutr. Food Res. 2014;58:1258–1268. doi: 10.1002/mnfr.201300830. [DOI] [PubMed] [Google Scholar]
- 206.Liu Y., Tian X., Gou L., Sun L., Ling X., Yin X. Luteolin attenuates diabetes-associated cognitive decline in rats. Brain Res. Bull. 2013;94:23–29. doi: 10.1016/j.brainresbull.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 207.Li M., Li Q., Zhao Q., Zhang J., Lin J. Luteolin improves the impaired nerve functions in diabetic neuropathy: Behavioral and biochemical evidences. Int. J. Clin. Exp. Pathol. 2015;8:10112–10120. [PMC free article] [PubMed] [Google Scholar]
- 208.Vinothiya K., AshokKumar N. Modulatory effect of vanillic acid on antioxidant status in high fat diet-induced changes in diabetic hypertensive rats. Biomed. Pharmacother. 2017;87:640–652. doi: 10.1016/j.biopha.2016.12.134. [DOI] [PubMed] [Google Scholar]
- 209.Roy S., Metya S.K., Sannigrahi S., Rahaman N., Ahmed F. Treatment with ferulic acid to rats with streptozotocin-induced diabetes: Effects on oxidative stress, pro-inflammatory cytokines, and apoptosis in the pancreatic β cell. Endocrine. 2013;44:369–379. doi: 10.1007/s12020-012-9868-8. [DOI] [PubMed] [Google Scholar]
- 210.Roy S., Metya S.K., Rahaman N., Sannigrahi S., Ahmed F. Ferulic acid in the treatment of post-diabetes testicular damage: Relevance to the down regulation of apoptosis correlates with antioxidant status via modulation of TGF-β1, IL-1β and Akt signalling. Cell Biochem. Funct. 2014;32:115–124. doi: 10.1002/cbf.2983. [DOI] [PubMed] [Google Scholar]
- 211.Amalan V., Natesan V., Indumathi D., Ramakrishnan A. Antidiabetic and antihyperlipidemic activity of p -coumaric acid in diabetic rats, role of pancreatic GLUT 2: In vivo approach. Biomed. Pharmacother. 2016;84:230–236. doi: 10.1016/j.biopha.2016.09.039. [DOI] [PubMed] [Google Scholar]
- 212.Abdel-Moneim A., Yousef A.I., El-Twab S.M.A., Reheim E.S.A., Ashour M.B. Gallic acid and p-coumaric acid attenuate type 2 diabetes-induced neurodegeneration in rats. Metab. Brain Dis. 2017;32:1279–1286. doi: 10.1007/s11011-017-0039-8. [DOI] [PubMed] [Google Scholar]
- 213.Adisakwattana S. Cinnamic acid and its derivatives: Mechanisms for prevention and management of diabetes and its complications. Nutrients. 2017;9:163. doi: 10.3390/nu9020163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Cherng Y.-G., Tsai C.-C., Chung H.-H., Lai Y.-W., Kuo S.-C., Cheng J.-T. Antihyperglycemic action of sinapic acid in diabetic rats. J. Agric. Food Chem. 2013;61:12053–12059. doi: 10.1021/jf403092b. [DOI] [PubMed] [Google Scholar]
- 215.Bhardwaj P., Deepa K., Balakumar P. Catechin averts experimental diabetes mellitus-induced vascular endothelial structural and functional abnormalities. Cardiovasc. Toxicol. 2014;14:41–51. doi: 10.1007/s12012-013-9226-y. [DOI] [PubMed] [Google Scholar]
- 216.Zhu D., Wang L., Zhou Q., Yan S., Li Z., Sheng J., Zhang W. (+)-Catechin ameliorates diabetic nephropathy by trapping methylglyoxal in type 2 diabetic mice. Mol. Nutr. Food Res. 2014;58:2249–2260. doi: 10.1002/mnfr.201400533. [DOI] [PubMed] [Google Scholar]
- 217.Abdulkhaleq L.A., Assi M.A., Noor M.H.M., Rasedee A., Zamri-Saad M., Taufiq-Yap Y.-H. Therapeutic uses of epicatechin in diabetes and cancer. Vet. World. 2017;10:869–872. doi: 10.14202/vetworld.2017.869-872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Fu Z., Yuskavage J., Liu D. Dietary flavonol epicatechin prevents the onset of type 1 diabetes in nonobese diabetic mice. J. Agric. Food Chem. 2013;61:4303–4309. doi: 10.1021/jf304915h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Taub P.R., Ramirez-Sanchez I., Ciaraldi T.P., Gonzalez-Basurto S., Coral-Vazquez R., Perkins G., Hogan M., Maisel A.S., Henry R.R., Ceballos G., et al. Perturbations in skeletal muscle sarcomere structure in patients with heart failure and Type 2 diabetes: Restorative effects of (−)-epicatechinrich cocoa. Clin. Sci. 2013;125:383–389. doi: 10.1042/CS20130023. [DOI] [PubMed] [Google Scholar]
