Abstract
Propyl-propane thiosulfinate (PTS) and propyl-propane thiosulfonate (PTSO) are two volatile compounds derived from Allium cepa with a widely documented antimicrobial activity. The aim of this study was to evaluate their anti-candidiasis activity and the ability of its gaseous phase to inhibit bacterial and yeast growth in vitro. The minimum inhibitory concentration of various antifungal products (including PTS and PTSO) was determined versus 203 clinical isolates of Candida spp. through broth microdilution assay. Additionally, the antimicrobial activity through aerial diffusion of PTS and PTSO was evaluated over the growth of a collection of bacteria and yeasts cultivated in agar plates. All yeasts were susceptible to the antifungals tested, except C. glabrata and C. krusei, that showed azole resistance. PTSO (MIC50 and MIC90 ranged from 4 to 16 mg/L and 8 to 32 mg/L, respectively) was significantly more active against yeasts than PTS (MIC50 and MIC90 ranged from 16 to 64 mg/L and 32 to 64 mg/L). Values were higher than those obtained for antifungal drugs. Gaseous phases of PTS and PTSO generated growth inhibition zones whose diameters were directly related to the substances concentration and inversely related to the microbial inoculum. The quantification of PTS and PTSO levels reached in the growth media through aerial diffusion displayed a concentration gradient from the central zone to the periphery. Only P. aeruginosa ATCC 27853 showed resistance, while yeasts (C. albicans ATCC 200955 and C. krusei ATCC 6258) presented the higher susceptibility to both compounds. These results suggest that PTS and PTSO display antibacterial and anti-candidiasis activity in vitro through aerial diffusion, having potential use in human therapy.
Keywords: propyl-propane-thiosulfinate, propyl-propane-thiosulfonate, antibacterial activity, antifungal activity, vapor
1. Introduction
In recent years, the antioxidative, hypolipidemic, hypocholesterolemia, antihypertensive, heart-protective, antithrombotic, anticancer, anti-inflammatory, immunomodulatory, and antimicrobial activities of different organosulfur products, such as thiosulfinates and thiosulfonates obtained from garlic (Allium sativum) and onion (Allium cepa), especially allicin (diallyl thiosulfinate), have been thoroughly studied [1,2,3].
While the precise mechanism of action has not yet been discovered, the main antimicrobial effect of these Allium-derived compounds has been reported to be due to its chemical reaction with thiol groups present in the main enzymes of the microbial metabolism, such as succinate dehydrogenase, alcohol dehydrogenase, thioredoxin reductase and ureases among others, via thiol-disulfide exchange reaction [4,5,6]. Additionally, they can react with thiols, such as glutathione, increasing the oxidized glutathione rate into a range that induces oxidative stress and cellular apoptosis [7]; they can interact with enzymes taking part of the microbial system of acetyl-CoA blocking the incorporation of acetate into fatty acids and inhibiting the development of a phospholipid bilayer of the membranes [5]; and they can inhibit RNA polymerase and, therefore, block the total synthesis of microbial RNA [8].
In onion, the most common sulfur compounds are isoalliin (S-propenyl-l-cysteine sulfoxide), methiin (S-methyl-l-cysteine sulfoxide) and propiin (S-propyl-l-cysteine sulfoxide). Propiin changes into propyl-propane thiosulfinate (PTS) due to the action of aliinase [9]. Although it is more stable than allicin, PTS is also a labile compound that, through dismutation or disproportionation reactions, changes into dipropyl disulfide and propyl-propane thiosulfonate (PTSO) [10].
In a previous study we compared the in vitro antibacterial activity of PTS and PTSO with other antibiotics. Both molecules showed broad spectrum antibacterial activity against multiresistant bacteria isolated from human clinical samples. These results contribute to the development and potential use of these compounds against human infections, such as oral, gastrointestinal, or skin infections, as well as for the treatment of urinary tract infections [11].
Candiduria is a common finding in immunosuppressed and hospitalized patients, which determines the clinical relevance of its detection and treatment. In recent decades, a remarkable increase of opportunistic candidiasis infections has been described, especially those affecting urinary tract produced by C. albicans, the most common among them. Nevertheless, an increase in the incidence of other different species of Candida has been described, some of them characterized by a higher resistance to the most common antifungals used in humans [12,13]. Therefore, in the same way as with bacteria, it would be interesting to compare the in vitro anticandidal activity of PTS and PTSO with that of other existing antifungals for potential application in medical therapy.
Both substances are volatile. Therefore, the study of their antimicrobial capacity through their gaseous phase would have great interest for the valorization of their possible use in the treatment of susceptible respiratory pathogens if PTS and PTSO reach appropriate concentrations in pulmonary epithelium administered by inhalation [3,14].
For all these reasons, the aim of this study was to evaluate anti-candidiasis activity of PTS and PTSO in vitro and the capacity of its gaseous phase to inhibit the growth of different bacteria and yeasts.
2. Results
2.1. Antifungal Susceptibility
The identification of the 203 clinical isolates of Candida spp. was as follows: C. albicans (n = 83), C. glabrata (n = 73), C. krusei (n = 12) and C. tropicalis (n = 35). A summary of the antifungal susceptibility data is presented in Table 1. All the isolates of C. glabrata and C. krusei were resistant to fluconazole. C. glabrata was also resistant to voriconazole. In the other cases, the isolates were susceptible to the assayed antifungals. Amphotericin B significantly showed more activity against C. albicans and C. tropicalis than against the two other species (p < 0.001), while C. glabrata was the species with the lowest MIC values (p < 0.001) to echinocandins (anidulafungin, micafungin, and caspofungin).
Table 1.
In vitro activity of different antifungal drugs, PTS, and PTSO against Candida spp.
| Species of Yeasts (Number of Isolates) | Range of MIC (in mg/L) |
MIC50
(in mg/L) |
MIC90
(in mg/L) |
Range of MFC (in mg/L) |
MFC50 (in mg/L) |
MFC90 (in mg/L) |
Number of Resistant Isolates |
|---|---|---|---|---|---|---|---|
| Candida albicans (n = 83) | |||||||
| Amphotericin B | 0.125–0.25 | 0.25 | 0.25 | 1–16 | 4 | 8 | 0 |
| Anidulafungin | ≤0.008–0.25 | 0.015 | 0.06 | 0.015–>4 | 0.25 | 1 | 0 |
| Micafungin | ≤0.008–0.25 | 0.03 | 0.125 | 0.03–4 | 0.5 | 2 | 0 |
| Caspofungin | ≤0.008–0.25 | 0.06 | 0.06 | 0.06–4 | 0.5 | 2 | 0 |
| Fluconazole | ≤0.125–2 | 0.25 | 2 | 8–>64 | >64 | >64 | 0 |
| Voriconazole | ≤0.008–0.06 | ≤0.008 | ≤0.008 | 0.03–0.5 | 0.5 | 0.5 | 0 |
| PTS | 16–64 | 32 | 32 | 16–>128 | 32 | 128 | - |
| PTSO | 8–64 | 16 | 32 | 8–128 | 32 | 64 | - |
| Candida glabrata (n = 73) | |||||||
| Amphotericin B | 0.06–1 | 0.25 | 0.5 | 1–>16 | 4 | 8 | 0 |
| Anidulafungin | ≤0.008–0.125 | 0.015 | 0.03 | 0.06–>4 | 0.25 | 0.5 | 0 |
| Micafungin | ≤0.008–0.06 | ≤0.008 | 0.03 | 0.008–4 | 0.06 | 0.25 | 0 |
| Caspofungin | ≤0.008–0.125 | 0.03 | 0.03 | 0.06–4 | 1 | 2 | 0 |
| Fluconazole | 0.25–≥64 | 4 | ≥64 | 16–>64 | >64 | >64 | 73 |
| Voriconazole | 0.03–≥4 | 0.5 | ≥4 | 2–>4 | >4 | >4 | 73 |
| PTS | 8–64 | 32 | 32 | 16–>128 | 64 | 128 | - |
| PTSO | 16–32 | 16 | 32 | 8–>128 | 32 | 64 | - |
| Candida krusei (n = 12) | |||||||
| Amphotericin B | 0.25–1 | 0.5 | 1 | 4–16 | 8 | 16 | 0 |
| Anidulafungin | ≤0.008–0.06 | 0.015 | 0.03 | 0.125–0.5 | 0.25 | 0.5 | 0 |
| Micafungin | 0.06–0.25 | 0.125 | 0.125 | 0.5–4 | 0.5 | 1 | 0 |
| Caspofungin | 0.06–0.25 | 0.125 | 0.25 | 1–4 | 1 | 2 | 0 |
| Fluconazole | 8–≥64 | 16 | ≥64 | >64 | >64 | >64 | 12 |
| Voriconazole | 0.03–0.5 | 0.125 | 0.5 | 2–>4 | >4 | >4 | 0 |
| PTS | 4–32 | 16 | 32 | 32–>128 | 32 | 128 | - |
| PTSO | 4–16 | 4 | 8 | 64–>128 | 128 | 128 | - |
| Candida tropicalis (n = 35) | |||||||
| Amphotericin B | ≤0.03–1 | 0.125 | 0.25 | 2–16 | 2 | 8 | 0 |
| Anidulafungin | ≤0.008–0.25 | 0.06 | 0.25 | 0.015–2 | 1 | 2 | 0 |
| Micafungin | ≤0.008–0.25 | 0.06 | 0.25 | 0.008–2 | 0.5 | 2 | 0 |
| Caspofungin | ≤0.008–0.25 | 0.06 | 0.25 | 0.06–4 | 1 | 4 | 0 |
| Fluconazole | ≤0.125–2 | ≤0.125 | 1 | 8–>64 | >64 | >64 | 0 |
| Voriconazole | ≤0.008–0.06 | ≤0.008 | 0.015 | 0.03–0.5 | 0.5 | 0.5 | 0 |
| PTS | 32–64 | 64 | 64 | 64–>128 | 128 | 128 | - |
| PTSO | 4–32 | 16 | 32 | 4–128 | 32 | 64 | - |
MIC: minimum inhibitory concentration. MFC: minimum fungicidal concentration.
