Abstract
Background: phenolic compounds are bioactive chemical species derived from fruits and vegetables, with a plethora of healthy properties. In recent years, there has been a growing interest in persimmon (Diospyros kaki L.f.) due to the presence of many different classes of phenolic compounds. However, the analysis of individual phenolic compounds is difficult due to matrix interferences. Methods: the aim of this research was the evaluation of individual phenolic compounds and antioxidant capacity of the pulp of two varieties of persimmon (Rojo Brillante and Triumph) by an improved extraction procedure together with a UPLC-Q-TOF-MS platform. Results: the phenolic compounds composition of persimmon was characterized by the presence of hydroxybenzoic and hydroxycinnamic acids, hydroxybenzaldehydes, dihydrochalcones, tyrosols, flavanols, flavanones, and flavonols. A total of 31 compounds were identified and 17 compounds were quantified. Gallic acid was the predominant phenolic compounds found in the Rojo Brillante variety (0.953 mg/100 g) whereas the concentration of p-hydroxybenzoic acid was higher in the Triumph option (0.119 mg/100 g). Conclusions: the results showed that the Rojo Brillante variety had higher quantities of phenolic compounds than the Triumph example. These data could be used as reference in future phenolic compound databases when individual health effects of phenolic compounds become available.
Keywords: phenolic compounds, antioxidant capacity, UPLC-QTOF-MS, Diospyros kaki, persimmon, Rojo Brillante, Triumph
1. Introduction
Persimmons (Diospyros kaki L.f.) belong to the Ebenaceae family and are a plant native to Asia, where 90% of the world’s production is located [1]. Persimmons have traditionally been used for juice or vinegar production [2,3,4], but also for medicinal purposes, to help the likes of cough relief, hypertension, dyspnea, paralysis, burns, and bleeding [5,6]. There are more than 1000 persimmon varieties differenced by astringency. Astringent persimmons are the most common class and are richer in tannins, while non-astringent varieties have less tannins, and are, therefore, the variety preferred by consumers [7,8]. The Rojo Brillante variety (astringent) is sweet at maturity but needs to be CO2 treated in order to reduce astringency [7]. On the contrary, the Triumph variety (non-astringent), mainly found in Andalusia (Spain), can be consumed as it is because the fruit matures on the tree [8,9]. However, the type of post-harvest treatment affects the final primary and secondary metabolite content of persimmon fruit with more bioactive compounds being found in astringent varieties than the non-astringent options [10].
Spain is the world’s second largest persimmon producer [1] and it is one of the crops with the highest growth rate within the country. In 2019, the harvest exceeded 400,000 tons, most of which was exported (70%), mainly thanks to the Kaki Ribera del Xúquer PDO (Protected Designation of Origin) variety (MAP 2019). On the contrary, the average consumption of this fruit in Europe is only 0.35 Kg/inhabitant compared to 24 Kg/inhabitant consumption of apples or pears.
Persimmons are rich in sugars and low in fats, while the composition of micronutrients, such as minerals, is interesting due to its high content in P and K [11]. Persimmons are also rich in many different types of phenolic compounds, which play a role in reducing different pathologies, such as diabetes, cardiovascular diseases, cancer, and Alzheimer’s disease [12,13,14,15,16]. Phenolic compounds are secondary metabolites of plant origin and considered the main antioxidant components in food [9,17], which could explain their healthy properties [18].
As far as we know, only a few studies have evaluated the profile of phenolic compounds in persimmon fruit [19,20,21,22,23]. The main analytical technique for the detection of these compounds is liquid chromatography (HPLC), coupled with a diode array detector [19], ultraviolet [24], fluorescence [6], and mass spectrometry [25]. For the purpose of this study, high-resolution mass spectrometry has been used to analyze of phenolic compounds in different fruits [26,27,28] due to its sensitivity-selectivity of detection and powerful structural elucidation of unknown phenolic compounds in complex samples. In addition, coupling of high-resolution platforms with chromatographic separation by ultra-performance liquid chromatography allows fast and reliable determination of more compounds in less time, minimizing the effect of coeluting interferences and increasing sensitivity [25]. However, such analytical platforms have not yet been used to analyze phenolic compounds in persimmon fruit. In this study a extraction technique coupled to an UPLC-QTOF-MS high-resolution mass spectrometer was used, having previously been tested by Esteban-Muñoz et al. [29] on other fruits.
Therefore, the aim of this study was to evaluate the antioxidant capacity and the individual phenolic compounds in two Spanish grown persimmon varieties (Rojo Brillante and Triumph), in order to evaluate and promote persimmon consumption as the most convenient functional food in the European diet compared to other more commonly consumed fruit. Moreover, the study aimed to update and complete the European food composition databases with indigenous products that more correctly reflect actual consumption.
2. Materials and Methods
2.1. Samples
Two varieties of persimmon were analyzed: Rojo Brillante and Triumph. The samples were obtained from various supermarkets in the cities of Granada and Malaga, from two different harvests. Each sample consisted of three different, randomly chosen pieces of fruit from either a basket or mesh packaging found within the fruit display. They were then immediately taken to the laboratory to be processed. All non-edible parts—skin, seeds, and/or pits—were removed; the separated edible part was then ground in a blender and the juice extracted from each fruit. The processed samples were stored at 4 °C until needed for analysis, which was performed as soon as possible. All analyses were conducted in triplicate.
2.2. Reagents
The following reagents were obtained from Sigma-Aldrich SL (Madrid, Spain): gallic acid, p-hydroxybenzoic acid, vanillic acid, protocatechuic acid, syringic acid, ellagic acid, pyrogallol, 3,4-dimethoxybenzoic acid, caffeic acid, dimethyl caffeic acid, p-coumaric acid, ferulic acid, 4-methoxycinnamic, sinapic acid, chlorogenic acid, tyrosol, and quercetin. Diethyl ether, acetic acid, anhydrous sodium sulfate, and methanol were from Panreac Química SL (Barcelona, Spain). Water was obtained from a Milli-Q purification system (Millipore, Bedford, MA, USA). Hydrogen peroxide (H2O2), anhydrous sodium carbonate (Na2CO3), hexane, and sodium hydroxide (NaOH) were all purchased from Carlo-Erba (Rodano, Milan, Italy). Methanol, Folin Ciocalteu reagent, 6-hydroxy-2,5,7,8-tetramethyl-chroman-2-carboxylic acid (Trolox), 2,2-azinobis-(3-ethylbensothiazoline)-6-sulfonic acid (ABTS), and 2,2-diphenyl-1-picrylhydrazyl (DPPH) were provided by Sigma-Aldrich (Milan, Italy) and potassium peroxodisulfate (K2S2O8) by Panreac (Barcelona, Spain). Gallic acid was obtained from Sigma (Milan, Italy). All reagents were of analytical grade.
2.3. Sample Treatment
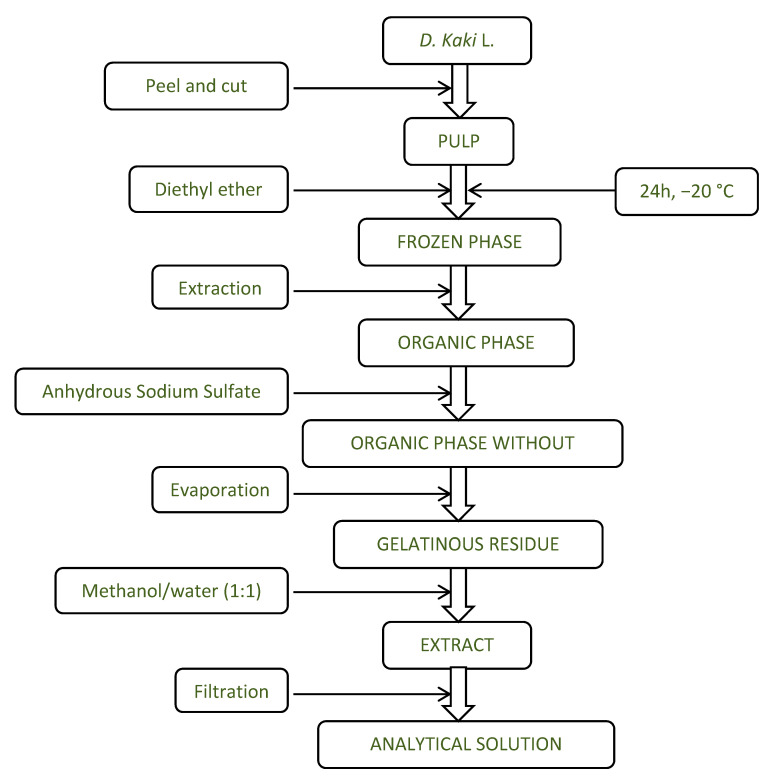
The method used for sample treatment was described by Moreno-Montoro et al. [30] with some changes described by Esteban-Muñoz et al. [29], which used diethyl ether instead of acidic methanol to extract phenolic compounds from different fruit matrixes. An amount of 20 mL of diethyl ether was briefly added to persimmon (100 g of homogenized pulp) and the resulting solution was frozen at −20 °C for 24 h. Then, the mixture was centrifuged for 10 min at 9000 rpm. The supernatant was transferred to a separatory funnel and three extractions were made with 20 mL diethyl ether. A spatula tip of anhydrous sodium sulfate was added to the organic extract, filtered, and evaporated under rotary evaporation at 30 °C to the smallest possible volume. The extracts were filled to 1 mL with a methanol/water mixture (1:1), filtered through a 0.20 μm membrane filter, and passed to the chromatography vial for analysis. The procedure is shown by Figure 1.
Figure 1.
Schematic process of the extraction of phenolic compounds.
2.4. Individual Phenolic Compounds
An UPLC-QTOF-MS method [31,32] was used to identify the different polyphenols with some modifications described by Esteban-Muñoz et al. [29].
2.4.1. Chromatographic and Mass Spectrometer Operating Conditions
The electrospray ionization (ESI)-MS experiments were performed on a liquid chromatography system with a hybrid mass spectrometer SYNAPT G2 HDMS Q-TOF model (Waters, Milford, CT, USA). The UPLC separation was performed using an ACQUITY UPLC™ HSS T3 2.1 × 100 mm, 1.8 µm C18 column (Waters, Milford, CT, USA). The program for chromatography was set with a binary gradient consisting of (A) water with 0.5% acetic acid and (B) acetonitrile, as follows: 0.0–15.0 min, 5% (B); 15.0–15.1 min, from 5 to 95% (B); and 15.1–18.0 min, from 95% to 5% (B), in this last part the column was reconditioned. Ten microliters of sample were injected and the flow rate was 0.4 mL/min. TOF conditions (measurement ranged from 50 to 1200 Da with the following mass spectrometer settings: nebulizer gas: 2 bar; drying temperature: 180 °C; capillary: 3100 V; drying gas: 6 L/min) consisted of a full MS and data-dependent scanning was performed in negative mode with electrospray ionization (ESI). The acquisition of fragment spectra for each mass was carried out using collision energy set to 40 eV with MS/MS mode.
