Abstract
OBJECTIVES:
Concern about falling is common among older hypertension patients and could impact decisions to intensify blood pressure therapy. Our aim was to determine whether intensive therapy targeting a systolic blood pressure (SBP) of 120 mm Hg is associated with greater changes in concern about falling when compared with standard therapy targeting an SBP of 140 mm Hg.
DESIGN:
Subsample analysis of participants randomized to either intensive or standard therapy in the Systolic Blood Pressure Intervention Trial (SPRINT).
SETTING:
Approximately 100 outpatient sites.
PARTICIPANTS:
A total of 2313 enrollees in SPRINT; participants were all age 50 or older (mean = 69 y) and diagnosed with hypertension.
MEASUREMENTS:
Concern about falling was described by the shortened version of the Falls Efficacy Scale International as measured at baseline, 6 months, 1 year, and annually thereafter.
RESULTS:
Concern about falling showed a small but significant increase over time among all hypertension patients. No differences were noted, however, among those randomized to intensive vs standard therapy (P = .95). Among participants younger than 75 years, no increase in concern about falling over time was noted, but among participants aged 75 years and older, the mean falls self-efficacy score increased by .3 points per year (P < .0001). No differences were observed between the intensive and standard treatment groups when stratified by age (P = .55).
CONCLUSION:
Intensive blood pressure therapy is not associated with increased concern about falling among older hypertension patients healthy enough to participate in SPRINT.
Keywords: hypertension, falls, concern about falling
The Systolic Blood Pressure Intervention Trial (SPRINT)1 published in 2015 and the 2017 American College of Cardiology/American Heart Association hypertension guideline2 emphasize treating hypertension in older people to a lower systolic blood pressure (SBP), with a guideline-recommended target of lower than 130 mm Hg. Such intensive therapy reduces cardiovascular morbidity, mortality, and the development of cognitive impairment,1–3 and it can be done without decrements in health-related quality of life.4,5 Many older people, however, may be concerned about falls and fall-related injuries6 as well as symptoms related to hypotension.7 Such concerns could limit decisions to intensify blood pressure therapy; in one study, nearly 50% of older people preferred not to intensify antihypertensive therapy because of concerns about falls and other adverse effects.8 A better understanding of concern about falling, also known as falls self-efficacy (FSE), may then be important in clinicians’ discussions with patients about hypertension therapy goals.
Concern about falling is common among community-dwelling older adults.9,10 A longitudinal study showed that concern about falling increases over time, especially in the presence of multiple falls.11 Less is known on concern about falling in older people with hypertension. Using baseline data from SPRINT, we showed that 47.2% of older hypertension patients have some concern about falling.12 However, neither lower blood pressures nor more antihypertensive medications was associated with greater concern. It is unknown whether intensive blood pressure therapy results in increased concern about falling.
We therefore used data from SPRINT to address the following questions. First, among older hypertension patients, does concern about falling change over time? Second, is intensive blood pressure therapy targeting an SBP of 120 mm Hg associated with greater changes in concern about falling when compared with standard therapy targeting 140 mm Hg? Finally, is the impact of intensive therapy on concern about falling different in patients older than 75 years when compared with younger patients?
METHODS
Study methods for SPRINT and the measure of FSE were previously described.12,13 Briefly, SPRINT is a multicenter randomized controlled trial comparing a standard care strategy targeting an SBP of 140 mm Hg with an intensive care strategy targeting 120 mm Hg. The study population consisted of patients 50 years or older with a screening visit SBP between 130 and 180 mm Hg. Participants were required to be at elevated risk for cardiovascular events as evidenced by clinical or subclinical cardiovascular disease, chronic kidney disease, an elevated Framingham risk score, or age 75 years or older. Individuals with diabetes, prior stroke, or an SBP after 1 minute of standing below 110 mm Hg were excluded. Enrollment began in November 2010 and ended in March 2013 following recruitment of 9361 subjects from more than 100 outpatient sites. The study was approved by each site’s institutional review board and registered with clinical trials.gov (NCT01206062).
