Abstract
Simple Summary
Filariasis is emerging as a public health concern for humans, dogs, cats, and other wildlife species, and is frequently found in southeast Asian countries. The present study confirmed the species of filarial nematodes in free-roaming dogs from temple communities. Two species were found: Dirofilaria immitis infection and, for the first time, Brugia pahangi. The occurrence of the two species was comparable. Geographic spatial distribution revealed the abundance of D. immitis and B. pahangi in the central areas at altitudes less than 400 m. However, at higher altitudes between 400 and 800 m, we found a significantly higher number of B. pahangi infections than D. immitis infections. In conclusion, D. immitis and B. pahangi were the most common filarial infections found in community dogs in Northern Thailand. Dogs might be an important reservoir for B. pahangi in that region. The population dynamics of the mosquito vector of B. pahangi across altitudinal gradients merits further study.
Abstract
Filariasis is emerging as a public health concern in tropical and subtropical areas. Filariasis is an endemic problem commonly found in southeast Asian countries. Using the PCR-restriction fragment length polymorphism (PCR-RFLP) of the ITS1 region with Vsp I, the overall prevalence rates of Dirofilaria immitis (12.2% (41/337); 95% confidence interval: 9.1–16.1%) and Brugia pahangi (8.3% (28/337); 95% confidence interval: 5.8–11.8%) were determined based on 337 free-roaming community dogs from 20 districts in Northern Thailand. Microfilaremia was found in only 6.2% of dogs (21/337). Co-infection with D. immitis and B. pahangi was observed in two dogs. Of the 215 blood samples examined using a Canine Heartworm Ag Kit, only 3.72% (eight dogs) were D. immitis antigen positive. Among these eight, six dogs had occult D. immitis infections. In terms of geographic distribution, we found the abundance of D. immitis and B. pahangi in the central areas at altitudes less than 400 m to be 12.1% and 10.3%, respectively. In contrast, at higher altitudes between 400 and 800 m, a significantly higher number of B. pahangi compared with D. immitis infected individuals were observed at 14.29% and 4.1%, respectively. In conclusion, D. immitis and B. pahangi were the most common filarial infections found in community dogs in Northern Thailand. Dogs might be an important reservoir of B. pahangi in that region. Increasing awareness and concern and including proper deworming programs for community dogs should be endorsed to reduce the transmission risk. Additionally, the population dynamics of the mosquito vector of B. pahangi across altitudinal gradients deserved further investigation.
Keywords: Brugia pahangi, Dirofilaria immitis, PCR-RFLP, community dogs, spatial distribution, altitude
1. Introduction
Filarial nematode infection is an important vector-borne disease in tropical countries. According to the World Health Organization (WHO) roadmap (WHO 2020), the goal of eliminating filariasis is expected to be achieved by 2030. Filarial infections are currently common in companion animals worldwide [1]. Specifically, in Southeast Asia, lymphatic filariasis caused by Brugia malayi (Brugian or Malayan filariasis), Wuchereria bancrofti (bancroftian filariasis) and Brugia timori is considered a significant human health problem. Filariasis in dogs is caused by various species, e.g., Dirofilaria spp., Acanthocheilonema spp., and Brugia spp. [2,3,4]. Typically, the life cycle of the filarial worm requires a bloodsucking insect such as mosquitoes as a transmission vector, known as an intermediate host. The ingested microfilariae (L1) from the dog develop into the infective stage larvae (L3) in the vector. However, the worm has a species–specific target organ within the final host [5].
Canine heartworm disease, D. immitis infection, is one of the serious types of filariasis observed in veterinary practice. Adult worms commonly live in pulmonary arteries and congestive heart failure can occur in severe cases. Dirofilaria repens and Acanthocheilonema reconditum are other [6] filarial worms usually located in subcutaneous tissues or subconjunctival areas. Brugia spp., such as Brugia pahangi and Brugia malayi, are known to cause lymphatic filariasis (LF), whereas B. malayi causes a serious lymphatic obstruction in humans. Whether B. pahangi is a causative agent of human disease in the natural environment is not yet known [7]. However, microfilaria in the blood, as well as signs and symptoms of LF, were detected in experimentally infected human volunteers [8]. A clinical description of lymphatic filariasis caused by natural infection with B. pahangi in Malaysia was recently published [9]. Dogs and cats are reservoir hosts of B. malayi, but they are the essential hosts of B. pahangi [10].
Currently, several methods are employed to diagnose filarial infections. The basic conventional technique generally uses microscopic examination to detect microfilariae. The microhematocrit centrifugation technique, or Woo’s test, is easy to perform, quick, and inexpensive [11,12], but it cannot identify the species of the parasites. An alternative approach for the identification of filarial worms is DNA technology. Polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) analysis is a molecular technique that involves cutting the specific target of the PCR product with a restriction endonuclease. According to Nuchprayoon et al. [13], differentiation of a wide variety of species of filarial worms was accomplished using PCR-RFLP analysis of the internal transcribed spacer 1 (ITS1). Rishniw et al. [3] reported that using the pan-filarial primers DIDR-F1 and DIDR-R1 in a single PCR test could discriminate among six discordant microfilaria species, including D. immitis, D. repens, A. reconditum, B. malayi, and B. pahangi. The species–specific (for D. immitis) PCR-targeting cytochrome c oxidase subunit 1 (cox1) gene has been used to confirm D. immitis infection.
In tropical countries, including Thailand, the endemic areas for D. immitis, B. malayi, and B. pahangi have been reported, including different species of filarial infection [14,15]. D. immitis infection was found throughout Thailand and has a higher prevalence in stray dogs than in pet dogs [16]. Brugia spp. infection was reported to be found mostly in the southern part of Thailand [17,18]. In the Chiang Mai province in Northern Thailand, canine filariasis showed a prevalence of 18.2% in 2008; however, this study lacked reliable filarial species identification [19]. In addition, Brugia infection in dogs has never been confirmed in Northern Thailand. The WHO recommended using a mapping method for delimiting areas requiring mass drug treatment to save resources [20]; however, to date, no spatial clustering of vector-borne diseases of dogs has been detected in this region. The present study was aimed at updating the occurrence of filarial infection in free-roaming community dogs in the province of Chiang Mai, confirming filarial species using molecular techniques and documenting the geographic distribution of D. immitis and B. pahangi using spatial mapping in an effort to augment disease control efforts in animals and humans, thus reducing public health concerns.
