Abstract
Temperature across the globe is increasing continuously at the rate of 0.15–0.17 °C per decade since the industrial revolution. It is influencing agricultural crop productivity. Therefore, thermotolerance strategies are needed to have sustainability in crop yield under higher temperature. However, improving thermotolerance in the crop is a challenging task for crop scientists. Therefore, this review work was conducted with the aim of providing information on the wheat response in three research areas, i.e., physiology, breeding, and advances in genetics, which could assist the researchers in improving thermotolerance. The optimum temperature for wheat growth at the heading, anthesis, and grain filling duration is 16 ± 2.3 °C, 23 ± 1.75 °C, and 26 ± 1.53 °C, respectively. The high temperature adversely influences the crop phenology, growth, and development. The pre-anthesis high temperature retards the pollen viability, seed formation, and embryo development. The post-anthesis high temperature declines the starch granules accumulation, stem reserve carbohydrates, and translocation of photosynthates into grains. A high temperature above 40 °C inhibits the photosynthesis by damaging the photosystem-II, electron transport chain, and photosystem-I. Our review work highlighted that genotypes which can maintain a higher accumulation of proline, glycine betaine, expression of heat shock proteins, stay green and antioxidant enzymes activity viz., catalase, peroxidase, super oxide dismutase, and glutathione reductase can tolerate high temperature efficiently through sustaining cellular physiology. Similarly, the pre-anthesis acclimation with heat treatment, inorganic fertilizer such as nitrogen, potassium nitrate and potassium chloride, mulches with rice husk, early sowing, presoaking of a 6.6 mM solution of thiourea, foliar application of 50 ppm dithiothreitol, 10 mg per kg of silicon at heading and zinc ameliorate the crop against the high temperature. Finally, it has been suggested that modern genomics and omics techniques should be used to develop thermotolerance in wheat.
Keywords: heat stress, photosynthesis, antioxidant enzymes, HSPs, QTLs, omics
1. Introduction
Climate change is the result of a higher level of greenhouse gases such carbon dioxide (CO2), nitrous oxide, and methane (CH4). These gases can entrap the sun rays leading towards the severity of extreme events for crops development [1,2]. It has been observed that CO2 was increased 0.6 ± 0.1 ppm/year in the early 1960s and 2.3 ± 0.6 ppm/year during the last decade. Meanwhile, the CH4 gas was doubled after the industrial revolution until the 1980s and it is increasing at the rate of 12 parts per billion per year. However, during the last three decades it was increasing 2–5 parts per billion per year. The nitrous oxide concentration was enhanced 18% more than the 1970s and increased 0.8 parts per billion per year [3,4].
The escalating global warming evokes an extreme weather pattern, increases disease incidences, insect pest survival, and ultimately influences crop productivity [5,6]. Global warming potential (GWP) is the contribution of one molecule of compound over 100 years to global warming as compared to CO2. It allows the comparison of different gas contributions to global warming and how much energy emissions of 1 ton of gas absorbs more than 1 ton of CO2 over a given time period. The larger global warming potential represents more potential of the given gas to persist and the ability to warm the Earth temperature over a given time period. The GWP of carbon dioxide is 1, CH4 28–36, and nitrous oxide 265–298 over 100 years. However, these gases possess more potential and persistency to entrap the sun rays than CO2 but a major contributor in global warming is CO2 [7].
Agricultural crop productivity depends on biotic (diseases and insect pest) and abiotic (heat, drought, and salinity) factors [8]. Among the abiotic stresses, the higher temperature is a major concern influencing crop growth and development. The global temperature roughly increased by 1.5 °C with the same accelerating trend in all regions from the 1970s, as reported by the intergovernmental panel of climatic change (IPCC) and was predicted to increase 2.5–5.8 °C until the 2100s [3]. The global average temperature annually increased by 0.04–0.07 °C and 0.15–0.17 °C per decade since the 1880s and 1970s, respectively according to the National Oceanic and Atmospheric Administration (NOAA, 2018). Therefore, global warming characterized by an extreme temperature possesses the challenge to improve the yield potential of crops.
Terminal and continual heat stresses are two major constraints influencing crops growth and development. The temperature threshold levels were reported at different stages for crops viz., cotton [9,10,11,12,13], rice [14,15,16,17,18,19], sorghum [20,21], barley [12], maize [9,22,23], and soybean [23]. Wheat is an imperative staple food, the cheapest energy source, provides 8–20% of protein, and 70–75% of calories in our average diet [24], but a high temperature restricts the wheat crop to express its full genetic potential. Therefore, there is a dire need to understand the wheat response against the high temperature and a suitable strategy to improve its productivity.
2. Impact of High Temperature on Wheat
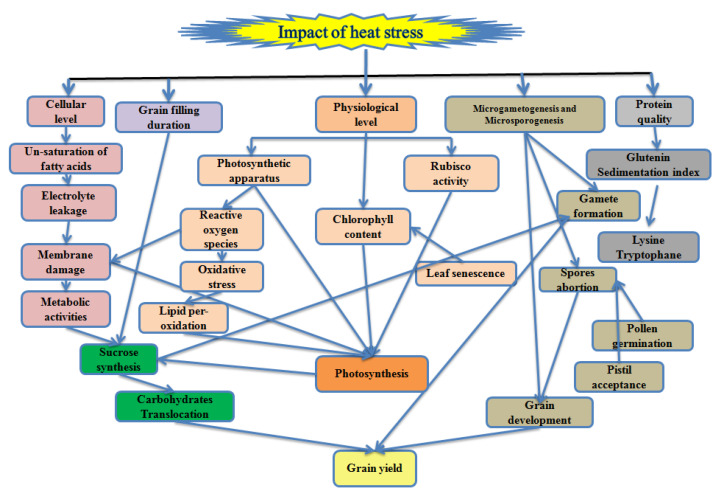
High temperature influences the wheat productivity in tropical, subtropical, arid and semi-arid regions of the world. The optimum temperature for wheat growth and development are given in Table 1. The high temperature in the tropical region is an inevitable constraint for wheat during germination and early growth stages, whereas in the Mediterranean region, the reproductive stage is highly sensitive [25]. A high temperature of 3–4 °C above the optimum temperature at grain filling reduces 10–50% of the wheat yield in Asia with the current production technology and varieties [26]. High temperature declines 0.07% per °C grain yield depending on the wheat variety [27]. Each degree increase in the temperature at the grain filling duration reduces 6% of wheat yield globally [28,29] and 3–17% in South Asia including India and Pakistan [30]. It accredited directly or indirectly the disturbance in different cellular, physiological functions and metabolic pathways associated with the grain yield in wheat (Figure 1).
Table 1.
Optimal temperature requirements of wheat at different growth stages.
| Stages | Optimum Temperature (°C) | Minimum Temperature (°C) | Maximum Temperature (°C) |
|---|---|---|---|
| Root growth | 17.2 ± 0.87 | 3.5 ± 0.73 | 24 ± 1.21 |
| Shoot growth | 18.5 ± 1.90 | 4.5 ± 0.76 | 20.1 ± 0.64 |
| Leaf initiation | 20.5 ± 1.25 | 1.5 ± 0.52 | 23.5 ± 0.95 |
| Terminal Spikelet | 16 ± 2.3 | 2.5 ± 0.49 | 20 ± 1.6 |
| Anthesis | 23 ± 1.75 | 10 ± 1.12 | 26 ± 1.01 |
| Grain Filling Duration | 26 ± 1.53 | 13 ± 1.45 | 30 ± 2.13 |
Figure 1.
Schematic illustration of the high temperature impact on wheat associated with the grain yield.
2.1. Cellular Metabolism
The plasma membrane is a highly organized structure composed of lipids and proteins. It regulates the enzymatic activity and transport of ions. High temperature alters the microtubules organization, expansion, elongation, and cell differentiation [48]. It increases the kinetic energy of hydrogen bonds between adjacent fatty acids, weakens the bonds, and leads to membrane fluidity. This fluidity, unsaturation of fatty acids, and disruption of different proteins trigger the electrolyte leakage [49,50]. High temperature causes 25–55% electrolyte leakage at 45 °C for 1 h [51], while 21–40% leakage at 40 °C for 30 min [52]. Therefore, the cell damages its internal composition and sustainable physiological processes (e.g., photosynthesis, respiration, and transpiration) associated with the synthesis and translocation of carbohydrates into the grains.
2.2. Grain Filling Duration
High temperature enforces the plant to complete the growing degree days earlier, which results in early maturity and shorter life cycle of plant, lesser biosynthetic products accumulation, and ultimately poor grain development [32,53,54]. Vernalization (VRN1, VRN2) and the photoperiodic (PPD-A1, PPD-D1) sensitive gene determines the developmental phases at volatile temperature events and triggers earliness in wheat by limiting various growth phases [55,56]. The longer grain filling duration determines the appropriate grains development associated with the grain yield [57]. However, high temperature reduces the duration to uptake the available nutrients and translocation of photosynthates.
2.3. Grain Formation and Development
Vital events at the reproductive stage such as flowering initiation, pollen germination, pistil receptiveness, and embryo development determine the florets fertility [58,59]. The embryo sac and embryo formation are sensitive to high temperature [60]. Microgametogenesis and microsporogenesis are sensitive to high temperature, which hinder the gametes development and cause spores abortion [61,62].
Wheat grain contains 60–70% starch content and gradually drops under high temperature [63,64]. High temperature inhibits the starch accumulation into grains ascribable to the enzymes inactivity viz., granule bound starch, soluble starch, and sucrose synthase during the grain filling phase [65,66]. It also declines the starch content synthesis [67,68], stem reserves carbohydrates translocation [69,70], alters the structure of aleurone layer, and endosperm of seed [71,72], which ultimately influences grain development.
2.4. Leaf Senescence
Leaf senescence is the reduction in green leaf area during the reproductive phase due to the retardation in the chlorophyll content and carotenoids [73,74]. The chlorophyll content and carotenoid have an indispensable role in harvesting sunlight for photosynthesis [75]. High temperature disturbs the chloroplast integrity, leaf senescence, and ultimately photosynthesis in wheat [76].
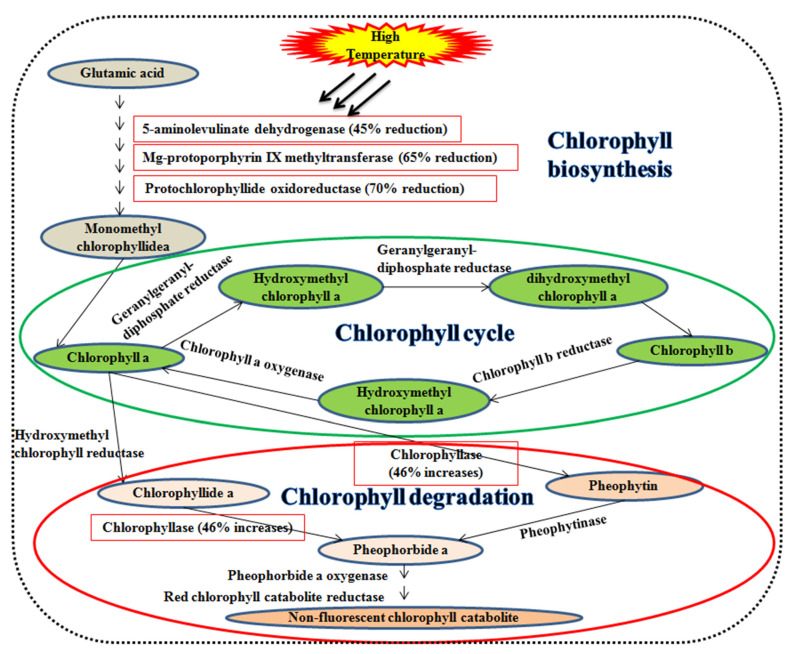
Leaf senescence during the grain filling duration degrades the leaf chlorophyll content. Initially, chlorophyll-b is converted into chlorophyll-a during the chlorophyll cycle (Figure 2). The chlorophyllase enzyme catalyzes chlorophyll-a into chlorophyllide-a or pheophytin and subsequently into pheophorbide-a. Pheophorbide-a monooxygenase catalyzes the pheophorbide-a and is converted to red chlorophyll catabolites ensuing fluorescent and non-fluorescent chlorophyll catabolites [77,78]. A high temperature of 42 °C declines the enzymes efficiency viz., 5-aminolevulinate dehydrogenase (45%), mg-protoporphyrin IX methyltransferase (65%), protochlorophyllide oxidoreductase (70%), and increases chlorophyllase (46%) in wheat [79].
Figure 2.
Impact on the high temperature of leaf senescence. Enzymes associated with chlorophyll synthesis viz., 5-aminolevulinate dehydrogenase, mg-protoporphyrin IX methyltransferase, and protochlorophyllide oxidoreductase, whereas chlorophyllase is responsible for chlorophyll degradation.
Chlorophyll deficiency reduces the absorbance of light energy and transfer to the reaction centers (RCs) of PS-II and PS-I at high temperature in wheat [80,81]. Carotenoids dissipate the excess light and protect the reaction centers against stress conditions [82]. Carotenoids viz., xanthophylls, and isoprene maintain the thylakoid membrane from leakage [83]. However, thylakoid components are sensitive at a temperature above 40 °C and inhibit the carotenoids biosynthesis pathways in the chloroplast [46,84], which interrupt the photosynthesis stability and ultimately reduce the grain yield in wheat [25].
2.5. Protein Quality
The protein content, protein quality, and glutenin/gliadin determine the backing quality of bakery products [85,86]. High temperature enhances the total protein content but reduces the end use of protein quality [87,88], which is more or less dependent on the grain protein concentration [89]. Protein fractions (albumin, globulin, gliadin, and glutenin) are important components for the end use quality of wheat grain [90]. High temperature at the grain filling duration decreases the albumin and globulin content [91], whereas it increases the gliadin content at the expense of glutenin content in wheat [92]. Furthermore, high temperature increases the protein content but reduces the production of glutenin, sedimentation index [71], and essential amino acids such as lysine, methionine, and tryptophan content, which determines the viscoelastic properties of wheat loaf [45].
2.6. Physiological Process
Heat stress inhibits the photosynthesis, damaging photosynthetic apparatus, and synthesis of ROS (reactive oxygen species) as discussed below.
