Abstract
Healthy lifestyles are associated with better health-related quality of life (HRQoL), favorable prognosis and lower mortality in breast cancer (BC) survivors. We investigated changes in HRQoL after a 12-month lifestyle modification program in 227 BC survivors participating in DEDiCa trial (Mediterranean diet, exercise, vitamin D). HRQoL was evaluated through validated questionnaires: EQ-5D-3L, EORTC-QLQ-C30 and EORTC QLQ-BR23. Baseline changes were tested using analysis of variance. Multiple regression analyses were performed to assess treatment effects on HRQoL. Increases were observed in global health status (p < 0.001), physical (p = 0.003), role (p = 0.002) and social functioning (p < 0.001), body image (p < 0.001), future perspective (p < 0.001), well-being (p = 0.001), and reductions in fatigue (p < 0.001), nausea and vomiting (p = 0.015), dyspnea (p = 0.001), constipation (p = 0.049), financial problems (p = 0.012), sexual functioning (p = 0.025), systematic therapy side effects (p < 0.001) and breast symptoms (p = 0.004). Multiple regression analyses found inverse associations between changes in BMI and global health status (p = 0.048) and between serum 25(OH)D levels and breast symptoms (p = 0.002). A healthy lifestyle treatment of traditional Mediterranean diet and exercise may impact positively on HRQoL in BC survivors possibly through reductions in body weight while vitamin D sufficiency may improve BC-related symptoms. These findings are relevant to BC survivors whose lower HRQoL negatively affects treatment compliance and disease outcomes.
Keywords: quality of life, mediterranean diet, breast cancer, lifestyle, physical activity, vitamin D
1. Introduction
A global increase in breast cancer (BC) incidence and mortality has been observed in 2018 [1]. Breast cancer (BC) is the most frequently diagnosed cancer in women in most regions of the world and the most frequent cause of death from cancer in 11 regions of the world [2]. BC survival rates are increasing globally reaching 86% at 5 years in Italy [3]. The increase in BC incidence and the high survival rates among BC patients indicate the importance of targeting health-related quality of life (HRQoL) and understanding its relationship with lifestyle including diet and physical activity. Healthy lifestyles have been associated with better quality of life [4] which is in turn associated with favorable prognosis and lower mortality [5,6,7,8,9].
Cancer patients report declines in physical functioning, increased pain and generally reduced quality of life [10,11,12]. This can reduce compliance to oncologic treatment with adverse consequences on cancer prognosis and mortality [13,14]. Diet has been shown to improve quality of life in BC survivors [6,15,16,17,18] and a greater adherence to the Mediterranean diet has been associated with higher physical functioning and health status in women recently diagnosed with BC [4].
Physical activity has also been found effective in improving overall quality of life in BC survivors [19,20] through direct physiologic effects or indirectly by reducing oncologic treatment side effects [8,21].
Few studies have examined the effects of vitamin D supplementation on HRQoL in BC survivors. However, some evidence suggests beneficial effects of vitamin D supplementation on musculoskeletal pain in BC patients with low vitamin D levels and receiving adjuvant anastrozole therapy [22].
Therefore, we investigated HRQoL in BC survivors after a 12-month lifestyle modification program, including higher adherence to the traditional Mediterranean diet, higher physical activity and vitamin D supplementation to reach sufficiency levels.
2. Materials and Methods
2.1. Study Design
This study is part of an ongoing multicenter randomized controlled trial on the efficacy of a treatment program including dietary modifications, physical activity and vitamin D supplementation (DEDiCa study) on BC relapse [23], approved by the Ministry of Health Italian, Italian Medicines Agency (AIFA) and the ethics committees of each recruiting hospital (ClinicalTrials.gov NCT02786875). Eligible participants were found through surgical lists of participating hospitals. They were contacted by phone and offered to learn more about the study during group information sessions. Informed consent was obtained at baseline from all participants included in the study. Eligible women were randomized to follow either one of two treatments: (a) low glycemic index traditional Mediterranean diet + daily brisk walking + vitamin D supplementation; (b) traditional Mediterranean diet + avoidance of physical inactivity + vitamin D supplementation. Our analyses included 227 participants with complete data at baseline and 12 months. Clinic visits included the evaluation of anthropometric, dietary and biochemical parameters at baseline and at 12 months. Furthermore, through specific questionnaires we obtained information at the baseline and at 12 months on adherence to the Mediterranean diet and on the health-related quality of life (HRQoL). Details of these questionnaires are explained below.
2.2. Anthropometric and Physical Activity Level Measurements
Anthropometric data of the participants were collected by the study staff at baseline and 12 months, specifically weight was measured to the nearest 0.5 Kg using a Seca scale (Seca 761) while height to the nearest 1 cm using a Seca stadiometer. Body mass index (BMI) was calculated using the formula weight (kg)/height (m2).
Physical activity level was assessing using a step counter (Omron Walking Style IV) provided by study staff prior to the baseline visit. Participants wore the step counter for at least 7 days before each visit. Four categories of activity were identified: sedentary (<5000 steps/day), low activity (5000 < 7500 steps/day), medium activity (7500 < 10,000 steps/day) and high activity (≥10,000 steps/day) [24].
2.3. Adherence to Mediterranean Diet
At baseline and 12-month follow-up all participants completed a 7-day food record and the 14-item questionnaire administered by study staff. The 14-point Mediterranean Diet Adherence Screener (MEDAS), developed to control for compliance with the dietary intervention of the study Prevención con Dieta Mediterránea (PREDIMED) [25], was used to assess dietary adherence in people living in a Mediterranean area [26]. MEDAS questionnaire includes 14 questions: 12 investigate the frequency and quantity of foods (olive oil, vegetables, fruit, red or processed meats, butter, soda drinks, legumes, fish, commercial sweets, nuts, wine, sofrito sauce), while two questions are related to the preference of olive oil and meat consumption. Each question has two possible answers and scores; 1 score for “yes” and 0 for “no”. The 14-point MEDAS can range from 0 to 14 where 14 represents the highest adherence to Mediterranean diet.
2.4. Dietary Assessment
Dietary data, including beverage intakes and alcohol consumption, were collected by a 7-day food records at baseline and 12-month follow-up. During clinic visits patients returned the food records to the nutritionist who asked supplemental questions where necessary. Data were stored and processed using a professional software (WinFood©) which utilized the CREA—Alimenti e Nutrizione nutritional database. Dietary fiber was calculated as g/1000 kcal, while saturated fatty acids (SFA) and monounsaturated fatty acids (MUFA) as percent of daily energy intake at baseline and 12-month follow-up.
2.5. Health Related Quality of Life (HRQoL)
To assess the quality of life at baseline and 12-months, the patients completed three validated questionnaires: the European Quality of Life 5 Dimensions 3 Level (EQ-5D-3L) [27], the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core 30 items (EORTC QLQ-C30) and Breast Cancer 23 items (EORTC QLQ-BR23) [28]. The EQ-5D-3L is one of most commonly used generic health-related quality-of-life questionnaire, made by the Euroqol group. The questionnaire comprises five dimensions (mobility, self-care, usual activities, pain or discomfort, and anxiety or depression) and three levels of perceived problems (no problems, some problems and extreme problems). Another part of the questionnaire includes a visual analogue scale (EQ-VAS) that measures patient self-perceived health status. We have used the Italian health states value set [29] to obtain a unique health index score (EQ-5D-index), where the value of 1 represents the best health status and below 1 worse health status; in addition, EQ-VAS evaluates the participant’s self-reported health state on a scale from 0 (worse health status) to 100 best health status.