The present analyses are based on a subsample of SPRINT selected to receive additional assessments including FSE, captured using the short version of the Falls Efficacy Scale-International (FES-I).14 Concern about falling in performing each of seven items is rated on a 1 (not at all concerned) to 4 (very concerned) scale (Supplementary Table S1). Scores can range from 7 to 28; 8 to 10 is considered mild concern, and 11 or higher is moderate to severe concern.12 Evaluations of the FES-I report good reliability and validity.11,15,16 Having a fall compared with not having had a fall was reported as associated with a small effect size of .2 standard deviations (SDs), corresponding to a 1 point higher score on the short FES-1.14 FSE was measured at baseline, 6 months, 1 year, and annually thereafter until study end. Information on the occurrence of injurious falls was collected quarterly and in the reporting of serious adverse events.7
Other data used in the analyses were captured at baseline. Demographic data included age, sex, and ethnicity. Physical examination findings included body mass index and SBP. Comorbid illnesses included chronic kidney disease and a count of the 30 conditions used in the Selim index.17 Health-related quality of life was measured using physical and mental component scores from the Veterans RAND 12 Item Questionnaire.18 Depressive symptoms were described using the Patient Health Questionnaire.19 Social supports were addressed with a single item on whether the participant lived alone. Frailty status was based on a 36-item index that classified people as frail, vulnerable, or not frail.20
Analyses
All analyses are based on data through the planned end of the trial in the summer of 2016. Descriptive statistics with means and SDs for continuous measures and frequency distributions for categorical variables were measured according to treatment intensity category. Linear mixed models were developed to compare the longitudinal trajectory of the FES-I score between the two treatment groups using Proc Mixed in SAS v.9.4 (SAS Institute, Inc, Cary, NC) under data missing at random assumptions. The initial model adjusted only for treatment group and time as fixed effects and subject as random effect. Next, independent patient variables from baseline were incorporated into the model as covariates. Models were subsequently repeated stratified by age. Interactions among time, treatment group, and age group were examined with P < .05 considered statistically significant. Other prespecified subgroups (sex, black race, cardiovascular disease history, chronic kidney disease, and SBP tertile) and frailty status were examined in a similar fashion as age. Finally, the occurrence of an injurious fall was entered into models as a time-dependent variable. Sensitivity analyses restricted to visits on or before August 20, 2015, the date the National Heart, Lung, and Blood Institute accepted the Data and Safety Monitoring Board’s recommendation to stop the intervention, produced similar results.
RESULTS
Participants were 2313 hypertension patients from the full SPRINT sample of 9361 people. Participants randomized to intensive blood pressure therapy were similar to those receiving standard therapy (Supplementary Table S2). The mean age of the sample was 69 ± 10.3 years, and 37.5% were 75 years or older. A history of cardiovascular disease was present in 21.8%, and 28.4% were classified as frail. Follow-up assessments were available on 2102 people at 1 year, 1996 at 2 years, and 1428 at 3 years. Few participants had an assessment at 4 years.
The mean FSE score at baseline for the full sample was 8.96 ± 3.46; 416 people (18.0%) had a score of 11 or higher. The FSE score significantly (P < .0001) increased to 9.07 ± 3.52 at 3 years; 20.1% had a score of 11 or higher. The mean increase in the FSE score in the regression analysis was .11 points per year, and there were no differences over time between the intensive and standard treatment groups (P for time × treatment interaction = .95) (Table 1, Model 1, and Figure 1). Results were similar after adjusting for covariates (P = .71) (Table 1, Model 2).
Table 1.
Regression Results Estimating Change in Falls Self-Efficacy Score Over Time and by Age and Treatment Group
| FSE change | ||||||
|---|---|---|---|---|---|---|
| Participants | Randomized group | Points per year | 95% CI | P value for time × treatment interaction | P value for time × age group interaction | |
| Model 1a | All | Intensive | .11 | (.05 to .17) | .95 | NA |
| Standard | .11 | (.05 to .17) | ||||
| <75 y | Intensive | .01 | (−.06 to .08) | .55 | <.0001 | |
| Standard | .05 | (−.02 to .12) | ||||
| ≥75 y | Intensive | .34 | (.24 to .45) | |||
| Standard | .25 | (.14 to .36) | ||||
| Model 2a | All | Intensive | .12 | (.06 to .18) | .71 | NA |
| Standard | .11 | (.05 to .17) | ||||
| <75 y | Intensive | .03 | (−.05 to .10) | .47 | <.0001 | |
| Standard | .05 | (−.02 to .12) | ||||
| ≥75 y | Intensive | .35 | (.24 to .46) | |||
| Standard | .26 | (.15 to .37) | ||||
Abbreviations: CI, confidence interval; FSE, falls self-efficacy score; NA, not applicable.
