Abstract
Cortical development in humans is a long and ongoing process that continuously modifies the neural circuitry into adolescence. This is well represented by the dynamic maturation of the corpus callosum, the largest white matter tract in the brain. Callosal projection neurons whose long-range axons form the main component of the corpus callosum are evolved relatively recently with a substantial, disproportionate increase in numbers in humans. Though the anatomy of the corpus callosum and cellular processes in its development have been intensively studied by experts in a variety of fields over several decades, the whole picture of its development, in particular, the molecular controls over the development of callosal projections, still has many missing pieces. This review highlights the most recent progress on the understanding of corpus callosum formation with a special emphasis on the novel molecular players in the development of axonal projections in the corpus callosum.
Keywords: corpus callosum, callosal projections, cerebral cortex, development, cortical neurons
1. Introduction—The Corpus Callosum
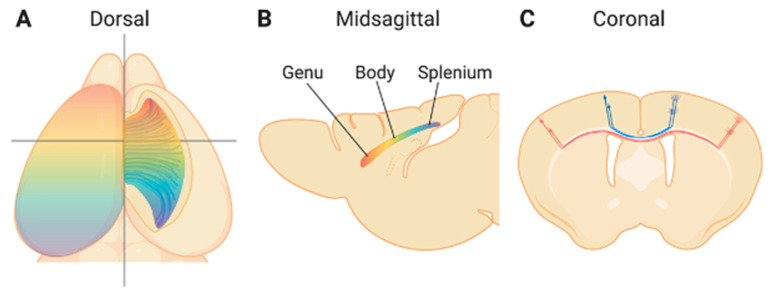
The corpus callosum (CC) is the largest white matter tract that connects two cerebral hemispheres of placental animals. The communication via approximately two hundred million callosal axons allows efficient information exchange between the two hemispheres, coordinating our higher-order motor, sensory, and cognitive tasks [1,2]. The human CC is anatomically divided into several regions that topographically connect two hemispheres: The anterior-most rostrum, genu, body, isthmus, and splenium at the posterior end, while the mouse CC is usually divided into three regions: genu, body, and splenium (Figure 1A,B). Along the anterior–posterior axis, the genu and rostrum connect the frontal and premotor regions of the cerebral cortex, the body conjoins the motor, somatosensory, and parietal regions, while the splenium links the temporal and occipital cortices on both sides [3,4,5]. Although the organization of the CC is defined anatomically, corresponding functional topography has been found based on imaging studies and studies on patients who underwent callosal resection, as well as studies using animal models. For example, neuronal signals for the motor function pass through the genu, while somatosensory inputs go through posterior body of CC. Axons in isthmus are in charge of transmitting auditory signals, and visual information via splenium [6,7]. Dorsal and ventral parts of the CC then connect the medial and lateral cortical regions, respectively [8,9,10] (Figure 1C). Different parts of the CC, therefore, consist of axonal projections from different cortical regions. In addition, callosal projections from each cortical region also include axons of cortical neurons in multiple cortical layers [11,12,13]. It is estimated that 80% of callosal axons come from neurons in layer II/III, and 20% are from neurons in layer V in the mature brain [11].
Figure 1.
The organization of the mouse corpus callosum (CC). Dorsal (A), midsagittal (B), and coronal (C) views of the CC. Callosal axon projections from each cortical hemisphere cross the midline and primarily connect with homotopic cortical regions in a topographic manner, by which projections from anterior and posterior cortical regions form anterior and posterior parts of the CC, respectively (represented by rainbow colors in A and B). Callosal projections from the medial and lateral regions of the cortex form the dorsal and ventral portions of the CC, respectively (C).
With these highly precise neuronal connections, the CC integrates inputs from left and right sides of the body for central processing and coordinates bimanual motor movements [14]. The presence of an intact, functional CC facilitates signal exchange between the two hemispheres [15]. The integrity and the efficiency of interhemispheric information exchange is also strongly correlated with social-cognitive functions [16,17,18]. As such, if the formation of CC is compromised (e.g., agenesis, dysgenesis, or hypoplasia) by genetic mutations or prenatal exposure to environmental insults [19,20,21,22,23], the abnormal CC leads to various transient and permanent symptoms. These include impairment in problem solving, dyslexia, ataxia, apraxia, alien limb, agraphia, paresis, and mutism [1,24]. Understanding the mechanisms of CC development is therefore critical to maintain the health and wellbeing of human life.
In the following sections, we will first review the findings on prenatal development of callosal projection, and move onto the less understood postnatal processes of callosal projection development. As this review focuses on the molecular mechanisms underlying these events, we leave out some important topics on CC development, including the formation of midline glial structure, myelination, and activity-dependent refinement, and instead only present informative reviews on these topics.
2. Prenatal Development of Callosal Projections
The formation of the CC begins with the projection of pioneer fibers from the cingulate and rostrolateral cortices [25,26,27] at around the 12th week of gestation (GW) in humans, followed by formation of the genu [20,28]. The fusion of these different parts of the CC completes at around GW14, and the CC expands as the brain increases in size [29,30]. Though each region grows at different rates, the overall volume of the CC increases rapidly in early development (GW19–21), and continues to grow at a slower rate until it reaches a plateau at around GW33 [29,31,32].
Our current understanding of prenatal development of the CC at the molecular and cellular levels have mainly come from studies using animal models such as mice, cats, and monkeys, while studies of fetal specimens have supplied the anatomical understanding of CC development in humans. Since there are many excellent reviews on the prenatal development of the CC available already (e.g., [11,33,34]), we will review this topic focusing on the latest discoveries.
2.1. Specification of Callosal Projection Neurons
Callosal projections in the mature brain consist of axons of pyramidal neurons in layer II/III and layer V, and to a lesser extent, of layer VI [21,35]. These cortical neurons that project axons to the contralateral hemisphere through the CC are callosal projection neurons (CPNs). CPNs project within the telencephalon, and are further classified based on their connecting target(s): (1) CPNs that only have one projection to the contralateral cortex, (2) CPNs that project to contralateral cortex and one of the striata, and (3) CPNs that have dual projections to contralateral cortex and ipsilateral frontal cortex [35]. Consistent with the general order of cortical layer development, CPNs in deeper layers (layer V/VI) are born and project to the contralateral target earlier than those in superficial layers (layer II/III) [11,26,36,37].
2.1.1. SATB2-mediated Specification
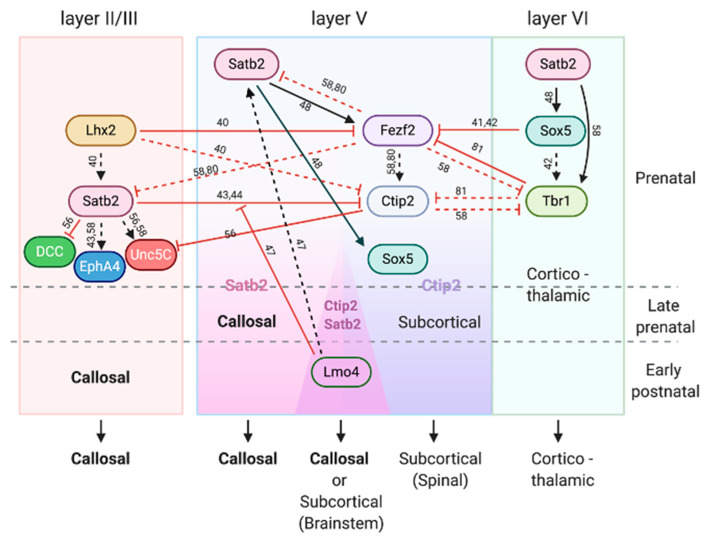
Specification of CPNs has been a topic of strong interest. SATB2, a protein involved in transcription regulation, is probably the most-studied molecule for CPN development. Many findings on the molecular mechanisms for specification of CPNs have been obtained through studies on the role and function of SATB2 [38,39]. Figure 2 summarizes the interactions between key transcription factors and axon guidance molecules (will be discussed in the next section) for the fate determination of CPNs and other projection neurons during the prenatal as well as early postnatal (will be discussed later) period [40,41,42].
Figure 2.
Interactions between key transcription factors and axon guidance molecules for CPN specification. SATB2-associated interactions during prenatal period define TBR1+ corticothalamic projection neurons in layer VI, SATB2+ CPNs in layer II/III, as well as SATB2+ CPNs and CTIP2+ subcortical (spinal) projection neurons in layer V. LMO4 further specifies CPNs or subcortical (brainstem) projection neurons from SATB2+/CTIP2+ populations during the early postnatal period in layer V. Regular arrows and flat-headed arrows indicate positive and negative regulations. Continuous lines indicate direct regulations, while dashed lines indicate suggested interactions demonstrated by knockout mouse and/or in utero electroporation experiments. Reference numbers supporting each interaction are indicated.
SATB2 plays a central role in defining the identity of CPNs in the developing brain. In seminal works by Britanova et al. and Alcamo et al., they independently utilized different Satb2 knockout (KO) mouse models to investigate the role of SATB2 on CPN specification and callosal projections [43,44]. Both groups reported that Satb2 KO mice failed to form the CC, and the loss of Satb2 in upper layer neurons led to expanded expression of CTIP2 (also known as BCL11B), a transcription factor that is expressed in subcortical projection neurons in deep layers and plays an essential role in their specification [43,44]. Such a transcriptional network is well conserved across mammalian species [45]. A protooncogene Ski cooperates with SATB2 in the direct transcriptional repression of Ctip2 [46]. Accordingly, Ski KO mice result in complete agenesis of the CC akin to Satb2 KO mice due to rerouted projection to the corticospinal tract or decreased CPN population [46].
Importantly, it has recently been shown that SATB2 is not necessary only for callosal projections, but also for sub-cerebral projections [47,48]. SATB2 promotes sub-cerebral projection neuron identity in layer V by directly activating transcription of Fezf2 and Sox5, while suppressing sub-cerebral projection neuron characters in CPNs in upper layers [48]. FEZF2 in turn negatively regulates SATB2 to suppress CPN characters in layer V sub-cerebral projection neurons [48]. Thus, SATB2 plays its roles in promoting these two different neuronal populations in a cell context-dependent manner.
