Abstract
Simple Summary
Although pullorum disease is endemic in many parts of the world, this avian disease responsible for high economic and commercial losses has been eliminated from organized poultry production in Europe and North America. However, it may still remain in backyards and reappear sporadically on conventional poultry farms. This study presents in detail a recurrent outbreak of pullorum disease on a quail farm. In this case report, we present how epidemiological and bacteriological investigations using molecular sequencing tools were carried out in order to identify the source of contamination. Finally, we identify high-risk sanitary practices, and propose recommendations to manage and control this poultry disease, which has become rare in European countries. Given the development of outdoor farms and the increase in self-consumption family farms, a resurgence of pullorum disease cannot be excluded in the coming years. It is essential to develop effective sanitary barriers to prevent transmission between the two coexisting populations of commercial and non-commercial poultry and to raise awareness among all those involved in the poultry industry to be able to detect any outbreak quickly.
Abstract
An outbreak of pullorum disease causing septicemia and high mortality was diagnosed in 2019 on a quail farm in western France. An initial episode had been detected in another building at the same site eight months earlier. Given the exceptional nature and the extent of the potential economic consequences of pullorum disease, epidemiological and bacteriological investigations using molecular sequencing tools were carried out. Salmonella Gallinarum and Salmonella Infantis were isolated (using the NF U 47-101 reference method) from samples taken from birds at the infected site. A resurgence of the initial episode by horizontal transmission of S. Gallinarum is the most likely hypothesis, supported by whole-genome sequencing (WGS) of the strains isolated during the two episodes. Risk health practices have been identified, including the rearing of animals of different ages and species on the same site. Recurrence is explained by the probable persistence of reservoirs of the pathogen on the site (manure, lesser mealworm beetles). The article also highlights the importance of decontamination measures, including pest control, as a key element in the success of the disease control protocol.
Keywords: epidemiological investigation, Salmonella, pullorum disease, quail, whole genome sequencing
1. Introduction
Pullorum disease is a septicemic bacterial disease caused by Salmonella enterica subsp. enterica serovar Gallinarum biovar Pullorum affecting different avian species [1]. This disease is distinct from fowl typhoid, a septicemic disease of adult poultry caused by Salmonella Gallinarum biovar Gallinarum (Salmonella Gallinarum). Chickens and turkeys are particularly affected, but other species such as quails, ducks, pheasants, partridges, and guinea fowls are not exempt [2,3]. In its acute form, Pullorum disease is almost exclusively a septicemic disease of young chickens causing serious general damage associated with mortality of 50% to up to 100%. However, the organism may also be associated with disease in other avian production and may be carried subclinically or lead to reduced egg production and hatchability in older birds. Game birds and backyard poultry flocks may act as reservoirs of infection and are important in the epidemiology of the disease [1]. Vertical transmission via egg contamination is the major route of transmission but horizontal transmission is also possible [1,2,3].
Although pullorum disease does not have a significant public health impact, it is especially important in animal health and for economic reasons including trade restrictions [2]. Pullorum disease is endemic in many parts of the world (Middle East, Africa, Asia, Central, and South America), where it still causes considerable economic losses [4,5]. The disease has been eliminated from organized poultry production in Europe and North America [5,6] and many countries are reported to be free [6]. Few cases have been identified in Europe over the last decade, notably in Hungary, Sweden, Greece, Netherlands, Serbia, and UK and more recently in Russia and Denmark.
The implementation of a drastic eradication policy, carried out in the 1970s, led to the elimination of pullorum disease in France. However, it may still remain in backyards and reappear sporadically on conventional poultry farms. The last outbreaks observed in France occurred in 1984 and 1985 in laying hens, 2003 and 2004 in guinea fowl, and more recently in 2011 in Gallus gallus. Three outbreaks of pullorum disease (S. Gallinarum) were identified in different production sectors (broilers, laying hens, and breeders) at that time in northwestern France [7]. The origin of the infection could not be formally identified but an epidemiological link between the farms could be shown, confirmed by the molecular profiles of S. Gallinarum strains isolated using the pulsed-field gel electrophoresis (PFGE) method.
In July 2019, a new outbreak was reported on a broiler quail farm in western France. The farm includes four separate rearing sites, with different poultry productions (breeding, broiler and laying quails, chicken broilers, ornamental poultry, pheasants, and partridges). The farmer has his own quail hatchery and slaughterhouse for personal production of chickens and quails. Most of the production is marketed via short circuits, on the farm and at local markets, with the exception of game production, which is sold for export by a production company.