The behavior of PTS and PTSO was quite homogeneous, regardless of the analyzed species (Table 1). MIC50 and MIC90 values of PTS ranged from 16 to 64 mg/L for the first and from 32 to 64 mg/L for the second. The MFC50 and MFC90 ranged from 32 to 128 mg/L and 128 mg/L, respectively. On the other hand, the values of MIC50 and MIC90 of PTSO ranged from 4 to 16 mg/L and 8 to 32 mg/L, while MFC50 y MFC90 ranged from 32 to 128 mg/L and from 64 to 128 mg/L, respectively. Considering all of the clinical isolates of Candida spp., the data indicated that PTSO was significantly more active than PTS (p < 0.001) and showed the fungicidal activity of these compounds since MIC and MFC values differed in only one dilution for both PTS (MIC50 = 32 mg/L and MFC50 = 64 mg/L; MIC90 = 64 mg/L and MFC90 = 128 mg/L) and PTSO (MIC50 = 16 mg/L and MFC50 = 32 mg/L; MIC90 = 32 mg/L and MFC90 = 64 mg/L).
2.2. Antimicrobial Activity of Vapor
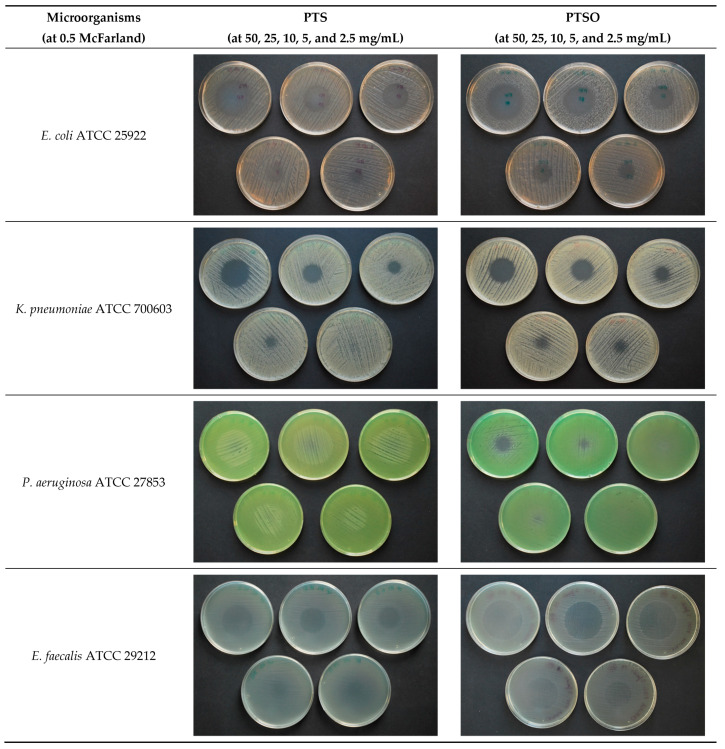
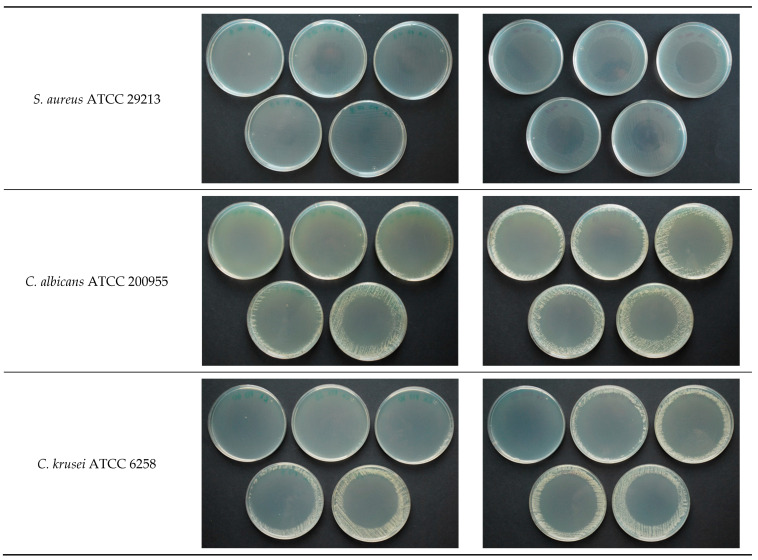
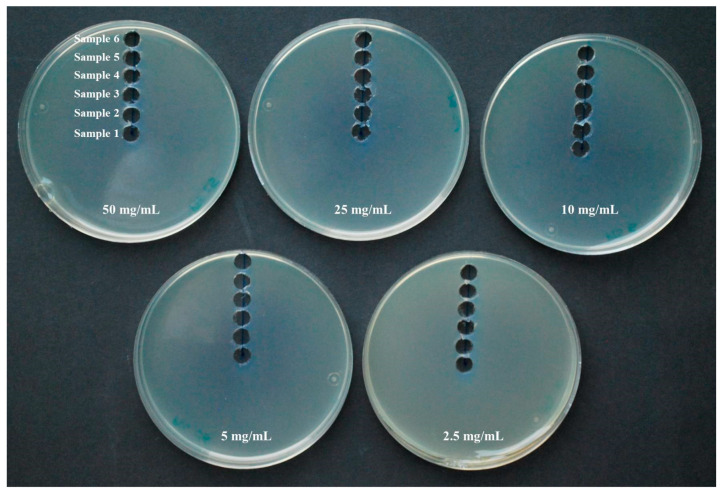
PTS and PTSO inhibited growth in six of the seven microorganisms tested in the present study through its gaseous phase without coming into contact with the medium and thus, with the microorganism, except for its aerial diffusion. The vapor produced by both substances reaches the agar medium, inhibiting microbial growth in a circular area above the drop placed in the lid of the Petri dish (Figure 1). The absence of any microbial growth in the inhibition area suggests a predominant biocidal effect of these substances. Only P. aeruginosa ATCC 27853 showed resistance to both compounds, showing absence of inhibition halo in the majority of the concentrations and bacterial inocula used. In the case of this bacterium, halos with a diameter below 10 mm were observed only when PTSO was used at a concentration of 50 mg/mL and 25 mg/mL against a bacterial inoculum at 0.5 of McFarland scale.
Figure 1.
Antimicrobial activity of PTS and PTSO via the gas phase. Photograph showing halos of inhibition at doses of 50 mg/mL, 25 mg/mL, 10 mg/mL, 5 mg/mL, and 2.5 mg/mL (from left to right and from top to bottom). Although assays were carried out at 0.5, 1, and 2 McFarland turbidity, only the results at 0.5 McFarland are shown.
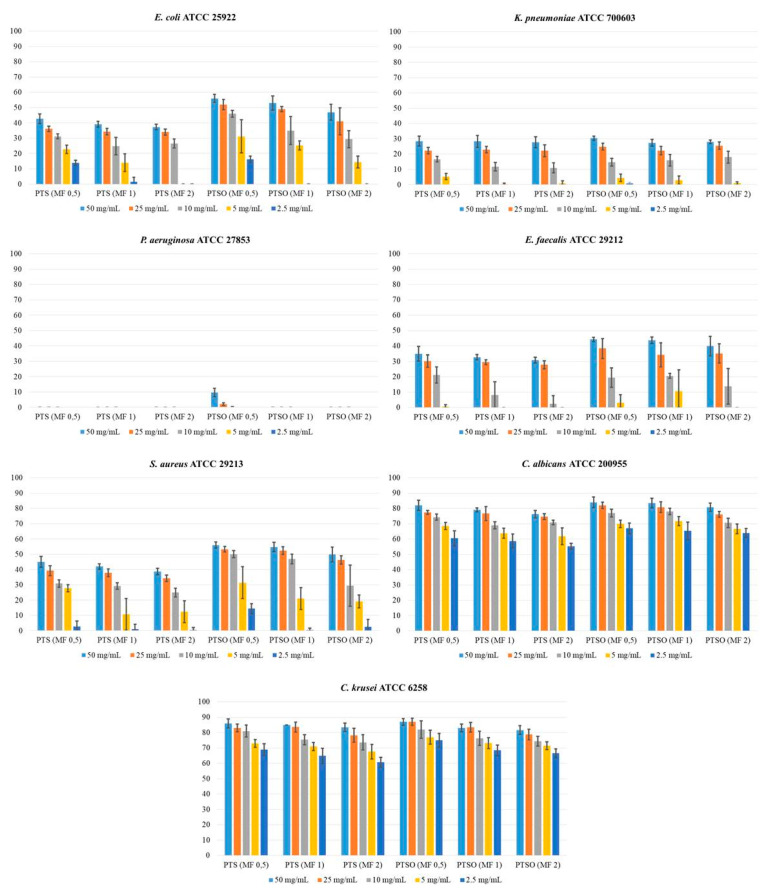
As it is shown in Figure 2 and Table 2, diameters from the inhibition of growth zones were directly related to the concentration of PTS or PTSO used, and inversely related to the microbial inoculum used: the higher the concentration of PTS or PTSO were and the lower the inoculum of microorganisms were, the larger the diameter of the halo was (p < 0.001). Greater halos and, consequently an increased susceptibility to these compounds were observed in the case of yeasts (C. albicans ATCC 200955 and C. krusei ATCC 6258).
