Abstract
Objectives: Data on clinical and biological tolerance of tedizolid (TZD) prolonged therapy are lacking. Methods: We conducted a prospective multicentre study including patients with prosthetic joint infections (PJIs) who were treated for at least 6 weeks but not more than 12 weeks. Results: Thirty-three adult patients of mean age 73.3 ± 10.5 years, with PJI including hip (n = 19), knee (n = 13) and shoulder (n = 1) were included. All patients were operated, with retention of the infected implants and one/two stage-replacements in 11 (33.3%) and 17/5 (51.5%/15.2%), respectively. Staphylococci and enterococci were the most prevalent bacteria identified. The mean duration of TZD therapy was 8.0 ± 3.27 weeks (6–12). TZD was associated with another antibiotic in 18 patients (54.5%), including rifampicin in 16 cases (48.5). Six patients (18.2%) had to stop TZD therapy prematurely because of intolerance which was potentially attributable to TZD (n = 2), early failure of PJI treatment (n = 2) or severe anaemia due to bleeding (n = 2). Regarding compliance with TZD therapy, no cases of two or more omissions of medication intake were recorded during the whole TZD treatment duration. Conclusions: These results suggest good compliance and a favourable safety profile of TZD, providing evidence of the potential benefit of the use of this agent for the antibiotic treatment of PJIs.
Keywords: tedizolid, prosthetic joint infections, prolonged oral treatment, tolerance, compliance
1. Introduction
Prosthetic joint infection (PJI) is a serious and complex complication following arthroplasty at an incidence rate after hip or knee replacement of 1 to 2% [1]. The aims of the management of patients with PJIs are to restore satisfactory joint function and to eliminate infection. Surgical options include debridement antibiotics and implant retention (DAIR), one- or two-stage replacement, arthroplastic resection and, sometimes, amputation. Given the increasing burden of these infections, especially among the elderly population, developing new therapies such as cell therapy to prevent the progression of osteo-arthritis, and thus, the need for total joint arthroplasty, is an important field of research [2,3,4,5]. The antibiotic treatment of patients with PJIs is limited by the tolerance of its prolonged administration and the resistance level of some pathogens [6,7]. Gram-positive cocci, especially coagulase-negative staphylococci (CoNS), are predominant bacteria which are responsible for infections in and around orthopaedic devices [8]. In this context, the use of the oxazolidinone agent linezolid (LZD) has been validated, but potential bone-marrow, neurologic, and metabolic toxicity limit treatment duration to no more than two to three weeks [9,10,11]. Additionally, the wide use of LZD has resulted in the emergence of CoNS carrying cfr genes which are responsible for high levels of LZD resistance [12]. The combination of rifampicin with LZD leads to a reduction in LZD blood concentration, which is associated with a lower rate of adverse hematologic effects but also with lower clinical remission rates [13,14]. Tedizolid (TZD) phosphate is a second generation oxazolidinone which is indicated for the treatment of acute bacterial skin and skin structure infections in adults [15,16,17,18]. In the Establish 1 and 2 studies, gastrointestinal disorders and bone marrow toxicity were less frequent in TZD than in LZD patients [19,20]. However, the duration of TZD treatment did not exceed six days. TZD has a high oral bioavailability and can be administered once-daily; furthermore, drug–drug interactions with mono-amine oxidase inhibitors (MAOI), serotonin-reuptake inhibitors (SRI) or rifampicin are unlikely, although the latter has recently been questioned [21,22]. Recent in vitro and animal studies suggested that the addition of rifampicin to TZD was likely to achieve a synergistic effect against methicillin-resistant Staphylococcus aureus (MRSA) and S. epidermidis, and prevent the emergence of rifampicin-resistant mutants [23,24]. While TZD appears to be an attractive candidate for the treatment of PJIs due to gram-positive cocci, and has shown satisfactory efficacy and tolerability in clinical trials, data about its tolerability and compliance in long-term treatments are lacking. The aim of the present multicentre prospective cohort study was to assess the long-term safety profile and compliance of oral TZD in monotherapy or in combination therapy for the treatment of PJIs.