In addition to these transcriptional regulations, expressions of SATB2 and CTIP2 are partially controlled by calcium signaling, histone methylation, and other mechanisms. Studies on Timothy Syndrome (TS), which is caused by a mutation in the L-type calcium channel Cav1.2, shed light on the importance of calcium on CPN differentiation. The TS mutation prohibits necessary expressional change of two Cav1.2 isoforms during early postnatal corticogenesis. Paşca et al. first pointed out the altered ratio of SATB2 and CTIP2 in neuronal cultures from TS patient-derived induced pluripotent stem (iPS) cells [49]. This observation has been followed up recently using in utero electroporation in mice [50]. The expression of mutated Cav1.2 in developing mouse brains reduced the expression of SATB2 and increased the expression of CTIP2. The altered ratio of SATB2 and CTIP2 expressions, therefore, may be due to the sustained calcium elevation resulting from the mutation.
Methyl transferase DOT1L has been previously reported to affect callosal development via interacting with AF9, a protein involved in transcription regulation [51]. It was recently shown that DOT1L-deficiency led to downregulation of SATB2 and upregulation of CTIP2 in embryonic mouse brains [52]. Chromatin remodeling mediated by an ATP-dependent chromatin remodeling factor SNF2H is required for embryonic expansion of intermediate progenitor cells and for the following specification of SATB2+ CPNs in the developing cerebral cortex. Telencephalon-specific knockout of Snf2h causes partial agenesis of the CC due to reduction of SATB2+ upper layer CNPs [53]. Inositol polyphosphate 4-phosphatase II (INPP4B), a PI (3, 4) P2 metabolizing 4-phosphatase, was another molecule found to regulate the formation of SATB2+ pyramidal neuron population and control callosal axon polarization, although the detailed mechanism has yet to be explored [54].
Timing of SATB2 expression may also be a critical factor for CPN fate specification. Premature SATB2 overexpression in the mouse cerebral cortex steers CPNs to acquire a marsupial-like projection fate, instead sending axons through the anterior commissure [45], which is phylogenetically the oldest of forebrain commissures. A recent study has similarly provided evidence that the peak birthdate of CTIP2+ laterally-projecting neurons in layer V is slightly earlier than that of SATB2+ medially-projection neurons within the same layer V, or even those in layer VI in the embryonic mouse lateral cortex. This observation has suggested a general sequential order in the generation of subcortical (lateral) projection neurons and callosal (medial) projection neurons [55].
The interactions of axon guidance cue Netrin-1 with its attractive and repulsive receptors DCC and UNC5C have been shown to be involved in the formation of callosal projections of deep layer CPNs in mice [56]. Likewise, mutations in DCC have been shown to cause agenesis of the CC in humans [57]. DCC is directly downregulated by SATB2 [56], while Unc5C is directly upregulated and downregulated by SATB2 and CTIP2, respectively [56,58]. SATB2 also directly upregulates the expression of EphA4, an Eph family receptor tyrosine kinase that mediates another major axon guidance pathway, Eph/ephrin signaling, in CPNs [58]. In summary, SATB2 and CTIP2 directly contribute to the establishment of specific neuronal connections of callosal and subcortical projection neurons by, at least in part, controlling downstream axon guidance molecules.
2.1.2. Other Players in CPN Specification
Despite decades of hard work described above and in literature, the mechanisms underlying CPN specifications remain to be further elucidated. There has been significant progress in the search for molecules potentially involved in CPN specification [11,35,59]. In pioneering studies by Molyneaux et al. and other groups, comparative microarray analyses on retrogradely labeled and isolated CPNs have identified a number of genes specifically expressed in mouse CPNs at several key embryonic and postnatal stages [36,60,61,62,63,64,65]. Enrichment of many of these genes in lower layer CPNs are highly conserved in the developing primate brain, while genes expressed in upper layer CPNs show greater variability in the degrees of conservation, supporting the hypothesis of evolutionary expansion of upper layer CPN subpopulations in primates [60].
The roles of multiple identified genes in CPN specification are just beginning to be elucidated. One such gene is a transcription factor, Foxg1 [66,67]. FOXG1 binds to an enhancer site of another transcription factor COUP-TF1, repressing its expression in the mouse somatosensory cortex [68]. Ectopic expression of FOXG1 in layer IV transforms local projection neurons in this layer to acquire pyramidal morphologies, SATB2 expression, and callosal projections, while removal of FOXG1 in layer II/III projection neurons de-represses COUP-TFI and converts them to layer IV neuron identity [68]. FOXG1 has been shown to also form a complex with another transcription factor, RP58, in these layer II/IIII neurons and directly repress the expression of Robo1 and Slit3, components of Slit/Robo signaling in axon guidance [66]. This repression is critical for guiding callosal axons to cross the midline, and inactivation of one allele of Foxg1 in cortical neurons is sufficient to cause agenesis of the CC [66].
Along with the progress in search of CPN-specifying genes, recent studies have revealed that CPNs in mature brains are more heterogenous than previously thought. RNA in situ hybridization combined with retrograde labeling of cortical neurons in mature mouse cortices has identified various molecules that are expressed in CPN subtypes [69]. More recent high-throughput screenings using bulk and single-cell RNA sequencing further advanced molecular identification of CPN subtypes [64,70]. Highly heterogenous transcriptional identities of both upper layer and lower layer CPNs have been found in these studies. The heterogeneity largely reflects their axonal targets rather than their birth dates or laminar positions [36]. This is in agreement with the finding that target specificity is a strong predictor of molecular identity of long-range projection neurons in layers V and VI [71]. Physiologically, upper layer CPNs have longer action potential duration and lower firing rate, while lower layer CPNs have higher firing rate, but smaller action potential width compared to upper layer CPNs, regardless of their localization [72]. Together, the specification of CPN subtypes during development determines complex combinations of shared and distinct cellular properties between them. Table 1 summarizes the recently reported molecules that are shown to affect CPN specification.
Table 1.
List of newly reported molecules that play roles in callosal projection neuron (CPN) specification.
| Molecule | Molecular Function | Cortical Expression | CPN Subgroup Identification | Roles in CPN Development | References |
|---|---|---|---|---|---|
| CAV1 | Lipid-bound scaffolding domain protein | Layer V in caudo-lateral cortex, late embryonic to early postnatal period | Dual projecting callosal/frontal projection neurons (CPN/FPN) | Not necessary for early specification of CPN/FPN; not necessary for dual axonal targeting; may function in postmitotic development and refinement | [62,63] |
| LMO4 | Probable transcriptional factor | Layer V during early differentiation (E15.5), then expands to all cortical layer by P0 and later stages | CPNs and subcerebral projection neurons in presumptive sensory-motor area; colocalized with SATB2 in layer V by P6 | Second backward projection development; molecular identity diversification of CPNs in rostral motor cortex | [73,74,75] |
| CITED2 | Transcriptional coactivator of the p300/CBP-mediated transcription complex | Subventricular zone at E15.5; layer II/III, V, and VI in postnatal somatosensory cortex | CPNs in somatosensory cortex | Necessary for acquiring molecular identity of upper layer CPNs in somatosensory cortex | [11,63,76] |
| CTIP1 | DNA-binding transcription factor | Embryonic callosal and corticothalamic projection neurons; high in all layers of somatosensory cortex and the most superficial aspect of layer II/III in motor cortex postnatally | Expressed by all CPNs | Repression of CTIP2 expression; specification of sensory area identity in CPNs and other neurons | [77,78,79] |
| FEZF2 | DNA-binding transcription factor | Forebrain progenitors and their progeny in layer V | No | Repression of SATB2 expression; specification of subcerebral neuron identity | [48,60,80,81,82] |
| SNF2H | ATP-dependent chromatin remodeling protein | Embryonic neural progenitors | No | Primes upper layer cortical neuron development | [53,83,84] |
| INPP4B | Enzyme involved in phosphatidylinositol signaling pathways | TBD | No | Controlling axon polarization and generation of SATB2+ pyramidal neuron population | [54] |
| DOT1L | Histone methyltransferase specific to H3K79 | Progenitor zone and cortical plate | TBD | Regulation of SATB2 and CTIP2 expression | [51,52,85] |
| ASCL1/NGN2 | Basic helix-loop-helix family transcription factors | Neural progenitors in the embryonic ventral and dorsal telencephalon, respectively | No | Regulate the generation of SATB2+ upper layer neurons | [86,87,88] |
| FOXG1 | Forked-head family transcription factor | Neural progenitors in embryonic cortex; high in layer II/III and lower layer V postnatally | No | Promotes SATB2 expression and layer II/III CPN specification; directly represses Robo1 and Slit3 expression; directly represses Coup-TF1 expression |
[66,68,89] |
| COUP-TF1 | Member of nuclear hormone receptor family of steroid hormone receptors | Superficial cortical plate in embryonic brain; layer IV and upper layer V postnatally | No | Promotes layer IV identity, while suppresses layer II/III and layer V specification | [68] |
2.2. Guidance of Callosal Axons
Guidance of callosal axons is a series of well-orchestrated interactions of attractive and repulsive cues that navigates extending axons to their targets on the contralateral side [21,90,91]. Callosal axons from lower cortical layers cross the midline during the embryonic period, while callosal axons from upper cortical layers do not reach the midline until postnatal stages [92]. The guidance cues for early callosal axon growth are mainly provided by midline glia and pioneering axons [27,93], but other brain tissues, e.g., meninges, or CPNs themselves, also participate in delivering axon guiding cues [94,95].
Guidance of callosal axons mediated by the midline glial structures has been most extensively studied [9,96,97,98,99,100]. The transient glial structure is formed at the midline of the embryonic brain [97,98,99,100,101,102], and consists of the radial glia-derived glial wedges (GW) located by the lateral ventricles [103], the indusium griseum (IG) at the dorsal midline, the midline zipper glia (MZG) at the ventral midline, and the glial slings (GS) that migrate from the ventricles and physically bridge the two hemispheres below the longitudinal cerebral fissure [100]. The GS is actually a migratory population of developing neurons in the subventricular zone [97]. Additionally, subgroups of GABAergic neurons from the ganglionic eminence also migrate into the midline, serving as guidepost cells and attracting projecting axons [97,104]. Since there are other excellent reviews on midline structure development [91,105], we will not discuss it in detail in this review.