2.6.1. Photosynthesis Response to High Temperature
A high temperature of 35/25 °C (day/night) at the grain filling duration inhibits the leaf photosynthesis up to 50% in wheat (Figure 3 and Figure 4). The net photosynthesis during the wheat crop cycle is essential in controlling the crop biomass and grain yield under a high temperature. The optimum temperature for net photosynthesis is 20–30 °C, but a high temperature above 32 °C declines the photosynthetic rate rapidly in wheat [46]. The photosynthesis in wheat leaves is more sensitive than those, which are associated with the synthesis and mobilization of stem reserves into developing grains during grain filling. Photosynthesis is associated with the activity of photosynthetic apparatus, Rubisco (Ribulose bisphosphate carboxylase/oxygenase) enzyme, and various green organs of the plants such as chlorophyll content and carotenoids [76,93].
Figure 3.
Photosynthetic (µmol m−2 s−1) response at the seedling and reproductive stage of 180 wheat genotypes with the grain yield per plant (g). Photosynthetic rate was recorded on a clear day between 10:00 a.m. to 12:00 p.m. with the help of infrared gas analyzer (IRGA ADC, LCA-4, Hoddesdon, UK). Data collected under normal and heat stress conditions at the vegetative (Zadoks scale 39) and reproductive stages (Zadoks scale 69) [94]. It represented that the photosynthesis is directly associated with the grain yield at both stages. As the photosynthetic rate decreases, it reduces the grain yield in wheat.
Figure 4.
Photosynthetic (µmol m−2 s−1) response of 180 wheat genotypes with the grain yield per plant (g) at the seedling and reproductive stages. Data collected under normal and heat stress (4–5 °C above normal) conditions [94].
2.6.2. Photosynthetic Apparatus
High temperature disturbs the photosystem-II (PS-II) and photosystem-I (PS-I) mediated electron transport chain (ETC). A high temperature of 35–40 °C at the grain filling phase directly damages the photosynthetic apparatus including the PS-II and PS-I mediated electron transport chain [46]. PS-II is a complex subunit of chlorophylls and proteins and is more sensitive than PS-I [73,95]. It harvests the light energy to oxidize the water molecule and transfer electrons to plastoquinone (PQ) ensuing the cytochrome b6f complex, but a high temperature declines the efficiency of PQ and Cytochrome b6f [96].
The light harvesting complex-II (LHC-II) is an assortment of proteins associated with the PS-II core complex. It harvests the sunlight energy and transfers it to the PS-II core complex to form multi-complex proteins [97]. High basal florescence separates the LHC-II from the PS-II core complex and alters the energy distribution to PS-I [98]. A high temperature of 32–38 °C also synthesizes the zeaxanthin, which destabilizes the thylakoid membrane composition and photosynthetic apparatus [48].
2.6.3. Rubisco Activity
Rubisco is an essential light activated enzyme, which possesses the binding sites for CO2 and Rubisco activase for the regulation of the Calvin cycle, but its efficiency gradually declines under a high temperature of 25–40 °C in wheat [99]. Sugar phosphate inhibitors viz., XuBP (D-xylulose-1, 5-bisphosphate), RuBP (Ribulose-1, 5-bisphosphate), CA1P (2-Carboxy-D-arabinitol 1-phosphate), and CTBP (2-Carboxytetritol-1, 4-bisphosphate) impaired with the active site, which modulate the Rubisco activity for photosynthesis [100,101]. Rubisco activase removes these inhibitors from the active site and facilitates the carboxylation reaction modulated by the Rubisco enzyme [102]. It also protects the nascent proteins from aggregation but it is heat labile. Therefore, a high temperature of >32 °C alters the composition for the accessibility of carbamylation [103,104].
High temperature declines the solubility of CO2 and enhances the O2 level from the compensation point due to the reduction in evapotranspiration [105,106,107] and specificity of the Rubisco enzyme activity, which is poor in discriminating O2 and CO2 [108,109] (Figure 5). These factors stimulate the photorespiration and consume ATPs, release the fixed CO2, and produce the photorespiratory metabolite (glyoxylate), which consume NADH2 [110,111] and ultimately reduce the yield up to 20% in wheat [112].
Figure 5.
Rubisco enzyme activity pathway alteration at a high temperature. Rubisco has a characteristic of both oxygenation and carboxylation activities. High temperature increases the synthesis of oxygen through photosynthesis, which enhanced the solubility of oxygen than carbon dioxide. Therefore, it promotes the oxygenase activity of Rubisco and stimulates photorespiration, which compartmentalized in chloroplast, peroxisomes, and mitochondria.
2.6.4. Reactive Oxygen Species
Reactive oxygen species (ROS) are synthesized during the malfunction of PS-II and the Calvin cycle of photosynthesis [113] causing lipid per-oxidation and cell membrane damage in wheat [114,115]. ROS such as super oxides (O-2), hydroxyl radical (OH-), and hydrogen peroxide (H2O2) commonly synthesize at high temperatures. The manganese superoxide dismutase (Mn-SOD) catalysis in mitochondria produces hydrogen peroxides, whereas the auto-oxidation of ubisemiquinone complex-I and complex-III generates super oxides radicals ensuing the oxidative stress in the cell, as well as DNA damage, protein modification, and membrane instability [48,116].
Super oxides synthesize by the reduction of one electron, whereas further electrons reduction generates peroxide, which is neutralized by two protons of hydrogen atom and form H2O2, as shown in Figure 6. Hydrogen peroxide is produced by incomplete water molecules oxidation, which is reduced by the manganese to form the hydroxyl radical [117]. The hydrogen peroxide concentration gradually increases from vegetative to milky dough stage at a high temperature and negatively influences the photosynthesis [118].
Figure 6.
Synthesis of the reactive oxygen species and their consequences.
3. Tolerance Mechanism against High Temperature
The plant’s tolerance to high temperature facilitates adaptation in adverse conditions through maintaining their physiology and ameliorate grain yield.
3.1. Phytohormones and Bioregulators
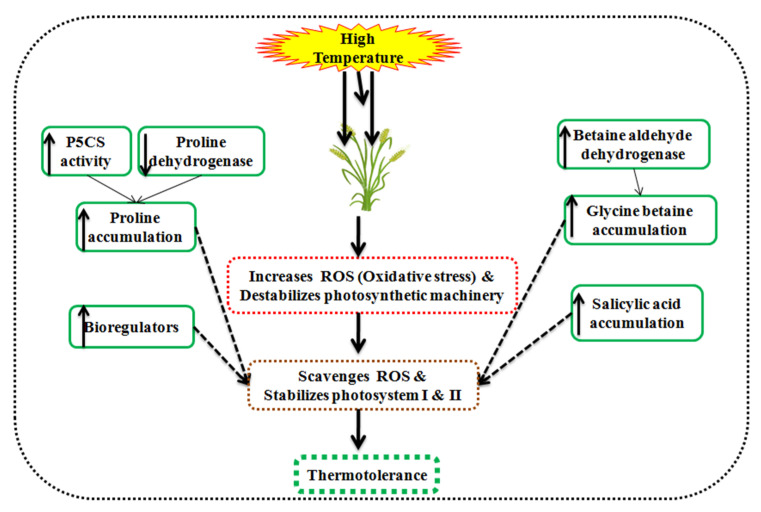
Phytohormones inevitably associated with the antioxidant enzymes activity and growth regulation under heat stress conditions [119]. Phytohormones viz., proline, glycine betaine, salicylic acid, abscisic acid, and ethylene maintain the physiology at a high temperature through soluble salts accumulation in the cell and reducing H2O2 production in wheat, as displayed in Figure 7.
Figure 7.
Schematic illustration of osmolytes associated with thermotolerance in wheat.
A high temperature of 30–35 °C discolorizes the chlorophyll, beta-carotene, and damages the photochemical activity. Glycine betaine accumulates in the chloroplast of leaves and stabilizes PS-II, reaction centers in the thylakoid membrane [120,121], Rubisco enzyme, and inhibits the ROS production [122]. It adjusts the osmotic pressure, ameliorate antioxidant enzymes activity, and photosynthesis under high temperature in wheat [123]. Salicylic acid acts as a phenolic hormone in plants and is responsible for osmoregulation, scavenges ROS, and maintains the photosynthesis in wheat [124]. It also triggers the osmolytes synthesis viz., glycine betaine, proline, and sugars under heat stress conditions [125,126,127].
Proline accumulation is determined by the proline dehydrogenase activity and Δ1-pyrroline-5-carboxylate synthetase/reductase (P5CS) [128]. High temperature increases the P5CS and decreases proline dehydrogenase in tolerant wheat seedlings. Proline dehydrogenase catalyzes the proline degeneration in mitochondria. However, glutamate acts as a precursor in the presence of P5CS1 for the proline synthesis and accumulates in plant under heat stress conditions [129,130]. The proline content is directly linked to a high temperature of 35–40 °C and ameliorates the defense mechanism in wheat seedlings [131]. A high temperature of 35 °C than 25 °C accumulates a higher proline content (up to 200%) and improves the photosynthetic efficiency and yield [132].
Bioregulators upregulate the antioxidant defense mechanism and maintains the PS-II under high temperature. Foliar application during the grain filling period and seed priming with a 6.6 mM solution of thiourea intensifies the antioxidant enzyme activity, chlorophyll content, total soluble protein, amino acid, and grain weight in wheat [133]. Foliar application of 50 ppm dithiothreitol also ameliorates the adverse effect of high temperature in wheat [134].
3.2. Stay Green
Stay green represents the chlorophyll retention and longevity of photosynthetic apparatus for the adaptation of wheat under high temperature [135,136,137]. Stay green associated with the stabilized photosynthetic apparatus of chloroplast viz., scavenges of ROS, and maintaining the photosynthetic apparatus indicates the slow degeneration of tissues in wheat.
The stay green trait has the potential to protect photosystem-II in the chloroplast and inhibits the ROS synthesis in wheat [138,139]. It maintains the green pigment at a high temperature of >30 °C during the grain filling phase. The short grain filling duration and high canopy temperature are associated with non-stay green genotypes in wheat [140]. Stay green is positively associated with the normal grain filling phase, membrane stability, photosynthesis, stem reserve carbohydrates, and grain development [141,142].
Chlorophyll biosynthesis enzymes determine the senescence in wheat, which influences the assimilation and translocation of photosynthates into grains during grain filling [37,143]. For example, the SGR mutant of Arabidopsis and rice exhibit the stay green phenotype due to the suppression of Mg dechelatase enzyme, which is responsible for chlorophyll degradation [144]. SGR mutants have also been reported in other species viz., pea, tomato, and pepper [142]. The NYC gene suppression also delays the senescence of crops that catalyzed the chlorophyll breakdown for the conversion of chlorophyll-b into chlorophyll-a [145]. The PPH genes mutant removes the phytol from phaeophytin in Arabidopsis and results in stay green [146]. Genes NYC, PPH, and SGR have a potential role for stay green in arbidopsis and rice that must be explored in wheat to improve thermotolerance.
3.3. Antioxidant Enzymes
Antioxidant enzymes protect the plant from ROS, convert the free radicals of oxygen and hydroxyl into H2O2 followed by the water molecule. These enzymes scavenge the ROS, balance the production/elimination of ROS from oxidative stress, maintain the growth, development, metabolism, and overall productivity [147]. Antioxidant enzymes viz., POD (peroxidase), SOD (superoxide dismutase), CAT (catalase), and GR (glutathione reductase) usually generate under a high temperature of 35/28 °C day/night in wheat [147,148,149].
The SOD enzyme converts the O−2 to H2O2, which is a less toxic form than the free radicals [150,151]. CAT and POD convert H2O2 into H2O, but the CAT activity is higher than other antioxidant enzymes in wheat [152,153]. CAT reduces several millions of H2O2 molecules into H2O and oxygen per minute [154,155]. GR protects the plant from oxidative stress by reducing oxidized glutathione [156,157]. Glutathione peroxidase (GPx) efficiency depends on high γ-glutamyl cysteine synthetase and glutathione synthetase activity for the reduction of H2O2 into H2O [158].
3.4. Heat Shock Proteins
Wheat plant produces heat shock proteins (HSPs) at 32–34 °C and provides protection against high temperature [159,160]. High temperature disturbs the membrane proteins in plants but upregulates the translation of heat shock genes, which encodes for HSPs [132,161,162]. These HSPs protect the cell from adverse effects of heat stress by maintaining photosynthesis, upregulation of other proteins, and cell metabolism [163]. There are different families of ATP dependent HSPs viz., HSP60, HSP70, HSP90, and HSP100 except small HSPs based on molecular weight.
The small HSP (smHSP) in wheat genome assembles with other homo-oligomers and facilitates binding in ATP independent manners. It assembles with HSP90 to prevent unfolding and refolding of proteins under high temperature [159,160]. HSP60 expresses constitutively in chloroplast and mitochondria [164,165]. The Rubisco large subunit binding protein (chHSP60) is a cofactor of HSP60, which regulates the Rubisco enzyme folding at high temperature [166].
HSP70 is a highly conserved protein, which recognizes only a short sequence of the polypeptide chain, temporal and inhibits aggregation of non-native protein at high temperature [167]. HSP110 is a sub family of HSP70 and inhibits the aggregation with a greater capability than HSP70 [168]. HSP90 regulates transcription, cellular signaling, and managing protein folding through assembling molecular proteins including HSP40 and HSP70 [118,168,169], whereas HSP100 interacts with different smHSPs and HSP70 to prevent the aggregation of protein [170].
4. Tolerance Strategies against High Temperature
Strategies against heat stress viz., crop management, conventional, non-conventional, and molecular approaches ameliorate the thermotolerance in wheat. These strategies are further elaborated below.
4.1. Crop Management
Agronomic practices including seed priming, organic mulches, inorganic fertilizers, and timely sowing with recommended management practices mitigate the heat stress in wheat. Wheat seed priming in the aerated solution of CaCl2 (1.2%) for 12 h improves the germination, growth, leaf area index, chlorophyll content, assimilation rate, and grain yield [171,172,173]. Mulching with rice husk conserves water, improves water use efficiency, maintains the water status in soil, and slows down the release of nitrogen for plant uptake [174,175].
The application of inorganic fertilizers viz., nitrogen, and potassium maintain the chlorophyll content, osmoregulation, cytokinin biosynthesis, protein stability, redox homeostasis, and photosynthesis at high temperature [25,176]. Zinc improves the superoxide dismutase activity, membrane integrity, chlorophyll content, chlorophyll florescence, and kernel growth at high temperature [27,177]. The silicon application at 10 mg/kg of soil at heading improves the osmotic potential (26%), photosynthetic rate (21%), catalase activity (38%), superoxide dismutase activity (35%), stomatal conductance (20%), and transpiration rate (32%) in wheat under high temperature [178,179].