2. Results
Thirty-three adult patients (sex ratio female/male 17/16) of mean age 73.3 ± 10.5 years were included from August 2018 to November 2019. A total of 17 patients (51.5%) were enrolled at the Tourcoing Centre, 13 (39.4%) at the Ambroise Paré Centre and 3 (9.1%) at the Lyon Centre. Patient characteristics are presented in Table 1. Despite chronic infection, three patients were treated with a debridement antibiotic and implant retention (DAIR) because of their age and general status which contraindicated the replacement of the implant. TZD use was used to avoid LZD potential toxicities or drug–drug interactions in 16 patients (48.5%), or because of previous, LZD-related adverse events in three patients (9.1%). Among these three patients, one had experienced thrombocytopenia, one anaemia and one gastro-intestinal intolerance. No included patients were receiving MAOI or SRI concomitantly with TZD therapy. Staphylococci were the most prevalent bacterium identified in our patients, accounting for 58% of the total number, and including 21 (42%) methicillin-resistant strains (Table 2). Infection was polymicrobial in 18 cases (54.5%) among which five were associated with gram-negative rods, all of which were susceptible to fluoroquinolones. Geometric mean MIC values for linezolid, as determined by E-test methods, were 1.24 ± 0.83 mg/L, 1.5 ± 0.7 mg/L and 1.64 ± 0.48 mg/L for Staphylococcus spp., Streptococcus spp. and Enterococcus spp., respectively. MIC measurements or TZD blood levels were not routinely performed for tedizolid in this study.
Table 1.
Demographic data of 33 patients with periprosthetic joint infections.
| Patient Characteristics | Values and Number of Patients (%) |
|---|---|
| Age, years in mean ± SD | 73.3 ± 10.5 |
| Sex ratio (female/male) | 17/16 |
| Body mass index, kg/m2 mean ± SD (>30) | 29.7 ± 6.2 (51.5) |
Comorbidities *
|
19 (57.6) 10 (30.3) 5 (15.2) 1 (3.0) 4 (12.1) 1 (3.0) 2 (6.0) |
| American Society of Anaesthesiologists score ≥2 [range] | 27 (81.2) [1–3] |
| Previous surgical revision of the prosthesis ≥1 [range] | 12 (36.4) [1–10] |
Total joint arthroplasty
|
19 (57.6) 13 (39.4) 1 (3.0) |
| Age of the prosthesis, months mean ± SD [range] | 24.5 ± 39.0 [1–180] |
Type of infection
|
6 (18.2) 25 (75.8) 2 (6.1) |
Surgical intervention
|
11 (33.3) 17 (51.5) 5 (15.2) |
| Fever (temperature > 38.0 °C) | 4 (12.1) |
| Fistula | 12 (36.4) |
| C-reactive protein at baseline, mg/L mean ± SD [range; IQR] | 42.16 ± 34.9 [5.8–111; 52] |
| White blood cells at baseline, G/L mean ± SD [range; IQR] | 8.34 ± 2.5 [4.3–15.6; 3.2] |
SD: standard deviation. *: 4 patients had ≥2 comorbidities.
Table 2.
Microbiology of 33 patients with periprosthetic joint infections.
| Bacteria | N° of Strains (%) |
|---|---|
| Gram positive cocci | 43 (86.0) |
| - Staphylococcus aureus (MRSA = 7) | 13 (26) |
| - Staphylococcus epidermidis (MRSE = 14) | 15 (30) |
| - Staphylococcus caprae (MR = 2) | 1 (2) |
| - Streptococcus agalactiae | 2 (4) |
| - Corynebacterium striatum | 4 (8) |
| - Enterococcus faecalis | 7 (14) |
| - Enterococcus gallinarum | 1 (2) |
| Gram negative bacilli | 5 (10) |
| - Escherichia coli | 2 (4) |
| - Klebsiella pneumoniae | 1 (2) |
| - Pseudomonas aeruginosa | 1 (2) |
| - Pasteurella multocida | 1 (2) |
| Anaerobes | 2 (4) |
| - Cutibacterium acnes | 2 (4) |
| Total number of bacterial strains | 50 (100) |
Legend: MRSA: Methicillin-resistant Staphylococcus aureus; MRSE: Methicillin-resistant Staphylococcus epidermidis; MR: Methicillin-resistant.
Following postoperative empirical antibiotic therapy (PEAT) of median duration of 7 days (range 4 to 14 days), the mean duration of TZD therapy was 8.0 ± 3.27 weeks (ranging from 6–12 weeks). Among the 27 out of 33 patients (81.8%) who completed the planned therapy, the mean duration of TZD was 8.77 weeks ± 2.79 (range 6–12 weeks). The mean total duration of the antibiotic treatment including PEAT and targeted therapy was 9.15 ± 3.43 weeks (7–12). TZD was associated with another antibiotic in 18 patients (54.5%), e.g., rifampicin in 16 cases (48.5%).