This case occurred following a previous case that had been reported eight months earlier (2018) in another building on the same farm. As this farm was located in the heart of a major free-range poultry production area, intensive epidemiological investigations were decided in close collaboration with the veterinarian, the veterinary officer, and the farmer. The objectives were to prevent the risk of spread to neighboring farms, and to propose effective sanitation measures.
Considering the low number of available epidemiological descriptions regarding pullorum disease, this case report study documents the identification, testing, and epidemiological investigations that were carried out. To our knowledge, this is the first case report using whole-genome sequencing, allowing a better discrimination of the strains, while the latest publications were supported by pulsed-field gel electrophoresis (PFGE) methods [7,8,9]. We also identify high-risk sanitary practices, and propose recommendations to manage and control this poultry disease, which has become rare in European countries.
2. Materials and Methods
2.1. Case Description
The case occurred on one of the four sites, equipped with two buildings containing exclusively broiler quails. Both buildings are separated into two rooms, each with a capacity of 12,000 animals of the same age. Only one of the two buildings was affected. Mortality started in one of the rooms in 8-day-old animals (cumulative mortality of 16% over 2 weeks). The animals in the other room were slightly older (15 days) and were also affected, but later and to a lesser extent.
At necropsy, septicemic lesions were found by the veterinarian and organs were removed for bacteriological analysis. Blood samples were also taken for serological analysis. Antimicrobial treatment (trimethoprim-sulfadiazine) was immediately started. At the same time, a declaration of suspicion was communicated to the veterinary authorities and commercial restrictions were imposed.
An initial outbreak of Salmonella pullorum had been discovered at the same site 8 months earlier (November 2018), but in the second building.
2.2. Epidemiological Investigations
The farm was the target of a collaborative in-depth epidemiological investigation using a questionnaire covering outbreak description, husbandry practices, biosecurity, animal movements, and possible farm-to-farm contacts. The background of the first Salmonella pullorum infection on the farm was documented.
About 10 poultry farms and a few backyards were identified within 3 km and were clinically monitored by their health veterinarian as specified in French regulations [10]. As part of this process, a close monitoring combining clinical surveillance and samples for bacteriological research of two free-range farms (one laying hen farm and one broiler farm) and two backyards located in the immediate vicinity of the infected farm was also carried out, as epidemiological links by geographical proximity could not be ruled out.
Close monitoring of the cleaning and disinfection operations following the depopulation, and a check on their effectiveness were carried out before restarting the farming activity at the site.
2.3. Sampling Protocol
Samples were taken by the veterinarian and the competent veterinary authorities at the three key stages of monitoring: (i) during clinical suspicion in the affected animals, (ii) as part of the epidemiological investigation, and (iii) during the cleaning and disinfection operations. Sampling was carried out at different places of interest: at the infected site to confirm the clinical diagnosis, at other farm sites to monitor the health status of other animals kept on the farm (in particular breeding quails and game), and on epidemiologically linked farms to check for possible spread to other production facilities. Several types of samples were sent to the laboratory for analysis: organs from dead animals (hearts, livers, caeca) for bacteriological testing, as well as blood for specific antibody detection by rapid slide agglutination (RSA), environmental swabs, and lesser mealworm (Alphitobius diaperinus) beetles. A summary of samples taken is presented in Table 1.
Table 1.
Summary of samples taken and serological and bacteriological results obtained.