Sowing time is a counteract strategy against high temperature. Delayed planting compels the plants to complete their growing degree days earlier, but they have to face high temperature during the anthesis and grain filling phase [53,180]. Wheat planted in normal sowing dates utilizes a longer duration to capture the available reserves/carbohydrates and improve the grain development [70,181,182].
4.2. Conventional Approaches for Thermotolerance
Thermotolerance is an inherited component stabilizing economic yield against heat stress. Tolerance to high temperature is characterized as the least effect on growth, development, and productivity. Screening of wheat genotypes is difficult in a spatial environment under natural heat stress conditions. This is due to the consistent selection criteria that have not been developed to screen diverse germplasm. The selection criteria based on traits directly associated with the grain yield facilitates better improvement in the genetic material for thermotolerance (Table 2).
Table 2.
Major desirable selection criteria for the screening heat tolerance in wheat.
| Traits | References |
|---|---|
| Cell membrane stability | [50,51,188,189] |
| Proline content | [131,190,191,192] |
| Heat susceptibility index for grain yield | [25,193,194,195,196] |
| Chlorophyll content | [76,131,188,189,197,198] |
| Photosynthesis | [48,106,107,117,199] |
| Stay green | [70,137,140,142,143,200] |
| Grain filling duration | [70,181,201] |
| Grains formation | [59,67,202,203,204] |
| Grain development | [67,71,203,204,205] |
| Early heading | [46,64,204,206,207] |
| Canopy temperature depression | [30,140,201,208,209,210,211,212] |
Breeding has made considerable advances in the genetic basis, diversity, and development of thermotolerant varieties. However, utilization and explorations of novel genetic diversity facilitates the genetic improvement for thermotolerance in the breeding program. However, the genetic gain is limited due to the narrow genetic basis [183,184]. Therefore, utilizing landraces and wild relatives increases the genetic variation in wheat for developing thermotolerance. Breeding for thermotolerance utilizing land races and wild relatives viz., Aegilops speltoides, Aegilops tauschii, Triticum turgidum, and Triticum durum have the ability to maintain chlorophyll content, canopy temperature depression, membrane stability, and photosynthesis under stress conditions [74,185,186,187].
4.3. Non-Conventional Approaches
Plants development utilizing genetic engineering or the indirect selection of traits through molecular markers or omics technology facilitates the improvement against heat stress in wheat.
4.3.1. Biotechnological Approach and Heat Shock Factors
Genetic engineering is the development of cultivar through incorporating the individual gene [213]. Advances in biotechnology enable the faster genetic gain than conventional breeding methods. Several genes encoding heat shock factors have been identified in wheat, but novel genes identification for thermotolerance remains a challenge (Table 3). The identification of novel genes and their altered expression under high temperature in wheat crop provides the molecular basis for improving thermotolerance.
Table 3.
List of genes encoding transcription factors related to thermotolerance.
| TFs/Genes | Source | Function | Reference |
|---|---|---|---|
| Hsf6A/wheat | HVA1s promoter of barley | Regulation of heat shock protein genes and improve thermotolerance | [214] |
| EF-Tu | Ubiquitin 1 promoter of maize | Overexpression reduces the thermal aggregation of leaf proteins, photosynthetic membrane, and increases CO2 fixation | [215] |
| HvSUT1 | Hordein B1 promoter of barley | Increase sucrose transport into grains | [216] |
| TaFER-5B | Ubiquitin 1 of maize | Reduces oxidative stress by scavenging ROS and improves leaf iron content | [217] |
| TaGASR1 | Wheat variety TM107 | Reduces ROS and hormonal signal transduction pathway | [218] |
| TaHsfC2a | Monocot-specific HsfC2 subclass | Thermotolerance development via the ABA-mediated regulatory pathway | [219] |
| TaHSP23.9 | Chinese wheat based on proteomic analysis | Upregulation under heat stress facilitates in seed development during the grain filling phase | [220] |
| TaFBA1 | F-box gene from wheat | Upregulation improves photosynthesis and the antioxidant enzyme activity | [221] |
| TaHsfA2-1 | Wheat | Overexpression of heat shock proteins and chlorophyll content | [222] |
| SGR | Arbidopsis and rice | Binding of light harvesting complex during photosystem-II | [142,144] |
| NYC | Arbidopsis and rice | Responsible for the activity of chlorophyll reductase to convert chl-b into chl-a | [142,145] |
| PPH | Arbidopsis and rice | Responsible for the activity pheophytinase for dephytylation to phaeophytin | [142,146] |
Quantitative Trait Loci (QTL)
Heat tolerance is under polygenic control and the QTL analysis enlightens the genetic basis of thermotolerance in wheat. It facilitates the indirect selection of traits rather than the selection based on phenotypic traits. Many QTLs have been identified for physio-morphic traits in wheat, but few were identified against heat stress (Table 4), which facilitates in gene pyramiding and marker assisted selection in wheat breeding programs for developing thermotolerance.
Table 4.
Major quantitative trait loci (QTL) identified for traits against heat stress.
| Traits | Chromosome | References |
|---|---|---|
| Chlorophyll content | 2A, 3A, 6A, 7A, 2B, 5B, 2D | [223,224] |
| Chlorophyll florescence | 1A, 2A, 3A, 3B, 2D, 1D | [224,225] |
| Plasma membrane damage | 7A, 2B | [223] |
| Thylakoid membrane damage | 6A, 7A, 1D | [223] |
| Canopy temperature | 7A, 1B, 2B, 3B | [226] |
| Grain weight | 1A, 2A, 4A, 1B, 2B, 3B, 4B, 6B, 6D | [226,227,228] |
| Grains formation | 1A, 4A, 2B, 3B, 5B | [228,229] |
| Chlorophyll florescence | 1A, 4A, 1B, 2B, 7D | [230] |
| Senescence | 2A, 3A, 6A, 7A, 3B, 6B | [231,232] |
| Stay green | 1A, 3B, 7D | [233,234] |
4.3.2. Omics Technology
Omics techniques facilitate the development of thermotolerance in wheat through the identification of transcriptional, translational, and post translational mechanisms (Figure 8). Transcriptomics represent the alteration in transcriptome factors under different environmental conditions through the DNA microarray technology [235,236]. It has already been used to study the glumes [237], grain development [238], and quality traits [239] for the identification of candidate gene expression under heat stress conditions. MicroRNAs (miRNAs) are non-coding small RNA that serve as the regulation of post-transcriptional gene expression in plants. Micromics assist in the candidate miRNA identification and their role in transcriptome homeostasis, developmental, and cellular tolerance of plants under high temperature [197].
Figure 8.
Schematic diagram representing the omics techniques associated with thermotolerance development in wheat at the molecular genetics level.
Proteomics is the analysis of candidate proteins, the expression when they translated from mRNA to functional proteins, and a further characterization of their role in the heat tolerance mechanism [240]. Proteomic analysis revealed heat shock proteins, protein synthesis, detoxification, photosynthesis, and protein quality under heat stress conditions [115,241,242,243,244,245]. Hence, the omics technology provides us with a novel opportunity for the identification of genes, their expression, and pathways linked to these genes. However, the further genetic network and their component identification will be a challenge to adapt plants in a high temperature environment.
5. Conclusions and Future Prospects
Temperature is gradually increasing and affecting crop productivity. The impact of high temperature on wheat crop has been extensively studied, but understanding the mechanism of thermotolerance remains elusive. High temperature disrupts membrane stability, declines grain filling duration, grain formation, and starch accumulation into grains. Inhibition in the physiological process has been observed due to the high temperature stress. It disturbs the photosynthetic apparatus and generates the reactive oxygen species leading towards oxidative stress. The strategy against high temperature requires systematically understanding the physiological, metabolic, and development process associated with thermotolerance. The tolerance mechanism including more accumulation of proline, glycine betaine, antioxidant enzyme activity, heat shock protein, and stay green could be a useful indicator for thermotolerance.
Crop management stabilizes the physiological process and metabolic pathways through mulches, extra irrigation, inorganic fertilizers, early sowing, exogenous application of micronutrient, osmoprotectants, and bioregulators. Integrating crop management practices with molecular genetics tools can ameliorate the adverse effects of high temperature, but need to further explore the strategies associated with high yield under heat stress [246,247,248,249,250,251,252,253,254,255,256,257,258]. The tolerance development can be achieved through a selection based on thermotolerant traits from existing germplasm and breeding utilizing land races and their wild relatives. The suitable selection criteria based on thermotolerant traits requires developing germplasm against heat stress. Recently, the canopy temperature depression at the reproductive stage, grain filling duration, heat susceptibility index for grain yield, and stay green have been established for screening germplasm against heat stress conditions. Stay green with other useful traits provide the solution of the burning problem due to the high temperature in wheat. Therefore, the contribution of the synthesis of chlorophyll turnover equation in photosynthesizing leaves for the stay green trait expression has a good future against high temperature stress.
The marker assisted breeding programs must be pooled with the transgenic approach for thermotolerance QTLs and genes. Understanding the QTLs and omics techniques pave the way to develop thermotolerance in wheat, but a further understanding of the genes network and their regulation of expression related to high temperature would be a challenge. There is a need to understand the molecular and biochemical basis of thermotolerance from the upcoming changing climate for crop improvement. Functional genomics also proved to be supportive against high temperature, but the alteration in transcriptomes and proteomes needs to be further investigated against high temperature. Noteworthy, molecular and genetic approaches facilitate crop adaptability coupled with the economic yield under high temperature, but the expression of yield potential requires the estimation of yield at the crop level. Therefore, the application of incorporating a future scenario into crop models provides model-based recommendations to improve thermotolerance in wheat.
Acknowledgments
All authors are thankful to the Higher Education Commission (HEC) Pakistan, for the financial support of this study. The authors are thankful to all the valuable reviewers who putted lot of efforts to improve this review work. Furthermore, the authors dedicate this work to the COVID-19 patients, front line doctors, paramedic staff, and all others who are directly or indirectly fighting against this disease.
Author Contributions
The research was designed by M.A. (Munir Ahmad) and M.A. (Mukhtar Ahmed); M.A. (Munir Ahmad), M.A. (Mukhtar Ahmed), A.K., and M.I.H. performed the review; A.K. analyzed the data with input from M.A. (Munir Ahmad) and M.A. (Mukhtar Ahmed); A.K., M.A. (Mukhtar Ahmed), and M.A. (Munir Ahmad) wrote the review. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Higher Education Commission (HEC) Pakistan grant number NRPU and the APC was funded through Discount Voucher: 1a55b19f0b99ca1b (1600.00 CHF) given to Mukhtar Ahmed for his review services to MDPI journals.
Data Availability Statement
The data presented in this study are available in this study as well as in the PhD thesis of Adeel Khan i.e., first author.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Knox J., Daccache A., Hess T., Haro D. Meta-analysis of climate impacts and uncertainty on crop yields in Europe. Environ. Res. Lett. 2016;11:113004. doi: 10.1088/1748-9326/11/11/113004. [DOI] [Google Scholar]
- 2.Xiao D., Bai H., Liu D.L. Impact of future climate change on wheat production: A simulated case for China’s wheat system. Sustainabilty. 2018;10:1277. doi: 10.3390/su10041277. [DOI] [Google Scholar]
- 3.IPCC Climate Change. Mitigation of Climate Change. Contribution of Working Group III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. [(accessed on 25 February 2019)];2014 Available online: https://www.ipcc.ch/site/assets/uploads/2018/02/ipcc_wg3_ar5_full.pdf.