In total, 20 patients (60.6%) experienced at least one adverse event during TZD therapy. A list of adverse events (AE) potentially attributable to tedizolid is shown in Table 3; the most frequent AE were anaemia (n = 4) and pruritus (n = 4). Six patients (18.2%) had to stop TZD therapy prematurely because of (i) intolerance which was potentially attributable to TZD (n = 2), (ii) early failure of PJI treatment (n = 2) or (iii) severe anaemia (n = 2). TZD-attributable discontinuation episodes consisted of inflammatory arthritis of the wrist and knee in one patient who also received doxycycline and did not improve after stopping doxycycline but partially recovered after discontinuation of TZD, and vomiting in another patient who received TZD alone (Appendix A Table A1). According to the definitions used to describe the bone marrow toxicity profile of TZD, 8 patients (24.2%) experienced haematological adverse events including anaemia in 4 cases, 2 of which presented acute haemorrhage, leukopenia in 2 cases and thrombocytopenia in 2 cases (Table A1). With the exception of the two patients with acute haemorrhage, none of these adverse events resulted in withdrawing TZD therapy. Although a gastric haemorrhage in one patient and a hematoma at the surgical site in another patient resulted in acute severe anaemia which was most probably unrelated to TZD therapy, the treatment was discontinued. Haematological adverse events were mild and resolved spontaneously during TZD therapy except in the two patients with severe anaemia who received a blood transfusion. Non haematological adverse events were recorded in 13 (39.4%) patients for whom no premature discontinuation of TZD therapy was required, and were mostly pruritus (n = 4), headache (n = 2) and insomnia (n = 2) (Table A1). There was no safety signal for TZD-associated optic or peripheral neurologic toxicity or metabolic disorder. Overall, the proportion of patients who experienced TZD-attributable adverse event did not differ significantly in patients treated with a combination of antibiotics or with TZD alone [13/18 (72.2%) versus 8/15 (52.3%), respectively; p = 0.45], nor did it vary according to the use of rifampicin in combination with TZD or the total duration of TZD therapy (Table 4).
Table 3.
Episodes of adverse effects reported in 33 patients during tedizolid therapy.
| Adverse Event (N° of Discontinuation of Tedizolid Therapy) |
N° of Episodes of Adverse Effects * |
|---|---|
| anemia (2) | 4 |
| asthenia | 1 |
| leukopenia | 2 |
| thrombocytopenia | 2 |
| headache | 2 |
| pruritus | 4 |
| abdominal pain | 1 |
| nausea/vomiting (1) | 2 |
| vertigo | 1 |
| xerosis | 1 |
| dysgeusia | 1 |
| epistaxis | 1 |
| arthralgia (1) | 2 |
| thrush | 1 |
| insomnia | 2 |
| intermittent blurred vision | 1 |
| Total | 28 |
* Five patients had more than one episode of adverse effects.
Table 4.
Adverse events according to the duration and the antibiotic regimen in 33 patients treated with tedizolid.
| Patients’ Characteristics | N° of Patients (%), Total = 33 | p |
|---|---|---|
| ≥1 adverse event | 20 (60.6) | |
Any combination therapy
|
11 (61.1) 9 (60) |
0.8 |
Rifampicin combination therapy
|
9 (56.3) 11 (64.7) |
0.9 |
Duration of treatment ≤6 weeks
|
7 (53.8) 13 (65) |
0.8 |
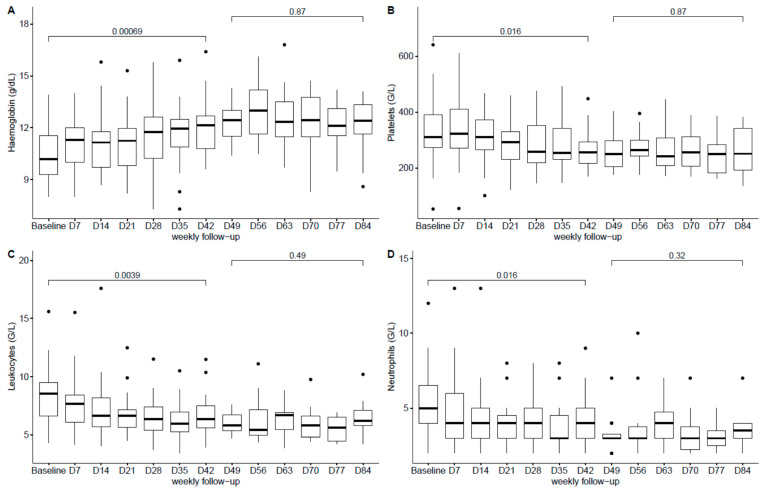
The follow-up of the haematological parameters showed a significant increase of haemoglobin blood levels between baseline and week 6 followed by stabilisation, as well as a significant decrease in platelets, leukocytes and neutrophils counts between baseline and week 6 followed by stabilisation until the end of the treatment (Figure 1A–D).
Figure 1.
Boxplot of the haematological parameters ((A): Haemoglobin, (B): Platelets, (C): Leukocytes, (D): Neutrophils) during Tedizolid therapy. Each box represents median, interquartile range, largest, smallest and outside (point) values.
Regarding compliance to TZD therapy, no cases of two or more omissions of medication intake during TZD treatment were recorded, in accordance with the number of pills present in the returned boxes. Six failures (18.2%), including two early cases, were recorded at one-year following the end of TZD therapy.