| Location | Type of Samples | Results |
|---|---|---|
| Suspected site - on clinically suspect quail |
Autopsies Bacteriology (heart, liver, caeca, etc.) Serology (60 blood samples/building) |
Septicaemia Salmonella Infantis Salmonella Gallinarum 55 RSA neg, 5 RSA pos |
| Other poultry present on the farm - breeding quails |
Autopsies Serology (2 × 60 blood samples taken 10 days apart) |
RSA neg |
| Poultry farms in the vicinity - backyards - laying hen farm - broiler chicken farm |
Serology (4 to 60 blood samples/building) | RSA neg |
| At the infected site after cleaning and disinfection operations | swabs lesser mealworm beetles |
Salmonella Infantis Salmonella Infantis |
2.4. Bacteriological Analysis
In this national study, dead animals and organ samples were analyzed by the national reference laboratory for Salmonella, according to the French Standard NF U 47-101 [11] (Animal health analysis methods—Isolation and identification of any Salmonella serotypes or of specified Salmonella serotypes among birds). As recommended by the reference method used in France (NF U 47-101), organs (liver, heart, spleen, and cecum samples) from the quails were diluted, homogenized in 1:10 with Buffered Peptone Water (Biomérieux, Marcy-l’Etoile, France) and incubated for 16 h to 20 h at 37 °C. In parallel, a direct isolation on rich agar medium of the organs presenting the lesions is carried out. Enrichment was done with three media (Muller Kauffmann tetrathionate broth (MKTTn, Biokar, Allonne, France), selenite cystine broth (SC, Oxoid, Dardilly, France), and modified semi-solid Rappaport-Vassiliadis (MSRV, Biokar, Allonne, France)). MKTTn and MSRV were incubated for 24 h at 41.5 °C, whereas SC broth was incubated at 37 °C for 24 h. After enrichment MKTTn suspension were plate on xylose lysine tergitol 4 (XLT4, Biokar, Allonne, France) and MSRV and SC suspension were plate on RAPID’Salmonella (R’S, BioRad, Marnes-La-Coquette, France) and xylose lysine desoxycholate (XLD, Biokar, Allonne, France). The agar plates were incubated for 24 to 48 h at 37 °C. Characteristic colonies were biochemically tested for glucose fermentation, lactose oxidation, hydrogen sulfide, and gas production on Kligler Hajna medium (KH, Biokar, Allonne, France) incubated for 24 h at 37 °C. All Salmonella isolates were confirmed by serotyping according to the Kauffmann–White scheme using slide agglutination test [12].
The detection of Salmonella-specific antibodies was carried out by official laboratories according to the French Standard NF U 47-034 [13] (Animal health analysis methods—Detection of antibodies specific for Salmonella Pullorum Gallinarum in the serum by rapid slide agglutination). This method consisted of a rapid slide agglutination (RSA) test, based on a reaction between half antigens (from standard (O: 1, 9, 121, and 123) and variant (O: 1, 9, 121, and 122)) and half serum (25 µL). The observation of an agglutination led to a positive result.
2.5. Molecular Analysis
Genomic DNA of Salmonella strains was extracted from one-day single-colony cultures using a QIAamp DNA Mini Kit (QIAGEN, Marseille, France), and quantified using a Qubit 2.0 fluorometer and the Qubit dsDNA (double-stranded DNA) HS (high-sensitivity) assay kit (Thermo Fisher Scientific, Saint Herblain, France). All S. Gallinarum strains were sequenced using Illumina technology by the Paris Brain Institute (Institut du Cerveau et de la Moelle épinière, ICM, Pitié-Salpêtrière Hospital, Paris; www.icm-institute.org). Libraries were prepared using the Nextera XT DNA Library Preparation Kit and Nextera XT Index Kit (96 indexes) (Illumina). Samples were then sequenced with a NextSeq 500 machine using the NextSeq 500 Mid Output Kit v2 (300 cycles) (Illumina, Evry, France). Paired-end raw reads were deposited on the public EnteroBase database platform for Salmonella (http://enterobase.warwick.ac.uk/), and were automatically de novo assembled using SPAdes [14] once the sequences had been uploaded. Further details on assembly pipelines are available on the EnteroBase website (https://enterobase.readthedocs.io/en/latest/pipelines/enterobase-pipelines.html). The genomic comparison of strains was carried out using the cgMLST scheme available on EnteroBase including 3002 genes [15]. Similarly to 7-gene mutlilocus sequence typing (MLST), a specific sequence type (ST) is attributed to each unique allelic combination, based on 3002 loci, hereafter defined as cgSTs. Similar but non-identical strains (strains showing different cgSTs) were identified in EnteroBase by using the hierarchical clustering method (HierCC) that allows clustering of strains which can differ up to a specified and fixed number of cgMLST alleles [15].
Due to the rarity of the serovar Gallinarum in France, 28 strain assemblies from international European countries were included in the comparison to assess the genetic similarity of the strains (Table S1). A neighbor-joining tree was created in EnteroBase using GrapeTree [16] and the RapidNJ algorithm [17]. Assemblies are publicly available from EnteroBase (S18LNRS01-09: enterobase barcode SAL_ZA0109AA; S19LNRS08-01: enterobase barcode SAL_ZA0111AA).
