Abstract
Tomato spotted wilt virus (TSWV; species Tomato spotted wilt orthotospovirus) is an economically important plant virus that infects multiple horticultural crops on a global scale. TSWV encodes a non-structural protein NSs that acts as a suppressor of host RNA silencing machinery during infection. Despite extensive structural and functional analyses having been carried out on TSWV NSs, its protein-interacting targets in host plants are still largely unknown. Here, we systemically investigated NSs-interacting proteins in Nicotiana benthamiana via affinity purification and mass spectrometry (AP-MS) analysis. Forty-three TSWV NSs-interacting candidates were identified in N. benthamiana. Gene Ontology (GO) and protein–protein interaction (PPI) network analyses were carried out on their closest homologs in tobacco (Nicotiana tabacum), tomatoes (Solanum lycopersicum) and Arabidopsis (Arabidopsis thaliana). The results showed that NSs preferentially interacts with plant defense-related proteins such as calmodulin (CaM), importin, carbonic anhydrase and two heat shock proteins (HSPs): HSP70 and HSP90. As two major nodes in the PPI network, CaM and importin subunit α were selected for the further verification of their interactions with NSs via yeast two-hybrid (Y2H) screening. Our work suggests that the downstream signaling, transportation and/or metabolic pathways of host-NSs-interacting proteins may play critical roles in NSs-facilitated TSWV infection.
Keywords: affinity purification, calmodulin, carbonic anhydrase, heat shock protein, importin, mass spectrometry, NSs, protein–protein interaction network, RNA silencing suppressor, tomato spotted wilt virus
1. Introduction
Tomato spotted wilt virus (TSWV; species Tomato spotted wilt orthotospovirus) is the best known member in Orthotospovirus, which is the only genus with plant-infecting viruses in the family Tospoviridae [1]. Belonging to the order Bunyavirales, tospoviruses contain segmented RNA genomes with three single-stranded (ss) RNAs packaged in enveloped virus particles [2]. The large (L) RNA is negative sense, while the medium (M) and the small (S) RNAs possess an ambisense genome organization [3]. As a well-studied and economically important plant virus [4], TSWV causes significant yield losses in a wide range of agronomic and horticultural crops such as beans, lettuce, peanuts (groundnuts), peppers, potatoes, tobacco and tomatoes [5,6].
The TSWV L RNA encodes an RNA-dependent RNA polymerase (RdRp). The M RNA encodes a non-structural movement protein NSm, and the precursor of two structural glycoproteins GN and GC. A nucleocapsid protein (N) and another non-structural protein (NSs) are encoded by the S RNA [7]. Both the M and S RNAs are organized in an ambisense manner [8]. The three genomic RNAs of TSWV and the N protein form ribonucleoproteins encapsulated by the glycoprotein (GN and GC) envelope. TSWV infects plants via the thrips vector in the field [9].
NSs proteins are widely found in plant- and vertebrate-infecting Bunyaviruses [10]. NSs proteins from different tospoviruses share a common feature of binding both small and long double-stranded (ds) RNAs [11]. As a non-structural protein, TSWV NSs acts as an RNA silencing suppressor for overcoming the host immunity barrier [12]. NSs is an avirulence determinant of the TSWV resistance gene Tsw in peppers [13,14]. Tsw-mediated resistance in peppers can be overcome by a single amino acid change in NSs at position 104 (T–A) [15]. The N-terminal domain in NSs is important for its avirulence and RNA silencing suppression functions [16]. Two conserved motifs, GKV/T at positions 181–183 and YL at positions 412–413, are critical for the silencing suppressor function of NSs [7].
Despite the advancement of structural and functional research on TSWV NSs, its protein-interacting targets in host plants are still largely unknown. In this research, we investigated the NSs-interacting proteins in Nicotiana benthamiana via affinity purification and mass spectrometry (AP-MS) analysis. Gene Ontology (GO) and protein–protein interaction (PPI) network analyses were carried out on their closest homologs in Arabidopsis (Arabidopsis thaliana), tobacco (Nicotiana tabacum) and tomatoes (Solanum lycopersicum). Network analysis was carried out, followed by experimental validation by using the yeast two-hybrid (Y2H) assay. This approach of using AP-MS and network analysis combined with experimental validation offers an efficient approach for understanding the PPIs underlying virus–host interactions.
2. Results
2.1. Affinity Purification—Mass Spectrometry Analysis Reveals NSs-Interacting Proteins in N. benthamiana
TSWV NSs was fused with an mGFP5 tag at its C-terminal (NSs-GFP) and was transiently expressed in N. benthamiana leaves at the four-leaf stage. Its binding proteins were extracted and analyzed by AP-MS. To identify the host proteins that specifically interact with TSWV NSs, overlapping candidates were selected from two independent AP-MS replicates. The list was then compared to the list of candidates that bind the V2 protein of Croton yellow vein mosaic virus (unpublished), to remove overlapping non-specific interactors. Eventually, 43 N. benthamiana proteins were found to specifically interact with TSWV NSs (Table 1). The list is arranged according to the numbers of peptide spectrum matches (#PSMs), posterior error probability (PEP) values of the PSMs (Sum PEP Score) and sums of the scores of the individual peptides from the Sequest HT search (Score SEQUEST HT) in Replicate 1 (R1).
Table 1.
N. benthamiana protein-interacting candidates for tomato spotted wilt virus (TSWV) NSs revealed by affinity purification and mass spectrometry (AP-MS). Two independent replicates (designated as R1 and R2) were performed. The list is arranged according to the values of the numbers of peptide spectrum matches (#PSMs), posterior error probability (PEP) values of the PSMs (Sum PEP Scores), and sums of the scores of the individual peptides from the Sequest HT search (Scores SEQUEST HT) in Replicate 1 (R1). Two bold candidates (importin subunit α and Calmodulin 3) were further confirmed to interact with NSs via yeast two-hybrid assays.