2.4.2. Identification and Quantification
Phenolic compounds were identified using the exact mass, isotopes, by comparing negative masses from previously recorded research and comparison of detected fragments ions with databases (ChemSpider, FooDB, and PubChem) using MassLynx V4 software (Waters, Milford, CT, USA) for instrument control, data acquisition, and data analysis. Individual phenolic compounds were quantified by obtaining a series of solutions, with a concentration of 0.1–40 mg/L of standards with different retention times. For each phenolic compound selected, we carried out a five-point calibration curve with R2 ≥ 0.93 in order to ensure the linearity of the method. The standards were analyzed under the same working conditions as the samples. An example of the identification of vanillic acid in a sample of Rojo Brillante is shown in Figure S1.
2.4.3. Analytical Validation
Validation was made by studying the analytical parameters of selectivity, linearity, limit of detection (LOD), limit of quantification (LOQ), and precision according to the Association of Official Analytical Chemists´ guidelines for the validation of analytical methods [33]. The LOD and LOQ of the method were determined from the background response of three blanks using 1.0 mL of water. They were calculated as three (LOD) and 10 times (LOQ) the standard deviation (SD) of the response against the slope of the calibration curves for each phenolic compound. The intra-assay precision was evaluated by calculating the relative coefficient of variation (CV) of the analysis of 10 aliquots of each sample on the same day.
Figure S2a,b show an example of a chromatogram of Rojo Brillante and Triumph persimmon respectively. A total of 31 phenolic compounds were identified by UPLC-QTOF-MS in the crushed pulp of both persimmon varieties.
Table S1 reports the tested amount ranges, retention times, the adjusted linear equations, correlation coefficients, LOD and LOQ. All correlation coefficients were ≥0.99, except for 3,5-dimethoxybenzoic acid (r = 0.98), tyrosol (r = 0.96) and vanillic acid (r = 0.96). The LOD and LOQ (as mg/L) obtained are shown in Table S1. This method allowed the detection of 31 phenolic compounds and the quantification of 17 phenolic compounds at concentrations <6.4 and <21.3 mg/L for LOD and LOQ, respectively. The highest LOD and LOQ corresponded to 3,5-dimethoxybenzoic acid (6.377 and 21.258) and the lowest to sinapic acid (0.082 and 0.273). Intra-assay precision (% CV) is reported in Table S2. Precision was lower than 1.38 for all the phenolic compounds analyzed and ranged from 0.26% (p-coumaric acid) to 1.38% (p-hydroxybenzoic acid). The intra-assay precision values comply with those indicated by the AOAC (Association of Official Agricultural Chemists) [33] for a single laboratory (<4%).
2.5. Determination of Antioxidant Capacity
2.5.1. Antioxidant Capacity (AC)
The AC of the samples was measured via three different methods, in a BMG Labtech FLUOstar Omega plate reader (Offenburg, Germany) using the Omega Control program and MARS Data Analysis Software (BMG LABTECH, Ortenberg, Germany). The DPPH scavenging assay was used as proposed by Cuvellier et al. [34]. The method derived the ferric reducing ability of FRAP according to the procedure described by Benzie and Strain [35] and the antioxidant equivalent capacity as radical scavenging activity, based on reduction of the radical cation ABTS assay following the procedure of Re et al. [36]. These assays were modified in our laboratory and these methods have been described in depth by our research group [37,38,39,40,41]. In all of the methods applied, we determined the dilution of persimmon methanolic extracts that gave a linear response. The absorbance signal was translated into antioxidant activity using Trolox as the external standard. Different calibration curve ranges were used depending on the method employed (Table S3). Results of the assays were expressed as micromoles of Trolox equivalents per gram (µmol TE/G). All experiments were conducted in triplicate and values expressed as averages ± standard deviation.
2.5.2. Total Phenolics (TP)
The concentration of total phenolics was determined by the Folin–Ciocalteu colorimetric method described by Singleton and Rossi et al. [30] and modified in our laboratory [31]. We added 2.5 mL of deionized water and 500 µL of Folin–Ciocalteu reagent to 500 µL of methanolic pulp extract. The mixture was allowed to stand for 5 min, and then 2 mL of a 10% aqueous Na2CO3 solution was added. The final volume was adjusted to 10 mL. Samples and the blank were allowed to stand at room temperature for 90 min before being measured at 700 nm using a BOECO S-22 UV–VIS spectrophotometer (Hamburg, Germany). The amount of total phenolics is expressed as gallic acid equivalents per gram of fresh weight (mg GAE/g FW) through the calibration curve of gallic acid. The calibration curve range was 0.5–7.5 µg/mL (r = 0.991) (Table S3).
2.6. Statistical Analyses
Results are expressed as means ± SD. The SPSS 20.0 statistical package (IBM Inc., Armonk, New York, NY, USA) was used to analyze the data. Normality of variables was assessed by the Kolmogorov–Smirnov test (p < 0.05) to establish p the use of a parametric or non-parametric test for variance analysis. In addition, the Bartlett test (p < 0.05) was used to check the homogeneity of variances. As a result, the parametric Student’s t-test (95% significance level, established at p < 0.05) and the non-parametric Kruskal–Wallis test (95% significance level, established at p < 0.05) were carried out. Statistical analyses were conducted using GraphPad Prism 8.0.1 software (San Diego, CA, USA). The mean and standard deviation of values were determined, as were the differences between groups by means of a t-Student analysis and an analysis of variance (ANOVA). Differences with a p value < 0.05 were considered significant.
3. Results and Discussion
3.1. Individual Phenolic Compounds
3.1.1. Qualitative Determination of Phenolic Compounds in Persimmon
Table S1 shows the 31 phenolic compounds identified by UPLC-QTOF-MS, including their molecular formula, mass retention times, the persimmon variety in which they were detected, theoretical, and experimental molecular mass and both errors in ppm, and the fragments. Compounds 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 13, and 26 of Table 1 were identified by comparing with authentic standards that were later used in quantification; those that were not quantified with authentic standards were identified by comparing with fragmentation standards and previous publications. The phenolic compounds compositions of the Rojo Brillante and Triumph varieties were characterized mainly by the presence of hydroxybenzoic acids, hydroxycinnamic acids, hydroxybenzaldehydes, tyrosols, dihydrochalcones, flavanols, flavanones, and flavonols.
Table 1.
Individual phenolic compounds identified in Rojo Brillante and Triumph.
| No. | Proposed Compounds |
Molecular Formula |
Rt (min) |
Extract | (M-H)− calc. |
(M-H)− exp. |
Error (ppm) |
MS Fragments |
|---|---|---|---|---|---|---|---|---|
| Phenolic acids/ Hydroxybenzoic Acids |
||||||||
| 1 | Gallic acid | C7H6O5 | 1.24 | Triumph | 169.0137 | 169.0139 | 1.0 | 125.0229, 123.0071 |
| Rojo Brillante | 169.0137 | 169.0135 | 1.0 | 125.0236, 123.0062, 107.0111 | ||||
| 2 | Vanillic acid | C8H8O4 | 2.88 | Triumph | 167.0344 | 167.0345 | 0.1 | 153.0145, 109.0310 |
| Rojo Brillante | 167.0344 | 167.0340 | 2.9 | 121.0285 | ||||
| 3 | p-Hydroxybenzoic acid | C7H6O3 | 2.53 | Triumph | 137.0239 | 137.0243 | 2.9 | 121.0268 |
| Rojo Brillante | 137.0239 | 137.0240 | 0.1 | 121.0270 | ||||
| 4 | Protocatechuic acid | C7H6O4 | 1.89 | Triumph | 153.0179 | 153.0188 | 2.0 | 109.0343 |
| Rojo Brillante | 153.0179 | 153.0199 | 2.6 | 109.0343, 107.0051 | ||||
| 5 | 3,4-Dimethoxybenzoic acid | C9H10O4 | 5.50 | Triumph | 181.0507 | 181.0501 | 1.1 | 121.0241, 107.0143 |
| Rojo Brillante | 181.0507 | 181.0496 | 3.9 | 137.0605 | ||||
| Phenolic acids/Hydroxycinnamic Acids | ||||||||
| 6 | Caffeic acid | C9H8O4 | 2.85 | Triumph | 179.0344 | 179.0351 | 3.7 | 135.0462, 133.0267, 123.0416 |
| Rojo Brillante | 179.0344 | 179.0342 | 1.2 | 135.0462, 121.0235, 107.0155 | ||||
| 7 | Dimethyl caffeic acid | C11H12O4 | 5.02 | Rojo Brillante | 207.0657 | 207.0659 | 1.0 | 191.0306, 163.0337, 133.0658 |
| 8 | Ferulic acid | C10H10O4 | 3.90 | Triumph | 193.0501 | 193.0502 | 0.8 | 147.0396, 133.0295, 105.0355 |
| Rojo Brillante | 193.0501 | 193.0504 | 1.7 | 177.0184, 133.0322, 107.0157 | ||||
| 9 | p-coumaric acid | C9H8O3 | 3.58 | Triumph | 163.0395 | 163.0394 | 1.0 | 119.0521 |
| Rojo Brillante | 163.0394 | 0.5 | 145.0258, 117.0308, 93.0340 | |||||
| 10 | Sinapic acid | C11H12O5 | 3.91 | Triumph | 223.0606 | 223.0609 | 1.3 | 193.0521, 149.0620, 121.0259 |
| Rojo Brillante | 223.0606 | 223.0608 | 0.9 | 193.0490, 191.0334, 121.0294 | ||||
