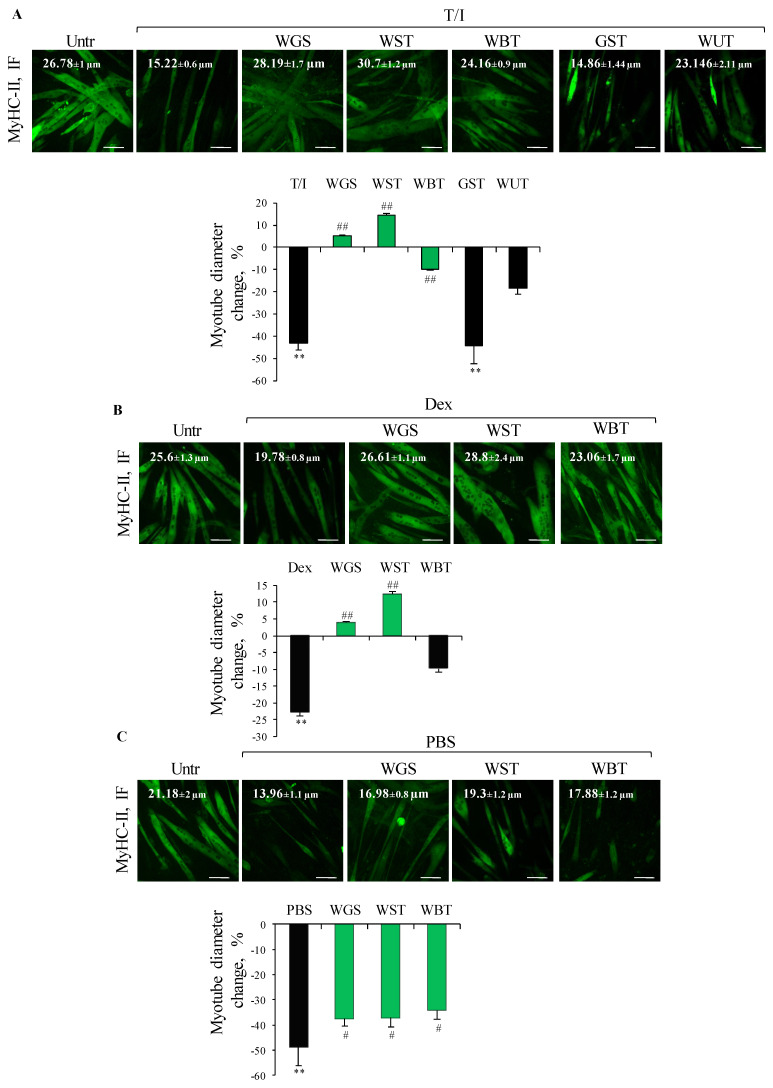
Figure 3.
(A–C) The best formulations were tested (100 µg/mL) on C2C12 myotubes treated or not with TNFα (tumor necrosis factor α, 20 ng/mL)/IFNγ (interferon γ, 100 U/mL) (T/I) or dexamethasone (Dex, 1 µM), or starved with PBS (phosphate buffered saline). After 48 h or 16 h (PBS), immunofluorescence (IF) staining for myosin heavy chain (MyHC)-II was performed and myotube diameters were measured. Reported are representative images with myotube diameters (µm) and the percent changes of myotube diameters with respect to untreated control. The green bars represent the formulations able to protect against myotube atrophy induced by different stimuli. Results are means ± SEM (standard error of the mean) (A–C). Statistical analysis was conducted using t-test ** p < 0.01 significantly different from untreated control; # p < 0.05 and ## p < 0.01 significantly different from T/I (A), Dex (B) or PBS (C). Bars, 100 µm.