Multiple signaling pathways coordinate the complex process of midline axon guidance. In addition to major axon guidance pathways such as Semaphorin/Plexin/Neuropilin, Slit/Robo, Eph/ephrin, and Netrin/DCC/Unc5 pathways, Wnt/Ryk and FGF8/MAPK pathways also partake in the process to a minor degree [106]. Recent studies have been continuously identifying novel components of each signaling pathway and links between them, filling in the missing pieces of the complex jigsaws of signaling required for developing precise interhemispheric connection. Table 2 provides the list of recently reported molecules that are involved in CPN axon guidance.
Table 2.
List of newly reported molecules that play roles in CPN axon guidance.
| Molecule | Molecular Function | Cortical Expression | Interacting Pathway | Roles in Callosal Axon Development | Reference |
|---|---|---|---|---|---|
| APP | Receptor-like membrane protein | Embryonic and neonatal CC and neuronal cell body in layer V | Slit/Robo | Serves as a Slit receptor and mediates axon repulsion | [107] |
| HSC70 | Molecular chaperone of the heat shock protein 70 (HSP70) family | Preferentially expressed in neurons | Netrin/DCC | Required for the stability of DCC/TRIO complex at the growth cone to mediate axon outgrowth and guidance | [108] |
| PLCγ1 | Signal transducer of receptor tyrosine kinases | Broadly expressed in the brain from embryonic to adult stages; strongly expressed in the cortex | Netrin/DCC | Triggers actin rearrangement for axonal growth | [109] |
| MARCKS | Cellular substrate for protein kinase C; F-actin crosslinking protein | Ubiquitous | Netrin/DCC | Mediates DCC activation via membrane recruitment of tyrosine kinases PTK2 and SRC | [110,111] |
| GPM6A and GPM6B | Glycoprotein localized in cholesterol-rich lipid rafts of the plasma membrane | Expressed in actively elongating axons in embryonic and neonatal brain | Extension and guidance of callosal axons | [112,113] |
2.2.1. Players in Semaphorin/Neuropilin/Plexin Pathway
Semaphorins are a family of secreted, transmembrane, or GPI-anchored proteins that are essential for axon guidance and other processes in neural development. Class 3 Semaphorins (SEMA3A and SEMA3C), play important roles in midline guidance of callosal axons through the signaling mediated by their coreceptors formed by Neuropilin-1 and Plexin-A1 [114,115]. In contrast to the classic view, a recent study has shown that SEMA3E signaling mediated by its receptor Plexin-D1 and the adaptor protein GIPC1 plays a role in layer positioning of CPNs, but not in projection of callosal axons [116,117]. Another update on Semaphorin signaling is its crosstalk with ephrin-B1 to control the navigation of post-crossing callosal axons [118]. After midline crossing, callosal axons switch off their response to axon guidance cues including SEMA3C that act as attractants for these axons before crossing the midline. This change is due to the inhibition of SEMA3C signaling by upregulated ephrin-B1 and its interaction with Neuropilin-1 independent of Eph receptors [118].
2.2.2. Players in Slit/Robo Pathway
Slit/Robo signaling plays a crucial role for proper guidance of both pre-crossing and post-crossing callosal axons [102,119,120]. Recently, amyloid precursor protein (APP), which is known to have a central role in Alzheimer’s disease, was identified as a novel receptor of Slit [107]. APP is strongly expressed in the embryonic and neonatal CC and layer V neurons along with other brain regions such as internal capsule, hippocampal commissure, and anterior commissure. Upon binding to Slit, APP transduces intracellular signaling to mediate axon repulsion. Double knockout of APP and its family member APLP2 in mice causes failure of callosal axons to cross the midline [107].
2.2.3. Players in Netrin/DCC/Unc5 Pathway
Netrin-1 can be of attractive or repulsive cue depending on the receptor it binds and downstream intracellular pathways [106,121,122]. Although an extensive amount of knowledge on the mechanisms of Netrin-1-mediated axon guidance has been gained through the research on neuronal projections other than callosal projections, several key findings have been brought from the studies on callosal axon guidance. For example, Netrin-1 has been shown to be attractive for callosal pioneering axons from the cingulate cortex to assist them cross the midline, but not for callosal axons from CPNs. Instead, Netrin-1 prevents SLIT2-mediated repulsion, allowing CPN axons to grow toward and across the midline [123]. Recent studies have added new players in the Netrin-1 pathway; the heat shock cognate protein HSC70 is required for the stability of the DCC/TRIO signaling complex at the growth cone to mediate Netrin-1-induced callosal axon outgrowth and guidance [108]. Similarly, myristoylated alanine-rich C-kinase substrate (MARCKS) mediates Netrin-1-induced DCC activation via membrane recruitment of tyrosine kinases PTK2 and SRC during CC formation [110]. It is also suggested that phospholipase C gamma1 (PLCγ1), a signal transducer of receptor tyrosine kinases, plays a key role in CC formation downstream of SRC kinase by triggering actin rearrangement for axonal growth [109].
3. Postnatal Development of Callosal Projections
The postnatal development of the CC is a process as elegant and complicated as the prenatal process. In primates, postnatal CC development completes at a much later stage compared to rodents [124], and this characteristic is considered to involve in the evolution of higher cognitive function [125]. Mass spectrometry-based proteomic profiling of developing mouse CC revealed that many proteins involved in axon growth and guidance reach their peak expression around P3, while proteins for neuronal maturation, glial development, myelin formation, and synapse formation increase their expression after P10, highlighting the dynamic and phased progress of postnatal CC development associated with the expression of a unique set of molecules at each stage [126]. During the early postnatal development of callosal projections, CPNs are further specified and additional callosal projections from upper layer CPNs continue to grow and cross the midline. This section will compile recent findings on the molecular mechanisms associated with these postnatal processes of callosal projection development. Although we do not discuss in this review, important processes of postnatal CC development include morphological and functional maturation of CPNs and their axons, myelination, and activity-dependent neurite and synapse growth and refinement as well [127,128,129,130].
3.1. Postnatal Specification of Callosal Projection Neurons
Specification of CPNs that starts from the prenatal period continues postnatally. Various molecules are involved in further establishing their identities, which include specific morphologies and gene expression. Transcription factors have a broad influence on CPN development, since their downstream gene regulation can collectively cover every single aspect of CPN identities. Transplantation of embryonic neurons that have already been fate-restricted to lower layer projection neurons or partially fate-restricted post-mitotic neuroblasts into early postnatal somatosensory cortex results in their integration as projection neuron subtypes with proper molecular and electrophysiological identities. They not only migrate into correct layer positions, but also form appropriate callosal and subcortical axonal projections [131], indicating that the regulations during prenatal period have predominant effects on the establishment of their final specificity.
On the other hand, embryonic and early postnatal CPNs in upper layers can be reprogrammed in vivo into layer V/VI neurons with molecular properties and axonal connectivity of corticofugal projection neurons, by forced expression of FEZF2 [132]. Efficiency of lineage reprograming is high by expressing FEZF2 from embryonic day (E) 17.5, but becomes lower by expressing FEZF2 from postnatal day (P) 3. Moreover, the reprogramming cannot be achieved by expressing FEZF2 after P21 [132]. This suggests that early post-mitotic upper layer CPNs still have plasticity to change their identity, but become progressively more fate-restricted during the postnatal period.
3.1.1. Transcription Factors
Transcription factors that are in effect during prenatal development continue their influence in postnatal stage. SATB2 is one such example. Although SATB2 was initially suggested to be a determinant of the CPN identity [12], later evidence indicates that SATB2 is required not only for CPNs, but also sub-cerebral projection neurons for their proper differentiation and axon pathfinding [80,133]. SATB2 and CTIP2 that respectively regulate the identity of callosal and sub-cerebral projection neurons are expressed in largely distinct neuronal populations during the prenatal period in layer V of the somatosensory cortex. However, the number of neurons co-expressing CTIP2 and SATB2 gradually increases after birth [47] (Figure 2). Neurons that postnatally co-express SATB2 and CTIP2 become two distinct neuronal subclasses projecting either contralaterally or to the brainstem, indicating that CTIP2/SATB2 co-expression plays a role in refining the neuronal property rather than specifying their identity [47]. Epigenetic modification of Ctip2 locus by a transcriptional adaptor molecule LMO4 underlies the CTIP2 expression in SATB2-positive neurons in a time- and area-specific manner [47].
Transcription factor CUX1 controls the formation of callosal projections of layer II/III CPNs by regulating the expression of Kv1 voltage-dependent potassium channels, which in turn modifies activity-dependent axon development [134]. CPNs with reduced CUX1 expression still project to the contralateral hemisphere normally until P8, but result in impaired axonal innervation into the contralateral cortical plate due to the inability to turn on Kv1-dependent firing responses. Restoring CUX1 expression from P8 rescues both the axonal and electrophysiological defects in these neurons [134].
Together, these findings demonstrate that postnatal expression of specific transcription factors is essential not only for establishing unique anatomical and electrophysiological properties of CPNs, but also for the activity-dependent formation of callosal neural circuit.
3.1.2. Other Players in CPN Specification
The roles of non-transcription-factor molecules in the development of callosal projections are also being characterized. The Cav1 gene that encodes a membrane-bound scaffolding protein Caveolin 1 (CAV1) is one of the genes identified in the aforementioned microarray study that described many genes enriched in isolated embryonic and postnatal CPNs [63]. CAV1 is specifically expressed in a unique subpopulation of layer V CPNs that maintain dual ipsilateral frontal projections with expression peaking early postnatally [62]. Although CAV1 is not required for the specification of these CPNs or formation of dual axonal projections, its unique expression pattern suggests its role in maturation and refinement of these neurons [62].