The EORTC QLQ-C30 (Questionnaire for Quality of Life Assessment in patients with cancer, version 3.0) is a tool for assessing quality of life in cancer patients, consisting of 30 questions subsequently transformed in 15 scales: five functional dimensions (physical, role, emotional, cognitive, and social), three symptom items (fatigue, nausea or vomiting, and pain), six single items (dyspnea, sleep disturbance, appetite loss, constipation, diarrhea, and financial impact) and a global health status/QoL scale [28]. The EORTC QLQ-BR23 (Quality of Life Questionnaire—Breast Cancer) assesses quality of life specifically in breast cancer patients and comprises of 23 questions that evaluate body image, sexual functioning, sexual enjoyment, future perspective, systemic therapy side effects, breast symptoms, arm symptoms and distress from hair loss. For each question there are four answers (not at all, a little, quite a bit, very much). All items are linearly transformed to a 0–100 scale according to a standardized process described in EORTC QLQ-C30 Scoring Manual 3rd Edition [30]. Higher scores for functioning and for global health status indicate better health. Conversely, higher scores for symptoms indicate worse health.
In our analyses we did not include the answers to the questions related to sexual enjoyment and feeling towards hair loss because at least 50% of participants did not respond to these questions at baseline.
2.6. Biochemical Measurements
Blood samples were collected at baseline and after 12 months. Serum 25(OH)D concentrations were measured using chemiluminescent immunoassay (CLIA) technology for the quantitative determination of 25-hydroxyvitamin D and other hydroxylated vitamin D metabolites in human serum (LIAISON® 25 OH Vitamin D TOTAL Assay) at the Laboratory Medicine Unit. All analytes were measured in the coordinating hospital routine analytical laboratory (Laboratory Medicine Unit, Istituto Nazionale Tumori—IRCCS “Fondazione G. Pascale”, Napoli, Italy) after quality control procedures.
2.7. Statistical Analyses
The present analysis includes data from the first 227 women enrolled in DEDiCa study with complete data at baseline and 12 months. Baseline characteristics were described as numbers (n) and percentages (%). Sociodemographic and clinical characteristics were summarized using tabulations for categorical variables and means and standard deviations (SD) for continuous variables. Means and SD were calculated for all HRQoL dimensions, as well as anthropometric parameters, step count, 25(OH)D levels, dietary measurement at baseline and at 12-month follow-up and changes from baseline were compared with the analysis of variance (ANOVA). These analyses were also performed in strata of BMI at baseline (<25 kg/m2 vs. ≥25 kg/m2) and in strata of hormone therapy use at 12 months (treatment vs no treatment). Considering there were no significant differences in HRQoL between randomization arms at 12 months, data from both groups were analyzed together. Multiple linear regression models adjusted for terms of age, civil status, education, time from surgery, BMI, step count, 25(OH)D level, MEDAS score, dietary fiber intake, monounsaturated fatty acids (MUFA) and saturated fatty acids (SFA) intakes were performed to assess the association between changes in HRQoL dimensions and selected covariates. The dependent variables were changes in the HRQoL domains and the independent variables were the lifestyle treatment components at baseline, 12 months and their time changes. The dependent variables were chosen based on significant ANOVA variation from baseline to 12 months. Results are reported as estimates beta coefficients, p value and R2 adjusted for the goodness of the model. The Statistical Package for the Social Sciences (SPSS) software, version 26.0 (Chicago, IL, USA) was used for all data analyses. Results were considered statistically significant at p-value < 0.05.
3. Results
Baseline characteristics of randomized participants (n = 227) are shown in Table 1. Mean age (±SD) was 52.3 ± 9.3 years (45% < 50 years), mean body mass index (BMI) was 27.3 kg/m2 ± 5.8 (43% normal weight, 31% overweight and 26% obese), 52.4% non-smokers, 76.3% were inactive and 62.6% showed adherence to the Mediterranean diet. The majority of participants, 96% were postmenopausal (natural or pharmacologically induced), 81.1% were married or common-law and 65.6% attained high school or higher education. Moreover, Table 1 shows cancer characteristics including staging, molecular subtypes and oncologic treatment. Patients undertaking hormone therapy were 54.6% while 16% were still receiving adjuvant chemotherapy at baseline. Participants who reported at least one comorbidity at baseline were 41%: 5.7% reported type 2 diabetes, 23.3% hypertension and 22.9% hypercholesterolemia.
Table 1.
Baseline characteristics of the participants (n = 227).
| n | (%) | |
|---|---|---|
| Age (years) | ||
| <50 yrs | 101 | 44.5 |
| ≥50 yrs | 126 | 55.5 |
| Education (years of school) | ||
| ≤11 yrs | 78 | 34.4 |
| ≥12 yrs | 149 | 65.6 |
| Civil status a | ||
| Single c | 43 | 18.9 |
| Married (or common law) | 184 | 81.1 |
| Menopausal status | ||
| Menopause | 217 | 95.6 |
| Premenopause | 10 | 4.4 |
| Smoking status | ||
| Non-smoker | 119 | 52.4 |
| Smoker | 40 | 17.6 |
| Former smoker | 68 | 30.0 |
| Body Mass Index (kg/m 2 ) b | ||
| Normal weight | 97 | 42.7 |
| Overweight/Obese | 129 | 57.3 |
| Physical activity (steps/day) c | ||
| Sedentary | 105 | 46.3 |
| Low active | 68 | 30.0 |
| Somewhat active | 37 | 16.3 |
| Active | 17 | 7.5 |
| Mediterranean diet adherence | ||
| Low (≤7)) | 85 | 37.4 |
| High (>7) | 142 | 62.6 |
| Number of comorbidities d | ||
| 0 | 134 | 59.0 |
| 1 | 59 | 26.0 |
| >1 | 34 | 15.0 |
| Time from surgery | ||
| <8 months | 105 | 46.3 |
| ≥8 months | 122 | 53.7 |
| Molecular subtype | ||
| Luminal A | 123 | 54.2 |
| Luminal B | 26 | 11.5 |
| HER2+ | 42 | 18.5 |
| Triple negative | 36 | 15.9 |
| Cancer Stage e | ||
| I | 67 | 29.5 |
| II | 128 | 56.4 |
| III | 32 | 14.1 |
| Cancer treatment | ||
| Adjuvant chemotherapy | ||
| Never | 78 | 34.4 |
| Not current | 112 | 49.3 |
| Current | 37 | 16.3 |
| Radiotherapy | ||
| Never | 98 | 43.2 |
| Not current | 112 | 49.3 |
| Current | 17 | 7.5 |
| Biological therapy | ||
| Never | 197 | 86.8 |
| Not current | 1 | 0.4 |
| Current | 29 | 12.8 |
| Hormone therapy | ||
| No | 103 | 45.4 |
| Yes (current) | 124 | 54.6 |
a Single are widow, divorced or maiden; b Normal weight < 25.0 kg/m2, Overweight 25.0–29.9 kg/m2, Obese ≥ 30.0 kg/m2; c Physical activity (steps/day): Sedentary (<5000), Low active (5000–7499), Somewhat active (7500–9999), Active (≥10,000); d Type 2 Diabetes, Hypertension, Hypertriglyceridemia, Hypercholesterolemia; e based on the TNM system (T, size of primary tumor; N, lymph nodes involved; M, metastasis).