3. Results
3.1. Epidemiological Investigations and Follow-Up of Cleaning and Disinfection Effectiveness
The simultaneous presence on the same farm of many poultry species and several ages on the same site (multi-age and multi-species farming) have been identified as major risk factors for the maintenance of infection on a farm. Under these conditions, crossing circuits of animals, equipment, or personnel is unavoidable.
The epidemiological investigation also provided information on the background to the first outbreak, including the management of the cleaning and disinfection operations following the slaughter of the birds. Assessment of these operations proved favorable and the site was authorized to resume activity at the beginning of 2019. The manure from this first outbreak had been limed, removed, and stored in piles near the farm premises for several months. The farmer did not observe any combustion of this manure pile. In early spring 2019, it was then picked up by another farmer for spreading on fields.
Concerning the investigation of epidemiological links, as quail production is conducted in self-sufficiency, forward and backward tracing showed limited contacts. None were considered relevant to investigate during this period. Investigation of neighborhood links and geographically nearby farms did not make it possible to detect any clinical cases. All the bacteriological and serological analyses carried out during the investigations on the farms geographically linked to the outbreak were negative.
As soon as the infected flock was eliminated, the site was immediately subjected to a deep cleaning and disinfection protocol. An assessment of the effectiveness of the cleaning and disinfection was carried out at the end of the process. During the visit, the premises were considered visually clean. However, the persistence of lesser mealworm beetles, in particular in the sanitary airlock where staff change clothes, wash, and disinfect their hands, was noted. The farmer reported that he was particularly concerned about the recurrence of these beetles at his premises.
3.2. Bacteriological Results
In 2018, the isolated strain of S. Gallinarum (S18LNRS01-09) sent to the French National Reference Laboratory (NRL) for Salmonella was confirmed by serotyping analysis (formula: 1,9,12:-:-). The diagnosis of pullorum disease was based on the isolation of Salmonella Gallinarum (Salmonella enterica subsp. enterica serovar Gallinarum) from samples taken from dead animals at the suspected site. In 2019, the NRL for Salmonella established the quail’s infection, by S. Gallinarum and Salmonella Infantis, by the detection of the strains in the heart and liver (strain S19LNRS08-01). Salmonella Infantis, a Salmonella that had remained on the farm for several years and was previously detected especially during the previous case, was also identified.
The detection of specific antibodies by rapid slide agglutination (RSA) revealed five positive sera (5/60) in the birds from the affected building.
All bacteriological and serological tests carried out in animals from other poultry productions on the farm were negative. Environmental samples taken at the infected site were positive for S. Infantis, but all were negative for S. Gallinarum.
The environmental swabs taken after the cleaning and disinfection of the premises revealed the presence of S. Infantis on the floor and walls. Salmonella Infantis was also found on lesser mealworm beetles still present in the room. Salmonella Gallinarum was not found. A summary of the analytical results is presented in Table 1.
3.3. Whole-Genome Sequencing Results
Fifty-nine and fifty-eight contigs were obtained for the S. Gallinarum sequenced genomes from 2018 and 2019, respectively. The total assembled sequence length was 4,781,993 bp for strain S18LNRS01-09 and 4,776,421 bp for strain S19LNRS08-01. To compare the genome of the two strains of S. Gallinarum, the core-genome MLST approach was used (cgMLST scheme available on the EnteroBase website). The S18LNRS01-09 and S19LNRS08-01 strains belonged to cgST199405 and cgST199407, respectively (Table 2).
Table 2.
Core genome MLST profile of Salmonella Gallinarum strains.