2. Materials and Methods
2.1. Study Area, Sample Collection, and Ethical Concerns
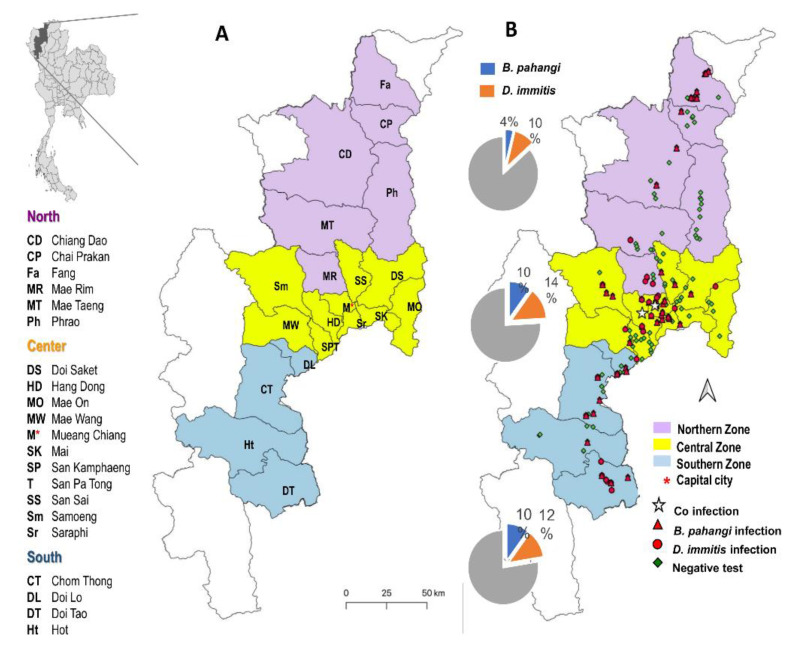
The study area was in the province of Chiang Mai in the upper northern region of Thailand, which is considered the province with the highest dog population density in the nation (278,943 dogs in Chiang Mai of 645,368 total dogs in the region, Bureau of Disease Control and Veterinary Services 2016). The study area was divided into three zones (northern, central, and southern; Figure 1A), encompassing 146 community temples as well as households distributed over 20 of 25 districts and 72 subdistricts to include a wide range of geographical features from north to south of Chiang Mai. The 20 districts included six districts in the northern part of the province (Chiang Dao, Chai Prakan, Fang, Mae Rim, Mae Taeng, and Phrao), 10 districts in the central part (Doi Saket, Hang Dong, Mae On, Mae Wang, Mueang Chiang Mai, Samoeng, San Kamphaeng, San Pa Tong, San Sai, and Saraphi), and four districts in the southern part (Chom Thong, Doi Lo, Doi Tao, and Hot). GPS location was determined (Map Plus TM version 2.4, mobile application) for each sampling site, including coordinates (latitude and longitude) and altitude (meters). Samples were collected from June to December 2019. According to a government announcement (Thai Meteorological Department 2018), this sampling period could be defined as spanning the rainy (June to October) and cold seasons (November to February).
Figure 1.
Study sites and geographic distribution of canine filariasis in three zones of Chiang Mai, Thailand. (A). The study districts; (B). Distribution of Brugia pahangi and Dirofilaria immitis positives and the proportion of prevalence of filarial nematode species in each zone.
A total of 337 blood samples were collected from the community dogs (the dogs owned by specific owners, able to be free ranging in the community, and taken care of by people in the communities), including 168 males and 169 females. Inclusion criteria included age >7 months and having not received heartworm prevention medication. From each dog, 2 mL of blood was collected from either the cephalic or saphenous vein which was kept in EDTA tubes. The first 0.5 mL of blood was used for Woo’s examination for the presence of microfilaria. The remaining 1.5 mL was kept in a freezer at −20 °C for further molecular analysis.
All dog owners (temple master or dog keepers) signed an informed consent form, and the treatment of the animals was approved by the Animal Care and Use Committee, Faculty of Veterinary Medicine, Chiang Mai University (R15/2562) on 12 June 2019.
2.2. Laboratory Examination
2.2.1. Detection of Circulating Microfilaria and D. immitis Antigens
The whole blood samples were examined for total circulating microfilariae using the microhematocrit centrifugation technique (Woo’s technique) [11]. In addition, the packed cell volume (PCV) was also measured as a percent. Measurement of the severity of anemia in this study followed the guidelines for classification of severity of anemia from the World Small Animal Veterinary Association (WSAVA) 2005 [21]. A total of 215 whole blood samples were randomly selected for additional study to detect the adult female D. immitis antigen using a Thinka Canine Heartworm Ag Test kit (Thinka CHW, Arkray, Kyoto, Japan) following the manufacturer’s instructions. For quality control, the blood samples were examined within 48 h after blood collection.
2.2.2. Molecular Techniques and Sequencing
Genomic DNA (gDNA) was extracted from 200 µL anticoagulated blood samples. A commercial DNA extraction kit (Nucleospin® Blood, Macherey-Nagel, Duren, Germany) was used following the manufacturer’s instructions.
Various diagnostic tools have been developed for the precise diagnosis of filaria infection. PCR-RFLP—which is based on a diagnostic method previously designed by Nuchprayoon et al. [13] and targets the ITS1 region (primer: ITS1-F and ITS1-R; Table 1)—was conducted. PCR amplification reactions were performed in a 20 μL reaction volume containing 100 ng gDNA (3–5 µL), 0.2 μM of each primer (0.4 μL of 10 μM), and 10 µL of 2 × Quick Taq® HS DyeMix (TOYOBO, Osaka, Japan). PCR products were analyzed on a 1.5% agarose gel electrophoresis. The positive ITS1 PCR products were then digested with five units of Vsp I (SibEnzyme, Novosibirsk, Russia) as a restriction endonuclease, according to the manufacturer’s protocol. One unit of Vsp I was used to digest 1 µg of the ITS1 PCR product for 1 h at 37 °C in a total reaction volume of 50 µL. DNA fragment analysis was conducted by 1.5% agarose gel electrophoresis, stained with RedSafe™ nucleic acid staining solution (iNtRON Biotechnology, Gyeonggi-do, Korea), and visualized using a GelMax™ Imager (Ultra-Violet Products, Cambridge, UK).
Table 1.
Primer pairs used for PCR amplification.
| Gene Target | Primer Pairs | Primer Sequence (5′-3′) | Filarial Species | Product Size (bp) |
|---|---|---|---|---|
| ITS1 | ITS1-F ITS1-R |
GGT GAA CCT GCG GAA GGA TC GAG TTA CGC AGA CGT TAA GCG |
W. bancrofti | 482 |
| B. malayi | 504 | |||
| B. pahangi | 510 | |||
| D. immitis | 595 | |||
| D. repens | 602 | |||
| 5.8S-ITS2-28S | DIDR-F1 DIDR-R1 |
AGT GCG AAT TGC AGA CGC ATT GAG AGC GGG TAA TCA CGA CTG AGT TGA |
D. immitis | 542 |
| A. reconditum | 578 | |||
| D. repens | 484 | |||
| A. dracunculoides | 584 | |||
| B. pahangi | 664 | |||
| B. malayi | 615 | |||
| COI | DI COI -F1 DI COI-R1 |
AGT GTA GAG GGT CAG CCT GAG TTA ACA GGC ACT GAC AAT ACC AAT |
D. immitis | 203 |
Two other PCR techniques were used to confirm the presence of filarial DNA in the suspicious samples, including PCR of pan-filarial primers (DIDR-F1 and DIDR-R1) to discriminate between A. reconditum and Amphiachyris dracunculoides, and primers specific for D. immitis (DI COI-F1 and DI COI-R1) to confirm D. immitis DNA in suspected occult infections. The PCR procedure was performed following previously published methods [3] and using the same reagent previously described for the PCR of ITS1. Distilled water (DW) as a negative control and the gDNA of D. immitis, B. pahangi, and B. malayi as positive controls were included in the analysis.
Species confirmation in the 10 previous D. immitis- and B. pahangi-positive samples was achieved by DNA sequencing of the 5.8s-ITS2-28S amplicons using pan-filarial primers given the clear and distinctive amplicon. The PCR product (50 μL) was purified using a NucleoSpin® PCR Clean-up Kit (Macherey-Nagel GmbH, Duren, Germany) and submitted for direct fluorescent dye-terminator sequencing in the sense and antisense directions by BioBasic Inc. (The Elitist, Singapore).