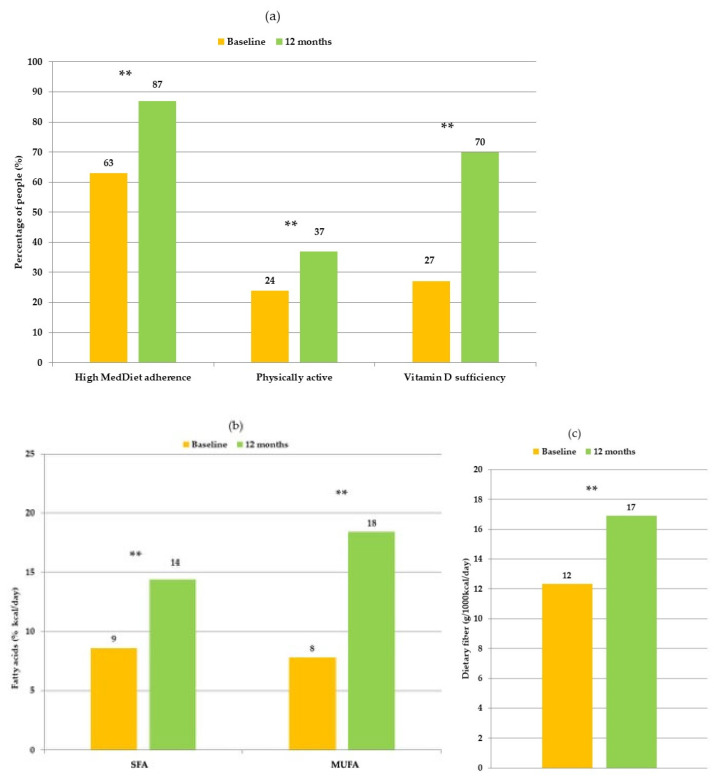
After 12 months, most participants showed high adherence to lifestyle treatment resulting in increased Mediterranean diet adherence, higher intakes of dietary fiber and MUFA and lower intakes of SFA, increased physical activity and circulating vitamin D levels increased reached sufficiency (≥30 ng/mL) in 70% of participants (Figure 1a–c).
Figure 1.
(a) Comparison of participants with high Mediterranean diet adherence (MEDAS score > 7), physically active (>7500 steps/day) and with vitamin D sufficiency (≥30 ng/mL) at baseline and at 12 months; (b) Comparison of participants’ fatty acids and (c) dietary fibers intakes at baseline and 12 months. (**) significance p < 0.001.
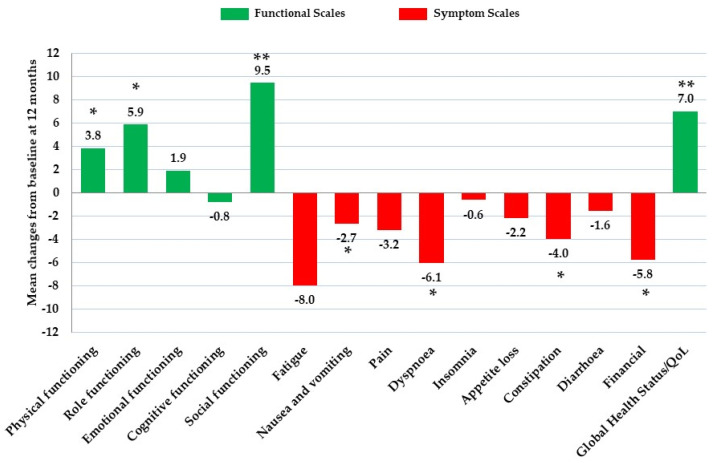
In the EORTC QLQ-C30 questionnaire, compared to baseline, at 12 months we observed that patients had significantly higher scores for physical functioning (p = 0.003), role functioning (p = 0.002), and social functioning (p < 0.001); among symptoms, at 12 months patients had significantly lower scores for fatigue (p < 0.001), nausea and vomiting (p = 0.015), dyspnea (p = 0.001), constipation (p = 0.049) and financial difficulties (p = 0.012). At 12 months general global health status significantly increased (p < 0.001) compared to baseline, indicating an improved HRQoL (Table 2, Figure 2). Pain symptom scores were also reduced at 12 months albeit not significantly (p = 0.099).
Table 2.
Comparison of European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core 30 items (EORTC QLQ-C30), Breast Cancer 23 items (EORTC QLQ-BR23), and European Quality of Life 5 Dimensions 3 Level (EQ 5D 3L) scores of participants at baseline and at 12 months.
| Baseline | 12 Months | Changes | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| EORTC QLQ-C30 | N | Mean | SD | N | Mean | SD | N | Mean | SD | p |
| Functioning | ||||||||||
| Physical functioning | 226 | 83.16 | 14.04 | 214 | 86.95 | 12.48 | 213 | 3.8 | 10.0 | 0.003 |
| Role functioning | 224 | 80.88 | 21.99 | 212 | 86.79 | 17.58 | 210 | 5.9 | 21.3 | 0.002 |
| Emotional functioning | 226 | 76.13 | 20.59 | 215 | 78.33 | 18.96 | 214 | 1.9 | 19.2 | 0.239 |
| Cognitive functioning | 225 | 82.37 | 21.14 | 213 | 81.77 | 18.25 | 211 | −0.8 | 17.9 | 0.750 |
| Social functioning | 226 | 78.25 | 24.84 | 216 | 88.12 | 17.02 | 215 | 9.5 | 22.9 | <0.001 |
| Symptoms | ||||||||||
| Fatigue | 227 | 31.68 | 22.28 | 213 | 24.15 | 18.00 | 213 | −8.0 | 21.9 | <0.001 |
| Nausea and vomiting | 226 | 7.15 | 12.93 | 214 | 4.36 | 10.79 | 213 | −2.7 | 12.9 | 0.015 |
| Pain | 226 | 22.58 | 21.42 | 213 | 19.33 | 19.51 | 212 | −3.2 | 19.5 | 0.099 |
| Dyspnoea | 226 | 18.73 | 22.40 | 215 | 12.25 | 19.06 | 214 | −6.1 | 23.5 | 0.001 |
| Insomnia | 226 | 28.17 | 27.36 | 211 | 27.01 | 26.87 | 210 | −0.6 | 28.0 | 0.656 |
| Appetite loss | 227 | 7.20 | 17.24 | 216 | 4.94 | 13.50 | 216 | −2.2 | 19.7 | 0.127 |
| Constipation | 200 | 15.00 | 22.85 | 208 | 10.74 | 20.65 | 185 | −4.0 | 24.7 | 0.049 |
| Diarrhoea | 225 | 8.15 | 16.30 | 216 | 6.48 | 14.70 | 215 | −1.6 | 19.3 | 0.261 |
| Financial | 224 | 16.96 | 25.25 | 216 | 11.42 | 20.67 | 213 | −5.8 | 24.3 | 0.012 |
| Global Health Status/QoL | 225 | 63.89 | 21.30 | 216 | 70.87 | 17.90 | 214 | 7.0 | 20.4 | <0.001 |
| EORTC QLQ-BR23 | ||||||||||
| Functioning | ||||||||||
| Body image | 226 | 65.04 | 29.98 | 215 | 77.17 | 23.39 | 214 | 11.8 | 24.0 | <0.001 |
| Sexual functioning | 222 | 81.83 | 22.09 | 213 | 77.0 | 22.73 | 208 | −4.2 | 21.0 | 0.025 |
| Future perspective | 226 | 45.58 | 34.61 | 216 | 57.10 | 30.00 | 215 | 11.0 | 29.3 | <0.001 |
| Symptoms | ||||||||||
| Systematic therapy side effects | 225 | 24.06 | 18.35 | 214 | 16.07 | 11.60 | 213 | −7.7 | 15.4 | <0.001 |
| Breast symptoms | 224 | 20.65 | 18.71 | 215 | 15.97 | 15.12 | 212 | −4.8 | 18.8 | 0.004 |
| Arm symptoms | 224 | 20.39 | 19.16 | 214 | 17.44 | 18.59 | 211 | −2.8 | 17.0 | 0.104 |
| EQ-5D-3L | ||||||||||
| EQ INDEX SCORE | 225 | 0.88 | 0.10 | 219 | 0.88 | 0.11 | 218 | 0.01 | 0.09 | 0.237 |
| EQ VAS SCORE | 222 | 68.61 | 15.91 | 222 | 74.55 | 15.30 | 218 | 5.80 | 14.82 | 0.001 |
SD: standard deviation; Changes: mean changes from baseline to 12 months; QoL: quality of life; VAS: visual analogue scale. Bold data indicate statistically significant p-values (significance p < 0.05).