3. Discussion
We report the first prospective cohort study to date providing data on the safety and compliance of prolonged use (i.e., ≥6 weeks) of oral TZD phosphate at a 200-mg, once-daily dose for the treatment of PJIs. As our safety results suggest, oral TZD therapy administered for 6 to 12 weeks according to the current recommendations [25] can be considered for the treatment of PJIs. The overall proportion of patients who experienced an adverse event (60.6%) may appear high, but this may be explained by the design of the study which allowed us to report an exhaustive list of adverse events. Despite the nonoptimal profile regarding the general status of our patients, the tolerance of prolonged oral TZD therapy allowed us to complete the therapy in more than 80% of the cases. Indeed, 19 patients (57.6%) had comorbidities and 27 (81.2%) had an ASA score ≥ 2, which is significantly different from the populations of patients evaluated in other, pivotal clinical trials [19,20]. Our results are close to those reported by Kim et al. on a series of 25 patients with nontuberculous mycobacterial infections treated with a median duration of TZD therapy of 91 days [26]. Eleven of their patients (44%) experienced an adverse event including gastrointestinal intolerance in five patients (20%) and thrombocytopenia in one (4%); no case of anaemia was recorded, while peripheral neuropathy was reported in five patients (20%). The attribution of an adverse event to TZD was, however, difficult, as almost all patients were receiving multidrug therapy. The mean age of our patients was 73.3 years, which is quite high with regard to the risk of developing bone marrow toxicity to LZD, as reported by several authors [9,10]. The correction of LZD-induced bone marrow toxicity after switching to TZD observed in one of our patients has already been reported elsewhere [27,28]. Overall, we only recorded a few significant haematological abnormalities which did not result in discontinuation of TZD therapy, except in patients with acute haemorrhage. There were no differences in safety, especially with regard to haematological laboratory changes, between patients receiving TZD in combination with rifampicin versus patients receiving TZD alone (full data are available upon request), as reported with LZD-rifampicin combination [9,10]. We hypothesise that the increase of haemoglobin values during TZD treatment represents a restoration process after blood spoliation secondary to the surgical intervention, while the decrease of platelets and WBC during TZD treatment might be related to the resolution of the infectious process. The incidence of digestive disorders reported in our patients is close to the results of a meta-analysis by Lan et al., noting, however, that the duration of TZD therapy in the studies included was six days, as recommended for the treatment of acute bacterial skin and skin structure infections [29].
The main limitations of the present pilot study are the small size of the studied population and the assessment of the patients’ adherence to TDZ treatment, which was based on the return of the pillboxes and on patient self-reporting. The strengths of the present study are its prospective design and the selection criteria which allowed investigators to include patients in a real-life setting. We strongly believe that the inclusion of patients, regardless of age and risk factors for bone marrow toxicity, enhanced the external validity of our conclusions regarding the tolerance of prolonged oral TZD therapy in patients treated with PJIs.
4. Materials and Methods
The purpose of the present study was to obtain reliable data on the tolerance, compliance and efficacy of prolonged (i.e., ≥6 weeks) use of TZD alone or in combination therapy for the treatment of PJIs. We present herein data about adherence and tolerance. As post-treatment follow-ups are currently underway, we present data only for the one-year follow-up. We conducted a prospective multicentre cohort study in three French national centres for the management of complex bone and joint infections (also called CRIOAc): Lille-Tourcoing, Paris-Ambroise Paré and Lyon [30].
4.1. Definitions
Adult patients with PJIs defined according to the MSIS 2018 [31] criteria and for whom TZD treatment was indicated according to the investigator’s decision were prospectively included. All patients gave their written informed consent after an explanation of the protocol by the investigating physician. PJIs were characterised according to: acute haematogenous (infection with three-week duration or less of symptoms after an uneventful postoperative period), early postinterventional (infection that manifested within one month after implantation) and chronic (infection with symptoms that persisted for >3 weeks, i.e., beyond the early postinterventional period) according to Zimmerli’s definition [32].
Patients demographic data (age, gender, body mass index), comorbidities, microbiology, prior use of LZD and reason for TZD use (e.g., failure and/or toxicity of previous treatment, need to avoid linezolid toxicity or drug–drug interactions), treatment duration, concomitant antibiotics, potential adverse events attributable to TZD were recorded. Laboratory data were recorded at baseline, weekly and at the end of treatment, including haemoglobin, white blood count (WBC), platelet count, alanine aminotransferase (ALT), aspartate aminotransferase (AST) and Protein-C reactive (PCR).
