Abstract
The application of exogenous plant growth regulator can improve plant resistance to drought stress. The effects of application of exogenous cytokinin (CTK), brassinolide (BR), or gibberellic acid (GA) at the silking time on the grain quality of two waxy maize hybrids under drought stress at grain formation stage were studied. Grain weight of both hybrids was unaffected by exogenous phytohormones under control conditions but increased under drought conditions with the application of BR. The grain starch content in response to drought varied with hybrid and phytohormone. Starch granule size and protein content in grains were increased by drought under all conditions, but various phytohormones exerted different forms of influence. The starch λmax in Yunuo7 was unaffected by single or interaction of phytohormones and water deficit, λmax in Jingkenuo2000 with BR was unaffected but with CTK or GA increased by drought. Relative crystallinity was reduced by drought without the application of phytohormone, but with phytohormones in response to drought it was different. Flour peak viscosity was reduced by drought. The value was increased with BR spraying under control and drought conditions. Retrogradation percentage under drought conditions was unaffected by exogenous phytohormones in Jingkenuo2000. In Yunuo7, retrogradation percentage was unaffected by BR but reduced by CTK and GA. In conclusion, spraying phytohormones at the silking stage can affect grain weight and starch quality, grains with a sticky taste can be improved by applying BR, and grains with low retrograde tendency can be produced by applying CTK.
Keywords: grain weight, pasting viscosity, plant growth regulator, retrogradation, starch granule size, water deficit, waxy maize
1. Introduction
Maize (Zea mays L.) plays an important role in global food security as it is a staple food and a feed crop for millions of people [1]. Rainfall mainly contributes to maize survival; unpredictable precipitation often induces drought or waterlogging, which decreases grain yield and quality [2]. Among the different abiotic stresses, drought is the vital adversity that affects maize growth and development [3]. Drought-prone maize regions will expand in the future and increase the frequencies of yield instability and food supply insecurity at local and even global levels [4].
Starch is the primary component (65–70%) of grains, followed by protein. In most cases, starch content decreases, and protein content increases after plants suffer post-silking drought stress [5,6,7]. Grain starch is gradually deposited after pollination and endosperm cell differentiation, and its composition and physicochemical properties are affected when plants are subjected to drought at this stage. Post-silking water deficit reduces the number of endosperm cells and starch granules [8], decreases starch granule size, and ultimately restricts total starch and amylopectin accumulation [9,10,11]. The small sizes of starch granules under drought conditions can improve starch pasting viscosity [12]. However, post-silking short-time water deficit increases pasting viscosity by enlarging starch granules [13], and irrigation can reduce the starch breakdown rate and setback viscosity [14]. Xia et al. [15] reported that water deficit reduces the numbers and volumes of starch granules and thereby lowers peak and trough viscosities. Zhang et al. [16] reported that moderate drought enlarges the size of starch granules and increases the proportion of long chains, which induces high gelatinization temperatures and retrogradation percentage, but severe drought exerts an opposite influence.
Maize is sensitive to drought at the flowering and grain developmental stages, and thus strategies for relieving stress and increasing yield sustainably are necessary [17]. The application of plant growth regulators is an important cropping practice for alleviating the negative influences of environmental stresses [18]. Brassinolide (BR) enhances maize drought tolerance by mediating physiological and metabolic activities, maintaining tissue water potential, and antioxidant enzyme activity under drought [19]. Gibberellic acid (GA) can improve maize drought tolerance by keeping membrane permeability and increasing the contents of chlorophyll, relative water, and macronutrients in leaves [20]. Application of exogenous 2-(3,4-Dichlorophenoxy) triethylamine [21], melatonin [22], glutathione [23], spermidine [24], and salicylic acid [25] are reported to improve the drought tolerance of maize. However, the influence of exogenous phytohormones on the maize grain quality remain poorly understood. The plant growth regulators (ethephon, chlormequat chloride, and trinexapac-ethyl) application reduce grain weight, but their effects on malting quality are generally inconsiderable [26]. Post-anthesis drought reduces the weight and starch content of grains and increases the percentage of large starch granules, and spermidine or aminoethoxyvinylglycine can increase the proportion of small starch granules [9]. Polyamines (spermidine or free spermine) mediate the effect of post-anthesis water shortage on starch deposition by regulating activities of starch biosynthetic enzymes, and improved polyamine levels under moderate drought levels increasing the volume of medium granules and the weight of inferior grains in wheat [27]. Paclobutrazol, GA, or 6-benzylaminopurine can improve the milling quality and nutrition traits of rice grains by increasing head rice rate and amylose content [28].
Starch is composed of nearly pure amylopectin in waxy maize, which endows its high viscosity, low retrograde, and easier to digest than normal maize starch, widely used in food or some non-food industries [29,30]. Drought is the main environmental stress in China. Previous studies reported that grain yield and starch quality are affected by post-silking water deficit, which restricts starch deposition, downregulates enzymatic activities related to starch synthesis, reduces the sizes of starch granules, decreases starch crystallinity, and shortens amylopectin chains [31,32,33]. However, little is known about the influence of exogenous phytohormones on maize grain quality under drought stress. Exogenous plant growth regulator is a simple and economical cropping practice for improving the maize drought tolerance [18]. We hypothesize that the foliar spraying of exogenous phytohormones can alleviate detrimental influences of drought on the grain starch quality of waxy maize. In the present study, three widely used phytohormones, namely, cytokinin (CTK), BR, and GA were sprayed at the silking stage, and their effects on grain quality of plants subjected to water deficit after pollination (lasting 15 days) were studied. These results offer a choice for using exogenous phytohormones in cultivating waxy maize under post-silking drought conditions.
2. Results and Discussion
2.1. Grain Weight
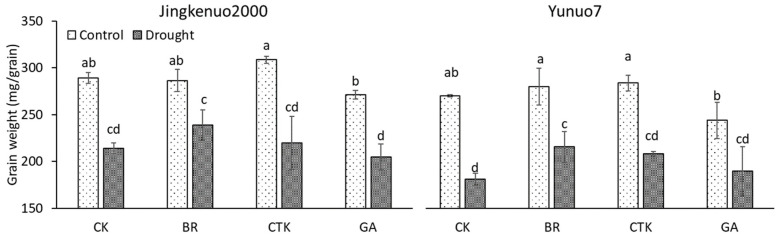
The grain weights of JKN2000 and YN7 significantly decreased by 26.0% and 33.0% under drought stress (Figure 1). Similar results were observed in fresh waxy maize [32], normal maize [34], barley [35], and wheat [9]. The decrease may be due to downregulated enzymatic activities related to starch synthesis and decreased IAA content [31]. Grain weight was unaffected by exogenous phytohormones under control conditions in both hybrids. Under drought conditions, grain weight in JKN2000 was also unaffected by phytohormones, the value in YN7 was increased only by exogenous BR and unaffected by CTK and GA. The foliar spraying of uniconazole with ethephon at the 12-leaf stage and 10 days after silking increased the contents of abscisic acid (ABA) and zeatin (Z) + zeatin riboside (ZR) but reduced the content of GA in grain, thereby increasing grain weight [36]. A study on rice observed that application of exogenous CTK (6-benzylaminopurine) at the late booting stage increases grain weight [37], but another study showed that grain weight in response to CTK (kinetin) under control and salt stresses varied among the tested hybrids [38]. A study on wheat showed that grain weight is unaffected by CTK (ZR) but is decreased by ABA [39].
Figure 1.
The grain weight with exogenous hormones under control and drought conditions in waxy maize. CK, water; CTK, cytokinin; BR, brassinolide; GA, gibberellic acid. Bars mean SE (n = 3). Different letters above the bars in each hybrid are significantly different at 5% probability level.