| 11 | Chlorogenic acid | C16H18O9 | 2.41 | Triumph | 353.0873 | 353.0873 | 5.4 | 307.0836, 191.0544, 179.0359 |
| Other/Hydroxybenzaldehydes | ||||||||
| 12 | Protocatechuic aldehyde | C7H6O3 | 2.53 | Triumph | 137.0239 | 137.0243 | 2.9 | 121.0268, 109.0289 |
| Tyrosols | ||||||||
| 13 | Tyrosol | C8H10O2 | 5.47 | Triumph | 137.0603 | 137.0604 | 0.7 | 107.0523, 105.0356 |
| Rojo Brillante | 137.0603 | 137.0601 | 1.5 | 107.0523, 105.0356 | ||||
| Flavonoids/Dihydrochalcones | ||||||||
| 14 | Phloretin | C15H14O5 | 5.35 | Rojo Brillante | 273.0763 | 273.0758 | 1.8 | 255.0659, 167.0377, 121.0623 |
| 15 | Phloridzin | C21H24O10 | 4.60 | Rojo Brillante | 435.1291 | 435.1299 | 1.7 | 273.0761, 151.0047, 125.0239 |
| Flavonoids/Flavanols | ||||||||
| 16 | Epicatechin | C15H14O6 | 2.45 | Triumph | 289.0712 | 289.0707 | 1.6 | 179.0369, 151.0414, 109.0287 |
| Rojo Brillante | 289.0712 | 289.0716 | 1.4 | 179.0369, 151.0414, 109.0287 | ||||
| 17 | Epigallocatechin | C15H14O7 | 1.67 | Triumph | 305.0661 | 305.0661 | 0.1 | 287.0493, 179.0411, 121.0336 |
| 18 | Epigallocatechin 7-O-glucuronide | C21H22O13 | 3.83 | Rojo Brillante | 481.0982 | 481.0985 | 0.6 | 447.0999, 313.0568, 165.0591 |
| Flavonoids/Flavanones | ||||||||
| 19 | Naringenin | C15H12O5 | 6.20 | Rojo Brillante | 271.0606 | 271.0619 | 2.0 | 177.0160, 151.0033, 119.0497 |
| 20 | Naringenin 7-O-glucuronide | C21H20O11 | 4.20 | Triumph | 447.0927 | 447.0935 | 1.7 | 269.0468, 255.0289, 227.0318 |
| 21 | Luteolin | C15H10O6 | 2.66 | Triumph | 285.0399 | 285.0401 | 0.8 | 229.0176, 201.0266, 150.9992 |
| Rojo Brillante | 285.0399 | 285.0398 | 0.3 | 201.0185, 177.0167, 153.0184 | ||||
| Flavonoids/Flavonols | ||||||||
| 22 | Kaempferol | C15H10O6 | 3.99 | Triumph | 285.0399 | 285.0401 | 0.8 | 165.0232, 153.0147, 150.9992 |
| Rojo Brillante | 285.0399 | 285.0398 | 0.3 | 165.0125, 153.0184, 133.0365 | ||||
| 23 | Kaempferol 3-O-galactoside | C21H20O11 | 4.20 | Triumph | 447.0927 | 447.0935 | 1.7 | 311.0517, 285.0447, 255.0289 |
| Rojo Brillante | 447.0927 | 447.0927 | 0.2 | 285.0412, 255.0306, 151.0020 | ||||
| 24 | Kaempferol 3-O-glucoside | C21H20O11 | 4.20 | Triumph | 447.0927 | 447.0935 | 1.7 | 311.0517, 285.0447, 255.0289 |
| Rojo Brillante | 447.0927 | 447.0927 | 0.2 | 255.0306, 151.0020 | ||||
| 25 | Kaempferol 3-O-acetyl-glucoside | C23H22O12 | 4.87 | Rojo Brillante | 489.1033 | 489.1012 | 4.2 | 490.1111, 327.0491 |
| 26 | Quercetin | C15H10O7 | 5.52 | Triumph | 301.0348 | 301.0349 | 0.4 | 283.0324, 165.0098, 151.0087 |
| Rojo Brillante | 301.0348 | 301.0355 | 3.1 | 175.0043, 151.0044, 137.0245 | ||||
| 27 | Quercetin 3-O-rhamnoside | C21H20O11 | 4.20 | Triumph | 447.0927 | 447.0935 | 1.7 | 285.0341 |
| 28 | Myricetin | C15H10O8 | 3.11 | Rojo Brillante | 317.0297 | 317.0297 | 0.1 | 193.0136, 165.0182, 151.0029 |
| 29 | Myricetin 3-O-glucoside | C21H20O13 | 3.33 | Rojo Brillante | 479.0826 | 479.0838 | 2.6 | 219.0306, 125.0249 |
| 30 | Myricetin 3-O-rhamnoside | C21H20O12 | 3.80 | Rojo Brillante | 463.0877 | 463.0873 | 0.8 | 301.0343, 245.0072, 151.0017 |
| 31 | Isorhamnetin 3-O-glucoside | C22H22O12 | 4.32 | Rojo Brillante | 477.1033 | 477.0994 | 8.2 | 285.0368, 161.0352, 125.0228 |
Other literature mentions the presence of simple phenolic compounds (hydroxybenzoic acids and hydroxycinnamic acids), tannins, procyanidins, flavonoids, and tyrosols [20,25,42] in persimmon fruit. Among hydroxybenzoic acids compounds, Table 1 shows that gallic acid was detected in both varieties, with a characteristic (M-H)]—experimental at m/z of 169.0139 and 169.0135 in Triumph and Rojo Brillante samples, respectively. The peak at 2.55 min in both extracts showed an (M-H)—experimental at m/z of 137.0239 and a molecular formula corresponding to p-hydroxybenzoic acid. Regarding hydroxycinnamic acids, major compounds p-coumaric, and caffeic acids were identified in both Rojo Brillante and Triumph samples, by means of distinctive (M-H)—experimental at m/z 163.0401 and m/z 179.0336, respectively. Figure S1 shows an example of a chromatogram of a Rojo Brillante sample, chromatogram extracted from vanillic acid, and a spectrum used for identification.
3.1.2. Quantitative Determination of Phenolic Compounds in Persimmon
In this study, the phenolic compounds species quantified by UPLC-QTOF-MS were seven hydroxybenzoic acids (gallic, vanillic, p-hydroxybenzoic, protocatechuic, syringic, ellagic and 3,5-dimethoxybenzoic acids) and seven hydroxycinnamic acids (caffeic, dimethyl caffeic, ferulic, p-coumaric, 4-methoxycinnamic, sinapic, and chlorogenic acid). All of these phenolic compounds were non-flavonoid phenols and other phenolic compounds such as pyrogallol. We also detected and quantified tyrosol and a flavonoid species (the flavonol quercetin). Table 2 shows the concentrations (mg/100 g fruit pulp) of the 17 phenolic compounds quantified in samples of Triumph (n = 10) and Rojo Brillante (n = 10) persimmon varieties.
Table 2.
Individual phenolic compounds quantified in persimmon varieties (mg/100 g fruit pulp).
| Phenolic Compounds |
Triumph Variety (n = 10) |
Rojo Brillante Variety (n = 10) |
|---|---|---|
| Hydroxybenzoic acids | ||
| Gallic acid | 0.033 ± 0.035 a | 0.953 ± 0.344 b |
| Vanillic acid | 0.021 ± 0.016 a | 0.056 ± 0.013 b |
| p-Hydroxybenzoic acid | 0.119 ± 0.030 a | 0.109 ± 0.039 a |
| Protocatechuic acid | 0.004 ± 0.002 a | 0.013 ± 0.010 b |
| Syringic acid | ND a | 0.037 ± 0.024 b |
| Ellagic acid | ND a | 0.327 ± 0.173 b |
| 3,4-Dimethoxybenzoic acid | 0.036 ± 0.048 a | 0.054 ± 0.025 a |
| Hydroxycinnamic Acids | ||
| Caffeic acid | 0.078 ± 0.069 a | 0.046 ± 0.060 a |
| Dimethyl caffeic acid | ND a | 0.022 ± 0.001 b |
| Ferulic acid | 0.008 ± 0.003 a | 0.017 ± 0.008 b |
| p-coumaric acid | 0.088 ± 0.046 a | 0.113 ± 0.055 a |
| 4-Methoxycinnamic | 0.001 ± 0.001 a | 0.002 ± 0.002 a |
| Sinapic acid | 0.002 ± 0.001 a | 0.002 ± 0.001 a |
| Chlorogenic acid | 0.007 ± 0.001 a | ND a |
| Tyrosols | ||
| Tyrosol | 0.020 ± 0.017 | 0.038 ± 0.021 a |
| Flavonols | ||
| Quercetin | 0.005 ± 0.001 | 0.007 ± 0.006 a |
| Others | ||
| Pyrogallol | 0.001 ± 0.001 a | 0.028 ± 0.009 b |
ND: not detected; different letters within the same phenolic compounds indicate statistically significant differences (p < 0.05).
Hydroxybenzoic Acids
There were seven hydroxybenzoic acids among the non-flavonoid phenolic compounds (Table 2). In this study, gallic acid was the most predominant phenolic compound found in extracts of the Rojo Brillante variety (0.953 ± 0.344 mg/100 g fruit pulp), whereas in Triumph extracts, the concentration was 30 times lower (p < 0.05). These values are in line with those reported by other authors [21,43], but below the concentrations stated by Gao et al. and Fu et al. [22,23]. On the contrary, some authors did not detect gallic acid in persimmon pulp, in varieties such as D. kaki Jiro and D. kaki Zenjimaru [21,44]. According to Macheix et al. [45], gallic acid is found in many fruits and vegetables in the range of 0.5–15 mg/100 g. Gallic acid can exert beneficial health effects, especially important for its anticarcinogenic and cardioprotective activities [46,47]. In this sense, in-vitro studies have shown gallic acid to have great effectiveness against human prostate cancer [48] and digestive cancers [49].
The levels of p-hydroxybenzoic acid were similar for the Triumph and Rojo Brillante varieties (Table 2). These results are in line with the observations of other authors regarding the presence of p-hydroxybenzoic acid in persimmon leaves [25] or different varieties like D. kaki Zenjimaru and D. kaki Zhouqumomoshi [21], but 2–7 lower than other varieties [21,43]. Higher concentrations of this phenolic compound have been reported in other fruit pulps, such as white grapes [30]. Moreover, p-hydroxybenzoic acid is of interest to agriculture as it regulates antioxidant enzyme activity and mitigates heat stress in cucumber leaves [50].
Vanillic acid is a very common phenolic compound that can be added to foods as flavoring and an olfactory agent giving a pleasant and creamy aroma [51]. It is an intermediate product in the two-stage bioconversion of ferulic acid to vanillin [52], and it selectively inhibits 5‘nucleotidase activity [53]. It was identified in the two varieties of persimmon, with a statistically higher concentration (p < 0.05) in Rojo Brillante (0.056 ± 0.013) than in Triumph (0.021 ± 0.016). The same tendency was observed for protocatechuic acid (Table 2) as the concentrations of the Rojo Brillante variety (0.013 mg/100 g) were higher (p < 0.05) than those obtained for the Triumph sample (0.004 mg/100 g). Higher concentrations were found in different persimmon extract obtained with different solvents [22], but the use of an HPLC equipped with ECD (electron capture detector) detection could overestimate the concentration. Protocatechuic acid has been demonstrated as an effective antiproliferative compound on HL-60 cells due to apoptotic effects [54].