Figure 2.
Mean changes of EORTC QLQ-C30 (European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core 30) scores from baseline at 12 months. (*) significance p < 0.05; (**) significance p < 0.001.
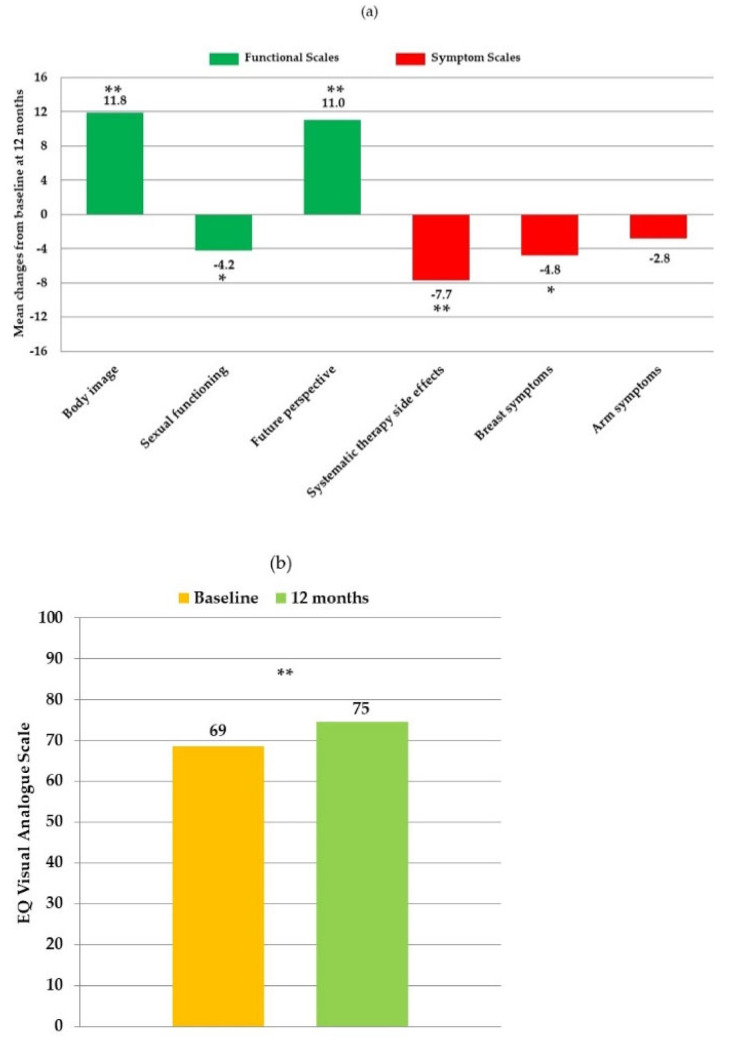
In the breast cancer specific questionnaire EORTC QLQ-BR23, at 12 months participants had significantly higher scores for body image (p < 0.001) and future perspective (p < 0.001) but lower scores for sexual functioning (p = 0.025). Among symptoms, at 12 months patients had significantly lower scores for systematic therapy side effects (p < 0.001) and breast symptoms (p = 0.004) (Table 2, Figure 3a). Reductions in arm symptom scores were also observed albeit not significantly (p = 0.104). The EQ-5D-3L questionnaire showed significantly higher scores for the visual analogue scale (VAS) (74.6 vs. 68.6; p = 0.001) at 12 months, indicating improved overall wellbeing (Table 2, Figure 3b).
Figure 3.
(a) Mean changes of European ORTC QLQ-BR23 (European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Breast 23) scores of participants. (b) Comparison of participants EQ VAS (European Quality of Life Visual Analogue Scale) at baseline and at 12 months. (*) significance p < 0.05; (**) significance p < 0.001.
When data were stratified by baseline BMI, changes in scores for HRQoL components did not differ significantly at 12 months compared to baseline, except for EQ-5D-3L index scores which significantly increased in normal weight patients indicating improved health status and decreased in overweight/obese (0.034 ± 0.077 vs. −0.013 ± 0.094; p < 0.001, respectively). When stratifying data by hormone therapy use at 12 months, we observed significantly higher scores for body image in patients not taking hormone therapy compared to those taking hormone therapy (18.0 ± 20.8 vs. 10.0 ± 24.6; p = 0.038, respectively; data not shown) while reductions in sexual functioning were significantly higher in patients not taking hormone therapy compared to those taking hormone therapy (−9.7 ± 22.8 vs. −2.6 ± 20.2; p =0.039, respectively; data not shown).
In multivariate analyses relevant changes (β > 0.20; p < 0.05) were reported only for breast symptoms changes which were inversely related to changes in serum 25(OH)D levels.
4. Discussion
Quality of life improved in women recently diagnosed with BC undertaking oncologic and lifestyle treatments. Compared to baseline, increases were found in global health status, self-perceived health status and functional scales, in most domains, while decreases were found in symptom scales for most items, after 12 months. Specifically, we observed improvements in physical functioning, role functioning and social functioning, and reductions in fatigue, nausea and vomiting, dyspnea, constipation and financial difficulties.
We found that scores improved in functional scales for body image and future prospective, and in systematic therapy side effects and breast symptoms, after 12 months of lifestyle modifications. The increased adherence to the Mediterranean diet measured by MEDAS questionnaire and by increased dietary fiber and MUFA with reduced SFA intakes, by the increased step count and by the normalization of vitamin D levels, confirm that lifestyle modifications were implemented.
Although our multivariate analyses did not show that many of the above treatment components singularly explained changes in HRQoL components (except for vitamin D) the model indicated that changes in BMI were inversely related to changes in global health status indicating improved HRQoL in those who lost weight. This finding has been confirmed by others [31]. Among women who completed the 12-month treatment, two-thirds were overweight/obese at baseline and showed an average weight loss of 1.9 kg despite the majority were taking oncological hormone therapy which is known to increase body weight [32]. Weight loss in a lifestyle modification trial could be due to dietary and exercise modifications which is in line with our findings of significantly improved diet quality and physical activity after 12 months.
We had previously demonstrated that higher adherence to the Mediterranean diet in BC survivors was associated with higher HRQoL, particularly for physical functioning, sleep, pain and overall well-being [4]. Generally, a healthy dietary pattern is associated with better HRQoL [17,18,33,34] including fatigue which is prevalent in BC survivors and is associated with shorter overall survival [35]. Dietary intervention rich in fruits, vegetables, whole grains, and omega-3 fatty acid-rich foods, which are staple foods of the Mediterranean diet, significantly decreased cancer-related fatigue and improved sleep quality in BC survivors [34]. A possible mechanism for the relationship between a healthy diet and HRQoL may be through inflammation [36,37] and the Mediterranean diet has been previously associated with reduced inflammation [38]. Considering the increased life expectancy of BC survivors, adherence to a healthy diet may offer multiple health benefits including reduced cardiovascular disease risk, the most common comorbidity and cause of death in BC survivors [9] which can also negatively impact on quality of life. Furthermore, a higher adherence to the Mediterranean diet has been shown to reduce the risk of chronic diseases and overall mortality [39,40].