Figure 2.
Average value ± standard deviation (in mm) of the growth inhibition halos for each concentration of PTS and PTSO, in the different inocula (0.5, 1, and 2 of McFarland) and for the different microorganisms. Diameters from the inhibition of growth zones were directly related to the concentration of PTS or PTSO used, and inversely related to the microbial inoculum used. Greater halos were observed in the case of yeasts. The diameters of the growth inhibition halos produced via the gas phase of PTSO were significantly larger than those obtained from PTS.
Table 2.
Average diameter ± standard deviation (in mm) of the growth inhibition halos for the different PTS and PTSO concentrations, in each inoculum (0.5, 1, and 2 from McFarland) and for the different microorganisms.
| Microorganisms | McFarland | PTS Concentration (Growth Inhibition in mm) |
PTSO Concentration (Growth Inhibition in mm) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 50 mg/mL | 25 mg/mL | 10 mg/m L | 5 mg/mL | 2.5 mg/mL | 50 mg/mL | 25 mg/mL | 10 mg/mL | 5 mg/mL | 2.5 mg/mL | ||
| E. coli ATCC 25922 | 0.5 | 43 ± 3.2 | 36 ± 1.9 | 31 ± 1.6 | 23 ± 2.7 | 14 ± 1.6 | 56 ± 2.7 | 52 ± 3.3 | 46 ± 2.2 | 31 ± 10.8 | 16 ± 2.1 |
| 1 | 39 ± 2.0 | 34 ± 2.1 | 25 ± 5.6 | 14 ± 5.7 | 1 ± 3.0 | 53 ± 4.5 | 49 ± 1.8 | 35 ± 9.2 | 25 ± 3.0 | 0 | |
| 2 | 37 ± 1.8 | 34 ± 2.0 | 27 ± 3.0 | 0 | 0 | 47 ± 5.1 | 42 ± 8.8 | 29 ± 5.5 | 14 ± 3.9 | 0 | |
| K. pneumoniae ATCC 700603 | 0.5 | 28 ± 3.3 | 22 ± 2.2 | 17 ± 1.8 | 5 ± 2.0 | 0 | 30 ± 1.3 | 25 ± 2.2 | 15 ± 2.5 | 5 ± 2.5 | 1 ± 0.7 |
| 1 | 28 ± 3.9 | 23 ± 2.2 | 12 ± 2.8 | 0 | 0 | 27 ± 2.3 | 22 ± 2.7 | 16 ± 3.8 | 3 ± 2.7 | 0 | |
| 2 | 28 ± 3.6 | 22 ± 3.8 | 11 ± 3.3 | 1 ± 1.8 | 0 | 28 ± 1.2 | 26 ± 2.4 | 18 ± 3.9 | 1 ± 0.9 | 0 | |
| P. aeruginosa ATCC 27853 | 0.5 | 0 | 0 | 0 | 0 | 0 | 10 ± 2.7 | 2 ± 0.9 | 0 | 0 | 0 |
| 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| E. faecalis ATCC 29212 | 0.5 | 35 ± 4.6 | 30 ± 4.0 | 21 ± 5.2 | 1 ± 1.2 | 0 | 44 ± 1.3 | 38 ± 6.5 | 20 ± 6.3 | 3 ± 5.3 | 0 |
| 1 | 33 ± 1.8 | 30 ± 1.6 | 8 ± 8.7 | 0 | 0 | 44 ± 2.1 | 34 ± 7.8 | 21 ± 1.6 | 11 ± 14.0 | 0 | |
| 2 | 31 ± 1.9 | 28 ± 2.7 | 3 ± 5.3 | 0 | 0 | 40 ± 6.3 | 35 ± 6.2 | 14 ± 11.6 | 0 | 0 | |
| S. aureus ATCC 29213 | 0.5 | 45 ± 3.6 | 39 ± 3.3 | 31 ± 2.4 | 28 ± 2.4 | 3 ± 3.5 | 56 ± 2.0 | 53 ± 1.8 | 50 ± 2.3 | 31 ± 10.4 | 14 ± 3.3 |
| 1 | 42 ± 1.8 | 38 ± 2.5 | 29 ± 2.0 | 11 ± 10.3 | 1 ± 3.2 | 55 ± 3.1 | 52 ± 2.5 | 47 ± 3.2 | 21 ± 7.1 | 0 | |
| 2 | 39 ± 2.1 | 34 ± 2.1 | 25 ± 2.8 | 12 ± 7.1 | 1 ± 1.6 | 50 ± 4.9 | 46 ± 2.7 | 29 ± 13.5 | 19 ± 4.3 | 3 ± 4.8 | |
| C. albicans ATCC 200955 | 0.5 | 82 ± 3.4 | 77 ± 1.2 | 74 ± 1.9 | 69 ± 2.3 | 61 ± 4.9 | 84 ± 3.4 | 82 ± 2.2 | 77 ± 2.5 | 70 ± 2.4 | 67 ± 3.5 |
| 1 | 79 ± 1.3 | 77 ± 4.5 | 69 ± 2.3 | 64 ± 3.3 | 59 ± 4.5 | 84 ± 3.2 | 81 ± 3.4 | 78 ± 2.1 | 72 ± 3.0 | 65 ± 5.8 | |
| 2 | 76 ± 2.5 | 75 ± 1.9 | 71 ± 1.5 | 62 ± 5.5 | 55 ± 1.9 | 81 ± 2.8 | 76 ± 1.9 | 71 ± 3.0 | 67 ± 3.2 | 64 ± 2.9 | |
| C. krusei ATCC 6258 | 0.5 | 86 ± 3.0 | 83 ± 2.5 | 81 ± 3.9 | 73 ± 2.5 | 69 ± 3.9 | 87 ± 2.1 | 87 ± 2.3 | 82 ± 5.6 | 77 ± 4.5 | 75 ± 4.5 |
| 1 | 85 ± 0.0 | 84 ± 3.3 | 75 ± 3.2 | 71 ± 2.6 | 65 ± 5.0 | 83 ± 2.5 | 84 ± 3.2 | 76 ± 4.7 | 73 ± 3.6 | 69 ± 3.4 | |
| 2 | 84 ± 2.7 | 78 ± 4.5 | 74 ± 4.9 | 68 ± 4.7 | 61 ± 3.2 | 82 ± 2.8 | 79 ± 3.5 | 75 ± 3.2 | 72 ± 2.5 | 67 ± 2.8 | |
Considering the set of microorganisms used in this study (with the exception of P. aeruginosa ATCC 27853 due to the demonstrated resistance to both compounds), the antimicrobial activity of PTSO was significantly higher than that of PTS, since, for the same microbial inoculum and substance concentration, the diameters of the growth inhibition halos produced by the gas phase of PTSO were significantly larger than those obtained from PTS (p < 0.01, in all cases).
Table 3 shows the concentration of PTS and PTSO detected in the Mueller–Hinton medium by HPLC-UV. The gas phase of both compounds, deposited on the lid of the Petri dish, reached the culture medium being able to be detected and quantified. The highest concentration in all cases was reached in sample 1, which coincided with the center of the plate, located in the most vertical position just above the drop deposited on the lid. As the sample moved away from the center (samples from 2 to 6), the concentration decreased, creating a gradient from the central area of the plate (highest concentration) towards its periphery (lowest concentration). In some cases, either because the concentration of the drop was small (2.5 mg/mL) and/or because the distance to the center was higher (sample 6), the concentration reached in the medium could not be quantified because it was below the limit of detection of the HPLC-UV technique.
Table 3.
Concentration ± standard deviation (in mg/L) of PTS and PTSO reached in Mueller–Hinton agar for each of the samples, in relation to the initial concentration of each substance in the drop deposited on the lid of the Petri dish.
| Organosulfur Compound | Initial Concentration | Sample 1 | Sample 2 | Sample 3 | Sample 4 | Sample 5 | Sample 6 |
|---|---|---|---|---|---|---|---|
| PTS | 50 mg/mL | 82.7 ± 0.1 | 70.8 ± 0.3 | 51.2 ± 0.1 | 30.2 ± 0.2 | 17.0 ± 0.3 | 9.4 ± 0.1 |
| 25 mg/mL | 68.6 ± 0.2 | 59.6 ± 0.1 | 42.4 ± 0.3 | 25.1 ± 0.2 | 12.4 ± 0.1 | 5.8 ± 0.2 | |
| 10 mg/mL | 57.3 ± 0.2 | 48.6 ± 0.1 | 35.5 ± 0.1 | 22.1 ± 0.2 | 4.2 ± 0.1 | <LD | |
| 5 mg/mL | 48.5 ± 0.2 | 36.4 ± 0.2 | 28.6 ± 0.3 | 8.5 ± 0.2 | <LD | <LD | |
| 2.5 mg/mL | <LD | <LD | <LD | <LD | <LD | <LD | |
| PTSO | 50 mg/mL | 28.6 ± 0.2 | 26.3 ± 0.2 | 20.9 ± 0.2 | 14.8 ± 0.1 | 9.9 ± 0.4 | 4.2 ± 0.2 |
| 25 mg/mL | 23.4 ± 0.2 | 18.6 ± 0.2 | 14.8 ± 0.2 | 12.7 ± 0.2 | 8.2 ± 0.2 | 2.6 ± 0.1 | |
| 10 mg/mL | 18.7 ± 0.1 | 15.8 ± 0.1 | 13.8 ± 0.1 | 12.9 ± 0.3 | 7.5 ± 0.1 | <LD | |
| 5 mg/mL | 17.9 ± 0.1 | 14.8 ± 0.3 | 13.4 ± 0.2 | 7.5 ± 0.1 | 2.8 ± 0.3 | <LD | |
| 2.5 mg/mL | <LD | <LD | <LD | <LD | <LD | <LD |
LD: Limit of detection of the HPLC-UV technique.
