Abstract
Nanotechnology is a new frontier of this century that finds applications in various fields of science with important effects on our life and on the environment. Nanoencapsulation of bioactive compounds is a promising topic of nanotechnology. The excessive use of synthetic compounds with antifungal activity has led to the selection of resistant fungal species. In this context, the use of plant essential oils (EOs) with antifungal activity encapsulated in ecofriendly nanosystems could be a new and winning strategy to overcome the problem. We prepared nanoencapsules containing the essential oils of Origanum vulgare (OV) and Thymus capitatus (TC) by the nanoprecipitation method. The colloidal suspensions were characterized for size, polydispersity index (PDI), zeta potential, efficiency of encapsulation (EE) and loading capacity (LC). Finally, the essential oil nanosuspensions were assayed against a panel of fourteen fungal strains belonging to the Ascomycota and Basidiomycota phyla. Our results show that the nanosystems containing thyme and oregano essential oils were active against various fungal strains from natural environments and materials. In particular, the minimum inhibitory concentration (MIC) and minimum fungicidal concentration (MFC) values were two to four times lower than the pure essential oils. The aqueous, ecofriendly essential oil nanosuspensions with broad-spectrum antifungal activity could be a valid alternative to synthetic products, finding interesting applications in the agri-food and environmental fields.
Keywords: antifungal activity, essential oils, nanoencapsulation, poly(ε-caprolactone), Origanum vulgare, Thymus capitatus
1. Introduction
Fungi are ubiquitous microorganisms that can colonize several natural environments, equipped with enzymatic machinery enabling the degradation of various types of materials and matrices [1]. Members of the genus Aspergillus, such as A. fumigatus and A. flavus, together with Penicillium rubens, can be isolated from indoor environments and can colonize and damage cellulolytic substrates such as archival documents and books [2,3]. The fungi of the genus Cladosporium are well known as phytopathogenic microorganisms able to infect different kinds of plants [4]. Penicillium citrinum is recognized mainly as a citrus fruit pathogen, but occasionally it also occurs in tropical spices and cereals [5]. Other fungi, such as members of the genera Geotrichum, Mucor and Fusarium, can be isolated from various foods where they can release dangerous mycotoxins [6,7].
There are fungi typically from soil, such as Purpureocillium and Exophiala, and certain types of them can successfully inhabit stones and be responsible for bioweathering phenomena [1,8]. Different species, mainly belonging to the phylum Basidiomycota, are considered to be wood-decay fungi such as Pleurotus eryngii, Bjerkandera adusta and Phanerochaete chrysosporium [9]. The wood-decay fungi can also lead to the biodeterioration of wood components commonly used in construction and may impose serious problems on building stability [10]. Finally, many of the fungi mentioned above can cause various fastidious health complications and, in some cases, also serious pathogenicity [11]. Therefore, it is necessary to implement a series of precautions for their control and inhibition on various kinds of surfaces (especially in an indoor environment) and in food processing.
Over the decades, the massive use of synthetic antifungal products induced resistance phenomena in a large number of fungal species. In this direction, the use of substances derived from plants, such as essential oils, can be considered a valid alternative that better meets the wishes of consumers increasingly oriented toward natural remedies [12]. Essential oils (EOs) are phytocomplexes obtained from aromatic plants by hydrodistillation or steam distillation. Their chemical composition is very complex due the presence of a large variety of volatile compounds, mainly terpenes. Monoterpenes and sesquiterpenes are present in the form of hydrocarbons, alcohols, aldehydes, ketones, esters, ethers, peroxides and phenols. Phenylpropane derivatives occur less frequently than the aforesaid terpenes [13]. The EO chemical composition is influenced by the geographical position, environment condition, stage of ripening and extraction technique. More than half of the essential oils from Lamiaceae family plants have good antifungal activity (minimum inhibitory concentrations (MICs) < 1000 µg/mL) [14]. As reported by Rao et al. [15], terpenes and terpenoids are known to exhibit intense antifungal activity. Their action mechanism is multitarget and does not favor the appearance of resistance fungal strains [16,17].
Unfortunately, EOs are lipophilic compounds, easily degradable by the effects of oxygen, light, moisture and temperature. Nanoencapsulation is a valid strategy to overcome these obstacles. This technology allows for the protection the essential oils from thermal and photodegradation phenomena, increasing their solubility in aqueous environments, masking their flavor and improving their bioaccessibility and bioavailability [18]. The subcellular size and relative larger surface area per unit volume enhances EO concentration in the zone where the microorganisms are preferentially located, such as water-rich phases or liquid–solid interfaces [19].
The nanocapsules, prepared by interfacial deposition of the preformed polymer (nanoprecipitation), represent an effective method to obtain robust nanosystems suitable (by a feasible scale-up process) for applications in various fields, ranging from medicine, health and agri-food to the environment [20]. In these systems, the EO is located in the inner core, surrounded by a polymeric wall.
In this study, nanocapsules based on the biodegradable and biocompatible poly(ε-caprolactone) (PCL) polymer were prepared. The nanocapsules were loaded with commercial EOs of Origanum vulgare (OV) and Thymus capitatus (TC), both having known antifungal activity [21,22,23]. The antifungal ability of the encapsulated and free EOs were assayed against a panel of fourteen different fungi belonging to the Ascomycota and Basidiomycota phyla. These fungi are usually responsible for the biodeterioration and biodegradation of different materials and for the contamination of food, causing damage to human health.
The aim of this work is the preparation of aqueous, ecofriendly nanosuspensions with effective and broad-spectrum antifungal activity for potential applications on different natural matrices and materials.