Model 1 adjusted only for treatment and time; model 2 adjusted for treatment, time, age > 75 years, sex, black race, Hispanic ethnicity, cardiovascular disease history, chronic kidney disease status, systolic blood pressure tertile, Veterans RAND 12 Item Health Survey physical and mental scores, Patient Health Questionnaire-9 score, number of chronic diseases, body mass index, living with others, and frailty status.
Figure 1.
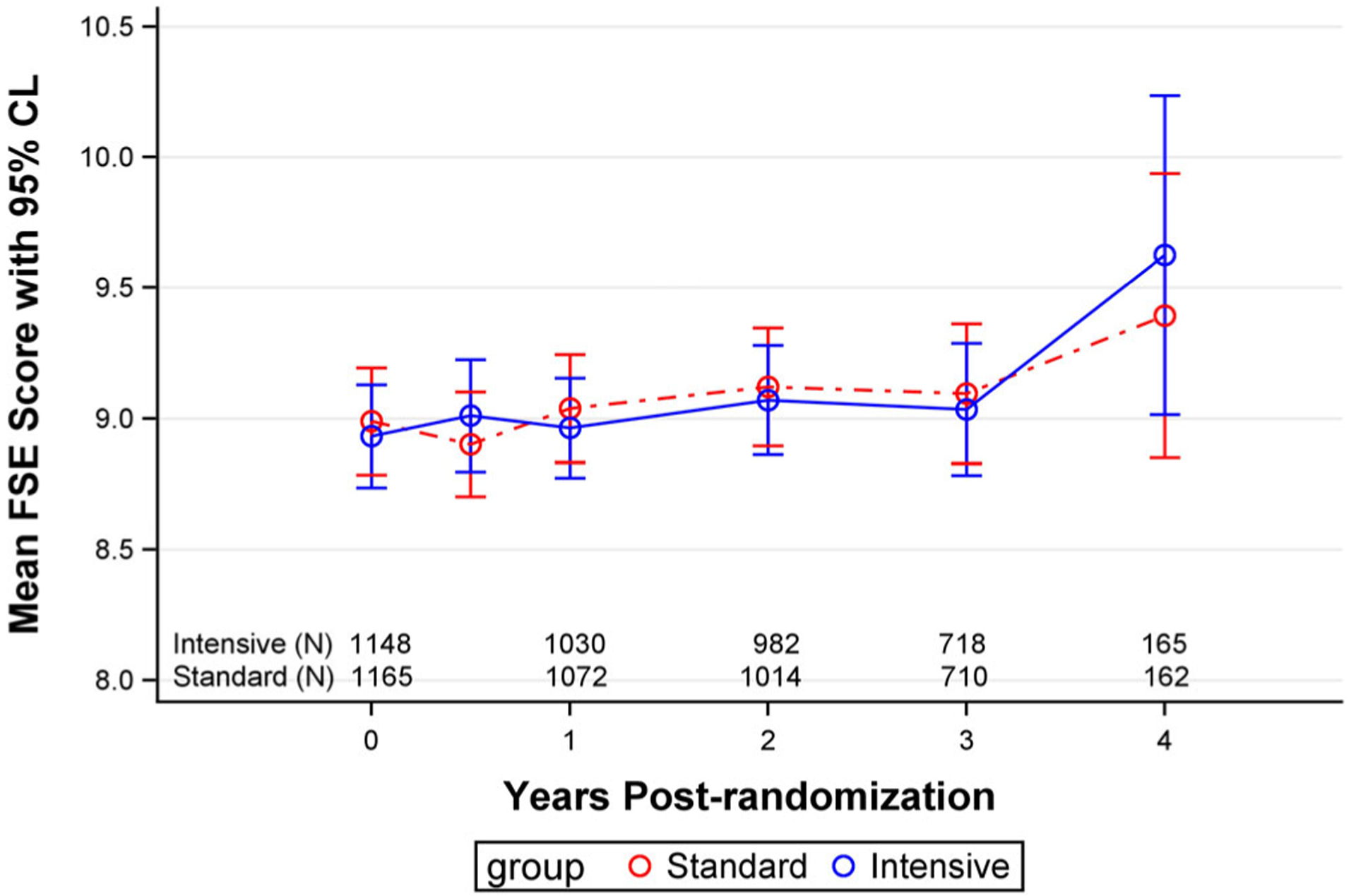
Falls self-efficacy (FSE) scores over time in the intensive and standard treatment groups. No differences were present between the two groups (P = .95). CL, confidence limit.