2.2. Starch Content
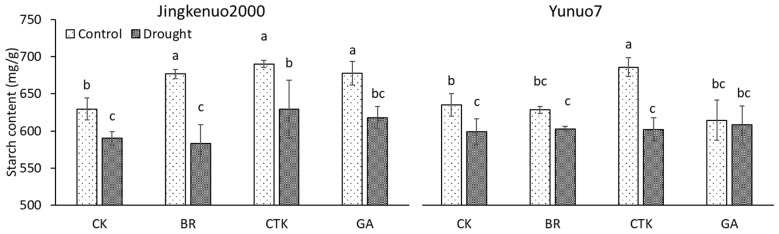
Grain starch content was affected by the interaction between water deficit and plant growth regulator. In JKN2000, starch content was decreased by drought with or without phytohormones, especially with BR. In YN7, starch content with GA or BR was unaffected by drought, but the value with CTK or water decreased (Figure 2). Drought reduced grain starch content [5,6,7], owing to down-regulated enzymatic activities related to starch biosynthesis [31,40]. The decreased starch content under drought condition without phytohormones may be due to enlarged starch granules, which cannot compensated the reduced numbers of starch granules [11]. The starch content in normal maize grain was unaffected by the application of ethephon and diethyl aminoethyl hexanoate [41].
Figure 2.
The grain starch content with exogenous hormones under control and drought conditions in waxy maize. CK, water; CTK, cytokinin; BR, brassinolide; GA, gibberellic acid. Bars mean SE (n = 3). Different letters above the bars in each hybrid are significantly different at 5% probability level.
Under control condition, grain starch content in JKN2000 was significantly increased by exogenous phytohormones, but the increase was similar among the three phytohormones. Grain starch content in YN7 was unaffected by BR and GA but increased by CTK. Grain starch content can be increased by CTK (kinetin) [38]. Grain starch content was increased by spermidine and reduced by ethephon under both control and water deficit conditions [9]. Under water deficit condition, grain starch content in YN7 was similar among different treatments, the value in JKN2000 was unaffected by BR and GA but increased by CTK.
2.3. Protein Content
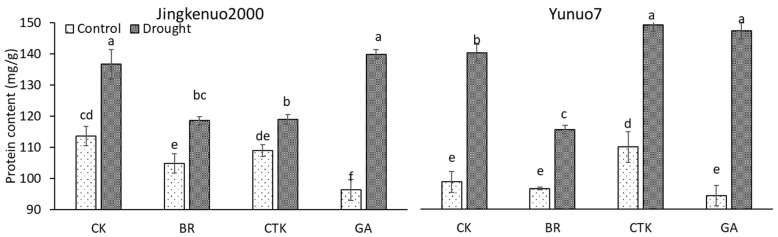
Protein content in grain was increased by drought in both hybrids with or without plant growth regulators (Figure 3). This was similar to the result on wheat, which reported that drought enhances the sizes and relative areas of protein bodies in dorsal and abdominal endosperms [42]. In JKN2000, protein content under control condition was decreased with exogenous phytohormones, the value under drought condition was unaffected by GA and decreased by BR and CTK. In YN7, protein content under control condition was increased by CTK and unaffected by BR and GA, the value under drought condition was decreased by BR and increased by CTK and GA. A study on wheat found that the protein content in grain was unchanged by the application of CTK (ZR) but was increased by ABA [15]. McMillan et al. [26] reported that application of ethephon, chlormequat chloride, and trinexapac-ethyl did not affect the grain protein content in barley.
Figure 3.
The grain protein content with exogenous hormones under control and drought conditions in waxy maize. CK, water; CTK, cytokinin; BR, brassinolide; GA, gibberellic acid. Bars mean SE (n = 3). Different letters above the bars in each hybrid are significantly different at 5% probability level.
2.4. Starch Granule Size (SGS)
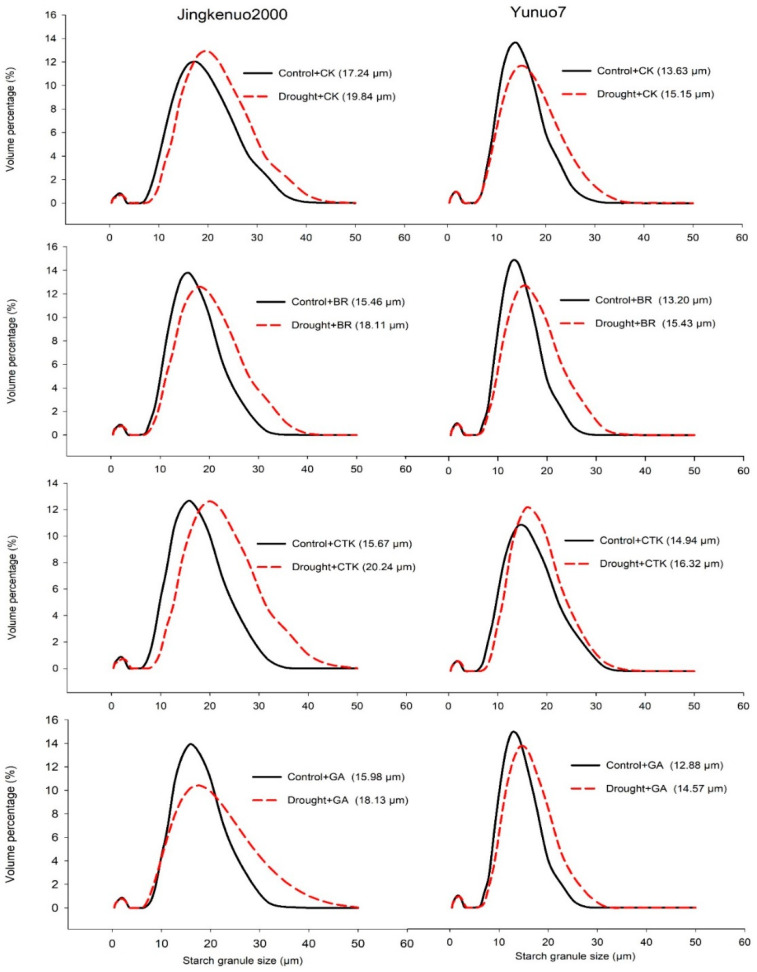
Drought stress greatly enlarged the average SGS with or without spraying exogenous phytohormones in both hybrids (Figure 4). Drought at whole grain developmental stage decreased the SGS [32]. A study on wheat found that the SGS in response to post-anthesis drought was different among various hybrids [43]. The development of starch granules in grain divided into two stages, increase the numbers of starch granules at the first stage, following by the enlargement of starch granules [44]. The enlarged size of starch granules under drought stress in present study may be caused by re-watering induced photoassimilates transferred to the surviving starch granules and enlarged its size, as drought at the grain formation stage reduced the number of endosperm cells and starch granules [8]. Short-term water deficit increases starch granule size in wheat [13]. The SGS in JKN2000 was significantly larger than that in YN7.
Figure 4.
The size distribution of grain starch granules with exogenous hormones under control and drought conditions in waxy maize. CK, water; CTK, cytokinin; BR, brassinolide; GA, gibberellic acid. Data in the bracket are the average starch granule size.