Specific neurotrophic factors have been shown to control the survival of CPNs at distinct postnatal stages using in vitro cultures [135]. A recent in vivo study has further demonstrated that layer V CPNs express IGF1R, the receptor of insulin-like growth factor 1 (IGF1). The survival of layer V CPNs requires microglia-derived IGF1 acting as a trophic factor during early postnatal development [136]. The authors suggest that this is because these neurons do not receive sufficient trophic signals from their targets in the early postnatal period, as they have not yet penetrated or started branching in their targets. Although how these neurons acquire IGF1R expression is unknown, it highlights one aspect of their identity required during postnatal development.
3.2. Callosal Axon Guidance During the Postnatal Period
Callosal axons from lower cortical layers cross the midline during the embryonic period, while callosal axons from upper cortical layers reach the midline during the postnatal stage [92]. Although the mechanisms underlying the axon guidance through the midline during prenatal period have been extensively studied [9,86,96,100,102], the mechanisms of postnatal callosal axon guidance remains to be addressed.
Fate-restricted embryonic CPNs that are transplanted into the early-postnatal cerebral cortex can migrate into correct cortical layers and form appropriate callosal connections [131], suggesting that early postnatal cortex provides an environment similar to that in prenatal brain, for postnatal axon guidance. Alternatively, later growing callosal axons may utilize priorly crossed axons as their guides [56]. Currently, there is no clear evidence for guidance deficits that are stage-specific to postnatally growing axons, or postnatal-specific axon guidance mechanisms that dissociate from prenatal mechanisms.
By developing a new approach which allows identification and quantification of local transcriptomes and proteomes from labelled growth cones of single projections in vivo, Poulopoulos et al. obtained paired subcellular proteomes and transcriptomes from single CPN subtypes in the P3 mouse brain [137]. Such systems-level approaches may provide new findings of subtype- and stage-specific molecular mechanisms of callosal axon guidance.
After crossing the midline and reaching the target cortical area in the contralateral hemisphere, callosal axons make a turn from the white matter toward the cortical plate, arborize, and establish synaptic connections. These late processes largely depend on activity-dependent mechanisms, and have been extensively reviewed elsewhere [129,138].
4. Conclusions and Future Directions
The development of the CC is a long, well-orchestrated and dynamic process that requires precise control of many different signaling molecules and pathways. During the prenatal and postnatal periods, CPN specification and axon growth/guidance are essential. These relatively well-studied processes still have many mysteries to be solved. High throughput transcriptomics and proteomics of developing CPNs and CC tissue identified hundreds more possible molecular players that require further verification and functional characterization. Whole-brain spatial transcriptomics revealed new area- and layer-specific subregions within the adult mouse cerebral cortex [139]. Cross-referencing these new findings with previous gene expression data would further validate and expand current understanding of CPN identities. Most studies on the specification of CPNs have been focused on the events in postmitotic neurons. Since neuronal identities can also be controlled during progenitor cell stage, such mechanisms need to be further studied. For example, CPNs in lower layers and upper layers have been shown to differentiate from the same fate-restricted pool of neural progenitors expressing transcription factor CUX2, sharing a common lineage irrespective of their final layer position [140] (although the fate-restricted nature of CUX2+ progenitor cells remains under debate: [141,142,143]).
There is also a large gap in our understanding of some important processes of CC development at the molecular level. These include the mechanisms of topographic targeting of callosal axons, postnatal control of callosal axon guidance, and the mechanisms of the refinement of the CC that involves massive elimination of initially overproduced callosal axons and maintenance/preservation of specific axons. Postnatal CC development may have higher demands for intracellular protein transport, correct protein folding, post-transcriptional processing, and cellular energy regulation [126,144], but these hypotheses remain to be tested. A recent mouse study has demonstrated that the elimination of developmentally transient callosal projections during postnatal refinement is more massive than previously expected, and the pruning even includes transient callosal axons from layer IV neurons that never form callosal connections in the mature brain [92].
It is also important to keep in mind the possible differences between species, as recent advance in gene expression profiling reveals differences in gene expression in brain cells between humans and other species [145,146].
In summary, research on the development of callosal projection has entered a new phase at the molecular level, and is expected to develop and progress rapidly in the near future.
Acknowledgments
We thank Kazue Hashimoto-Torii for critical reading.
Author Contributions
R.Y.K. and M.T. conceived the idea and wrote the manuscript. Both authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by NIH/NIAAA F32AA028163 (R.Y.K), NIH/NIMH R01MH111674, NIH/NIAAA R01AA026272, and Scott-Gentle Foundation (M.T).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no competing or financial interests.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Paul L.K., Brown W.S., Adolphs R., Tyszka J.M., Richards L.J., Mukherjee P., Sherr E.H. Agenesis of the corpus callosum: Genetic, developmental and functional aspects of connectivity. Nat. Rev. Neurosci. 2007;8:287–299. doi: 10.1038/nrn2107. [DOI] [PubMed] [Google Scholar]
- 2.Paul L.K. Developmental malformation of the corpus callosum: A review of typical callosal development and examples of developmental disorders with callosal involvement. J. Neurodev. Disord. 2011;3:3–27. doi: 10.1007/s11689-010-9059-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park H.J., Kim J.J., Lee S.K., Seok J.H., Chun J., Kim D.I., Lee J.D. Corpus callosal connection mapping using cortical gray matter parcellation and DT-MRI. Hum. Brain Mapp. 2008;29:503–516. doi: 10.1002/hbm.20314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Putnam M.C., Steven M.S., Doron K.W., Riggall A.C., Gazzaniga M.S. Cortical projection topography of the human splenium: Hemispheric asymmetry and individual differences. J. Cogn. Neurosci. 2010;22:1662–1669. doi: 10.1162/jocn.2009.21290. [DOI] [PubMed] [Google Scholar]
- 5.Wahl M., Lauterbach-Soon B., Hattingen E., Jung P., Singer O., Volz S., Klein J.C., Steinmetz H., Ziemann U. Human motor corpus callosum: Topography, somatotopy, and link between microstructure and function. J. Neurosci. Off. J. Soc. Neurosci. 2007;27:12132–12138. doi: 10.1523/JNEUROSCI.2320-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Funnell M.G., Corballis P.M., Gazzaniga M.S. Cortical and subcortical interhemispheric interactions following partial and complete callosotomy. Arch. Neurol. 2000;57:185–189. doi: 10.1001/archneur.57.2.185. [DOI] [PubMed] [Google Scholar]
- 7.Blaauw J., Meiners L.C. The splenium of the corpus callosum: Embryology, anatomy, function and imaging with pathophysiological hypothesis. Neuroradiology. 2020;62:563–585. doi: 10.1007/s00234-019-02357-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Niquille M., Garel S., Mann F., Hornung J.P., Otsmane B., Chevalley S., Parras C., Guillemot F., Gaspar P., Yanagawa Y., et al. Transient neuronal populations are required to guide callosal axons: A role for semaphorin 3C. PLoS Biol. 2009;7:e1000230. doi: 10.1371/journal.pbio.1000230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nishikimi M., Oishi K., Tabata H., Torii K., Nakajima K. Segregation and pathfinding of callosal axons through EphA3 signaling. J. Neurosci. Off. J. Soc. Neurosci. 2011;31:16251–16260. doi: 10.1523/JNEUROSCI.3303-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou J., Wen Y., She L., Sui Y.N., Liu L., Richards L.J., Poo M.M. Axon position within the corpus callosum determines contralateral cortical projection. Proc. Natl. Acad. Sci. USA. 2013;110:E2714–E2723. doi: 10.1073/pnas.1310233110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fame R.M., MacDonald J.L., Macklis J.D. Development, specification, and diversity of callosal projection neurons. Trends Neurosci. 2011;34:41–50. doi: 10.1016/j.tins.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meissirel C., Dehay C., Berland M., Kennedy H. Segregation of callosal and association pathways during development in the visual cortex of the primate. J. Neurosci. Off. J. Soc. Neurosci. 1991;11:3297–3316. doi: 10.1523/JNEUROSCI.11-11-03297.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wise S.P., Jones E.G. The organization and postnatal development of the commissural projection of the rat somatic sensory cortex. J. Comp. Neurol. 1976;168:313–343. doi: 10.1002/cne.901680302. [DOI] [PubMed] [Google Scholar]