| Accession | Description | #PSMs | Sum PEP Scores | Scores SEQUEST HT | |||
|---|---|---|---|---|---|---|---|
| R1 | R2 | R1 | R2 | R1 | R2 | ||
| A0A286RNF7 | Carbonic anhydrase | 33 | 28 | 65.105 | 67.837 | 86.19 | 71.41 |
| A0A0M3SBS3 | Heat shock protein 90-3 | 28 | 26 | 50.052 | 58.461 | 63.66 | 48.87 |
| A4D0J9 | Carbonic anhydrase (fragment) | 19 | 22 | 49.892 | 67.834 | 53.63 | 68.49 |
| I3QHX5 | Adenosylhomocysteinase | 15 | 9 | 23.165 | 17.085 | 27.23 | 11.48 |
| I0B7J2 | Chloroplast photosystem II subunit O2 (PSBO2) | 12 | 13 | 25.788 | 36.984 | 35.48 | 25.76 |
| I0B7J5 | Chloroplast photosystem II subunit P1 (PSBP1) | 10 | 9 | 29.376 | 31.322 | 28.37 | 29.27 |
| U5PY93 | MP-Interacting Protein (MIP) 1.2 | 10 | 6 | 18.593 | 13.573 | 23.1 | 12.96 |
| Q769C6 | Heat shock protein 70 (fragment) | 9 | 4 | 9.018 | 7.495 | 18.44 | 6.57 |
| U3MY90 | Proteinase inhibitor (fragment) | 8 | 10 | 19.765 | 31.157 | 20.94 | 26 |
| A0A0A7EAV4 | Ankyrin repeat containing protein 2 (AKR2) | 6 | 2 | 10.561 | 4.414 | 12.68 | 2.11 |
| F2Z9R2 | Glucose-6-phosphate 1-dehydrogenase (G6PD) | 6 | 2 | 7.83 | 2.791 | 10.65 | 1.79 |
| A1YUL9 | Importin subunit α | 5 | 5 | 13.326 | 9.718 | 17.15 | 6.01 |
| A0A0C4Y3N1 | RabG3c protein | 5 | 6 | 7.097 | 6.486 | 8.22 | 2.17 |
| A0A0S3ANR1 | NB-LRR HR-associated cell death (NRC) 2a | 5 | 3 | 6.318 | 2.992 | 1.87 | 1.63 |
| Q5YLB4 | DNA gyrase subunit B | 4 | 1 | 4.471 | 0.71 | 2.38 | 0 |
| U3MW48 | Calmodulin 3 (fragment) | 3 | 1 | 8.901 | 2.829 | 10.37 | 2.46 |
| Q5XPZ0 | Adenosine kinase (fragment) | 3 | 3 | 5.692 | 5.426 | 7.01 | 4.12 |
| R4S2V6 | Lipoxygenase (fragment) | 3 | 1 | 3.467 | 0.731 | 2.21 | 0 |
| A0A387K491 | Ran binding protein RanBP1-1b | 3 | 1 | 2.618 | 1.051 | 1.96 | 0 |
| A0A0K1U1X9 | Clade XV lectin receptor kinase | 3 | 8 | 1.266 | 1.145 | 4.89 | 7.08 |
| F8WQS4 | Quinone reductase (fragment) | 2 | 1 | 4.126 | 0.807 | 4.73 | 0 |
| A0A172WC56 | Defensin-like protein 1 | 2 | 1 | 3.97 | 0.732 | 2.84 | 0 |
| A2PYH3 | Nascent polypeptide associated complex α | 2 | 3 | 3.392 | 4.614 | 2.27 | 4.41 |
| Q6XX16 | Glutathione S-transferase U2 (fragment) | 2 | 3 | 2.565 | 1.677 | 3.44 | 0 |
| D6QX33 | Plastid RNA-binding protein | 2 | 1 | 2.326 | 0.695 | 0 | 0 |
| A0A0C5LA06 | Mitogen-activated protein kinase | 2 | 2 | 2.143 | 1.889 | 1.65 | 0 |
| F8WQS2 | Acetylglutamate kinase (fragment) | 1 | 2 | 3.648 | 2.075 | 3.27 | 0 |
| A0A0A7HDA5 | Epi-aristolochene dihydroxylase | 1 | 2 | 3.296 | 2.77 | 3.53 | 1.72 |
| Q18NX4 | Nitrate reductase | 1 | 1 | 2.896 | 1.947 | 2.65 | 0 |
| B0CN62 | Myosin VIII-1 | 1 | 1 | 2.364 | 1.331 | 2.55 | 0 |
| W6JJ90 | Nuclear pore complex protein Sec13d | 1 | 1 | 1.971 | 0.754 | 2.69 | 0 |
| Q20KN2 | Metacaspase type II (fragment) | 1 | 1 | 1.969 | 2.13 | 0 | 1.72 |
| Q5D1L7 | Serine/threonine protein kinase (fragment) | 1 | 1 | 1.888 | 1.185 | 2.11 | 0 |
| Q2QFR2 | Cysteine proteinase glycinain type (fragment) | 1 | 1 | 1.627 | 1.227 | 2.28 | 0 |
| C9DFC0 | Phytophthora-inhibited protease 1 (fragment) | 1 | 1 | 1.605 | 0.698 | 1.97 | 1.78 |
| A0A4Y5QRT8 | Serine/threonine protein kinase PBS1a | 1 | 2 | 1.396 | 1.303 | 0 | 1.77 |
| Q2QFR3 | Cysteine proteinase aleurain type | 1 | 1 | 1.191 | 1.367 | 0 | 1.62 |
| A0A024B875 | Dihydrolipoamide acetyltransferase component | 1 | 1 | 1.003 | 1.596 | 2.28 | 0 |
| D5JXY5 | Calcium-transporting ATPase | 1 | 1 | 0.754 | 0.754 | 0 | 0 |
| A0A1V1H6S6 | Calcium-dependent protein kinase isoform 2 | 1 | 1 | 0.732 | 0.801 | 2.11 | 0 |
| Q52JJ5 | Glutamyl-tRNA synthetase | 1 | 1 | 0.7 | 0.789 | 0 | 0 |
| A7L4B4 | Histone H3 | 1 | 2 | 0.509 | 1.589 | 1.76 | 2.54 |
| V5KY72 | Ubiquitin-conjugating enzyme variant | 1 | 1 | 0.503 | 0.766 | 0 | 0 |
Multiple signaling-relevant proteins can be found in the NSs-interacting list, including a lectin receptor kinase (LecRK; A0A0K1U1X9), a mitogen-activated protein kinase (MAPK; A0A0C5LA06), a calcium-dependent protein kinase (CDPK; A0A1V1H6S6), a calmodulin (CaM) (U3MW48) and two serine/threonine protein kinases (STPKs; A0A4Y5QRT8 and Q5D1L7). Two heat shock proteins (HSPs), HSP70 (Q769C6) and HSP90 (A0A0M3SBS3), also interact with NSs. For these N. benthamiana interactors, their closest homologs in tobacco, tomatoes and Arabidopsis were found by BLASTP and are listed in Table 2. Both A0A286RNF7 and A4D0J9 are carbonic anhydrases with LOC107768773, Solyc02g086820 and AT3G01500 being their closest homologs in tobacco, tomatoes and Arabidopsis, respectively. Therefore, only 42 inferred homologous proteins in each species are listed (Table 2).
Table 2.
The closest homologs of TSWV NSs-interacting candidates in tobacco (Nicotiana tabacum), tomatoes (Solanum lycopersicum) and Arabidopsis (Arabidopsis thaliana). Five bold candidates (HSP70, importin subunit α, CaM, MAPK and STPK) are major nodes in the protein–protein interaction (PPI) network.
| Description | Closest Homologs in | ||
|---|---|---|---|
| Tobacco | Tomato | Arabidopsis | |
| Carbonic anhydrase | LOC107768773 | Solyc02g086820 | AT3G01500 |
| Heat shock protein 90 (HSP90) | LOC107768797 | Solyc12g015880 | AT5G56000 |
| Adenosylhomocysteinase | LOC107783029 | Solyc09g092380 | AT4G13940 |
| Chloroplast photosystem II subunit O2 (PSBO2) | LOC107766588 | Solyc02g065400 | AT3G50820 |
| Chloroplast photosystem II subunit P1 (PSBP1) | LOC107830202 | Solyc07g044860 | AT1G06680 |
| MP-interacting protein (MIP) 1.2 | LOC107801992 | Solyc04g009770 | AT3G44110 |
| Heat shock protein 70 (HSP70) | LOC107803414 | Solyc11g066060 | AT3G12580 |
| Proteinase inhibitor | LOC107799889 | Solyc03g019690 | AT1G17860 |
| Ankyrin repeat containing protein 2 (AKR2) | LOC107793888 | Solyc01g104170 | AT2G17390 |
| Glucose-6-phosphate 1-dehydrogenase (G6PD) | LOC107794892 | Solyc07g045540 | AT5G35790 |
| Importin subunit α | LOC107810574 | Solyc01g060470 | AT4G16143 |
| RabG3 protein | LOC107815360 | Solyc03g120750 | AT1G52280 |
| NB-LRR HR-associated cell death (NRC) 2 | LOC107792680 | Solyc10g047320 | AT1G53350 |
| DNA gyrase subunit B | LOC107786139 | Solyc12g021230 | AT5G04130 |
| Calmodulin (CaM) | LOC107761764 | Solyc10g081170 | AT3G43810 |
| Adenosine kinase | LOC107790330 | Solyc09g007940 | AT5G03300 |
| Lipoxygenase | LOC107830099 | Solyc01g099160 | AT1G55020 |
| Ran binding protein RanBP | LOC107771336 | Solyc08g062660 | AT5G58590 |