In YN7, the SGS under control condition was decreased by BR and GA but enlarged by CTK, and the SGS under drought condition was enlarged by BR and CTK but reduced by GA. In JKN2000, the SGS under control condition was decreased with all the exogenous phytohormones; under drought condition, it was reduced by BR and GA but enlarged by CTK. However, a research on wheat showed that the percentage of large starch granules was reduced by ABA but increased by GA3 [45]. Studies on rice [37] and wheat [46] demonstrated that the application of exogenous CTK increases endosperm cells and promotes nutrients to developing endosperms. GA has an important role in early grain development [47]. The distributions of starch granules in response to exogenous phytohormones varies among hybrid and water status [9]. To understand the effect of the application of exogenous phytohormones on grain development, accurate information on the enzymatic activities and contents of phytohormones related to starch synthesis and starch granule development need further consideration.
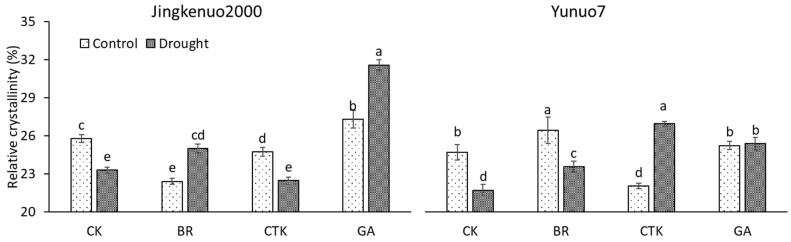
2.5. X-ray Diffraction (XRD) Pattern
The XRD profiles of all the samples present a typical A-type pattern (data not shown), but the relative crystallinity (RC) was affected by drought and phytohormone (Figure 5). In JKN2000, the RC was increased by drought with BR and GA and decreased with CTK and water, respectively. The RC under the control condition was increased by GA and decreased by BR and CTK, the value under drought condition was not infected by CTK but improved by BR and GA. In YN7, the RC was decreased by drought with water and BR, increased with CTK, and unaffected with GA. The RC under the control condition was increased by BR, decreased by CTK, and unaffected by GA; it under drought condition was increased with all the phytohormones. The decreased RC under drought conditions similar to the reports on fresh waxy maize [32], Trimezia juncifolia [48] and wheat [15]. Changes in RC is a part of strategy to adapt to different growth environments. Considering the effects of different exogenous phytohormones, selecting a suitable plant growth regulator is a feasible cropping practice based on utilizations.
Figure 5.
The flour relative crystallinity with exogenous hormones under control and drought conditions in waxy maize. CK, water; CTK, cytokinin; BR, brassinolide; GA, gibberellic acid. Bars mean SE (n = 3). Different letters above the bars in each hybrid are significantly different at 5% probability level.
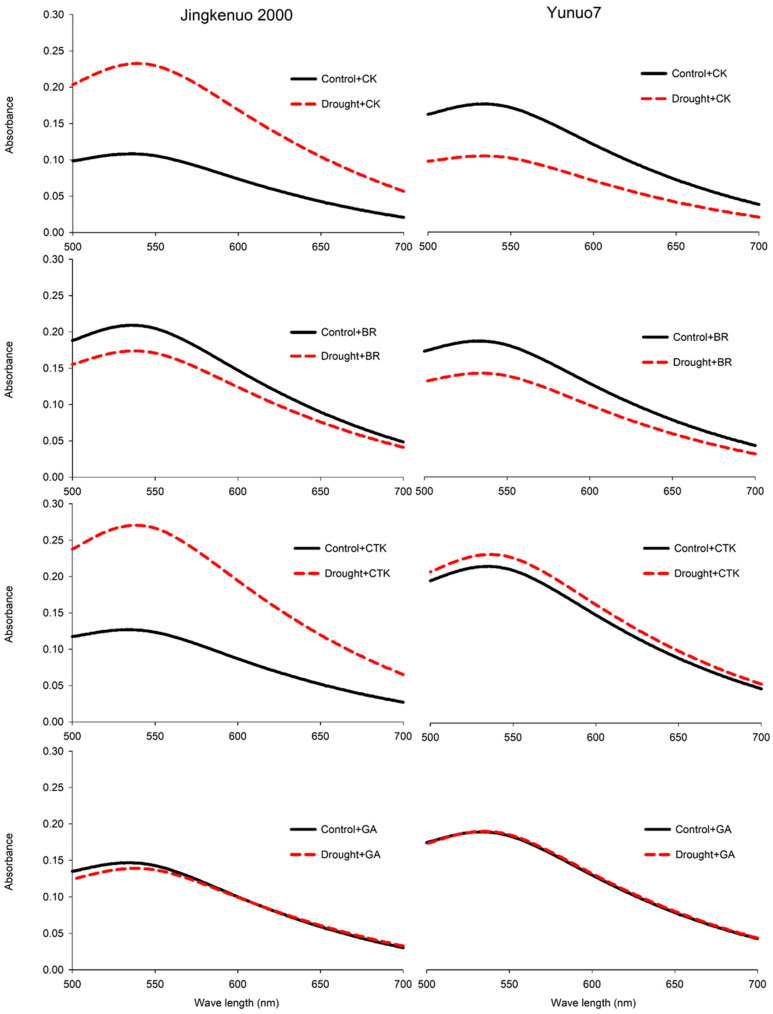
2.6. Iodine Staining
Iodine can form a helical complex with amylopectin chains, and a high λmax indicates that starch has a high proportion of long chains [49]. The λmax of starch from waxy rice and maize was approximately 530–540 nm, present typical waxy character [32,49]. The starch λmax in JKN2000 ranged from 534.7 nm to 535.3 nm and from 535.3 to 540.3 nm under control and drought conditions, respectively (Figure 6). The λmax with BR was unaffected by drought, but it increased under other treatments. The starch λmax in YN7 ranged from 533.3 nm to 535.7 nm, and it was unaffected by phytohormone and water deficit. The starch λmax in this hybrid was unaffected by drought during total grain developmental stage [32]. The starch λmax was positively correlated to the SGS, indicating that a large SGS has a high proportion of long chains. Those results are consistent with the earlier report [32].
Figure 6.
The starch wavelength distributions with exogenous hormones under control and drought conditions in waxy maize. CK, water; CTK, cytokinin; BR, brassinolide; GA, gibberellic acid.
2.7. Pasting Property
Grain pasting characteristics were affected by drought, phytohormone, and hybrid (Table 1). YN7 had a higher pasting characteristic than JKN2000 in general. Drought reduced the peak (PV) and breakdown (BD) viscosities and increased pasting temperature (Ptemp) in both hybrids with or without exogenous phytohormones. In JKN2000, the trough (TV) and final (FV) viscosities were increased by drought only with GA and unaffected by drought in the other three treatments; setback (SB) viscosity was increased by drought under all treatments. In YN7, the TV and FV with CTK were decreased but with GA and BR were increased by drought. The SB with water and CTK was increased but with BR and GA was decreased by drought. Our early study observed that the PV and BD of flour and starch in waxy maize were decreased by post-silking water deficit [32,33]. However, studies on wheat reported that starch pasting viscosities were improved by post-anthesis drought though the changes of starch granule size were discordant [12,13,14,47]. In this work, the low pasting viscosities may be caused by large granule size and high proportion of long chains in starch, as those viscosities were negatively correlated to SGS and λmax. In addition, BD was negatively correlated to protein content, indicating that proteins coated on starch granules restricted the starch granules broken during heating [50].
Table 1.