- 4.Portner H.O., Roberts D.C., Masson-Delmotte V., Zhai P., Tignor M., Poloczanska E., Mintenbeck K., Alegría A., Nicolai M., Okem A., et al. IPCC Special Report on the Ocean and Cryosphere in a Changing Climate. Intergovernmental Panel on Climate Change (IPCC); Geneva, Switzerland: 2019. [Google Scholar]
- 5.Grace M.A., Achick T.-F.E., Bonghan B.E., Bih M.E., Ngo N.V., Ajeck M.J., Prudence G.T.B., Ntungwen F.C. An overview of the impact of climate change on pathogens, pest of crops on sustainable food biosecurity. Int. J. Ecotoxicol. Ecobiol. 2019;4:114–119. doi: 10.11648/j.ijee.20190404.15. [DOI] [Google Scholar]
- 6.Porfirio L.L., Newth D., Finnigan J.J., Cai Y. Economic shifts in agricultural production and trade due to climate change. Palgrave Commun. 2018;4:1–9. doi: 10.1057/s41599-018-0164-y. [DOI] [Google Scholar]
- 7.Skytt T., Nielsen S.N., Jonsson B.G. Global warming potential and absolute global temperature change potential from carbon dioxide and methane fluxes as indicators of regional sustainability-A case study of Jamtland, Sweden. Ecol. Ind. 2020;110:105831. doi: 10.1016/j.ecolind.2019.105831. [DOI] [Google Scholar]
- 8.Pareek A., Dhankher O.P., Foyer C.H. Mitigating the impact of climate change on plant productivity and ecosystem sustainability. J. Exp. Bot. 2020;71:451–456. doi: 10.1093/jxb/erz518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schlenker W., Roberts M.J. Nonlinear temperature effects indicate severe damages to US crop yields under climate change. Proc. Natl. Acad. Sci. USA. 2009;106:15594–15598. doi: 10.1073/pnas.0906865106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conaty W., Burke J., Mahan J., Neilsen J., Sutton B. Determining the optimum plant temperature of cotton physiology and yield to improve plant-based irrigation scheduling. Crop Sci. 2012;52:1828–1836. doi: 10.2135/cropsci2011.11.0581. [DOI] [Google Scholar]
- 11.Sawan Z.M. Cotton production and climatic factors: Studying the nature of its relationship by different statistical methods. Cogent Biol. 2017;3:1292882. doi: 10.1080/23312025.2017.1292882. [DOI] [Google Scholar]
- 12.Fromme D.D. Effect of an Upper Temperature Threshold on Heat Unit Calculations, Defoliation Timing, Lint Yield, and Fiber Quality in Cotton. J. Cotton Sci. 2014;18:108–121. [Google Scholar]
- 13.Jans Y., von Bloh W., Schaphoff S., Muller C. Global cotton production under climate change-implications for yield and water consumption. Hydrol. Earth Syst. Sci. Discuss. 2020:1–27. doi: 10.5194/hess-2019-595. [DOI] [Google Scholar]
- 14.Wu Y.C., Chang S.J., Lur H.S. Effects of field high temperature on grain yield and quality of a subtropical type japonica rice-Pon-Lai rice. Plant Prod. Sci. 2016;19:145–153. doi: 10.1080/1343943X.2015.1128091. [DOI] [Google Scholar]
- 15.Rathnayake W., De Silva R., Dayawansa N. Assessment of the suitability of temperature and relative humidity for rice cultivation in rainfed lowland paddy fields in Kurunegala district. Trop. Agric. Res. 2016;27:370–388. doi: 10.4038/tar.v27i4.8214. [DOI] [Google Scholar]
- 16.Hatfield J.L., Prueger J.H. Temperature extremes: Effect on plant growth and development. Weather Clim. Extrem. 2015;10:4–10. doi: 10.1016/j.wace.2015.08.001. [DOI] [Google Scholar]
- 17.Yang Z., Zhang Z., Zhang T., Fahad S., Cui K., Nie L., Peng S., Huang J. The effect of season-long temperature increases on rice cultivars grown in the central and southern regions of China. Front. Plant Sci. 2017;8:e01908. doi: 10.3389/fpls.2017.01908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mukamuhirwa A., Persson Hovmalm H., Bolinsson H., Ortiz R., Nyamangyoku O., Johansson E. Concurrent drought and temperature stress in rice-a possible result of the predicted climate change: Effects on yield attributes, eating characteristics, and health promoting compounds. Int. J. Environ. Res. Public Health. 2019;16:1043. doi: 10.3390/ijerph16061043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumar R.R., Pathak H., Sharma S.K., Kala Y.K., Nirjal M.K., Singh G.P., Goswami S., Rai R.D. Novel and conserved heat-responsive microRNAs in wheat (Triticum aestivum L.) Funct. Integr. Genom. 2015;15:323–348. doi: 10.1007/s10142-014-0421-0. [DOI] [PubMed] [Google Scholar]
- 20.Wiebbecke C.E., Graham M.A., Cianzio S.R., Palmer R.G. Day temperature influences the male-sterile locus ms9 in soybean. Crop Sci. 2012;52:1503–1510. doi: 10.2135/cropsci2011.08.0410. [DOI] [Google Scholar]
- 21.Prasad P.V., Boote K.J., Allen Jr L.H. Adverse high temperature effects on pollen viability, seed-set, seed yield and harvest index of grain-sorghum [Sorghum bicolor (L.) Moench] are more severe at elevated carbon dioxide due to higher tissue temperatures. Agric. Forest Meteorol. 2006;139:237–251. doi: 10.1016/j.agrformet.2006.07.003. [DOI] [Google Scholar]
- 22.Crafts-Brandner S.J., Salvucci M.E. Sensitivity of photosynthesis in a C4 plant, maize, to heat stress. Plant Physiol. 2002;129:1773–1780. doi: 10.1104/pp.002170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao C., Liu B., Piao S., Wang X., Lobell D.B., Huang Y., Huang M., Yao Y., Bassu S., Ciais P. Temperature increase reduces global yields of major crops in four independent estimates. Proc. Natl. Acad. Sci. USA. 2017;114:9326–9331. doi: 10.1073/pnas.1701762114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Day L. Proteins from land plants–potential resources for human nutrition and food security. Trends Food Sci. Tech. 2013;32:25–42. doi: 10.1016/j.tifs.2013.05.005. [DOI] [Google Scholar]
- 25.Akter N., Islam M.R. Heat stress effects and management in wheat. A review. Agron. Sustain. Dev. 2017;37:1–19. doi: 10.1007/s13593-017-0443-9. [DOI] [Google Scholar]
- 26.Hussain J., Khaliq T., Ahmad A., Akhter J., Asseng S. Wheat responses to climate change and its adaptations: A focus on arid and semi-arid environment. Int. J. Environ. Res. 2018;12:117–126. doi: 10.1007/s41742-018-0074-2. [DOI] [Google Scholar]
- 27.Nuttall J.G., Barlow K.M., Delahunty A.J., Christy B.P., O’Leary G.J. Acute high temperature response in wheat. Agron. J. 2018;110:1296–1308. doi: 10.2134/agronj2017.07.0392. [DOI] [Google Scholar]
- 28.Asseng S., Ewert F., Martre P., Rotter R.P., Lobell D., Cammarano D., Kimball B., Ottman M., Wall G., White J.W. Rising temperatures reduce global wheat production. Nat. Clim. Chang. 2015;5:143–147. doi: 10.1038/nclimate2470. [DOI] [Google Scholar]
- 29.Lobell D.B., Burke M.B., Tebaldi C., Mastrandrea M.D., Falcon W.P., Naylor R.L. Prioritizing climate change adaptation needs for food security in 2030. Science. 2008;319:607–610. doi: 10.1126/science.1152339. [DOI] [PubMed] [Google Scholar]
- 30.Pask A., Joshi A., Manes Y., Sharma I., Chatrath R., Singh G., Sohu V., Mavi G., Sakuru V., Kalappanavar I. A wheat phenotyping network to incorporate physiological traits for climate change in South Asia. Field Crops Res. 2014;168:156–167. doi: 10.1016/j.fcr.2014.07.004. [DOI] [Google Scholar]
- 31.Tack J., Barkley A., Nalley L.L. Effect of warming temperatures on US wheat yields. Proc. Natl. Acad. Sci. USA. 2015;112:6931–6936. doi: 10.1073/pnas.1415181112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mondal S., Singh R.P., Mason E.R., Huerta-Espino J., Autrique E., Joshi A.K. Grain yield adaptation and progress in breeding for early-maturing and heat-tolerant wheat lines in South Asia. Field Crops Res. 2016;192:78–85. doi: 10.1016/j.fcr.2016.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Farooq M., Bramley H., Palta J.A., Siddique K.H. Heat stress in wheat during reproductive and grain-filling phases. Crit. Rev. Plant Sci. 2011;30:491–507. doi: 10.1080/07352689.2011.615687. [DOI] [Google Scholar]
- 34.Ortiz R., Braun H.J., Crossa J., Crouch J.H., Davenport G., Dixon J., Joshi A.K. Wheat genetic resources enhancement by the International Maize and Wheat Improvement Center (CIMMYT) Genet. Resour. Crop Evol. 2008;55:1095–1140. doi: 10.1007/s10722-008-9372-4. [DOI] [Google Scholar]
- 35.Tahir M., Ali A., Nadeem M.A., Hussain A., Khalid F. Effect of different sowing dates on growth and yield of wheat (Triticum aestivum L.) varieties in District Jhang, Pakistan. Pak. J. Life Soc. Sci. 2009;7:66–69. [Google Scholar]
- 36.Zhao H., Dai T., Jiang D., Cao W. Effects of high temperature on key enzymes involved in starch and protein formation in grains of two wheat cultivars. J. Agron. Crop Sci. 2008;194:47–54. doi: 10.1111/j.1439-037X.2007.00283.x. [DOI] [Google Scholar]
- 37.Zhao H., Dai T.B., Jing Q., Jiang D., Cao W.X. Leaf senescence and grain filling affected by post-anthesis high temperatures in two different wheat cultivars. Plant Growth Regul. 2007;51:149–158. doi: 10.1007/s10725-006-9157-8. [DOI] [Google Scholar]
- 38.Spiertz J.H.J., Hamer R.J., Xu H., Martin C.P., Don C., Putten P.E.L.V. Heat stress in wheat (Triticum aestivum L.) effects on grain growth and quality traits. Eur. J. Agron. 2006;25:89–95. doi: 10.1016/j.eja.2006.04.012. [DOI] [Google Scholar]
- 39.Acevedo E., Silva P., Silva H. Wheat growth and physiology. In: Curtis B.C., editor. Bread Wheat. FAO Plant Production and Protection Series; Rome, Italy: 2002. [Google Scholar]
- 40.Stone P.J., Nicolas M.E. Wheat cultivars vary widely in their responses of grain-yield and quality to short periods of postanthesis heat-stress. Aust. J. Plant Physiol. 1994;21:887–900. [Google Scholar]
- 41.Asseng S., Foster I., Turner N.C. The impact of temperature variability on wheat yields. Glob. Chang. Biol. 2011;17:997–1012. doi: 10.1111/j.1365-2486.2010.02262.x. [DOI] [Google Scholar]
- 42.Buriro M., Oad F.C., Keerio M.I., Tunio S., Gandahi A.W., Hassan S.W.U., Oad S.M. Wheat seed germination under the influence of temperature regimes. Sarhad J. Agric. 2011;27:539–543. [Google Scholar]
- 43.Tahir I., Nakata N., Yamaguchi T., Nakano J., Ali A. Influence of high shoot and root-zone temperatures on growth of three wheat genotypes during early vegetative stages. J. Agron. Crop Sci. 2008;194:141–151. doi: 10.1111/j.1439-037X.2008.00298.x. [DOI] [Google Scholar]
- 44.Porter J.R., Gawith M. Temperatures and the growth and development of wheat: A review. Eur. J. Agron. 1999;10:23–36. doi: 10.1016/S1161-0301(98)00047-1. [DOI] [Google Scholar]
- 45.Nuttall J., O’Leary G., Panozzo J., Walker C., Barlow K., Fitzgerald G. Models of grain quality in wheat-A review. Field Crops Res. 2017;202:136–145. doi: 10.1016/j.fcr.2015.12.011. [DOI] [Google Scholar]
- 46.Narayanan S. Effects of high temperature stress and traits associated with tolerance in wheat. Open Access J. Sci. 2018;2:177–186. doi: 10.15406/oajs.2018.02.00067. [DOI] [Google Scholar]
- 47.Mukherjee A., Wang S.-Y.S., Promchote P. Examination of the climate factors that reduced wheat yield in northwest India during the 2000s. Water. 2019;11:343. doi: 10.3390/w11020343. [DOI] [Google Scholar]
- 48.Djanaguiraman M., Boyle D., Welti R., Jagadish S., Prasad P. Decreased photosynthetic rate under high temperature in wheat is due to lipid desaturation, oxidation, acylation, and damage of organelles. BMC Plant Biol. 2018;18:55–63. doi: 10.1186/s12870-018-1263-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bita C.E., Gerats T. Plant tolerance to high temperature in a changing environment scientific fundamentals and production of heat stress-tolerant crops. Front. Plant Sci. 2013;4:273–286. doi: 10.3389/fpls.2013.00273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Niu Y., Xiang Y. An overview of biomembrane functions in plant responses to high-temperature stress. Front. Plant Sci. 2018;9:915–923. doi: 10.3389/fpls.2018.00915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.ElBasyoni I., Saadalla M., Baenziger S., Bockelman H., Morsy S. Cell membrane stability and association mapping for drought and heat tolerance in a worldwide wheat collection. Sustainaibilty. 2017;9:1606. doi: 10.3390/su9091606. [DOI] [Google Scholar]
- 52.Khan S.U., Gurmani A.R., Qayyum A., Khan H. Heat tolerance evaluation of wheat (Triticum aestivum L.) genotypes based on some potential heat tolerance indicators. J. Chem. Soc. Pak. 2013;35:647–653. [Google Scholar]
- 53.Aslam M.A., Ahmed M., Stöckle C.O., Higgins S.S., Hayat R. Can growing degree days and photoperiod predict spring wheat phenology. Front. Environ. Sci. 2017;5:57–68. doi: 10.3389/fenvs.2017.00057. [DOI] [Google Scholar]
- 54.Prajapat A.L., Saxena R. Thermal requirements of wheat (Triticum aestivum L.) cultivars under different growing environments. Int. J. Chem. Stud. 2018;6:17–22. [Google Scholar]
- 55.Dubcovsky J., Loukoianov A., Fu D., Valarik M., Sanchez A., Yan L. Effect of photoperiod on the regulation of wheat vernalization genes VRN1 and VRN2. Plant Mol. Biol. 2006;60:469–480. doi: 10.1007/s11103-005-4814-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Whittal A., Kaviani M., Graf R., Humphreys G., Navabi A. Allelic variation of vernalization and photoperiod response genes in a diverse set of North American high latitude winter wheat genotypes. PLoS ONE. 2018;13:e0203068. doi: 10.1371/journal.pone.0203068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moshatati A., Siadat S., Alami-Saeid K., Bakhshandeh A., Jalal-Kamali M. The impact of terminal heat stress on yield and heat tolerance of bread wheat. Int. J. Plant Prod. 2017;11:549–560. [Google Scholar]