2.3. Data and Statistical Analysis
2.3.1. Determination of the Presence and Prevalence of Filariasis
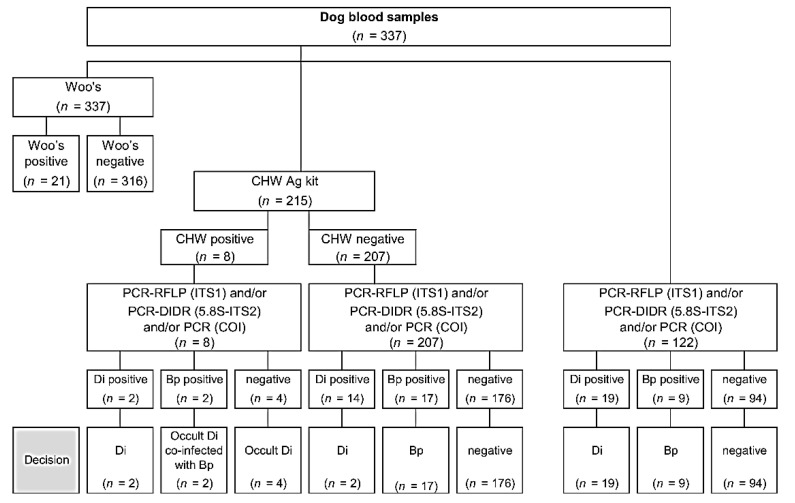
Diagnostic steps for identification and determination of the prevalence of filariasis are presented in Figure 2. The determination of the presence of filariasis was based on three diagnostic techniques: (1) D. immitis infection when positive with CHW Ag kit and/or D. immitis-DNA-positive with any molecular technique; (2) occult D. immitis infection when positive with CHW Ag kit but negative with any PCR; (3) B. pahangi infection when B. pahangi-DNA-positive with any molecular technique. Descriptive statistics summarizing the prevalence of filarial worm infection are presented as a percentage of each species. Differences in prevalence between groups or categories were analyzed using proportion tests. All statistical analyses were performed using R software [22] and p-values < 0.05 were considered statistically significant.
Figure 2.
Diagnostic diagram of canine filariasis using CHW Ag test kit and three molecular techniques: PCR-RFLP of ITS1 region, PCR of 5.8S-ITS2-28S region, and PCR of cox1 gene. Di: D. immitis; Bp: Brugia pahangi.
2.3.2. Sequencing and Phylogenetic Analyses
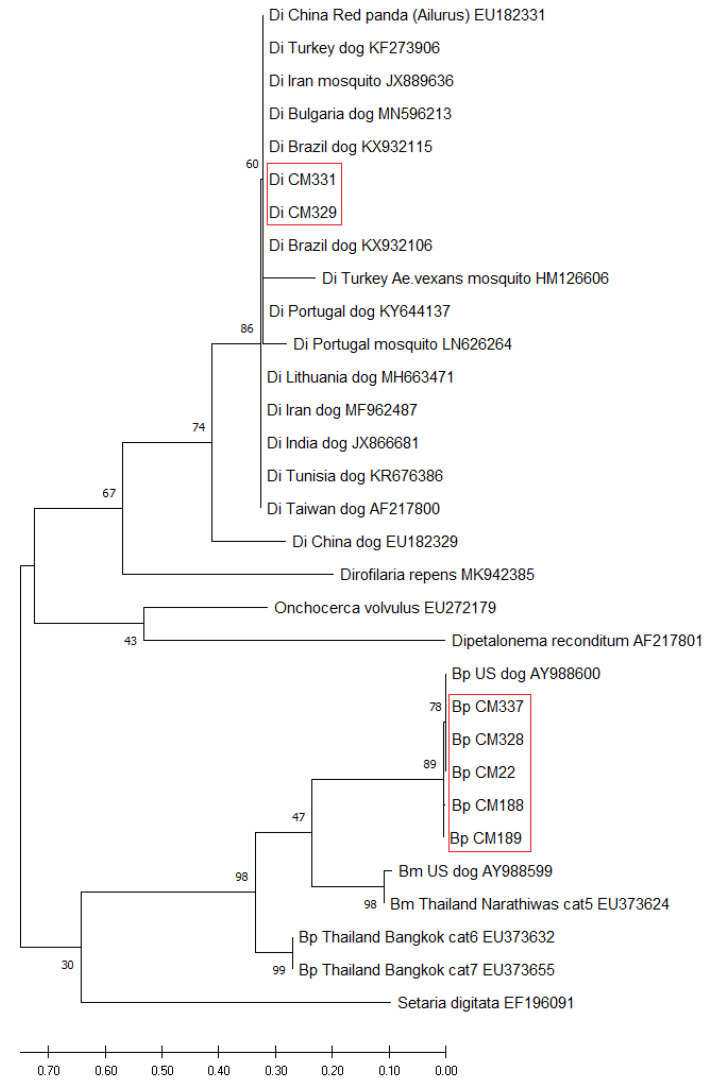
The obtained sequences were compared with the previously deposited ones in GenBank using the BLASTn program [23] for specific diagnosis. Seven successful 5.8S-ITS2 nucleotide sequences originating from two D. immitis isolates (Di CM329 and CM331) and five isolates of B. pahangi (Bp-CM22, CM188, CM189, CM328, and CM337) (Table S1) were used to analyze the phylogenetic relationship among the regions. In addition, the available sequences of D. immitis and B. pahangi from other countries (Brazil, Bulgaria, China, India, Iran, Lithuania, Portugal, Taiwan, Thailand, Turkey, Tunisia, and the U.S.; Table S1) were compared with the sequences obtained in this study for the construction of a phylogenetic tree. The sequences of B. malayi, Onchocerca volvulus, A. reconditum (previously Dipetalonema reconditum) and D. repens (GenBank accession: EU373624, EU272179, AF217801, and MK942385, respectively), and the sequence of Setaria digitata (EF196091) as the outgroup were included for evolution analysis. All sequences obtained in this study were submitted to the DDBJ/EMBL/GenBank database (Table S1).
Evolutionary analyses were conducted using MEGA X [24]. Multiple sequences were aligned using ClustalW and a phylogenetic tree using a maximum likelihood (ML) method based on the Tamura–Nei model [25]. The Neighbor-Join and BioNJ algorithms were applied to a matrix of pairwise distances estimated using the Tamura-Nei model, and then the topology was selected with superior log likelihood value. A consensus tree was obtained after bootstrap analysis of 100 replications. The tree is drawn to scale, with branch lengths indicating the number of substitutions per site. Thirty nucleotide sequences were included in this analysis. Codon positions included were first, second, third, and noncoding.
2.3.3. Geographical Information System Mapping and Distribution by Altitude
Geographical Information System (GIS) locations (latitude, longitude, and altitude) from a mobile phone application were input to a computer. The digital elevation model (DEM) was obtained from the NASA Earth data website and used to create a raster image and to convert the model into contour lines representing altitude in the GIS application (QGIS software version 3.6, GNU General Public License). The altitudes of the infected sites were measured using a GPS. Altitudes of the positive prevalence spots were divided into three classes modified from those used by Devi and Jauhari [26]: below 400, 400–800, and over 800 m. A proportional test was used to determine differences in prevalence of B. pahangi and D. immitis among altitude classes using R software [22] and p-values < 0.05 were considered statistically significant. Geographic distribution by altitude was generated from geographic information systems and GPS tracking data using QGIS software version 3.6. Notably, NASA Earth data indicates that all NASA data are available without restrictions.
2.3.4. Geographic Spatial Cluster Analysis
Spatial cluster analyses of the positive D. immitis and B. pahangi cases were attained using a Bernoulli model and SaTScan™ v9.6 software [27]. Generally, this model requires data from cases and controls. The definition of a case was a positive filarial nematode using any of the three diagnostic techniques (Figure 2), and a negative individual was the control. In the study area, 50% of the total population was set up in the spatial scanning window. The Monte Carlo hypothesis testing technique (number of replications = 999) was used to determine the statistical significance of the cluster. The primary clusters of D. immitis and B. pahangi were classified based on the highest log-likelihood ratio (LLR), in which the likelihood function of the Bernoulli model is:
| (1) |
where is the total number of cases, is the combined total number of cases, the control is the observed number of cases within the window, is the total number of cases and controls within the window, and is the indicator of function . As the analysis was only focused on detecting clusters with higher than expected rates, was set to 1 [27]. The illustrated spatial cluster of each species combined with the altitude and the geographic functional land use was generated by QGIS software version 3.6.