Clinically significant laboratory changes were defined as: (1) anaemia, decrease in haemoglobin ≥2 g/L from baseline after TZD initiation and classified as severe if haemoglobin was <8 g/dL, (2) leukopenia, white blood count (WBC) of <4 G/L after TZD initiation and classified as mild (low limit normal to 3 G/L), moderate (≥2-<3 G/L) and severe (<2 G/L), (3), neutropenia, absolute neutrophil count of <1.5 G/L after TZD initiation, (4) thrombocytopenia, platelet count of <150 G/L after TZD initiation and classified as mild (75–150 G/L), moderate (50-<75 G/L) or severe (<50 G/L); for patients with a baseline platelet count of <150 G/L, thrombocytopenia was defined as a reduction of 25% from the baseline, and (5) elevated AST or ALT 3 times above the upper limit of normal.
Microbiological documentation was based on joint aspiration and/or intraoperative culture samples. During surgical procedures, at least three tissue samples were taken in different areas suspected of infection, using a separate sterile instrument for each sample. The antibiotic susceptibility profile of all pathogens was assessed either by the Vitek 2 cards (BioMérieux, Marcy l’Etoile, France) or by agar diffusion technique using the procedure and interpretation criteria proposed by the Comité de l’Antibiogramme de la Société Française de Microbiologie (CA-SFM EUCAST 2018) (http://www.sfm-microbiologie.org). Methicillin resistance was confirmed by the detection of the mecA gene if required.
Adverse events (AEs) were identified from patients’ medical records and laboratory data. Associations of adverse events with TZD and related antibiotics were assessed as suggested by the patient’s physician and confirmed by the principal investigator according to the chronology of events, as were the need to reduce the daily dosage of the potentially problematic antibiotic, data from any attempt to reintroduce such a mode of treatment, and the type of recorded toxicity (e.g., anaemia, thrombocytopenia and peripheral neuropathy for TZD, tendonitis, and myalgia for levofloxacin and drug–drug interaction for rifampicin). To be attributable to a given antibiotic, a reduction in the daily dosage and/or discontinuation due to intolerance had to be recorded as well as temporal association with event resolution after discontinuation or dose reduction of the agent in question.
4.2. Antibiotic Treatment
TZD was administered orally at a once daily dose of 200 mg (i.e., one tablet) as a single antibiotic therapy or in combination therapy with another agent with proven activity against the involved pathogen(s) according to the physician’s choice. The duration of antibiotic therapy ranged from 6 to 12 weeks. Exclusion criteria were pregnant women or women of childbearing age who were not using contraception, breastfeeding intolerance to TZD, allergy to oxazolidinone, the detection of bacteria which were nonsusceptible to TZD, patients with uncertainty regarding the possibility of achieving a one-year follow-up after the end of treatment or the absence of written consent. Patients were examined during consultations every 3 weeks during treatment and at 6 months and one year after the EOT. During treatment, special attention was paid to potential neurological and optical side effects, as well as to possible drug–drug interactions.
4.3. Statistics
Data are presented as numbers (percentages) for qualitative variables and as medians (interquartile range: IQR) or means (SD) for quantitative variables. We compared biological variable that might have been affected by the use of oxazolidinone between baseline and day 42 and between day 49 and day 84 using the Student t-test, with p = 0.05 being set as the threshold of significance.
4.4. Ethics
Research was conducted in accordance with the Declaration of Helsinki and national and institutional standards. The study was recorded on clinicaltrial.gov under the number NCT03378427, in the EudraCT database under the number 2017-001238-24 and was approved by the French Sud Mediterranean IV Committee of Protection of the People in Biomedical Research on 21 November 2017 under the number 17 10 09. This interventional survey was declared to the National Agency for Medicines and Safety of Health Products under the number 17060A-43. Tedizolid was supplied by Merck Sharp & Dohme, Inc.
5. Conclusions
The results of the present study suggest good compliance and a favourable safety profile of TZD, providing evidence of the potential benefit of the use of this agent for the antibiotic treatment of PJIs.
Acknowledgments
We greatly thank the G. Dron hospital clinical research unit, especially Solange Tréhoux for the technical assistance.
Appendix A
Table A1.
Details on tolerance of TZD therapy in 33 patients treated for periprosthetic joint infections.