Syringic and ellagic acids were detected and quantified only in the Rojo Brillante variety (0.037 and 0.327 mg/100 g fruit pulp, respectively). In the case of syringic acid, the results are in line with those reported for other varieties (Table 2), ranging from 0.07 to 0.32 mg/100 g [21]. It has been reported as a product of the microbial metabolism of anthocyanins and other phenolic compounds [55] related to the positive role of wine phenolic compounds against oxidation of low-density lipoprotein [56]. On the other hand, to the best of our knowledge, this is the first time ellagic acid has been identified in persimmon. It is a phenolic compounds with interesting antioxidant, estrogenic and/or antiestrogenic, anti-inflammatory, antimicrobial, and prebiotic effects [57].
Hydroxycinnamic Acids
Among the non-flavonoid phenolic compounds, seven hydroxycinnamic acids were identified and quantified. The second most abundant hydroxycinnamic phenolic compound in both varieties was caffeic acid, with 0.078 and 0.046 mg/100 g for the Triumph and Rojo Brillante varieties, respectively (Table 2). These levels are in line with those reported for other persimmon varieties [21,22]. On the contrary, there are no data available about the levels of dimethylcaffeic acid concentrations in persimmon, which was quantified only in the Rojo Brillante variety (0.022 mg/100 g). Caffeic acid has been described as a potent antioxidant and reducing species compared to other antioxidants like coumaric or ferulic acids, BHA (butylhydroxyanisole), BHT (butylhydroxytoluene) or α-tocopherol (Gulcin, n.d.), and has also shown to have a protective effect against ultraviolet radiation [58].
p-coumaric acid was the hydroxycinnamic acid with highest concentrations in both varieties of persimmon (not statistically different), ranging from 0.088 to 0.113 mg/100 g fruit pulp. These values are slightly higher (Table 2) than those reported by other authors [21,22].
Ferulic acid is a natural antioxidant abundant in the cell walls of plants and is commonly found in fruit and vegetables [59]. It has been described as having many physiological functions, including antimicrobial, anti-inflammatory, anti-thrombosis, and anticarcinogenic effects [60]. As stated in Table 2, Rojo Brillante persimmon had a statistically higher (p < 0.05) concentration of ferulic acid than the Triumph variety (0.011 vs. 0.008 mg/100 g). These levels are in line with (or slightly lower than) those reported for different persimmon varieties by other authors [21,43] (Table 2).
In the case of 4-methoxycinnamic and sinapic acids, their levels (Table 2) were quite low (0.001–0.002 mg/100 g fruit pulp) but this is the first time that these phenolic compounds have been found in persimmon (Table 2). In a recent study by Zare et al. [61], it was shown that sinapic acid has neuroprotective potential in Parkinson’s disease in rats.
Finally, chlorogenic acid was only quantified in the Triumph variety (0.007 mg/100 g). Some authors have detected chlorogenic acids at higher concentrations in other varieties of persimmon [21], whereas other authors have not detected it at all in different types of persimmon pulp [43]. Chlorogenic acid anticarcinogenic activity in rats is due to its high antioxidant activity [62].
Tyrosols
Tyrosol is a natural antioxidant with the effect of preventing protein damage caused by hydrogen peroxide but not the superoxide anion released during the respiratory burst [63]. Tyrosol was detected at similar levels in both persimmon varieties (Table 2), but no other studies discussing tyrosol have been found.
Flavonols
According to Macheix et al. [45], the usual range of flavonols in fruits is 0.5–25 mg/100 g. As a result, it is expected that persimmon would have flavonols in its chemical composition. In general, the extracts of other varieties of persimmon described gallic acid and quercetin as the most predominant phenolic compounds [21]. Quercetin was quantified in similar proportions in the Triumph and Rojo Brillante varieties (Table 2) in the range of 0.005–0.007 mg/100 g fruit pulp. Although these values are lower than those reported for other persimmon varieties [21], some other authors did not detect any quercetin in persimmon [43]. Quercetin has been reported to effectively recycle vitamin E and is also known to reduce inflammation, tumorigenesis, and cell damage caused by oxidation [64].
Other
Pyrogallol was quantified in both persimmon varieties, being higher in the Rojo Brillante variety (p < 0.05). The presence of pyrogallol in persimmon has not been previously reported in other papers, but it was found in other persimmon species, such as Diospyros Castanea. Its crude extract exerted high toxicity and selectivity against HepG2 and HeLa cells due to the production of intracellular superoxide anion cells [4,48]. The levels of 3,5-dimethoxybenzoic acid were similar in both the Triumph and Rojo Brillante varieties.
Comparing these values (using a simulate analytical method with other studies of our group) [29] mainly with tropical fruits, much higher levels of hydroxybenzoic acids and especially gallic acid (0.953 mg/100 g) are clearly observed in the Rojo Brillante variety. Three types of fresh fruit were compared with this variety of persimmon and were found to have compatible high tannin content (epicatechin gallate and epigallocatechin gallate): cherimoya 0.0735 mg/100 g, papaya 0.0341 mg/100 g, and pitaya 0.0546 mg/100 g. These compounds are commonly called catechins and form a group of bioactive compounds with strong antioxidant capacity involved in many oxidative stress-based chronic diseases.
The effects of persimmon on human lymphoid leukemia Molt 4B cells have also been studied. In this sense, a persimmon extract and some of its individual polyphenols (catechin, epicatechin, epigallocatechin and epicatechin gallate) were investigated [65]. These authors found that persimmon extract, as well as epigallocatechin and epicatechin gallate inhibited the growth of these cells in a dose dependent manner. After three days of treatment, they observed severe cell damage, such as DNA fragmentation. These findings could indicate a possible use for therapeutic purposes. Additionally, as part of a case-controlled study of thyroid cancer in Korean women, an inverse correlation was found between persimmon consumption and malignant, as well as benign, thyroid cancer risk [66].
3.2. Determination of Antioxidant Activity
3.2.1. Antioxidant Capacity (AC)
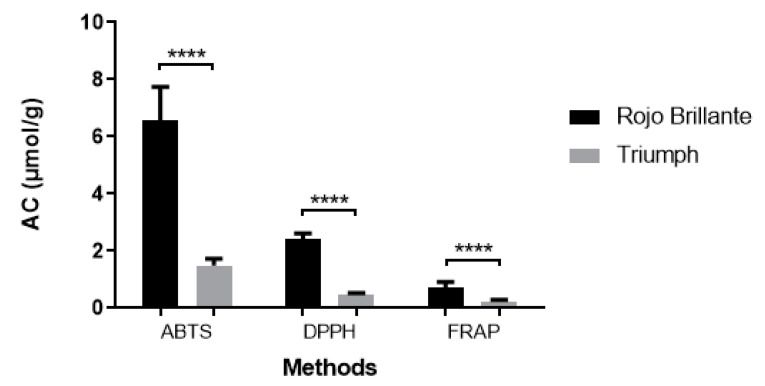
The ABTS and DPPH radical scavenging activities and FRAP ferric reducing ability of the persimmon varieties were determined, and the results are shown in Table 3. Figure 2 summarizes the antioxidant capacities of persimmon measured by the different methods. The antioxidant activity values expressed in micromoles Trolox per gram ranged from 1.280 ± 0.069 to 8.865 ± 0.056 μmol TE/g when measured by ABTS, 0.458 ± 0.05 to 2.633 ± 0.03 μmol TE/g when measured by DPPH, and 0.206 ± 0.01 to 0.965 ± 0.005 μmol TE/g when the FRAP method was used.
Table 3.
Statistical analysis relating to antioxidant activity as measured by DPPH, ABTS, and FRAP assays, and total phenolics as measured by the Folin method in Rojo Brillante and Triumph varieties.
| Method | Equation | Sample | n | Mean (SD) |
|---|---|---|---|---|
| ABTS | y = 130.94x + 4.6699 | Rojo Brillante | 10 | 6.572 (1.164) |
| (µmol TE/G) | r2 = 0.9995 | Triumph | 10 | 1.484 (0.249) |
| DPPH | y = 0.1706x + 6.7611 | Rojo Brillante | 10 | 2.417 (0.200) |
| (µmol TE/G) | r2 = 0.9958 | Triumph | 10 | 0.492 (0.039) |
| FRAP | y = 0.0043x +0.0333 | Rojo Brillante | 10 | 0.731 (0.178) |
| (µmol TE/G) | r2 = 0.9943 | Triumph | 10 | 0.242 (0.057) |
| TP | y = 0.0915x + 0.0931 | Rojo Brillante | 10 | 380.786 (158.539) |
| (µg GAE/G) | r2 = 0.9991 | Triumph | 10 | 81.568 (34.989) |
SD: standard deviation; ABTS: 2-2′-azino-bis (3-ethylbenzthiazoline-6-sulphonic acid) radical method; DPPH: 1,1-diphenyl-2-picrylhydrazyl radical method; FRAP: ferric reducing antioxidant power assay; TP: Total phenolics; TE: Trolox equivalent; GAE: Gallic acid equivalent.
Figure 2.
Evaluation of the antioxidant activity (AC) of the Rojo Brillante and Triumph varieties measured by ABTS, DPPH, and FRAP assay. **** Indicates p < 0.0001.
The greatest ABTS·+ scavenging capacity was detected in Rojo Brillante (6.572 μmol TE/g), while the lowest was found in the non-astringent variety Triumph (41.484 μmol TE/g). The ABTS·+ scavenging capacity in Rojo Brillante was almost six fold higher than that of the Triumph.
The radical scavenging of varieties on DPPH (Table 3) showed similar diversity to the results of the ABTS·+ assay, with the astringent variety almost twofold higher than the non-astringent variety. The antioxidant activities of all extracts determined as DPPH radical scavenging ability ranged from 2.633 to 0.458 μmol TE/g.
The ferric reducing antioxidant power of Rojo Brillante and Triumph extracts was similar to the DPPH scavenging activity and ABTS·+ scavenging capacity, with higher values for the astringent variety (0.965 ± 0.005 μmol TE/g).
In general, the astringent variety (Rojo Brillante) showed much higher antioxidant activity than the non-astringent variety (Triumph), for both ABTS (6.572 µmol TE/g and 1.484 µmol TE/g, respectively); DPPH (2.417 µmol TE/g and 0.492 µmol TE/G, respectively) and FRAP assay (0.731 µmol TE/g and 0.242 µmol TE/g, respectively). The Rojo Brillante variety also has the highest values of total phenol content as measured by the Folin method (380.786 µg GAE/g and 81.568 786 µg GAE/g, respectively) (Table 3).
For persimmon samples, these results were similar to those of other authors [67]. Their results ranged from 1027.03 to 1667.65 µmol/kg), concentrations very close to those reported in our study, although very few papers discussed antioxidants for the fruits considered in this study. Our results are similar and consistent with data from research, which investigated diverse persimmon genotypes.