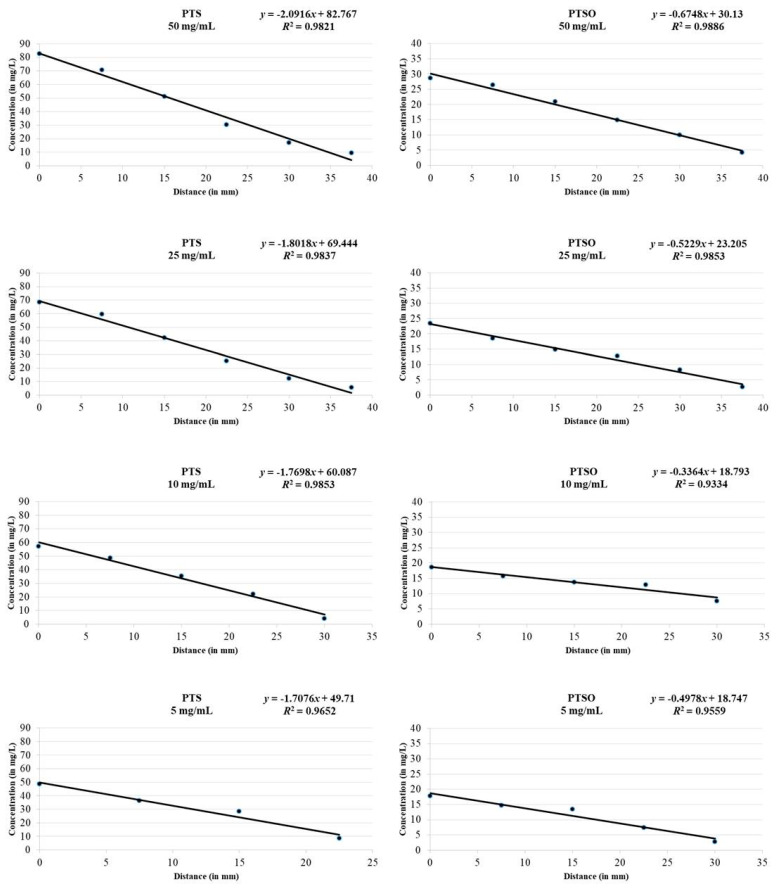
From the results shown in Table 3, the linear regression lines were drawn (Figure 3), relating the distance to the center of the plate (measured in mm) to the concentration that PTS or PTSO reached at a given point (in mg/L) according to each of the concentrations of the drop deposited on the lid. As it can be seen, there was a high correlation in all cases, which determined the quality of the fit.
Figure 3.
Relationship between the distance from the center of the Petri dish and the concentration reached via the gas phase at a defined point of the Mueller–Hinton medium for each concentration of PTS and PTSO deposited on the lid of the Petri dish. There is an inverse linear relationship between the distance to the center of the culture medium and the concentration reached via the gas phase at a given point.
Theoretical concentration of PTS and PTSO reached at the limit of the microbial growth inhibition zone for each microorganism and inoculum concentration is shown in Table 4. This concentration was determined by measuring the radius (in mm) of the inhibition areas obtained for each microorganism and inoculum concentration and extrapolating the results in the linear regression line for the corresponding concentrations of PTS and PTSO showed in Figure 3. The concentrations of PTSO at which microbial growth was inhibited were lower than those of PTS (p < 0.01), showing a greater antimicrobial activity. Furthermore, the inhibition of yeast growth compared to that of the bacteria occurred with lower concentrations, with either of the two compounds.
Table 4.
Average value ± standard deviation (in mg/L) of the concentration of PTS and PTSO reached at the limit of the microbial growth inhibition zone.
| Microorganism | McFarland | Limit of the Microbial Growth | PTS Concentration | PTSO Concentration | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 50 mg/mL | 25 mg/mL | 10 mg/mL | 5 mg/mL | 50 mg/mL | 25 mg/mL | 10 mg/mL | 5 mg/mL | |||
| E. coli ATCC 25922 | 0.5 | Radius | 21.5 | 18 | 15.5 | 11.5 | 28 | 26 | 23 | 15.5 |
| Concentration | 38.1 ± 3.3 | 36.9 ± 1.7 | 32.5 ± 1.4 | 30.3 ± 2.3 | 11.3 ± 0.9 | 9.5 ± 0.9 | 11.1 ± 0.4 | 11.0 ± 2.7 | ||
| 1 | Radius | 19.5 | 17 | 12.5 | 7 | 26.5 | 24.5 | 17.5 | 12.5 | |
| Concentration | 41.9 ± 2.1 | 38.5 ± 1.9 | 38.1 ± 5.0 | 37.8 ± 4.9 | 12.2 ± 1.5 | 10.4 ± 0.5 | 12.9 ± 1.5 | 12.4 ± 0.7 | ||
| 2 | Radius | 18.5 | 17 | 13.5 | 0 | 23.5 | 21 | 14.5 | 7 | |
| Concentration | 43.8 ± 1.8 | 38.8 ± 1.8 | 36.5 ± 2.6 | ≥48.5 | 14.2 ± 1.7 | 12.2 ± 2.3 | 13.8 ± 0.9 | 15.2 ± 1.0 | ||
| K. pneumoniae ATCC 700603 | 0.5 | Radius | 14 | 11 | 8.5 | 2.5 | 15 | 12.5 | 7.5 | 2.5 |
| Concentration | 53.1 ± 3.4 | 49.4 ± 1.9 | 45.3 ± 1.6 | 45.2 ± 1.7 | 19.9 ± 0.5 | 16.7 ± 0.6 | 16.3 ± 0.4 | 17.6 ± 0.6 | ||
| 1 | Radius | 14 | 11.5 | 6 | 0 | 13.5 | 11 | 8 | 1.5 | |
| Concentration | 53.2 ± 4.0 | 48.8 ± 2.0 | 49.6 ± 2.5 | ≥48.5 | 20.9 ± 0.8 | 17.4 ± 0.7 | 16.1 ± 0.6 | 18.0 ± 0.7 | ||
| 2 | Radius | 14 | 11 | 5.5 | 0.5 | 14 | 13 | 9 | 0.5 | |
| Concentration | 53.7 ± 3.7 | 49.4 ± 3.4 | 50.4 ± 2.9 | ≥48,5 | 20.7 ± 0.4 | 16.5 ± 0.6 | 15.8 ± 0.6 | ≥17.9 | ||
| E. faecalis ATCC 29212 | 0.5 | Radius | 17.5 | 15 | 10.5 | 0.5 | 22 | 19 | 10 | 1.5 |
| Concentration | 46.2 ± 4.9 | 42.2 ± 3.6 | 41.3 ± 4.6 | ≥48.5 | 15.2 ± 0.5 | 13.2 ± 1.7 | 15.5 ± 1.1 | 18.0 ± 1.3 | ||
| 1 | Radius | 16.5 | 15 | 4 | 0 | 22 | 17 | 10.5 | 5.5 | |
| Concentration | 48.5 ± 1.8 | 42.8 ± 1.4 | 52.9 ± 7.7 | ≥48.5 | 15.4 ± 0.7 | 14.3 ± 2.0 | 15.3 ± 0.3 | 16.1 ± 3.5 | ||
| 2 | Radius | 15.5 | 14 | 1.5 | 0 | 20 | 17.5 | 7 | 0 | |
| Concentration | 50.5 ± 2.0 | 44.3 ± 2.4 | 57.9 ± 4.7 | ≥48.5 | 16.7 ± 2.1 | 14.0 ± 1.6 | 16.5 ± 2.0 | ≥17.9 | ||
| S. aureus ATCC 29213 | 0.5 | Radius | 22.5 | 19.5 | 15.5 | 14 | 28 | 26.5 | 25 | 15.5 |
| Concentration | 36.1 ± 3.7 | 34.0 ± 2.9 | 32.8 ± 2.1 | 26.1 ± 2.0 | 11.3 ± 0.7 | 9.3 ± 0.5 | 10.4 ± 0.4 | 10.9 ± 2.6 | ||
| 1 | Radius | 21 | 19 | 14.5 | 5.5 | 27.5 | 26 | 23.5 | 10.5 | |
| Concentration | 39.4 ± 1.9 | 35.4 ± 2.2 | 34.2 ± 1.8 | 40.6 ± 8.8 | 11.7 ± 1.0 | 9.5 ± 0.7 | 10.9 ± 0.5 | 13.5 ± 1.8 | ||
| 2 | Radio | 19.5 | 17 | 12.5 | 6 | 25 | 26 | 14.5 | 9.5 | |
| Concentration | 42.3 ± 2.2 | 38.6 ± 1.9 | 38.0 ± 2.5 | 39.1 ± 6.1 | 13.3 ± 1.7 | 11.1 ± 0.7 | 13.8 ± 2.3 | 14.0 ± 1.1 | ||
| C. albicans ATCC 200955 | 0.5 | Radius | 41 | 38.5 | 37 | 34.5 | 42 | 41 | 38.5 | 35 |
| Concentration | ≤9.4 | ≤5.8 | ≤ 4.2 | ≤8.5 | ≤4.2 | ≤2.6 | ≤7.5 | ≤2.8 | ||
| 1 | Radius | 39.5 | 38.5 | 34.5 | 32 | 42 | 40.5 | 39 | 36 | |
| Concentration | ≤9.4 | ≤5.8 | ≤4.2 | ≤8.5 | ≤4.2 | ≤2.6 | ≤7.5 | ≤2.8 | ||
| 2 | Radius | 38 | 37.5 | 35.5 | 31 | 40.5 | 38 | 35.5 | 33.5 | |
| Concentration | ≤9.4 | ≤5.8 | ≤4.2 | ≤8.5 | ≤4.2 | ≤2.6 | ≤7.5 | ≤2.8 | ||
| C. krusei ATCC 6258 | 0.5 | Radius | 43 | 41.5 | 40.5 | 36.5 | 43.5 | 43.5 | 41 | 38.5 |
| Concentration | ≤9.4 | ≤5.8 | ≤4.2 | ≤8.5 | ≤4.2 | ≤2.6 | ≤7.5 | ≤2.8 | ||
| 1 | Radius | 42.5 | 42 | 37.5 | 35.5 | 41.5 | 42 | 38 | 36.5 | |
| Concentration | ≤9.4 | ≤5.8 | ≤4.2 | ≤8.5 | ≤4.2 | ≤2.6 | ≤7.5 | ≤2.8 | ||
| 2 | Radius | 42 | 36 | 37 | 34 | 41 | 39.5 | 37.5 | 36 | |
| Concentration | ≤9.4 | ≤5.8 | ≤4.2 | ≤8.5 | ≤4.2 | ≤2.6 | ≤7.5 | ≤2.8 | ||
Radius: average value (in mm) of the radius of the growth inhibition halos for the 10 assays carried out with each microorganism, inoculum, concentration and organosulfur compound (see Table 2).