Physical exercise is another lifestyle component that can positively influence cancer outcomes and treatment-related side effects, quality of life, BC recurrence, and overall survival in BC patients [8]. During our 12-month lifestyle treatment participants increased physical activity level and the number of physically active patients increased from 54 to 86 while the number of sedentary patients decreased from 104 to 71 after 12 months. Physical activity is one of the mainstays of cancer prevention and it is also included in guidelines for BC survivors [41]. Higher physical activity was related to better quality of life in BC patients shortly after adjuvant treatments [42] and it positively influenced cancer- and treatment-related side effects including fatigue and peripheral neuropathy as well as aromatase inhibitor-related arthralgia [8,20,42,43].
Vitamin D may be another treatment component in our study influencing HRQoL. In our study we found significant increases in circulating vitamin D after 12-month supplementation, with 70% of patients reaching sufficiency from 27% at baseline. Multivariate analyses indicated that increased serum vitamin D levels were indeed associated with lower scores for systematic therapy side effects. Although we did not find a significant reduction in pain, there was a trend towards less pain severity at 12 months, although worsening of pain symptoms was expected over time due to side effects of oncologic hormonal therapy (e.g., tamoxifen and aromatase inhibitors) considering the higher number of patients taking hormone therapy at 12-month (77%) compared to baseline (55%). Vitamin D is known to exert a wide range of health effects, including those relating to musculoskeletal structure. Considering the high prevalence of vitamin D deficiency in BC survivors, it is likely that suboptimal vitamin D levels may contribute to the increased incidence and severity of musculoskeletal symptoms experienced by aromatase inhibitor-treated women. It has been reported that non-deficient vitamin D levels and vitamin D supplement use were associated with higher self-reported quality of life in BC survivors [44]. Other studies found beneficial effects of vitamin D supplementation on musculoskeletal pain in BC patients receiving aromatase inhibitor therapy [22,45,46]. Among adverse effects of aromatase inhibitors, pain has a negative impact on quality of life, on treatment compliance and on survival of BC patients [47,48,49,50]. A possible mechanism for the beneficial effect of vitamin D arthralgia and myalgia may be its anti-inflammatory activity.
The present study has some strengths and limitations. Data derives from a multicenter clinical trial where participants were closely followed with quarterly visits, all measurements were collected from centrally trained research staff and all analyses were performed in a central research location. This allowed to collect a large number of covariates which were used in our analyses. Limitations include HRQoL as secondary end point of DEDiCa trial which has a different aim [23] and we did not compare the intervention to the control group for the purpose of the current analysis. Patients were enrolled at different stages of cancer treatment which may have differently affected HRQoL. Although response-shift may affect quality of life outcomes [51] our study included patients enrolled within one year of surgery. At baseline only 16% of participants in the study were still receiving chemotherapy, 50% completed chemotherapy treatment and 34% did not receive chemotherapy treatment, therefore it is unlikely that chemotherapy influenced HRQoL significantly. When we repeated analysis stratifying patients by hormone therapy use at 12 months we found similar results for most components of the HRQoL questionnaires. Finally, we repeated analysis stratifying patients by BMI at baseline and we found similar results for most components of the HRQoL questionnaires, except for EQ-5D-3L index scores (mobility, self-care, usual activities, pain/discomfort and anxiety/depression) where higher scores were seen in normal weight patients and lower scores in overweight and obese patients. Finally, reporting findings at 12 months, instead of 24 or 36 months, may decrease the likelihood of significant HRQoL changes due to lifestyle modifications which may be shadowed by the strong therapy side effects in the first year which tend to subside afterwards.
5. Conclusions
To date, there are few studies evaluating the effects of a combined lifestyle program on HRQoL in BC survivors. Our study analyzed and reported in details quality of life aspects in BC patients after a lifestyle program of dietary modification, daily walking and vitamin D supplementation. In conclusion, a healthy lifestyle modification of traditional Mediterranean diet and exercise may impact positively on HRQoL in BC survivors possibly through reductions in body weight while correcting vitamin D sufficiency may improve BC-related symptoms. These findings are relevant to BC survivors whose lower HRQoL negatively affects treatment compliance and disease outcomes. Future studies on BC survivors should be carried out considering these findings to determine the relationship between lifestyle factors and HRQoL in BC survivors and to investigate mechanisms.
Acknowledgments
We would like to express our special thanks to all patients who participated in the study and to Maurizio Montella († 2 May 2019), PI of DEDiCa study, for his long-term contribution to the investigation of the role of lifestyle factors in breast cancer risk, for his ideas and support of this current manuscript. We would like to thank: Italian Ministry of Health, Barilla Spa (Parma, Italy) for providing participants with pasta, Panificio Giacomo Luongo (Naples, Italy) for providing fresh whole wheat bread, The Almond Board of California (Modesto, California, USA) and Consorzio Mandorle di Avola (Avola, Italy) for providing dry almonds, SunRice (Sydney, Australia) for providing low glycemic index rice, Roberto Alimentare (Treviso, Italy) for providing low glycemic index bread, DietaDoc (Trieste, Italy) for providing ready-to-eat food portions, Ello Frutta (Naples, Italy) for providing dehydrated fruit, Perrotta Montella for providing chestnuts (Avellino, Italy), Naturamica health food store (Naples, Italy) for providing bio-degradable food bags, Abiogen Pharma for providing vitamin D and Universita’ di Parma for GI testing of selected foods.
Author Contributions
Conceptualization, C.M., G.P., M.L. and L.S.A.A.; methodology, A.C., M.L. and L.S.A.A.; software, S.V. and E.P.; validation, L.S.A.A.; formal analysis, C.M., G.P. and A.C.; investigation C.M., G.P., S.V., E.P., I.C., S.C. (Serena Cubisino), V.M. and L.S.A.A.; resources, M.G., M.L., R.P., L.F., L.P., G.T., D.C., M.P. (Monica Pinto), M.D.L., C.P., M.R., M.D., D.S., S.M., L.C., C.E., A.S., F.C., G.L.B., F.F., R.R., G.A., G.G., A.F., F.M., A.M., B.G., M.C., E.C. (Ernesta Cavalcanti), E.C. (Egidio Celentano), G.B. and L.S.A.A.; data curation, C.M., G.P., A.C., S.V., E.P. R.P., I.C., M.P. (Melania Prete), N.E., F.N., and S.C. (Sergio Coluccia); writing—original draft preparation, C.M., G.P. and L.S.A.A.; writing—review and editing, C.M., G.P., A.C., M.G., M.L., S.V., E.P., I.C., S.C. (Serena Cubisino), L.F., L.P., V.M., M.P. (Melania Prete), N.E., S.C. (Sergio Coluccia), G.T., D.C., M.P. (Monica Pinto), M.D.L., C.P., M.R., M.D., D.S., S.M., L.C., A.S., F.C., G.L.B., G.S., F.F., R.R., G.A., G.G., A.F., F.M., G.R., D.G., D.J.A.J., A.M., B.G., P.C.F., E.C. (Ernesta Cavalcanti), E.C. (Egidio Celentano), G.B. and L.S.A.A.; visualization, C.M., G.P., M.P. (Melania Prete) and N.E.; supervision, A.C., M.G., M.L., D.S., F.F., G.R., D.G., D.J.A.J. and L.S.A.A.; project administration, M.G., R.P., L.P. and L.S.A.A.; funding acquisition, M.L. and L.S.A.A. All authors have read and agreed to the published version of the manuscript.