Li et al. reported much higher values for the ABTS and DPPH assays in the astringent variety than the non-astringent option, for both pulp and peel. However, the FRAP assay did not show any great difference as in our study. The pulp obtained higher values in the astringent variety rather than the non-astringent option while the opposite occurred in the peel [68]. Lucas-González et al. also reported values higher by ABTS, DPPH, and FRAP assays for Rojo Brillante than Triumph persimmon [10].
Two authors determined AC with ABTS, DPPH, and FRAP assays. Loizo et al. determined AC in a variety of astringent persimmon Diospyros lotus L., (72.6 ± 1.5 µg/mL for DPPH, 0.21 ± 0.07 µM/g for FRAP and 0.36 ± 0.01 TEAC for the ABTS method) [69]. Pu et al. obtained higher AC values in the astringent varieties for ABTS (648.79, 889.14, and 1110.13 µmol/100 g); DPPH (898.91, 1588.26, and 1209.13 µmol/100 g) and FRAP assay (354.61, 699.43, and 613.82 µmol/100 g) than for the non-astringent varieties by three methods (47.86 and 106.59 µmol/100 g; 190.83 and 560.77 µmol/100 g; 111.78 and 90.10 µmol/100 g, respectively) [21]; persimmon-based dairy products activity was measured by DPPH. Their results ranged from 1027.03 to 1667.65 µmol/kg), concentrations very close to those reported in our study
Chen et al. determined antioxidant activity by using the ABTS and DPPH methods. The Mopan persimmon extract exhibited the strongest inhibiting activities against both ABTS (23.512 μmol/g) and DPPH (22.485 μmol/g) radicals [70]. This study did not find great differences between the two methods like our study did; but it did report higher values than in ours. In another study, in which AC was measured by the three methods, results contrary to ours were determined, obtaining higher AC values in non-astringent varieties (Triumph) than in an astringent option (Rojo Brillante) [71].
Furthermore, tannin concentration is one of the major antioxidants in persimmon pulp and this is where astringent and non-astringent persimmons differ the most. The greater presence of tannins in the astringent varieties could explain why higher AC can be found in them rather than in non-astringent varieties [72].
The variations found between the different studies may be due to the fact that antioxidant properties of fruit are strongly affected by the species, variety, and cultivation conditions of the particular plant [73].
Results of this study showed that the astringent persimmon extracts had stronger ABTS, DPPH radical scavenging activities, and ferric reducing antioxidant power, than the non-astringent extract. The diversity of ABTS·+ and DPPH radical scavenging activities could be explained by the different stoichiometry reactions between fruits and the two radicals. Moreover, the antioxidant properties of fruit greatly depend on the type of fruit (species and variety within species), and cultivation conditions (environmental and cultivation techniques) [73].
Our results also show that the antioxidant activity of persimmon can be significantly correlated with total phenolic compounds. In this sense, the greater capacity of Rojo Brillante variety can be attributed to having more individual phenolic compounds than the Triumph variety, as it has, regardless of the method used, a lower total, and individual phenolic compounds content, and a lower antioxidant activity. Other authors, such as Chen et al., have also described this relationship, which showed a positive and highly significant correlation between total phenolics content and radical scavenging activity against ABTS and DPPH radicals [70].
According to the aforementioned study on tropical fruits [29] the antioxidant capacity for the Rojo Brillante variety, measured by the ABTS method, was superior to avocado (5455 µmol/g), mango (4889 µmol/g), papaya (4221 µmol/g) and carambola (2271 µmol/g) but lower than that of cherimoya (52,142 µmol/g), and kiwi (45,627 µmol/g). According to Park et al. (2015), the antioxidant capacity of persimmon is higher than that of banana, grape, grapefruit, and lemon, but lower than that of strawberry, apple pulp, kiwi, and mangosteen [44].
With reference to other widely consumed fruits, according to the data of Fu et al., using the ABTS method [74], where all values are expressed as µmol Trolox/g fruit, the Rojo Brillante variety of persimmon presents a higher antioxidant capacity than apple (36.0%), banana (176.0%), grape (168.2%), peach (176,%), and pear (178.4%). Likewise and according to Scalzo et al., again using the same method (ABTS) and units, the Rojo Brillante variety presents a higher antioxidant capacity (6.572 µmol/g) than apple (1.60 µmol/g), apricot (1.39 µmol/g) or peach (1.22 µmol/g), with only strawberry (113.17) presenting less [73].
To a certain extent, this high antioxidant capacity, together with the functional activities already mentioned, also highlights diabetes prevention, or reduces oxidative damage caused by diabetes when free radicals are released. Furthermore, due to its high antioxidant content, the persimmon could help prevent or reduce LDL oxidation, and, thus, the development of atherosclerosis [75]. Additionally, tannins have shown the ability to trap bile acids, which could lead to a reduction in plasma cholesterol levels, resulting in a reduction of cholesterol levels and risk of cardiovascular disease [76].
3.2.2. Total Phenolics (TP)
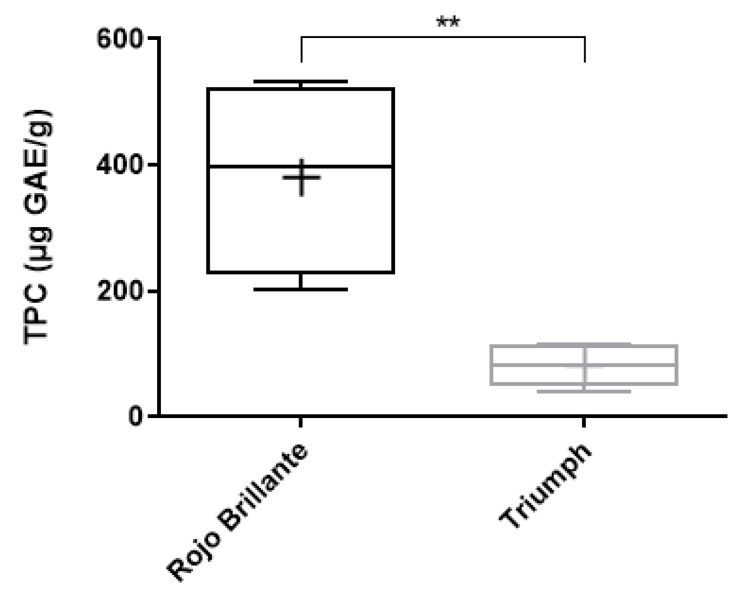
The total phenolics in both persimmon varieties were evaluated with the Folin–Ciocalteu method. Rojo Brillante samples showed high total phenol contents ranging from 202.163 μg/g to 532.089 μg/g gallic acid (µg GAE/g). The lowest total phenolic contents (ranging from 41.070 to 116.073 μg GAE/g) were found in the Triumph sample, which also has the lowest values for antioxidant capacity as measured by the direct methods (Table 3).
Thus, the Rojo Brillante variety (380.786 ± 158.539 µg GAE/g) showed a significantly (p < 0.01) higher TP than the Triumph variety (84.568 ± 34.989 µg GAE/g.), as shown in Figure 3. It has been reported that the TP may vary in different persimmon varieties and can vary significantly depending on the ripening stage or the astringency of the specific cultivar studied [77,78].
Figure 3.
Quantification of the total phenolic compounds (TPC) in µg GAE/g of two persimmon fruit varieties (Rojo Brillante and Triumph). ** Indicates p < 0.01.
Similar or higher values have been reported in the other scientific literature; however, any information regarding TP is rather scarce. A recent study reported similar results, with a TP (mg GAE/100 g FW) of 59.2 ± 0.4 [79].
In the study of Suzuki et al., the TP of three astringent persimmons (HN; 84.6, TW; 68.3 and IW; 76.4 mg/100 g) were four to six times higher than those of two non-astringent persimmons (MJ; 18.4 and MF; 14.8 mg/100 g) [80], presenting very similar values to those of our study. According to the study by Lucas-González et al., 2018 [10], total phenolic compound (TPC) was also higher in the Rojo Brillante than the Triumph variety. Ercisli et al. (2008) reported a similar variation of TP from 157 to 423 mg GAE/g, and differences were observed among persimmon genotypes [81].
Li et al. reported higher TP values for the astringent variety of persimmon, both for flesh and peel samples, than the non-astringent variety [68]. Lucas-González et al. determined TP in both the Rojo Brillante and Triumph varieties (undigested, oral digested, gastric digested, and intestinal digested samples). Undigested samples presented the same values in both varieties (≈100 mg/g) [71].
Veberic et al. determined TP in 11 cultivars of persimmon fruit, three astringent varieties (Fuji, ToneWase, and Triumph) and eight non-astringent varieties (Amankaki, Cal Fuyu, Hana Fuyu, Jiro, O’Gosho, Tenjin O’Gosho, Thiene, and Tipo). The Fuji variety obtained the highest TP concentration (295 ± 31.3 mg GAE/kg). However, the other two astringent varieties, including the Triumph variety (127 ± 17.9 mg GAE/kg) did not present higher values with respect to the rest of the non-astringent varieties [20].
Pu et al. also reported higher concentrations of TP for the astringent varieties analyzed (538.30 mg GAE/100 g for D. kaki. Cv. Xingyangshuishi; 905.53 mg GAE/100 g for D. kaki. Cv. Zhouqumomoshi and 1055.02 mg GAE/100 g for D. kaki. Cv. Xiuningbianshi) than the non-astringent varieties (168.22 mg GAE/100 g for D. kaki. Cv. Jiro and 347.77 mg GAE/100 g for D. kaki. Cv. Zenjimaru) [21]. Nevertheless, these authors obtained higher TP content than our study.
Chen et al. determined total amounts of phenolic in the astringent persimmons (Diospyros kaki L. Cv. Mopan) and other fruits (grape, apple, and tomato). The Mopan persimmon showed the highest TP (168.15 ± 0.12 mg GAE/100 g) [19]. In another study that determined TP in 62 fruits, persimmon was one of the fruits with the highest content of these compounds [23] showing a value of 112.09 ± 4.60 mg/100 g. However, both these studies reported three times higher values than the varieties in our study.
In summary, this study showed that astringent persimmons (Rojo Brillante) has significantly higher concentrations of total phenolic and greater capacities than non-astringent persimmons (Triumph).
3.3. Nutritional Impact of Persimmon as an Antioxidant
In recent years, there has been increasing interest in dietary phytochemicals including isothiocyanates, phenolic compounds, flavonoids, isoflavones, lignans, saponins and coumestrol—due to their possible protective action against cardio A. vascular disease and certain types of cancer [82].