The flour pasting properties with spraying exogenous phytohormones under post-silking control and drought conditions.
| Hybrid | Soil Moisture | Exogenous Hormones | PV (cP) |
TV (cP) |
BD (cP) |
FV (cP) |
SB (cP) |
Ptemp (°C) |
|---|---|---|---|---|---|---|---|---|
| JKN2000 | Control | CK | 413 c | 234 b | 179 c | 353 b | 119 cd | 76.8 c |
| BR | 701 a | 308 a | 389 a | 434 a | 123 bcd | 74.9 d | ||
| CTK | 418 c | 237 b | 182 c | 351 b | 114 d | 76.7 c | ||
| GA | 498 b | 204 b | 291 b | 320 b | 114 d | 75.1 d | ||
| Drought | CK | 246 d | 235 b | 85 de | 347 b | 138 a | 79.3 a | |
| BR | 402 c | 291 a | 138 cd | 421 a | 131 abc | 76.9 bc | ||
| CTK | 282 d | 212 b | 71 e | 332 b | 120 cd | 77.0 bc | ||
| GA | 381 c | 293 a | 91 de | 421 a | 135 ab | 78.3 ab | ||
| YN7 | Control | CK | 1516 b | 731 ef | 771 b | 913 c | 177 cd | 76.7 cd |
| BR | 1650 a | 811 c | 839 a | 1009 b | 198 a | 75.9 d | ||
| CTK | 1473 b | 880 ab | 593 c | 1062 a | 182 bc | 78.3 b | ||
| GA | 1512 b | 709 f | 803 ab | 883 c | 174 cd | 76.2 d | ||
| Drought | CK | 979 d | 770 de | 209 e | 962 b | 191 ab | 79.4 a | |
| BR | 1492 b | 882 a | 610 c | 1063 a | 181 bc | 77.5 bc | ||
| CTK | 981 d | 801 cd | 179 e | 999 b | 198 a | 79.7 a | ||
| GA | 1354 c | 841 bc | 513 d | 1007 b | 167 d | 78.3 b | ||
| F-value | ||||||||
| Hybrid | 6690.3 ** | 6865.8** | 2145.3 ** | 6025.3 ** | 722.3 ** | 19.1 ** | ||
| Soil moisture | 492.0 ** | 15.6 ** | 1037.9 ** | 13.0 ** | 11.5 ** | 95.4 ** | ||
| Hormone | 128.1 ** | 26.7 ** | 166.7 ** | 24.0 ** | 4.7 ** | 16.5 ** | ||
| Hybrid × Soil moisture | 45.3 ** | 4.7 ** | 157.7 ** | 2.5 | 7.1 ** | 0.0 | ||
| Hybrid × Hormone | 8.1 ** | 11.5 ** | 34.6 ** | 10.6 ** | 6.5 ** | 5.2 ** | ||
| Soil moisture × Hormone | 16.8 ** | 24.8 ** | 6.0 ** | 15.9 ** | 4.1 * | 4.5 * | ||
| Hybrid × Soil moisture × Hormone | 28.9 ** | 5.0 ** | 43.0 ** | 2.4 | 4.0 * | 1.3 | ||
JKN2000, Jingkenuo2000; YN7, Yunuo7; CK, control; BR, brassinolide; CTK, cytokinin; GA, gibberellic acid; PV, peak viscosity; TV, trough viscosity; BD, breakdown viscosity; FV, final viscosity; SB, setback viscosity; Ptemp, pasting temperature; cP, centipoise. Mean value in the same column within each hybrid followed by different letters are significantly different (p < 0.05). *, p < 0.05; **, p < 0.01.
Under control condition, BR increased the pasting characteristics in both hybrids. The pasting characteristics in JKN2000 were unaffected by application of CTK, this phytohormone did not affect PV and SB, decrease BD, and increase TV, FV, and Ptemp in YN7. The application of GA increased PV and BD and decreased Ptemp but did not affect TV and FV in JKN2000, it did not affect all the pasting parameters in YN7. Under drought condition, SB and Ptemp were decreased by the application of phytohormones, PV, TV, BD, and FV were increased by BR and GA but unaffected by CTK in JKN2000. In YN7, the parameters were unaffected by CTK, but PV, TV, BD, FV were increased and SB and Ptemp were decreased with spraying BR and GA. Study on rice observed that the application of exogenous hormones under ambient temperature unaffected the starch pasting viscosities but it under heat stress significantly improved the viscosities, alleviate the negative influence of high temperature [51].
2.8. Thermal Property
Gelatinization properties were affected by drought and exogenous phytohormones (Table 2). In JKN2000, ΔHgel was decreased by drought under different treatments. The ΔHgel under control condition was lowest with GA and similar among the other three treatments, and the difference disappeared under drought condition. In YN7, the ΔHgel was unaffected and decreased with exogenous phytohormones under control and water shortage conditions, respectively. The ΔHgel was increased without phytohormones, with BR and CTK were unaffected, and with GA was decreased by drought. In JKN2000, the To with water, CTK, and GA increased, but the value with BR decreased by drought. The Tp with BR was unaffected by drought and increased in the other three treatments. The Tc with CTK was unaffected, with BR was decreased, but with GA and water was increased by drought. In YN7, the gelatinization temperatures increased by drought under all conditions. The ΔHgel of flour or starch was unaffected by drought during whole grain filling stage, but gelatinization temperatures in response to water deficit were different among different waxy maize hybrids [32,33]. Zhang et al. [16] observed that moderate drought during grain filling increased starch gelatinization temperatures and reduced ΔHgel in wheat, but severe drought produced a contrary influence.
Table 2.
The flour thermal properties with spraying exogenous phytohormones under post-silking control and drought conditions.
| Hybrid | Soil Moisture | Exogenous Hormones | ΔHgel (J/g) |
To (°C) |
Tp (°C) |
Tc (°C) |
ΔHret (J/g) |
%R (%) |
|---|---|---|---|---|---|---|---|---|
| JKN2000 | Control | CK | 9.2 a | 68.7 e | 73.9 e | 83.1 c | 3.6 a | 39.1 a |
| BR | 9.3 a | 69.5 cd | 74.8 d | 83.9 b | 3.2 b | 33.9 b | ||
| CTK | 9.0 a | 69.4 d | 74.7 d | 84.1 b | 2.1 e | 23.2 d | ||
| GA | 8.1 b | 69.9 c | 75.4 c | 84.4 b | 2.1 de | 26.3 c | ||
| Drought | CK | 7.3 c | 70.8 b | 76.1 b | 84.4 b | 2.5 c | 34.6 b | |
| BR | 7.0 c | 68.8 e | 74.7 d | 82.4 d | 2.3 de | 32.4 b | ||
| CTK | 6.9 c | 69.7 cd | 75.5 c | 84.3 b | 2.2 de | 32.3 b | ||
| GA | 7.1 c | 72.0 a | 77.1 a | 85.7 a | 2.3 cd | 32.8 b | ||
| YN7 | Control | CK | 7.4 bc | 68.1 f | 73.4 f | 81.5 d | 3.2 b | 42.9 d |
| BR | 7.8 ab | 68.6 e | 74.5 e | 82.5 c | 3.1 bc | 39.5 e | ||
| CTK | 7.8 ab | 69.6 d | 75.0 d | 82.4 c | 3.0 bc | 39.0 e | ||
| GA | 7.7 b | 68.8 e | 73.7 f | 82.1 cd | 4.0 a | 51.8 a | ||
| Drought | CK | 8.2 a | 71.4 b | 76.9 ab | 84.2 a | 4.1 a | 49.4 b | |
| BR | 7.8 ab | 70.1 c | 76.1 c | 83.4 b | 3.8 a | 48.4 b | ||
| CTK | 7.4 bc | 71.8 a | 77.2 a | 84.6 a | 2.8 c | 37.1 f | ||
| GA | 7.1 c | 71.1 b | 76.5 b | 84.8 a | 3.3 b | 46.0 c | ||
| F-value | ||||||||
| Hybrid | 15.0 ** | 1.3 | 5.6 * | 71.5 ** | 307.9 ** | 1332.4 ** | ||
| Soil moisture | 105.3 ** | 639.4 ** | 1052.2 ** | 159.9 ** | 7.2 * | 39.7 ** | ||
| Hormone | 7.3 ** | 62.2 ** | 39.9 ** | 31.5 ** | 49.2 ** | 115.4 ** | ||
| Hybrid × Soil moisture | 101.3 ** | 101.3 ** | 151.1 ** | 85.1 ** | 34.1 ** | 0.4 | ||
| Hybrid × Hormone | 0.3 | 48.7 ** | 65.5 ** | 7.9 ** | 14.8 ** | 46.4 ** | ||
| Soil moisture × Hormone | 2.9 | 64.3 ** | 69.0 ** | 33.0 ** | 0.9 | 6.6 ** | ||
| Hybrid × Soil moisture × Hormone | 7.2 ** | 11.8 ** | 1.3 | 1.6 | 54.9 ** | 89.9 ** | ||
JKN2000, Jingkenuo2000; YN7, Yunuo7; CK, control; BR, brassinolide; CTK, cytokinin; GA, gibberellic acid; ΔHgel, gelatinization enthalpy; To, onset temperature; Tp, peak gelatinization temperature; Tc, conclusion temperature; ΔHret, retrogradation enthalpy; %R, retrogradation percentage. Mean value in the same column within each hybrid followed by different letters are significantly different (p < 0.05). *, p < 0.05; **, p < 0.01.