3. Discussion
3.1. Antifungal Susceptibility
Invasive fungal diseases of nosocomial origin or associated to health care, especially those caused by Candida spp., have become a major health problem as they are associated with high rates of morbidity and mortality. Although candida urinary tract infections and vulvovaginal infections are, a priori, milder processes, they have a higher incidence among the population and can be the origin of more serious and disseminated infections in patients with underlying diseases [15]. Similarly, yeast colonization of the skin and mucosal surfaces is also a risk factor for the development of invasive candidiasis in patients, especially those admitted in the intensive care unit as a consequence of risk factors such as handling by colonized healthcare personnel, central venous and urinary catheters, use of broad-spectrum antibiotics, prolonged lengths of stay, mechanical ventilation, parenteral feeding, etc. [16].
On the other hand, alongside the increase of the prevalence of yeast infections by genus Candida, a significant increase in the rates of resistance to antifungals commonly used in human clinics (mainly polyenes, azoles, and echinocandins) is currently described both for C. albicans as in other non-albicans species. Therefore, it is difficult to establish a preventive and therapeutic approach to these infections, which makes advisable to research new alternative therapies to conventional treatments with different and/or synergistic mechanisms of fungicide action and fewer side effects [17,18,19,20].
In this context, the thiosulfinates derived from Allium spp., such as allicin, have demonstrated broad antifungal activity in numerous in vitro studies against yeasts of the genus Candida [21,22,23]. In the present work, the MICs obtained for antifungals were similar to those obtained in previous research, both in our geographical area [24,25] and in more remote areas [19,26,27,28,29]. The lowest MICs were obtained for C. albicans and C. tropicalis; the highest for C. glabrata and C. krusei, especially in the case of azoles, which indicates that Candida species remain susceptible to commonly used antifungals and do not represent a problem for the therapeutic approach. The usual resistance of C. glabrata and C. krusei to azoles would be an exception.
Similarly, our results demonstrate that PTS and PTSO have a significant antifungal activity against different isolates of Candida spp. from human clinical samples, although their activity is not as strong as that of some antifungals, such as amphotericin B, echinocandins or azoles, especially if we consider the most susceptible species to them. In addition, our findings related to the antifungal effects of other organosulfur compounds against yeast isolates are in compliance with those already described in the literature [21,30,31].
In order to explain the antifungal effect of these molecules, different mechanisms of action have been proposed. The most directly associated to cell damage and decreased growth capacity of the fungus are the ability to modify essential enzymes in fungal metabolism [32], induce oxidative stress [7], alter lipids by damaging the integrity of cell membranes (including cytoplasmatic organelles, such as mitochondria or vacuoles) [33] and modify the expression of some genes [34].
Even if our preliminary results provide useful information about the potential use of PTS and PTSO for the prevention or treatment of candidiasis infections caused by multidrug resistant yeasts, further research is needed to demonstrate the effectiveness of these compounds with a wider group of fungi and in vivo models [35]. Even though these results may support their therapeutic use, the absence of cut-off points defined by international committees for this kind of substances does not allow relevant conclusions to be drawn. Further investigation regarding their pharmacokinetic and toxicological characteristics is required before considering safe clinical use.
3.2. Antimicrobial Activity of Vapor
One of the main characteristics of the organosulfur compounds obtained from plants of the genus Allium is their volatility, which is the main reason of the characteristic aroma that these plants exude, especially when they are mashed or crushed. Given the scarcity of volatile antimicrobials available for clinical use in humans, these molecules could be considered an alternative (alone or in combination with other antimicrobials) for the treatment of lung infections via inhalation, instead of oral or parenteral administration [3]. However, there are still few studies evaluating their antimicrobial capacity through their gas phase and their potential applicability for the treatment of infectious diseases [3,14,36].
In the present work, PTS and PTSO showed high bactericidal and fungicidal activity through their gas phase, inhibiting the growth of six of the seven microorganisms assayed (E. coli ATCC 25922, K. pneumoniae ATCC 700603, E. faecalis ATCC 29212, S. aureus ATCC 29213, C. albicans ATCC 200955 and C. krusei ATCC 6258, but not P. aeruginosa ATCC 27853), which might suggest that these substances are likely to be less active against P. aeruginosa than against other pathogens. Various studies have demonstrated that, against these bacteria and other related ones, very high concentrations of these compounds should be used to inhibit bacterial growth, which may not be feasible from a therapeutic point of view [11,37,38].
However, the microbicidal activity of these compounds was not the same against both types of microorganisms. The antifungal effect was higher than the antibacterial effect (the growth inhibition halos were significantly higher in C. albicans ATCC 200955 and C. krusei ATCC 6258 in comparison to those obtained against bacteria). The higher activity of these compounds on yeasts is also observed if we compare the results obtained in the present work on antifungal susceptibility, in terms of MIC, with a previous work of our group with clinical bacterial isolates from human origin: MIC50 and MIC90 values of PTS and PTSO are lower against yeasts than against bacteria [11]. According to some studies, it is possible that the presence of a fungal cell wall, more permeable to these compounds than the peptidoglycan wall of the bacteria, allows this cytotoxic effect at lower doses [39]. On the other hand, the gas phase of PTSO was more active than that of PTS against all the microorganisms evaluated (with the exception of P. aeruginosa), since the growth inhibition halos were significantly higher for PTSO than for PTS in all cases. In fact, the higher antimicrobial activity of PTSO in comparison to PTS has already been described previously by our group [11].
As shown by Leontiev et al. [14], despite of the lack of direct contact of the organosulfur compounds with the agar and with the microorganism itself, when the antimicrobial effect via the gas phase is studied, it is worth noting how clear are the microbial growth inhibition halos and how well defined are the edges. His interpretation, as well as ours, is that this could be due to the existence of a concentration gradient from the closest area of the agar to the drop, precisely at its zenith, to the periphery. In our study, the quantification of the concentration of PTS and PTSO in the agar allowed to confirm this suspicion, showing that there is an inverse linear relationship between the distance to the center of the culture medium and the concentration reached via the gas phase at a given point.
From the results obtained in the present study, as well as from previous works [3,14,40] it can be deduced that organosulfur compounds derived from Allium spp., such as PTS and especially PTSO could be used for the treatment of lung infections, due to its high volatility by inhalation, producing an effect directly on the lung. An advantage that would facilitate their clinical use is that these substances are perceived as innocuous as they are present naturally in food such as onion, widely consumed and included in the diet in most cultures. Among the disadvantages, we could highlight the need to improve the extraction procedures in order to preserve the biological properties of these substances avoiding their loss as a consequence of events such as heating, long-term preservation, etc. In addition, there is a need to diminish the strong impact of their smell and aroma.
However, since the microbicidal effect is directly proportional to the concentration of PTS and PTSO used, a definitive conclusion on the efficacy of this treatment cannot be drawn without evaluating the potential toxicity on human lung cells. It may be possible that the dose required to exert the expected effect in vivo is so high that it produces undesirable toxic consequences. As indicated by Reiter et al. [3], the use of lower and less toxic concentrations concomitantly with other antibiotics or antifungals could be considered if a synergistic effect is demonstrated. In this sense, it has been proven that thiosulfinates have a synergistic effect with antibiotics, such as ampicillin, piperacillin-tazobactam, levofloxacin, gentamicin, amikacin, azithromycin, vancomycin, doxycycline, or polymyxin B [41,42,43,44,45] and with antifungals such as azoles [22,46] or amphotericin B [21]. The potential clinical usefulness of these substances at low concentrations, via inhalation and in combination with other antimicrobials, requires further studies in vitro and with animal and/or human models.