2. Results
2.1. Chemical Composition of Essential Oils
Thyme and oregano essential oils are, generally, characterized by a large amount of monoterpenes (both hydrocarbons and oxygenated), reaching almost 90% of the whole oil. The two main phenolic monoterpenes, thymol and carvacrol, occur more frequently, often accompanied by their biogenetically correlated compounds p-cymene and γ-terpinene [13,24]. Thymus is probably one of the most taxonomically complex genera of its family. Several studies confirmed that the two most common chemotypes are thymol and carvacrol [25], followed by less common non-phenolic chemotypes [26]. As reported in materials and methods section, the two EO samples of this study were commercial. The chemical composition of our oregano sample has already been published in a previous study with different purposes from this one [27], while the chemical composition of our commercial thyme EO is published here for the first time. The two analytical results are listed together in Table 1 for easier comparison by readers. The samples subjected to this study had chemical profiles quite typical of the Origanum vulgare thymol and carvacrol chemotype and the Thymus capitatus carvacrol chemotype of essential oils. Gas chromatography (GC) techniques, coupled with a flame ionization detector (FID) and a mass spectrometer (MS), allowed the identification of more than 35 components, covering up to 99% and 98% of the total oil compositions, respectively. Table 1 shows the details of the chemical compositions, listing only the components with a % > 0.05. The most represented class for both samples was that of oxygenated monoterpenes (68.7% and 72.9%, respectively), followed by hydrocarbon monoterpenes (30.0% and 22.3%, respectively). The sum of these classes in the oregano sample reached 96.7%, while in the thyme sample it reached 95.1%. For both samples, sesquiterpenes and other components were below 4%. The common feature of these two oils was to have three main components which alone covered more than 80% of the whole composition. The oregano essential oil profile was characterized by the presence of carvacrol (36%) and thymol (25%) as the main compounds, followed by p-cymene (22%). All other compounds were below 5%. The thyme essential oil profile was dominated by a high amount of carvacrol (almost 70%), followed by p-cymene (9%) and γ-terpinene (8%). The thymol percentage was negligible (0.5%), as were the percentages of all other compounds, with the exception of β-caryophyllene, which reached 2.6%.
Table 1.
Chemical composition of commercial Origanum vulgare and Thymus capitatus essential oils (EOs).
| # a | RI Lit b |
RI Exp c |
Class or Compound d |
O. vulgaree % f |
T. capitatus % f |
|---|---|---|---|---|---|
| Monoterpene Hydrocarbons | 27.99 | 22.26 | |||
| 1 | 930 | 924 | α-Thujene | 0.07 | 0.61 |
| 2 | 939 | 931 | α-Pinene | 1.37 | 0.79 |
| 3 | 954 | 947 | Camphene | 0.56 | 0.16 |
| 4 | 979 | 974 | β-Pinene | 0.43 | N.D. |
| 7 | 991 | 986 | β-Myrcene | 0.66 | 1.29 |
| 9 | 1002 | 999 | Δ2-Carene | 0.06 | N.D. |
| 10 | 1003 | 1003 | α-Phellandrene | N.D. | 0.18 |
| 11 | 1004 | 1009 | p-Mentha-1(7),8-diene | N.D. | 0.07 |
| 12 | 1017 | 1013 | α-Terpinene | 0.36 | 1.03 |
| 13 | 1025 | 1027 | p-Cymene | 21.54 | 9.26 |
| 14 | 1029 | 1029 | Limonene | 0.78 | 0.46 |
| 16 | 1060 | 1058 | γ-Terpinene | 2.16 | 8.22 |
| 18 | 1089 | 1085 | Terpinolene | N.D. | 0.19 |
| Oxygenated Monoterpenes | 68.66 | 72.88 | |||
| 15 | 1031 | 1031 | 1,8-Cineole | 0.84 | 0.13 |
| 17 | 1070 | 1067 | cis Sabinene hydrate | N.D. | 0.14 |
| 19 | 1097 | 1098 | Linalool | 4.26 | 0.86 |
| 20 | 1146 | 1144 | Camphor | 0.36 | N.D. |
| 21 | 1156 | 1158 | Isoborneol | 0.27 | N.D. |
| 22 | 1169 | 1167 | Borneol | 0.90 | 0.41 |
| 23 | 1177 | 1177 | Terpinen-4-ol | 0.35 | 0.59 |
| 24 | 1189 | 1190 | α-Terpineol | 0.61 | N.D. |
| 25 | 1208 | 1238 | trans-Piperitol | N.D. | 0.06 |
| 26 | 1245 | 1243 | Carvacrol methyl ether | 0.16 | 0.06 |
| 27 | 1253 | 1259 | Geraniol | N.D. | 0.08 |
| 28 | 1290 | 1303 | Thymol | 25.02 | 0.58 |
| 29 | 1299 | 1319 | Carvacrol | 35.95 | 69.91 |
| 31 | 1373 | 1364 | Carvacrol acetate | N.D. | 0.06 |
| Sesquiterpenes | 1.94 | 3.19 | |||
| 32 | 1419 | 1425 | β-Caryophyllene | 1.70 | 2.56 |
| 33 | 1455 | 1459 | α-Humulene | 0.24 | 0.10 |
| 34 | 1506 | 1492 | β-Bisabolene | N.D. | 0.20 |
| 35 | 1507 | 1525 | (Z)-α-Bisabolene | N.D. | 0.16 |
| 36 | 1583 | 1569 | Caryophyllene oxide | N.D. | 0.23 |
| Others | 0.47 | 0.22 | |||
| 5 | 979 | 976 | 1-Octen-3-ol | 0.21 | 0.16 |
| 6 | 984 | 982 | 3-Octanone | 0.10 | N.D. |
| 8 | 978 | 993 | 3-Octanol | 0.05 | N.D. |
| 30 | 1359 | 1361 | Eugenol | 0.11 | 0.06 |
| TOTAL | 99.06 | 98.55 |
a The numbering refers to the elution order. b Literature retention index (RI). c Retention index (RI) relative to the standard mixture of n-alkanes on the SPB-5 column. d Identified compounds (those < 0.05% have not been reported). e Data previously reported (see [27]). f Relative peak area percent, representing the averages of three determinations. N.D. = not detected.
2.2. Physicochemical Characterization of Essential Oil-Loaded Nanocapsules (EO-NCs)
In a previous work, we reported the encapsulation of thyme and oregano essential oils in PCL nanocapsules [28]. Unlike the previous work, here we used commercial essential oils having different compositions of volatile components. This offered numerus advantages, including the lower cost of preparation and the standardization in the compositions of the EOs. In this manner, the obtained nanocapsules could potentially be more attractive for industrial sectors. Table 2 shows the reported values of the physicochemical parameters (z-average diameter, polydispersity index (PDI), zeta potential (ζ), encapsulation efficiency (EE) and loading capacity (LC)) which allowed us to characterize the nanocapsules containing thyme and oregano essential oils (TC-NCs and OV-NCs, respectively).
Table 2.