| cgMLST | ||||||||
|---|---|---|---|---|---|---|---|---|
| Strain | Serotype | Year | Sector | HC0 | HC2 | HC5 | HC10 | HC20 |
| S18LNRS01-09 | Gallinarum | 2018 | Quails | 199405 | 199405 | 199405 | 199405 | 199405 |
| S19LNRS08-01 | Gallinarum | 2019 | Quails | 199407 | 199407 | 199407 | 199405 | 199405 |
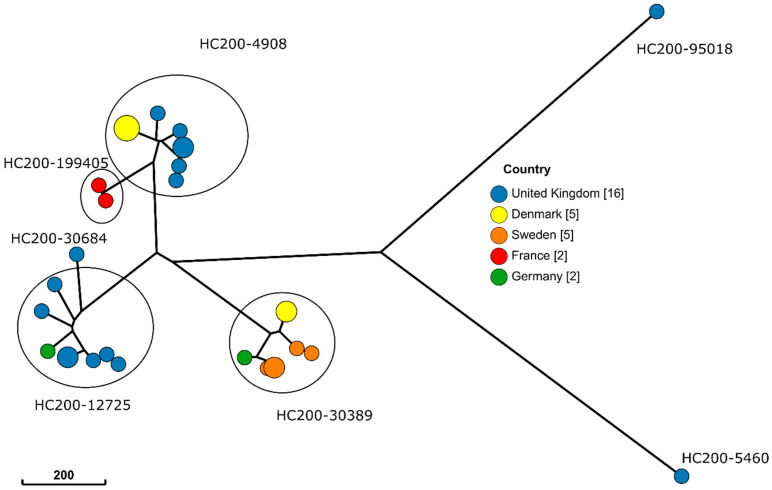
Hierarchical clustering of cgMLST (HierCC) defines clusters based on cgMLST. The distances between genomes were calculated using the number of shared cgMLST alleles. Both strains have a different core-genome sequence type but they are grouped in the same hierarchical cluster (HC) using 10 different cgMLST allele distances. The comparison of their allelic profiles on 2989 loci highlighted six differences: allelic variations on five loci between strains, and one locus was missing or truncated in one strain while present in the second strain. Therefore, these strains seem to be phylogenetically closely related and may be considered (according to the European Union Reference Laboratory (EURL) for Salmonella recommendation) to have a common source of contamination. These results were emphasized by the gene-by-gene comparison of strains from France with all the international European S. Gallinarum strains available from the EnteroBase data collection. Importantly, while strains from France harbored five allelic variations between each other, they showed significant differences with international European strains (>200 allelic variations) (Figure 1).
Figure 1.
Neighbor-joining tree (NJTree) of European Salmonella Gallinarum strains, available from the EnteroBase data collection, compared using the EnteroBase cgMLST scheme.
Strains are colored on the NJtree according to their country. Clusters generated using the hierarchical clustering method from EnteroBase and using a 200 cgMLST allele distance (HC200) are represented by circles. S. Gallinarum strains from France sequenced in this study showed five allelic differences between them, while more than 200 differences were observed between French and other European strains.
4. Discussion
The epidemiological investigation did not enable us to identify retrospectively the origin of the contamination that occurred in November 2018. A major issue was to determine whether this second outbreak was a new introduction or a resurgence of the disease. Resurgence is defined as the reappearance of the disease on a previously infected and subsequently sanitized farm, without further introduction of the pathogen [18]. Several epidemiological findings support this hypothesis.
The first relates to the maintenance at the site of manure previously contaminated during the first outbreak, which may have been a source of persistence of infection. Despite lime application, the farmer did not see any reduction in the volume of the pile or any real combustion of the manure. The resistance of Salmonella Gallinarum biovar Pullorum is similar to other Salmonella bacteria. Incineration or composting ensuring a rise in temperature to 65 °C in the center (55 °C on the surface) for several days is required to obtain their destruction and secure the effluents [19,20,21,22]. Probably insufficiently humidified, the manure would not have risen sufficiently in temperature to allow its sanitization. As a result, run-off during the winter period as well as the removal operation of the contaminated manure pile in early spring could have been the cause of spread of Salmonella still present.
The presence of a large number of lesser mealworm beetles was detected on this farm. The observation of these beetles inside the premises and in the airlock after the desensitization, rodent removal, cleaning, and disinfection operations alerted the veterinarian. The hypothesis of a possible persistence of infection via the beetles was reinforced by the results of environmental samples taken after cleaning and disinfection on which S. Infantis was detected in areas usually easy to clean (walls and floor of the room). The collected beetles also tested positive for S. Infantis. Lesser mealworm beetles are known to be reservoirs and mechanical vectors for many pathogens, including zoonotic agents such as Salmonella [23]. Under experimental conditions, Salmonella can be detected in their larvae for up to 7 days from a contaminated substrate [24]. In such cases, transmission of the infection by ingestion is the most likely pathway. Other passive vectors are known to transmit S. Pullorum, including red mites [1] and rodents. For example, Anderson et al. [8] reported the presence of an S. Pullorum strain in the intestine of a rat trapped in an outbreak, which was very similar to the strain identified in poultry. In our case, although S. Gallinarum could not be isolated directly on the lesser mealworm beetles, the detection of S. Infantis is a good indicator of the maintenance of Salmonella contamination in this facility.