- 220.Wang Q.-Q., Cheng N., Yi W.-B., Peng S., Zou X.-Q. Synthesis, nitric oxide release, and α-glucosidase inhibition of nitric oxide donating apigenin and chrysin derivatives. Bioorganic Med. Chem. 2014;22:1515–1521. doi: 10.1016/j.bmc.2014.01.038. [DOI] [PubMed] [Google Scholar]
- 221.Samarghandian S., Azimi-Nezhad M., Samini F., Farkhondeh T. Chrysin treatment improves diabetes and its complications in liver, brain, and pancreas in streptozotocin-induced diabetic rats. Can. J. Physiol. Pharmacol. 2015;94:388–393. doi: 10.1139/cjpp-2014-0412. [DOI] [PubMed] [Google Scholar]
- 222.Li R., Zang A., Zhang L., Zhang H., Zhao L., Qi Z., Wang H. Chrysin ameliorates diabetes-associated cognitive deficits in Wistar rats. Neurol. Sci. 2014;35:1527–1532. doi: 10.1007/s10072-014-1784-7. [DOI] [PubMed] [Google Scholar]
- 223.Ahad A., Ganai A.A., Mujeeb M., Siddiqui W.A. Chrysin, an anti-inflammatory molecule, abrogates renal dysfunction in type 2 diabetic rats. Toxicol. Appl. Pharmacol. 2014;279:1–7. doi: 10.1016/j.taap.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 224.Szkudelski T., Szkudelska K. Resveratrol and diabetes: From animal to human studies. Biochim. Biophys. Acta BBA—Mol. Basis Dis. 2015;1852:1145–1154. doi: 10.1016/j.bbadis.2014.10.013. [DOI] [PubMed] [Google Scholar]
- 225.Franceschi S., Parpinel M., La Vecchia C., Favero A., Talamini R., Negri E. Role of different types of vegetables and fruit in the prevention of cancer of the colon, rectum, and breast. Epidemiology. 1998;9:338–341. doi: 10.1097/00001648-199805000-00020. [DOI] [PubMed] [Google Scholar]
- 226.Choudhari A.S., Mandave P.C., Deshpande M., Ranjekar P., Prakash O. Phytochemicals in cancer treatment: From preclinical studies to clinical practice. Front. Pharmacol. 2020;10:1614. doi: 10.3389/fphar.2019.01614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Wall-Medrano A., Olivas-Aguirre F.J. Functional Foods in Cancer Prevention and Therapy. Academic Press; Cambridge, MA, USA: 2020. Antioxidant phytochemicals in cancer prevention and therapy—An update; pp. 195–220. [Google Scholar]
- 228.Khan N., Afaq F., Saleem M., Ahmad N., Mukhtar H. Targeting multiple signaling pathways by green tea polyphenol (−)-epigallocatechin-3-gallate. Cancer Res. 2006;66:2500–2505. doi: 10.1158/0008-5472.CAN-05-3636. [DOI] [PubMed] [Google Scholar]
- 229.Corona G., Deiana M., Incani A., Vauzour D., Dessì M.A., Spencer J.P. Inhibition of p38/CREB phosphorylation and COX-2 expression by olive oil polyphenols underlies their anti-proliferative effects. Biochem. Biophys. Res. Commun. 2007;362:606–611. doi: 10.1016/j.bbrc.2007.08.049. [DOI] [PubMed] [Google Scholar]
- 230.Adams L.S., Chen S. Phytochemicals for breast cancer prevention by targeting aromatase. Front. Biosci. 2009;14:3846–3863. doi: 10.2741/3493. [DOI] [PubMed] [Google Scholar]
- 231.Singh N., Sharma B. Role of toxicants in oxidative stress mediated DNA damage and protection by phytochemicals. EC Pharmacol. Toxicol. 2019;7:1–7. [Google Scholar]
- 232.Ezzat S.M., El-Halawany A.M., Hamed A.R., Abdel-Sattar E.A. Studies in Natural Products Chemistry. Volume 60. Elsevier; Amsterdam, The Netherlands: 2019. Role Phytochemicals Play in the Activation of Antioxidant Response Elements (AREs) and Phase II Enzymes and Their Relation to Cancer Progression and Prevention; pp. 345–369. [Google Scholar]
- 233.Chikara S., Nagaprashantha L.D., Singhal J., Horne D., Awasthi S., Singhal S.S. Oxidative stress and dietary phytochemicals: Role in cancer chemoprevention and treatment. Cancer Lett. 2018;413:122–134. doi: 10.1016/j.canlet.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 234.Liskova A., Kubatka P., Samec M., Zubor P., Mlyncek M., Bielik T., Samuel S.M., Zulli A., Kwon T.K., Büsselberg D. Dietary phytochemicals targeting cancer stem cells. Molecules. 2019;24:899. doi: 10.3390/molecules24050899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Franceschi S., Bidoli E., La Vecchia C., Talamini R., D’Avanzo B., Negri E. Tomatoes and risk of digestive-tract cancers. Int. J. Cancer. 1994;59:181–184. doi: 10.1002/ijc.2910590207. [DOI] [PubMed] [Google Scholar]