Among participants younger than 75 years, there was no increase in concern about falling over time (Figure 2 and Table 1). However, among participants aged 75 years and older, the mean FSE score increased by .3 points per year, and this difference between age groups was significant even after adjusting for covariates (P < .0001). No differences were observed between the intensive and standard treatment groups when stratified by age (P for time × treatment interaction = .55) (Figure 2). Similarly, there were no differences over time on concern about falling when stratified for other prespecified subgroups (P > .05 for all).
Figure 2.
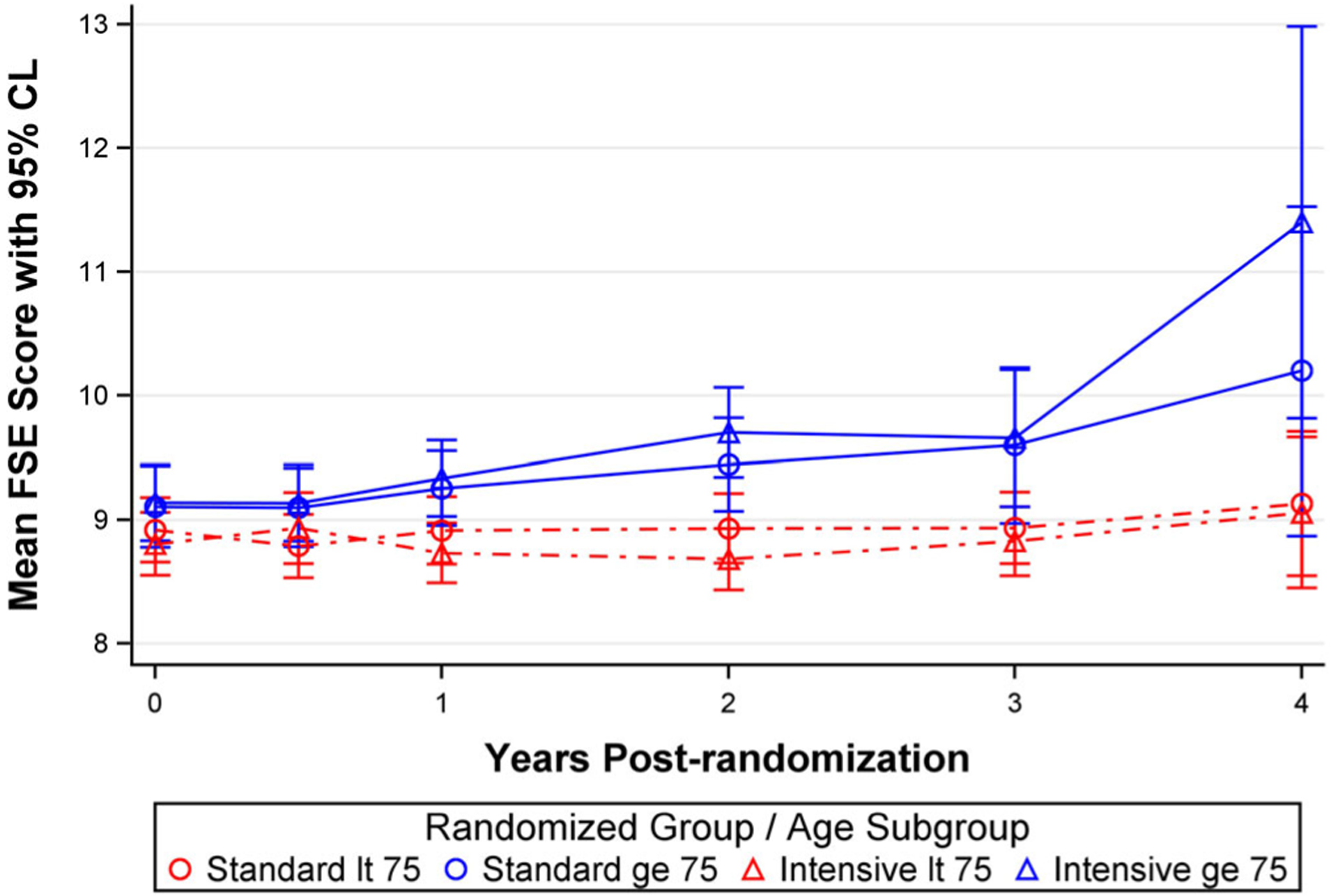
Falls self-efficacy (FSE) scores over time in the intensive and standard treatment groups stratified by age less than (lt) 75 years versus greater than or equal (ge) to 75 years. No differences were present between the two groups (P = .55). CL, confidence limit.