3. Results
3.1. Prevalence of Filariasis Using a Combination of Conventional and Molecular Techniques
Various diagnostic techniques were employed to identify filarial species infection and molecular techniques were applied to identify the species of filarial nematodes. Of the total of 337 blood samples, microfilaria was detected in only 21 dogs (6.23%) using Woo’s method and most of those were from the central zone (Table 2). Of the 215 blood samples examined using a CHW Ag kit, only eight dogs (3.72%) were D. immitis antigen positive (Figure 2). Only three CHW Ag-positive dogs had microfilaremia, of which molecular techniques could discriminate one positive with D. immitis and two positives with B. pahangi. The overall prevalence of D. immitis infection was 12.17% (41/337 dogs), higher than the prevalence of 8.31% for B. pahangi (28/337 dogs). However, the difference in infection rate between the two species was not significant (p > 0.05). The distributions of D. immitis and B. pahangi infection in the three zones of Chiang Mai province are shown in Table 2, Table S2, and Figure 1B. The observed D. immitis and B. pahangi infection incidence was highest in the central zone at 22.96% (31/135), followed by the south zone at 22.45% (22/98). However, the prevalence rates of D. immitis and B. pahangi infection among the three zones were not significantly different (p > 0.05). Two dogs in the central zone had dual infections with D. immitis and B. pahangi. No adult D. immitis antigens were detected in the two D. immitis DNA-positive samples. Finally, an important proportion of occult heartworm infections (6/8; 75%) was distinguished (Figure 2).
Table 2.
Prevalence of microfilariae and filarial nematode species in the community dogs among three zones of Chiang Mai, Thailand.
| Zone | No. of Examined | Microfilariae (Woo’s Method) |
Filarial DNA (Molecular Techniques) |
|||
|---|---|---|---|---|---|---|
| % Positive (no.) |
B. pahangi Infection (n1) |
D. immitis Infection (n2) |
Dual Infection (n3) |
Total (n1 + n2 − n3) |
||
| North | 104 | 2.88 (3) a, b | 3.85 (4) | 9.62 (10) | 0 | 13.46 (14) |
| Central | 135 | 11.11 (15) b | 10.37 (14) | 14.07 (19) | 2 | 22.96 (31) |
| South | 98 | 3.06 (3) a | 10.20 (10) | 12.24 (12) | 0 | 22.45 (22) |
| Total | 337 | 6.23 (21) | 8.31 (28) | 12.17 (41) | 2 | 19.88 (67) |
a, b values in the same column with different superscripts are statistically different (p < 0.05); n1, n2, n3 are the number of positive cases.
The range of the percent PCV (%PCV) of dogs with positive microfilariae was 15–46%, with a mean ± SD of 32.48 ± 8.38 and a mode of 31%. Fourteen dogs with positive microfilariae (71.43%; 15/21) had a %PCV lower than 37%, which is considered anemic. Anemic status was categorized as mild (30–37% PCV; 9/21, 42.86%), moderate (20–29% PCV; 4/21, 19.05%), and severe (13–19% PCV; 2/21, 9.52%). No icteric plasma was observed in any of the samples.
3.2. Phylogenetic Relationship of D. immitis and B. pahangi
The nucleotide sequences of the partial 5.8S rRNA and ITS2 region contained approximately 300–350 bp that allowed the filarial nematodes to be classified as D. immitis or B. pahangi. The nucleotide sequences of five isolates of B. pahangi were identical and grouped in a haplotype that showed 100% identity with B. pahangi (AY988600; [3]) and 95.67% with isolates from cats from the Narathiwat province in Southern Thailand (EU373655; [28]). However, two nucleotide sequences of D. immitis had some nucleotide differences, which showed 95–100% identity with the D. immitis references (Table S1). The nucleotide sequences of D. immitis and B. pahangi in this study were deposited in the DDBJ/EMBL/GenBank database, including D. immitis (accession No. LC554219-554220) and B. pahangi (accession No. LC554214-554218 (Table S1)).
A comparative genomic analysis of D. immitis and B. pahangi was performed to investigate the phylogenetic relationship among the different geographic regions (Figure 3). The phylogenetic tree based on the partial 5.8S and ITS2 sequence showed that all isolated samples from D. immitis clustered together in one group and were similar to D. immitis from China (EU182331), Brazil (KX932106), Iran (JX889636), Bulgaria (MN596213), and Turkey (KF273906 and HM126606). Five isolates from B. pahangi were clustered together in the same clade with B. pahangi published by Rishniw et al. [3], in which the worm was from an unknown region. Comparison of nucleotide sequences with other species of filarial nematode found D. immitis and B. pahangi could be distinguished from B. malayi, D. repens, O. volvulus, and A. reconditum (Dipetalonema reconditum in Figure 3).
Figure 3.
Evolutionary analysis of D. immitis and B. pahangi 5.8S-ITS2 region. A phylogenetic tree based on the 5.8S-ITS2 region of D. immitis (Di) and B. pahangi (Bp) from Thailand and other countries was inferred using the maximum likelihood method and the Tamura–Nei model. The tree with the highest log likelihood (−2536.04) is shown. The percentage of trees in which the associated taxa clustered together is shown next to the branches. There was a total of 344 positions in the final dataset. Sequences in the red outline boxes originated from the present study.
3.3. Geographical Distribution of Filarial Infection
3.3.1. Altitudinal Distribution of Filarial Infection
There was no difference in the filarial positivity of either B. pahangi or D. immitis among the different altitude classes (Table 3). The range of B. pahangi infection was 4.08–10.27%, with the highest occurrence observed in the low areas <400 m altitude (10.27%; 23 cases). The range of D. immitis infection was 12.05–14.29%, with the highest occurrence observed at 400–800 m (14.29%; 14 cases). The prevalence of D. immitis was significantly higher than B. pahangi in the middle altitude class of 400–800 m, at 14.29% and 4.08%, respectively (p < 0.05; Table 3). The altitudinal distribution of filarial positive cases is illustrated on a GIS map of Chiang Mai, Thailand (Figure 4C and Figure 5C).
Table 3.
The altitudinal distribution and prevalence of canine filariasis in Chiang Mai, Thailand.
| Level | Altitude Level (m.) | No. of Examined | B. pahangi Infection (n) | D. immitis Infection (n) | Total Canine Filariasis (n) |
|---|---|---|---|---|---|
| 1 | <400 | 224 | 10.27% (23) | 12.05% (27) | 21.43% (48) |
| 2 | 400–800 | 98 | 4.08% (4) a | 14.29% (14) b | 18.37% (18) |
| 3 | >800 | 15 | 6.67% (1) | 0 | 6.67% (1) |
| Total | 337 | 8.31% (28) | 12.17% (41) | 19.88% (67) |
a, b Values in the same column with different superscripts are statistically different (p < 0.05).