| Lectin receptor kinase | LOC107782584 | Solyc03g080060 | AT5G55830 |
| Quinone reductase (fragment) | LOC107761412 | Solyc10g006650 | AT4G27270 |
| Defensin-like protein 1 | LOC107831752 | Solyc07g006380 | AT1G61070 |
| Nascent polypeptide associated complex α | LOC107791866 | Solyc10g081030 | AT3G12390 |
| Glutathione S-transferase U2 | LOC107782951 | Solyc07g056490 | AT1G78380 |
| Plastid RNA-binding protein | LOC107787150 | Solyc03g111050 | AT3G48500 |
| Mitogen-activated protein kinase (MAPK) | LOC107794128 | Solyc01g094960 | AT4G01370 |
| Acetylglutamate kinase | LOC107803486 | Solyc11g005620 | AT3G57560 |
| Epi-aristolochene dihydroxylase; CYP71B35 | LOC107759261 | Solyc04g083140 | AT3G26310 |
| Nitrate reductase | LOC107785409 | Solyc11g013810 | AT1G37130 |
| Myosin | LOC107806983 | Solyc02g020910 | AT3G19960 |
| Nuclear pore complex protein SEC13 | LOC107777830 | Solyc02g087300 | AT2G30050 |
| Metacaspase type II | LOC107824366 | Solyc09g098150 | AT1G79330 |
| Serine/threonine protein kinase (STPK) | LOC107808522 | Solyc02g067030 | AT3G01090 |
| Cysteine proteinase glycinain type | LOC107760226 | Solyc04g080960 | AT4G39090 |
| PIP1; cysteine endopeptidase | LOC107774651 | Solyc02g077040 | AT3G48340 |
| Serine/threonine protein kinase PBS1 | LOC107830934 | Solyc05g024290 | AT5G13160 |
| Cysteine proteinase aleurain type | LOC107784768 | Solyc07g041900 | AT5G60360 |
| Lipoamide acetyltransferase component | LOC107820956 | Solyc01g066520 | AT3G06850 |
| Calcium-transporting ATPase | LOC107814306 | Solyc04g016260 | AT3G57330 |
| Calcium-dependent protein kinase | LOC107805386 | Solyc07g064610 | AT3G20410 |
| Glutamyl-tRNA synthetase | LOC107774917 | Solyc01g112290 | AT5G64050 |
| Histone H3 | LOC107759185 | Solyc01g073970 | AT5G65360 |
| Ubiquitin-conjugating enzyme variant | LOC107831808 | Solyc04g007960 | AT1G70660 |
2.2. Gene Ontology Overrepresentation/Enrichment Tests of NSs-Interacting Proteins
To facilitate GO analysis, the closest tobacco, tomato and Arabidopsis homologs inferred from the N. benthamiana NSs-interacting proteins (Table 2) were used for overrepresentation/enrichment tests. Only the Arabidopsis homologs generated meaningful results in the GO biological process test that classified proteins according to the cellular activities in which they were involved (Table 3). Defense-responsive proteins were found to be enriched by about 10 fold (Table 3), which is consistent with the virulent nature of NSs. The defense-related proteins in the list include a LecRK (AT5G55830), a carbonic anhydrase (AT3G01500), chloroplast photosystem II subunit P1 (PSBP1; AT1G06680), a CaM (AT3G43810), a lipoxygenase (AT1G55020), STPK PBS1 (AT5G13160), a MAPK (AT4G01370), a cysteine proteinase (AT4G39090), a defensin-like protein (AT1G61070), a calcium-transporting ATPase (AT3G57330) and a NB-LRR protein required for hypersensitive response (HR)-associated cell death (NRC) (AT1G53350). The host immunity responses triggered by these defense proteins may be suppressed by the binding of NSs during TSWV infection.
Table 3.
PANTHER overrepresentation test of Gene Ontology (GO) biological processes using Arabidopsis homologs inferred from NSs-interacting proteins. A total of 27,416 proteins (GO Ontology database, doi:10.5281/zenodo.3980761) were included in the Arabidopsis reference list. Fisher’s exact test with Bonferroni correction for multiple testing was adopted. Only results with Bonferroni-corrected p < 0.05 are displayed.
| GO Biological Process Complete |
Arabidopsis Reference # |
NSs-Interacting Proteins | ||||
|---|---|---|---|---|---|---|
| # | Expected | Fold Enrichment | +/− | p Value | ||
| Defense response to bacterium | 413 | 7 | 0.63 | 11.06 | + | 9.04 × 10−3 |
| Response to bacterium | 506 | 8 | 0.78 | 10.32 | + | 2.75 × 10−3 |
| Response to other organisms | 1092 | 12 | 1.67 | 7.17 | + | 1.74 × 10−4 |
| Interspecies interaction between organisms | 1120 | 12 | 1.72 | 6.99 | + | 2.28 × 10−4 |
| Response to external biotic stimulus | 1092 | 12 | 1.67 | 7.17 | + | 1.74 × 10−4 |
| Response to biotic stimulus | 1093 | 12 | 1.67 | 7.17 | + | 1.75 × 10−4 |
| Response to stimulus | 5567 | 22 | 8.53 | 2.58 | + | 1.20 × 10−2 |
| Response to external stimulus | 1509 | 15 | 2.31 | 6.49 | + | 9.27 × 10−6 |
| Defense response to other organisms | 805 | 9 | 1.23 | 7.30 | + | 8.95 × 10−3 |
| Defense response | 952 | 10 | 1.46 | 6.86 | + | 4.03 × 10−3 |
| Response to stress | 3091 | 18 | 4.74 | 3.80 | + | 6.09 × 10−4 |
| Cellular process | 11,979 | 33 | 18.35 | 1.80 | + | 1.67 × 10−2 |
| Unclassified | 5450 | 5 | 8.35 | 0.60 | − | 0.00 |
Arabidopsis reference #: number of proteins that are classified in the category out of 27,416 Arabidopsis reference proteins. NSs-interacting protein candidate #: number of NSs-interacting proteins that are classified in the category out of 42 candidates; Expected: expected number of NSs-interacting proteins that are classified in the category out of 42 candidates; Fold enrichment: fold enrichment of NSs-interacting proteins that are classified in the category, calculated as #/Expected; +/−: significantly enriched/diluted.
Meaningful results were obtained when using tobacco and Arabidopsis homologs in the overrepresentation test of GO molecular functions (Table 4). In tobacco homologs, proteins that bind unfolded proteins were found to be enriched by more than 20 fold (Table 4), including the nascent polypeptide-associated complex subunit α (LOC107791866), two HSPs (LOC107768797 and LOC107803414) and a DnaJ protein homolog (LOC107801992). NSs may interact with them to prevent the correct folding of host proteins. In Arabidopsis homologs, cysteine-type proteinases (also called proteases or endopeptidases) were found to be enriched by about 30 fold (Table 4), including an aleurain-type cysteine proteinase (AT5G60360), a type-II metacaspase (AT1G79330), a KDEL-tailed cysteine endopeptidase (AT3G48340) and a glycinain-type cysteine proteinase (AT4G39090).
Table 4.
PANTHER overrepresentation test of GO molecular function using tobacco and Arabidopsis homologs inferred from N. benthamiana NSs-interacting proteins. A total of 61,238 proteins (GO Ontology database, doi:10.5281/zenodo.4033054) were included in the tobacco reference list. All other test parameters are the same as those in Table 3.