In YN7, To under control condition was increased by exogenous phytohormones, and Tp and Tc were unaffected by GA and increased by BR and CTK. Under drought condition, the gelatinization temperatures were unaffected by GA, decreased by BR, and increased by CTK, respectively. In JKN2000, the gelatinization temperatures under control condition were increased by exogenous phytohormones. Under drought conditions, the To and Tp were increased by GA and decreased by BR and CTK, Tc was increased by GA, decreased by BR, and unaffected by CTK. The gelatinization temperatures were positively correlated with protein content (Table 3), demonstrating that flour under drought conditions produced higher protein contents affected the gelatinization, similar to the influences on Ptemp.
Table 3.
Correlation coefficients between various characteristics (n = 16).
| PC | SC | SGS | RC | λmax | ΔHgel | T o | T p | T c | ΔHret | %R | PV | TV | BD | FV | SB | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SC | −0.56 * | |||||||||||||||
| SGS | 0.43 | −0.25 | ||||||||||||||
| RC | 0.11 | −0.08 | 0.02 | |||||||||||||
| λ max | 0.32 | −0.32 | 0.83 ** | 0.05 | ||||||||||||
| ΔHgel | 0.39 | −0.20 | −0.26 | 0.01 | −0.43 | |||||||||||
| T o | −0.38 | 0.58 * | 0.32 | −0.21 | 0.34 | −0.28 | ||||||||||
| T p | 0.86 ** | −0.31 | 0.36 | 0.25 | 0.31 | −0.34 | 0.97 ** | |||||||||
| T c | 0.85 ** | −0.36 | 0.51 * | 0.18 | 0.46 | −0.07 | 0.85 ** | 0.82 ** | ||||||||
| ΔHret | 0.69 ** | −0.06 | −0.59 * | 0.33 | −0.52 * | 0.25 | −0.09 | −0.13 | −0.37 | |||||||
| %R | −0.04 | −0.27 | −0.52 * | −0.34 | −0.36 | −0.16 | 0.01 | 0.00 | −0.35 | 0.91 ** | ||||||
| PV | 0.10 | −0.49 * | −0.85 ** | −0.26 | −0.60 * | −0.11 | −0.22 | −0.19 | −0.54 * | 0.65 ** | 0.73 ** | |||||
| TV | −0.25 | −0.03 | −0.70 ** | −0.15 | −0.51 * | −0.22 | 0.08 | 0.12 | −0.34 | 0.65 ** | 0.76 ** | 0.92 ** | ||||
| BD | 0.10 | −0.21 | −0.86 ** | −0.18 | −0.57 * | 0.00 | −0.49 * | −0.49 * | −0.67 ** | 0.54 * | 0.58 * | 0.92 ** | 0.70 ** | |||
| FV | −0.56 * | 0.13 | −0.70 ** | −0.10 | −0.52 * | −0.22 | 0.08 | 0.12 | −0.34 | 0.65 ** | 0.76 ** | 0.92 ** | 1.00 ** | 0.70 ** | ||
| SB | 0.10 | −0.21 | −0.60 * | −0.18 | −0.43 | −0.29 | 0.14 | 0.18 | −0.31 | 0.60 * | 0.73 ** | 0.84 ** | 0.95 ** | 0.61 ** | 0.95 ** | |
| P temp | 0.86 ** | −0.54 * | 0.29 | −0.06 | 0.34 | −0.43 | 0.72 ** | 0.70 ** | 0.37 | 0.10 | 0.27 | −0.01 | 0.33 | −0.34 | 0.33 | 0.43 |
PC, protein content; SC, starch content; SGS, starch granule size; RC, relative crystallinity; λmax, maximum wavelength; PV, peak viscosity; TV, trough viscosity; BD, breakdown viscosity; FV, final viscosity; SB, setback viscosity; Ptemp, pasting temperature; ΔHgel, gelatinization enthalpy; To, onset temperature; Tp, peak gelatinization temperature; Tc, conclusion temperature; ΔHret, retrogradation enthalpy; %R, retrogradation percentage. * Correlation is significant (p < 0.05). ** Correlation is significant (p < 0.01).
In JKN2000, the ΔHret with water and BR was decreased, but with CTK and GA was unaffected by drought. In comparison with control, the %R decreased without exogenous phytohormones, unaffected with BR, but increased with CTK and GA under drought condition. The ΔHret of YN7 under drought increased with BR and water, decreased with GA, and unaffected by CTK, the %R was decreased with CTK and GA and increased with BR and water. Our previous studies reported that ΔHret and %R were increased by post-silking water deficit [32,33]. Zhang et al. [16] found that the ΔHret and %R of wheat starch were increased and decreased by moderate and severe drought stresses, respectively. The discrepancy may due to the different drought tolerance, drought degree, and duration. The different responses to exogenous hormones provide a reference for the selection of hybrids and exogenous hormone based on utilization.
In YN7, the ΔHret under control condition was unaffected by BR and CTK but increased by GA, the value under drought condition was unaffected by BR but decreased by CTK and GA. The %R under control was increased by GA and decreased by BR and CTK, and %R under drought condition was unaffected by BR but was decreased by CTK and GA. In JKN2000, the ΔHret under both conditions decreased with exogenous phytohormones, the %R was reduced with exogenous phytohormones under control condition but the influence was disappeared under drought condition. The ΔHret and %R were negatively correlated to starch content, SGS and λmax, indicated that high starch content in grains, large granule size, and high proportion of long chains can reduce the retrogradation. For both hybrids under both water levels, the %R was lowest with CTK, indicated that the application of CTK could produce grains with low retrograde tendency.
3. Materials and Methods
3.1. Experimental Design
A pot trial was conducted at the Experimental Farm of Yangzhou University in 2019. Two waxy maize hybrids, namely Jingkenuo2000 (JKN2000) and Yunuo7 (YN7), were used as materials. The pots, 38 cm in height and 43 cm in diameter, were loaded with 30 kg of sieved sandy loam soil from field. To each pot, 1.5 g of N, P2O5, and K2O was applied at the transplanting time, and 3.0 g of N was applied at the jointing stage.