Among the main limitations of our study, was that the methodologies used, both for the study of the antifungal effect of PTS and PTSO, as well as for the antimicrobial effect that their gas phase could exert, are not standardized. Therefore, it is not possible to establish a direct correlation between the results obtained and the potential human therapeutic use. Although their antimicrobial activity seems obvious as numerous studies have shown, international committees for the study of antimicrobial susceptibility have not yet defined cut-off points for these compounds. Thus, no final conclusion can be drawn. Furthermore, the present work has not evaluated the volatility of the organosulfur derivatives and, therefore, the possible loss of activity of PTS and PTSO over time or with increasing temperature, which other authors have described previously [36]. These factors could reduce their clinical usefulness. Finally, further studies should be focused on standardizing the methods to evaluate the antimicrobial activity, understanding how these substances are distributed or removed from the organism, determining the administration routes, and evaluating their effect in different dosages, organs and systems and evaluating the safety of their administration in humans, before safe clinical use is considered.
4. Materials and Methods
4.1. Antifungal Susceptibility Testing
4.1.1. Antifungals, PTS, and PTSO
Amphotericin B, anidulafungin, micafungin, caspofungin, fluconazole and voriconazole were purchased from Sigma-Aldrich (Madrid, Spain). PTS and PTSO are organosulfur compounds present in onion extracts (AlioCareTM) which were supplied with high purity (97%) by Enzim-Orbita Agroalimentares LDA (Tavira, Portugal) and dissolved in polysorbate-80 to a final concentration of 500,000 mg/L.
4.1.2. Candida Isolates and Identification
Two hundred and three clinical isolates of Candida spp. obtained from urine samples processed in the Laboratory of Microbiology of the Virgen de las Nieves University Hospital (Granada, Spain) were selected. CHROMagar Orientation medium (Becton Dickinson, Franklin Lakes, NJ, USA) was used for the growth of isolates. All colonies with yeast compatible morphology were subcultured using a CHROMagar Candida medium (Becton Dickinson). Species were identified using filamentation test and ASM Vitek system (bioMérieux, Madrid, Spain) or MALDI Biotyper system (Bruker Daltonics, Billerica, MA, USA). All isolates were stored at −40° C until the susceptibility study.
4.1.3. In Vitro Antifungal Assay
Standard broth microdilution method was carried out according to the guidelines of the Clinical and Laboratory Standards Institute (CLSI) [47]. A series of two-fold final dilutions of each drug, PTS, and PTSO were prepared in RPMI-1640 medium with L-glutamine but without bicarbonate. Glucose was added to a final concentration of 0.2% and pH was adjusted to 7.0 with acid morpholine propane sulfonic (0.165 M) buffer.
First, all isolates were subcultured onto Sabouraud dextrose agar. Twenty-four hours after the incubation, standard 0.5 McFarland fungal suspensions were prepared with saline solution. Microdilution testing was carried out in 96-well, flat-bottom microtiter plates with a final concentration of the yeast cell suspension equal to 1–5 × 103 cells/mL in each well. Each plate contained 10 serial two-fold dilutions of each antifungal compound, PTS or PTSO. The range of concentrations (in mg/L) assayed for each compound were as follows: amphotericin B (0.03–16), anidulafungin (0.008–4), micafungin (0.008–4), caspofungin (0.008–4), fluconazole (0.125–64) and voriconazole (0.008–4). The concentration ranges of both PTS and PTSO were 0.25–128 mg/L. The positive controls (yeast suspension without antifungal) and negative control (RPMI medium) were added in the last two wells of the plate.
Microtiter plates were incubated at 35 °C and the minimum inhibitory concentration (MIC) values were assessed visually after 24 h (48 h in case of azoles). For amphotericin B, the MIC was determined as the lowest concentration of drug which produced a total inhibition of visual growth. For azoles and echinocandins, the MICs were defined as the lowest concentration of drug that produced ≥50% reduction of visual growth in comparison with the growth of control wells.
The clinical isolates were considered to be susceptible (S), intermediate (I) or susceptible dose-dependent (SDD, only for fluconazole), or resistant (R) to anidulafungin, micafungin, caspofungin, fluconazole and voriconazole according to the recommendations of the CLSI [48]. For amphotericin B, European Committee on Antimicrobial Susceptibility Testing (EUCAST) guidelines were followed [49]. CLSI clinical breakpoints for the susceptibility patterns of C. albicans, C. tropicalis, and C. krusei to anidulafungin, micafungin, and caspofungin were S ≤ 0.25 mg/L, I = 0.5 mg/L, and R ≥ 1 mg/L. Breakpoints for the susceptibility patterns of C. glabrata to anidulafungin and caspofungin were S ≤ 0.12 mg/L, I = 0.25 mg/L, and R ≥ 0.5 mg/L; and to micafungin were S ≤ 0.06 mg/L, I = 0.12 mg/L, and R ≥ 0.25 mg/L. CLSI clinical breakpoints for the susceptibility patterns of C. albicans and C. tropicalis to fluconazole were S ≤ 2 mg/L, SDD = 4 mg/L, and R ≥ 8 mg/L; and for C. glabrata SDD ≤ 32 mg/L, and R ≥ 64 mg/L. For voriconazole, S, I, and R breakpoints for C. albicans and C. tropicalis were ≤ 0.12 mg/L, 0.25–0.5 mg/L, and ≥ 1 mg/L, respectively, and for C. krusei ≤ 0.5 mg/L, 1 mg/L, and ≥ 2 mg/L, respectively. With regard to amphotericin B, the following cutoff values were used for all yeasts: S < 1 mg/L and R ≥ 1 mg/L. There are no cut-off points defined in CLSI or EUCAST for C. krusei to fluconazole neither C. glabrata to voriconazole. All isolates of C. krusei and C. glabrata were then considered resistant to fluconazole or voriconazole, respectively, regardless of the MICs. The values of MIC50 and MIC90 were determined as the lowest concentration of the antifungal at which 50% and 90% of the isolates were inhibited, respectively.
To determine the minimal fungicidal concentration (MFC), after mixing the contents of each well, 100 μL were inoculated onto a plate with Sabouraud dextrose agar and incubated at 35 °C for 48 h. The lowest concentrations that showed no growth after incubation gave the MFC value. MFC50 and MFC90 values were defined as the concentration of antifungal that kills 50% and 90% of the isolates, respectively.
Following the CLSI and EUCAST guidelines, C. krusei ATCC 6258 was used as quality control in the procedures.
4.2. Antimicrobial Activity of Gaseous PTS and PTSO
Seven microorganisms from the ATCC collection (American Type Culture Collection, Manassas, VA, USA) were used. Escherichia coli ATCC 25922, Klebsiella pneumoniae ATCC 700603, and Pseudomonas aeruginosa ATCC 27853 were used as representative Gram-negative bacteria. Enterococcus faecalis ATCC 29212 and Staphylococcus aureus ATCC 29213 were used as representative Gram-positive bacteria. Finally, Candida albicans ATCC 200955 and Candida krusei ATCC 6258 were used as representative yeast.
Bacteria and yeasts were grown over night at 36 ± 1 °C on sheep blood agar and Sabouraud dextrose agar plates, respectively. Colonies were suspended in 5 mL saline solution at 0.5, 1, and 2 McFarland turbidity. Bacteria were spread on Mueller-Hinton agar and yeast on Mueller–Hinton agar supplemented with 2% glucose. Drops of 20 μL of different concentrated PTS and PTSO solutions (50 mg/mL, 25 mg/mL, 10 mg/mL, 5 mg/mL, and 2.5 mg/mL) were placed in the center of a 9-cm diameter Petri dish lid and the solidified agar plates with bacteria or yeasts were placed inverted over the lid according to a previously described procedure [3,14]. Thus, the test solution and the agar itself did not come into contact except by diffusion through the air. After incubation for 24 h at 36 ± 1 °C the diameter of the inhibition zone was measured. Each trial was repeated 10 times.
In the same way as in the previous procedure but without the use of any microorganism, drops of 20 μL of different concentrations of PTS and PTSO solutions (50 mg/mL, 25 mg/mL, 10 mg/mL, 5 mg/mL and 2.5 mg/mL) were placed in the center of the Petri dishes lid and the solidified Mueller–Hinton agar plates were placed inverted over the lid. After incubation for 24 h at 36 ± 1 °C samples of the growth media with a diameter of 7.5 mm were extracted from the center of the plate to the periphery in order to determine the concentration of PTS and PTSO reached on the agar as a consequence of its evaporation (Figure 4). Each trial was repeated three times. The PTS and PTSO concentration achieved in each of the Mueller–Hinton agar samples was determined by high-performance liquid chromatography using a UV detector (HPLC-UV).
Figure 4.
Procedure for obtaining samples from the Mueller–Hinton agar growth medium to establish the concentration of PTS and PTSO reached on it via the gas phase of both compounds by HPLC-UV. For each organosulfur compound and concentration, after incubation for 24 h at 36 ± 1 °C, samples with a diameter of 7.5 mm were extracted from the center of the plate to the periphery in order to determine the concentration of PTS and PTSO reached on the agar as a consequence of its evaporation.