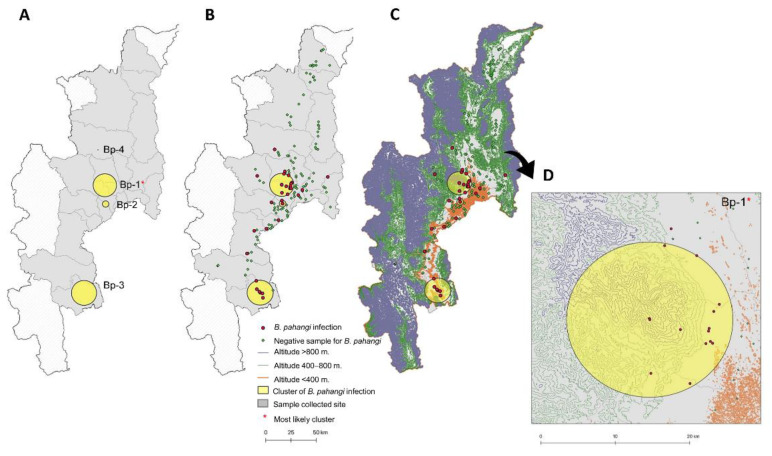
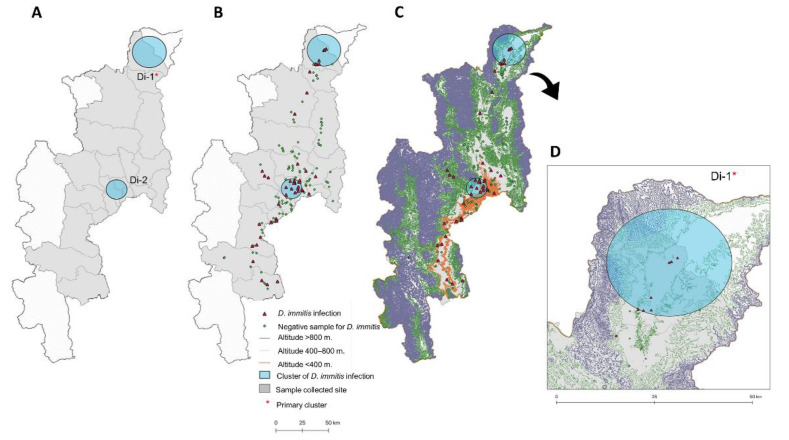
Figure 4.
Geographic spatial clusters of B. pahangi infection obtained using the Bernoulli scan statistic model. (A). The distribution of four clusters of B. pahangi infection; (B). the distribution of B. pahangi cases in each cluster; (C). the altitudinal distribution of B. pahangi cases in each cluster; (D). the distribution of B. pahangi cases in the most likely cluster.
Figure 5.
Geographic spatial clusters of D. immitis infection obtained using the Bernoulli scan statistic model. (A). the distribution of four clusters of D. immitis infection; (B). the distribution of D. immitis cases in each cluster; (C). the altitudinal distribution of D. immitis cases in each cluster; (D). the distribution of D. immitis cases in the most likely cluster.
3.3.2. Spatial Cluster Distribution of Filarial Infection
The canine filariasis spatial clusters were analyzed using the Bernoulli special scanning statistic method.Tthe relative risk (RR) and size of each cluster are outlined in Table 4. Four and two spatial clusters were identified for B. pahangi and D. immitis, respectively. Regarding B. pahangi distribution, a significant cluster (cluster Bp-1; p < 0.001) was identified with RR at 6.69 and a clustering radius of 10.55 km. This most likely cluster was distributed in three districts: Hang Dong, Mae Rim, and Mueang. The primary cluster of B. pahangi infection was found to occur in various altitudinal areas (0 to >800 m Figure 4C,D). Replacement of forests by agricultural activities and irrigated crops occurred in scattered locations in this cluster area. However, no significant spatial patterns were identified for D. immitis infection (Table 4), although the number of cases observed was higher than with B. pahangi. The most likely cluster of D. immitis infection (Di-1) was located in the Fang district (18.737609° N, 98.931619° E) where the clustering radius was 9.32 km, and the RR was 4.03. Sites of positive identification of D. immitis in this cluster were distributed in the low areas at altitudes of 400 to 800 m (Figure 5C,D) and were found mainly in areas with irrigated agricultural crops.
Table 4.
Geographic spatial clusters obtained by Bernoulli scan statistic model on canine filariasis in Chiang Mai, Thailand.
| Cluster Number | Cluster Type | Centroid (X,Y)/Radius (km) | Cases | Expected Cases | Observed to Expected Cases | RR a | LLR b | p-Value | Districts |
|---|---|---|---|---|---|---|---|---|---|
| B. pahangi infection | |||||||||
| Bp-1 | Most likely | 18.815706 N, 98.883035 E/10.55 | 8 | 1.58 | 5.07 | 6.69 | 8.848 | 0.010 | Hang Dong, Mae Rim, Mueang, Samoeng |
| Bp-2 | Secondary | 18.660241 N, 98.889764 E/2.91 | 2 | 0.17 | 12.04 | 12.88 | 5.043 | 0.439 | Hang Dong, San Pa Tong |
| Bp-3 | Secondary | 17.924869 N, 98.704168 E/11.42 | 5 | 1.41 | 3.54 | 4.09 | 3.457 | 0.754 | Doi Tao |
| Bp-4 | Secondary | 19.108108 N, 98.824182 E/0 | 2 | 0.25 | 8.02 | 8.56 | 3.214 | 0.883 | Mae Taeng |
| D. immitis infection | |||||||||
| Di-1 | Most likely | 18.737609 N, 98.931619 E/9.32 | 9 | 2.68 | 3.36 | 4.03 | 6.386 | 0.143 | Fang |
| Di-2 | Secondary | 19.922648 N, 99.208706 E/15.01 | 6 | 1.70 | 3.52 | 3.96 | 4.392 | 0.393 | Hang Dong, Mueang, Saraphi |
a RR = relative risk, b LLR = log likelihood ratio.
4. Discussion
The current study updates our understanding of the situation of D. immitis infection and proven B. pahangi infection in community dogs in Northern Thailand. B. malayi was not found in Chiang Mai, although the province does have a large free-roaming dog population.
Heartworm disease caused by D. immitis infection is widespread in tropical and subtropical regions and is a primary life-threatening disease of dogs and cats worldwide. The prevalence of D. immitis infection in dogs worldwide was 10.91% (95% CI = 10.18–11.65) in 2020. The prevalence of D. immitis in dogs varies across countries and continents, e.g., 22.68% in Australia, 12.07% in Asia, 11.60% in the Americas, 10.45% in Europe, and 7.57% in Africa [1]. Over the past few decades, the regional prevalence of D. immitis infection in dogs in Thailand has been reported as 10–58% in Bangkok [16,29,30,31], 23–25% in the southern region [32], and 6–25% in the northern region [19,33,34,35]. In Chiang Mai, the prevalence gradually decreased from 45.76% in 1987 to 24.71% in 1992, to 18.20% in 2008, and then to 12.17% in 2020. The reduction of dirofilariasis is possibly due to effective regular preventive chemotherapy of dogs and to vector control by dog owners in collaboration with the active support of District Administration Organizations, which should be extended to other high prevalence districts such as Fang.
B. pahangi, a lymphatic filarial worm that infests mammals, is commonly found in cats, dogs, and wild carnivores [36], and is closely related to B. malayi. Recently, five cases of clinically typical LF caused by B. pahangi were reported in suburban areas in the city of Kuala Lumpur, Malaysia [9]. Additionally, there was a report of a human subconjunctival infection with B. pahangi in Malaysia, which was identified by cyclooxygenase-1 (COX-1) PCR [37]. To the best of our knowledge, that study was the first to confirm B. pahangi infection in an animal in Chiang Mai. Elsewhere in Thailand, the B. pahangi infection rate range of 4–25% in dogs and cats in Bangkok has been reported [16,31,38]. In addition, in Western and Southern Thailand, accidental zoonotic filariasis of B. pahangi infections in children were reported, and Armigeres subalbatus was found to be a common mosquito in the infected areas [39]. The recently discovered parasite B. pahangi exhibits novel aspects and adaptations. For that reason, Brugia infections in humans in this region should be closely monitored, especially the environment and geographical distribution in areas with a high vector density and densely crowded human populations.