Physicochemical Characterization of essential oil (EO)-NCs.
| EO-NCs | Z-Average (nm) | PDI | ζ (mV) | EE% | LC% |
|---|---|---|---|---|---|
| TC-NCs | 198 ± 3 | 0.09 ± 0.02 | −11 ± 1 | 84 ± 6 | 52 ± 3 |
| OV-NCs | 200 ± 3 | 0.05 ± 0.03 | −10 ± 2 | 80 ± 9 | 51 ± 4 |
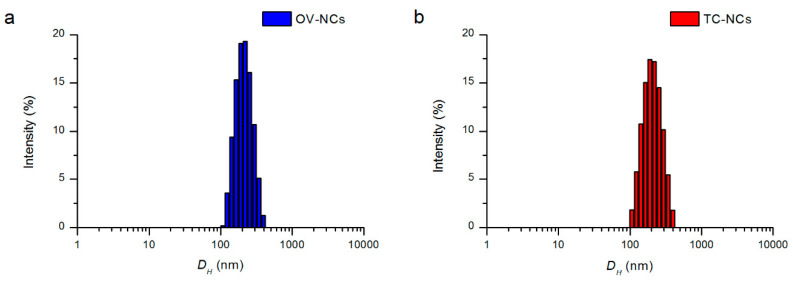
The z-average diameters of 198 ± 3 nm and 200 ± 3 nm for the TC-NCs and OV-NCs, respectively, were in agreement with the nanometric structures of the nanocapsules. Low PDI values for both nanocapsules pointed out a narrow size distribution of nanoparticles and the presence in aqueous solution of monodisperse nanosystems (Figure 1). Negative zeta potentials of −11 and −10 mV for the TC-NCs and OV-NCs, respectively, were very similar to those obtained for other stable PCL nanocapsules [28,29]. The percentages of EE and LC were high, with values of 84 ± 6 and 52 ± 3 for the TC-NCs and 80 ± 9 and 51 ± 4 for the OV-NCs. The prepared TC-NC and OV-NC aqueous nanosuspensions showed a total essential oil content of 5.7 ± 0.3 and 5.8 ± 0.4 mg/mL, respectively.
Figure 1.
I-weighted distribution of the hydrodynamic diameter (DH) of (a) OV-NCs and (b) TC-NCs.
2.3. Antifungal Activities of Pure EOs and EO-NCs
The screening of encapsulated essential oils (EO-NCs) against a panel of fungal strains (Table 3) showed marked antifungal activity (Table 4 and Figure 2). The obtained results evidenced that EO-NCs inhibited the growth of assayed fungi at s MIC values ranging from 0.125 to 0.25 mg/mL. These concentrations were two to four times lower than those observed for pure EOs. For both EO-NCs, an MIC of 0.125 mg/mL was effective for inhibiting the growth of A. fumigatus, C. aggregatocicatricatum, C. herbarum and P. eryngii. In addition, this concentration for OV-NCs was also enough to inhibit the fungus Bjerkandera adusta.
Table 3.
Characteristics of the assayed fungal strains.
| Fungal Strain | Environment of Isolation | Source |
|---|---|---|
| Aspergillus fumigatus | Indoor air | IMB-SAS |
| Aspergillus flavus | Indoor air | IMB-SAS |
| Penicillium rubens | Indoor air | IMB-SAS |
| Penicillium citrinum | Fruit | IMB-SAS |
| Cladosporium aggregatocicatricatum | Wax seal | IMB-SAS |
| Cladosporium herbarum | Wax seal | IMB-SAS |
| Fusarium oxysporum | Cheese | IMB-SAS |
| Geotrichum candidum | Cheese | IMB-SAS |
| Mucor circinelloides | Cheese | IMB-SAS |
| Exophiala xenobiotica | Soil | IMB-SAS |
| Purpureocillium lilacinum | Stone | IMB-SAS |
| Pleurotus eryngii | Tree | CCBAS |
| Bjerkandera adusta | Tree | CCBAS |
| Phanerochaete chrysosporium | Tree | CCBAS |
IMB-SAS = fungal collection of the Institute of Molecular Biology (Slovak Academy of Sciences). CCBAS = Culture collection of Basidiomycetes, Institute of Microbiology, Academy of Sciences of the Czech Republic.
Table 4.
Minimum inhibitory concentration (MIC) and minimum fungicidal concentration (MFC) values of encapsulated essential oils and pure essential oils.
| Fungal Strain | TC-NCs | OV-NCs | TC-EO | OV-EO | ||||
|---|---|---|---|---|---|---|---|---|
| MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC | |
| Aspergillus fumigatus | 0.125 | 0.25 | 0.125 | 0.25 | 0.25 | 0.5 | 0.25 | 0.5 |
| Aspergillus flavus | 0.25 | 0.5 | 0.25 | 0.5 | 0.5 | 1 | 0.5 | 1 |
| Penicillium rubens | 0.25 | 0.5 | 0.25 | 0.5 | 0.5 | 1 | 0.5 | 1 |
| Penicillium citrinum | 0.25 | 0.5 | 0.25 | 0.5 | 0.5 | 1 | 0.5 | 1 |
| Cladosporium aggregatocicatricatum | 0.125 | 0.25 | 0.125 | 0.25 | 0.25 | 0.5 | 0.25 | 0.5 |
| Cladosporium herbarum | 0.125 | 0.25 | 0.125 | 0.25 | 0.5 | 1 | 0.25 | 0.5 |
| Fusarium oxysporum | 0.25 | 0.5 | 0.25 | 0.5 | 0.5 | 1 | 0.5 | 1 |
| Geotrichum candidum | 0.25 | 0.5 | 0.25 | 0.5 | 0.5 | 1 | 0.5 | 1 |
| Mucor circinelloides | 0.25 | 0.5 | 0.25 | 0.5 | 0.5 | 1 | 0.5 | 1 |
| Exophiala xenobiotica | 0.25 | 0.5 | 0.25 | 0.5 | 0.5 | 1 | 0.5 | 1 |
| Purpureocillium lilacinum | 0.25 | 0.5 | 0.25 | 0.5 | 0.5 | 1 | 0.5 | 1 |
| Pleurotus eryngii | 0.125 | 0.25 | 0.125 | 0.25 | 0.25 | 0.5 | 0.25 | 0.5 |
| Bjerkandera adusta | 0.25 | 0.5 | 0.125 | 0.5 | 0.5 | 1 | 0.5 | 1 |
| Phanerochaete chrysosporium | 0.25 | 0.5 | 0.25 | 0.5 | 0.5 | 1 | 0.5 | 1 |
MIC and MFC are expressed in mg/mL. TC-NCs = nanoencapsulated thyme essential oil; OV-NCs = nanoencapsulated oregano essential oil; TC-EO = thyme essential oil; and OV-EO = oregano essential oil.