Funding
This trial is funded by a grant of the Italian Ministry of Health (Grant no. PE-2013-02358099) and Lega Italiana per la Lotta Contro i Tumori (LILT Nazionale) Progetto Cinque Per Mille 2013 and Progetto Cinque Per Mille 2015. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by Ethics Committee of of each recruiting hospital (ClinicalTrials.gov NCT02786875; 17 March 2016).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
L.S.A.A. is a founding member of the International Carbohydrate Quality Consortium (ICQC) and has received honoraria from the Nutrition Foundation of Italy (NFI), research grants from LILT (a non-profit organization for the fight against cancer) and in-kind research support from Abiogen Pharma, the Almond Board of California (USA), Barilla (Italy), Consorzio Mandorle di Avola (Italy), DietaDoc (Italy), Ello Frutta (Italy), Panificio Giacomo Luongo (Italy), Perrotta (Italy), Roberto Alimentare (Italy), SunRice (Australia). However, no funding that she has received has been involved in the current project. G.L.B. reports personal fees from Janssen-Cilag, personal fees from Boehringer Ingelheim, personal fees from Roche, non-financial support from Bristol-Myers Squibb, non-financial support from AstraZeneca/MedImmune, non-financial support from Pierre Fabre, non-financial support from Ipsen, outside the submitted work. D.G. has received speaking and/or consulting fees from Abiogen Pharma, Amgen, Eli-Lilly, Janssen-Cilag, Merck and Mundipharma. M.P. has received research support from Amgen. G.R. has received research grants from the Barilla Company to his University Department and is member of the scientific advisory boards of the foundation “Barilla Center for Food and Nutrition” and of “Nutrition Foundation of Italy”. D.J.A.J. has received research grants from Saskatchewan & Alberta Pulse Growers Associations, the Agricultural Bioproducts Innovation Program through the Pulse Research Network, the Advanced Foods and Material Network, Loblaw Companies Ltd., Unilever Canada and Netherlands, Barilla, the Almond Board of California, Agriculture and Agri-food Canada, Pulse Canada, Kellogg’s Company, Canada, Quaker Oats, Canada, Procter & Gamble Technical Centre Ltd., Bayer Consumer Care, Springfield, NJ, Pepsi/Quaker, International Nut & Dried Fruit (INC), Soy Foods Association of North America, the Coca-Cola Company (investigator initiated, unrestricted grant), Solae, Haine Celestial, the Sanitarium Company, Orafti, the International Tree Nut Council Nutrition Research and Education Foundation, the Peanut Institute, Soy Nutrition Institute (SNI), the Canola and Flax Councils of Canada, the Calorie Control Council, the Canadian Institutes of Health Research (CIHR), the Canada Foundation for Innovation (CFI) and the Ontario Research Fund (ORF). He has received in-kind supplies for trials as a research support from the Almond board of California, Walnut Council of California, American Peanut Council, Barilla, Unilever, Unico, Primo, Loblaw Companies, Quaker (Pepsico), Pristine Gourmet, Bunge Limited, Kellogg Canada, WhiteWave Foods. He has been on the speaker’s panel, served on the scientific advisory board and/or received travel support and/or honoraria from the Almond Board of California, Canadian Agriculture Policy Institute, Loblaw Companies Ltd., the Griffin Hospital (for the development of the NuVal scoring system), the Coca-Cola Company, EPICURE, Danone, Diet Quality Photo Navigation (DQPN), Better Therapeutics (FareWell), Verywell, True Health Initiative (THI), Heali AI Corp, Institute of Food Technologists (IFT), Soy Nutrition Institute (SNI), Herbalife Nutrition Institute (HNI), Saskatchewan & Alberta Pulse Growers Associations, Sanitarium Company, Orafti, the American Peanut Council, the International Tree Nut Council Nutrition Research and Education Foundation, the Peanut Institute, Herbalife International, Pacific Health Laboratories, Nutritional Fundamentals for Health (NFH), Barilla, Metagenics, Bayer Consumer Care, Unilever Canada and Netherlands, Solae, Kellogg, Quaker Oats, Procter & Gamble, Abbott Laboratories, Dean Foods, the California Strawberry Commission, Haine Celestial, PepsiCo, the Alpro Foundation, Pioneer Hi-Bred International, DuPont Nutrition and Health, Spherix Consulting and WhiteWave Foods, the Advanced Foods and Material Network, the Canola and Flax Councils of Canada, Agri-Culture and Agri-Food Canada, the Canadian Agri-Food Policy Institute, Pulse Canada, the Soy Foods Association of North America, the Nutrition Foundation of Italy (NFI), Nutra-Source Diagnostics, the McDougall Program, the Toronto Knowledge Translation Group (St. Michael’s Hospital), the Canadian College of Naturopathic Medicine, The Hospital for Sick Children, the Canadian Nutrition Society (CNS), the American Society of Nutrition (ASN), Arizona State University, Paolo Sorbini Foundation and the Institute of Nutrition, Metabolism and Diabetes. He received an honorarium from the United States Department of Agriculture to present the 2013 W.O. Atwater Memorial Lecture. He received the 2013 Award for Excellence in Research from the International Nut and Dried Fruit Council. He received funding and travel support from the Canadian Society of Endocrinology and Metabolism to produce mini cases for the Canadian Diabetes Association (CDA). He is a member of the International Carbohydrate Quality Consortium (ICQC). His wife, Alexandra L Jenkins, is a director and partner of INQUIS Clinical Research for the Food Industry, his 2 daughters, Wendy Jenkins and Amy Jenkins, have published a vegetarian book that promotes the use of the foods described here, The Portfolio Diet for Cardiovascular Risk Reduction (Academic Press/Elsevier 2020 ISBN:978-0-12-810510-8) and his sister, Caroline Brydson, received funding through a grant from the St. Michael’s Hospital Foundation to develop a cookbook for one of his studies. He has had close contact with the food industry to produce plant based diets. However, no funding that he has received has been involved in the current project. All other authors declare no competing interests.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J., Soerjomataram I., Dikshit R., Eser S., Mathers C., Rebelo M., Parkin D.M., Forman D., Bray F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 3.Dafni U., Tsourti Z., Alatsathianos I. Breast Cancer Statistics in the European Union: Incidence and Survival across European Countries. Breast Care. 2019;14:344–353. doi: 10.1159/000503219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Porciello G., Montagnese C., Crispo A., Grimaldi M., Libra M., Vitale S., Palumbo E., Pica R., Calabrese I., Cubisino S., et al. Mediterranean diet and quality of life in women treated for breast cancer: A baseline analysis of DEDiCa multicentre trial. PLoS ONE. 2020;15:e0239803. doi: 10.1371/journal.pone.0239803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balneaves L.G., Van Patten C., Truant T.L.O., Kelly M.T., Neil S.E., Campbell K.L. Breast cancer survivors’ perspectives on a weight loss and physical activity lifestyle intervention. Support. Care Cancer. 2014;22:2057–2065. doi: 10.1007/s00520-014-2185-4. [DOI] [PubMed] [Google Scholar]