A persimmon´s functional properties are related to its bioactive compounds content, which exerts a protective role on hypercholesterolemia, diabetes, cancer, hypertension and some dermic disorders [44]. In a wider sense, the functional properties of persimmon have been related to its antioxidant capacity. For example, a randomized controlled trial studied the plasmatic antioxidant capacity and urinary excretion of 8-isoprostane in individuals with a high intake of persimmon vinegar [82]. A significant increase in plasma antioxidant activity and reduced excretion of 8-isoprostane (a urinary biomarker of oxidative stress) were found.
To try to establish the nutritional value of introducing persimmon consumption into the Spanish diet, some estimates were made regarding polyphenolic compounds intake according to the studies by Saura-Calixto et al. They estimated its intake at 1209 mg/person/day, these being the main source of exogenous antioxidants [83]. From the consumption data, Román Martínez et al. calculated the total antioxidant capacity (TAC) of the diet as 10,577.9 micromoles of Trolox equivalent (water-soluble vitamin E analog) per day. Based on the previous results, fruit (the group with the highest contribution) [84] contributes 37.20% of the total.
A daily intake of persimmon (200–400 g) over a period of 3 to 5 months of the year would suppose 300 g/day × 6.57 µg Trolox/g = 1971 µg Trolox/day = 18.60% of the total dietary antioxidant capacity intake, with approximately 50% of that contributed by the fruit.
According to the ORAC method (Román Martínez et al., 2012), which is based on real consumption (kg/person/day), this contribution is considerably higher than the values in μmol Trolox/day of CAT (total antioxidant capacity) of more commonly consumed fruits in the Spanish diet (MAP 2018): apple (690.54.70), pear (261.81), orange (846.86), banana (282.95), peach (156.6), grape (72.49), melon (65.13), and watermelon (30.42) [84,85].
A joint FAO/WHO expert consultation published a report recommending a minimum consumption of 400 g of fruit and vegetables per day (excluding starchy tubers, such as potatoes) to prevent chronic diseases, particularly heart disease, cancer, type 2 diabetes, and obesity.
Both organizations agree that consuming a wide variety of fruit and vegetables helps ensure sufficient intake of most micronutrients, dietary fiber, and other health beneficial non-nutrient substances. A higher consumption of fruit and vegetables can also help replace excessive consumption of less healthy foods (e.g., those rich in fat, sugar, or salt) [1].
4. Conclusions
The aforementioned UPLC-Q-TOF-MS adequately described the phenolic composition of two Diospyros kaki varieties (Triumph and Rojo Brillante). These fruits were characterized by the presence of hydroxybenzoic and hydroxycinnamic acids, flavanols, flavonols, flavones, flavanones, tyrosols, and other compounds. Using the UPLC-Q-TOF-MS platform, a total of 31 different phenolic compounds were identified, of which 17 were quantified. The results show the Rojo Brillante variety is richer in many different phenolic compounds with bioactive properties. Therefore, the potential beneficial effects of persimmon, especially Rojo Brillante, could be evaluated in the future.
Furthermore, this study showed that astringent persimmons (Rojo Brillante) had significantly higher concentrations of phenolic compounds and greater capacities than non-astringent persimmons (Triumph). Daily persimmon consumption would provide a greater amount of phenolic compounds (catechins) and a higher antioxidant capacity (taking into account the current consumption/person) than the majority of fruits consumed by most. This makes us propose it as a functional food to improve oxidative stress levels and the current high rates of cardiovascular disease, diabetes, or cancer in the general public.
Acknowledgments
This paper will form part of the doctoral thesis by Adelaida Esteban-Muñoz, conducted within the context of the “Nutrition and Food Sciences Programme” at the University of Granada.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-3921/10/1/31/s1, Figure S1: Chromatogram of a Rojo Brillante sample, chromatogram extracted from vanillic acid and spectrum of vanillic acid, Figure S2: UPLC-QTOF-MS chromatogram Rojo Brillante sample (a) Triumph sample (b), Table S1: Quality parameters for the chromatographic determination of phenolic compounds in persimmon, Table S2: Precision parameters of the method used to determine individual phenolic compounds in persimmon, Table S3: Statistical analysis relating to antioxidant activity as measured by DPPH, ABTS and FRAP assays, and total phenolic compound content as measured by the FOLIN method.
Author Contributions
Conceptualization, A.E.-M. and M.O.-H.; methodology, A.E.-M.; software, A.E.-M. and S.S.-H.; validation, A.E.-M.; formal analysis, A.E.-M.; investigation, A.E.-M. and C.S.-S.; resources, C.S.-S. and M.O.-H.; data curation, A.E.-M., R.G.-M., and C.S.-S.; writing—original draft preparation, A.E.-M. and S.S.-H.; writing—review and editing, A.E.-M., S.S.-H., C.S.-S., R.G.-M., and M.O.-H.; visualization, M.O.-H. and A.E.-M.; supervision, M.O.-H.; project administration, M.O.-H.; funding acquisition, M.O.-H. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by project AGL2014-53895-R from the Spanish Ministry of Economy and Competitiveness and by the European Regional Development Fund (FEDER).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.FAO Food and Agricultural Organization of the United Nation FAO. [(accessed on 10 November 2020)]; Available online: http://www.fao.org/faostat/en/#data.
- 2.Imai K., Shiozaki S., Tsuda T., Nakao Y., Ogata T., Horiuchi S. Changes in Sugar, Organic Acid Contents and Characteristics of Kaki-shibu during Fermentation. Engei Gakkai Zasshi. 2001;70:95–101. doi: 10.2503/jjshs.70.95. [DOI] [Google Scholar]
- 3.Gao H., Liu B., Yang J., Fu S. Optimization of the prescription of persimmon vinegar-tea beverage by response surface methodology; Proceedings of the ICIME 2010–2010 2nd IEEE International Conference on Information Management and Engineering; Chengdu, China. 16–18 April 2010; pp. 121–123. [Google Scholar]
- 4.Han L., Qi S., Lu Z., Li L. Effects of immature persimmon (diospyros kaki linn. f.) juice on the pasting, textural, sensory and color properties of rice noodles. J. Texture Stud. 2012;43:187–194. doi: 10.1111/j.1745-4603.2011.00328.x. [DOI] [Google Scholar]
- 5.Mowat A. Charting the future, proceedings of the first national non-astringent persimmon industry workshop. Gatt. Coll. Univ. Queensl. 1990;56:425–430. [Google Scholar]
- 6.Gorinstein S., Zemser M., Weisz M., Halevy S., Deutsch J., Tilis K., Feintuch D., Guerra N., Fishman M., Bartnikowska E. Fluorometric Analysis of Phenolics in Persimmons. Biosci. Biotechnol. Biochem. 1994;58:1087–1092. doi: 10.1271/bbb.58.1087. [DOI] [Google Scholar]
- 7.Plaza L., Colina C., De Ancos B., Sánchez-Moreno C., Pilar Cano M. Influence of ripening and astringency on carotenoid content of high-pressure treated persimmon fruit (Diospyros kaki L.) Food Chem. 2012;130:591–597. doi: 10.1016/j.foodchem.2011.07.080. [DOI] [Google Scholar]
- 8.ILLESCAS. José L., Bacho O., Ferrer S. Frutas y Hortalizas: Guía Práctica. Empresa Nacional Mercasa; Madrid, Spain: 2008. [Google Scholar]
- 9.Chang Y.L., Lin J.T., Lin H.L., Liao P.L., Wu P.J., Yang D.J. Phenolic compositions and antioxidant properties of leaves of eight persimmon varieties harvested in different periods. Food Chem. 2019;289:74–83. doi: 10.1016/j.foodchem.2019.03.048. [DOI] [PubMed] [Google Scholar]
- 10.Lucas-González R., Fernández-López J., Pérez-Álvarez J.Á., Viuda-Martos M. Effect of particle size on phytochemical composition and antioxidant properties of two persimmon flours from Diospyros kaki Thunb. vars. ‘Rojo Brillante’ and ‘Triumph’ co-products. J. Sci. Food Agric. 2018;98:504–510. doi: 10.1002/jsfa.8487. [DOI] [PubMed] [Google Scholar]
- 11.Barea-Álvarez M., Delgado-Andrade C., Haro A., Olalla M., Seiquer I., Rufián-Henares J.Á. Subtropical fruits grown in Spain and elsewhere: A comparison of mineral profiles. J. Food Compos. Anal. 2016;48:34–40. doi: 10.1016/j.jfca.2016.02.001. [DOI] [Google Scholar]
- 12.Subar A., Patterson B. Fruit, Vegetables, and Cancer Prevention: A Review of the Epidemiological Evidence. Nutr. Cancer. 1992;18:1–29. doi: 10.1080/01635589209514201. [DOI] [PubMed] [Google Scholar]
- 13.Ames B.N., Shigenaga M.K., Hagen T.M. Oxidants, antioxidants, and the degenerative diseases of aging. Proc. Natl. Acad. Sci. USA. 1993;90:7915–7922. doi: 10.1073/pnas.90.17.7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duthie G.G., Duthie S.J., Kyle J.A.M. Plant polyphenols in cancer and heart disease: Implications as nutritional antioxidants. Nutr. Res. Rev. 2000;13:79–106. doi: 10.1079/095442200108729016. [DOI] [PubMed] [Google Scholar]
- 15.Willett W.C. Balancing life-style and genomics research for disease prevention. Sciences. 2002;296:695–698. doi: 10.1126/science.1071055. [DOI] [PubMed] [Google Scholar]