Plants were grown in the field environment before the silking. The plants with similar appearance were sampled and manual pollinated on the same day, the exogenous phytohormones were sprayed on the plants at the afternoon (16:00–18:00). The treatments including water (CK), 0.1 g/L CTK [52], 0.025 g/L BR [53], or 0.02 g/L of GA [45], all solutions contains Tween 20 (0.05%) as the surfactant and the spraying volume was 100 mL/plant. The pots were moved to a mobile rain shelter for water treatments in the next day. Well-watered (75%‒80% of field moisture capacity) and drought (50%–55% of field moisture capacity), were imposed from silking. Soil moisture content was monitoring by a soil moisture sensor and evaporated water was supplied through a weighing method every morning. The period of drought stress lasted 15 days (grain formation stage) and then the stressed plants were re-watered normally until maturity (40 d after pollination). Each treatment included 20 pots.
3.2. Grain Weight
Three independent ears of each treatment were harvested at maturity. The kernels were stripped off ears, sun-dried and the weight (mg/grain) was weighed. After weighing, the grains (100 g, dry basis) were ground using a disintegrator (FW-100, Taisite, Tianjin, China), made to pass through a sieve with diameter 0.149 mm, and dried to constant weight at 60 °C.
3.3. Grain Starch and Protein Contents Determination
Starch content in grain was measured by the anthrone-sulfuric acid method [54]. Nitrogen content in grain was determined following the procedure of AACC 46–10.01 [55], and protein content was calculated by measuring nitrogen content (protein content = nitrogen content × 6.25).
3.4. Starch Isolation
The starch in mature grains (100 g) was isolated using the method described in [29] after steeping in 500 mL of 1 g/L NaHSO3 solution at room temperature for 48 h.
3.5. Granule Size Distribution
Size distributions of starch granule were detected according to the protocol in [56].
3.6. Iodine Staining
The maximum absorption wavelength (λmax) of starch was measured according to the procedure in [56].
3.7. X-ray Diffraction Pattern
The X-ray diffraction (XRD) patterns of starch were obtained by an X-ray diffractometer (D8 Advance, Bruker-AXS, Germany), and the relative crystallinity (RC, %) was calculated according to the procedure in [56].
3.8. Pasting Properties
The pasting properties of flours (28 g total weight; 10% dry basis, w/w) were measured according to the procedure in [29].
3.9. Thermal Properties
The thermal properties of flours were determined with a differential scanning calorimetry (200 F3 Maia, NETZSCH, Bavaria, Germany) according to the procedure in [29]. The thermal transitions were defined as onset temperature (To), peak gelatinization temperature (Tp), conclusion temperature (Tc), and gelatinization enthalpy (ΔHgel). Samples were stored at 4 °C for 7 days after thermal analysis for retrogradation investigations. Retrogradation enthalpy (ΔHret) was automatically calculated, and retrogradation percentage (%R) was calculated as %R = 100 × ΔHret /ΔHgel.
3.10. Statistical Analysis
Data presented in the tables and figures were the mean value of three replications. ANOVA was used to determine the LSD at p < 0.05 level by a data processing system (version 7.05) [57].
4. Conclusions
The grain weight under normal condition was unaffected by spraying exogenous phytohormones at silking stage, but it under drought condition was increased by spraying BR in both hybrids. The grain starch and protein contents, starch granule sizes, λmax, and starch relative crystallinity were affected by the water deficit at 1–15 day after pollination, but exogenous phytohormones changed these parameters. The correlation results indicated that large size of starch granules, high proportion of long chains reduced the pasting viscosities, and ΔHret and %R. Starch content in grain was negatively correlated to %R. High protein content results in high gelatinization temperatures. Among the three widely used plant growth regulators, the application of BR could improve the grain with sticky taste, while the application of CTK could improve the grain with low retrograde tendency. Between the two hybrids, the high viscosities in YN7 and low %R in JKN2000 endows its advantages on different utilizations.
Acknowledgments
The authors highly acknowledge the Priority Academic Program Development of Jiangsu Higher Education Institutions, and High-end Talent Support Program of Yangzhou University, China for its support.
Author Contributions
Conceptualization, W.L. and D.L.; methodology, D.L.; software, D.L.; validation, D.L.; formal analysis, L.W.; investigation, L.W. and Y.Y.; resources, D.L.; data curation, L.W.; writing—original draft preparation, L.W. and Y.Y.; writing—review and editing, L.W. and Y.Y.; visualization, D.L.; supervision, W.L. and D.L.; project administration, D.L.; funding acquisition, W.L. and D.L. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (31771709, 32071958), National Key Research and Development Program of China (2018YFD0200703, 2016YFD0300109), Earmarked Fund for Jiangsu Agricultural Industry Technology System (JATS[2020]444), Jiangsu Agriculture Science and Technology Innovation Fund (CX[19]3056, CX[20]3147).
Institutional Review Board Statement
“Not applicable” for studies not involving humans or animals.
Informed Consent Statement
“Not applicable” for studies not involving humans or animals.
Data Availability Statement
The data presented in this study is contained within the article.
Conflicts of Interest
The authors have declared no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Boomsma C.R., Vyn T.J. Maize drought tolerance: Potential improvements through arbuscular mycorrhizal symbiosis? Field Crop. Res. 2008;108:14–31. doi: 10.1016/j.fcr.2008.03.002. [DOI] [Google Scholar]
- 2.Zaidi P.H., Rafique S., Singh N.N. Response of maize (Zea mays L.) genotypes to excess soil moisture stress: Morpho-physiological effects and basis of tolerance. Eur. J. Agron. 2003;19:383–399. doi: 10.1016/S1161-0301(02)00090-4. [DOI] [Google Scholar]
- 3.Daryanto S., Wang L.X., Jacinthe P.A. Global synthesis of drought effects on maize and wheat production. PLoS ONE. 2016;11:e0156362. doi: 10.1371/journal.pone.0156362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Daryanto S., Wang L.X., Jacinthe P.A. Global synthesis of drought effects on cereal, legume, tuber and root crops production: A review. Agric. Water Manag. 2017;179:18–33. doi: 10.1016/j.agwat.2016.04.022. [DOI] [Google Scholar]
- 5.Wang Y.X., Frei M. Stressed food—The impact of abiotic environmental stresses on crop quality. Agric. Ecosyst. Environ. 2011;141:271–286. doi: 10.1016/j.agee.2011.03.017. [DOI] [Google Scholar]