Injurious falls occurred in 63 participants (2.7%). The occurrence of an injurious fall resulted in a significant 2.1-point increase in the FSE score (Supplementary Table S3, Model 1). Thirteen injurious falls occurred in participants younger than 75 years. A significant fall by age group interaction (P = .01) indicated that much of the increase in the FSE score following an injurious fall occurred in those younger than 75 years with little change in those 75 years or older. Results were similar after adjusting for covariates (Supplementary Table S3, Model 2).
DISCUSSION
Achieving recommended treatment goals in the management of hypertension may increasingly require more intensive use of antihypertensive medications. Such increases in therapy can only be achieved through meaningful discussions between patients and providers that consider benefits and risks. Concern about falling was identified as one reason why older hypertension patients may be unwilling to increase medications.8 Yet little is known about concern regarding falling and the impact of intensive therapy in older hypertension patients. We previously demonstrated that concern about falling was highly prevalent among older hypertension patients.12 We now used longitudinal data from SPRINT to supply additional information about this common concern.
Our results demonstrate that among older hypertension patients, concern about falling significantly increases over time. But the change in the FSE score was relatively small and clinically insignificant. Few studies have examined changes in FSE over time. In one study of 500 community-dwelling people aged 70 to 90 years, the FES-I score increased over 1 year from a mean of 9.4 to 10.0 points.11 These results are comparable with SPRINT.
Importantly, intensive therapy targeting an SBP of 120 mm Hg was not associated with a greater increase in concern about falling when compared with 140 mm Hg. Even among people aged 75 years or older, no difference was found between intensive and standard therapy. These results add to the literature demonstrating that lower blood pressure targets can be safe and are unassociated with declines in patient-reported outcomes. In both SPRINT and the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial, intensive therapy was not associated with meaningful changes in health-related quality of life.4,5 As with other clinical trials,21,22 the lower blood pressure target in SPRINT was unaccompanied by increases in injurious falls.7
Having an injurious fall was associated with an increase in concern about falling. This is consistent with another study that showed multiple falls were associated with an increase in the FSE score.11 The impact of an injurious fall, however, was mostly seen in people younger than 75 years, perhaps because they feel less vulnerable than older people until they actually fall.
Several limitations should be noted. Assessments were performed at fixed time points. It could be that the greatest impact on concern about falling may occur shortly after intensifying therapy. The risk of hip fracture is greatest in the 45 days following initiation of antihypertensive therapy,23 but we were limited to a 6-month assessment to capture the effect of the initial therapy intensification. Some participants were lost to follow-up or were censored following a study end point. We cannot be certain how this impacted study conclusions. It is likely that people lost to follow-up were sicker and might have had greater concern about falling. More detailed information on all falls, not just injurious falls, may be important in understanding concern about falling. Finally, SPRINT recruited many community-dwelling older people with multiple comorbidities and reduced health-related quality of life. Yet older people with multiple comorbidities are often under-represented in clinical trials,24 and the SPRINT results may then not apply to many of the frailest individuals seen in geriatrics practice.25
SPRINT has added significant new information about concern about falling in older hypertension patients. We now know it is highly prevalent but not associated with lower blood pressure or more medications.11 Moreover, intensive therapy, on average, does not lead to worsening concern about falling. This finding should not be interpreted as implying that blood pressure therapy can be increased without any consideration of concern about falling because individual patients may respond differently. Additionally, SPRINT was designed with an SBP target so that people receiving intensive therapy who developed side effects could have their medications tapered back despite not being at target.