| GO Molecular Function Complete | Tobacco Reference # |
NSs-Interacting Proteins | ||||
|---|---|---|---|---|---|---|
| # | Expected | Fold Enrichment | +/− | p Value | ||
| Unfolded protein binding | 284 | 4 | 0.16 | 24.64 | + | 4.15 × 10−2 |
| Binding | 21,517 | 26 | 12.30 | 2.11 | + | 5.53 × 10−3 |
| ATP binding | 4591 | 12 | 2.62 | 4.57 | + | 9.61 × 10−3 |
| Adenyl ribonucleotide binding | 4708 | 12 | 2.69 | 4.46 | + | 1.24 × 10−2 |
| Adenyl nucleotide binding | 4734 | 12 | 2.71 | 4.44 | + | 1.32 × 10−2 |
| Purine nucleotide binding | 5258 | 13 | 3.01 | 4.33 | + | 6.22 × 10−3 |
| Nucleotide binding | 5870 | 16 | 3.35 | 4.77 | + | 6.52 × 10−5 |
| Small molecule binding | 6512 | 17 | 3.72 | 4.57 | + | 3.66 × 10−5 |
| Nucleoside phosphate binding | 5870 | 16 | 3.35 | 4.77 | + | 6.52 × 10−5 |
| Purine ribonucleotide binding | 5217 | 13 | 2.98 | 4.36 | + | 5.70 × 10−3 |
| Ribonucleotide binding | 5285 | 14 | 3.02 | 4.63 | + | 9.62 × 10−4 |
| Carbohydrate derivative binding | 5332 | 14 | 3.05 | 4.59 | + | 1.07 × 10−3 |
| Purine ribonucleoside triphosphate binding | 5100 | 13 | 2.91 | 4.46 | + | 4.43 × 10−3 |
| Anion binding | 6438 | 16 | 3.68 | 4.35 | + | 2.38 × 10−4 |
| Ion binding | 11,853 | 20 | 6.77 | 2.95 | + | 1.59 × 10−3 |
| Unclassified | 26,668 | 2 | 15.24 | 0.13 | − | 0.00 |
| GO Molecular Function Complete |
Arabidopsis
Reference # |
NSs-Interacting Proteins | ||||
| # | Expected | Fold Enrichment | +/− | p Value | ||
| Cysteine-type endopeptidase activity | 72 | 4 | 0.11 | 36.26 | + | 9.91 × 10−3 |
| Cysteine-type peptidase activity | 102 | 4 | 0.16 | 25.60 | + | 3.71 × 10−2 |
| Catalytic activity | 8305 | 27 | 12.72 | 2.12 | + | 1.06 × 10−2 |
| Cation binding | 1647 | 11 | 2.52 | 4.36 | + | 5.00 × 10−2 |
| Ion binding | 3071 | 16 | 4.70 | 3.40 | + | 1.04 × 10−2 |
| Binding | 9721 | 31 | 14.89 | 2.08 | + | 1.26 × 10−3 |
| Protein binding | 5109 | 23 | 7.83 | 2.94 | + | 3.25 × 10−4 |
| Unclassified | 5502 | 1 | 8.43 | 0.12 | − | 0.00 |
Tobacco reference #: number of proteins that are classified in the category out of 61,238 Nicotiana tabacum reference proteins. All other column descriptions are the same as those in Table 3.
Meaningful results were obtained when using tomato and Arabidopsis homologs in the GO cellular component overrepresentation test (Table 4). In tomato homologs, lysosomal enzymes localized in the extracellular space were enriched by about 40 to 50 fold (Table 5), which include three cysteine proteases: Solyc07g041900, Solyc02g077040 and Solyc04g080960. Similarly, in Arabidopsis homologs, lysosome- and chloroplast-localized proteins were found to be enriched by more than 40 and 7 fold, respectively (Table 5). The three lysosome-localized Arabidopsis homologs include an aleurain-type cysteine proteinase (AT5G60360), a KDEL-tailed cysteine endopeptidase (AT3G48340) and a glycinain-type cysteine proteinase (AT4G39090). Actually, all four Arabidopsis cysteine proteinases characterized in the GO molecular function test are localized in the lysosome, except the type-II metacaspase (AT1G79330). The chloroplast-localized Arabidopsis proteins in the list include a carbonic anhydrase (AT3G01500), PSBP1 (AT1G06680), a glutamate-tRNA ligase (AT5G64050), chloroplast photosystem II subunit O2 (PSBO2; AT3G50820), a glutathione S-transferase (GST; AT1G78380), HSP90 (AT5G56000), a plastid RNA-binding protein (AT3G48500) and glucose-6-phosphate 1-dehydrogenase (G6PD1; AT5G35790).
Table 5.
PANTHER overrepresentation test of GO cellular components using tomato and Arabidopsis homologs inferred from N. benthamiana NSs-interacting proteins. A total of 34,637 proteins (GO Ontology database, doi:10.5281/zenodo.4033054) were included in the tomato reference list. All other parameters are the same as those in Table 3.
| GO Cellular Component Complete | Tomato Reference # |
NSs-Interacting Proteins | ||||
|---|---|---|---|---|---|---|
| # | Expected | Fold Enrichment | +/− | p Value | ||
| Lysosome | 49 | 3 | 0.06 | 50.49 | + | 1.71 × 10−2 |
| Lytic vacuole | 52 | 3 | 0.06 | 47.58 | + | 2.02 × 10−2 |
| Intracellular membrane-bounded organelle | 5532 | 18 | 6.71 | 2.68 | + | 1.61 × 10−2 |
| Membrane-bounded organelle | 5782 | 19 | 7.01 | 2.71 | + | 7.25 × 10−3 |
| Organelle | 6262 | 19 | 7.59 | 2.50 | + | 2.30 × 10−2 |
| Cellular anatomical entity | 9174 | 26 | 11.12 | 2.34 | + | 7.57 × 10−4 |
| Intracellular organelle | 6130 | 19 | 7.43 | 2.56 | + | 1.70 × 10−2 |
| Intracellular | 7723 | 25 | 9.36 | 2.67 | + | 1.09 × 10−4 |
| Cytoplasm | 5053 | 21 | 6.13 | 3.43 | + | 3.26 × 10−5 |
| Extracellular space | 62 | 3 | 0.08 | 39.90 | + | 3.34 × 10−2 |
| Unclassified | 25,226 | 16 | 30.59 | 0.52 | − | 0.00 |
| GO Cellular Component Complete |
Arabidopsis
Reference # |
NSs-Interacting Proteins | ||||
| # | Expected | Fold Enrichment | +/− | p Value | ||
| Lysosome | 46 | 3 | 0.07 | 42.57 | + | 3.98 × 10−2 |
| Vacuole | 1084 | 10 | 1.66 | 6.02 | + | 3.03 × 10−3 |
| Cytoplasm | 14,776 | 38 | 22.64 | 1.68 | + | 3.25 × 10−4 |
| Chloroplast stroma | 718 | 8 | 1.10 | 7.27 | + | 8.34 × 10−3 |
| Plastid stroma | 730 | 8 | 1.12 | 7.15 | + | 9.39 × 10−3 |
| Whole membrane | 830 | 8 | 1.27 | 6.29 | + | 2.33 × 10−2 |
| Membrane | 5495 | 22 | 8.42 | 2.61 | + | 2.28 × 10−3 |
| Bounding membrane of organelle | 921 | 8 | 1.41 | 5.67 | + | 4.82 × 10−2 |
| Cytosol | 3242 | 22 | 4.97 | 4.43 | + | 1.35 × 10−7 |
| Plasma membrane | 3529 | 18 | 5.41 | 3.33 | + | 1.05 × 10−3 |
| Cell periphery | 4001 | 19 | 6.13 | 3.10 | + | 1.35 × 10−3 |
| Unclassified | 1877 | 1 | 2.88 | 0.35 | − | 0.00 |
Tomato reference #: number of proteins that are classified in the category out of 34,637 Solanum lycopersicum reference proteins. All other column descriptions are the same as those in Table 3.