Natural infections of canine dirofilariasis enhance the risk of transmission to humans [40]. The relative risk of canine dirofilariasis has been found to be related to various factors, e.g., outdoor living, lack of heartworm prevention, and infected vector exposure [19,40]. The present study observed D. immitis cases in all altitude classes (0 to >800 m), probably due to the broad range of mosquito species, which are potential transmission vectors. Although Mansonia uniformis is an important natural mosquito vector of D. immitis in Chiang Mai [33], Culex pipiens quinquefasciatus, Aedes albopictus, and Ae. aegypti are additional vector species for D. immitis transmission. The majority of Aedes, Anopheles, Armigeres, and Culex mosquitoes have been found at altitudes between 300 and 900 m [26]. Ae. albopictus is distributed in a wide range of elevations, between 300 and 1300 m, whereas Cx. quinquefasciatus has been found at elevations of 500 m and above [26] and Mansonia species are spread across different altitudes [41]. The number of Mansonia species is likely to be higher in areas with floating aquatic plants.
B. pahangi is considered to be a new causative agent of urban Brugia filariasis in Thailand [42]. Infective-stage larvae of B. pahangi microfilariae can develop in An. quadrimaculatus and Ae. aegypti [43]. Development and transmission of B. pahangi microfilariae to a vertebrate host can occur via natural vectors such as Ar. subalbatus [44,45]. Ar. subalbatus has also been found to be a common mosquito in infected areas and is suspected of being the main vector for zoonotic B. pahangi infection of children in Thailand [39]. Chaves et al. [46] demonstrated that density of adult Ar. subalbatus decreases with altitude. Its preferred range is 109–330 m, but it tends to increase in areas with abundant leaf litter on the ground. However, other mosquitoes, including the genera Culex, Aedes, Mansonia, Anopheles, and Armigeres, are potential mosquito vectors of Brugia spp. [47] which might explain the distribution of B. pahangi cases at all altitudes from 0 to >800 m. Climatic and geographic conditions, as well as environmental factors and changes in land use, may support the high prevalence of B. pahangi at low altitudes. The occurrence of more hotspots of D. immitis and B. pahangi in suburban areas of Chiang Mai, such as the Fang and Mae Rim districts (Figure 4), could be influenced by factors driving transmission such as urbanization, rural–urban migration, and expansion of irrigated agricultural land, which is common in this area and provides suitable humidity for mosquitoes to thrive. Spatial mapping of the mosquito vector distribution and breeding habitats in canine filarial-infected areas on finer scales should be conducted to complete our current understanding of transmission dynamics and to help with the establishment of appropriate control strategies.
Although results of analyses of the correlation among diagnostic techniques for filariasis have so far been inconclusive [48,49], a combination of various diagnostic methods remains crucial for precise canine filariasis mapping of the region. Although the sensitivity of microhematocrit centrifugation for microfilaria detection was reported to be only 30% [50], this concentration technique is commonly applied in clinical practice to screen for microfilaria prior to confirmation using a heartworm antigen kit. We did not differentiate the microfilaria species by microscopic examination because this is labor-intensive and because of the difficulty in resolving the identification of species using blood smears. However, molecular techniques such as PCR can successfully detect microfilaria from blood samples. Additionally, PCR can confirm species identification using specific primers or by sequencing, making it more precise than microscopic procedures, especially when many filarial species and coinfection are involved. In the present study, two D. immitis DNA-positive dogs did not present with adult D. immitis antigen in their blood. This may be linked to the existence of an immune complex that blocks the antigen detection process or to the low antigen secretion by adult worms [51]. However, one occult infection was apparently observed in the current study. This detection problem might be due to many factors, including detection in dogs with prepatent infection, immature female worms, unisexual worm infection, drug-induced sterility of adult heartworms, senile infertility of female worms, host immune responses, and treatment with macrocyclic lactones [6,52,53].
This study has some limitations. First, although we covered a wide range of geographical features in Northern Thailand, the results should be interpreted with caution as they cannot be generalized to all endemic settings. More variations in geographical characteristics should be included in future studies. Second, as sampling was conducted mainly among free-roaming dogs in the studied communities, information about the dogs, e.g., housing, routine drugs, and vaccination, was limited; thus, an analysis of risk factors associated with the risk of B. pahangi could not be determined in this study. Last, the sampling strategy in this study was from an unknown prevalence; thus, it may pose a challenge, especially for B. pahangi, possibly contributing to an underestimation of number of samplings. However, for a follow-up study, the prevalence obtained from this study might be used and modified for a sample size determination.
5. Conclusions
In this study, we covered the detection and confirmation of species of canine filarial infection in free-roaming community dogs found in Chiang Mai, Northern Thailand, using a combination of diagnostic methods. The infection rates of B. pahangi were found to be comparable to those of D. immitis. Both types of filarial nematodes had a high prevalence in the low altitude suburban agricultural areas. The residents of the region should be made aware that lymphatic filariasis is caused by B. pahangi. For practicing veterinarians, filarial species identification is necessary for determination of the proper heartworm treatment as well as for public health efforts related to zoonotic transmission. Spatial distribution of filariasis in consecutive areas as well as mosquito vector distribution and breeding habitats of D. immitis and B. pahangi in infected areas should be determined at finer scales to identify and implement appropriate control strategies.
Acknowledgments
The authors appreciate the cooperation of the monks and animal keepers at all temples and community locations where blood samples and data were collected. We would also like to thank Piyanan Taweethavonsawat (Faculty of Veterinary Science, Chulalongkorn University, Thailand) and Atiporn Saeung (Faculty of Medicine, Chiang Mai University, Thailand) for the positive control of Brugia spp.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-2615/11/1/33/s1, Table S1: The 5.8S-ITS2 sequence list of D. immitis, B. pahangi, and other filarial nematodes used in the phylogenetic analysis, Table S2: Prevalence of canine filariasis in different districts in Chiang Mai Province, Thailand.
Author Contributions
Conceptualization, S.T.; methodology, M.K., D.K., S.F. and S.T.; software, O.A., V.P. and S.T.; validation, S.T.; formal analysis, M.K., O.A., V.P. and S.T.; investigation, M.K., D.K. and S.T.; resources, M.K., K.T., C.B. and S.T.; data curation, M.K., V.P. and S.T.; writing—original draft preparation, M.K., D.K. and S.T.; writing—review and editing, M.K., S.F., V.P. and S.T.; visualization, M.K., V.P. and S.F.; supervision, K.T., C.B., V.P. and S.T.; project administration, S.T.; funding acquisition, V.P. All authors have read and agreed to the published version of the manuscript.