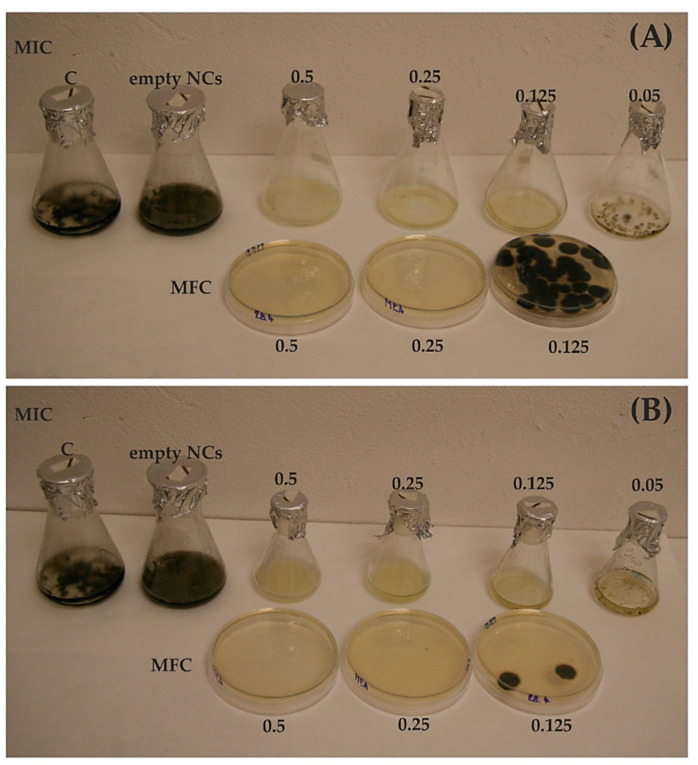
Figure 2.
Example of MIC and MFC assays with the isolate Cladosporium herbarum. (A) Results using a nanoencapsulated oregano EO. (B) Results using a nanoencapsulated thyme EO. C = control. The range of nanoencapsulated EO concentrations used was 0.05–0.5 mg/mL.
We also evaluated the fungicidal effect of EO-NCs versus the panel of fourteen fungal strains. In particular, both the TC-NCs and OV-NCs had minimum fungicidal concentrations (MFCs) of 0.25 mg/mL against A. fumigatus, C. aggregatocicatricatum, C. herbarum and P. eryngii, while having an MFC of 0.5 mg/mL against A. flavus, P. rubens, P. citrinum, F. oxysporum, G. candidum, M. circinelloides, E. xenobiotica, P. lilacinum and P. chrysosporium. The MFC values of the EOs were two to four times higher than the MFCs of the EO-NCs on the tested strains. The empty NCs revealed no activity on the fungal growth.
3. Discussion
The current study evaluated the antifungal activity of TC-NCs and OV-NCs against selected fungal strains that contaminate different natural matrices, indoor air and materials (Table 3). The results evidenced that both TC-NCs and OV-NCs showed in vitro antifungal activity against all tested isolates with an MIC range of 0.125–0.25 mg/mL (Table 4). This activity is two to four times greater than that of pure oils. These results highlight the effectiveness of EO encapsulation in a nanometric structure. In fact, the nanocapsules were able to protect essential oils from degradation phenomena and increase the transport and diffusion mechanisms of essential oils through the cell membrane [30].
Moreover, the same MIC and MFC values were observed for both nanocapsule suspensions in respect to the different strains, with the one exception of the Bjerkandera adusta strain, against which the OV-NCs showed more activity than the TC-NCs. The results were imputable to the chemical compositions of commercial essential oils. Although the main bioactive oxygenated monoterpenes (thymol and carvacrol) were present in different quantities in the two essential oils, the sum of their relative percentages was of the same order of magnitude (Table 1). Generally, the growth-inhibiting effects were attributable to the major components, thymol and carvacrol, but the bioactive monoterpene hydrocarbons, p-cymene and γ-terpinene present in both essential oils in non-negligible quantities could have given a valuable contribution to the antifungal properties [31].
Regarding the physicochemical characterization of the nanocapsules, the TC-NCs and OV-NCs showed nanodimensions and good PDIs indicating the presence of monodisperse species. This characteristic is crucial for achieving optimal biological activity results [32]. Acidic residues on the PCL polymer and the presence of tween 80 (a neutral surfactant covering the nanoparticle wall) were in agreement with the observed low and negative values of the zeta potential. Moreover, these values were very similar to those previously reported by us [28,29] and by other authors [33,34] for stable poly(ε-caprolactone) nanocapsules.
The good encapsulation in the range of 80–84% and the loading capacity of 51–52% highlighted the ability of the PCL to effectively encapsulate essential oils. Furthermore, the ease of preparation of these nanosystems and their stability in aqueous environments could make them useful in different application fields.
PCL is a biocompatible and biodegradable polymer approved by the Food and Drug Administration for drug delivery application. It is a polymer with a low cost and high hydrophobicity and stability. Moreover, PCL is characterized by slow degradation, which makes it potentially more suitable than other polymers, such as polyglycolide (PGA) and poly D, L-lactide (PDLA), when a slow release of the bioactive is required [35].
We have reported here the first example of PCL-based nanocapsules containing essential oils with more enhanced antifungal activity than pure essential oils against a panel of fourteen fungal strains. In the literature, the antifungal activity of essential oils encapsulated in different nanocarriers was also reported. There are many examples of encapsulated EOs in chitosan nanostructures with antifungal activity [36], the majority of which were obtained by the emulsion ionic gelation technique. In a recent work, Hasheminejad et al. [37] demonstrated that clove essential oil encapsulated by chitosan nanoparticles inhibited the growth of Aspergillus niger, isolated from spoiled pomegranate, at a concentration of 1.5 mg/mL. Instead, against Aspergillus flavus, Khalili et al. [38] reported an MIC value of 300 mg/L by using a chitosan benzoic acid nanogel containing thyme essential oil under sealed conditions. Fusarium graminearum was inhibited by a chitosan-encapsulating Cymbopogon martini essential oil [39], with an MIC value of around 0.42 mg/mL.
Nanoemulsions of oregano EO, prepared by Bedoia-Serna et al. [40], showed antifungal effects against Cladosporium sp., Fusarium sp. and Penicillium sp. The authors found that for Fusarium sp., unlike with Penicillium sp., the encapsulation of the oregano essential oil enhanced its antifungal effect.
β-Cyclodextrin was also used to entrap essential oils with antifungal activity through the formation of an inclusion complex. Capsules of β-cyclodextrin containing clove and Mexican oregano EO were active against Fusarium oxysporum [41]. An inclusion complex based on β-cyclodextrin and Litsea cubeba EO presented effective antifungal activity against three main fungi in citrus, including Penicillium digitatum, Penicillium italicum and Geotrichum citri-aurantii [42].