4.3. HPLC-UV Analysis
For the analysis of PTS and PTSO in agar samples, an Agilent 1260 Infinity HPLC (Agilent Technologies Inc., Waldbron, Germany) system was used. The system is equipped with an online degasser, an autosampler, a column thermostat, a diode array detector, and a quaternary pump. The technology used to determine PTS and PTSO was previously described by our group [50,51]. The analysis was carried out in a C18 column (Zorbax Eclipse Plus 50 mm × 4.6 mm, 1.8 μm). Solvents used were 30 mM perchloric acid and MeCN (solvent A and B, respectively) dissolved in water at a flow rate of 0.85 mL min−1. The injection volume was 10 μL and the gradient elution program was: 0 min, 50% B; 2.2 min, 50% B; 4.5 min, 100% B; 6.8 min, 100% B; 8 min, 50% B; 10.5 min, 50% B. The wavelength of detection was set at 200 nm. Agar samples were individually weighed and extracted in 500 μL of methanol through 5 min in vortex. The extract was filtered and directly injected into the HPLC-UV.
4.4. Statistical Analysis
In order to compare the distribution of MIC values of antifungals, PTS and PTSO in the different groups of yeasts studied, the Wilcoxon rank-sum test was used. The differences in the diameters of the growth inhibition halos and in the concentrations reached in the limits of the zone of microbial growth inhibition of the microorganisms after exposure to the gaseous phase of PTS and PTSO were compared using the Wilcoxon signed-rank test. p-values < 0.05 were considered statistically significant. Data analysis was performed using the software IBM SPSS Statistics, version 25.0. (IBM Corporation, Armonk, NY, USA).
5. Conclusions
PTS, and especially PTSO, showed antibacterial and anti-candida activity, even during their gaseous phase, which makes them potentially useful molecules for human therapy. However, it would be necessary to establish its efficacy in human trials, and to know the concentrations that they achieve in the lung tissue when they are administered by inhalation.
Acknowledgments
We would like to acknowledge the Laboratory of Microbiology, Virgen de las Nieves University Hospital and the work of Jose Manuel Garcia-Madero in editing the text.
Author Contributions
Conceptualization: A.S.-P., J.G.-F., and A.B.-A.; methodology: A.S.-P., M.A.-C., I.L.-M., and L.G.-M.; validation: J.J.A.-R. and A.B.-A.; formal analysis: M.A.-C., I.L.-M., and L.G.-M.; investigation: A.S.-P. and M.A.-C.; resources: A.M.-T. and J.G.-F.; writing—original draft preparation: A.S.-P. and M.A.-C.; writing—review and editing: A.B.-A. and J.G.-F.; visualization: A.S.-P.; supervision: A.S.-P. and J.G.-F. All authors have read and agreed to the published version of the manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or non-profit sectors.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the main text.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Borlinghaus J., Albrecht F., Gruhlke M.C., Nwachukwu I.D., Slusarenko A.J. Allicin: Chemistry and biological properties. Molecules. 2014;19:12591–12618. doi: 10.3390/molecules190812591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chan J.Y., Yuen A.C., Chan R.Y., Chan S.W. A review of the cardiovascular benefits and antioxidant properties of allicin. Phytother. Res. 2013;27:637–646. doi: 10.1002/ptr.4796. [DOI] [PubMed] [Google Scholar]
- 3.Reiter J., Levina N., van der Linden M., Gruhlke M., Martin C., Slusarenko A.J. Diallylthiosulfinate (Allicin), a volatile antimicrobial from garlic (Allium sativum), kills human lung pathogenic bacteria, including MDR strains, as a vapor. Molecules. 2017;22:1711. doi: 10.3390/molecules22101711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ankri S., Mirelman D. Antimicrobial properties of allicin from garlic. Microbes Infect. 1999;1:125–129. doi: 10.1016/S1286-4579(99)80003-3. [DOI] [PubMed] [Google Scholar]
- 5.Focke M., Feld A., Lichtenthaler K. Allicin, a naturally occurring antibiotic from garlic, specifically inhibits acetyl-CoA synthetase. FEBS Lett. 1990;261:106–108. doi: 10.1016/0014-5793(90)80647-2. [DOI] [PubMed] [Google Scholar]
- 6.Rabinkov A., Miron T., Konstantinovski L., Wilchek M., Mirelman D., Weiner L. The mode of action of allicin: Trapping of radicals and interaction with thiol containing proteins. Biochim. Biophys. Acta. 1998;1379:233–244. doi: 10.1016/S0304-4165(97)00104-9. [DOI] [PubMed] [Google Scholar]
- 7.Gruhlke M.C., Portz D., Stitz M., Anwar A., Schneider T., Jacob C., Schlaich N.L., Slusarenko A.J. Allicin disrupts the cell’s electrochemical potential and induces apoptosis in yeast. Free Radic. Biol. Med. 2010;49:1916–1924. doi: 10.1016/j.freeradbiomed.2010.09.019. [DOI] [PubMed] [Google Scholar]
- 8.Feldberg R.S., Chang S.C., Kotik A.N., Nadler M., Neuwirth Z., Sundstrom D.C., Thompson N.H. In vitro mechanism of inhibition of bacterial cell growth by allicin. Antimicrob. Agents Chemother. 1988;32:1763–1768. doi: 10.1128/AAC.32.12.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keusgen M., Schulz H., Glodek J., Krest I., Krüger H., Herchert N., Keller J. Characterization of some Allium hybrids by aroma precursors, aroma profiles, and alliinase activity. J. Agric. Food Chem. 2002;50:2884–2890. doi: 10.1021/jf011331d. [DOI] [PubMed] [Google Scholar]
- 10.Guillamón E. Effect of phytochemical compounds of the genus Allium on the immune system and the inflammatory response. Ars Pharm. 2018;59:185–196. doi: 10.30827/ars.v59i3.7479. [DOI] [Google Scholar]
- 11.Sorlozano-Puerto A., Albertuz-Crespo M., Lopez-Machado I., Ariza-Romero J.J., Baños-Arjona A., Exposito-Ruiz M., Gutierrez-Fernandez J. In vitro antibacterial activity of propyl-propane-thiosulfinate and propyl-propane-thiosulfonate derived from Allium spp. against gram-negative and gram-positive multidrug-resistant bacteria isolated from human samples. Biomed Res. Int. 2018;2018:7861207. doi: 10.1155/2018/7861207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singla N., Gulati N., Kaistha N., Chander J. Candida colonization in urine samples of ICU patients: Determination of etiology, antifungal susceptibility testing and evaluation of associated risk factors. Mycopathologia. 2012;174:149–155. doi: 10.1007/s11046-011-9514-7. [DOI] [PubMed] [Google Scholar]
- 13.Sobel J.D., Fisher J.F., Kauffman C.A., Newman C.A. Candida urinary tract infections—Epidemiology. Clin. Infect. Dis. 2011;52:S433–S436. doi: 10.1093/cid/cir109. [DOI] [PubMed] [Google Scholar]
- 14.Leontiev R., Hohaus N., Jacob C., Gruhlke M., Slusarenko A.J. A comparison of the antibacterial and antifungal activities of thiosulfinate analogues of Allicin. Sci. Rep. 2018;8:6763. doi: 10.1038/s41598-018-25154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miceli M.H., Díaz J.A., Lee S.A. Emerging opportunistic yeast infections. Lancet Infect. Dis. 2011;11:142–151. doi: 10.1016/S1473-3099(10)70218-8. [DOI] [PubMed] [Google Scholar]
- 16.Kucukates E., Gultekin N., Alisan Z., Hondur N., Ozturk R. Identification of Candida species and susceptibility testing with Sensititre YeastOne microdilution panel to 9 antifungal agents. Saudi Med. J. 2016;37:750–757. doi: 10.15537/smj.2016.7.13412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alexander B.D., Johnson M.D., Pfeiffer C.D., Jiménez-Ortigosa C., Catania J., Booker R., Castanheira M., Messer S.A., Perlin D.S., Pfaller M.A. Increasing echinocandin resistance in Candida glabrata: Clinical failure correlates with presence of FKS mutations and elevated minimum inhibitory concentrations. Clin. Infect. Dis. 2013;56:1724–1732. doi: 10.1093/cid/cit136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Castanheira M., Messer S.A., Jones R.N., Farrell D.J., Pfaller M.A. Activity of echinocandins and triazoles against a contemporary (2012) worldwide collection of yeast and moulds collected from invasive infections. Int. J. Antimicrob. Agents. 2014;44:320–326. doi: 10.1016/j.ijantimicag.2014.06.007. [DOI] [PubMed] [Google Scholar]
- 19.Cretella D., Barber K.E., King S.T., Stover K.R. Comparison of susceptibility patterns using commercially available susceptibility testing methods performed on prevalent Candida spp. J. Med. Microbiol. 2016;65:1445–1451. doi: 10.1099/jmm.0.000383. [DOI] [PubMed] [Google Scholar]
- 20.Wang E., Farmakiotis D., Yang D., McCue D.A., Kantarjian H.M., Kontoyiannis D.P., Mathisen M.S. The ever-evolving landscape of candidaemia in patients with acute leukaemia: Non-susceptibility to caspofungin and multidrug resistance are associated with increased mortality. J. Antimicrob. Chemother. 2015;70:2362–2368. doi: 10.1093/jac/dkv087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.An M., Shen H., Cao Y., Zhang J., Cai Y., Wang R., Jiang Y. Allicin enhances the oxidative damage effect of amphotericin B against Candida albicans. Int. J. Antimicrob. Agents. 2009;33:258–263. doi: 10.1016/j.ijantimicag.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 22.Khodavandi A., Alizadeh F., Aala F., Sekawi Z., Chong P.P. In vitro investigation of antifungal activity of allicin alone and in combination with azoles against Candida species. Mycopathologia. 2010;169:287–295. doi: 10.1007/s11046-009-9251-3. [DOI] [PubMed] [Google Scholar]
