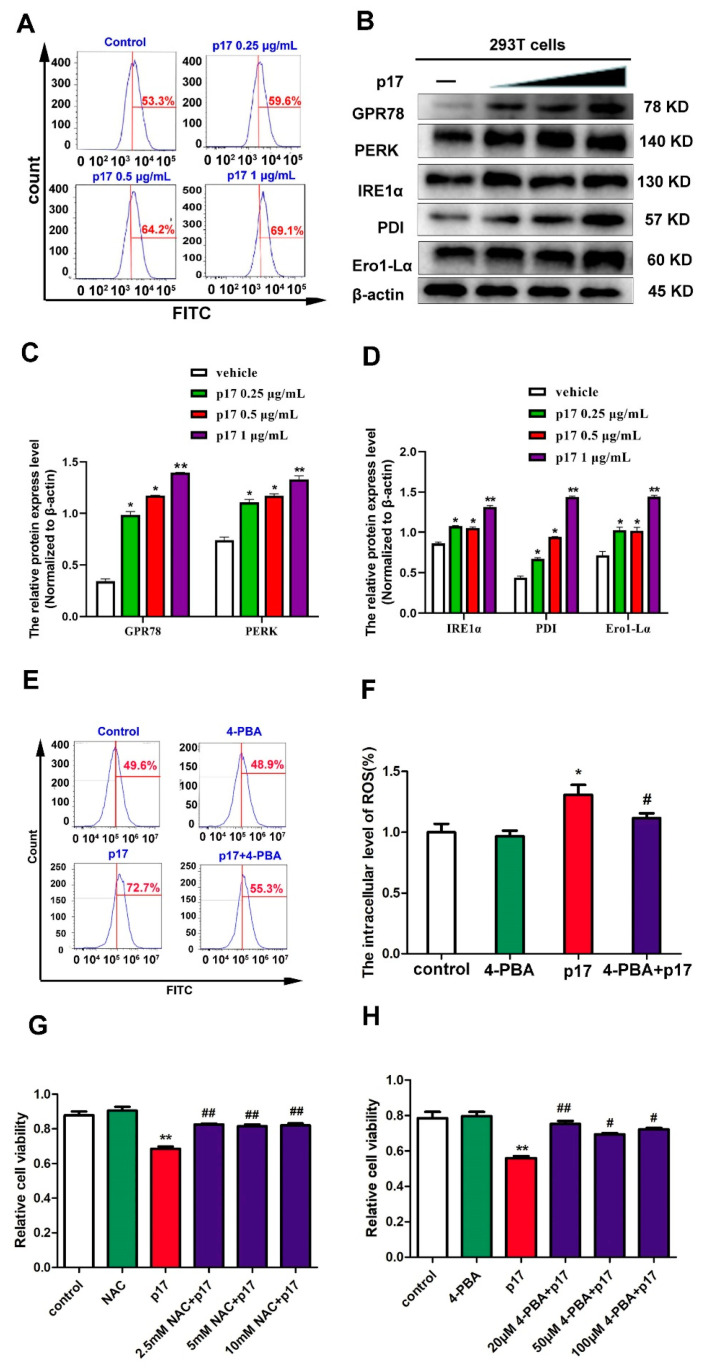
Figure 5.
P17 could induce ER stress, and ER stress was responsible for the p17-inhibited cell proliferation. (A) The effect of p17 on the level of intracellular Ca2+ was analyzed using Fluo-3 AM staining followed by flow cytometry. P17 groups were transfected with FLAG-p17 plasmids (0.25, 0.5, and 1 μg/mL) for 24 h. (B) The effect of p17 on the expressions of GPR78, PERK, IRE1α, PDI, and Ero1-Lα. P17 groups were transfected with FLAG-p17 plasmids (0.25, 0.5, and 1 μg/mL) for 24 h. (C,D) The intensity of the western blot bands was quantified by densitometric analysis. (E,F) 4-PBA pre-treatment could decrease the production of ROS by p17. 293T cells were pretreated with 4-PBA (20 μM) for 30 min, and then transfected with p17 gene in the presence of 4-PBA for another 24 h. (G) The role of ROS in the decrease of cell proliferation induced by p17 was analyzed by using the CCK8 assay. Using NAC (2.5 mM) to alleviate the production of ROS could reverse the decrease of cell proliferation induced by p17 in 293T cells. 293T cells were pretreated with NAC for 30 min, and then transfected with P17 gene in the presence of 4-PBA for another 24 h. (H) The role of ER stress in the decrease of cell proliferation induced by p17 was analyzed by using the CCK8 assay. Using 4-PBA (20 μM) to alleviate ER stress could also prevent the cell proliferation decrease induced by p17. Control groups were transfecting with empty plasmids. Values represent the mean ± SD; * p < 0.05, ** p < 0.01 versus control groups; # p < 0.05, ## p < 0.01 versus p17 groups.