- 58.Cleland E.E., Chuine I., Menzel A., Mooney H.A., Schwartz M.D. Shifting plant phenology in response to global change. Trends Ecol. Evol. 2007;22:357–365. doi: 10.1016/j.tree.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 59.Raja M.M., Vijayalakshmi G., Naik M.L., Basha P.O., Sergeant K., Hausman J.F., Khan P.S.S.V. Pollen development and function under heat stress: From effects to responses. Acta Physiol. Planta. 2019;41:1–20. doi: 10.1007/s11738-019-2835-8. [DOI] [Google Scholar]
- 60.García C.C., Nepi M., Pacini E. It is a matter of timing: Asynchrony during pollen development and its consequences on pollen performance in angiosperms-a review. Protoplasma. 2017;254:57–73. doi: 10.1007/s00709-016-0950-6. [DOI] [PubMed] [Google Scholar]
- 61.Thakur P., Kumar S., Malik J.A., Berger J.D., Nayyar H. Cold stress effects on reproductive development in grain crops. Environ. Exp. Bot. 2010;67:429–443. doi: 10.1016/j.envexpbot.2009.09.004. [DOI] [Google Scholar]
- 62.Hyun Y., Richter R., Coupland G. Competence to flower: Age-controlled sensitivity to environmental cues. Plant Physiol. 2017;173:36–46. doi: 10.1104/pp.16.01523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sterbova L., Bradova J., Sedlacek T., Holasova M., Fiedlerova V., Dvoracek V., Smrckova P. Influence of technological processing of wheat grain on starch digestibility and resistant starch content. Starch Starke. 2016;68:593–602. doi: 10.1002/star.201500162. [DOI] [Google Scholar]
- 64.Balla K., Karsai I., Bónis P., Kiss T., Berki Z., Horváth Á., Mayer M., Bencze S., Veisz O. Heat stress responses in a large set of winter wheat cultivars (Triticum aestivum L.) depend on the timing and duration of stress. PLoS ONE. 2019;14:e0222639. doi: 10.1371/journal.pone.0222639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lu H., Hu Y., Wang C., Liu W., Ma G., Han Q., Ma D. Effects of high temperature and drought stress on the expression of gene encoding enzymes and the activity of key enzymes involved in starch biosynthesis in wheat grains. Front. Plant Sci. 2019;10:1414. doi: 10.3389/fpls.2019.01414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zi Y., Ding J., Song J., Humphreys G., Peng Y., Li C., Zhu X., Guo W. Grain yield, starch content and activities of key enzymes of waxy and non-waxy wheat (Triticum aestivum L.) Sci. Rep. 2018;8:1–12. doi: 10.1038/s41598-018-22587-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Khatun S., Ahmed J.U., Hossain T., Islam M.R., Mohi-Ud-Din M. Variation of wheat cultivars in their response to elevated temperature on starch and dry matter accumulation in grain. Int. J. Agron. 2016;2016:1–6. doi: 10.1155/2016/9827863. [DOI] [Google Scholar]
- 68.Thalmann M., Santelia D. Starch as a determinant of plant fitness under abiotic stress. New Phytol. 2017;214:943–951. doi: 10.1111/nph.14491. [DOI] [PubMed] [Google Scholar]
- 69.MacNeill G.J., Mehrpouyan S., Minow M.A., Patterson J.A., Tetlow I.J., Emes M.J., Raines C. Starch as a source, starch as a sink: The bifunctional role of starch in carbon allocation. J. Exp. Bot. 2017;68:4433–4453. doi: 10.1093/jxb/erx291. [DOI] [PubMed] [Google Scholar]
- 70.Schittenhelm S., Langkamp-Wedde T., Kraft M., Kottmann L., Matschiner K. Effect of two-week heat stress during grain filling on stem reserves, senescence, and grain yield of European winter wheat cultivars. J. Agron. Crop Sci. 2020;206:722–733. doi: 10.1111/jac.12410. [DOI] [Google Scholar]
- 71.Dias A.S., Bagulho A.S., Lidon F.C. Ultrastructue and biochemical traits of bread and durum wheat grains under heat stress. Brazz. J. Plant Physiol. 2008;20:323–333. doi: 10.1590/S1677-04202008000400008. [DOI] [Google Scholar]
- 72.Tetlow I.J., Emes M.J. Starch biosynthesis in the developing endosperms of grasses and cereals. Agronomy. 2017;7:81. doi: 10.3390/agronomy7040081. [DOI] [Google Scholar]
- 73.Wang X., Cai J., Jiang D., Liu F., Dai T., Cao W. Pre-anthesis high-temperature acclimation alleviates damage to the flag leaf caused by post-anthesis heat stress in wheat. J. Plant Physiol. 2011;168:585–593. doi: 10.1016/j.jplph.2010.09.016. [DOI] [PubMed] [Google Scholar]
- 74.Pradhan G.P., Prasad P.V., Fritz A.K., Kirkham M.B., Gill B.S. Effects of drought and high temperature stress on synthetic hexaploid wheat. Funct. Plant Biol. 2012;39:190–198. doi: 10.1071/FP11245. [DOI] [PubMed] [Google Scholar]
- 75.Zhang X., Wollenweber B., Jiang D., Liu F., Zhao J. Water deficits and heat shock effects on photosynthesis of a transgenic Arabidopsis thaliana constitutively expressing ABP9, a bZIP transcription factor. J. Exp. Bot. 2008;59:839–848. doi: 10.1093/jxb/erm364. [DOI] [PubMed] [Google Scholar]
- 76.Haque M.S., Kjaer K.H., Rosenqvist E., Sharma D.K., Ottosen C.O. Heat stress and recovery of photosystem II efficiency in wheat (Triticum aestivum L.) cultivars acclimated to different growth temperatures. Environ. Exp. Bot. 2014;99:1–8. doi: 10.1016/j.envexpbot.2013.10.017. [DOI] [Google Scholar]
- 77.Ohmiya A., Hirashima M., Yagi M., Tanase K., Yamamizo C. Identification of genes associated with chlorophyll accumulation in flower petals. PLoS ONE. 2014;9:e113738. doi: 10.1371/journal.pone.0113738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yang L.Y., Yang S.L., Li J.Y., Ma J.H., Pang T., Zou C.M., He B., Gong M. Effects of different growth temperatures on growth, development, and plastid pigments metabolism of tobacco (Nicotiana tabacum L.) plants. Bot. Stud. 2018;59:1–13. doi: 10.1186/s40529-018-0221-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tewari A.K., Tripathy B.C. Temperature-stress-induced impairment of chlorophyll biosynthetic reactions in cucumber and wheat. Plant Physiol. 1998;117:851–858. doi: 10.1104/pp.117.3.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Almeselmani M., Abdullah F., Hareri F., Naaesan M., Ammar M.A., ZuherKanbar O., Saud A.A. Effect of drought on different physiological characters and yield component in different varieties of Syrian durum wheat. J. Agric. Sci. 2011;3:127–134. doi: 10.5539/jas.v3n3p127. [DOI] [Google Scholar]
- 81.Balouchi H. Screening wheat parents of mapping population for heat and drought tolerance detection of wheat genetic variation. Int. J. Biol. Life Sci. 2010;6:56–66. [Google Scholar]
- 82.Nagy L., Kiss V., Brumfeld V., Osvay K., Börzsönyi Á., Magyar M., Szabó T., Dorogi M., Malkin S. Thermal effects and structural changes of photosynthetic reaction centers characterized by wide frequency band hydrophone: Effects of carotenoids and terbutryn. Photochem. Photobiol. 2015;91:1368–1375. doi: 10.1111/php.12511. [DOI] [PubMed] [Google Scholar]
- 83.Shah S.H., Houborg R., McCabe M.F. Response of chlorophyll, carotenoid and SPAD-502 measurement to salinity and nutrient stress in wheat (Triticum aestivum L.) Agronomy. 2017;7:61. doi: 10.3390/agronomy7030061. [DOI] [Google Scholar]
- 84.Ristic Z., Bukovnik U., Momcilovic I., Fu J., Prasad P.V.V. Heat-induced accumulation of chloroplast protein synthesis elongation factor, EF-Tu, in winter wheat. J. Plant Physiol. 2008;165:192–202. doi: 10.1016/j.jplph.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 85.Labuschagne M.T., Moloi J., Biljon A.V. Abiotic stress induced changes in protein quality and quantity of two bread wheat cultivars. J. Cereal Sci. 2016;69:259–263. doi: 10.1016/j.jcs.2016.03.018. [DOI] [Google Scholar]
- 86.Balla K., Rakszegi M., Li Z., Bekes F., Bencze S., Veisz O. Quality of winter wheat in relation to heat and drought shock after anthesis. Czech J. Food Sci. 2011;29:117–128. doi: 10.17221/227/2010-CJFS. [DOI] [Google Scholar]
- 87.Li Y., Wu Y., Espinosa H., Pena R.J. The influence of drought and heat stress on the expression of end-use quality parameters of common wheat. J. Cereal Sci. 2013;57:73–78. doi: 10.1016/j.jcs.2012.09.014. [DOI] [Google Scholar]
- 88.Castro M., Peterson C., Dalla Rizza M., Dellavalle P.D., Vázquez D., Ibanez V., Ross A. Wheat Production in Stressed Environments. Springer; Berlin/Heidelberg, Germany: 2007. pp. 365–371. [Google Scholar]
- 89.Xue C., Matros A., Mock H.P., Mühling K.H. Protein composition and baking quality of wheat flour as affected by split nitrogen application. Front. Plant Sci. 2019;10:642–650. doi: 10.3389/fpls.2019.00642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang Y., Hu X., Juhasz A., Islam S., Yu Z., Zhao Y., Li G., Ding W., Ma W. Characterising avenin-like proteins (ALPs) from albumin/globulin fraction of wheat grains by RP-HPLC, SDS-PAGE, and MS/MS peptides sequencing. BMC Plant Biol. 2020;20:45–55. doi: 10.1186/s12870-020-2259-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hurkman W.J., Vensel W.H., Tanaka C.K., Whitehand L., Altenbach S.B. Effect of high temperature on albumin and globulin accumulation in the endosperm proteome of the developing wheat grain. J. Cereal Sci. 2009;49:12–23. doi: 10.1016/j.jcs.2008.06.014. [DOI] [Google Scholar]
- 92.Branlard G., Lesage V.S., Bancel E., Martre P., Méleard B., Rhazi L. Advances in Wheat Genetics: From Genome to Field. Springer; Tokyo, Japan: 2015. pp. 255–264. [Google Scholar]
- 93.Xu X.L., Zhang Y.H., Wang Z.M. Effect of heat stress during grain filling on phosphoenolpyruvate carboxylase and ribulose-1,5-bisphosphate carboxylase/oxygenase activities of various green organs in winter wheat. Photosynthetica. 2003;42:317–320. doi: 10.1023/B:PHOT.0000040608.97976.a3. [DOI] [Google Scholar]
- 94.Khan A. Ph.D. Thesis. PMAS-Arid Agriculture University Rawalpindi; Rawalpindi, Pakistan: 2020. Plant Breeding and Genetics. [Google Scholar]
- 95.Mathur S., Allakhverdiev S.I., Jajoo A. Analysis of high temperature stress on the dynamics of antenna size and reducing side heterogeneity of Photosystem II in wheat leaves (Triticum aestivum L.) Biochim. Biophys. Acta. 2011;1807:22–29. doi: 10.1016/j.bbabio.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 96.Mathur S., Agrawal D., Jajoo A. Photosynthesis: Response to high temperature stress. J. Photochem. Photobio. 2014;137:116–126. doi: 10.1016/j.jphotobiol.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 97.Drop B., Webber-Birungi M., Yadav S.K., Filipowicz-Szymanska A., Fusetti F., Boekema E.J., Croce R. Light-harvesting complex II (LHCII) and its supramolecular organization in Chlamydomonas reinhardtii. Biochim. Biophys. Acta BBA Bioenergetics. 2014;1837:63–72. doi: 10.1016/j.bbabio.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 98.Zhang S., Scheller H.V. Light-harvesting complex II binds to several small subunits of photosystem I. J. Biol. Chem. 2004;279:3180–3187. doi: 10.1074/jbc.M311640200. [DOI] [PubMed] [Google Scholar]
- 99.Sage R.F., Kubien D.S. The temperature response of C3 and C4 photosynthesis. Plant Cell Environ. 2007;30:1086–1106. doi: 10.1111/j.1365-3040.2007.01682.x. [DOI] [PubMed] [Google Scholar]
- 100.Hobson P.D., Keys A.J., Parry M.A., Lea P.J. Regulation of Rubisco during drought and heat stress in wheat. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2002;132:173–180. [Google Scholar]
- 101.Yin Z., Zhang Z., Deng D., Chao M., Gao Q., Wang Y., Yang Z., Bian Y., Hao D., Xu C. Characterization of RuBisCo activase genes in maize: An α-isoform gene functions alongside a β-isoform gene. Plant Physiol. 2014;164:2096–2106. doi: 10.1104/pp.113.230854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Portis A.R., Li C., Wang D., Salvucci M.E. Regulation of Rubisco activase and its interaction with Rubisco. J. Exp. Bot. 2007;59:1597–1604. doi: 10.1093/jxb/erm240. [DOI] [PubMed] [Google Scholar]
- 103.Kumar R.R., Goswami S., Dubey K., Singh K., Singh J.P., Kumar A., Rai G.K., Singh S.D., Bakshi S., Singh B. RuBisCo activase-a catalytic chaperone involved in modulating the RuBisCo activity and heat stress-tolerance in wheat. J. Plant Biochem. Biotech. 2019;28:63–75. doi: 10.1007/s13562-018-0463-9. [DOI] [Google Scholar]
- 104.Scafaro A.P., Bautsoens N., den Boer B., Van Rie J., Gallé A. A conserved sequence from heat-adapted species improves Rubisco activase thermostability in wheat. Plant Physiol. 2019;181:43–54. doi: 10.1104/pp.19.00425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Aliyev J.A. Photosynthesis, photorespiration and productivity of wheat and soybean genotypes. Physiol. Plant. 2012;145:369–383. doi: 10.1111/j.1399-3054.2012.01613.x. [DOI] [PubMed] [Google Scholar]
- 106.Posch B.C., Kariyawasam B.C., Bramley H., Coast O., Richards R.A., Reynolds M.P., Trethowan R., Atkin O.K. Exploring high temperature responses of photosynthesis and respiration to improve heat tolerance in wheat. J. Exp. Bot. 2019;70:5051–5069. doi: 10.1093/jxb/erz257. [DOI] [PubMed] [Google Scholar]
- 107.Morales F., Ancín M., Fakhet D., González-Torralba J., Gámez A.L., Seminario A., Soba D., Ben Mariem S., Garriga M., Aranjuelo I. Photosynthetic metabolism under stressful growth conditions as a bases for crop breeding and yield improvement. Plants. 2020;9:88. doi: 10.3390/plants9010088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tabita F.R., Hanson T.E., Li H., Satagopan S., Singh J., Chan S. Function, structure, and evolution of the RubisCO-like proteins and their RubisCO homologs. Microbiol. Mol. Biol. Rev. 2007;71:576–599. doi: 10.1128/MMBR.00015-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Badger M.R., Bek E.J. Multiple Rubisco forms in proteobacteria: Their functional significance in relation to CO2 acquisition by the CBB cycle. J. Exp. Bot. 2008;59:1525–1541. doi: 10.1093/jxb/erm297. [DOI] [PubMed] [Google Scholar]