| Pt | Age | Surgery | ASA Score | Type of Infection | Microbiology | Duration of TZD Therapy (Weeks) | Antibiotic Associated with TZD | Premature Discontinuation of TZD (Origin) | No Adverse Events without Premature Discontinuation of TZD |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 88 | DAIR | 2 | Chronic | EC, SB | 6 | RIF | No | anaemia, asthenia |
| 2 | 74 | 1-SR | 2 | Chronic | SE * | 6 | None | No | thrush |
| 3 | 66 | DAIR | 2 | EPO | EF | 12 | RIF | No | leukopenia |
| 4 | 62 | 1-SR | 3 | Chronic | CA | 1 | None | Yes (anaemia due to gastric haemorrhage) | |
| 5 | 80 | DAIR | 3 | Chronic | EF, SA, PM | 7 | RIF, LEV | No | thrombocytopenia |
| 6 | 80 | 1-SR | 2 | Chronic | SE * | 6 | None | No | |
| 7 | 64 | 2-SR | 2 | AH | CS, EF, EC | 11 | None | No | thrombocytopenia |
| 8 | 73 | 1-SR | 3 | AH | SA, SE * | 6 | DALB | No | anaemia |
| 9 | 71 | 2-SR | 2 | Chronic | SA * | 11 | None | No | intermittent blurred vision |
| 10 | 80 | DAIR | 1 | EPO | SE * | 11 | RIF | No | |
| 11 | 75 | DAIR | 2 | EPO | CS | 11 | RIF | No | leukopenia |
| 12 | 88 | DAIR | 3 | EPO | EF, SE * | 12 | RIF | No | |
| 13 | 67 | DAIR | 1 | EPO | EF, SE | 6 | DOX | Yes (arthralgia) | |
| 14 | 79 | 2-SR | 2 | Chronic | SE * | 11 | None | No | xerosis, pruritus |
| 15 | 75 | 1-SR | 2 | Chronic | SA | 6 | None | Yes (vomiting) | headache, dysgeusia |
| 16 | 78 | 2-SR | 3 | Chronic | EF, SA | 5 | None | Yes (early failure) | |
| 17 | 77 | 1-SR | 2 | Chronic | SE * | 11 | None | No | abdominal pain, headache, vertigo |
| 18 | 45 | 2-SR | 1 | Chronic | Sterile | 10 | None | No | pruritus |
| 19 | 86 | DAIR | 3 | EPO | SA * | 6 | None | No | |
| 20 | 70 | 1-SR | 1 | Chronic | KP, SA, SE * | 6 | RIF, LEV | No | tinnitus, insomnia nausea pruritus |
| 21 | 55 | 1-SR | 1 | Chronic | EG | 6 | None | No | |
| 22 | 79 | DAIR | 3 | EPO | SA * | 6 | RIF | No | vomiting |
| 23 | 83 | 1-SR | 3 | Chronic | SE * | 7 | RIF | No | insomnia |
| 24 | 78 | 1-SR | 3 | Chronic | SE * | 6 | RIF | No | pruritus |
| 25 | 67 | 1-SR | 2 | Chronic | CS, SA, SE * | 6 | RIF | No | |
| 26 | 81 | DAIR | 2 | Chronic | CA, SB | 6 | None | No | |
| 27 | 45 | DAIR | 1 | AH | SA, SE | 9 | None | No | |
| 28 | 87 | 1-SR | 2 | EPO | SA *, CA, EF, PA | 1 | RIF | Yes (anaemia due to a haematoma at the operated site) | |
| 29 | 74 | 1-SR | 1 | Chronic | SA * | 12 | RIF | No | |
| 30 | 76 | 1-SR | 1 | Chronic | SE | 11 | RIF, CIP | No | |
| 31 | 66 | 1-SR | 2 | EPO | SE * | 9 | RIF | Yes (early failure) | |
| 32 | 69 | 1-SR | 2 | Chronic | SA * | 8 | RIF | No | epistaxis |
| 33 | 82 | 1-SR | 3 | Chronic | SC * | 12 | None | No |
Pt: patient; ASA: American Society of Anaesthesiologists, TZD: TZD, DAIR: debridement antibiotic and implant retention, 1-SR: one stage replacement, 2-SR two-stage replacement, EPO: early postoperative, AH; acute haematogenous, RIF: rifampicin, LEV: levofloxacin, DALB: dalbavancin, DOX: doxycycline, CIP: ciprofloxacin, EC: Escherichia coli, SB: group B streptococci, SA: Staphylococcus aureus, SE: Staphylococcus epidermidis, EF: Enterococcus faecalis, CA: Cutibacterium acnes, PM: Pasteurella multocida, CS: Corynebacterium striatum, KP: Klebsiella pneumoniae, EG: Enterococcus gallinarum, PA: Pseudomonas aeruginosa, CA: Staphylococcus caprae, *: methicillin-resistant.
Author Contributions
E.S., E.B. and O.R. conceived the design of the study and wrote the manuscript; A.D. and T.F. reviewed the manuscript, all but N.B. participated in the inclusions and management of the patients; N.B. reviewed the microbiology part of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
No external funding was received except that tedizolid pills were provided by Merck Sharp and Dohme.
Data Availability Statement
Data are available upon request to Pr Eric Senneville (esenneville@ch-tourcoing.fr); the French authorities do not authorize the sharing of this type of data without prior consent and infor-mation of the patients on their final use.