- 14.Schulte T., Müller-Oehring E.M. Contribution of callosal connections to the interhemispheric integration of visuomotor and cognitive processes. Neuropsychol. Rev. 2010;20:174–190. doi: 10.1007/s11065-010-9130-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Forster B., Corballis M.C. Interhemispheric transmission times in the presence and absence of the forebrain commissures: Effects of luminance and equiluminance. Neuropsychologia. 1998;36:925–934. doi: 10.1016/S0028-3932(98)00016-5. [DOI] [PubMed] [Google Scholar]
- 16.Koshiyama D., Fukunaga M., Okada N., Morita K., Nemoto K., Yamashita F., Yamamori H., Yasuda Y., Fujimoto M., Kelly S., et al. Role of frontal white matter and corpus callosum on social function in schizophrenia. Schizophr. Res. 2018;202:180–187. doi: 10.1016/j.schres.2018.07.009. [DOI] [PubMed] [Google Scholar]
- 17.Shafer A.T., Benoit J.R., Brown M.R.G., Greenshaw A.J., Van Vliet K.J., Vohra S., Dolcos F., Singhal A. Differences in attentional control and white matter microstructure in adolescents with attentional, affective, and behavioral disorders. Brain Imaging Behav. 2020;14:599–614. doi: 10.1007/s11682-019-00211-7. [DOI] [PubMed] [Google Scholar]
- 18.Jiang W., Shi F., Liu H., Li G., Ding Z., Shen H., Shen C., Lee S.W., Hu D., Wang W., et al. Reduced White Matter Integrity in Antisocial Personality Disorder: A Diffusion Tensor Imaging Study. Sci. Rep. 2017;7:43002. doi: 10.1038/srep43002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mathews M.S., Linskey M.E., Binder D.K. William P. van Wagenen and the first corpus callosotomies for epilepsy. J. Neurosurg. 2008;108:608–613. doi: 10.3171/JNS/2008/108/3/0608. [DOI] [PubMed] [Google Scholar]
- 20.Barkovich A.J., Norman D. Anomalies of the corpus callosum: Correlation with further anomalies of the brain. Ajr. Am. J. Roentgenol. 1988;151:171–179. doi: 10.2214/ajr.151.1.171. [DOI] [PubMed] [Google Scholar]
- 21.Richards L.J., Plachez C., Ren T. Mechanisms regulating the development of the corpus callosum and its agenesis in mouse and human. Clin. Genet. 2004;66:276–289. doi: 10.1111/j.1399-0004.2004.00354.x. [DOI] [PubMed] [Google Scholar]
- 22.Kale A., Joshi P., Kelkar A.B. Restricted diffusion in the corpus callosum: A neuroradiological marker in hypoxic-ischemic encephalopathy. Indian J. Radiol. Imaging. 2016;26:487–492. doi: 10.4103/0971-3026.195795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jarmasz J.S., Basalah D.A., Chudley A.E., Del Bigio M.R. Human brain abnormalities associated with prenatal alcohol exposure and fetal alcohol spectrum disorder. J. Neuropathol. Exp. Neurol. 2017;76:813–833. doi: 10.1093/jnen/nlx064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Graham D., Tisdall M.M., Gill D. Corpus callosotomy outcomes in pediatric patients: A systematic review. Epilepsia. 2016;57:1053–1068. doi: 10.1111/epi.13408. [DOI] [PubMed] [Google Scholar]
- 25.Koester S.E., O’Leary D.D. Connectional distinction between callosal and subcortically projecting cortical neurons is determined prior to axon extension. Dev. Biol. 1993;160:1–14. doi: 10.1006/dbio.1993.1281. [DOI] [PubMed] [Google Scholar]
- 26.Ozaki H.S., Wahlsten D. Prenatal formation of the normal mouse corpus callosum: A quantitative study with carbocyanine dyes. J. Comp. Neurol. 1992;323:81–90. doi: 10.1002/cne.903230107. [DOI] [PubMed] [Google Scholar]
- 27.Rash B.G., Richards L.J. A role for cingulate pioneering axons in the development of the corpus callosum. J. Comp. Neurol. 2001;434:147–157. doi: 10.1002/cne.1170. [DOI] [PubMed] [Google Scholar]
- 28.Rakic P., Yakovlev P.I. Development of the corpus callosum and cavum septi in man. J. Comp. Neurol. 1968;132:45–72. doi: 10.1002/cne.901320103. [DOI] [PubMed] [Google Scholar]
- 29.Cignini P., Padula F., Giorlandino M., Brutti P., Alfò M., Giannarelli D., Mastrandrea M.L., D’Emidio L., Vacca L., Aloisi A., et al. Reference charts for fetal corpus callosum length: A prospective cross-sectional study of 2950 fetuses. J. Ultrasound Med. Off. J. Am. Inst. Ultrasound Med. 2014;33:1065–1078. doi: 10.7863/ultra.33.6.1065. [DOI] [PubMed] [Google Scholar]
- 30.Horgos B., Mecea M., Boer A., Szabo B., Buruiana A., Stamatian F., Mihu C.-M., Florian I.Ş., Susman S., Pascalau R. White Matter Dissection of the Fetal Brain. Front. Neuroanat. 2020;14 doi: 10.3389/fnana.2020.584266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Achiron R., Achiron A. Development of the human fetal corpus callosum: A high-resolution, cross-sectional sonographic study. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2001;18:343–347. doi: 10.1046/j.0960-7692.2001.00512.x. [DOI] [PubMed] [Google Scholar]
- 32.Harreld J.H., Bhore R., Chason D.P., Twickler D.M. Corpus callosum length by gestational age as evaluated by fetal MR imaging. AJNR Am. J. Neuroradiol. 2011;32:490–494. doi: 10.3174/ajnr.A2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De León Reyes N.S., Bragg-Gonzalo L., Nieto M. Development and plasticity of the corpus callosum. Development. 2020;147:dev189738. doi: 10.1242/dev.189738. [DOI] [PubMed] [Google Scholar]
- 34.Raybaud C. The corpus callosum, the other great forebrain commissures, and the septum pellucidum: Anatomy, development, and malformation. Neuroradiology. 2010;52:447–477. doi: 10.1007/s00234-010-0696-3. [DOI] [PubMed] [Google Scholar]
- 35.Molyneaux B.J., Arlotta P., Menezes J.R., Macklis J.D. Neuronal subtype specification in the cerebral cortex. Nat. Rev. Neurosci. 2007;8:427–437. doi: 10.1038/nrn2151. [DOI] [PubMed] [Google Scholar]
- 36.Klingler E., De la Rossa A., Fièvre S., Devaraju K., Abe P., Jabaudon D. A Translaminar Genetic Logic for the Circuit Identity of Intracortically Projecting Neurons. Curr. Biol. 2019;29:332–339.e335. doi: 10.1016/j.cub.2018.11.071. [DOI] [PubMed] [Google Scholar]
- 37.Ozaki H.S., Wahlsten D. Timing and origin of the first cortical axons to project through the corpus callosum and the subsequent emergence of callosal projection cells in mouse. J. Comp. Neurol. 1998;400:197–206. doi: 10.1002/(SICI)1096-9861(19981019)400:2<197::AID-CNE3>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 38.Leone D.P., Srinivasan K., Chen B., Alcamo E., McConnell S.K. The determination of projection neuron identity in the developing cerebral cortex. Curr. Opin. Neurobiol. 2008;18:28–35. doi: 10.1016/j.conb.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leyva-Díaz E., López-Bendito G. In and out from the cortex: Development of major forebrain connections. Neuroscience. 2013;254:26–44. doi: 10.1016/j.neuroscience.2013.08.070. [DOI] [PubMed] [Google Scholar]
- 40.Muralidharan B., Khatri Z., Maheshwari U., Gupta R., Roy B., Pradhan S.J., Karmodiya K., Padmanabhan H., Shetty A.S., Balaji C., et al. LHX2 Interacts with the NuRD Complex and Regulates Cortical Neuron Subtype Determinants Fezf2 and Sox11. J. Neurosci. Off. J. Soc. Neurosci. 2017;37:194–203. doi: 10.1523/JNEUROSCI.2836-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shim S., Kwan K.Y., Li M., Lefebvre V., Sestan N. Cis-regulatory control of corticospinal system development and evolution. Nature. 2012;486:74–79. doi: 10.1038/nature11094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kwan K.Y., Lam M.M., Krsnik Z., Kawasawa Y.I., Lefebvre V., Sestan N. SOX5 postmitotically regulates migration, postmigratory differentiation, and projections of subplate and deep-layer neocortical neurons. Proc. Natl. Acad. Sci. USA. 2008;105:16021–16026. doi: 10.1073/pnas.0806791105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alcamo E.A., Chirivella L., Dautzenberg M., Dobreva G., Fariñas I., Grosschedl R., McConnell S.K. Satb2 regulates callosal projection neuron identity in the developing cerebral cortex. Neuron. 2008;57:364–377. doi: 10.1016/j.neuron.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 44.Britanova O., de Juan Romero C., Cheung A., Kwan K.Y., Schwark M., Gyorgy A., Vogel T., Akopov S., Mitkovski M., Agoston D., et al. Satb2 is a postmitotic determinant for upper-layer neuron specification in the neocortex. Neuron. 2008;57:378–392. doi: 10.1016/j.neuron.2007.12.028. [DOI] [PubMed] [Google Scholar]