Finally, whole-genome sequencing allows better discrimination than pulsed-field gel electrophoresis (PFGE) usually used as the gold standard method for Salmonella investigation. In this study, WGS results provided strong evidence for the epidemiological link between the strains in the two episodes (a single cluster at HC10) and confirmed the recurrence hypothesis of the epidemiological investigation. These tools allow for a more comprehensive investigation to identify the strains linked to each other, and thus confirm or refute the existence of an epidemiological link between outbreaks. No epidemiological link could be demonstrated with other European strains of S. Gallinarum (Pullorum) sequenced in EnteroBase, supporting the hypothesis of a resurgence in the quails farm.
Comprehensive investigations were carried out in the breeding quails reared at another site. Transmission of pullorum disease can result from vertical or horizontal contamination, but vertical transmission through eggs due to contamination of the ova is the most common mode of transmission [2], or indirectly through contact from chick to chick in the hatchery. Direct or indirect horizontal transmission appears to be epidemiologically less common, but it is also described in particular between epidemiologically linked farms, via litter, water or feed, animal movements [9], and rodents [8]. In our present case, all the samples taken from the breeders were negative, focusing on horizontal contamination, as manure and lesser mealworm beetles may have acted as a reservoir and ensured the maintenance and spread of the bacteria at the site.
Evidence from countries where the disease remains enzootic shows that multi-age livestock production is an important factor for the persistence and spread of the disease. Non-commercial farms are often cited as potential reservoirs of the disease [8,25]. Since these farms are poorly monitored from a health point of view, they may represent a greater risk because Salmonella often circulates there subclinically, without any signs being detected in the birds. According to several authors, many cases are likely to occur in backyard flocks and disease occurrences are under-reported in official data. Therefore, the prevalence of pullorum disease is probably underestimated [6,9]. It is thus important to detect and eliminate the disease from backyard premises in order to prevent its introduction on commercial farms.
In this context, backyards identified near the affected site were monitored with clinical and sampling visits in order to check their status and rule out their potential role as a reservoir of the disease.
S. Gallinarum was not isolated from any of the environmental samples. In contrast to classical (motile and H2S+) Salmonella, which can be detected in the environment using swabs, dust, or droppings samples, it is really difficult to detect S. Gallinarum in this way [26]. The method of selenite-cystine enrichment at 37 °C, which can be used to detect S. Gallinarum, is not very sensitive and not particularly selective for environmental samples. However, the identification of S. Infantis on a recurrent basis for several years and during the control carried out following cleaning and disinfection is a good indicator of the possible maintenance of Salmonella-like pathogens on the farm.
5. Conclusions
The detection of an outbreak of Salmonella disease on a commercial farm is an event that has now become exceptional in France. Cases observed are rare and not recent. However, the development of outdoor farms and the increase in self-consumption family farms are likely to lead to a recrudescence of this disease, as well as other diseases that are currently considered historical. Therefore, it is essential to raise awareness among all those involved in the poultry industry (farmers, health and technical staff, veterinarians, and diagnostic laboratories) to be able to detect any outbreak quickly.
The co-existence of many poultry species and multi-ages on the same farm have been identified as major risk practices for the persistence of infection on the farm. The management of cleaning and disinfection operations is a key element of the control measures. In our case, the implementation of an additional decontamination step and a reinforced pest control protocol were necessary to eradicate the disease.
Acknowledgments
The authors are grateful to the farmer and the regional competent veterinary authorities who supported us in our investigations. This work benefited from equipment and services from the iGenSeq core facility, at the ICM.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-2615/11/1/29/s1. Table S1 List of the 30 Salmonella enterica subsp. enterica Gallinarum strains available from EnteroBase and included in the genomic comparison to assess genetic similarity between French isolates.