Conflicts of Interest
E.S., A.D., O.R. and T.F. declare congress support, speaker invitation, participation to scientific board with MSD; E.B. and N.B. have nothing to declare in relation with the present study. E.S., A.D., O.R. and T.F. declare congress support, speaker invitation, and participation to scientific board with MSD; E.B. and N.B. have nothing to declare in relation with the present study.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Li C., Renz N. Management of Periprosthetic Joint Infection. Hip. Pelvis. 2018;30:138–146. doi: 10.5371/hp.2018.30.3.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rabini A., Boccia G. Effects of focal muscle vibration on physical functioning in patients with knee osteoarthritis: A randomized controlled trial. Eur. J. Phys. Rehabil. Med. 2015;51:513–520. [PubMed] [Google Scholar]
- 3.De Sire A., Stagno D. Long-term effects of intra-articular oxygen-ozone therapy versus hyaluronic acid in older people affected by knee osteoarthritis: A randomized single-blind extension study. J. Back Musculoskelet Rehabil. 2020;33:347–354. doi: 10.3233/BMR-181294. [DOI] [PubMed] [Google Scholar]
- 4.McAlindon T.E., Bannuru R.R. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthr. Cartil. 2014;22:363–388. doi: 10.1016/j.joca.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 5.Migliore A., Paoletta M. The perspectives of intra-articular therapy in the management of osteoarthritis. Expert Opin. Drug Deliv. 2020;17:1213–1226. doi: 10.1080/17425247.2020.1783234. [DOI] [PubMed] [Google Scholar]
- 6.Del Pozo J.L., Patel R. Clinical practice. Infection associated with prosthetic joints. N. Engl. J. Med. 2009;361:787–794. doi: 10.1056/NEJMcp0905029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zimmerli W., Trampuz A. Prosthetic-joint infections. N. Engl. J. Med. 2004;351:1645–1654. doi: 10.1056/NEJMra040181. [DOI] [PubMed] [Google Scholar]
- 8.Titécat M., Senneville E. Bacterial epidemiology of osteoarticular infections in a referent center: 10-year study. Orthop. Traumatol. Surg. Res. 2013;99:653–658. doi: 10.1016/j.otsr.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 9.Soriano A., Gomez J. Efficacy and tolerability of prolonged linezolid therapy in the treatment of orthopedic implant infections. Eur. J. Clin. Microbiol. Infect. Dis. 2007;26:353–356. doi: 10.1007/s10096-007-0289-1. [DOI] [PubMed] [Google Scholar]
- 10.Senneville E., Legout L. Risk factors for anaemia in patients on prolonged linezolid therapy for chronic osteomyelitis: A case-control study. J. Antimicrob. Chemother. 2004;54:798–802. doi: 10.1093/jac/dkh409. [DOI] [PubMed] [Google Scholar]
- 11.Bassetti M., Vitale F. Linezolid in the treatment of Gram-positive prosthetic joint infections. J. Antimicrob. Chemother. 2005;55:387–390. doi: 10.1093/jac/dki016. [DOI] [PubMed] [Google Scholar]
- 12.Ruiz-Ripa L., Feßler A.T. Mechanisms of Linezolid Resistance Among Clinical Staphylococcus sin Spain: Spread of Methicillin- and Linezolid-Resistant, S. epidermidis ST2. Microb. Drug Resist. 2020 doi: 10.1089/mdr.2020.0122. [DOI] [PubMed] [Google Scholar]
- 13.Legout L., Valette M. Tolerability of prolonged linezolid therapy in bone and joint infection: Protective effect of rifampicin on the occurrence of anaemia? J. Antimicrob. Chemother. 2010;65:2224–2230. doi: 10.1093/jac/dkq281. [DOI] [PubMed] [Google Scholar]
- 14.Tornero E., Morata L. Importance of selection and duration of antibiotic regimen in prosthetic joint infections treated with debridement and implant retention. J. Antimicrob. Chemother. 2016;71:1395–1401. doi: 10.1093/jac/dkv481. [DOI] [PubMed] [Google Scholar]
- 15.Schmidt-Malan S.M., Greenwood Quaintance K.E. In vitro activity of tedizolid against staphylococci isolated from prosthetic joint infections. Diagn. Microbiol. Infect. Dis. 2016;85:77–79. doi: 10.1016/j.diagmicrobio.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 16.Littorin C., Hellmark B. In vitro activity of tedizolid and linezolid against Staphylococcus epidermidis isolated from prosthetic joint infections. Eur. J. Clin. Microbiol. Infect. Dis. 2017;36:1549–1552. doi: 10.1007/s10096-017-2966-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ract P., Piau-Couapel C. In vitro activity of tedizolid and comparator agents against Gram-positive pathogens responsible for bone and joint infections. J. Med Microbiol. 2017;66:1374–1378. doi: 10.1099/jmm.0.000595. [DOI] [PubMed] [Google Scholar]