- 6.Lofterød T., Frydenberg H., Flote V., Eggen A.E., McTiernan A., Mortensen E.S., Akslen L.A., Reitan J.B., Wilsgaard T., Thune I. Exploring the effects of lifestyle on breast cancer risk, age at diagnosis, and survival: The EBBA-Life study. Breast Cancer Res. Treat. 2020;182:1–13. doi: 10.1007/s10549-020-05679-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hashemi S.H.B., Karimi S., Mahboobi H. Lifestyle changes for prevention of breast cancer. Electron. Physician. 2014;6:894–905. doi: 10.14661/2014.894-905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wirtz P., Baumann F.T. Physical Activity, Exercise and Breast Cancer—What Is the Evidence for Rehabilitation, Aftercare, and Survival A Review. Breast Care. 2018;13:92–100. doi: 10.1159/000488717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weigl J., Hauner H., Hauner D. Can Nutrition Lower the Risk of Recurrence in Breast Cancer? Breast Care. 2018;13:86–91. doi: 10.1159/000488718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dodd M.J., Miaskowski C., Paul S.M. Symptom clusters and their effect on the functional status of patients with cancer. Oncol. Nurs. Forum. 2001;28:465–470. [PubMed] [Google Scholar]
- 11.Kurtz M.E., Kurtz J.C., Stommel M., Given C.W., Given B. Physical functioning and depression among older persons with cancer. Cancer Pract. 2001;9:11–18. doi: 10.1046/j.1523-5394.2001.91004.x. [DOI] [PubMed] [Google Scholar]
- 12.Serlin R.C., Mendoza T., Nakamura Y., Edwards K.R., Cleeland C.S. When is cancer pain mild, moderate or severe? Grading pain severity by its interference with function. Pain. 1995;61:277–284. doi: 10.1016/0304-3959(94)00178-H. [DOI] [PubMed] [Google Scholar]
- 13.Chim K., Xie S.X., Stricker C.T., Li Q.S., Gross R., Farrar J.T., DeMichele A., Mao J.J. Joint pain severity predicts premature discontinuation of aromatase inhibitors in breast cancer survivors. BMC Cancer. 2013;13:401. doi: 10.1186/1471-2407-13-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Humphries B., Collins S., Guillaumie L., Lemieux J., Dionne A., Provencher L., Moisan J., Lauzier S. Women’s Beliefs on Early Adherence to Adjuvant Endocrine Therapy for Breast Cancer: A Theory-Based Qualitative Study to Guide the Development of Community Pharmacist Interventions. Pharmacy. 2018;6:53. doi: 10.3390/pharmacy6020053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gong X.-H., Wang J.-W., Li J., Chen X.-F., Sun L., Yuan Z.-P., Yu J. Physical exercise, vegetable and fruit intake and health-related quality of life in Chinese breast cancer survivors: A cross-sectional study. Qual. Life Res. 2017;26:1541–1550. doi: 10.1007/s11136-017-1496-6. [DOI] [PubMed] [Google Scholar]
- 16.Inoue-Choi M., Lazovich D., Prizment A.E., Robien K. Adherence to the World Cancer Research Fund/American Institute for Cancer Research Recommendations for Cancer Prevention Is Associated with Better Health-Related Quality of Life Among Elderly Female Cancer Survivors. J. Clin. Oncol. 2013;31:1758–1766. doi: 10.1200/JCO.2012.45.4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim N.-H., Lee J.E., Jung S.-Y., Lee E., Kim Z., Moon H.-G., Noh D.-Y., Lee J.E. Dietary pattern and health-related quality of life among breast cancer survivors. BMC Women’s Health. 2018;18:1–10. doi: 10.1186/s12905-018-0555-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galilea-Zabalza I., Buil-Cosiales P., Salas-Salvadó J., Toledo E., Ortega-Azorín C., Díez-Espino J., Vázquez-Ruiz Z., Zomeño M.D., Vioque J., Martínez J.A., et al. Mediterranean diet and quality of life: Baseline cross-sectional analysis of the PREDIMED-PLUS trial. PLoS ONE. 2018;13:e0198974. doi: 10.1371/journal.pone.0198974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duncan M., Moschopoulou E., Herrington E., Deane J., Roylance R., Jones L., Bourke L., Morgan A., Chalder T., A Thaha M., et al. Review of systematic reviews of non-pharmacological interventions to improve quality of life in cancer survivors. BMJ Open. 2017;7:e015860. doi: 10.1136/bmjopen-2017-015860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mirandola D., Miccinesi G., Muraca M.G., Sgambati E., Monaci M., Marini M. Evidence for Adapted Physical Activity as an Effective Intervention for Upper Limb Mobility and Quality of Life in Breast Cancer Survivors. J. Phys. Act. Health. 2014;11:814–822. doi: 10.1123/jpah.2012-0119. [DOI] [PubMed] [Google Scholar]
- 21.Zhu G., Zhang X., Wang Y., Xiong H., Zhao Y., Sun F. Effects of exercise intervention in breast cancer survivors: A meta-analysis of 33 randomized controlled trails. OncoTargets Ther. 2016;9:2153–2168. doi: 10.2147/OTT.S97864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rastelli A.L., Taylor M.E., Gao F., Armamento-Villareal R., Jamalabadi-Majidi S., Napoli N., Ellis M. Vitamin D and aromatase inhibitor-induced musculoskeletal symptoms (AIMSS): A phase II, double-blind, placebo-controlled, randomized trial. Breast Cancer Res. Treat. 2011;129:107–116. doi: 10.1007/s10549-011-1644-6. [DOI] [PubMed] [Google Scholar]
- 23.Augustin L.S.A., Libra M., Crispo A., Grimaldi M., De Laurentiis M., Rinaldo M., D’Aiuto M., Catalano F., Banna G.L., Ferraù F., et al. Low glycemic index diet, exercise and vitamin D to reduce breast cancer recurrence (DEDiCa): Design of a clinical trial. BMC Cancer. 2017;17:1–13. doi: 10.1186/s12885-017-3064-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tudor-Locke C., Bassett D.R. How many steps/day are enough? Preliminary pedometer indices for public health. Sports Med. 2004;2004. 34:1–8. doi: 10.2165/00007256-200434010-00001. [DOI] [PubMed] [Google Scholar]
- 25.Schröder H., Fitó M., Estruch R., Martínez-González M.A., Corella D., Salas-Salvadó J., Lamuela-Raventós R., Ros E., Salaverría I., Fiol M., et al. A Short Screener Is Valid for Assessing Mediterranean Diet Adherence among Older Spanish Men and Women. J. Nutr. 2011;141:1140–1145. doi: 10.3945/jn.110.135566. [DOI] [PubMed] [Google Scholar]
- 26.Martinez-Gonzalez M.A., Garcia-Arellano A., Toledo E., Salas-Salvado J., Buil-Cosiales P., Corella D., Covas M.I., Schroder H., Aros F., Gomez-Gracia E., et al. A 14-item Mediterranean diet assessment tool and obesity indexes among high-risk subjects: The PREDIMED trial. PLoS ONE. 2012;7:e43134. doi: 10.1371/journal.pone.0043134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Devlin N., Brooks R. EQ-5D and the EuroQol Group: Past, Present and Future. Appl. Health Econ. Health Policy. 2017;15:127–137. doi: 10.1007/s40258-017-0310-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aaronson N.K., Ahmedzai S., Bergman B., Bullinger M., Cull A., Duez N.J., Filiberti A., Flechtner H., Fleishman S.B., De Haes J.C., et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A Quality-of-Life Instrument for Use in International Clinical Trials in Oncology. J. Natl. Cancer Inst. 1993;85:365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 29.Scalone L., Cortesi P.A., Ciampichini R., Belisari A., D’Angiolella L.S., Cesana G., Mantovani L.G. Italian Population-Based Values of EQ-5D Health States. Value Health. 2013;16:814–822. doi: 10.1016/j.jval.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 30.Fayers P., Aaronson N.K., Bjordal K., Groenvold M., Curran D., Bottomley A. EORTC QLQ-C30 Scoring Manual. 3rd ed. European Organisation for Research and Treatment of Cancer; Brussels, Belgium: 2001. [Google Scholar]