Author Contributions
Conceptualization, S.L.B., R.S., and L.B.; Methodology, S.L.B., R.S., L.B., A.T., and M.C.; Validation S.L.B., R.S., L.B., A.T., and M.C.; Investigation, S.L.B., R.S., T.L., L.B., and M.C.; Formal analysis L.B., A.T., and S.R.; Resources, all co-authors; Writing—original draft preparation, S.L.B., L.B., and A.T.; Writing—review and editing, all co-authors; Supervision and project administration, S.L.B., M.C., and F.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This paper presents non-experimental clinical veterinary studies. Animals on farms were treated in accordance with the European and French regulation on farmed animal protection. Birds killed on the farm were killed in accordance with Regulation EC/1099/2009 and Directive 2010/63/EU.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.OIE . Manual of Diagnostic Tests and Vaccines for Terrestrial Animals. Volume 8. OIE; Paris, France: 2018. Fowl_Typhoid And Pullorum Disease; pp. 914–930. [Google Scholar]
- 2.Shivaprasad H. Fowl typhoid and pullorum disease. Rev. Sci. Tech. Off. Int. Epiz. 2000;19:405–424. doi: 10.20506/rst.19.2.1222. [DOI] [PubMed] [Google Scholar]
- 3.Shivaprasad H.L., Barrow P.A. Pullorum disease and fowl typhoid. In: Swayne D.E., Glisson J.R., McDougald L.R., Nolan L.K., Suarez D.L., Nair V., editors. Diseases of Poultry. Wiley; Hoboken, NJ, USA: 2016. pp. 678–693. [Google Scholar]
- 4.Tadesse S., Ashenafi H., Aschalew Z. Seroprevalence study of Newcastle disease in local chickens in central Ethiopia. Int. J. Appl. Res. Vet. Med. 2005;3:25–29. [Google Scholar]
- 5.Teferi M., Nejash A. Epidemiology and Economic Importance of Pullorum Disease in Poultry: A Review. Glob. Vet. 2016;17:228–237. [Google Scholar]
- 6.Barrow P.A., Freitas N.O.C. Pullorum disease and fowl typhoid—New thoughts on old diseases: A review. Avian Pathol. 2011;40:1–13. doi: 10.1080/03079457.2010.542575. [DOI] [PubMed] [Google Scholar]
- 7.El Hassimiou D.M., Le Bouquin S., Vignaud M.L., Bonin E., Sadonès H., Michel V., Granier S., Moury F., Brisabois A. Investigations épidémiologiques et microbiologiques de récents foyers de typhose et de pullorose chez les volailles en France. Bull. Épidémiol. Santé Anim. Aliment. 2012;48:10–13. [Google Scholar]
- 8.Anderson L.A., Miller D.A., Trampel D.W. Epidemiological investigation, cleanup, and eradication of pullorum disease in adult chickens and ducks in two small-farm flocks. Avian. Dis. 2006;50:142–147. doi: 10.1637/7397-062105R.1. [DOI] [PubMed] [Google Scholar]
- 9.Eriksson H., Soderlund R., Ernholm L., Melin L., Jansson D.S. Diagnostics, epidemiological observations and genomic subtyping in an outbreak of pullorum disease in non-commercial chickens. Vet. Microbiol. 2018;217:47–52. doi: 10.1016/j.vetmic.2018.02.025. [DOI] [PubMed] [Google Scholar]
- 10.Ministerial Decree Dated 29 March 2011, fixant les mesures techniques et administratives relatives à la lutte contre la pullorose, NOR AGRG1108929A. [(accessed on 30 March 2011)]; Available online: https://www.legifrance.gouv.fr/eli/arrete/2011/3/29/AGRG1108929A/jo/texte.
- 11.NF U47-101, Méthodes D’analyse en Santé Animale—Isolement et identification de tout sérovar ou de sérovar(s) spécifié(s) de salmonelles chez les oiseaux. [(accessed on 1 November 2007)]; Available online: https://www.boutique.afnor.org/norme/nf-u47-102/methodes-d-analyse-en-sante-animale-isolement-et-identification-de-tout-serovar-ou-de-serovars-specifies-de-salmonelles-chez-les/article/779142/fa155328.
- 12.Grimont P.A., Weill F.X. Antigenic Formulae of the Salmonella Serovars. 9th ed. WHO Collaborating Center for Reference and Research on Salmonella; Paris, France: 2007. [Google Scholar]
- 13.NF U47-034, Méthodes D’analyse en Santé Animale—Recherche d’anticorps spécifiques de Salmonella Pullorum Gallinarum dans le sérum par agglutination rapide sur lame. [(accessed on 1 February 2009)]; Available online: https://www.boutique.afnor.org/norme/nf-u47-034/methodes-d-analyse-en-sante-animale-recherche-d-anticorps-specifiques-de-salmonella-pullorum-gallinarum-dans-le-serum-par-aggl/article/703819/fa159217.