The antifungal activity of inorganic nanoparticles loaded with essential oils was reported by Weisany et al. In particular, the authors showed that encapsulation of Thymus daenensis and Anethum graveolens in silver nanoparticles enhanced their fungicidal activity against the plant pathogen Colletotrichum nymphaeae [43]. The same authors proved that copper nanoparticles containing thyme and dill EOs were able to reduce the mycelium growth and strongly inhibit the germination of conidia of Colletotrichum nymphaeae [44].
Unexpectedly, in the literature, no example of nanostructured systems containing essential oils effective against the fungi Mucor circinelloides, Exophiala xenobiotica, Purpureocillium lilacinum, Pleurotus eryngii, Bjerkandera adusta or Phanerochaete chrysosporium has been reported.
Although the antibacterial activity of EO nanocapsules based on PCL have been evaluated [28,45,46], less interest has been paid to their antifungal activity. The co-encapsulation of chloramphenicol with lemongrass essential oil in PCL-Pluronic composite nanocapsules was reported by Wang et al. [47]. This nanosystem showed considerably enhanced activity against methicillin-resistant Staphylococcus aureus and Candida sp. Tea tree essential oil loaded in PCL nanocapsules exhibited antifungal activity against Trichophyton rubrum, an etiological agent of superficial human mycosis [48].
In light of this, our results could provide a valuable contribution to the use of encapsulated essential oils as effective natural fungicides, as an alternative to the synthetic ones responsible for increasing resistant fungal strains.
4. Materials and Methods
All solvents were of analytic grade. In order to prepare the essential oil-loaded nanocapsules, sorbitan monostearate (SM) and poly(ε-caprolactone) (PCL) (Mn 45000) were obtained from Sigma-Aldrich (Milan, Italy) and polysorbate 80 (Tween 80) was obtained from Fisher Chemical (Fisher Scientific, Geel, Belgium). Water Chromasolv Plus for HPLC (Honeywell Riedel-de-Haën, Seelze, Germany) was used. Commercial essential oils of oregano and thyme were provided by Esperis S.p.A., (Milan, Italy) and by Flora s.r.l. (Lorenzana, Pisa, Italy), respectively. A standard mix of n-alkanes C9–C22 was purchased by Alltech (Italy).
4.1. Characterization of Essential Oils by Gas Chromatography–Flame Ionization Detection (GC–FID) and Gas Chromatography–Mass Spectrometry (GC–MS)
GC–FID and GC–MS analysis were performed on a Shimadzu GC-17A and a Shimadzu GCMS-QP5050A, respectively. For both analyses, the same fused silica capillary column (Supelco SPBTM-5 15 m, 0.1 mm, 0.1 mm) was used.
The operating conditions for both runs were as follows: injector temperature 250 °C; detector temperature 280 °C; carrier gas helium (1 mL/min); split mode (1:200); and volume of injection 1 mL (4% essential oil/CH2Cl2 v/v). The heating ramp was as follows: 60 °C for 1 min, 60–280 °C at 10 °C/min, then 280 °C for 1 min. The relative percentages of compounds in each essential oil were determined from the peak areas in the GC–FID profiles. The mass spectrometer operating conditions were as follows: ionization at 70 eV and an ion source temperature of 180 °C. Mass spectral data were acquired in the scan mode in the m/z range of 40–400. Oil solutions were injected with the split mode (1:96) [49].
The identity of components was determined, comparing their retention indices relative to the C9–C22 n-alkanes on the SPB-5 column, computer matching of spectral MS data with those from National Institute of Standard and Technology (NIST) Mass Spectral Library (MS) 107 and 21 [50] and the comparison of the fragmentation patterns with those reported in the literature [51].
4.2. Preparation of Essential Oil-Loaded Nanocapsules (EO-NCs)
The EO-loaded nanocapsules were prepared according to the preparation described in Granata et al. [28], but with slight changes, above all concerning scaling up by about three times. A solution of sorbitan monostearate (112 mg), PCL (320 mg), and EO (1.0 g) in acetone (80 mL) at 30 °C was poured into an aqueous solution of polysorbate 80 (275 mg in 160 mL of pure water) while stirring. The suspension was stirred for another 10 min at 25 °C. Then, the organic solvent was removed carefully under vacuum conditions (bath at 30 °C, pressure gradually reduced from 550 to 100 mbar in 1 h, and then reduced at 90 mBar and kept constant for 10 min). Finally, in order to complete the solvent evaporation, the mixture was fluxed by N2 (1 h, atmospheric pressure), obtaining the EO-NC suspension (160 mL). The empty NC suspension was achieved under the same conditions but without essential oil.
4.3. Physicochemical Characterization of EO-NCs
4.3.1. Encapsulation Efficiency (EE) and Loading Capacity (LC) of EO-NCs
The total amount of essential oil in the EO-NC suspensions was determined by UV−vis spectroscopy (8453 UV-Visible Spectrophotometer, Agilent Technologies, Milan, Italy) over wavelengths ranging from 250 to 450 nm (λmax 274). A 20 μL EO-NC suspension was diluted with 2 mL of acetonitrile, and the absorbance at 274 nm was registered and corrected from the small absorbance due to the other components. The OV or TC concentration was determined from the value of the correct absorbance compared to the linear calibration curve of respective EO, obtained by plotting the absorbance at 274 nm of twelve solutions containing different concentrations of OV (from 11 to 118 µg/mL, R2 = 0.9999) or eleven solutions containing TC (from 10 to 102 µg/mL, R2 = 0.9999). The ultrafiltration centrifugation technique (Nanosep 30K Omega, Pall Life Science, Milan, Italy; 90 min at 3500× g) was used to estimate the free essential oil. The suspension (500 µL) of the EO-NC was ultrafiltrated, and then an aliquot (60 µL) of the filtrate was diluted with 2 mL of acetonitrile. As described above, the free essential oil amount was determined by the absorbance at 274 nm of the resulting solution. From the total and free amounts of essential oil, the encapsulation efficiency was calculated:
| EE (%) = ([EO]tot − [EO]free)/[EO]tot × 100 | (1) |
where [EO]tot and [EO]free are the total and free amounts of essential oil in the EO-NC suspensions, respectively.