In conclusion, shared decision making is necessary when considering intensifying hypertension therapy to achieve recommended blood pressure targets. Risks and benefits of treatment intensification identified in the literature need to be applied to individual patients. These discussions should incorporate this new information, so patients fully understand all implications of treatment intensification.
Supplementary Material
Supplementary Table S1: Items on the Short Falls Efficacy Scale International. Each item is scored on a 1 to 4 scale with higher scres indication greater concern about falling.
Supplementary Table S2: Baseline characteristics of the study sample.
Supplementary Table S3: Impact of Injurious Falls on Falls Self-Efficacy (FSE) Score.
ACKNOWLEDGMENTS
Financial Disclosure: The Systolic Blood Pressure Intervention Trial (SPRINT) is funded with federal funds from the National Heart, Lung, and Blood Institute, the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute on Aging, and the National Institute of Neurological Disorders and Stroke under contract numbers HHSN268200900040C, HHSN268200900046C, HHSN26 8200900047C, HHSN268200900048C, HHSN268200900 049C, and Inter-Agency Agreement A-HL-13-002-001. Some of this material is the result of work supported with resources and the use of facilities through the Department of Veterans Affairs. The SPRINT investigators acknowledge the contribution of study medications (azilsartan and azilsartan combined with chlorthalidone) from Takeda Pharmaceuticals Inc.
We also acknowledge the support from the following Clinical and Translational Science Awards funded by the National Center for Advancing Translational Sciences: Case Western Reserve University: UL1TR000439; Ohio State University: UL1RR025755; University of Pennsylvania: UL1 RR024134 and UL1TR000003; Boston: UL1RR025771; Stanford: UL1TR000093; Tufts: UL1RR025752, UL1TR 000073, and UL1TR001064; University of Illinois: UL1TR0 00050; University of Pittsburgh: UL1TR000005; UT Southwestern: 9U54TR000017-06; University of Utah: UL1TR00 0105-05; Vanderbilt University: UL1 TR000445; George Washington University: UL1TR000075; University of California, Davis: UL1 TR000002; University of Florida: UL1 TR000064; University of Michigan: UL1TR000433; Tulane University: P30GM103337 COBRE Award NIGMS.
Sponsor’s Role: Scientists at the National Institutes of Health (the sponsor) participated in the design of the study and as a group had one vote on the steering committee of the trial.
Footnotes
Conflict of Interest: None declared. All components of the SPRINT study protocol were designed and implemented by the investigators. The investigative team collected, analyzed, and interpreted the data. All aspects of manuscript writing and revision were carried out by the coauthors. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, the US Department of Veterans Affairs, or the US government. For a full list of contributors to SPRINT, see the supplementary acknowledgment list: ClinicalTrials.gov identifier: NCT01206062.
SUPPORTING INFORMATION
Additional Supporting Information may be found in the online version of this article.