- 45.Paolino A., Fenlon L.R., Kozulin P., Haines E., Lim J.W.C., Richards L.J., Suárez R. Differential timing of a conserved transcriptional network underlies divergent cortical projection routes across mammalian brain evolution. Proc. Natl. Acad. Sci. USA. 2020;117:10554–10564. doi: 10.1073/pnas.1922422117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baranek C., Dittrich M., Parthasarathy S., Bonnon C.G., Britanova O., Lanshakov D., Boukhtouche F., Sommer J.E., Colmenares C., Tarabykin V., et al. Protooncogene Ski cooperates with the chromatin-remodeling factor Satb2 in specifying callosal neurons. Proc. Natl. Acad. Sci. USA. 2012;109:3546–3551. doi: 10.1073/pnas.1108718109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Harb K., Magrinelli E., Nicolas C.S., Lukianets N., Frangeul L., Pietri M., Sun T., Sandoz G., Grammont F., Jabaudon D., et al. Area-specific development of distinct projection neuron subclasses is regulated by postnatal epigenetic modifications. eLife. 2016;5:e09531. doi: 10.7554/eLife.09531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McKenna W.L., Ortiz-Londono C.F., Mathew T.K., Hoang K., Katzman S., Chen B. Mutual regulation between Satb2 and Fezf2 promotes subcerebral projection neuron identity in the developing cerebral cortex. Proc. Natl. Acad. Sci. USA. 2015;112:11702–11707. doi: 10.1073/pnas.1504144112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Paşca S.P., Portmann T., Voineagu I., Yazawa M., Shcheglovitov A., Paşca A.M., Cord B., Palmer T.D., Chikahisa S., Nishino S., et al. Using iPSC-derived neurons to uncover cellular phenotypes associated with Timothy syndrome. Nat. Med. 2011;17:1657–1662. doi: 10.1038/nm.2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Panagiotakos G., Haveles C., Arjun A., Petrova R., Rana A., Portmann T., Paşca S.P., Palmer T.D., Dolmetsch R.E. Aberrant calcium channel splicing drives defects in cortical differentiation in Timothy syndrome. eLife. 2019;8:e51037. doi: 10.7554/eLife.51037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Büttner N., Johnsen S.A., Kügler S., Vogel T. Af9/Mllt3 interferes with Tbr1 expression through epigenetic modification of histone H3K79 during development of the cerebral cortex. Proc. Natl. Acad. Sci. USA. 2010;107:7042–7047. doi: 10.1073/pnas.0912041107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Franz H., Villarreal A., Heidrich S., Videm P., Kilpert F., Mestres I., Calegari F., Backofen R., Manke T., Vogel T. DOT1L promotes progenitor proliferation and primes neuronal layer identity in the developing cerebral cortex. Nucleic Acids Res. 2019;47:168–183. doi: 10.1093/nar/gky953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alvarez-Saavedra M., Yan K., De Repentigny Y., Hashem L.E., Chaudary N., Sarwar S., Yang D., Ioshikhes I., Kothary R., Hirayama T., et al. Snf2h Drives Chromatin Remodeling to Prime Upper Layer Cortical Neuron Development. Front. Mol. Neurosci. 2019;12:243. doi: 10.3389/fnmol.2019.00243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ji L., Kim N.H., Huh S.O., Rhee H.J. Depletion of Inositol Polyphosphate 4-Phosphatase II Suppresses Callosal Axon Formation in the Developing Mice. Mol. Cells. 2016;39:501–507. doi: 10.14348/molcells.2016.0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hatanaka Y., Namikawa T., Yamauchi K., Kawaguchi Y. Cortical Divergent Projections in Mice Originate from Two Sequentially Generated, Distinct Populations of Excitatory Cortical Neurons with Different Initial Axonal Outgrowth Characteristics. Cereb. Cortex. 2016;26:2257–2270. doi: 10.1093/cercor/bhv077. [DOI] [PubMed] [Google Scholar]
- 56.Srivatsa S., Parthasarathy S., Britanova O., Bormuth I., Donahoo A.L., Ackerman S.L., Richards L.J., Tarabykin V. Unc5C and DCC act downstream of Ctip2 and Satb2 and contribute to corpus callosum formation. Nat. Commun. 2014;5:3708. doi: 10.1038/ncomms4708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Marsh A.P., Heron D., Edwards T.J., Quartier A., Galea C., Nava C., Rastetter A., Moutard M.L., Anderson V., Bitoun P., et al. Mutations in DCC cause isolated agenesis of the corpus callosum with incomplete penetrance. Nat Genet. 2017;49:511–514. doi: 10.1038/ng.3794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Srinivasan K., Leone D.P., Bateson R.K., Dobreva G., Kohwi Y., Kohwi-Shigematsu T., Grosschedl R., McConnell S.K. A network of genetic repression and derepression specifies projection fates in the developing neocortex. Proc. Natl. Acad. Sci. USA. 2012;109:19071–19078. doi: 10.1073/pnas.1216793109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Greig L.C., Woodworth M.B., Galazo M.J., Padmanabhan H., Macklis J.D. Molecular logic of neocortical projection neuron specification, development and diversity. Nat. Rev. Neurosci. 2013;14:755–769. doi: 10.1038/nrn3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fame R.M., Dehay C., Kennedy H., Macklis J.D. Subtype-Specific Genes that Characterize Subpopulations of Callosal Projection Neurons in Mouse Identify Molecularly Homologous Populations in Macaque Cortex. Cereb Cortex. 2017;27:1817–1830. doi: 10.1093/cercor/bhw023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Heavner W.E., Ji S., Notwell J.H., Dyer E.S., Tseng A.M., Birgmeier J., Yoo B., Bejerano G., McConnell S.K. Transcription factor expression defines subclasses of developing projection neurons highly similar to single-cell RNA-seq subtypes. Proc. Natl. Acad. Sci. USA. 2020;117:25074–25084. doi: 10.1073/pnas.2008013117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.MacDonald J.L., Fame R.M., Gillis-Buck E.M., Macklis J.D. Caveolin1 Identifies a Specific Subpopulation of Cerebral Cortex Callosal Projection Neurons (CPN) Including Dual Projecting Cortical Callosal/Frontal Projection Neurons (CPN/FPN) eNeuro. 2018;5 doi: 10.1523/ENEURO.0234-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Molyneaux B.J., Arlotta P., Fame R.M., MacDonald J.L., MacQuarrie K.L., Macklis J.D. Novel subtype-specific genes identify distinct subpopulations of callosal projection neurons. J. Neurosci. Off. J. Soc. Neurosci. 2009;29:12343–12354. doi: 10.1523/JNEUROSCI.6108-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Molyneaux B.J., Goff L.A., Brettler A.C., Chen H.H., Hrvatin S., Rinn J.L., Arlotta P. DeCoN: Genome-wide analysis of in vivo transcriptional dynamics during pyramidal neuron fate selection in neocortex. Neuron. 2015;85:275–288. doi: 10.1016/j.neuron.2014.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Paolino A., Fenlon L.R., Suárez R., Richards L.J. Transcriptional control of long-range cortical projections. Curr. Opin. Neurobiol. 2018;53:57–65. doi: 10.1016/j.conb.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 66.Cargnin F., Kwon J.S., Katzman S., Chen B., Lee J.W., Lee S.K. FOXG1 Orchestrates Neocortical Organization and Cortico-Cortical Connections. Neuron. 2018;100:1083–1096.e1085. doi: 10.1016/j.neuron.2018.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kumamoto T., Toma K., Gunadi, McKenna W.L., Kasukawa T., Katzman S., Chen B., Hanashima C. Foxg1 coordinates the switch from nonradially to radially migrating glutamatergic subtypes in the neocortex through spatiotemporal repression. Cell Rep. 2013;3:931–945. doi: 10.1016/j.celrep.2013.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hou P.S., Miyoshi G., Hanashima C. Sensory cortex wiring requires preselection of short- and long-range projection neurons through an Egr-Foxg1-COUP-TFI network. Nat. Commun. 2019;10:3581. doi: 10.1038/s41467-019-11043-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sorensen S.A., Bernard A., Menon V., Royall J.J., Glattfelder K.J., Desta T., Hirokawa K., Mortrud M., Miller J.A., Zeng H., et al. Correlated gene expression and target specificity demonstrate excitatory projection neuron diversity. Cereb Cortex. 2015;25:433–449. doi: 10.1093/cercor/bht243. [DOI] [PubMed] [Google Scholar]
- 70.Tasic B., Menon V., Nguyen T.N., Kim T.K., Jarsky T., Yao Z., Levi B., Gray L.T., Sorensen S.A., Dolbeare T., et al. Adult mouse cortical cell taxonomy revealed by single cell transcriptomics. Nat. Neurosci. 2016;19:335–346. doi: 10.1038/nn.4216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pfeffer C.K., Beltramo R. Correlating Anatomy and Function with Gene Expression in Individual Neurons by Combining in Vivo Labeling, Patch Clamp, and Single Cell RNA-seq. Front. Cell. Neurosci. 2017;11:376. doi: 10.3389/fncel.2017.00376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ramos R.L., Tam D.M., Brumberg J.C. Physiology and morphology of callosal projection neurons in mouse. Neuroscience. 2008;153:654–663. doi: 10.1016/j.neuroscience.2008.02.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Azim E., Shnider S.J., Cederquist G.Y., Sohur U.S., Macklis J.D. Lmo4 and Clim1 progressively delineate cortical projection neuron subtypes during development. Cereb Cortex. 2009;19(Suppl. S1):i62–i69. doi: 10.1093/cercor/bhp030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cederquist G.Y., Azim E., Shnider S.J., Padmanabhan H., Macklis J.D. Lmo4 establishes rostral motor cortex projection neuron subtype diversity. J. Neurosci. Off. J. Soc. Neurosci. 2013;33:6321–6332. doi: 10.1523/JNEUROSCI.5140-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kashani A.H., Qiu Z., Jurata L., Lee S.K., Pfaff S., Goebbels S., Nave K.A., Ghosh A. Calcium activation of the LMO4 transcription complex and its role in the patterning of thalamocortical connections. J. Neurosci. Off. J. Soc. Neurosci. 2006;26:8398–8408. doi: 10.1523/JNEUROSCI.0618-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fame R.M., MacDonald J.L., Dunwoodie S.L., Takahashi E., Macklis J.D. Cited2 Regulates Neocortical Layer II/III Generation and Somatosensory Callosal Projection Neuron Development and Connectivity. J. Neurosci. Off. J. Soc. Neurosci. 2016;36:6403–6419. doi: 10.1523/JNEUROSCI.4067-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Greig L.C., Woodworth M.B., Greppi C., Macklis J.D. Ctip1 Controls Acquisition of Sensory Area Identity and Establishment of Sensory Input Fields in the Developing Neocortex. Neuron. 2016;90:261–277. doi: 10.1016/j.neuron.2016.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wiegreffe C., Simon R., Peschkes K., Kling C., Strehle M., Cheng J., Srivatsa S., Liu P., Jenkins N.A., Copeland N.G., et al. Bcl11a (Ctip1) Controls Migration of Cortical Projection Neurons through Regulation of Sema3c. Neuron. 2015;87:311–325. doi: 10.1016/j.neuron.2015.06.023. [DOI] [PubMed] [Google Scholar]