- 23.Khodavandi A., Alizadeh F., Harmal N.S., Sidik S.M., Othman F., Sekawi Z., Jahromi M.A., Ng K.P., Chong P.P. Comparison between efficacy of allicin and fluconazole against Candida albicans in vitro and in a systemic candidiasis mouse model. FEMS Microbiol. Lett. 2011;315:87–93. doi: 10.1111/j.1574-6968.2010.02170.x. [DOI] [PubMed] [Google Scholar]
- 24.Heras-Cañas V., Ros L., Sorlózano A., Gutiérrez-Soto B., Navarro-Marí J.M., Gutiérrez-Fernández J. Isolated yeast species in urine samples in a Spanish regional hospital. Rev. Argent. Microbiol. 2015;47:331–334. doi: 10.1016/j.ram.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 25.Jiménez-Guerra G., Casanovas Moreno-Torres I., Gutiérrez-Soto M., Vazquez-Alonso F., Sorlózano-Puerto A., Navarro-Marí J.M., Gutiérrez-Fernández J. Inpatient candiduria: Etiology, susceptibility to antifungal drugs and risk factors. Rev. Esp. Quimioter. 2018;31:323–328. [PMC free article] [PubMed] [Google Scholar]
- 26.Aigner M., Erbeznik T., Gschwentner M., Lass-Flörl C. Etest and Sensititre YeastOne susceptibility testing of echinocandins against Candida species from a single center in Austria. Antimicrob. Agents Chemother. 2017;61:e00512-17. doi: 10.1128/AAC.00512-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alfouzan W., Al-Enezi T., AlRoomi E., Sandhya V., Chandy R., Khan Z.U. Comparison of the VITEK 2 antifungal susceptibility system with Etest using clinical isolates of Candida species. Rev. Iberoam. Micol. 2017;34:171–174. doi: 10.1016/j.riam.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 28.Oz Y., Gokbolat E. Evaluation of direct antifungal susceptibility testing methods of Candida spp. from positive blood culture bottles. J. Clin. Lab. Anal. 2018;32:e22297. doi: 10.1002/jcla.22297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Siqueira R.A., Doi A.M., de Petrus Crossara P.P., Koga P., Marques A.G., Nunes F.G., Pasternak J., Martino M. Evaluation of two commercial methods for the susceptibility testing of Candida species: Vitek 2® and Sensititre YeastOne®. Rev. Iberoam. Micol. 2018;35:83–87. doi: 10.1016/j.riam.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 30.Diba A., Alizadeh F. In vitro and in vivo antifungal activity of Allium hirtifolium and Allium sativum. Avicenna J. Phytomed. 2018;8:465–474. [PMC free article] [PubMed] [Google Scholar]
- 31.Shams-Ghahfarokhi M., Shokoohamiri M.R., Amirrajab N., Moghadasi B., Ghajari A., Zeini F., Sadeghi G., Razzaghi-Abyaneh M. In vitro antifungal activities of Allium cepa, Allium sativum and ketoconazole against some pathogenic yeasts and dermatophytes. Fitoterapia. 2006;77:321–323. doi: 10.1016/j.fitote.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 32.Gruhlke M., Schlembach I., Leontiev R., Uebachs A., Gollwitzer P., Weiss A., Delaunay A., Toledano M., Slusarenko A.J. Yap1p, the central regulator of the S. cerevisiae oxidative stress response, is activated by allicin, a natural oxidant and defence substance of garlic. Free Radic. Biol. Med. 2017;108:793–802. doi: 10.1016/j.freeradbiomed.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 33.Li W.R., Shi Q.S., Dai H.Q., Liang Q., Xie X.B., Huang X.M., Zhao G.Z., Zhang L.X. Antifungal activity, kinetics and molecular mechanism of action of garlic oil against Candida albicans. Sci. Rep. 2016;6:22805. doi: 10.1038/srep22805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khodavandi A., Alizadeh F., Harmal N.S., Sidik S.M., Othman F., Sekawi Z., Chong P.P. Expression analysis of SIR2 and SAPs1-4 gene expression in Candida albicans treated with allicin compared to fluconazole. Trop. Biomed. 2011;28:589–598. [PubMed] [Google Scholar]
- 35.Ebrahimy F., Dolatian M., Moatar F., Majd H.A. Comparison of the therapeutic effects of Garcin® and fluconazole on Candida vaginitis. Singap. Med. J. 2015;56:567–572. doi: 10.11622/smedj.2015153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Curtis H., Noll U., Störmann J., Slusarenko A.J. Broad-spectrum activity of the volatile phytoanticipin allicin in extracts of garlic (Allium sativum L.) against plant pathogenic bacteria, fungi and Oomycetes. Physiol. Mol. Plant Pathol. 2004;65:79–89. doi: 10.1016/j.pmpp.2004.11.006. [DOI] [Google Scholar]
- 37.Abubakar E.-m.M. Efficacy of crude extracts of garlic (Allium sativum Linn.) against nosocomial Escherichia coli, Staphylococcus aureus, Streptococcus pneumoniae and Pseudomonas aeruginosa. J. Med. Plant Res. 2009;3:179–185. [Google Scholar]
- 38.Müller A., Eller J., Albrecht F., Prochnow P., Kuhlmann K., Bandow J.E., Slusarenko A.J., Leichert L.I. Allicin induces thiol stress in bacteria through S-allylmercapto modification of protein cysteines. J. Biol. Chem. 2016;291:11477–11490. doi: 10.1074/jbc.M115.702308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lemar K.M., Turner M.P., Lloyd D. Garlic (Allium sativum) as an anti-Candida agent: A comparison of the efficacy of fresh garlic and freeze-dried extracts. J. Appl. Microbiol. 2002;93:398–405. doi: 10.1046/j.1365-2672.2002.01707.x. [DOI] [PubMed] [Google Scholar]
- 40.Shadkchan Y., Shemesh E., Mirelman D., Miron T., Rabinkov A., Wilchek M., Osherov N. Efficacy of allicin, the reactive molecule of garlic, in inhibiting Aspergillus spp. in vitro, and in a murine model of disseminated aspergillosis. J. Antimicrob. Chemother. 2004;53:832–836. doi: 10.1093/jac/dkh174. [DOI] [PubMed] [Google Scholar]
- 41.Abouelfetouh A.Y., Moussa N.K. Enhancement of antimicrobial activity of four classes of antibiotics combined with garlic. Asian J. Plant Sci. 2012;11:148–152. doi: 10.3923/ajps.2012.148.152. [DOI] [Google Scholar]
- 42.Jonkers D., Sluimer J., Stobberingh E. Effect of garlic on vancomycin-resistant enterococci. Antimicrob. Agents Chemother. 1999;43:3045. doi: 10.1128/AAC.43.12.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ogita A., Nagao Y., Fujita K., Tanaka T. Amplification of vacuole-targeting fungicidal activity of antibacterial antibiotic polymyxin B by allicin, an allyl sulfur compound from garlic. J. Antibiot. 2007;60:511–518. doi: 10.1038/ja.2007.65. [DOI] [PubMed] [Google Scholar]
- 44.Pillai R., Trivedi N.A., Bhatt J.D. Studies on in vitro interaction of ampicillin and fresh garlic extract against Staphylococcus aureus by checkerboard method. Anc. Sci. Life. 2013;33:114–118. doi: 10.4103/0257-7941.139053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yalindag-Ozturk N., Ozdamar M., Cengiz P. Trial of garlic as an adjunct therapy for multidrug resistant Pseudomonas aeruginosa pneumonia in a critically ill infant. J. Altern. Complement. Med. 2011;17:379–380. doi: 10.1089/acm.2010.0445. [DOI] [PubMed] [Google Scholar]
- 46.Guo N., Wu X., Yu L., Liu J., Meng R., Jin J., Lu H., Wang X., Yan S., Deng X. In vitro and in vivo interactions between fluconazole and allicin against clinical isolates of fluconazole-resistant Candida albicans determined by alternative methods. FEMS Immunol. Med. Microbiol. 2010;58:193–201. doi: 10.1111/j.1574-695X.2009.00620.x. [DOI] [PubMed] [Google Scholar]
- 47.Clinical and Laboratory Standards Institute (CLSI) Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts. CLSI; Wayne, PA, USA: 2012. Fourth Informational Supplement M27-S4. [Google Scholar]
- 48.Clinical and Laboratory Standards Institute (CLSI) Performance Standards for Antifungal Susceptibility Testing of Yeasts. 1st ed. CLSI; Wayne, PA, USA: 2017. Supplement M60. [Google Scholar]
- 49.European Committee on Antimicrobial Susceptibility Testing (EUCAST) Antifungal Agents-Breakpoint Tables for Interpretation of MICs. Version 8.1. [(accessed on 24 October 2017)];2017 Available online: http://www.eucast.org/
- 50.Abad P., Lara F.J., Arroyo-Manzanares N., Baños A., Guillamón E., García-Campaña A.M. High-performance liquid chromatography method for the monitoring of the Allium derivative propyl propane thiosulfonate used as natural additive in animal feed. Food Anal. Methods. 2015;8:916–921. doi: 10.1007/s12161-014-9952-1. [DOI] [Google Scholar]
- 51.Abad P., Arroyo-Manzanares N., Gil L., García-Campaña A.M. Use of onion extract as a dairy cattle feed supplement: Monitoring propyl propane thiosulfonate as a marker of its effect on milk attributes. J. Agric. Food Chem. 2017;65:793–799. doi: 10.1021/acs.jafc.6b04395. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available in the main text.