- 6.Thitisaksakul M., Jimenez R.C., Arias M.C., Beckles D.M. Effects of environmental factors on cereal starch biosynthesis and composition. J. Cereal Sci. 2012;56:67–80. doi: 10.1016/j.jcs.2012.04.002. [DOI] [Google Scholar]
- 7.Beckles D.M., Thitisaksakul M. How environmental stress affects starch composition and functionality in cereal endosperm. Starch Stärke. 2014;66:58–71. doi: 10.1002/star.201300212. [DOI] [Google Scholar]
- 8.Nicolas M., Gleadow R., Dalling M. Effect of postanthesis drought on cell-division and starch accumulation in developing wheat grains. Ann. Bot. 1985;55:433–444. doi: 10.1093/oxfordjournals.aob.a086922. [DOI] [Google Scholar]
- 9.Yang W.B., Li Y., Yin Y.P., Jiang W.W., Peng D.L., Cui Z.Y., Yang D.Q., Wang Z.L. Ethylene and spermidine in wheat grains in relation to starch content and granule size distribution under water deficit. J. Integr. Agric. 2014;13:2141–2153. doi: 10.1016/S2095-3119(13)60726-1. [DOI] [Google Scholar]
- 10.Lu H.F., Wang C.Y., Guo T.C., Xie Y.X., Feng W., Li S.Y. Starch composition and its granules distribution in wheat grains in relation to post- anthesis high temperature and drought stress treatments. Starch Stärke. 2014;66:419–428. doi: 10.1002/star.201300070. [DOI] [Google Scholar]
- 11.Yu X.R., Li B., Wang L.L., Chen X.Y., Wang W.J., Gu Y.J., Wang Z., Xiong F. Effect of drought stress on the development of endosperm starch granules and the composition and physicochemical properties of starches from soft and hard wheat. J. Sci. Food Agric. 2016;96:2746–2754. doi: 10.1002/jsfa.7439. [DOI] [PubMed] [Google Scholar]
- 12.Li C., Li C.Y., Zhang R.Q., Liang W., Kang X.L., Jia Y., Liao Y.C. Effects of drought on the morphological and physicochemical characteristics of starch granules in different elite wheat varieties. J. Cereal Sci. 2015;66:66–73. doi: 10.1016/j.jcs.2015.10.005. [DOI] [Google Scholar]
- 13.Singh S., Singh G., Singh P., Singh N. Effect of water stress at different stages of grain development on the characteristics of starch and protein of different wheat varieties. Food Chem. 2008;108:130–139. doi: 10.1016/j.foodchem.2007.10.054. [DOI] [Google Scholar]
- 14.Singh S., Gupta A.K., Gupta S.K., Kaur N. Effect of sowing time on protein quality and starch pasting characteristics in wheat (Triticum aestivum L.) genotypes grown under irrigated and rain-fed conditions. Food Chem. 2010;122:559–565. doi: 10.1016/j.foodchem.2010.03.010. [DOI] [Google Scholar]
- 15.Xia J., Zhu D., Chang H.M., Yan X., Yan Y.M. Effects of water-deficit and high-nitrogen treatments on wheat resistant starch crystalline structure and physicochemical properties. Carbohyd. Polym. 2020;234:115905. doi: 10.1016/j.carbpol.2020.115905. [DOI] [PubMed] [Google Scholar]
- 16.Zhang W., Gu J., Wang Z., Wei C., Yang J., Zhang J. Comparison of structural and functional properties of wheat starch under different soil drought conditions. Sci. Rep. 2017;7:12312. doi: 10.1038/s41598-017-10802-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trivedi K., Anand K.G.V., Vaghela P., Ghosh A. Differential growth, yield and biochemical responses of maize to the exogenous application of Kappaphycus alvarezii seaweed extract, at grain-filling stage under normal and drought conditions. Algal. Res. 2018;35:236–244. doi: 10.1016/j.algal.2018.08.027. [DOI] [Google Scholar]
- 18.Ullah A., Manghwar H., Shaban M., Khan A.H., Akbar A., Ali U., Ali E., Fahad S. Phytohormones enhanced drought tolerance in plants: A coping strategy. Environ. Sci. Pollut. R. 2018;25:33103–33118. doi: 10.1007/s11356-018-3364-5. [DOI] [PubMed] [Google Scholar]
- 19.Anjum S.A., Wang L.C., Farooq M., Hussain M., Xue L.L., Zou C.M. Brassinolide application improves the drought tolerance in maize through modulation of enzymatic antioxidants and leaf gas exchange. J. Agron. Crop Sci. 2011;197:177–185. doi: 10.1111/j.1439-037X.2010.00459.x. [DOI] [Google Scholar]
- 20.Kaya C., Tuna A.L., Alves A.A.C. Gibberellic acid improves water deficit tolerance in maize plants. Acta Physiol. Plant. 2006;28:331–337. doi: 10.1007/s11738-006-0029-7. [DOI] [Google Scholar]
- 21.Xie T.L., Gu W.R., Li C.F., Li J., Wei S. Exogenous DCPTA increases the tolerance of maize seedlings to PEG-simulated drought by regulating nitrogen metabolism-related enzymes. Agron. Basel. 2019;9:676. doi: 10.3390/agronomy9110676. [DOI] [Google Scholar]
- 22.Zhang C.X., Guo B.W., Tang J., Xu F.F., Xu K., Hu Y.J., Xing Z.P., Zhang H.C., Dai Q.G., Huo Z.Y., et al. Combined effects of low temperature and weak light at grain-filling stage on rice grain quality. Acta Agron. Sin. 2019;45:1208–1220. [Google Scholar]
- 23.Pei L.M., Che R.H., He L.L., Gao X.X., Li W.J., Li H. Role of exogenous glutathione in alleviating abiotic stress in maize (Zea mays L.) J. Plant Growth Regul. 2019;38:199–215. doi: 10.1007/s00344-018-9832-9. [DOI] [Google Scholar]
- 24.Li L.J., Gu W.R., Li C.F., Li W.H., Li C.F., Li J., Wei S. Exogenous spermidine improves drought tolerance in maize by enhancing the antioxidant defence system and regulating endogenous polyamine metabolism. Crop. Pasture Sci. 2018;69:1076–1091. doi: 10.1071/CP18271. [DOI] [Google Scholar]
- 25.Maswada H.F., Abd El-Razek U.A., El-Sheshtawy A.N.A., Elzaawely A.A. Morpho-physiological and yield responses to exogenous moringa leaf extract and salicylic acid in maize (Zea mays L.) under water stress. Arch. Agron. Soil Sci. 2018;64:994–1010. doi: 10.1080/03650340.2017.1406079. [DOI] [Google Scholar]
- 26.McMillan T., Tidemann B.D., O’Donovan J.T., Izydorczyk M.S. Effects of plant growth regulator application on the malting quality of barley. J. Sci. Food Agric. 2020;100:2082–2089. doi: 10.1002/jsfa.10231. [DOI] [PubMed] [Google Scholar]
- 27.Xu Y.J., Qiu M.T., Li Y.Y., Qian X.Y., Gu J.F., Yang J.C. Polyamines mediate the effect of post-anthesis soil drying on starch granule size distribution in wheat kernels. Crop J. 2016;4:444–458. doi: 10.1016/j.cj.2016.05.004. [DOI] [Google Scholar]
- 28.Pan S.G., Rasul F., Li W., Tian H., Mo Z.W., Duan M.Y., Tang X.R. Roles of plant growth regulators on yield, grain qualities and antioxidant enzyme activities in super hybrid rice (Oryza sativa L.) Rice. 2013;6:1–10. doi: 10.1186/1939-8433-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu D.L., Lu W.P. Effects of protein removal on the physicochemical properties of waxy maize flours. Starch Stärke. 2012;64:874–881. doi: 10.1002/star.201200038. [DOI] [Google Scholar]
- 30.Hsieh C.F., Liu W.C., Whaley J.K., Shi Y.C. Structure and functional properties of waxy starches. Food Hydrocolloid. 2019;94:238–254. doi: 10.1016/j.foodhyd.2019.03.026. [DOI] [Google Scholar]
- 31.Yang H., Gu X.T., Ding M.Q., Lu W.P., Lu D.L. Activities of starch synthetic enzymes and contents of endogenous hormones in waxy maize grains subjected to post-silking water deficit. Sci. Rep. 2019;9:7059. doi: 10.1038/s41598-019-43484-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu D.L., Cai X.M., Zhao J.Y., Shen X., Lu W.P. Effects of drought after pollination on grain yield and quality of fresh waxy maize. J. Sci. Food Agric. 2015;95:210–215. doi: 10.1002/jsfa.6709. [DOI] [PubMed] [Google Scholar]