- 79.Woodworth M.B., Greig L.C., Liu K.X., Ippolito G.C., Tucker H.O., Macklis J.D. Ctip1 Regulates the Balance between Specification of Distinct Projection Neuron Subtypes in Deep Cortical Layers. Cell Rep. 2016;15:999–1012. doi: 10.1016/j.celrep.2016.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen B., Wang S.S., Hattox A.M., Rayburn H., Nelson S.B., McConnell S.K. The Fezf2-Ctip2 genetic pathway regulates the fate choice of subcortical projection neurons in the developing cerebral cortex. Proc. Natl. Acad. Sci. USA. 2008;105:11382–11387. doi: 10.1073/pnas.0804918105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.McKenna W.L., Betancourt J., Larkin K.A., Abrams B., Guo C., Rubenstein J.L., Chen B. Tbr1 and Fezf2 regulate alternate corticofugal neuronal identities during neocortical development. J. Neurosci. Off. J. Soc. Neurosci. 2011;31:549–564. doi: 10.1523/JNEUROSCI.4131-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Molyneaux B.J., Arlotta P., Hirata T., Hibi M., Macklis J.D. Fezl is required for the birth and specification of corticospinal motor neurons. Neuron. 2005;47:817–831. doi: 10.1016/j.neuron.2005.08.030. [DOI] [PubMed] [Google Scholar]
- 83.Alvarez-Saavedra M., De Repentigny Y., Lagali P.S., Raghu Ram E.V., Yan K., Hashem E., Ivanochko D., Huh M.S., Yang D., Mears A.J., et al. Snf2h-mediated chromatin organization and histone H1 dynamics govern cerebellar morphogenesis and neural maturation. Nat. Commun. 2014;5:4181. doi: 10.1038/ncomms5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lazzaro M.A., Picketts D.J. Cloning and characterization of the murine Imitation Switch (ISWI) genes: Differential expression patterns suggest distinct developmental roles for Snf2h and Snf2l. J. Neurochem. 2001;77:1145–1156. doi: 10.1046/j.1471-4159.2001.00324.x. [DOI] [PubMed] [Google Scholar]
- 85.Roidl D., Hellbach N., Bovio P.P., Villarreal A., Heidrich S., Nestel S., Grüning B.A., Boenisch U., Vogel T. DOT1L Activity Promotes Proliferation and Protects Cortical Neural Stem Cells from Activation of ATF4-DDIT3-Mediated ER Stress In Vitro. Stem Cells (Dayt. Ohio) 2016;34:233–245. doi: 10.1002/stem.2187. [DOI] [PubMed] [Google Scholar]
- 86.Dennis D.J., Wilkinson G., Li S., Dixit R., Adnani L., Balakrishnan A., Han S., Kovach C., Gruenig N., Kurrasch D.M., et al. Neurog2 and Ascl1 together regulate a postmitotic derepression circuit to govern laminar fate specification in the murine neocortex. Proc. Natl. Acad. Sci. USA. 2017;114:e4934–e4943. doi: 10.1073/pnas.1701495114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fode C., Ma Q., Casarosa S., Ang S.L., Anderson D.J., Guillemot F. A role for neural determination genes in specifying the dorsoventral identity of telencephalic neurons. Genes Dev. 2000;14:67–80. [PMC free article] [PubMed] [Google Scholar]
- 88.Nieto M., Schuurmans C., Britz O., Guillemot F. Neural bHLH genes control the neuronal versus glial fate decision in cortical progenitors. Neuron. 2001;29:401–413. doi: 10.1016/S0896-6273(01)00214-8. [DOI] [PubMed] [Google Scholar]
- 89.Tao W., Lai E. Telencephalon-restricted expression of BF-1, a new member of the HNF-3/fork head gene family, in the developing rat brain. Neuron. 1992;8:957–966. doi: 10.1016/0896-6273(92)90210-5. [DOI] [PubMed] [Google Scholar]
- 90.Izzi L., Charron F. Midline axon guidance and human genetic disorders. Clin. Genet. 2011;80:226–234. doi: 10.1111/j.1399-0004.2011.01735.x. [DOI] [PubMed] [Google Scholar]
- 91.Suarez R., Gobius I., Richards L.J. Evolution and development of interhemispheric connections in the vertebrate forebrain. Front. Hum. Neurosci. 2014;8:497. doi: 10.3389/fnhum.2014.00497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.De León Reyes N.S., Mederos S., Varela I., Weiss L.A., Perea G., Galazo M.J., Nieto M. Transient callosal projections of L4 neurons are eliminated for the acquisition of local connectivity. Nat. Commun. 2019;10:4549. doi: 10.1038/s41467-019-12495-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Koester S.E., O’Leary D.D. Axons of early generated neurons in cingulate cortex pioneer the corpus callosum. J. Neurosci. Off. J. Soc. Neurosci. 1994;14:6608–6620. doi: 10.1523/JNEUROSCI.14-11-06608.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Choe Y., Siegenthaler J.A., Pleasure S.J. A cascade of morphogenic signaling initiated by the meninges controls corpus callosum formation. Neuron. 2012;73:698–712. doi: 10.1016/j.neuron.2011.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hand R., Polleux F. Neurogenin2 regulates the initial axon guidance of cortical pyramidal neurons projecting medially to the corpus callosum. Neural Dev. 2011;6:30. doi: 10.1186/1749-8104-6-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Norris C.R., Kalil K. Guidance of callosal axons by radial glia in the developing cerebral cortex. J. Neurosci. Off. J. Soc. Neurosci. 1991;11:3481–3492. doi: 10.1523/JNEUROSCI.11-11-03481.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shu T., Li Y., Keller A., Richards L.J. The glial sling is a migratory population of developing neurons. Development. 2003;130:2929–2937. doi: 10.1242/dev.00514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shu T., Richards L.J. Cortical axon guidance by the glial wedge during the development of the corpus callosum. J. Neurosci. Off. J. Soc. Neurosci. 2001;21:2749–2758. doi: 10.1523/JNEUROSCI.21-08-02749.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Silver J., Edwards M.A., Levitt P. Immunocytochemical demonstration of early appearing astroglial structures that form boundaries and pathways along axon tracts in the fetal brain. J. Comp. Neurol. 1993;328:415–436. doi: 10.1002/cne.903280308. [DOI] [PubMed] [Google Scholar]
- 100.Silver J., Lorenz S.E., Wahlsten D., Coughlin J. Axonal guidance during development of the great cerebral commissures: Descriptive and experimental studies, in vivo, on the role of preformed glial pathways. J. Comp. Neurol. 1982;210:10–29. doi: 10.1002/cne.902100103. [DOI] [PubMed] [Google Scholar]
- 101.Hankin M.H., Schneider B.F., Silver J. Death of the subcallosal glial sling is correlated with formation of the cavum septi pellucidi. J. Comp. Neurol. 1988;272:191–202. doi: 10.1002/cne.902720204. [DOI] [PubMed] [Google Scholar]
- 102.Unni D.K., Piper M., Moldrich R.X., Gobius I., Liu S., Fothergill T., Donahoo A.L., Baisden J.M., Cooper H.M., Richards L.J. Multiple Slits regulate the development of midline glial populations and the corpus callosum. Dev. Biol. 2012;365:36–49. doi: 10.1016/j.ydbio.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 103.Shu T., Puche A.C., Richards L.J. Development of midline glial populations at the corticoseptal boundary. J. Neurobiol. 2003;57:81–94. doi: 10.1002/neu.10252. [DOI] [PubMed] [Google Scholar]
- 104.Niquille M., Minocha S., Hornung J.P., Rufer N., Valloton D., Kessaris N., Alfonsi F., Vitalis T., Yanagawa Y., Devenoges C., et al. Two specific populations of GABAergic neurons originating from the medial and the caudal ganglionic eminences aid in proper navigation of callosal axons. Dev. Neurobiol. 2013;73:647–672. doi: 10.1002/dneu.22075. [DOI] [PubMed] [Google Scholar]
- 105.Jovanov-Milosevic N., Culjat M., Kostovic I. Growth of the human corpus callosum: Modular and laminar morphogenetic zones. Front. Neuroanat. 2009;3:6. doi: 10.3389/neuro.05.006.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lindwall C., Fothergill T., Richards L.J. Commissure formation in the mammalian forebrain. Curr. Opin. Neurobiol. 2007;17:3–14. doi: 10.1016/j.conb.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 107.Wang B., Li H., Mutlu S.A., Bowser D.A., Moore M.J., Wang M.C., Zheng H. The Amyloid Precursor Protein Is a Conserved Receptor for Slit to Mediate Axon Guidance. eNeuro. 2017;4 doi: 10.1523/ENEURO.0185-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.DeGeer J., Kaplan A., Mattar P., Morabito M., Stochaj U., Kennedy T.E., Debant A., Cayouette M., Fournier A.E., Lamarche-Vane N. Hsc70 chaperone activity underlies Trio GEF function in axon growth and guidance induced by netrin-1. J. Cell Biol. 2015;210:817–832. doi: 10.1083/jcb.201505084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kang D.S., Yang Y.R., Lee C., Park B., Park K.I., Seo J.K., Seo Y.K., Cho H., Lucio C., Suh P.G. Netrin-1/DCC-mediated PLCγ1 activation is required for axon guidance and brain structure development. EMBO Rep. 2018;19:e46250. doi: 10.15252/embr.201846250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Brudvig J.J., Cain J.T., Schmidt-Grimminger G.G., Stumpo D.J., Roux K.J., Blackshear P.J., Weimer J.M. MARCKS Is Necessary for Netrin-DCC Signaling and Corpus Callosum Formation. Mol. Neurobiol. 2018;55:8388–8402. doi: 10.1007/s12035-018-0990-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Weimer J.M., Yokota Y., Stanco A., Stumpo D.J., Blackshear P.J., Anton E.S. MARCKS modulates radial progenitor placement, proliferation and organization in the developing cerebral cortex. Development. 2009;136:2965–2975. doi: 10.1242/dev.036616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Alvarez Juliá A., Frasch A.C., Fuchsova B. Neuronal filopodium formation induced by the membrane glycoprotein M6a (Gpm6a) is facilitated by coronin-1a, Rac1, and p21-activated kinase 1 (Pak1) J. Neurochem. 2016;137:46–61. doi: 10.1111/jnc.13552. [DOI] [PubMed] [Google Scholar]
- 113.Mita S., de Monasterio-Schrader P., Fünfschilling U., Kawasaki T., Mizuno H., Iwasato T., Nave K.A., Werner H.B., Hirata T. Transcallosal Projections Require Glycoprotein M6-Dependent Neurite Growth and Guidance. Cereb Cortex. 2015;25:4111–4125. doi: 10.1093/cercor/bhu129. [DOI] [PubMed] [Google Scholar]