- 236.Colditz G.A., Branch L.G., Lipnick R.J., Willett W.C., Rosner B.A., Posner B.M., Hennekens C.H. Increased green and yellow vegetable intake and lowered cancer deaths in an elderly population. Am. J. Clin. Nutr. 1985;41:32–36. doi: 10.1093/ajcn/41.1.32. [DOI] [PubMed] [Google Scholar]
- 237.Freudenhieim J.L., Graham S., Marshall J.R., Haughey B.P., Wilkinson G. A case-control study of diet and rectal cancer in western new york. Am. J. Epidemiol. 1990;131:612–624. doi: 10.1093/oxfordjournals.aje.a115545. [DOI] [PubMed] [Google Scholar]
- 238.Centonze S., Boeing H., Leoci C., Guerra V., Misciagna G. Dietary habits and colorectal cancer in a low-risk area. Results from a population-based case-control study in southern Italy. Nutr. Cancer. 1994;21:233–246. doi: 10.1080/01635589409514322. [DOI] [PubMed] [Google Scholar]
- 239.Hu J., Liu Y., Yu Y., Zhao T., Liu S., Wang Q. Diet and cancer of the colon and rectum: A case-control study in China. Int. J. Epidemiol. 1991;20:362–367. doi: 10.1093/ije/20.2.362. [DOI] [PubMed] [Google Scholar]
- 240.Franceschi S., Favero A., La Vecchia C., Negri E., Conti E., Montella M., Giacosa A., Nanni O., Decarli A. Food groups and risk of colorectal cancer in Italy. Int. J. Cancer. 1997;72:56–61. doi: 10.1002/(SICI)1097-0215(19970703)72:1<56::AID-IJC8>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 241.Kwatra B. A review on potential properties and therapeutic applications of lycopene. Int. J. Med. Biomed. Stud. 2020;4 doi: 10.32553/ijmbs.v4i4.1081. [DOI] [Google Scholar]
- 242.Lu Q.-Y., Hung J.C., Heber D., Go V.L., Reuter V.E., Cordon-Cardo C., Scher H.I., Marshall J.R., Zhang Z. Inverse associations between plasma lycopene and other carotenoids and prostate cancer. Cancer Epidemiol. Biomark. Prev. 2001;10:749–756. [PubMed] [Google Scholar]
- 243.Siler U., Barella L., Spitzer V., Schnorr J., Lein M., Goralczyk R., Wertz K. Lycopene and Vitamin E interfere with autocrine/paracrine loops in the Dunning prostate cancer model. FASEB J. 2004;18:1019–1021. doi: 10.1096/fj.03-1116fje. [DOI] [PubMed] [Google Scholar]
- 244.Vijayababu M., Kanagaraj P., Arunkumar A., Ilangovan R., Aruldhas M., Arunakaran J. Quercetin-induced growth inhibition and cell death in prostatic carcinoma cells (PC-3) are associated with increase in p21 and hypophosphorylated retinoblastoma proteins expression. J. Cancer Res. Clin. Oncol. 2005;131:765–771. doi: 10.1007/s00432-005-0005-4. [DOI] [PubMed] [Google Scholar]
- 245.Gulati N., Laudet B., Zohrabian V.M., Murali R., Jhanwar-Uniyal M. The antiproliferative effect of Quercetin in cancer cells is mediated via inhibition of the PI3K-Akt/PKB pathway. Anticancer Res. 2006;26:1177–1181. [PubMed] [Google Scholar]
- 246.Park C.H., Chang J.Y., Hahm E.R., Park S., Kim H.-K., Yang C.H. Quercetin, a potent inhibitor against β-catenin/Tcf signaling in SW480 colon cancer cells. Biochem. Biophys. Res. Commun. 2005;328:227–234. doi: 10.1016/j.bbrc.2004.12.151. [DOI] [PubMed] [Google Scholar]
- 247.Kim J.-H., Jin Y.-R., Park B.-S., Kim T.-J., Kim S.-Y., Lim Y., Hong J.-T., Yoo H.-S., Yun Y.-P. Luteolin prevents PDGF-BB-induced proliferation of vascular smooth muscle cells by inhibition of PDGF β-receptor phosphorylation. Biochem. Pharmacol. 2005;69:1715–1721. doi: 10.1016/j.bcp.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 248.Ujiki M.B., Ding X.-Z., Salabat M.R., Bentrem D.J., Melstrom L.G., Milam B., Talamonti M.S., Belljr R.H., Iwamura T., Adrian T. Apigenin inhibits pancreatic cancer cell proliferation through G2/M cell cycle arrest. Mol. Cancer. 2006;5:1–76. doi: 10.1186/1476-4598-5-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Szabo K., Cătoi A.-F., Vodnar D. Bioactive compounds extracted from Tomato Processing by-Products as a Source of Valuable Nutrients. Plant Foods Hum. Nutr. 2018;73:268–277. doi: 10.1007/s11130-018-0691-0. [DOI] [PubMed] [Google Scholar]
- 250.Nour V., Panainte T.D., Ropota M., Turcu R., Trandafir I., Corbu A.R. Nutritional and bioactive compounds in dried tomato processing waste. CyTA—J. Food. 2018;16:222–229. doi: 10.1080/19476337.2017.1383514. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.