- 110.Long S.P., Ainsworth E.A., Rogers A., Ortiz D.R. Rising atmospheric carbon dioxide: Plants face the future. Annu. Rev. Plant Biol. 2004;55:591–628. doi: 10.1146/annurev.arplant.55.031903.141610. [DOI] [PubMed] [Google Scholar]
- 111.Dusenge M.E., Duarte A.G., Way D.A. Plant carbon metabolism and climate change: Elevated CO2 and temperature impacts on photosynthesis, photorespiration and respiration. New Phytol. 2019;221:32–49. doi: 10.1111/nph.15283. [DOI] [PubMed] [Google Scholar]
- 112.Walker B.J., VanLoocke A., Bernacchi C.J., Ort D.R. The costs of photorespiration to food production now and in the future. Annu. Rev. Plant Biol. 2016;67:107–129. doi: 10.1146/annurev-arplant-043015-111709. [DOI] [PubMed] [Google Scholar]
- 113.Asada K. Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol. 2006;141:391–396. doi: 10.1104/pp.106.082040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Miller G., Suzuki N., Yilmaz S.C., Mittler R. Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ. 2010;33:453–467. doi: 10.1111/j.1365-3040.2009.02041.x. [DOI] [PubMed] [Google Scholar]
- 115.Wang X., Dinler B.S., Vignjevic M., Jacobsen S., Wollenweber B. Physiological and proteome studies of responses to heat stress during grain filling in contrasting wheat cultivars. Plant Sci. 2015;230:33–50. doi: 10.1016/j.plantsci.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 116.Pospíšil P. Production of reactive oxygen species by photosystem II as a response to light and temperature stress. Front. Plant Sci. 2016;7:e01950. doi: 10.3389/fpls.2016.01950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Foyer C.H. Reactive oxygen species, oxidative signaling and the regulation of photosynthesis. Environ. Exp. Bot. 2018;154:134–142. doi: 10.1016/j.envexpbot.2018.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kumar R.R., Goswami S., Sharma S.K., Singh K., Gadpayle K.A., Singh S.D., Pathak H., Rai R.D. Differential expression of heat shock protein and alteration in osmolyte accumulation under heat stress in wheat. J. Plant Biochem. Biotechnol. 2013;22:16–26. doi: 10.1007/s13562-012-0106-5. [DOI] [Google Scholar]
- 119.Hasanuzzaman M., Nahar K., Alam M.M., Roychowdhury R., Fujita M. Physiological, biochemical and molecular mechanisms of heat stress tolerance in plants. Int. J. Mol. Sci. 2013;14:9643–9684. doi: 10.3390/ijms14059643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Allakhverdiev S.I., Kreslavski V.D., Klimov V.V., Los D.A., Carpentier R., Mohanty P. Heat stress: An overview of molecular responses in photosynthesis. Photosyn. Res. 2008;98:541–550. doi: 10.1007/s11120-008-9331-0. [DOI] [PubMed] [Google Scholar]
- 121.Park E.-J., Jeknic Z., Pino M.T., Murata N., Chen T.H.-H. Glycinebetaine accumulation is more effective in chloroplasts than in the cytosol for protecting transgenic tomato plants against abiotic stress. Plant Cell Environ. 2007;30:994–1005. doi: 10.1111/j.1365-3040.2007.01694.x. [DOI] [PubMed] [Google Scholar]
- 122.Annunziata M.G., Ciarmiello L.F., Woodrow P., Dell’Aversana E., Carillo P. Spatial and temporal profile of glycine betaine accumulation in plants under abiotic stresses. Front. Plant Sci. 2019;10:230. doi: 10.3389/fpls.2019.00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wang Y., Liu S., Zhang H., Zhao Y., Zhao H., Liu H. Glycine betaine application in grain filling wheat plants alleviates heat and high light-induced photoinhibition by enhancing the psbA transcription and stomatal conductance. Acta Physiol. Planta. 2014;36:2195–2202. doi: 10.1007/s11738-014-1596-7. [DOI] [Google Scholar]
- 124.Sukumar T., Neeraj J., Samal S., Mishra B. Salicylic acid and high temperature stress. Ann. Biol. 2015;31:18–23. [Google Scholar]
- 125.Chen T.H., Murata N. Glycinebetaine protects plants against abiotic stress: Mechanisms and biotechnological applications. Plant Cell Environ. 2011;34:1–20. doi: 10.1111/j.1365-3040.2010.02232.x. [DOI] [PubMed] [Google Scholar]
- 126.Aldesuquy H.S., Abbas M.A., Abo-Hamed S.A., Elhakem A.H., Alsokari S.S. Glycine betaine and salicylic acid induced modification in productivity of two different cultivars of wheat grown under water stress. J. Stress Physiol. Biochem. 2012;8:72–89. [Google Scholar]
- 127.Khan M.I.R., Iqbal N., Masood A., Per T.S., Khan N.A. Salicylic acid alleviates adverse effects of heat stress on photosynthesis through changes in proline production and ethylene formation. Plant Signal. Behav. 2013;8:e26374. doi: 10.4161/psb.26374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sharma A., Shahzad B., Kumar V., Kohli S.K., Sidhu G.P.S., Bali A.S., Handa N., Kapoor D., Bhardwaj R., Zheng B. Phytohormones regulate accumulation of osmolytes under abiotic stress. Biomolecules. 2019;9:285. doi: 10.3390/biom9070285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hare P., Cress W., Van Staden J. Proline synthesis and degradation: A model system for elucidating stress-related signal transduction. J. Exp. Bot. 1999;50:413–434. doi: 10.1093/jxb/50.333.413. [DOI] [Google Scholar]
- 130.Fichman Y., Gerdes S.Y., Kovács H., Szabados L., Zilberstein A., Csonka L.N. Evolution of proline biosynthesis: Enzymology, bioinformatics, genetics, and transcriptional regulation. Biol. Rev. 2015;90:1065–1099. doi: 10.1111/brv.12146. [DOI] [PubMed] [Google Scholar]
- 131.Sattar A., Sher A., Ijaz M., Ul-Allah S., Rizwan M.S., Hussain M., Jabran K., Cheema M.A. Terminal drought and heat stress alter physiological and biochemical attributes in flag leaf of bread wheat. PLoS ONE. 2020;15:e0232974. doi: 10.1371/journal.pone.0232974. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 132.Gupta N.K., Agarwal S., Agarwal V.P., Nathawat N.S., Gupta S., Singh G. Effect of short-term heat stress on growth, physiology and antioxidative defence system in wheat seedlings. Acta Physiol. Plant. 2013;35:1837–1842. doi: 10.1007/s11738-013-1221-1. [DOI] [Google Scholar]
- 133.Asthir B., Thapar R., Farooq M., Bains N.S. Exogenous application of thiourea improves the performance of late sown wheat by inducing terminal heat resistance. Int. J. Agric. Biol. 2013;15:1337–1342. [Google Scholar]
- 134.Agarwal V., Gupta N., Gupta P., Rizwan M., Singh G. Sulfhydral compounds mitigate the adverse effect of high temperature stress in contrasting wheat genotypes. Vegetos Int. J. Plant Res. 2017;30:87–91. doi: 10.5958/2229-4473.2017.00018.0. [DOI] [Google Scholar]
- 135.Lopes M.S., Reynolds M.P. Stay-green in spring wheat can be determined by spectral reflectance measurements (normalized difference vegetation index) independently from phenology. J. Exp. Bot. 2012;63:3789–3798. doi: 10.1093/jxb/ers071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Adu M.O., Sparkes D.L., Parmar A., Yawson D.O. Stay green in wheat: Comparative study of modern bread wheat and ancient wheat cultivars. ARPN J. Agric. Biol. Sci. 2011;6:16–24. [Google Scholar]
- 137.Kamal N.M., Gorafi Y.S.A., Abdelrahman M., Abdellatef E., Tsujimoto H. Stay-green trait: A prospective approach for yield potential, and drought and heat stress adaptation in globally important cereals. Int. J. Mol. Sci. 2019;20:5837. doi: 10.3390/ijms20235837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Guo M., Liu J.-H., Ma X., Luo D.-X., Gong Z.-H., Lu M.-H. The plant heat stress transcription factors (HSFs): Structure, regulation, and function in response to abiotic stresses. Front. Plant Sci. 2016;7:e00114. doi: 10.3389/fpls.2016.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gururani M.A., Venkatesh J., Tran L.S.P. Regulation of photosynthesis during abiotic stress-induced photoinhibition. Mol. Plant. 2015;8:1304–1320. doi: 10.1016/j.molp.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 140.Kumari M., Pudake R.N., Singh V.P., Joshi A.K. Association of staygreen trait with canopy temperature depression and yield traits under terminal heat stress in wheat (Triticum aestivum L.) Euphytica. 2013;190:87–97. doi: 10.1007/s10681-012-0780-3. [DOI] [Google Scholar]
- 141.Jagadish K.S., Kavi Kishor P.B., Bahuguna R.N., von Wirén N., Sreenivasulu N. Staying alive or going to die during terminal senescence-an enigma surrounding yield stability. Front. Plant Sci. 2015;6:e01070. doi: 10.3389/fpls.2015.01070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Thomas H., Ougham H. The stay-green trait. J. Exp. Bot. 2014;65:3889–3900. doi: 10.1093/jxb/eru037. [DOI] [PubMed] [Google Scholar]
- 143.Shantanu D., Nabarun R., Indrani C., Monoj S., Debojit S. Significance of stay-green to foster crop production under stress environment-a mini-review. J. Exp. Biol. Agric. Sci. 2018;6:639–651. [Google Scholar]
- 144.Sakuraba Y., Schelbert S., Park S.-Y., Han S.-H., Lee B.-D., Andrès C.B., Kessler F., Hörtensteiner S., Paek N.-C. STAY-GREEN and chlorophyll catabolic enzymes interact at light-harvesting complex II for chlorophyll detoxification during leaf senescence in Arabidopsis. Plant Cell. 2012;24:507–518. doi: 10.1105/tpc.111.089474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Horie Y., Ito H., Kusaba M., Tanaka R., Tanaka A. Participation of chlorophyll b reductase in the initial step of the degradation of light-harvesting chlorophyll a/b-protein complexes in Arabidopsis. J. Biol. Chem. 2009;284:17449–17456. doi: 10.1074/jbc.M109.008912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Morita R., Sato Y., Masuda Y., Nishimura M., Kusaba M. Defect in non-yellow coloring 3, an α/β hydrolase-fold family protein, causes a stay-green phenotype during leaf senescence in rice. Plant J. 2009;59:940–952. doi: 10.1111/j.1365-313X.2009.03919.x. [DOI] [PubMed] [Google Scholar]
- 147.Caverzan A., Casassola A., Brammer S.P. Antioxidant responses of wheat plants under stress. Genet. Mol. Biol. 2016;39:1–6. doi: 10.1590/1678-4685-GMB-2015-0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Almeselmani M., Deshmukh P., Sairam R. High temperature stress tolerance in wheat genotypes: Role of antioxidant defence enzymes. Acta Agron. Hungarica. 2009;57:1–14. doi: 10.1556/AAgr.57.2009.1.1. [DOI] [Google Scholar]
- 149.Wang C., Wen D., Sun A., Han X., Zhang J., Wang Z., Yin Y. Differential activity and expression of antioxidant enzymes and alteration in osmolyte accumulation under high temperature stress in wheat seedlings. J. Cereal Sci. 2014;60:653–659. doi: 10.1016/j.jcs.2014.05.004. [DOI] [Google Scholar]
- 150.Xu L., Han L., Huang B. Antioxidant enzyme activities and gene expression patterns in leaves of Kentucky bluegrass in response to drought and postdrought recovery. J. Am. Soc. Hortic. Sci. 2011;136:247–255. doi: 10.21273/JASHS.136.4.247. [DOI] [Google Scholar]
- 151.Awasthi R., Bhandari K., Nayyar H. Temperature stress and redox homeostasis in agricultural crops. Front. Environ. Sci. 2015;3:e00011. doi: 10.3389/fenvs.2015.00011. [DOI] [Google Scholar]
- 152.Tripathy B.C., Oelmuller R. Reactive oxygen species generation and signaling in plants. Plant Signal Behav. 2012;7:1621–1633. doi: 10.4161/psb.22455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Jing J., Guo S., Li Y., Li W. The alleviating effect of exogenous polyamines on heat stress susceptibility of different heat resistant wheat (Triticum Aestivum L.) varieties. Sci. Rep. 2020;10:1–12. doi: 10.1038/s41598-020-64468-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wu K., Xiao S., Chen Q., Wang Q., Zhang Y., Li K., Yu Y., Chen L. Changes in the activity and transcription of antioxidant enzymes in response to Al stress in black soybeans. Plant Mol. Biol. Rep. 2013;31:141–150. doi: 10.1007/s11105-012-0487-6. [DOI] [Google Scholar]
- 155.Sarfraz-Ardakani M.R., Khavari-Nejad R.A., Moradi F., Najafi F. Abscisic acid and cytokinin-induced osmotic and antioxidant regulation in two drought-tolerant and drought-sensitive cultivars of wheat during grain filling under water deficit in field conditions. Not. Sci. Biol. 2014;6:354–362. doi: 10.15835/nsb639301. [DOI] [Google Scholar]
- 156.Gill S.S., Anjum N.A., Hasanuzzaman M., Gill R., Trivedi D.K., Ahmad I., Tuteja N. Glutathione and glutathione reductase: A boon in disguise for plant abiotic stress defense operations. Plant Physiol. Biochem. 2013;70:204–212. doi: 10.1016/j.plaphy.2013.05.032. [DOI] [PubMed] [Google Scholar]
- 157.Sairam R.K., Srivastava G.C., Saxena D.C. Increased antioxidant activity under elevated temperatures: A mechanism of heat stress tolerance in wheat genotypes. Biol. Plant. 2000;43:245–251. doi: 10.1023/A:1002756311146. [DOI] [Google Scholar]
- 158.Weydert C.J., Cullen J.J. Measurement of superoxide dismutase, catalase and glutathione peroxidase in cultured cells and tissue. Nat. Protoc. 2010;5:51–66. doi: 10.1038/nprot.2009.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Grigorova B., Vaseva I., Demirevska K., Feller U. Combined drought and heat stress in wheat changes in some heat shock proteins. Biol. Planta. 2011;55:105–111. doi: 10.1007/s10535-011-0014-x. [DOI] [Google Scholar]
- 160.Wang W., Vinocur B., Shoseyov O., Altman A. Role of plant heat-shock proteins and molecular chaperones in the abiotic stress response. Trends Plant Sci. 2004;9:244–252. doi: 10.1016/j.tplants.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 161.Lin Q., Wang Y.M., Nose A., Hong H.T.K., Agarie S. Effect of high night temperature on lipid and protein compositions in tonoplasts isolated from Ananas comosus and Kalanchoe pinnata leaves. Biol. Plant. 2008;52:59–65. doi: 10.1007/s10535-008-0008-5. [DOI] [Google Scholar]