- 16.Leifert W.R., Abeywardena M.Y. Grape seed and red wine polyphenol extracts inhibit cellular cholesterol uptake, cell proliferation, and 5-lipoxygenase activity. Nutr. Res. 2008;28:729–737. doi: 10.1016/j.nutres.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 17.Rinaldo D., Mbéguié-A-Mbéguié D., Fils-Lycaon B. Advances on polyphenols and their metabolism in sub-tropical and tropical fruits. Trends Food Sci. Technol. 2010;21:599–606. doi: 10.1016/j.tifs.2010.09.002. [DOI] [Google Scholar]
- 18.Cai Y., Luo Q., Sun M., Corke H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci. 2004;74:2157–2184. doi: 10.1016/j.lfs.2003.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen X.J., Ji H., Zhang Q.W., Tu P.F., Wang Y.T., Guo B.L., Li S.P. A rapid method for simultaneous determination of 15 flavonoids in Epimedium using pressurized liquid extraction and ultra-performance liquid chromatography. J. Pharm. Biomed. Anal. 2008;46:226–235. doi: 10.1016/j.jpba.2007.09.016. [DOI] [PubMed] [Google Scholar]
- 20.Veberic R., Jurhar J., Mikulic-Petkovsek M., Stampar F., Schmitzer V. Comparative study of primary and secondary metabolites in 11 cultivars of persimmon fruit (Diospyros kaki L.) Food Chem. 2010;119:477–483. doi: 10.1016/j.foodchem.2009.06.044. [DOI] [Google Scholar]
- 21.Pu F., Ren X.L., Zhang X.P. Phenolic compounds and antioxidant activity in fruits of six Diospyros kaki genotypes. Eur. Food Res. Technol. 2013;237:923–932. doi: 10.1007/s00217-013-2065-z. [DOI] [Google Scholar]
- 22.Gao H., Cheng N., Zhou J., Wang B., Deng J., Cao W. Antioxidant activities and phenolic compounds of date plum persimmon (Diospyros lotus L.) fruits. J. Food Sci. Technol. 2014;51:950–956. doi: 10.1007/s13197-011-0591-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fu L., Lu W., Zhou X.M. Phenolic Compounds and In Vitro Antibacterial and Antioxidant Activities of Three Tropic Fruits: Persimmon, Guava, and Sweetsop. Biomed. Res. Int. 2016;2016:4287461. doi: 10.1155/2016/4287461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grigoras C.G., Destandau E., Fougère L., Elfakir C. Evaluation of apple pomace extracts as a source of bioactive compounds. Ind. Crops Prod. 2013;49:794–804. doi: 10.1016/j.indcrop.2013.06.026. [DOI] [Google Scholar]
- 25.Heras R.M.L., Quifer-Rada P., Andrés A., Lamuela-Raventós R. Polyphenolic profile of persimmon leaves by high resolution mass spectrometry (LC-ESI-LTQ-Orbitrap-MS) J. Funct. Foods. 2016;23:370–377. doi: 10.1016/j.jff.2016.02.048. [DOI] [Google Scholar]
- 26.Vallverdú-Queralt A., Jáuregui O., Di Lecce G., Andrés-Lacueva C., Lamuela-Raventós R.M. Screening of the polyphenol content of tomato-based products through accurate-mass spectrometry (HPLC-ESI-QTOF) Food Chem. 2011;129:877–883. doi: 10.1016/j.foodchem.2011.05.038. [DOI] [PubMed] [Google Scholar]
- 27.Balázs A., Tóth M., Blazics B., Héthelyi É., Szarka S., Ficsor E., Ficzek G., Lemberkovics É., Blázovics A. Investigation of dietary important components in selected red fleshed apples by GC-MS and LC-MS. Fitoterapia. 2012;83:1356–1363. doi: 10.1016/j.fitote.2012.04.017. [DOI] [PubMed] [Google Scholar]
- 28.Vallverdú-Queralt A., Boix N., Piqué E., Gómez-Catalan J., Medina-Remon A., Sasot G., Mercader-Martí M., Llobet J.M., Lamuela-Raventos R.M. Identification of phenolic compounds in red wine extract samples and zebrafish embryos by HPLC-ESI-LTQ-Orbitrap-MS. Food Chem. 2015;181:146–151. doi: 10.1016/j.foodchem.2015.02.098. [DOI] [PubMed] [Google Scholar]
- 29.Esteban Muñoz A., Barea Álvarez M., Oliveras-López M.-J., Giménez Martínez R., Henares J.Á.R., Olalla Herrera M. Determination of Polyphenolic Compounds by Ultra-Performance Liquid Chromatography Coupled to Tandem Mass Spectrometry and Antioxidant Capacity of Spanish Subtropical Fruits. Agric. Sci. 2018;9:180–199. doi: 10.4236/as.2018.92014. [DOI] [Google Scholar]
- 30.Moreno-Montoro M., Olalla-Herrera M., Gimenez-Martinez R., Navarro-Alarcon M., Rufián-Henares J.A. Phenolic compounds and antioxidant activity of Spanish commercial grape juices. J. Food Compos. Anal. 2015;38:19–26. doi: 10.1016/j.jfca.2014.10.001. [DOI] [Google Scholar]
- 31.Rivera-Dominguez M., Wlodarchak N., Kushad M., Yahia E.M. Identification and quantification of phenolic compounds in grapes. In Proceedings of the Acta Horticulturae. Int. Soc. Hortic. Sci. 2010;877:1233–1240. [Google Scholar]
- 32.Rueda A., Cantarero S., Seiquer I., Cabrera-Vique C., Olalla M. Bioaccessibility of individual phenolic compounds in extra virgin argan oil after simulated gastrointestinal process. LWT Food Sci. Technol. 2017;75:466–472. doi: 10.1016/j.lwt.2016.09.028. [DOI] [Google Scholar]
- 33.AOAC International . Official Methods of Analysis of AOAC International. AOAC International; Gaithersburg, MD, USA: 2012. [(accessed on 10 November 2020)]. Available online: http://www.aoac.org. [Google Scholar]
- 34.Cuvelier M.E., Berset C. 4A Standard Calibration Techniques. Microflown E-B. 1995;28:25–30. [Google Scholar]
- 35.Benzie I.F.F., Strain J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 36.Re R., Pellegrini N., Proteggente A., Pannala A., Yang M., Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999;26:1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 37.Samaniego Sánchez C., Troncoso González A.M., García-Parrilla M.C., Quesada Granados J.J., López García de la Serrana H., López Martínez M.C. Different radical scavenging tests in virgin olive oil and their relation to the total phenol content. Anal. Chim. Acta. 2007;593:103–107. doi: 10.1016/j.aca.2007.04.037. [DOI] [PubMed] [Google Scholar]
- 38.Samaniego-Sánchez C., Oliveras-López M.J., Quesada-Granados J.J., Villalón-Mir M., Serrana H.L.-G. Alterations in picual extra virgin olive oils under different storage conditions. Eur. J. Lipid Sci. Technol. 2012;114:194–204. doi: 10.1002/ejlt.201100191. [DOI] [Google Scholar]
- 39.Samaniego-Sánchez C., Stagno C., Quesada-Granados J.J., Blanca-Herrera R., Brandolini V. HPLC Method and Antioxidant Activity for Bioactive Component Determination of Lycopersicon esculentum Mill. Varieties from a Coastal Area of Southern Spain. Food Anal. Methods. 2014;7:660–668. doi: 10.1007/s12161-013-9670-0. [DOI] [Google Scholar]
- 40.Ramírez-Anaya J.d.P., Samaniego-Sánchez C., Castañeda-Saucedo M.C., Villalón-Mir M., de la Serrana H.L.-G. Phenols and the antioxidant capacity of Mediterranean vegetables prepared with extra virgin olive oil using different domestic cooking techniques. Food Chem. 2015;188:430–438. doi: 10.1016/j.foodchem.2015.04.124. [DOI] [PubMed] [Google Scholar]
- 41.Ramírez-Anaya J.D.P., Castañeda-Saucedo M.C., Olalla-Herrera M., Villalón-Mir M., Serrana H.L.-G.D.L., Samaniego-Sánchez C. Changes in the Antioxidant Properties of Extra Virgin Olive Oil after Cooking Typical Mediterranean Vegetables. Antioxidants. 2019;8:246. doi: 10.3390/antiox8080246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Akagi T., Katayama-Ikegami A., Yonemori K. Proanthocyanidin biosynthesis of persimmon (Diospyros kaki Thunb.) fruit. Sci. Hortic. 2011;130:373–380. doi: 10.1016/j.scienta.2011.07.021. [DOI] [Google Scholar]
- 43.Lee J.H., Lee Y.B., Seo W.D., Kang S.T., Lim J.W., Cho K.M. Comparative studies of antioxidant activities and nutritional constituents of persimmon juice (Diospyros kaki L. cv. Gapjubaekmok) Prev. Nutr. Food Sci. 2012;17:141–151. doi: 10.3746/pnf.2012.17.2.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Park Y.S., Cvikrová M., Martincová O., Ham K.S., Kang S.G., Park Y.K., Namiesnik J., Rombolà A.D., Jastrzebski Z., Gorinstein S. In vitro antioxidative and binding properties of phenolics in traditional, citrus and exotic fruits. Food Res. Int. 2015;74:37–47. doi: 10.1016/j.foodres.2015.04.021. [DOI] [PubMed] [Google Scholar]
- 45.Macheix J.J., Fleuriet A., Billot J. Fruit Phenolics. CRC Press; Boca Raton, FL, USA: 2018. [Google Scholar]
- 46.Décordé K., Teissèdre P.L., Auger C., Cristol J.P., Rouanet J.M. Phenolics from purple grape, apple, purple grape juice and apple juice prevent early atherosclerosis induced by an atherogenic diet in hamsters. Mol. Nutr. Food Res. 2008;52:400–407. doi: 10.1002/mnfr.200700141. [DOI] [PubMed] [Google Scholar]
- 47.Priscilla D.H., Prince P.S.M. Cardioprotective effect of gallic acid on cardiac troponin-T, cardiac marker enzymes, lipid peroxidation products and antioxidants in experimentally induced myocardial infarction in Wistar rats. Chem. Biol. Interact. 2009;179:118–124. doi: 10.1016/j.cbi.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 48.Saxena H.O., Faridi U., Srivastava S., Kumar J.K., Darokar M.P., Luqman S., Chanotiya C.S., Krishna V., Negi A.S., Khanuja S.P.S. Gallic acid-based indanone derivatives as anticancer agents. Bioorg. Med. Chem. Lett. 2008;18:3914–3918. doi: 10.1016/j.bmcl.2008.06.039. [DOI] [PubMed] [Google Scholar]
- 49.Singh G.K., Miller B.A., Hankey B.F., Edwards B.K. Persistent area socioeconomic disparities in U.S. incidence of cervical cancer, mortality, stage, and survival, 1975–2000. Cancer. 2004;101:1051–1057. doi: 10.1002/cncr.20467. [DOI] [PubMed] [Google Scholar]
- 50.Zhang J., Li D.M., Sun W.J., Wang X.J., Bai J.G. Exogenous p-hydroxybenzoic acid regulates antioxidant enzyme activity and mitigates heat stress of cucumber leaves. Sci. Hortic. 2012;148:235–245. doi: 10.1016/j.scienta.2012.10.013. [DOI] [Google Scholar]