2.3. The Protein-Protein Interaction Network of NSs-Interacting Proteins
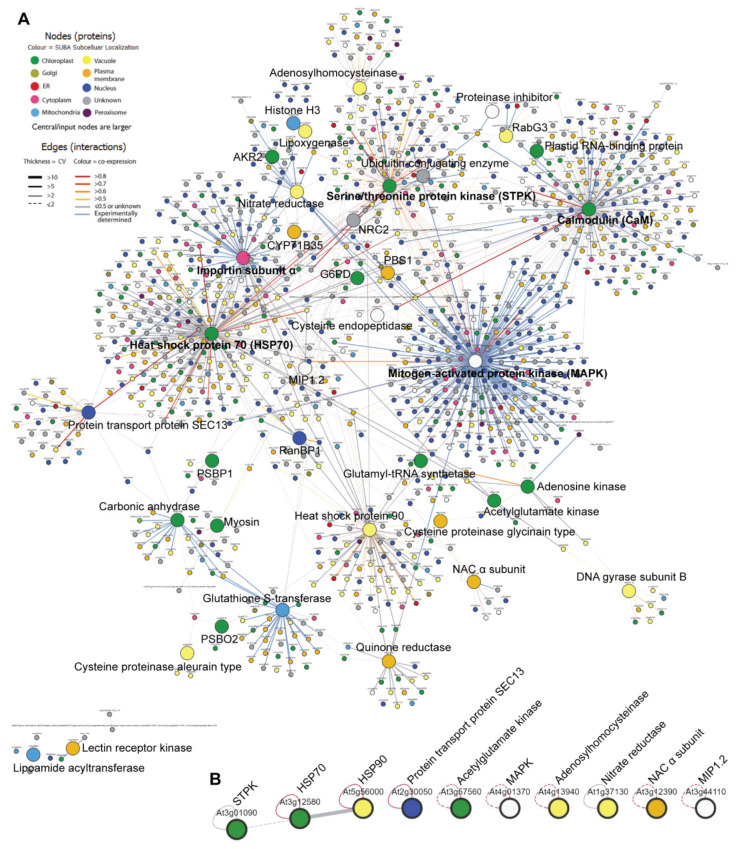
To explore the indirect and expanded consequences of physical interactions between NSs and plant proteins, a PPI network was constructed for 42 Arabidopsis homologs inferred from N. benthamiana NSs-interacting proteins (Figure 1A and Figure S1; Table S1). A total of 1346 interactions were predicted. Five major node proteins can be found in the PPI network, including HSP70 (At3G12580), CaM (AT3G43810), MAPK (AT4G01370), STPK (AT3G01090) and importin subunit α (AT4G16143) (Figure 1A). Interactions between NSs and these five plant signaling, chaperone and transporter proteins may play significant roles in TSWV infection. We further investigated interactions within the 42 Arabidopsis homologs (Figure 1B). The most reliable interaction was predicted to occur between HSP70 and HSP90 (Figure 1B). Ten proteins including HSP70 and HSP90 were predicted to have self-interactions (Figure 1B). As two major nodes in the PPI network, CaM and importin subunit α were selected for the further verification of their interactions with TSWV NSs.
Figure 1.
(A) The protein–protein interaction (PPI) network of 42 Arabidopsis homologs inferred from N. benthamiana NSs-interacting proteins. A total of 1346 interactions were predicted. HSP70, CaM, MAPK, STPK and importin subunit α are five major nodes found in the PPI network. (B) Predicted interactions within the 42 Arabidopsis homologs. The most reliable interaction occurs between HSP70 and HSP90. Ten proteins including HSP70 and HSP90 have self-interactions.
2.4. Importin Subunit α and Calmodulin 3 Interact with NSs in Targeted Yeast Two-Hybrid Assays
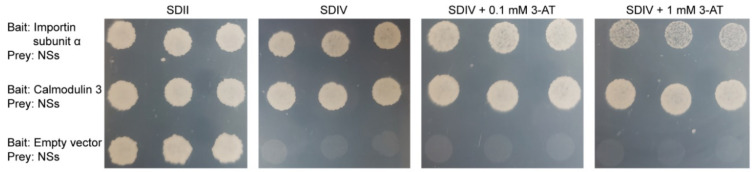
N. benthamiana importin subunit α (A1YUL9) and CaM 3 (U3MW48) were selected to verify their interactions with NSs via targeted Y2H. Both proteins interacted with NSs in the assays (Figure 2), which demonstrates that the AP-MS approach is effective and reliable in identifying host-NSs-interacting proteins.
Figure 2.
N. benthamiana proteins importin subunit α and Calmodulin 3 (CaM 3) were verified to interact with tomato spotted wilt virus NSs via targeted yeast two-hybrid (Y2H). Positive interactions were implied by the yeast’s ability to grow on synthetic defined (SD) selection medium minus four elements of uracil, histidine, leucine and tryptophan (SDIV) and its tolerance to the His3p enzyme inhibitor 3-aminotriazole (3-AT). All yeast clones grow normally on the SDII medium which only lacks leucine and tryptophan. Two concentrations of 3-AT (0.1 and 1 mM) were used in the test.
3. Discussion
Although NSs is well-known for its RNA silencing suppressor function during the TSWV infection process, the direct protein-interacting targets of NSs in plant hosts are still largely unknown. There is a report that TSWV NSs can suppress jasmonate signaling in plants [17] via direct interactions with three basic-helix-loop-helix (bHLH) transcription factors (TFs): MYC2, MYC3 and MYC4 [18]. In this work, we significantly expanded the reservoir of NSs’ physical interactors in plants. The interactions may be critical for TSWV virulence.
Multiple NSs-interacting proteins identified in this research have been demonstrated to regulate plant defenses. For example, cysteine proteinases play prominent roles in plant–pathogen interactions [19]. Notably, tomato aleurain-type cysteine proteinase can be inhibited by the pathogenic oomycete Phytophthora [20]. NSs-interacting cysteine proteinases are critical for lysosome-mediated autophagy function, which acts as an antiviral defense mechanism in plants. Viruses counteract host defenses by hijacking the autophagy pathway [21]. Interactions between NSs and lysosome-localized cysteine proteinases may contribute to the TSWV-induced suppression of autophagy.
N. benthamiana CaM 3 and importin subunit α are two NSs interactors verified by both AP-MS and Y2H assays. CaMs are significant components in plant immunity signaling networks [22]. There are multiple lines of evidence showing that CaMs participate in plant defenses against bacterial [23], fungal [24] and viral [25,26,27,28] pathogens. A tobacco CaM can bind the RNA silencing suppressor encoded by Cucumber mosaic virus and thereby trigger its degradation via the autophagy pathway [25]. On the contrary, an N. benthamiana CaM is required for the RNA silencing suppressor function of βC1, which is encoded by the geminivirus Tomato yellow leaf curl China virus [26]. Thus, the interaction between N. benthamiana CaM 3 and TSWV NSs may lead to either NSs degradation or the activation of its RNA silencing suppressor activity. Further investigations would reveal whether CaM 3 plays a positive or negative role in the NSs-mediated suppression of plant RNA silencing.
Importins are critical for the nuclear import of Agrobacterium virulence proteins [29]. There are multiple reports demonstrating that plant importin subunit α facilitates the nuclear transportation of viral proteins. N. plumbaginifolia importin subunit α can bind the coat/capsid proteins (CPs) of Rice tungro bacilliform virus and Mungbean yellow mosaic virus and transport them into the nucleus [30]. Similarly, tobacco importin subunit α mediates the nuclear import of Cauliflower mosaic virus translational transactivator protein P6, which suppresses plant RNA silencing in the nucleus [31]. N. benthamiana importin subunit α has a similar function of transporting viral proteins. For example, it interacts with the CP of Beet black scorch virus and transports it into the nucleus [32]. The nuclear localization of the Potato mop-top virus Triple Gene Block1 (TGB1) protein is mediated by N. benthamiana importin subunit α, which facilitates systemic virus movement [33]. The Pelargonium line pattern virus p37 protein acts as an RNA silencing suppressor whose nuclear localization is also mediated by N. benthamiana importin subunit α [34]. Taken together, we postulate that the physical interaction between TSWV NSs and importin subunit α may facilitate the nuclear transportation of NSs and the following exertion of its RNA silencing suppressor activity.
Since many plant virus infection events occur in the chloroplast [35] and are regulated by host photosynthetic and photomorphogenic activities [36], it is not surprising that NSs interacts with multiple chloroplast-localized proteins. Chloroplast-localized carbonic anhydrases appeared twice in our refined N. benthamiana NSs interactor list (Table 1). Their antioxidant activity is involved in plant HR defenses [37]. For example, carbonic anhydrase expression is indispensable for potato resistance to the late blight pathogen Phytophthora infestans [38]. It is possible that NSs interacts with plant carbonic anhydrases to suppress their antioxidant function, thereby promoting TSWV infection.
Both HSP70 and HSP90 were found to interact with TSWV NSs in our AP-MS analysis (Table 1). HSP70 is a major node in the PPI network of NSs-interacting proteins (Figure 1A). Functional and physical interactions between HSP70 and HSP90 exist ubiquitously in bacteria, yeasts [39] and plants [40]. In Arabidopsis, HSP70 expression can be induced by infections by multiple virus species [41]. In tomatoes, the Tomato yellow leaf curl virus CP interacts with HSP70 to facilitate virus infection [42]. In N. benthamiana, HSP90 is indispensable for plant resistance against Potato virus X and Tobacco mosaic virus [43]. Based on these reports, HSP70 and HSP90 may interact with NSs to positively or negatively regulate TSWV infection.