- 31.Swisher A.K., Abraham J., Bonner D., Gilleland D., Hobbs G., Kurian S., Yanosik M.A., Vona-Davis L. Exercise and dietary advice intervention for survivors of triple-negative breast cancer: Effects on body fat, physical function, quality of life, and adipokine profile. Support. Care Cancer. 2015;23:2995–3003. doi: 10.1007/s00520-015-2667-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Makari-Judson G., Braun B., Jerry D.J., Mertens W.C. Weight gain following breast cancer diagnosis: Implication and proposed mechanisms. World J. Clin. Oncol. 2014;5:272–282. doi: 10.5306/wjco.v5.i3.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koh D., Song S., Moon S.-E., Cho J., Yoo Y.B., Lee S.K., Lee J.E. Abstract 5061: Adherence to guidelines for cancer survivors and health-related quality of life among Korean breast cancer survivors. Epidemiology. 2019;7:10307–10319. doi: 10.1158/1538-7445.am2019-5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zick S., Colacino J.A., Cornellier M., Khabir T., Surnow K., Djuric Z. Fatigue reduction diet in breast cancer survivors: A pilot randomized clinical trial. Breast Cancer Res. Treat. 2017;161:299–310. doi: 10.1007/s10549-016-4070-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Groenvold M., Petersen M.A., Idler E., Bjorner J.B., Fayers P.M., Mouridsen H.T. Psychological distress and fatigue predicted recurrence and survival in primary breast cancer patients. Breast Cancer Res. Treat. 2007;105:209–219. doi: 10.1007/s10549-006-9447-x. [DOI] [PubMed] [Google Scholar]
- 36.Cleeland C.S., Bennett G.J., Dantzer R., Dougherty P.M., Dunn A.J., Meyers C.A., Miller A.H., Payne R., Reuben J.M., Wang X.S., et al. Are the symptoms of cancer and cancer treatment due to a shared biologic mechanism? A cytokine-immunologic model of cancer symptoms. Cancer. 2003;97:2919–2925. doi: 10.1002/cncr.11382. [DOI] [PubMed] [Google Scholar]
- 37.Orchard T., Andridge R.R., Yee L.D., Lustberg M. Diet Quality, Inflammation, and Quality of Life in Breast Cancer Survivors: A Cross-Sectional Analysis of Pilot Study Data. J. Acad. Nutr. Diet. 2018;118:578–588. doi: 10.1016/j.jand.2017.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sofi F., Abbate R., Gensini G.F., Casini A. Accruing evidence on benefits of adherence to the Mediterranean diet on health: An updated systematic review and meta-analysis. Am. J. Clin. Nutr. 2010;92:1189–1196. doi: 10.3945/ajcn.2010.29673. [DOI] [PubMed] [Google Scholar]
- 39.Eleftheriou D., Benetou V., Trichopoulou A., La Vecchia C., Bamia C. Mediterranean diet and its components in relation to all-cause mortality: Meta-analysis. Br. J. Nutr. 2018;120:1081–1097. doi: 10.1017/s0007114518002593. [DOI] [PubMed] [Google Scholar]
- 40.Dinu M., Pagliai G., Casini A., Sofi F. Mediterranean diet and multiple health outcomes: An umbrella review of meta-analyses of observational studies and randomised trials. Eur. J. Clin. Nutr. 2018;72:30–43. doi: 10.1038/ejcn.2017.58. [DOI] [PubMed] [Google Scholar]
- 41.Runowicz C.D., Leach C.R., Henry N.L., Henry K.S., Mackey H.T., Cowens-Alvarado R.L., Cannady R.S., Pratt-Chapman M.L., Edge S.B., Jacobs L.A., et al. American Cancer Society/American Society of Clinical Oncology Breast Cancer Survivorship Care Guideline. J. Clin. Oncol. 2016;34:611–635. doi: 10.1200/jco.2015.64.3809. [DOI] [PubMed] [Google Scholar]
- 42.Penttinen H.M., Saarto T., Kellokumpu-Lehtinen P., Blomqvist C., Huovinen R., Kautiainen H., Järvenpää S., Nikander R., Idman I., Luoto R., et al. Quality of life and physical performance and activity of breast cancer patients after adjuvant treatments. Psycho Oncol. 2010;20:1211–1220. doi: 10.1002/pon.1837. [DOI] [PubMed] [Google Scholar]
- 43.Mustian K.M., Alfano C.M., Heckler C., Kleckner A.S., Kleckner I.R., Leach C.R., Mohr D., Palesh O.G., Peppone L.J., Piper B.F., et al. Comparison of Pharmaceutical, Psychological, and Exercise Treatments for Cancer-Related Fatigue: A Meta-analysis. JAMA Oncol. 2017;3:961–968. doi: 10.1001/jamaoncol.2016.6914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Andersen M.R., Sweet E., Hager S., Gaul M., Dowd F., Standish L.J. Effects of Vitamin D Use on Health-Related Quality of Life of Breast Cancer Patients in Early Survivorship. Integr. Cancer Ther. 2019;18:1534735418822056. doi: 10.1177/1534735418822056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Khan Q.J., Reddy P.S., Kimler B.F., Sharma P., Baxa S.E., O’Dea A.P., Klemp J.R., Fabian C.J. Effect of vitamin D supplementation on serum 25-hydroxy vitamin D levels, joint pain, and fatigue in women starting adjuvant letrozole treatment for breast cancer. Breast Cancer Res. Treat. 2009;119:111–118. doi: 10.1007/s10549-009-0495-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Waltman N.L., Ott C.D., Twiss J.J., Gross G.J., Lindsey A.M. Vitamin D Insufficiency and Musculoskeletal Symptoms in Breast Cancer Survivors on Aromatase Inhibitor Therapy. Cancer Nurs. 2009;32:143–150. doi: 10.1097/01.NCC.0000339262.44560.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Laroche F., Perrot S., Medkour T., Cottu P.-H., Pierga J.-Y., Lotz J.-P., Beerblock K., Tournigand C., Chauvenet L., Bouhassira D., et al. Quality of life and impact of pain in women treated with aromatase inhibitors for breast cancer. A multicenter cohort study. PLoS ONE. 2017;12:e0187165. doi: 10.1371/journal.pone.0187165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meggetto O., Maunsell E., Chlebowski R., Goss P., Tu D., Richardson H. Factors Associated With Early Discontinuation of Study Treatment in the Mammary Prevention.3 Breast Cancer Chemoprevention Trial. J. Clin. Oncol. 2017;35:629–635. doi: 10.1200/JCO.2016.68.8895. [DOI] [PubMed] [Google Scholar]
- 49.Adami G., Saag K. Osteoporosis Pathophysiology, Epidemiology, and Screening in Rheumatoid Arthritis. Curr. Rheumatol. Rep. 2019;21:34. doi: 10.1007/s11926-019-0836-7. [DOI] [PubMed] [Google Scholar]
- 50.Niravath P. Aromatase inhibitor-induced arthralgia: A review. Ann. Oncol. 2013;24:1443–1449. doi: 10.1093/annonc/mdt037. [DOI] [PubMed] [Google Scholar]
- 51.Ilie G., Bradfield J., Moodie L., Lawen T., Ilie A., Lawen Z., Blackman C., Gainer R., Rutledge R.D.H. The Role of Response-Shift in Studies Assessing Quality of Life Outcomes among Cancer Patients: A Systematic Review. Front. Oncol. 2019;9:783. doi: 10.3389/fonc.2019.00783. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.