The loading capacity was calculated by the following equation:
| LC (%) = (mass of loaded EO)/(mass of loaded nanocapsules) × 100 | (2) |
4.3.2. Particle Size, Polydispersity and Zeta Potential Measurements
The mean diameter (z-average), the polydispersity index (PDI) and the I-weighted distribution of the EO-NCs were obtained at 25 °C by dynamic light scattering (DLS). The zeta potential (ζ) values were determined by electrophoretic light scattering (ELS). DLS and ELS experiments were performed on a Zetasizer Nano ZS-90 (Malvern Instruments, Cambridge, UK), and data were analyzed using Zetasizer Version 7.02 software. For these purposes, the EO-NC suspensions were previously diluted (1:200, v/v) with pure water or with a pre-filtered (0.45 µm) 10 mM NaCl aqueous solution to carry out DLS or ELS, respectively.
Each experiment was replicated at least twice, and measurements performed at least three times. All data are expressed as mean ± standard deviation (SD).
4.4. Microorganisms and Growth Conditions
The fungal strains used in this study, their environment of isolation and sources are detailed described in Table 3 (Aspergillus fumigatus, Aspergillus flavus, Penicillium rubens, Penicillium citrinum, Cladosporium aggregatocicatricatum, Cladosporium herbarum, Fusarium oxysporum, Geotrichum candidum, Mucor circinelloides, Exophiala xenobiotica, Purpureocillium lilacinum, Pleurotus eryngii CCBAS471, Bjerkandera adusta CCBAS232 and Phanerochaete chrysosporium CCBAS570). The fungal strains were grown at 26 °C on malt extract agar (MEA) for 5 days.
4.5. Minimum Inhibitory Concentration (MIC) and Minimum Fungicidal Concentration (MFC)
The MIC and MFC values of the nanoencapsulated essential oils (EO-NCs) and pure EOs were evaluated with a panel of fourteen fungal strains (Table 3). Fungal suspensions were prepared according to de Lira Mota et al. [52] by washing the surface of the MEA slant culture with 5 mL of sterile saline and shaking the suspensions for 5 min. The resulting mixture of sporangiospores and hyphal fragments was withdrawn and transferred to a sterile tube. After heavy particles were allowed to settle for 3–5 min, the upper suspension was collected and vortexed for 15 s. Fungal suspensions were adjusted to a final concentration of 106 conidia mL−1 in malt extract broth (MEB). Nine milliliters (9 mL) of MEB containing one hundred microliters of the fungal suspension was distributed into incubation flasks. Encapsulated essential oils of oregano (OV-NC) and thyme (TC-NC) at different concentrations (0.05 to 0.5 mg/mL) and pure oregano (OV-EO) and thyme (TC-EO) at concentrations ranging from 0.05 to 5 mg/mL were added individually to the flasks. The flasks were then incubated at 26 °C for 7 days. After incubation, those fungal strains that did not show any growth were transferred to fresh MEA plates without EO-NCs for an additional 7 days at 26 °C to confirm which concentration had a fungicidal effect. The concentration unfavorable for growth revival during the transfer experiment was taken as the MFC, and this effect was identified as fungicidal. The lowest concentration of EOs or EO-NCs that prevented visible fungal growth and allowed a revival of fungal growth during the transfer experiment was considered the MIC.
5. Conclusions
We prepared essential oil-loaded nanocapsules with broad-spectrum antifungal activity. The obtained results suggest the efficacy of nanoencapsulation for enhancing pure essential oil activity. These ecofriendly nanosytems could be a valid alternative to synthetic antifungals and could find effective applications in various industrial sectors concerning health, agri-food and the environment.
Acknowledgments
We thank Tonia Strano for the GC and GC–MS technical support.
Author Contributions
Conceptualization, C.G. and D.P.; methodology, G.G., A.P. and M.B.; formal analysis, G.G., E.N. and M.K.; investigation, G.G., E.N., M.K., A.P. and M.B.; data curation, E.N., M.K., A.P. and M.B.; writing—original draft preparation, C.G., E.N., G.G., M.K. and D.P.; writing—review and editing, C.G. and D.P.; visualization, G.G. and M.K.; supervision, C.G. and D.P.; project administration, C.G. and D.P.; funding acquisition, D.P. and M.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by VEGA projects 2/0061/17 (Innovative disinfection strategies: the essential oils effect on microflora and materials of cultural heritage objects) and 2/0059/19 (Combination of nanoparticles and essential oils for mitigating the biodeterioration on various types of building materials).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in this article.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sterflinger K. Fungi: Their role in deterioration of cultural heritage. Fungal Biol. Rev. 2010;24:47–55. doi: 10.1016/j.fbr.2010.03.003. [DOI] [Google Scholar]
- 2.Skóra J., Sulyok M., Nowak A., Otlewska A., Gutarowska B. Toxinogenicity and cytotoxicity of Alternaria, Aspergillus and Penicillium moulds isolated from working environments. Int. J. Environ. Sci. Technol. 2016;14:595–608. doi: 10.1007/s13762-016-1172-3. [DOI] [Google Scholar]
- 3.Pinheiro A.C., Sequeira S.O., Macedo M.F. Fungi in archives, libraries, and museums: A review on paper conservation and human health. Crit. Rev. Microbiol. 2019;45:686–700. doi: 10.1080/1040841X.2019.1690420. [DOI] [PubMed] [Google Scholar]
- 4.Bensch K., Braun U., Groenewald J., Crous P. The genus Cladosporium. Stud. Mycol. 2012;72:1–401. doi: 10.3114/sim0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reddy K.R., Abbas H.K., Abel C.A., Shier W.T., Oliveira C., Raghavender C.R. Mycotoxin contamination of commer-cially important agricultural commodities. Toxin Rev. 2009;28:154–168. doi: 10.1080/15569540903092050. [DOI] [Google Scholar]