Overall, the NSs-interacting proteins identified via AP-MS provide multiple clues for dissecting the roles of NSs in TSWV–host interaction. CaM-triggered defense signaling, importin-facilitated protein nuclear transportation, carbonic anhydrase-catalyzed antioxidation and HSP70/HSP90-mediated stress tolerance emerged as principal plant cellular activities in response to NSs invasion. In the future, the molecular mechanisms of how TSWV NSs interacts with these defense-related proteins (e.g., time and spatial patterns); the genetic, biochemical and physiological outcomes of the interactions; the expression changes of downstream genes triggered by the interactions; and the regulatory/regulated proteins up-/downstream of the interaction cascades should be investigated to obtain more details. The obtained results would provide a comprehensive portrait of NSs’ activities in the plant cell.
4. Materials and Methods
4.1. Plasmids and Gene Cloning
The TSWV NSs coding sequence (CDS) was previously described [8]. The NSs CDS was amplified using the PCR primers 5′-GGGGACAAGTTTGTACAAAAAAGCAGGCTATGTCTTCAAGTGTTTATGAG-3′ and 5′-GGGGACCACTTTGTACAAGAAAGCTGGGTGTTTTGATCCTGAAGCATA-3′. The amplified NSs CDS was cloned into the Gateway Donor vector pDONR 207 (Invitrogen) via a BP reaction (insertion of the att-B-sequence-containing PCR product into the att P recombination sites) and then inserted into the destination expression vector pEarleyGate 103 [44] from pDONR 207 via an LR reaction (insertion of the att-L-sequence-containing DNA into the att R recombination sites). In pEarleyGate 103, NSs was fused with an mGFP5 tag at its C-terminal (NSs-GFP). All the PCR-amplified sequences used in this research were verified by sequencing.
4.2. Affinity Purification—Mass Spectrometry Analysis of NSs-Interacting Proteins
NSs-GFP was transiently expressed in N. benthamiana leaves by agroinfiltration. Leaf samples were collected two days after infiltration, and the expression of NSs-GFP was verified by Western blotting. AP-MS was carried out using the GFP-Trap beads (Chromotek, Germany) as previously described [45,46]. Briefly, infiltrated leaves were ground into fine powder in liquid nitrogen, mixed with protein extraction buffer (1 mL per 500 mg of tissue) and then thawed on ice. After incubation and centrifugation at 4 °C, the extract supernatant was cleaned by filtration and then mixed with the GFP-Trap beads. After 1 h of incubation at 4 °C, the mixture was subsequently washed with wash buffer 3–5 times. The mass spectrometry (MS) analysis of the immunoprecipitated proteins was performed at the BGI Americas MS Service Center. The MS data were searched against the most updated Uniprot N. benthamiana database (2020_05) [47] using SEQUEST HT 2013 [48].
4.3. Refinement of the NSs-Interacting Protein Candidate List
Two NSs-GFP AP-MS biological replicates as well as two non-NSs AP-MS control replicates were performed for NSs-GFP. Since the Gateway-compatible pEarleyGate 103 cannot express mGFP5 without gene insertion, a pEarleyGate 103 construct expressing an mGFP5-fused V2 protein from Croton yellow vein mosaic virus was used as the non-NSs AP-MS control. Overlapping NSs-interacting protein candidates were identified from the two NSs-GFP AP-MS replicates. Non-specific NSs interactors were then removed from the list, including mGFP5, ubiquitin and proteins that were found to also interact with V2 in the control samples. This step helped to exclude non-specific NSs-interacting proteins that are expressed at high levels in N. benthamiana.
4.4. Verification of NSs-Interacting Proteins by Targeted Yeast Two-Hybrid Assay
Two N. benthamiana proteins in the interacting list, importin subunit α (A1YUL9) and CaM 3 (U3MW48), were selected to verify their interactions with NSs via targeted Y2H. The Y2H procedure has been described previously [49]. In brief, the NSs CDS was cloned into the Gateway-compatible prey vector pACT2-GW (pACT2-GW-NSs, with leucine selection marker) and then used to transform yeast strain A. After testing the transformed yeast clones for self-activation, the importin subunit α or CaM 3 CDS was cloned into the Gateway-compatible bait vector pBTM116-D9 with tryptophan selection marker and then used to transform a selected yeast line harboring pACT2-GW-NSs. Empty pBTM116-D9 was used as a negative control. Positive interactions were implied by the observation of the yeast’s growth on synthetic defined (SD) selection medium minus four elements of uracil, histidine, leucine and tryptophan (SDIV) and its tolerance to the His3p enzyme inhibitor 3-aminotriazole (3-AT).
4.5. Gene Ontology Analysis of Inferred Tobacco, Tomato and Arabidopsis Homologs
Since there is currently no available ontology data and analysis tool for N. benthamiana, the GO analysis of NSs-interacting proteins was performed using their closest homologs in tobacco, tomatoes and Arabidopsis. Tobacco is a close relative of N. benthamiana, and the genome of tomatoes has been well-studied compared to other species in the Solanaceae family. However, neither tobacco nor tomatoes have annotation information sufficient for a comprehensive GO analysis. Thus, Arabidopsis homologs were still used for all the GO enrichment tests for biological processes, molecular functions and cellular components. The test results for tobacco and tomato homologs were included if they contained meaningful information. All the tests were performed using the PANTHER (Version 15.0) GO Term Enrichment tools [50].
4.6. Protein-Protein Interaction Network Analysis of Inferred Arabidopsis Homologs
Arabidopsis homologs were used for the protein–protein interaction (PPI) network analysis due to the availability of PPI data and analysis tools. The PPI analysis of the Arabidopsis homologs inferred from the N. benthamiana NSs-interacting proteins was performed using the Bio-Analytic Resource (BAR) Arabidopsis Interactions Viewer [51]. The queries included interaction data and predictions from BioGrid (Version 4.1) [52], IntAct (Version 4.2.16) [53] and BAR (Version 20-04) [51]. The results of protein–DNA interactions from the BAR were also included.
4.7. Mass Spectrometry Data Deposit
The original AP-MS dataset (RAW files) and results of NSs-interacting candidates in N. benthamiana were deposited in the ProteomeXchange Consortium via the PRoteomics IDEntifications (PRIDE) [54] partner repository, with the dataset identifier PXD022401.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-0817/10/1/27/s1. Figure S1: Protein–protein interaction (PPI) map of 42 Arabidopsis homologs inferred from N. benthamiana NSs-interacting proteins (the original map of Figure 2A). Table S1: Protein–protein interaction (PPI) network of 42 Arabidopsis homologs inferred from N. benthamiana NSs-interacting proteins.