- 162.Caeiro A.S., Ramos P.C., Teixeira A.R., Ferreira R.B. The ubiquitin/proteasome pathway from Lemna minor subjected to heat shock. Biol. Plant. 2008;52:695–702. doi: 10.1007/s10535-008-0134-0. [DOI] [Google Scholar]
- 163.Nadeem M., Li J., Wang M., Shah L., Lu S., Wang X., Ma C. Unraveling field crops sensitivity to heat stress: Mechanisms, approaches, and future prospects. Agronomy. 2018;8:128. doi: 10.3390/agronomy8070128. [DOI] [Google Scholar]
- 164.Kotak S., Larkindale J., Lee U., von Koskull-Döring P., Vierling E., Scharf K.-D. Complexity of the heat stress response in plants. Plant Biol. 2007;10:310–316. doi: 10.1016/j.pbi.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 165.Hemantaranjan A., Nishant Bhanu A., Singh M., Yadav D., Patel P. Heat stress responses and thermotolerance. Adv. Plants Agric. Res. 2014;1:e00012. doi: 10.15406/apar.2014.01.00012. [DOI] [Google Scholar]
- 166.Xu Y., Zhan C., Huang B. Heat shock proteins in association with heat tolerance in grasses. Int. J. Proteom. 2011 doi: 10.1155/2011/529648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ye J., Yang X., Hu G., Liu Q., Li W., Zhang L., Song X. Genome-wide investigation of heat shock transcription factor family in wheat (Triticum aestivum L.) and possible roles in anther development. Int. J. Mol. Sci. 2020;21:608. doi: 10.3390/ijms21020608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Sumesh K.V., Sharma N.P., Ghildiyal M.C. Starch synthase activity and heat shock protein in relation to thermal tolerance of developing wheat grains. Biol. Plant. 2008;52:749–753. doi: 10.1007/s10535-008-0145-x. [DOI] [Google Scholar]
- 169.Lu Y., Zhao P., Zhang A., Ma L., Xu S., Wang X. Alternative splicing diversifies the heat response and evolutionary strategy of conserved Heat Shock Protein 90 in bread wheat (Triticum aestivum L.) Res. Square. 2019 doi: 10.21203/rs.2.17636/v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kumar A., Sharma S., Chunduri V., Kaur A., Kaur S., Malhotra N., Kumar A., Kapoor P., Kumari A., Kaur J. Genome-wide identification and cCharacterization of heat shock protein family reveals role in development and stress conditions in Triticum aestivum L. Sci. Rep. 2020;10:1–12. doi: 10.1038/s41598-020-64746-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wajid M., Khan M., Shirazi M., Summiya F., Saba M. Seed priming induced high temperature tolerance in wheat by regulating germination metabolism and physio-biochemical properties. Int. J. Agric. Biol. 2018;20:2140–2148. [Google Scholar]
- 172.Banerjee A., Roychoudhury A. Seed priming as a method to generate heat-stress tolerance in plants: A minireview. Heat Stress Toler. Plants Physiol. Mol. Genet. Perspect. 2020:23–32. doi: 10.1002/9781119432401.ch2. [DOI] [Google Scholar]
- 173.Hussain I., Ahmad R., Farooq M., Wahid A. Seed priming improves the performance of poor quality wheat seed. Int. J. Agric. Biol. 2013;15:1343–1348. [Google Scholar]
- 174.Rummana S., Amin A., Islam M., Faruk G. Effect of irrigation and mulch materials on growth and yield of wheat. Bangladesh Agron. J. 2018;21:71–76. doi: 10.3329/baj.v21i1.39362. [DOI] [Google Scholar]
- 175.Humphreys E., Gaydon D., Eberbach P. Evaluation of the effects of mulch on optimum sowing date and irrigation management of zero till wheat in central Punjab, India using APSIM. Field Crops Res. 2016;197:83–96. doi: 10.1016/j.fcr.2016.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Kajla M., Yadav V.K., Chhokar R.S., Sharma R.K. Management practices to mitigate the impact of high temperature on wheat. J. Wheat Res. 2015;7:1–12. [Google Scholar]
- 177.Tao Z., Wang D.M., Chang X.H., Wang Y.J., Yang Y.S., Zhao G.C. Effects of zinc fertilizer and short-term high temperature stress on wheat grain production and wheat flour proteins. J. Integrat. Agric. 2018;17:1979–1990. doi: 10.1016/S2095-3119(18)61911-2. [DOI] [Google Scholar]
- 178.Fahad S., Bajwa A.A., Nazir U., Anjum S.A., Farooq A., Zohaib A., Sadia S., Nasim W., Adkins S., Saud S. Crop production under drought and heat stress: Plant responses and management options. Front. Plant Sci. 2017;8:e01147. doi: 10.3389/fpls.2017.01147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Cooke J., Leishman M.R. Consistent alleviation of abiotic stress with silicon addition: A meta-analysis. Funct. Ecol. 2016;30:1340–1357. doi: 10.1111/1365-2435.12713. [DOI] [Google Scholar]
- 180.Baloch M., Baloch A., Shaikh M., Baloch M., Asad M., Depar M., Baloch G., Gandahi N., Mallano I., Baloch A. Evaluation of wheat genotypes for earliness and growth traits exposed to high temperature through delayed planting. Sindh Univ. Res. J. 2015;47:81–89. [Google Scholar]
- 181.Wu X., Tang Y., Li C., Wu C. Characterization of the rate and duration of grain filling in wheat in southwestern China. Plant Prod. Sci. 2018;21:358–369. doi: 10.1080/1343943X.2018.1518722. [DOI] [Google Scholar]
- 182.Khan A., Ahmad M., Shah M.K.N., Ahmed M. Genetic manifestation of physio-morphic and yield related traits conferring thermotolerance in wheat. Pak. J. Bot. 2020;52:1545–1552. doi: 10.30848/PJB2020-5(27). [DOI] [Google Scholar]
- 183.Driedonks N., Rieu I., Vriezen W.H. Breeding for plant heat tolerance at vegetative and reproductive stages. Plant Reprod. 2016;29:67–79. doi: 10.1007/s00497-016-0275-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Janni M., Gullì M., Maestri E., Marmiroli M., Valliyodan B., Nguyen H.T., Marmiroli N. Molecular and genetic bases of heat stress responses in crop plants and breeding for increased resilience and productivity. J. Exp. Bot. 2020;71:3780–3802. doi: 10.1093/jxb/eraa034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Gupta S., Kaur S., Sehgal S., Sharma A., Chhuneja P., Bains N.S. Genotypic variation for cellular thermotolerance in Aegilops tauschii Coss., the D genome progenitor of wheat. Euphytica. 2010;175:373–381. doi: 10.1007/s10681-010-0185-0. [DOI] [Google Scholar]
- 186.Rampino P., Gullì M., De Pascali M., De Caroli M., Marmiroli N., Perrotta C. Wild and cultivated Triticum species differ in thermotolerant habit and HSP gene expression. Plant Biosyst. Int. J. Deal. Asp. Plant Biol. 2019;153:337–343. doi: 10.1080/11263504.2018.1473304. [DOI] [Google Scholar]
- 187.Kishii M. An update of recent use of Aegilops species in wheat breeding. Front. Plant Sci. 2019;10:e00585. doi: 10.3389/fpls.2019.00585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Bala P., Sikder S. Evaluation of heat tolerance of wheat genotypes through membrane thermostability test. MAYFEB J. Agric. Sci. 2017;2:1–6. [Google Scholar]
- 189.Rehman S.U., Bilal M., Rana R.M., Tahir M.N., Shah M.K.N., Ayalew H., Yan G. Cell membrane stability and chlorophyll content variation in wheat (Triticum aestivum) genotypes under conditions of heat and drought. Crop Past. Sci. 2016;67:712–718. doi: 10.1071/CP15385. [DOI] [Google Scholar]
- 190.Ahmed M., Qadir G., Shaheen F.A., Aslam M.A. Response of proline accumulation in bread wheat (Triticum aestivum L.) under rainfed conditions. J. Agric. Meteorol. 2017;73:147–155. doi: 10.2480/agrmet.D-14-00047. [DOI] [Google Scholar]
- 191.Saeedipour S. Relationship of grain yield, ABA and proline accumulation in tolerant and sensitive wheat cultivars as affected by water stress. Proc. Natl. Acad. Sci. USA. 2013;83:311–315. doi: 10.1007/s40011-012-0147-5. [DOI] [Google Scholar]
- 192.Qaseem M.F., Qureshi R., Shaheen H. Effects of pre-anthesis drought, heat and their combination on the growth, yield and physiology of diverse wheat (Triticum aestivum L.) genotypes varying in sensitivity to heat and drought stress. Sci. Rep. 2019;9:1–12. doi: 10.1038/s41598-019-43477-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Suresh S., Bishnoi O.P., Behl R.K. Use of heat susceptibility index and heat response index as a measure of heat tolerance in wheat and triticale. Ekin J. Crop Breed. Genet. 2018;4:39–44. [Google Scholar]
- 194.Elbashir A.A.E., Gorafi Y.S.A., Tahir I.S.A., Kim J.S., Tsujimoto H. Wheat multiple synthetic derivatives: A new source for heat stress tolerance adaptive traits. Breed. Sci. 2017;67:248–256. doi: 10.1270/jsbbs.16204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Tricker P.J., ElHabti A., Schmidt J., Fleury D. The physiological and genetic basis of combined drought and heat tolerance in wheat. J. Exp. Bot. 2018;69:3195–3210. doi: 10.1093/jxb/ery081. [DOI] [PubMed] [Google Scholar]
- 196.Poudel A., Thapa D.B., Sapkota M. Cluster analysis of wheat (Triticum aestivum L.) genotypes based upon response to terminal heat stress. Int. J. App. Sci. Biotech. 2017;5:188–193. doi: 10.3126/ijasbt.v5i2.17614. [DOI] [Google Scholar]
- 197.Ni Z., Li H., Zhao Y., Peng H., Hu Z., Xin M., Sun Q. Genetic improvement of heat tolerance in wheat: Recent progress in understanding the underlying molecular mechanisms. Crop J. 2018;6:32–41. doi: 10.1016/j.cj.2017.09.005. [DOI] [Google Scholar]
- 198.Pandey G.C., Mamrutha H., Tiwari R., Sareen S., Bhatia S., Siwach P., Tiwari V., Sharma I. Physiological traits associated with heat tolerance in bread wheat (Triticum aestivum L.) Physiol. Mol. Biol. Plants. 2015;21:93–99. doi: 10.1007/s12298-014-0267-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Chovancek E., Zivcak M., Botyanszka L., Hauptvogel P., Yang X., Misheva S., Hussain S., Brestic M. Transient heat waves may affect the photosynthetic capacity of susceptible wheat genotypes due to insufficient photosystem I photoprotection. Plants. 2019;8:282. doi: 10.3390/plants8080282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Abdelrahman M., El-Sayed M., Jogaiah S., Burritt D.J., Tran L.S.P. The “STAY-GREEN” trait and phytohormone signaling networks in plants under heat stress. Plant Cell Rep. 2017;36:1009–1025. doi: 10.1007/s00299-017-2119-y. [DOI] [PubMed] [Google Scholar]
- 201.Thapa S., Jessup K.E., Pradhan G.P., Rudd J.C., Liu S., Mahan J.R., Devkota R.N., Baker J.A., Xue Q. Canopy temperature depression at grain filling correlates to winter wheat yield in the US Southern High Plains. Field Crops Res. 2018;217:11–19. doi: 10.1016/j.fcr.2017.12.005. [DOI] [Google Scholar]
- 202.Gong W., Han R., Li H., Song J., Yan H., Li G., Liu A., Cao X., Guo J., Zhai S. Agronomic traits and molecular marker identification of Wheat-Aegilops caudata addition lines. Front. Plant Sci. 2017;8:e01743. doi: 10.3389/fpls.2017.01743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Sehgal A., Sita K., Siddique K.H., Kumar R., Bhogireddy S., Varshney R.K., HanumanthaRao B., Nair R.M., Prasad P., Nayyar H. Drought or/and heat-stress effects on seed filling in food crops: Impacts on functional biochemistry, seed yields, and nutritional quality. Front. Plant Sci. 2018;9:e01705. doi: 10.3389/fpls.2018.01705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Khan A., Khaliq I., Ahmad M., Ahmed H.G.M.D., Khan A.G., Farooq M.S. Comparative performance of spring wheat (Triticum aestivum L.) through heat stress indices. Pak. J. Bot. 2018;50:481–488. [Google Scholar]
- 205.Baloch M.S., Nadim M.A., Zubair M., Awan I.U., Khan E.A., Ali S. Evaluation of wheat under normal and late sowing conditions. Pak. J. Bot. 2012;44:1727–1732. [Google Scholar]
- 206.Mondal S., Singh R.P., Crossa J., Huerta-Espino J., Sharma I., Chatrath R., Singh G.P., Sohu V.S., Mavi G.S., Sukuru V.S.P., et al. Earliness in wheat: A key to adaptation under terminal and continual high temperature stress in South Asia. Field Crops Res. 2013;151:19–26. doi: 10.1016/j.fcr.2013.06.015. [DOI] [Google Scholar]
- 207.Hossain A., Silva J.A.T.D. Phenology, growth and yield of three wheat (Triticum aestivum L.) varieties as affected by high temperature stress. Notulae Sci. Biol. 2012;4:97–109. doi: 10.15835/nsb437879. [DOI] [Google Scholar]
- 208.Deery D.M., Rebetzke G., Jimenez-Berni J.A., Bovill B., James R.A., Condon A.G., Furbank R., Chapman S., Fischer R. Evaluation of the phenotypic repeatability of canopy temperature in wheat using continuous-terrestrial and airborne measurements. Front. Plant Sci. 2019;10:875. doi: 10.3389/fpls.2019.00875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Neukam D., Ahrends H., Luig A., Manderscheid R., Kage H. Integrating wheat canopy temperatures in crop system models. Agronomy. 2016;6:7. doi: 10.3390/agronomy6010007. [DOI] [Google Scholar]
- 210.Gautam A., Prasad S.S., Jajoo A., Ambati D. Canopy temperature as a selection parameter for grain yield and its components in durum wheat under terminal heat stress in late sown conditions. Agric. Res. 2015;4:238–244. doi: 10.1007/s40003-015-0174-6. [DOI] [Google Scholar]
- 211.Mason R.E., Singh R.P. Considerations when deploying canopy temperature to select high yielding wheat breeding lines under drought and heat stress. Agronomy. 2014;4:191–201. doi: 10.3390/agronomy4020191. [DOI] [Google Scholar]
- 212.Khan A., Ahmad M., Shah M.K.N., Ahmed M. Performance of wheat genotypes for Morpho-Physiological traits using multivariate analysis under terminal heat stress. Pak. J. Bot. 2020;52:1981–1988. doi: 10.30848/PJB2020-6(30). [DOI] [Google Scholar]