- 6.Richard E., Heutte N., Sage L., Pottier D., Bouchart V., LeBailly P., Garon D. Toxigenic fungi and mycotoxins in mature corn silage. Food Chem. Toxicol. 2007;45:2420–2425. doi: 10.1016/j.fct.2007.06.018. [DOI] [PubMed] [Google Scholar]
- 7.Hymery N., Vasseur V., Coton M., Mounier J., Jany J.-L., Barbier G., Coton E. Filamentous Fungi and Mycotoxins in Cheese: A Review. Compr. Rev. Food Sci. Food Saf. 2014;13:437–456. doi: 10.1111/1541-4337.12069. [DOI] [PubMed] [Google Scholar]
- 8.Topalović O., Hussain M., Heuer H. Plants and associated soil microbiota cooperatively suppress plant-parasitic nema-todes. Front. Microbiol. 2020;11:313. doi: 10.3389/fmicb.2020.00313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lundell T.K., Mäkelä M.R., Hildén K. Lignin-modifying enzymes in filamentous basidiomycetes-ecological, functional and phylogenetic review. J. Basic Microbiol. 2010;50:5–20. doi: 10.1002/jobm.200900338. [DOI] [PubMed] [Google Scholar]
- 10.Schmidt O. Indoor wood-decay basidiomycetes: Damage, causal fungi, physiology, identification and characterization, prevention and control. Mycol. Prog. 2007;6:261–279. doi: 10.1007/s11557-007-0534-0. [DOI] [Google Scholar]
- 11.Gostinčar C., Grube M., Gunde-Cimerman N. Evolution of Fungal Pathogens in Domestic Environments? Fungal Biol. 2011;115:1008–1018. doi: 10.1016/j.funbio.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 12.Stević T., Berić T., Šavikin K., Soković M., Gođevac D., Dimkić I., Stanković S. Antifungal activity of selected essential oils against fungi isolated from medicinal plant. Ind. Crop. Prod. 2014;55:116–122. doi: 10.1016/j.indcrop.2014.02.011. [DOI] [Google Scholar]
- 13.Napoli E.M., Ruberto G. Sicilian aromatic plants: From traditional heritage to a new agro-industrial exploitation. In: Kralis J.F., editor. Spices: Types, Uses and Health Benefits. Nova Science Publisher; Hauppauge, NY, USA: 2012. pp. 1–56. [Google Scholar]
- 14.Karpiński T.M. Essential Oils of Lamiaceae Family Plants as Antifungals. Biomolecules. 2020;10:103. doi: 10.3390/biom10010103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rao A., Zhang Y., Muend S., Rao R. Mechanism of Antifungal Activity of Terpenoid Phenols Resembles Calcium Stress and Inhibition of the TOR Pathway. Antimicrob. Agents Chemother. 2010;54:5062–5069. doi: 10.1128/AAC.01050-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.D’Agostino M., Tesse N., Frippiat J., Machouart M., Debourgogne A., Agostino D. Essential Oils and Their Natural Active Compounds Presenting Antifungal Properties. Molecules. 2019;24:3713. doi: 10.3390/molecules24203713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gucwa K., Milewski S., Dymerski T., Szweda P. Investigation of the Antifungal Activity and Mode of Action of Thymus vulgaris, Citrus limonum, Pelargonium graveolens, Cinnamomum cassia, Ocimum basilicum, and Eugenia caryophyllus Essential Oils. Molecules. 2018;23:1116. doi: 10.3390/molecules23051116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gupta S., Variyar P.S. Encapsulations. Elsevier; Amsterdam, The Netherlands: 2016. Nanoencapsulation of essential oils for sustained release: Application as therapeutics and antimicrobials; pp. 641–672. [Google Scholar]
- 19.Weiss J., Gaysinsky S., Davidson M., McClements J. Global Issues in Food Science and Technology. Academic Press; Cambridge, MA, USA: 2009. Nanostructured encapsulation systems: Food antimicrobials; pp. 425–479. [Google Scholar]
- 20.Ephrem E., Greige-Gerges H., Fessi H., Charcosset C. Optimisation of rosemary oil encapsulation in polycaprolactone and scale-up of the process. J. Microencapsul. 2014;31:746–753. doi: 10.3109/02652048.2014.918669. [DOI] [PubMed] [Google Scholar]
- 21.Kohiyama C.Y., Ribeiro M.M., Mossini S.A., Bando E., da Silva Bomfim N., Nerilo S.B., Rocha G.H., Grespan R., Mikcha J.M., Machinski M., Jr. Antifungal properties and inhibitory effects upon aflatoxin production of Thymus vulgaris L. by Aspergillus flavus Link. Food Chem. 2015;173:1006–1010. doi: 10.1016/j.foodchem.2014.10.135. [DOI] [PubMed] [Google Scholar]
- 22.Puškárová A., Bučková M., Kraková L., Pangallo D., Kozics K. The antibacterial and antifungal activity of six essential oils and their cyto/genotoxicity to human HEL 12469 cells. Sci. Rep. 2017;7:8211. doi: 10.1038/s41598-017-08673-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Božik M., Cisarova M., Tančinová D., Kouřimská L., Hleba L., Klouček P. Selected essential oil vapours inhibit growth of Aspergillus spp. in oats with improved consumer acceptability. Ind. Crop. Prod. 2017;98:146–152. doi: 10.1016/j.indcrop.2016.11.044. [DOI] [Google Scholar]
- 24.Saija A., Speciale A., Trombetta D., Leto C., Tuttolomondo T., La Bella S., Licata M., Virga G., Bonsangue G., Gennaro M.C., et al. Phytochemical, Ecological and Antioxidant Evaluation of Wild Sicilian Thyme: Thymbra capitata (L.) Cav. Chem. Biodivers. 2016;13:1641–1655. doi: 10.1002/cbdv.201600072. [DOI] [PubMed] [Google Scholar]
- 25.Napoli E., Siracusa L., Ruberto G. New tricks for old guys: Recent developments in the chemistry, biochemistry, appli-cations and exploitation of selected species from the Lamiaceae family. Chem. Biodiver. 2020;17:e1900677. doi: 10.1002/cbdv.201900677. [DOI] [PubMed] [Google Scholar]
- 26.György Z., Incze N., Pluhár Z. Differentiating Thymus vulgaris chemotypes with ISSR molecular markers. Biochem. Syst. Ecol. 2020;92:104118. doi: 10.1016/j.bse.2020.104118. [DOI] [Google Scholar]
- 27.Avola R., Granata G., Geraci C., Napoli E.M., Graziano A.C.E., Cardile V. Oregano (Origanum vulgare L.) essential oil provides anti-inflammatory activity and facilitates wound healing in a human keratinocytes cell model. Food Chem. Toxicol. 2020;144:111586. doi: 10.1016/j.fct.2020.111586. [DOI] [PubMed] [Google Scholar]