Author Contributions
Conceptualization: Y.Z. and H.R.P.; data curation: Y.Z.; formal analysis: Y.Z.; funding acquisition: H.R.P.; investigation: Y.Z.; methodology: Y.Z.; project administration: Y.Z. and H.R.P.; supervision: H.R.P.; validation: Y.Z. and H.R.P.; writing—original draft: Y.Z.; writing—review and editing: Y.Z., P.G. and H.R.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research and the APC were funded by the USDA National Institute of Food and Agriculture, Hatch project, Accession #1016563, “Reducing the Impact of Pests and Diseases Affecting Washington Agriculture” (award to H.R.P.), and the Carl F. and James J. Chuey Endowment for Dahlia Research through the Scheetz Chuey Foundation (to H.R.P.).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Siddell S.G., Walker P.J., Lefkowitz E.J., Mushegian A.R., Adams M.J., Dutilh B.E., Gorbalenya A.E., Harrach B., Harrison R.L., Junglen S., et al. Additional changes to taxonomy ratified in a special vote by the International Committee on Taxonomy of Viruses (October 2018) Arch. Virol. 2019;164:943–946. doi: 10.1007/s00705-018-04136-2. [DOI] [PubMed] [Google Scholar]
- 2.Zhu M., van Grinsven I.L., Kormelink R., Tao X. Paving the way to tospovirus infection: Multilined interplays with plant innate immunity. Annu. Rev. Phytopathol. 2019;57:41–62. doi: 10.1146/annurev-phyto-082718-100309. [DOI] [PubMed] [Google Scholar]
- 3.Adkins S. Tomato spotted wilt virus—Positive steps towards negative success. Mol. Plant Pathol. 2000;1:151–157. doi: 10.1046/j.1364-3703.2000.00022.x. [DOI] [PubMed] [Google Scholar]
- 4.Scholthof K.B., Adkins S., Czosnek H., Palukaitis P., Jacquot E., Hohn T., Hohn B., Saunders K., Candresse T., Ahlquist P., et al. Top 10 plant viruses in molecular plant pathology. Mol. Plant Pathol. 2011;12:938–954. doi: 10.1111/j.1364-3703.2011.00752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pappu H.R., Jones R.A., Jain R.K. Global status of tospovirus epidemics in diverse cropping systems: Successes achieved and challenges ahead. Virus Res. 2009;141:219–236. doi: 10.1016/j.virusres.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 6.Oliver J.E., Whitfield A.E. The genus Tospovirus: Emerging Bunyaviruses that threaten food security. Annu. Rev. Virol. 2016;3:101–124. doi: 10.1146/annurev-virology-100114-055036. [DOI] [PubMed] [Google Scholar]
- 7.Zhai Y., Bag S., Mitter N., Turina M., Pappu H.R. Mutational analysis of two highly conserved motifs in the silencing suppressor encoded by Tomato spotted wilt virus (genus Tospovirus, family Bunyaviridae) Arch. Virol. 2014;159:1499–1504. doi: 10.1007/s00705-013-1928-8. [DOI] [PubMed] [Google Scholar]
- 8.Zhai Y., Peng H., Neff M.M., Pappu H.R. Putative auxin and light responsive promoter elements from the Tomato spotted wilt tospovirus genome, when expressed as cDNA, are functional in Arabidopsis. Front. Plant Sci. 2019;10:804. doi: 10.3389/fpls.2019.00804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Turina M., Kormelink R., Resende R.O. Resistance to tospoviruses in vegetable crops: Epidemiological and molecular aspects. Annu. Rev. Phytopathol. 2016;54:347–371. doi: 10.1146/annurev-phyto-080615-095843. [DOI] [PubMed] [Google Scholar]
- 10.Hedil M., Kormelink R. Viral RNA silencing suppression: The enigma of Bunyavirus NSs proteins. Viruses. 2016;8:208. doi: 10.3390/v8070208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hedil M., de Ronde D., Kormelink R. Biochemical analysis of NSs from different tospoviruses. Virus Res. 2017;242:149–155. doi: 10.1016/j.virusres.2017.09.020. [DOI] [PubMed] [Google Scholar]
- 12.Takeda A., Sugiyama K., Nagano H., Mori M., Kaido M., Mise K., Tsuda S., Okuno T. Identification of a novel RNA silencing suppressor, NSs protein of Tomato spotted wilt virus. FEBS Lett. 2002;532:75–79. doi: 10.1016/S0014-5793(02)03632-3. [DOI] [PubMed] [Google Scholar]
- 13.Margaria P., Ciuffo M., Pacifico D., Turina M. Evidence that the nonstructural protein of Tomato spotted wilt virus is the avirulence determinant in the interaction with resistant pepper carrying the Tsw gene. Mol. Plant Microbe Interact. 2007;20:547–558. doi: 10.1094/MPMI-20-5-0547. [DOI] [PubMed] [Google Scholar]
- 14.De Ronde D., Butterbach P., Lohuis D., Hedil M., van Lent J.W., Kormelink R. Tsw gene-based resistance is triggered by a functional RNA silencing suppressor protein of the Tomato spotted wilt virus. Mol. Plant Pathol. 2013;14:405–415. doi: 10.1111/mpp.12016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Almási A., Nemes K., Csömör Z., Tóbiás I., Palkovics L., Salánki K. A single point mutation in Tomato spotted wilt virus NSs protein is sufficient to overcome Tsw-gene-mediated resistance in pepper. J. Gen. Virol. 2017;98:1521–1525. doi: 10.1099/jgv.0.000798. [DOI] [PubMed] [Google Scholar]
- 16.De Ronde D., Pasquier A., Ying S., Butterbach P., Lohuis D., Kormelink R. Analysis of Tomato spotted wilt virus NSs protein indicates the importance of the N-terminal domain for avirulence and RNA silencing suppression. Mol. Plant Pathol. 2014;15:185–195. doi: 10.1111/mpp.12082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao L., Hu Z., Li S., Zhou X., Li J., Su X., Zhang L., Zhang Z., Dong J. Diterpenoid compounds from Wedelia trilobata induce resistance to Tomato spotted wilt virus via the JA signal pathway in tobacco plants. Sci. Rep. 2019;9:2763. doi: 10.1038/s41598-019-39247-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu X., Xu S., Zhao P., Zhang X., Yao X., Sun Y., Fang R., Ye J. The Orthotospovirus nonstructural protein NSs suppresses plant MYC-regulated jasmonate signaling leading to enhanced vector attraction and performance. PLoS Pathog. 2019;15:e1007897. doi: 10.1371/journal.ppat.1007897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thomas E.L., van der Hoorn R.A.L. Ten prominent host proteases in plant-pathogen interactions. Int. J. Mol. Sci. 2018;19:639. doi: 10.3390/ijms19020639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tian M., Win J., Song J., van der Hoorn R., van der Knaap E., Kamoun S. A Phytophthora infestans cystatin-like protein targets a novel tomato papain-like apoplastic protease. Plant Physiol. 2007;143:364–377. doi: 10.1104/pp.106.090050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang M., Ismayil A., Liu Y. Autophagy in plant-virus interactions. Annu. Rev. Virol. 2020;7:403–419. doi: 10.1146/annurev-virology-010220-054709. [DOI] [PubMed] [Google Scholar]
- 22.Cheval C., Aldon D., Galaud J.P., Ranty B. Calcium/calmodulin-mediated regulation of plant immunity. Biochim. Biophys. Acta. 2013;1833:1766–1771. doi: 10.1016/j.bbamcr.2013.01.031. [DOI] [PubMed] [Google Scholar]
- 23.Chiasson D., Ekengren S.K., Martin G.B., Dobney S.L., Snedden W.A. Calmodulin-like proteins from Arabidopsis and tomato are involved in host defense against Pseudomonas syringae pv. Tomato. Plant Mol. Biol. 2005;58:887–897. doi: 10.1007/s11103-005-8395-x. [DOI] [PubMed] [Google Scholar]
- 24.Takabatake R., Karita E., Seo S., Mitsuhara I., Kuchitsu K., Ohashi Y. Pathogen-induced calmodulin isoforms in basal resistance against bacterial and fungal pathogens in tobacco. Plant Cell Physiol. 2007;48:414–423. doi: 10.1093/pcp/pcm011. [DOI] [PubMed] [Google Scholar]
- 25.Nakahara K.S., Masuta C., Yamada S., Shimura H., Kashihara Y., Wada T.S., Meguro A., Goto K., Tadamura K., Sueda K., et al. Tobacco calmodulin-like protein provides secondary defense by binding to and directing degradation of virus RNA silencing suppressors. Proc. Natl. Acad. Sci. USA. 2012;109:10113–10118. doi: 10.1073/pnas.1201628109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li F., Huang C., Li Z., Zhou X. Suppression of RNA silencing by a plant DNA virus satellite requires a host calmodulin-like protein to repress RDR6 expression. PLoS Pathog. 2014;10:e1003921. doi: 10.1371/journal.ppat.1003921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bhattacharjee S., Lee L.Y., Oltmanns H., Cao H., Veena , Cuperus J., Gelvin S.B. IMPa-4, an Arabidopsis importin α isoform, is preferentially involved in Agrobacterium-mediated plant transformation. Plant Cell. 2008;20:2661–2680. doi: 10.1105/tpc.108.060467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chung Y.H., Lacatus G., Sunter G. Geminivirus AL2 protein induces expression of, and interacts with, a calmodulin-like gene, an endogenous regulator of gene silencing. Virology. 2014;460–461:108–118. doi: 10.1016/j.virol.2014.04.034. [DOI] [PubMed] [Google Scholar]