- 213.Asthir B. Protective mechanisms of heat tolerance in crop plants. J. Plant Int. 2015;10:202–210. doi: 10.1080/17429145.2015.1067726. [DOI] [Google Scholar]
- 214.Xue G.-P., Drenth J., McIntyre C.L. TaHsfA6f is a transcriptional activator that regulates a suite of heat stress protection genes in wheat (Triticum aestivum L.) including previously unknown Hsf targets. J. Exp. Bot. 2015;66:1025–1039. doi: 10.1093/jxb/eru462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Fu J., Momčilović I., Clemente T.E., Nersesian N., Trick H.N., Ristic Z. Heterologous expression of a plastid EF-Tu reduces protein thermal aggregation and enhances CO2 fixation in wheat (Triticum aestivum) following heat stress. Plant Mol. Biol. 2008;68:277–288. doi: 10.1007/s11103-008-9369-6. [DOI] [PubMed] [Google Scholar]
- 216.Weichert H., Högy P., Mora-Ramirez I., Fuchs J., Eggert K., Koehler P., Weschke W., Fangmeier A., Weber H. Grain yield and quality responses of wheat expressing a barley sucrose transporter to combined climate change factors. J. Exp. Bot. 2017;68:5511–5525. doi: 10.1093/jxb/erx366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Zang X., Geng X., Wang F., Liu Z., Zhang L., Zhao Y., Tian X., Ni Z., Yao Y., Xin M. Overexpression of wheat ferritin gene TaFER-5B enhances tolerance to heat stress and other abiotic stresses associated with the ROS scavenging. BMC Plant Biol. 2017;17:14. doi: 10.1186/s12870-016-0958-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Zhang L., Geng X., Zhang H., Zhou C., Zhao A., Wang F., Zhao Y., Tian X., Hu Z., Xin M. Isolation and characterization of heat-responsive gene TaGASR1 from wheat (Triticum aestivum L.) J. Plant Biol. 2017;60:57–65. doi: 10.1007/s12374-016-0484-7. [DOI] [Google Scholar]
- 219.Hu X.J., Chen D., Lynne Mclntyre C., Fernanda Dreccer M., Zhang Z.B., Drenth J., Kalaipandian S., Chang H., Xue G.P. Heat shock factor C2a serves as a proactive mechanism for heat protection in developing grains in wheat via an ABA-mediated regulatory pathway. Plant Cell Environ. 2018;41:79–98. doi: 10.1111/pce.12957. [DOI] [PubMed] [Google Scholar]
- 220.Wang J., Gao X., Dong J., Tian X., Wang J., Palta J.A., Xu S., Fang Y., Wang Z. Over-expression of the heat-responsive wheat gene TaHSP23.9 in transgenic Arabidopsis conferred tolerance to heat and salt stress. Front. Plant Sci. 2020;11:e00243. doi: 10.3389/fpls.2020.00243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Li Q., Wang W., Wang W., Zhang G., Liu Y., Wang Y., Wang W. Wheat F-box protein gene TaFBA1 is involved in plant tolerance to heat stress. Front. Plant Sci. 2018;9:e00521. doi: 10.3389/fpls.2018.00521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Liu Z., Li G., Zhang H., Zhang Y., Zhang Y., Duan S., Sheteiwy M.S.A., Zhang H., Shao H., Guo X. TaHsfA2-1, a new gene for thermotolerance in wheat seedlings: Characterization and functional roles. J. Plant Physiol. 2020:153135. doi: 10.1016/j.jplph.2020.153135. [DOI] [PubMed] [Google Scholar]
- 223.Talukder S.K., Babar M.A., Vijayalakshmi K., Poland J., Prasad P.V.V., Bowden R., Fritz A. Mapping QTL for the traits associated with heat tolerance in wheat (Triticum aestivum L.) BMC Genet. 2014;15:1–13. doi: 10.1186/s12863-014-0097-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Bhusal N., Sharma P., Sareen S., Sarial A. Mapping QTLs for chlorophyll content and chlorophyll fluorescence in wheat under heat stress. Biol. Plant. 2018;62:721–731. doi: 10.1007/s10535-018-0811-6. [DOI] [Google Scholar]
- 225.Sharma D.K., Torp A.M., Rosenqvist E., Ottosen C.O., Andersen S.B. QTLs and potential candidate genes for heat stress tolerance identified from the mapping populations specifically segregating for Fv/Fm in wheat. Front. Plant Sci. 2017;8:e01668. doi: 10.3389/fpls.2017.01668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Pinto R.S., Reynolds M.P., Mathews K.L., McIntyre C.L., Villegas J.O., Chapman S.C. Heat and drought adaptive QTL in a wheat population designed to minimize confounding agronomic effects. Theor. Appl. Genet. 2010;121:1001–1021. doi: 10.1007/s00122-010-1351-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Shirdelmoghanloo H., Taylor J.D., Lohraseb I., Rabie H., Brien C., Timmins A., Martin P., Mather D.E., Emebiri L., Collins N.C. A QTL on the short arm of wheat (Triticum aestivum L.) chromosome 3B affects the stability of grain weight in plants exposed to a brief heat shock early in grain filling. BMC Plant Biol. 2016;16:1–15. doi: 10.1186/s12870-016-0784-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Mason R.E., Mondal S., Beecher F.W., Pacheco A., Jampala B., Ibrahim A.M., Hays D.B. QTL associated with heat susceptibility index in wheat (Triticum aestivum L.) under short-term reproductive stage heat stress. Euphytica. 2010;174:423–436. doi: 10.1007/s10681-010-0151-x. [DOI] [Google Scholar]
- 229.Shi W., Hao C., Zhang Y., Cheng J., Zhang Z., Liu J., Yi X., Cheng X., Sun D., Xu Y. A combined association mapping and linkage analysis of kernel number per spike in common wheat (Triticum aestivum L.) Front. Plant Sci. 2017;8:e01412. doi: 10.3389/fpls.2017.01412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Azam F., Chang X., Jing R. Mapping QTL for chlorophyll fluorescence kinetics parameters at seedling stage as indicators of heat tolerance in wheat. Euphytica. 2015;202:245–258. doi: 10.1007/s10681-014-1283-1. [DOI] [Google Scholar]
- 231.Vijayalakshmi K., Fritz A.K., Paulsen G.M., Bai G., Pandravada S., Gill B.S. Modeling and mapping QTL for senescence-related traits in winter wheat under high temperature. Mol. Breed. 2010;26:163–175. doi: 10.1007/s11032-009-9366-8. [DOI] [Google Scholar]
- 232.Shi S., Azam F.I., Li H., Chang X., Li B., Jing R. Mapping QTL for stay-green and agronomic traits in wheat under diverse water regimes. Euphytica. 2017;213:246–254. doi: 10.1007/s10681-017-2002-5. [DOI] [Google Scholar]
- 233.Kumar U., Joshi A.K., Kumari M., Paliwal R., Kumar S., Roder M.S. Identification of QTLs for stay green trait in wheat (Triticum aestivum L.) in the ‘Chirya 3’בSonalika’population. Euphytica. 2010;174:437–445. doi: 10.1007/s10681-010-0155-6. [DOI] [Google Scholar]
- 234.Yang D., Li M., Liu Y., Chang L., Cheng H., Chen J., Chai S. Identification of quantitative trait loci and water environmental interactions for developmental behaviors of leaf greenness in wheat. Front. Plant Sci. 2016;7:e00273. doi: 10.3389/fpls.2016.00273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Sita K., Sehgal A., HanumanthaRao B., Nair R.M., Vara Prasad P., Kumar S., Gaur P.M., Farooq M., Siddique K.H., Varshney R.K. Food legumes and rising temperatures: Effects, adaptive functional mechanisms specific to reproductive growth stage and strategies to improve heat tolerance. Front. Plant Sci. 2017;8:e01658. doi: 10.3389/fpls.2017.01658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Gonzalez-Schain N., Dreni L., Lawas L.M., Galbiati M., Colombo L., Heuer S., Jagadish K.S., Kater M.M. Genome-wide transcriptome analysis during anthesis reveals new insights into the molecular basis of heat stress responses in tolerant and sensitive rice varieties. Plant Cell Physiol. 2016;57:57–68. doi: 10.1093/pcp/pcv174. [DOI] [PubMed] [Google Scholar]
- 237.Zou H., Tzarfati R., Hubner S., Krugman T., Fahima T., Abbo S., Saranga Y., Korol A.B. Transcriptome profiling of wheat glumes in wild emmer, hulled landraces and modern cultivars. BMC Genom. 2015;16:1–21. doi: 10.1186/s12864-015-1996-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Wan Y., Poole R.L., Huttly A.K., Toscano-Underwood C., Feeney K., Welham S., Gooding M.J., Mills C., Edwards K.J., Shewry P.R. Transcriptome analysis of grain development in hexaploid wheat. BMC Genom. 2008;9:1–16. doi: 10.1186/1471-2164-9-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Singh A., Mantri S., Sharma M., Chaudhury A., Tuli R., Roy J. Genome-wide transcriptome study in wheat identified candidate genes related to processing quality, majority of them showing interaction (quality x development) and having temporal and spatial distributions. BMC Genom. 2014;15:1–26. doi: 10.1186/1471-2164-15-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Keller M., Simm S., Consortium S.I. The coupling of transcriptome and proteome adaptation during development and heat stress response of tomato pollen. BMC Genom. 2018;19:447. doi: 10.1186/s12864-018-4824-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Rollins J., Habte E., Templer S., Colby T., Schmidt J., Von Korff M. Leaf proteome alterations in the context of physiological and morphological responses to drought and heat stress in barley (Hordeum vulgare L.) J. Exp. Bot. 2013;64:3201–3212. doi: 10.1093/jxb/ert158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Liu G.-T., Ma L., Duan W., Wang B.-C., Li J.-H., Xu H.-G., Yan X.-Q., Yan B.-F., Li S.-H., Wang L.-J. Differential proteomic analysis of grapevine leaves by iTRAQ reveals responses to heat stress and subsequent recovery. BMC Plant Biol. 2014;14:110. doi: 10.1186/1471-2229-14-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Lu Y., Li R., Wang R., Wang X., Zheng W., Sun Q., Tong S., Dai S., Xu S. Comparative proteomic analysis of flag leaves reveals new insight into wheat heat adaptation. Front. Plant Sci. 2017;8:e01086. doi: 10.3389/fpls.2017.01086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Kumar R.R., Singh K., Ahuja S., Tasleem M., Singh I., Kumar S., Grover M., Mishra D., Rai G.K., Goswami S. Quantitative proteomic analysis reveals novel stress-associated active proteins (SAAPs) and pathways involved in modulating tolerance of wheat under terminal heat. Funct. Int. Genom. 2019;19:329–348. doi: 10.1007/s10142-018-0648-2. [DOI] [PubMed] [Google Scholar]
- 245.Wang X., Xu Y., Hu Z., Xu C. Genomic selection methods for crop improvement: Current status and prospects. Crop J. 2018;6:330–340. doi: 10.1016/j.cj.2018.03.001. [DOI] [Google Scholar]
- 246.Ahmed M., Stockle C.O. Quantification of Climate Variability, Adaptation and Mitigation for Agricultural Sustainability. Springer; Berlin/Heidelberg, Germany: 2016. [Google Scholar]
- 247.Zahid A., Ali S., Ahmed M., Iqbal N. Improvement of Soil Health through Residue Management and Conservation Tillage in Rice-Wheat Cropping System of Punjab, Pakistan. Agronomy. 2020;10:1844. doi: 10.3390/agronomy10121844. [DOI] [Google Scholar]
- 248.Sheirdil R.A., Hayat R., Zhang X.X., Abbasi N.A., Ali S., Ahmed M., Khattak J.Z.K., Ahmad S. Exploring potential soil bacteria for sustainable wheat (Triticum aestivum L.) Production. Sustainability. 2019;11:3361. doi: 10.3390/su11123361. [DOI] [Google Scholar]
- 249.Qadeer U., Ahmed M., Hassan F.U., Akmal M. Impact of nitrogen addition on physiological, crop total nitrogen, efficiencies and agronomic traits of the wheat crop under rainfed conditions. Sustainability. 2019;11:6486. doi: 10.3390/su11226486. [DOI] [Google Scholar]
- 250.Asseng S., Martre P., Maiorano A., Rotter R.P., O’Leary G.J., Fitzgerald G.J., Girousse C., Motzo R., Giunta F., Babar M.A., et al. Climate change impact and adaptation for wheat protein. Glob. Chang. Biol. 2019;25:155–173. doi: 10.1111/gcb.14481. [DOI] [PubMed] [Google Scholar]
- 251.Ahmed M. Introduction to Modern Climate Change. Sci. Total Environ. 2020;734:139397. doi: 10.1016/j.scitotenv.2020.139397. [DOI] [Google Scholar]
- 252.Ahmad S., Abbas G., Ahmed M., Fatima Z., Anjum M.A., Rasul G., Khan M.A., Hoogenboom G. Climate warming and management impact on the change of phenology of the rice-wheat cropping system in Punjab, Pakistan. Field Crop. Res. 2019;230:46–61. doi: 10.1016/j.fcr.2018.10.008. [DOI] [Google Scholar]
- 253.Fatima Z., Ahmed M., Hussain M., Abbas G., Ul-Allah S., Ahmad S., Ahmed N., Ali M.A., Sarwar G., Haque E.U., et al. The fingerprints of climate warming on cereal crops phenology and adaptation options. Sci. Rep. 2020;10:18013. doi: 10.1038/s41598-020-74740-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Ahmed M., Qadeer U., Fayayz-ul-Hassan, Fahad S., Naseem W., Duangpan S., Ahmad S. Abiotic stress tolerance in wheat and the role of silicon: An experimental evidence. In: Hasanuzzaman M., editor. Agronomic Crops: Volume 3: Stress Responses and Tolerance. Springer; Singapore: 2020. pp. 443–479. [DOI] [Google Scholar]
- 255.Ahmed M., Hassan F.U., Aslam M., Aslam M.A. Physiological attributes based resilience of wheat to climate change. Int. J. Agric. Biol. 2012;14:407–412. [Google Scholar]
- 256.Ahmed M., Aslam M.A., Hassan F.U., Hayat R., Ahmad S. Biochemical, physiological and agronomic response of wheat to changing climate of rainfed Pakistan. Pak. J. Bot. 2019;51:535–551. doi: 10.30848/PJB2019-2(10). [DOI] [Google Scholar]
- 257.Ahmed M., Akram M.N., Asim M., Aslam M., Hassan F.-U., Higgins S., Stöckle C.O., Hoogenboom G. Calibration and validation of APSIM-Wheat and CERES-Wheat for spring wheat under rainfed conditions: Models evaluation and application. Comput. Electron. Agric. 2016;123:384–401. doi: 10.1016/j.compag.2016.03.015. [DOI] [Google Scholar]
- 258.Ahmed K., Shabbir G., Ahmed M., Shah K.N. Phenotyping for drought resistance in bread wheat using physiological and biochemical traits. Sci. Total Environ. 2020;729:139082. doi: 10.1016/j.scitotenv.2020.139082. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available in this study as well as in the PhD thesis of Adeel Khan i.e., first author.