- 18.Carvalhaes C.G., Sader H.S. Tedizolid in vitro activity against Gram-positive clinical isolates causing bone and joint infections in hospitals in the USA and Europe (2014-17) J. Antimicrob. Chemother. 2019;74:1928–1933. doi: 10.1093/jac/dkz120. [DOI] [PubMed] [Google Scholar]
- 19.Prokocimer P., De Anda C. Tedizolid Phosphate vs Linezolid for Treatment of Acute Bacteria Skin Structure Infections. The ESTABLISH-1 Randomized Trial. JAMA. 2013;309:559–569. doi: 10.1001/jama.2013.241. [DOI] [PubMed] [Google Scholar]
- 20.Moran G.J., Fang E. Tedizolid for 6 days versus linezolid for 10 days for acute bacterial skin and skin-structure infections (ESTABLISH-2): A randomised, double-blind, phase 3, non-inferiority trial. Lancet Infect. Dis. 2014;14:696–705. doi: 10.1016/S1473-3099(14)70737-6. [DOI] [PubMed] [Google Scholar]
- 21.Sivextro Package Insert. Merck & Co., Inc.; Kenilworth, NJ, USA: 2016. [Google Scholar]
- 22.Lee L., Kor Hee K. Rifampicin Reduces tedizolid Concentrations When Co-Administered in Healthy Volunteers. Open Forum Infect. Dis. 2019;6:S576. doi: 10.1093/ofid/ofz360.1442. [DOI] [Google Scholar]
- 23.Werth B.J. Exploring the Pharmacodynamic Interactions Between tedizolid and Other Orally Bioavailable Antimicrobials Against Staphylococcus aureus and Staphylococcus epidermidis. J. Antimicrob. Chemother. 2017;72:1410–1414. doi: 10.1093/jac/dkw588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park K.H., Greenwood-Quaintance K.E. Activity of tedizolid in Methicillin-Resistant Staphylococcus epidermidis Experimental Foreign Body-Associated Osteomyelitis. Antimicrob. Agents Chemother. 2017;61:e01644-16. doi: 10.1128/AAC.01644-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anemüller R., Belden K. Hip and Knee Section, Treatment, Antimicrobials: Proceedings of International Consensus on Orthopedic Infections. J. Arthroplast. 2019;34(Suppl. S2):463–475. doi: 10.1016/j.arth.2018.09.032. [DOI] [PubMed] [Google Scholar]
- 26.Kim T., Wills A.B. Abstracts of the IDWeek Conference, New Orleans, LA. 2016. [(accessed on 23 December 2020)]. Safety and Tolerability of Long Term Use of tedizolid for Treatment of Nontuberculous Mycobacterial Infections. Abstract 577. Available online: www.idweek.org. [Google Scholar]
- 27.Khatchatourian L., Le Bourgeois A. Correction of myelotoxicity after switch of linezolid to tedizolid for prolonged treatments. J. Antimicrob. Chemother. 2017;72:2135–2136. doi: 10.1093/jac/dkx097. [DOI] [PubMed] [Google Scholar]
- 28.Ferry T., Batailler C. Correction of Linezolid-Induced Myelotoxicity After Switch to tedizolid in a Patient Requiring Suppressive Antimicrobial Therapy for Multidrug-Resistant Staphylococcus epidermidis Prosthetic-Joint Infection. Open Forum Infect. Dis. 2018;5 doi: 10.1093/ofid/ofy246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lan S.H., Lin W.T. Tedizolid Versus Linezolid for the Treatment of Acute Bacterial Skin and Skin Structure Infection: A Systematic Review and Meta-Analysis. Antibiotics. 2019;8:137. doi: 10.3390/antibiotics8030137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferry T., Seng P., Mainard D., Jenny J.Y., Laurent F., Senneville E., Grare M., Jolivet-Gougeon A., Bernard L., Marmor S. The CRIOAc healthcare network in France: A nationwide Health Ministry program to improve the management of bone and joint infection. Orthop. Traumatol. Surg. Res. 2019;105:185–190. doi: 10.1016/j.otsr.2018.09.016. [DOI] [PubMed] [Google Scholar]
- 31.Parvizi J., Tan T.L. The 2018 Definition of Periprosthetic Hip and Knee Infection: An Evidence-Based and Validated Criteria. J. Arthroplast. 2018;33:1309–1314. doi: 10.1016/j.arth.2018.02.078. [DOI] [PubMed] [Google Scholar]
- 32.Zimmerli W., Sendi P. Orthopedic-implant associated infections. In: Bennett J.E., Dolin R., Blaser M., editors. Mandell, Douglas, and Bennett’s principles and Practice of Infectious Diseases. 8th ed. Elsevier; Philadelphia, PA, USA: 2014. pp. 1328–1340. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon request to Pr Eric Senneville (esenneville@ch-tourcoing.fr); the French authorities do not authorize the sharing of this type of data without prior consent and infor-mation of the patients on their final use.