Funding
This work was partially supported by Chiang Mai University (CMU), the research fund of Faculty of Veterinary Medicine, CMU (Grant Number R000016307 to V.P.), Research Grants for Young Researchers, CMU Y2014 (Grant Number H5701 to S.T.), and the partially supported by JSPS KAKENHI (Grant Numbers 19KK0175, 19H03121 to S.F.).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Institutional Animal Care and Use Committee (IACUC), and approved by the Faculty of Veterinary Medicine, Chiang Mai University Animal Care and Use Committee (FVM, ACUC; protocol code R15/2562, approved on 12 June 2019).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data available on request due to privacy for sequential projects.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ghasemi E., Shamsinia S., Taghipour A., Anvari D., Bahadory S., Shariatzadeh S.A., Kordi B., Majidiani H., Borji H., Chaechi Nosrati M., et al. Filarial worms: A systematic review and meta-analysis of diversity in animals from Iran with emphasis on human cases. Parasitology. 2020;147:909–921. doi: 10.1017/S003118202000058X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Irwin P.J. Companion animal parasitology: A clinical perspective. Int. J. Parasitol. 2002;32:581–593. doi: 10.1016/S0020-7519(01)00361-7. [DOI] [PubMed] [Google Scholar]
- 3.Rishniw M., Barr S.C., Simpson K.W., Frongillo M.F., Franz M., Dominguez Alpizar J.L. Discrimination between six species of canine microfilariae by a single polymerase chain reaction. Vet. Parasitol. 2006;135:303–314. doi: 10.1016/j.vetpar.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 4.Simón F., Kramer L.H., Román A., Blasini W., Morchón R., Marcos-Atxutegi C., Grandi G., Genchi C. Immunopathology of Dirofilaria immitis infection. Vet. Res. Commun. 2007;31:161–171. doi: 10.1007/s11259-006-3387-0. [DOI] [PubMed] [Google Scholar]
- 5.Bowman D.D. Georgis’ Parasitology for Veterinarians. Saunders, Elsevier; St. Louis, MO, USA: 2009. [Google Scholar]
- 6.Simón F., Siles-Lucas M., Morchón R., González-Miguel J., Mellado I., Carretón E., Montoya-Alonso J.A. Human and animal dirofilariasis: The emergence of a zoonotic mosaic. Clin. Microbiol. Rev. 2012;25:507–544. doi: 10.1128/CMR.00012-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson R.C. Nematode Parasites of Vertebrates: Their Development and Transmission. 2nd ed. CABI; London, UK: 2000. [Google Scholar]
- 8.Edeson J.F., Wilson T., Wharton R.H., Laing A.B. Experimental transmission of Brugia malayi and B. pahangi to man. Trans. R. Soc. Trop. Med. Hyg. 1960;54:229–234. doi: 10.1016/0035-9203(60)90066-3. [DOI] [PubMed] [Google Scholar]
- 9.Tan L.H., Fong M.Y., Mahmud R., Muslim A., Lau Y.L., Kamarulzaman A. Zoonotic Brugia pahangi filariasis in a suburbia of Kuala Lumpur City, Malaysia. Parasitol. Int. 2011;60:111–113. doi: 10.1016/j.parint.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 10.Melrose W.D. Lymphatic filariasis: New insights into an old disease. Int. J. Parasitol. 2002;32:947–960. doi: 10.1016/S0020-7519(02)00062-0. [DOI] [PubMed] [Google Scholar]
- 11.Woo P.T. Evaluation of the haematocrit centrifuge and other techniques for the field diagnosis of human trypanosomiasis and filariasis. Acta Trop. 1971;28:298–303. [PubMed] [Google Scholar]
- 12.Kawthalkar S.M. Essentials of Clinical Pathology. Jaypee Brothers Medical Publishers; New Delhi, India: 2018. [Google Scholar]
- 13.Nuchprayoon S., Junpee A., Poovorawan Y., Scott A.L. Detection and differentiation of filarial parasites by universal primers and polymerase chain reaction-restriction fragment length polymorphism analysis. Am. J. Trop. Med. Hyg. 2005;73:895–900. doi: 10.4269/ajtmh.2005.73.895. [DOI] [PubMed] [Google Scholar]
- 14.Dickson B.F.R., Graves P.M., McBride W.J. Lymphatic filariasis in mainland Southeast Asia: A systematic review and meta-analysis of prevalence and disease burden. Trop. Med. Infect. Dis. 2017;2:32. doi: 10.3390/tropicalmed2030032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaikuntod M., Thongkorn K., Tiwananthagorn S., Boonyapakorn C. Filarial worms in dogs in Southeast Asia. Vet. Integr. Sci. 2008;16:1–17. [Google Scholar]
- 16.Nithiuthai S., Chungpivat S. Lymphatic filaria (Brugia pahangi) in dogs. Thai J. Vet. Med. 1992;4:123–133. [Google Scholar]
- 17.Iyengar M.O. Filariasis in Thailand. Bull World Health Organ. 1953;9:731–766. [PMC free article] [PubMed] [Google Scholar]
- 18.Yokmek S., Warunyuwong W., Rojanapanus S., Jiraamornimit C., Boitano J.J., Wongkamchai S. A case report of Brugian filariasis outside an endemic area in Thailand. J Helminthol. 2013;87:510–514. doi: 10.1017/S0022149X12000533. [DOI] [PubMed] [Google Scholar]
- 19.Boonyapakorn C., Srikitjakarn L., Morakote N., Hoerchner F. The epidemiology of Dirofilaria immitis infection in outpatient dogs at Chiang Mai university small animal hospital, Thailand. Southeast Asian J. Trop. Med. Public. Health. 2008;39:33–38. [PubMed] [Google Scholar]
- 20.World Health Organization . UNDP-World Bank-WHO Special Programme for Research and Training in Tropical Diseases WHO-UNICEF Joint Programme for Health Mapping World Health Organization: Division of Tropical Diseases. Research on Rapid Geographical Assessment of Bancroftian Filariasis. World Health Organization; Geneva, Switzerland: 1998. [Google Scholar]
- 21.Tvedten H. Basic approach to anemia diagnosis; Proceedings of the World Small Animal Veterinary Association World Congress Proceedings; Mexico city, Mexico. 11–14 May 2005; [(accessed on 15 May 2019)]. Available online: https://www.vin.com/apputil/content/defaultadv1.aspx?id=3854233&pid=11196#:~:text=One%20documents%20the%20presence%20and,and%20how%20severe%20it%20is. [Google Scholar]
- 22.R Core Team R: A Language and Environment for Statistical Computing. [(accessed on 15 May 2019)]; Available online: http://www.R-project.org/
- 23.Altschul S.F., Gish W., Miller W., Myers E.W., Lipman D.J. Basic local alignment search tool. J. Mol. Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 24.Kumar S., Stecher G., Li M., Knyaz C., Tamura K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018;35:1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tamura K., Nei M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol. Biol. Evol. 1993;10:512–526. doi: 10.1093/oxfordjournals.molbev.a040023. [DOI] [PubMed] [Google Scholar]
- 26.Devi N.P., Jauhari R.K. Altitudinal distribution of mosquitoes in mountainous area of Garhwal region: Part-I. J Vector Borne Dis. 2004;41:17–26. [PubMed] [Google Scholar]
- 27.Kulldorff M. SaTScan™ User Guide for Version 9.6. [(accessed on 15 May 2019)]; Available online: https://www.satscan.org/cgi-bin/satscan/register.pl/SaTScan_Users_Guide.pdf?todo=process_userguide_download.