- 33.Lu D.L., Sun S.L., Wang X., Yan F.B., Lu W.P. Effects of water stress during grain filling on physicochemical properties of waxy maize flour. Sci. Agric. Sin. 2013;46:30–36. [Google Scholar]
- 34.El-Sabagh A., Barutcular C., Hossain A., Islam M.S. Response of maize hybrids to drought tolerance in relation to grain weight. Fresen Environ. Bull. 2018;27:2476–2482. [Google Scholar]
- 35.Rajala A., Hakala K., Makela P., Peltonen-Sainio P. Drought effect on grain number and grain weight at spike and spikelet level in six-row spring barley. J. Agron. Crop. Sci. 2011;197:103–112. doi: 10.1111/j.1439-037X.2010.00449.x. [DOI] [Google Scholar]
- 36.Ahmad I., Kamran M., Meng X.P., Ali S., Bilegjargal B., Cai T., Liu T.N., Han Q.F. Effects of plant growth regulators on seed filling, endogenous hormone contents and maize production in semiarid regions. J. Plant Growth Regul. 2019;38:1467–1480. doi: 10.1007/s00344-019-09949-2. [DOI] [Google Scholar]
- 37.Panda B.B., Sekhar S., Dash S.K., Behera L., Shaw B.P. Biochemical and molecular characterisation of exogenous cytokinin application on grain filling in rice. BMC Plant Biol. 2018;18:89. doi: 10.1186/s12870-018-1279-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Javid M.G., Sorooshzadeh A., Sanavy S.A.M.M., Allahdadi I., Moradi F. Effects of the exogenous application of auxin and cytokinin on carbohydrate accumulation in grains of rice under salt stress. Plant Growth Regul. 2011;65:305–313. doi: 10.1007/s10725-011-9602-1. [DOI] [Google Scholar]
- 39.Xie Z.J., Jiang D., Dai T.B., Jing Q., Cao W.X. Effects of exogenous ABA and cytokinin on leaf photosynthesis and grain protein accumulation in wheat ears cultured in vitro. Plant Growth Regul. 2004;44:25–32. doi: 10.1007/s10725-004-1880-4. [DOI] [Google Scholar]
- 40.Prathap V., Ali K., Singh A., Vishwakarma C., Krishnan V., Chinnusamy V., Tyagi A. Starch accumulation in rice grains subjected to drought during grain filling stage. Plant Physiol. Bioch. 2019;142:440–451. doi: 10.1016/j.plaphy.2019.07.027. [DOI] [PubMed] [Google Scholar]
- 41.Zhang Q., Zhang L.Z., Evers J.C., van der Werf W., Zhang W.Q., Duan L.S. Maize yield and quality in response to plant density and application of a novel plant growth regulator. Field Crop. Res. 2014;164:82–89. doi: 10.1016/j.fcr.2014.06.006. [DOI] [Google Scholar]
- 42.Chen X.Y., Li B., Shao S.S., Wang L.L., Zhu X.W., Yang Y., Wang W.J., Yu X.R., Xiong F. Accumulation characteristic of protein bodies in different regions of wheat endosperm under drought stress. J. Integr. Agric. 2016;15:2921–2930. doi: 10.1016/S2095-3119(16)61332-1. [DOI] [Google Scholar]
- 43.Zhang T., Wang Z., Yin Y., Cai R., Yan S., Li W. Starch content and granule size distribution in grains of wheat in relation to post-anthesis water deficits. J. Agron. Crop Sci. 2010;196:1–8. doi: 10.1111/j.1439-037X.2009.00388.x. [DOI] [Google Scholar]
- 44.Li L., Blanco M., Jane J. Physicochemical properties of endosperm and pericarp starches during maize development. Carbohyd. Polym. 2007;67:630–639. doi: 10.1016/j.carbpol.2006.08.013. [DOI] [Google Scholar]
- 45.Peng D.L., Cai T., Yin Y.P., Yang W.B., Ni Y.L., Yang D.Q., Wang Z.L. Exogenous application of abscisic acid or gibberellin acid has different effects on starch granule size distribution in grains of wheat. J. Integr. Agric. 2013;12:1551–1559. doi: 10.1016/S2095-3119(13)60557-2. [DOI] [Google Scholar]
- 46.Yang D.Q., Li Y., Shi Y.H., Cui Z.Y., Luo Y.L., Zheng M.J., Chen J., Li Y.X., Yin Y.P., Wang Z.L. Exogenous cytokinins increase grain yield of winter wheat cultivars by improving stay-green characteristics under heat stress. PLoS ONE. 2016;11:e0155437. doi: 10.1371/journal.pone.0155437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Basunia M.A., Nonhebel H.M. Hormonal regulation of cereal endosperm development with a focus on rice (Oryza sativa) Funct. Plant Biol. 2019;46:493–506. doi: 10.1071/FP18323. [DOI] [PubMed] [Google Scholar]
- 48.Almeida V.O., Batista K.A., Di-Medeiros M.C.B., Moraes M.G., Fernandes K.F. Effect of drought stress on the morphological and physicochemical properties of starches from Trimezia juncifolia. Carbohyd. Polym. 2019;212:304–311. doi: 10.1016/j.carbpol.2019.02.015. [DOI] [PubMed] [Google Scholar]
- 49.Bertofta E., Annor G.A., Shen X.Y., Rumpagaporn P., Seetharaman K., Hamaker B.R. Small differences in amylopectin fine structure may explain large functional differences of starch. Carbohyd. Polym. 2016;140:113–121. doi: 10.1016/j.carbpol.2015.12.025. [DOI] [PubMed] [Google Scholar]
- 50.Lu D.L., Sun X.L., Yan F.B., Wang X., Xu R.C., Lu W.P. Effects of high temperature during grain filling under control conditions on the physicochemical properties of waxy maize flour. Carbohyd. Polym. 2013;98:302–310. doi: 10.1016/j.carbpol.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 51.Fahad S., Hussain S., Saud S., Hassan S., Chauhan B.S., Khan F., Ihsan M.Z., Ullah A., Wu C., Bajwa A.A., et al. Responses of rapid viscoanalyzer profile and other rice grain qualities to exogenously applied plant growth regulators under high day and high night temperatures. PLoS ONE. 2016;11:e0159590. doi: 10.1371/journal.pone.0159590. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 52.Ren B.Z., Hu J., Zhang J.W., Dong S.T., Liu P., Zhao B. Spraying exogenous synthetic cytokinin 6-benzyladenine following the waterlogging improves grain growth of waterlogged maize in the field. J. Agron. Crop. Sci. 2019;205:616–624. doi: 10.1111/jac.12355. [DOI] [Google Scholar]
- 53.Gao Z., Liang X.G., Zhang L., Lin S., Zhao X., Zhou L.L., Shen S., Zhou S.L. Spraying exogenous 6-benzyladenine and brassinolide at maize yield by enhancing source and sink capacity tasseling increases. Field Crop. Res. 2017;211:1–9. doi: 10.1016/j.fcr.2017.05.027. [DOI] [Google Scholar]
- 54.Hansen J., Moller I. Percolation of starch and soluble carbohydrates from plant tissue for quantitative determination with anthrone. Anal. Biochem. 1975;68:87–94. doi: 10.1016/0003-2697(75)90682-X. [DOI] [PubMed] [Google Scholar]
- 55.Chemists A.A.O.C. Approved Methods of the AACCI. The Association; St. Paul, MN, USA: 1999. [Google Scholar]
- 56.Gu X.T., Huang T.Q., Ding M.Q., Lu W.P., Lu D.L. Effects of short-term heat stress at the grain formation stage on physicochemical properties of waxy maize starch. J. Sci. Food Agric. 2018;98:1008–1015. doi: 10.1002/jsfa.8549. [DOI] [PubMed] [Google Scholar]
- 57.Tang Q.Y., Feng M.G. Data Processing System: Experimental Design, Statistical Analysis and Data Mining. Science Press; Beijing, China: 2007. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study is contained within the article.