- 14.Bankevich A., Nurk S., Antipov D., Gurevich A.A., Dvorkin M., Kulikov A.S., Lesin V.M., Nikolenko S.I., Pham S., Prjibelski A.D., et al. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012;19:455–477. doi: 10.1089/cmb.2012.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alikhan N.F., Zhou Z., Sergeant M.J., Achtman M.A. Genomic overview of the population structure of Salmonella. PLoS Genet. 2018;14:e1007261. doi: 10.1371/journal.pgen.1007261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou Z., Alikhan N.-F., Sergeant M.J., Luhmann N., Vaz C., Francisco A.P., Carriço J.A., Achtman M. GrapeTree: Visualization of core genomic relationships among 100,000 bacterial pathogens. Genome Res. 2018;9:1395–1404. doi: 10.1101/gr.232397.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simonsen M., Mailund T., Pedersen C.N.S. Rapid Neighbour-Joining; Proceedings of the Algorithms in Bioinformatics, 8th International Workshop; Berlin/Heidelberg, Germany. 14–19 September 2008; Berlin/Heidelberg, Germany: Springer; 2008. pp. 113–122. [Google Scholar]
- 18.Toma B., Dufour B., Sanaa M., Bénet J.-J., Shaw A., Moutou F., Louza A. Epidémiologie Appliquée à la Lutte Collective Contre les Maladies Animales Transmissibles Majeures. Volume 1. AEEMA; Maisons-Alfort, France: 2001. p. 697. [Google Scholar]
- 19.Andino A., Hanning I. Salmonella enterica: Survival, Colonization, and Virulence Differences among Serovars. Sci. World J. 2015;2015:520179. doi: 10.1155/2015/520179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anses 2011 Opinion, AVIS de l’Agence Nationale de Sécurité Sanitaire de L’alimentation de L’environnement et du Travail Relatif à la Gestion Sanitaire D’un Foyer de Pullorose. Anses—Saisine n° 2011-SA-0059, 1–10. [(accessed on 16 March 2011)]; Available online: https://www.anses.fr/fr/system/files/SANT2011sa0059.pdf.
- 21.Podolak R., Enache E., Stone W., Black D.G., Elliott P.H. Sources and risk factors for contamination, survival, persistence, and heat resistance of Salmonella in low-moisture foods. J. Food Prot. 2010;73:1919–1936. doi: 10.4315/0362-028X-73.10.1919. [DOI] [PubMed] [Google Scholar]
- 22.Pui C.F., Wong W.C., Chai L.C., Robin T., Ponniah J., Hidayah M.S., Anyi U., Farinazleen M.G., Cheah Y.K., Son R. Review Article Salmonella: A foodborne pathogen. Int. Food Res. J. 2011;18:465–473. [Google Scholar]
- 23.Crippen T.L., Sheffield C.L., Beier R.C., Nisbet D.J. The horizontal transfer of Salmonella between the lesser mealworm (Alphitobius diaperinus) and poultry manure. Zoonoses Pub. Health. 2018;65:e23–e33. doi: 10.1111/zph.12404. [DOI] [PubMed] [Google Scholar]
- 24.Wynants E., Frooninckx L., Van Miert S., Geeraerd A., Claes J., Van Campenhout L. Risks related to the presence of Salmonella sp. during rearing of mealworms (Tenebrio molitor) for food or feed: Survival in the substrate and transmission to the larvae. Food Control. 2019;100:227–234. doi: 10.1016/j.foodcont.2019.01.026. [DOI] [Google Scholar]
- 25.Erbeck D.H., McLaughlin B.G., Singh S.N. Pullorum disease with unusual signs in two backyard chicken flocks. Avian Dis. 1993;37:895–897. doi: 10.2307/1592049. [DOI] [PubMed] [Google Scholar]
- 26.Shivaprasad H.L. Pullorum disease and Fowl typhoid. In: Brugère-Picoux J., Vaillancourt J.P., Shivaprasad H.L., Venne D., Bouzouaia M., editors. Manual of Poultry Diseases. AFAS; Paris France: 2015. pp. 287–292. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.