- 114.Hossain M.M., Tsuzuki T., Sakakibara K., Imaizumi F., Ikegaya A., Inagaki M., Takahashi I., Ito T., Takamatsu H., Kumanogoh A., et al. PlexinA1 is crucial for the midline crossing of callosal axons during corpus callosum development in BALB/cAJ mice. PLoS ONE. 2019;14:e0221440. doi: 10.1371/journal.pone.0221440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wu K.Y., He M., Hou Q.Q., Sheng A.L., Yuan L., Liu F., Liu W.W., Li G., Jiang X.Y., Luo Z.G. Semaphorin 3A activates the guanosine triphosphatase Rab5 to promote growth cone collapse and organize callosal axon projections. Sci. Signal. 2014;7:ra81. doi: 10.1126/scisignal.2005334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Velona T., Altounian M., Roque M., Hocine M., Bellon A., Briz C.G., Salin P., Nieto M., Chauvet S., Mann F. PlexinD1 and Sema3E determine laminar positioning of heterotopically projecting callosal neurons. Mol. Cell. Neurosci. 2019;100:103397. doi: 10.1016/j.mcn.2019.103397. [DOI] [PubMed] [Google Scholar]
- 117.Burk K., Mire E., Bellon A., Hocine M., Guillot J., Moraes F., Yoshida Y., Simons M., Chauvet S., Mann F. Post-endocytic sorting of Plexin-D1 controls signal transduction and development of axonal and vascular circuits. Nat. Commun. 2017;8:14508. doi: 10.1038/ncomms14508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mire E., Hocine M., Bazellières E., Jungas T., Davy A., Chauvet S., Mann F. Developmental Upregulation of Ephrin-B1 Silences Sema3C/Neuropilin-1 Signaling during Post-crossing Navigation of Corpus Callosum Axons. Curr. Biol. 2018;28:1768–1782.e1764. doi: 10.1016/j.cub.2018.04.026. [DOI] [PubMed] [Google Scholar]
- 119.Lopez-Bendito G., Flames N., Ma L., Fouquet C., Di Meglio T., Chedotal A., Tessier-Lavigne M., Marin O. Robo1 and Robo2 cooperate to control the guidance of major axonal tracts in the mammalian forebrain. J. Neurosci. Off. J. Soc. Neurosci. 2007;27:3395–3407. doi: 10.1523/JNEUROSCI.4605-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Shu T., Sundaresan V., McCarthy M.M., Richards L.J. Slit2 guides both precrossing and postcrossing callosal axons at the midline in vivo. J. Neurosci. Off. J. Soc. Neurosci. 2003;23:8176–8184. doi: 10.1523/JNEUROSCI.23-22-08176.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Meijers R., Smock R.G., Zhang Y., Wang J.H. Netrin Synergizes Signaling and Adhesion through DCC. Trends Biochem. Sci. 2020;45:6–12. doi: 10.1016/j.tibs.2019.10.005. [DOI] [PubMed] [Google Scholar]
- 122.Boyer N.P., Gupton S.L. Revisiting Netrin-1: One Who Guides (Axons) Front. Cell. Neurosci. 2018;12:221. doi: 10.3389/fncel.2018.00221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Fothergill T., Donahoo A.L., Douglass A., Zalucki O., Yuan J., Shu T., Goodhill G.J., Richards L.J. Netrin-DCC signaling regulates corpus callosum formation through attraction of pioneering axons and by modulating Slit2-mediated repulsion. Cereb. Cortex. 2014;24:1138–1151. doi: 10.1093/cercor/bhs395. [DOI] [PubMed] [Google Scholar]
- 124.Charvet C.J., Das A., Song J.W., Tindal-Burgess D.J., Kabaria P., Dai G., Kane T., Takahashi E. High Angular Resolution Diffusion MRI Reveals Conserved and Deviant Programs in the Paths that Guide Human Cortical Circuitry. Cereb Cortex. 2020;30:1447–1464. doi: 10.1093/cercor/bhz178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Manger P.R., Hemingway J., Haagensen M., Gilissen E. Cross-sectional area of the elephant corpus callosum: Comparison to other eutherian mammals. Neuroscience. 2010;167:815–824. doi: 10.1016/j.neuroscience.2010.02.066. [DOI] [PubMed] [Google Scholar]
- 126.Hendrickson T.J., Mueller B.A., Sowell E.R., Mattson S.N., Coles C.D., Kable J.A., Jones K.L., Boys C.J., Lim K.O., Riley E.P., et al. Cortical gyrification is abnormal in children with prenatal alcohol exposure. Neuroimage. Clin. 2017;15:391–400. doi: 10.1016/j.nicl.2017.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Innocenti G.M., Price D.J. Exuberance in the development of cortical networks. Nat. Rev. Neurosci. 2005;6:955–965. doi: 10.1038/nrn1790. [DOI] [PubMed] [Google Scholar]
- 128.Hand R.A., Khalid S., Tam E., Kolodkin A.L. Axon Dynamics during Neocortical Laminar Innervation. Cell Rep. 2015;12:172–182. doi: 10.1016/j.celrep.2015.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Mizuno H., Hirano T., Tagawa Y. Pre-synaptic and post-synaptic neuronal activity supports the axon development of callosal projection neurons during different post-natal periods in the mouse cerebral cortex. Eur. J. Neurosci. 2010;31:410–424. doi: 10.1111/j.1460-9568.2009.07070.x. [DOI] [PubMed] [Google Scholar]
- 130.Fenlon L.R., Richards L.J. Contralateral targeting of the corpus callosum in normal and pathological brain function. Trends Neurosci. 2015;38:264–272. doi: 10.1016/j.tins.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 131.Wuttke T.V., Markopoulos F., Padmanabhan H., Wheeler A.P., Murthy V.N., Macklis J.D. Developmentally primed cortical neurons maintain fidelity of differentiation and establish appropriate functional connectivity after transplantation. Nat. Neurosci. 2018;21:517–529. doi: 10.1038/s41593-018-0098-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Rouaux C., Arlotta P. Direct lineage reprogramming of post-mitotic callosal neurons into corticofugal neurons in vivo. Nat. Cell Biol. 2013;15:214–221. doi: 10.1038/ncb2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Leone D.P., Heavner W.E., Ferenczi E.A., Dobreva G., Huguenard J.R., Grosschedl R., McConnell S.K. Satb2 Regulates the Differentiation of Both Callosal and Subcerebral Projection Neurons in the Developing Cerebral Cortex. Cereb Cortex. 2015;25:3406–3419. doi: 10.1093/cercor/bhu156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Rodríguez-Tornos F.M., Briz C.G., Weiss L.A., Sebastián-Serrano A., Ares S., Navarrete M., Frangeul L., Galazo M., Jabaudon D., Esteban J.A., et al. Cux1 Enables Interhemispheric Connections of Layer II/III Neurons by Regulating Kv1-Dependent Firing. Neuron. 2016;89:494–506. doi: 10.1016/j.neuron.2015.12.020. [DOI] [PubMed] [Google Scholar]
- 135.Catapano L.A., Arnold M.W., Perez F.A., Macklis J.D. Specific neurotrophic factors support the survival of cortical projection neurons at distinct stages of development. J. Neurosci. Off. J. Soc. Neurosci. 2001;21:8863–8872. doi: 10.1523/JNEUROSCI.21-22-08863.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ueno M., Fujita Y., Tanaka T., Nakamura Y., Kikuta J., Ishii M., Yamashita T. Layer V cortical neurons require microglial support for survival during postnatal development. Nat Neurosci. 2013;16:543–551. doi: 10.1038/nn.3358. [DOI] [PubMed] [Google Scholar]
- 137.Poulopoulos A., Murphy A.J., Ozkan A., Davis P., Hatch J., Kirchner R., Macklis J.D. Subcellular transcriptomes and proteomes of developing axon projections in the cerebral cortex. Nature. 2019;565:356–360. doi: 10.1038/s41586-018-0847-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tagawa Y., Hirano T. Activity-dependent callosal axon projections in neonatal mouse cerebral cortex. Neural Plast. 2012;2012:797295. doi: 10.1155/2012/797295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ortiz C., Navarro J.F., Jurek A., Märtin A., Lundeberg J., Meletis K. Molecular atlas of the adult mouse brain. Sci. Adv. 2020;6:eabb3446. doi: 10.1126/sciadv.abb3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Franco S.J., Gil-Sanz C., Martinez-Garay I., Espinosa A., Harkins-Perry S.R., Ramos C., Muller U. Fate-restricted neural progenitors in the mammalian cerebral cortex. Science. 2012;337:746–749. doi: 10.1126/science.1223616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Eckler M.J., Nguyen T.D., McKenna W.L., Fastow B.L., Guo C., Rubenstein J.L.R., Chen B. Cux2-positive radial glial cells generate diverse subtypes of neocortical projection neurons and macroglia. Neuron. 2015;86:1100–1108. doi: 10.1016/j.neuron.2015.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Gil-Sanz C., Espinosa A., Fregoso S.P., Bluske K.K., Cunningham C.L., Martinez-Garay I., Zeng H., Franco S.J., Muller U. Lineage Tracing Using Cux2-Cre and Cux2-CreERT2 Mice. Neuron. 2015;86:1091–1099. doi: 10.1016/j.neuron.2015.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Guo C., Eckler M.J., McKenna W.L., McKinsey G.L., Rubenstein J.L., Chen B. Fezf2 expression identifies a multipotent progenitor for neocortical projection neurons, astrocytes, and oligodendrocytes. Neuron. 2013;80:1167–1174. doi: 10.1016/j.neuron.2013.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Luarte A., Cornejo V.H., Bertin F., Gallardo J., Couve A. The axonal endoplasmic reticulum: One organelle-many functions in development, maintenance, and plasticity. Dev. Neurobiol. 2018;78:181–208. doi: 10.1002/dneu.22560. [DOI] [PubMed] [Google Scholar]
- 145.Silbereis J.C., Pochareddy S., Zhu Y., Li M., Sestan N. The Cellular and Molecular Landscapes of the Developing Human Central Nervous System. Neuron. 2016;89:248–268. doi: 10.1016/j.neuron.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sousa A.M.M., Zhu Y., Raghanti M.A., Kitchen R.R., Onorati M., Tebbenkamp A.T.N., Stutz B., Meyer K.A., Li M., Kawasawa Y.I., et al. Molecular and cellular reorganization of neural circuits in the human lineage. Science. 2017;358:1027–1032. doi: 10.1126/science.aan3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.