- 51.Zheng W., Wang S.Y. Antioxidant activity and phenolic compounds in selected herbs. J. Agric. Food Chem. 2001;49:5129–5170. doi: 10.1021/jf010697n. [DOI] [PubMed] [Google Scholar]
- 52.Lesage-Meessen L., Delattre M., Haon M., Thibault J.F., Ceccaldi B.C., Brunerie P., Asther M. A two-step bioconversion process for vanillin production from ferulic acid combining Aspergillus niger and Pycnoporus cinnabarinus. J. Biotechnol. 1996;50:107–113. doi: 10.1016/0168-1656(96)01552-0. [DOI] [PubMed] [Google Scholar]
- 53.Dhananjaya B.L., Nataraju A., Rajesh R., Raghavendra Gowda C.D., Sharath B.K., Vishwanath B.S., D’Souza C.J.M. Anticoagulant effect of Naja naja venom 5′nucleotidase: Demonstration through the use of novel specific inhibitor, vanillic acid. Toxicon. 2006;48:411–421. doi: 10.1016/j.toxicon.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 54.Tseng T.H., Kao T.W., Chu C.Y., Chou F.P., Lin W.L., Wang C.J. Induction of apoptosis by Hibiscus protocatechuic acid in human leukemia cells via reduction of retinoblastoma (RB) phosphorylation and Bcl-2 expression. Biochem. Pharm. 2000;60:307–315. doi: 10.1016/S0006-2952(00)00322-1. [DOI] [PubMed] [Google Scholar]
- 55.Forester S.C., Waterhouse A.L. Identification of cabernet sauvignon anthocyanin gut microflora metabolites. J. Agric. Food Chem. 2008;56:9299–9304. doi: 10.1021/jf801309n. [DOI] [PubMed] [Google Scholar]
- 56.Yildirim H.K., Akçay Y.D., Güvenç U., Sözmen E.Y. Protection capacity against low-density lipoprotein oxidation and antioxidant potential of some organic and non-organic wines. Int. J. Food Sci. Nutr. 2004;55:351–362. doi: 10.1080/09637480412331319781. [DOI] [PubMed] [Google Scholar]
- 57.Landete J.M. Updated Knowledge about Polyphenols: Functions, Bioavailability, Metabolism, and Health. Crit. Rev. Food Sci. Nutr. 2012;52:936–948. doi: 10.1080/10408398.2010.513779. [DOI] [PubMed] [Google Scholar]
- 58.Prasad N.R., Jeyanthimala K., Ramachandran S. Caffeic acid modulates ultraviolet radiation-B induced oxidative damage in human blood lymphocytes. J. Photochem. Photobiol. B Biol. 2009;95:196–203. doi: 10.1016/j.jphotobiol.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 59.Buranov A.U., Mazza G. Extraction and purification of ferulic acid from flax shives, wheat and corn bran by alkaline hydrolysis and pressurised solvents. Food Chem. 2009;115:1542–1548. doi: 10.1016/j.foodchem.2009.01.059. [DOI] [Google Scholar]
- 60.Ou S., Kwok K.C. Ferulic acid: Pharmaceutical functions, preparation and applications in foods. J. Sci. Food Agric. 2004;84:1261–1269. doi: 10.1002/jsfa.1873. [DOI] [Google Scholar]
- 61.Zare K., Eidi A., Roghani M., Rohani A.H. The neuroprotective potential of sinapic acid in the 6-hydroxydopamine-induced hemi-parkinsonian rat. Metab. Brain Dis. 2014;30:205–213. doi: 10.1007/s11011-014-9604-6. [DOI] [PubMed] [Google Scholar]
- 62.Lafay S., Gil-Izquierdo A. Bioavailability of phenolic acids. Phytochem. Rev. 2008;7:301–311. doi: 10.1007/s11101-007-9077-x. [DOI] [Google Scholar]
- 63.O’Dowd C.D., Facchini M.C., Cavalli F., Ceburnis D., Mircea M., Decesari S., Fuzzi S., Young J.Y., Putaud J.P. Biogenically driven organic contribution to marine aerosol. Nature. 2004;431:676–680. doi: 10.1038/nature02959. [DOI] [PubMed] [Google Scholar]
- 64.Jovanovic S.V., Steenken S., Hara Y., Simic M.G. Reduction potentials of flavonoid and model phenoxyl radicals. Which ring in flavonoids is responsible for antioxidant activity? J. Chem. Soc. Perkin Trans. 2. 1996:2497–2504. doi: 10.1039/p29960002497. [DOI] [Google Scholar]
- 65.Achiwa Y., Hibasami H., Katsuzaki H., Imai K., Komiya T. Inhibitory effects of persimmon (diospyros kaki) extract and related polyphenol compounds on growth of human lymphoid leukemia cells. Biosci. Biotechnol. Biochem. 1997;61:1099–1101. doi: 10.1271/bbb.61.1099. [DOI] [PubMed] [Google Scholar]
- 66.Jung S.K., Kim K., Tae K., Kong G., Kim M.K. The effect of raw vegetable and fruit intake on thyroid cancer risk among women: A case-control study in South Korea. Br. J. Nutr. 2013;109:118–128. doi: 10.1017/S0007114512000591. [DOI] [PubMed] [Google Scholar]
- 67.García-Cayuela T., Nuño-Escobar B., Welti-Chanes J., Cano M.P. In vitro bioaccessibility of individual carotenoids from persimmon (Diospyros kaki, cv. Rojo Brillante) used as an ingredient in a model dairy food. J. Sci. Food Agric. 2018;98:3246–3254. doi: 10.1002/jsfa.8827. [DOI] [PubMed] [Google Scholar]
- 68.Li P.M., Du G.R., Ma F.W. Phenolics concentration and antioxidant capacity of different fruit tissues of astringent versus non-astringent persimmons. Sci. Hortic. 2011;129:710–714. doi: 10.1016/j.scienta.2011.05.024. [DOI] [Google Scholar]
- 69.Loizzo M.R., Said A., Tundis R., Hawas U.W., Rashed K., Menichini F., Frega N.G., Menichini F. Antioxidant and antiproliferative activity of Diospyros lotus L. extract and isolated compounds. Plant Foods Hum. Nutr. 2009;64:264–270. doi: 10.1007/s11130-009-0133-0. [DOI] [PubMed] [Google Scholar]
- 70.Chen X.N., Fan J.F., Yue X., Wu X.R., Li L.T. Radical scavenging activity and phenolic compounds in persimmon (Diospyros kaki L. cv. Mopan) J. Food Sci. 2008;73:C24–C28. doi: 10.1111/j.1750-3841.2007.00587.x. [DOI] [PubMed] [Google Scholar]
- 71.Lucas-González R., Viuda-Martos M., Pérez Álvarez J.A., Fernández-López J. Changes in bioaccessibility, polyphenol profile and antioxidant potential of flours obtained from persimmon fruit (Diospyros kaki) co-products during in vitro gastrointestinal digestion. Food Chem. 2018;256:252–258. doi: 10.1016/j.foodchem.2018.02.128. [DOI] [PubMed] [Google Scholar]
- 72.Gu H.F., Li C.M., Xu Y.J., Hu W.F., Chen M.H., Wan Q.H. Structural features and antioxidant activity of tannin from persimmon pulp. Food Res. Int. 2008;41:208–217. doi: 10.1016/j.foodres.2007.11.011. [DOI] [Google Scholar]
- 73.Scalzo J., Politi A., Pellegrini N., Mezzetti B., Battino M. Plant genotype affects total antioxidant capacity and phenolic contents in fruit. Nutrition. 2005;21:207–213. doi: 10.1016/j.nut.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 74.Fu L., Xu B.T., Xu X.R., Gan R.Y., Zhang Y., Xia E.Q., Li H. Bin Antioxidant capacities and total phenolic contents of 62 fruits. Food Chem. 2011;129:345–350. doi: 10.1016/j.foodchem.2011.04.079. [DOI] [PubMed] [Google Scholar]
- 75.Yaqub S., Farooq U., Shafi A., Akram K., Murtaza M.A., Kausar T., Siddique F. Chemistry and Functionality of Bioactive Compounds Present in Persimmon. J. Chem. 2016;2016:3424025. doi: 10.1155/2016/3424025. [DOI] [Google Scholar]
- 76.Gato N., Kadowaki A., Hashimoto N., Yokoyama S., Matsumoto K. Persimmon Fruit Tannin-Rich Fiber Reduces Cholesterol Levels in Humans. Ann. Nutr. Metab. 2013;62:1–6. doi: 10.1159/000343787. [DOI] [PubMed] [Google Scholar]
- 77.Giordani E., Doumett S., Nin S., Del Bubba M. Selected primary and secondary metabolites in fresh persimmon (Diospyros kaki Thunb.): A review of analytical methods and current knowledge of fruit composition and health benefits. Food Res. Int. 2011;44:1752–1767. doi: 10.1016/j.foodres.2011.01.036. [DOI] [Google Scholar]
- 78.Denev P., Yordanov A. Total polyphenol, proanthocyanidin and flavonoid content, carbohydrate composition and antioxidant activity of persimmon (Diospyros kaki L.) fruit in relation to cultivar and maturity stage. Bulg. J. Agric. Sci. 2013;19:981–988. [Google Scholar]
- 79.Conesa C., Laguarda-Miró N., Fito P., Seguí L. Evaluation of Persimmon (Diospyros kaki Thunb. cv. Rojo Brillante) Industrial Residue as a Source for Value Added Products. Waste Biomass Valorization. 2020;11:3749–3760. doi: 10.1007/s12649-019-00621-0. [DOI] [Google Scholar]
- 80.Suzuki T., Someya S., Hu F., Tanokura M. Comparative study of catechin compositions in five Japanese persimmons (Diospyros kaki) Food Chem. 2005;93:149–152. doi: 10.1016/j.foodchem.2004.10.017. [DOI] [Google Scholar]
- 81.Ercisli S., Akbulut M., Ozdemir O., Sengul M., Orhan E. Phenolic and antioxidant diversity among persimmon (Diospyrus kaki L.) genotypes in Turkey. Int. J. Food Sci. Nutr. 2008;59:477–482. doi: 10.1080/09637480701538262. [DOI] [PubMed] [Google Scholar]
- 82.Mure K., Takeshita T., Morioka I., Arita M. Effects of kakisu (persimmon vinegar) on plasma antioxidant power and urinary 8-isoprostane level. Nippon Eiseigaku Zasshi. Jpn. J. Hyg. 2007;62:32–38. doi: 10.1265/jjh.62.32. [DOI] [PubMed] [Google Scholar]
- 83.Saura-Calixto F., Goni I. Definition of the mediterranean diet based on bioactive compounds. Crit. Rev. Food Sci. Nutr. 2009;49:145–152. doi: 10.1080/10408390701764732. [DOI] [PubMed] [Google Scholar]
- 84.Román Martínez J., Villarino A., de Arpe C. Avances en Alimentación, Nutrición y Dietética—SEDCA 2012|Sociedad Española de Dietética y Ciencias de la Alimentación (S.E.D.C.A.) [(accessed on 10 November 2020)]; Available online: https://nutricion.org/job-offer/avances-en-alimentacion-nutricion-y-dietetica-sedca-2012/
- 85.Ministerio de Agricultura, Pesca y Alimentación Últimos datos de Consumo Alimentario. [(accessed on 10 November 2020)]; Available online: https://www.mapa.gob.es/es/alimentacion/temas/consumo-tendencias/panel-de-consumo-alimentario/ultimos-datos/default.aspx.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.