- 28.Areekit S., Singhaphan P., Khuchareontaworn S., Kanjanavas P., Sriyaphai T., Pakpitchareon A., Khawsak P., Chansiri K. Intraspecies variation of Brugia spp. in cat reservoirs using complete ITS sequences. Parasitol. Res. 2009;104:1465–1469. doi: 10.1007/s00436-009-1352-x. [DOI] [PubMed] [Google Scholar]
- 29.Jittapalapong S., Pinyopanuwat N., Chimnoi W., Nimsupan B., Saengow S., Simking P., Kaewmongkol G. Prevalence of heartworm infection of stray dogs and cats in Bangkok metropolitan areas. Kasetsart J. Nat. Sci. 2005;39:30–34. [Google Scholar]
- 30.Tiawsirisup S., Thanapaisarnkit T., Varatorn E., Apichonpongsa T., Bumpenkiattikun N., Rattanapuchpong S., Chungpiwat S., Sanprasert V., Nuchprayoon S. Canine heartworm (Dirofilaria immitis) infection and immunoglobulin G antibodies against Wolbachia (Rickettsiales: Rickettsiaceae) in stray dogs in Bangkok, Thailand. Thai J. Vet. Med. 2015;40:165–170. [Google Scholar]
- 31.Satjawongvanit H., Phumee A., Tiawsirisup S., Sungpradit S., Brownell N., Siriyasatien P., Preativatanyou K. Molecular analysis of canine filaria and its Wolbachia endosymbionts in domestic dogs collected from two animal university hospitals in Bangkok metropolitan region, Thailand. Pathogens. 2019;8:114. doi: 10.3390/pathogens8030114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kamyingkird K., Junsiri W., Chimnoi W., Kengradomkij C., Saengow S., Sangchuto K., Ka jeerum W., Pangjai D., Nimsuphan B., Inpankeaw T., et al. Prevalence and risk factors associated with Dirofilaria immitis infection in dogs and cats in Songkhla and Satun provinces, Thailand. Agric. Nat. Resour. 2017;51:299–302. doi: 10.1016/j.anres.2017.05.003. [DOI] [Google Scholar]
- 33.Choochote W., Sukhavat K., Keha P., Somboon P., Khamboonruang C., Suwanpanit P. The prevalence of Dirofilaria immitis in stray dog and its potential vector in Amphur Muang Chiang Mai, Northern Thailand. Southeast Asian J. Trop. Med. Public Health. 1987;18:131–134. [PubMed] [Google Scholar]
- 34.Choochote W., Suttajit P., Rongsriyam Y., Likitvong K., Tookyang B., Pakdicharoen A. The prevalence of Dirofilaria immitis in domestic dogs and their natural vectors in amphur Muang Chiang Mai, Northern Thailand. J. Trop. Med. Parasitol. 1992;15:11–17. [Google Scholar]
- 35.Colella V., Nguyen V.L., Tan D.Y., Lu N., Fang F., Zhijuan Y., Wang J., Liu X., Chen X., Dong J., et al. Zoonotic vectorborne pathogens and ectoparasites of dogs and cats in eastern and southeast Asia. Emerg. Infect. Dis. 2020;26:1221–1233. doi: 10.3201/eid2606.191832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Denham D.A., McGreevy P.B. Brugian filariasis: Epidemiological and experimental studies. Adv. Parasitol. 1977;15:243–309. doi: 10.1016/s0065-308x(08)60530-8. [DOI] [PubMed] [Google Scholar]
- 37.Chang K.M., Shamala R., Mangalam S., Rohela M., Fong M.Y., Sivanandam S., Azdayanti M., Jamaiah I., Noraishah M.A.A. Live Brugia pahangi immature female worm recovered from the subconjunctiva of a patient in Malaysia; Proceedings of the 46th MSPTM Annual Scientific Conference; Kuala Lumpur, Malaysia. 11–12 March 2020; p. 101. [Google Scholar]
- 38.Chungpivat S., Sucharit S. Microfilariae in cats in Bangkok. Thai J. Vet. Med. 1993;23:75–87. [Google Scholar]
- 39.Klinklom A., Santarattiwong P. Proceedings of PIDST Gazette. Royal Cliff Hotel Pattaya; Chon Buri, Thailand: Update on emerging infectious disease: Zoonotic filariasis; pp. 18–20. [Google Scholar]
- 40.Capelli G., Frangipane di Regalbono A., Simonato G., Cassini R., Cazzin S., Cancrini G., Otranto D., Pietrobelli M. Risk of canine and human exposure to Dirofilaria immitis infected mosquitoes in endemic areas of Italy. Parasit Vectors. 2013;6:60. doi: 10.1186/1756-3305-6-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Anwar C., Ghiffari A., Kuch U., Taviv Y. The Abundance of Mosquitoes (Family: Culicidae) Collected in an altitudinal gradient In South Sumatra, Indonesia; Proceedings of the International Conference on Agricultural, Ecological and Medical Sciences (AEMS-2015); Phuket, Thailand. 10 February 2015; pp. 28–30. [Google Scholar]
- 42.Zielke E., Hinz E., Sucharit S. Lymphatic filariasis in Thailand a review on distribution and transmission. Trop. Med. Parasitol. 1993;15:141–148. [Google Scholar]
- 43.Nayar J.K., Knight J.W., Vickery A.C. Susceptibility of Anopheles quadrimaculatus (Diptera: Culicidae) to subperiodic Brugia malayi and Brugia pahangi (Nematoda: Filarioidea) adapted to nude mice and jirds. J. Med. Entomol. 1990;27:409–411. doi: 10.1093/jmedent/27.3.409. [DOI] [PubMed] [Google Scholar]
- 44.Beerntsen B.T., Luckhart S., Christensen B.M. Brugia malayi and Brugia pahangi: Inherent difference in immune activation in the mosquitoes Armigeres subalbatus and Aedes aegypti. J. Parasitol. 1989;75:76–81. doi: 10.2307/3282940. [DOI] [PubMed] [Google Scholar]
- 45.Muslim A., Fong M.Y., Mahmud R., Lau Y.L., Sivanandam S. Armigeres subalbatus incriminated as a vector of zoonotic Brugia pahangi filariasis in suburban Kuala Lumpur, Peninsular Malaysia. Parasit Vectors. 2013;6:219. doi: 10.1186/1756-3305-6-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chaves L.F., Imanishi N., Hoshi T. Population dynamics of Armigeres subalbatus (Diptera: Culicidae) across a temperate altitudinal gradient. Bull. Entomol. Res. 2015;105:589–597. doi: 10.1017/S0007485315000474. [DOI] [PubMed] [Google Scholar]
- 47.Carton Y., Nappi A.J., Poirie M. Genetics of anti-parasite resistance in invertebrates. Dev. Comp. Immunol. 2005;29:9–32. doi: 10.1016/j.dci.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 48.Cano J., Moraga P., Nikolay B., Rebollo M.P., Okorie P.N., Davies E., Njenga S.M., Bockarie M.J., Brooker S.J. An investigation of the disparity in estimates of microfilaraemia and antigenaemia in lymphatic filariasis surveys. Trans. R. Soc. Trop. Med. Hyg. 2015;109:529–531. doi: 10.1093/trstmh/trv048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Irvine M.A., Njenga S.M., Gunawardena S., Wamae C.N., Cano J., Brooker S.J., Hollingsworth T.D. Understanding the relationship between prevalence of microfilariae and antigenaemia using a model of lymphatic filariasis infection. Trans. R. Soc. Trop. Med. Hyg. 2016;110:317. doi: 10.1093/trstmh/trw024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gadahi J.A., Arijo A.G., Abubakar M., Javaid S.B., Arshed M.J. Prevalence of blood parasites in stray and pet dogs in Hyderabad Area: Comparative sensitivity of different diagnostic techniqes for the detection of microfilaria. Vet. World. 2008;1:229–232. [Google Scholar]
- 51.Little S., Saleh M., Wohltjen M., Nagamori Y. Prime detection of Dirofilaria immitis: Understanding the influence of blocked antigen on heartworm test performance. Parasit Vectors. 2018;11:186. doi: 10.1186/s13071-018-2736-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.American Heartworm Society, Current Canine Guidelines for the Prevention, Diagnosis, and Management of Heartworm (Dirofilaria Immitis) Infection in Dogs. [(accessed on 15 May 2019)]; Available online: https://www.heartwormsociety.org/veterinary-550resources/american-heartworm-society-guidelines.
- 53.Prieto G., Cancrini G., Muro A., Genchi C., Simon F. Seroepidemiology of Dirofilaria immitis and Dirofilaria repens in humans from three areas of Southern Europe. Res. Rev. Parasitol. 2000;60:95–98. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data available on request due to privacy for sequential projects.