REFERENCES
- 1.SPRINT Study Research Group. A randomized trial of intensive versus standard blood pressure control. N Engl J Med. 2015;373:2103–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:1269–1324. [DOI] [PubMed] [Google Scholar]
- 3.Williamson JD, Pajewski NM, Auchus AP, et al. Effect of intensive vs standard blood pressure control on probable dementia: a randomized clinical trial. JAMA. 2019;321:553–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Connor PJ, Venkat Narayan KM, Anderson R, et al. Effect of intensive versus standard blood pressure control on depression and health-related quality of life in type-2 diabetes. Diabetes Care. 2012;35:1479–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berlowitz DR, Foy CG, Kazis LE, et al. Impact of intensive blood pressure therapy on patient-reported outcomes. N Engl J Med. 2017;377:733–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tinetti ME, Han L, Lee DSH, et al. Antihypertensive medications and serious falls injuries in a nationally representative sample of older adults. JAMA Intern Med. 2014;175:588–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sink KM, Evans GW, Shorr RI, et al. Syncope, hypotension, and falls in the treatment of hypertension: results from the randomized clinical Systolic Blood Pressure Intervention Trial. J Am Geriatr Soc. 2018;66:679–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tinetti ME, McAvay GJ, Fried TR, et al. Health outcome priorities among competing cardiovascular, fall injury, and medication-related symptom outcomes. J Am Geriatr Soc. 2008;56:1409–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scheffer AC, Schuurmans MJ, van Dijk N, van der Hooft T, de Rooij SE. Fear of falling: measurement strategy, prevalence, risk factors and consequences among older persons. Age Ageing. 2008;37:19–24. [DOI] [PubMed] [Google Scholar]
- 10.Kumar A, Carpenter H, Morris R, Iliffe S, Kendrick D. Which factors are associated with fear of falling in community-dwelling older people? Age Ageing. 2014;43:76–84. [DOI] [PubMed] [Google Scholar]
- 11.Delbaere K, Close JCT, Mikolaizak AS, et al. The Fall Efficacy Scale International (FES-I). A comprehensive longitudinal validation study. Age Ageing. 2010;39:210–216. [DOI] [PubMed] [Google Scholar]
- 12.Berlowitz DR, Breaux-Shropshire T, Foy C, et al. Hypertension treatment and concern about falling: baseline data from the SPRINT study. J Am Geriatr Soc. 2016;64:2302–2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ambrosius WT, Sink KM, Foy CG, et al. The design and rationale of a multicenter clinical trial comparing two strategies for control of systolic blood pressure: the systolic blood pressure intervention trial (SPRINT). Clin Trials. 2014;11:532–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kempen GIJM, Yardley L, van Haastregt JCM, et al. The short FES-I: a shortened version of the Falls Efficacy Scale-International to assess fear of falling. Age Ageing. 2008;37:45–50. [DOI] [PubMed] [Google Scholar]
- 15.Yardley L, Beyer N, Hauer K, et al. Development and initial validation of the Falls Efficacy Scale International (FES-I). Age Ageing. 2005;34:614–619. [DOI] [PubMed] [Google Scholar]
- 16.Fiqueiredo D, Neves M. Falls Efficacy Scale-International: exploring psychometric properties with adult day care users. Arch Gerontol Geriatr. 2018;79:145–150. [DOI] [PubMed] [Google Scholar]
- 17.Selim AJ, Fincke BG, Ren XS, et al. Comorbidity assessments based on patient report: results from the Veterans Health Study. J Ambul Care Manage. 2004;27:281–295. [DOI] [PubMed] [Google Scholar]
- 18.Selim A, Rogers W, Fleishman J, et al. Updated U.S. population standard for the Veterans RAND 12 Item Health Survey (VR-12). Qual Life Res. 2009; 18:43–52. [DOI] [PubMed] [Google Scholar]
- 19.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pajewski NM, Williamson JD, Applegate WB, et al. Characterizing frailty status in the systolic blood pressure intervention trial. J Gerontol A Biol Sci Med Sci. 2016;71:649–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Curb J, Applegate W, Vogt T, et al. Systolic Hypertension in the Elderly Program Cooperative Group. Antihypertensive therapy and falls and fractures in the systolic hypertension in the elderly program. J Am Geriatr Soc. 1993;41:SA15. [Google Scholar]
- 22.Margolis KL, Palermo L, Vittinghoff E, et al. Intensive blood pressure control, falls, and fractures in patients with type 2 diabetes: the ACCORD trial. J Gen Intern Med. 2014;29:1599–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Butt DA, Mandami M, Austin PC, et al. The risk of hip fracture after initiating antihypertensive drugs in the elderly. Arch Intern Med. 2012;172:1739–1744. [DOI] [PubMed] [Google Scholar]
- 24.Kuchel GA. Inclusion of older adults in research: ensuring relevance, feasibility, and rigor. J Am Geriatr Soc. 2019;67:203–204. [DOI] [PubMed] [Google Scholar]
- 25.Rich MW, Ouslander JO. Hypertension in older adults in the wake of the systolic blood pressure intervention trial. J Am Geriatr Soc. 2018;66:652–654. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table S1: Items on the Short Falls Efficacy Scale International. Each item is scored on a 1 to 4 scale with higher scres indication greater concern about falling.
Supplementary Table S2: Baseline characteristics of the study sample.
Supplementary Table S3: Impact of Injurious Falls on Falls Self-Efficacy (FSE) Score.