- 29.Li F., Zhao N., Li Z., Xu X., Wang Y., Yang X., Liu S.S., Wang A., Zhou X. A calmodulin-like protein suppresses RNA silencing and promotes geminivirus infection by degrading SGS3 via the autophagy pathway in Nicotiana benthamiana. PLoS Pathog. 2017;13:e1006213. doi: 10.1371/journal.ppat.1006213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guerra-Peraza O., Kirk D., Seltzer V., Veluthambi K., Schmit A.C., Hohn T., Herzog E. Coat proteins of Rice tungro bacilliform virus and Mungbean yellow mosaic virus contain multiple nuclear-localization signals and interact with importin alpha. J. Gen. Virol. 2005;86:1815–1826. doi: 10.1099/vir.0.80920-0. [DOI] [PubMed] [Google Scholar]
- 31.Haas G., Azevedo J., Moissiard G., Geldreich A., Himber C., Bureau M., Fukuhara T., Keller M., Voinnet O. Nuclear import of CaMV P6 is required for infection and suppression of the RNA silencing factor DRB4. EMBO J. 2008;27:2102–2112. doi: 10.1038/emboj.2008.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Y., Zhang X., Niu S., Han C., Yu J., Li D. Nuclear localization of Beet black scorch virus capsid protein and its interaction with importin α. Virus Res. 2011;155:307–315. doi: 10.1016/j.virusres.2010.10.029. [DOI] [PubMed] [Google Scholar]
- 33.Lukhovitskaya N.I., Cowan G.H., Vetukuri R.R., Tilsner J., Torrance L., Savenkov E.I. Importin-α-mediated nucleolar localization of Potato mop-top virus TRIPLE GENE BLOCK1 (TGB1) protein facilitates virus systemic movement, whereas TGB1 self-interaction is required for cell-to-cell movement in Nicotiana benthamiana. Plant Physiol. 2015;167:738–752. doi: 10.1104/pp.114.254938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pérez-Cañamás M., Hernández C. New insights into the nucleolar localization of a plant RNA virus-encoded protein that acts in both RNA packaging and RNA silencing suppression: Involvement of importins α and relevance for viral infection. Mol. Plant Microbe Interact. 2018;31:1134–1144. doi: 10.1094/MPMI-02-18-0050-R. [DOI] [PubMed] [Google Scholar]
- 35.Bhattacharyya D., Chakraborty S. Chloroplast: The Trojan horse in plant-virus interaction. Mol. Plant Pathol. 2018;19:504–518. doi: 10.1111/mpp.12533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhai Y., Peng H., Neff M.M., Pappu H.R. Emerging molecular links between plant photomorphogenesis and virus resistance. Front. Plant Sci. 2020;11:920. doi: 10.3389/fpls.2020.00920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Slaymaker D.H., Navarre D.A., Clark D., del Pozo O., Martin G.B., Klessig D.F. The tobacco salicylic acid-binding protein 3 (SABP3) is the chloroplast carbonic anhydrase, which exhibits antioxidant activity and plays a role in the hypersensitive defense response. Proc. Natl. Acad. Sci. USA. 2002;99:11640–11645. doi: 10.1073/pnas.182427699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Restrepo S., Myers K.L., del Pozo O., Martin G.B., Hart A.L., Buell C.R., Fry W.E., Smart C.D. Gene profiling of a compatible interaction between Phytophthora infestans and Solanum tuberosum suggests a role for carbonic anhydrase. Mol. Plant Microbe Interact. 2005;18:913–922. doi: 10.1094/MPMI-18-0913. [DOI] [PubMed] [Google Scholar]
- 39.Kravats A.N., Hoskins J.R., Reidy M., Johnson J.L., Doyle S.M., Genest O., Masison D.C., Wickner S. Functional and physical interaction between yeast Hsp90 and Hsp70. Proc. Natl. Acad. Sci. USA. 2018;115:E2210–E2219. doi: 10.1073/pnas.1719969115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hahn A., Bublak D., Schleiff E., Scharf K.D. Crosstalk between Hsp90 and Hsp70 chaperones and heat stress transcription factors in tomato. Plant Cell. 2011;23:741–755. doi: 10.1105/tpc.110.076018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aparicio F., Thomas C.L., Lederer C., Niu Y., Wang D., Maule A.J. Virus induction of heat shock protein 70 reflects a general response to protein accumulation in the plant cytosol. Plant Physiol. 2005;138:529–536. doi: 10.1104/pp.104.058958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gorovits R., Moshe A., Ghanim M., Czosnek H. Recruitment of the host plant heat shock protein 70 by Tomato yellow leaf curl virus coat protein is required for virus infection. PLoS ONE. 2013;8:e70280. doi: 10.1371/journal.pone.0070280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lu R., Malcuit I., Moffett P., Ruiz M.T., Peart J., Wu A.J., Rathjen J.P., Bendahmane A., Day L., Baulcombe D.C. High throughput virus-induced gene silencing implicates heat shock protein 90 in plant disease resistance. EMBO J. 2003;22:5690–5699. doi: 10.1093/emboj/cdg546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Earley K.W., Haag J.R., Pontes O., Opper K., Juehne T., Song K., Pikaard C.S. Gateway-compatible vectors for plant functional genomics and proteomics. Plant J. 2006;45:616–629. doi: 10.1111/j.1365-313X.2005.02617.x. [DOI] [PubMed] [Google Scholar]
- 45.Wang L., Ding X., Xiao J., Jiménez-Gόngora T., Liu R., Lozano-Durán R. Inference of a geminivirus-host protein-protein interaction network through affinity purification and mass spectrometry analysis. Viruses. 2017;9:275. doi: 10.3390/v9100275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sang Y., Wang Y., Ni H., Cazalé A.C., She Y.M., Peeters N., Macho A.P. The Ralstonia solanacearum type III effector RipAY targets plant redox regulators to suppress immune responses. Mol. Plant Pathol. 2018;19:129–142. doi: 10.1111/mpp.12504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.UniProt Consortium UniProt: A worldwide hub of protein knowledge. Nucleic Acids Res. 2019;47:D506–D515. doi: 10.1093/nar/gky1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brodbelt J.S., Russell D.H. Focus on the 20-year anniversary of SEQUEST. J. Am. Soc. Mass Spectrom. 2015;26:1797–1798. doi: 10.1007/s13361-015-1264-1. [DOI] [PubMed] [Google Scholar]
- 49.Peng H., Neff M.M. Circadian Clock Associated 1 and ATAF2 differentially suppress cytochrome P450-mediated brassinosteroid inactivation. J. Exp. Bot. 2020;71:970–985. doi: 10.1093/jxb/erz468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mi H., Muruganujan A., Ebert D., Huang X., Thomas P.D. PANTHER version 14: More genomes, a new PANTHER GO-slim and improvements in enrichment analysis tools. Nucleic Acids Res. 2019;47:D419–D426. doi: 10.1093/nar/gky1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Geisler-Lee J., O’Toole N., Ammar R., Provart N.J., Millar A.H., Geisler M. A predicted interactome for Arabidopsis. Plant Physiol. 2007;145:317–329. doi: 10.1104/pp.107.103465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Oughtred R., Stark C., Breitkreutz B.J., Rust J., Boucher L., Chang C., Kolas N., O’Donnell L., Leung G., McAdam R., et al. The BioGRID interaction database: 2019 update. Nucleic Acids Res. 2019;47:D529–D541. doi: 10.1093/nar/gky1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kerrien S., Aranda B., Breuza L., Bridge A., Broackes-Carter F., Chen C., Duesbury M., Dumousseau M., Feuermann M., Hinz U., et al. The IntAct molecular interaction database in 2012. Nucleic Acids Res. 2012;40:D841–D846. doi: 10.1093/nar/gkr1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Perez-Riverol Y., Csordas A., Bai J., Bernal-Llinares M., Hewapathirana S., Kundu D.J., Inuganti A., Griss J., Mayer G., Eisenacher M., et al. The PRIDE database and related tools and resources in 2019: Improving support for quantification data. Nucleic Acids Res. 2019;47:D442–D450. doi: 10.1093/nar/gky1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.