- 28.Granata G., Stracquadanio S., Leonardi M., Napoli E., Consoli G.M.L., Cafiso V., Stefani S., Geraci C. Essential oils encapsulated in polymer-based nanocapsules as potential candidates for application in food preservation. Food Chem. 2018;269:286–292. doi: 10.1016/j.foodchem.2018.06.140. [DOI] [PubMed] [Google Scholar]
- 29.Granata G., Consoli G.M., Nigro R.L., Geraci C. Hydroxycinnamic acids loaded in lipid-core nanocapsules. Food Chem. 2018;245:551–556. doi: 10.1016/j.foodchem.2017.10.106. [DOI] [PubMed] [Google Scholar]
- 30.Bilia A.R., Guccione C., Isacchi B., Righeschi C., Firenzuoli F., Bergonzi M.C. Essential Oils Loaded in Nanosystems: A Developing Strategy for a Successful Therapeutic Approach. Evid. Based Complement. Altern. Med. 2014;2014:1–14. doi: 10.1155/2014/651593. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 31.Nazzaro F., Fratianni F., Coppola R., De Feo V. Essential Oils and Antifungal Activity. Pharmaceuticals. 2017;10:86. doi: 10.3390/ph10040086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Danaei M., Dehghankhold M., Ataei S., Davarani F.H., Javanmard R., Dokhani A., Khorasani S., Mozafari M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceuticals. 2018;10:57. doi: 10.3390/pharmaceutics10020057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dos Santos P.P., Paese K., Guterres S.S., Pohlmann A.R., Costa T.M.H., Jablonski A., Flôres S.H., Rios A.D.O. Development of lycopene-loaded lipid-core nanocapsules: Physicochemical characterization and stability study. J. Nanoparticle Res. 2015;17:1–11. doi: 10.1007/s11051-015-2917-5. [DOI] [Google Scholar]
- 34.Lobato K.B.D.S., Paese K., Forgearini J.C., Guterres S.S., Jablonski A., Rios A.D.O. Characterisation and stability evaluation of bixin nanocapsules. Food Chem. 2013;141:3906–3912. doi: 10.1016/j.foodchem.2013.04.135. [DOI] [PubMed] [Google Scholar]
- 35.Woodruff M.A., Hutmacher D.W. The return of a forgotten polymer—Polycaprolactone in the 21st century. Prog. Polym. Sci. 2010;35:1217–1256. doi: 10.1016/j.progpolymsci.2010.04.002. [DOI] [Google Scholar]
- 36.Detsi A., Kavetsou E., Kostopoulou I., Pitterou I., Pontillo A.R.N., Tzani A., Christodoulou P., Siliachli A., Zoumpoulakis P. Nanosystems for the Encapsulation of Natural Products: The Case of Chitosan Biopolymer as a Matrix. Pharamceutics. 2020;12:669. doi: 10.3390/pharmaceutics12070669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hasheminejad N., Khodaiyan F., Safari M. Improving the antifungal activity of clove essential oil encapsulated by chitosan nanoparticles. Food Chem. 2019;275:113–122. doi: 10.1016/j.foodchem.2018.09.085. [DOI] [PubMed] [Google Scholar]
- 38.Khalili S.T., Mohsenifar A., Beyki M., Zhaveh S., Rahmani-Cherati T., Abdollahi A., Bayat M., Tabatabaei M. En-capsulation of Thyme essential oils in chitosan-benzoic acid nanogel with enhanced antimicrobial activity against Aspergillus flavus. Lebensm Wiss Technol. 2015;60:502–508. doi: 10.1016/j.lwt.2014.07.054. [DOI] [Google Scholar]
- 39.Kalagatur N.K., Nirmal Ghosh O.S., Sundararaj N., Mudili V. Antifungal activity of chitosan nanoparticles encapsu-lated with Cymbopogon martinii essential oil on plant pathogenic fungi Fusarium graminearum. Front. Pharmacol. 2018;9:610. doi: 10.3389/fphar.2018.00610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bedoya-Serna C.M., Dacanal G.C., Fernandes A.M., Pinho S.C. Antifungal activity of nanoemulsions encapsulating oregano (Origanum vulgare) essential oil: In vitro study and application in Minas Padrão cheese. Braz. J. Microbiol. 2018;49:929–935. doi: 10.1016/j.bjm.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Estrada-Cano C., Castro M.A., Muñoz-Castellanos L.N., García-Triana N.A., Hernández-Ochoa L. Antifungal activity of microcapsulated clove (Eugenia caryophyllata) and Mexican oregano (Lippia berlandieri) essential oils against Fusarium ox-ysporum. J. Microb. Biochem. Technol. 2017;9:567–571. doi: 10.4172/1948-5948.1000342. [DOI] [Google Scholar]
- 42.Wang Y., Yin C., Cheng X., Li G., Yang S., Zhu X. β-Cyclodextrin Inclusion Complex Containing Litsea cubeba Essential Oil: Preparation, Optimization, Physicochemical, and Antifungal Characterization. Coatings. 2020;10:850. doi: 10.3390/coatings10090850. [DOI] [Google Scholar]
- 43.Weisany W., Amini J., Samadi S., Hossaini S., Yousefi S., Struik P.C. Nano silver-encapsulation of Thymus daenensis and Anethum graveolens essential oils enhances antifungal potential against strawberry anthracnose. Ind. Crop. Prod. 2019;141:111808. doi: 10.1016/j.indcrop.2019.111808. [DOI] [Google Scholar]
- 44.Weisany W., Samadi S., Amini J., Hossaini S., Yousefi S., Maggi F. Enhancement of the antifungal activity of thyme and dill essential oils against Colletotrichum nymphaeae by nano-encapsulation with copper NPs. Ind. Crop. Prod. 2019;132:213–225. doi: 10.1016/j.indcrop.2019.02.031. [DOI] [Google Scholar]
- 45.Jummes B., Sganzerla W.G., Da Rosa C.G., Noronha C.M., Nunes M.R., Bertoldi F.C., Barreto P.L.M. Antioxidant and antimicrobial poly-ε-caprolactone nanoparticles loaded with Cymbopogon martinii essential oil. Biocatal. Agric. Biotechnol. 2020;23:101499. doi: 10.1016/j.bcab.2020.101499. [DOI] [Google Scholar]
- 46.da Silva N.P., Pereira E.D., Duarte L.M., de Oliveira Freitas J.C., de Almeida C.G., da Silva T.P., Melo R.C., Apolônio A.C., de Oliveira M.A., de Mello Brandão H., et al. Improved an-ti-Cutibacterium acnes activity of tea tree oil-loaded chitosan-poly (ε-caprolactone) core-shell nanocapsules. Colloids Surf. B Biointerfaces. 2020;196:111371. doi: 10.1016/j.colsurfb.2020.111371. [DOI] [PubMed] [Google Scholar]
- 47.Kalita S., Kandimalla R., Devi B., Kalita B., Kalita K., Deka M., Kataki A.C., Sharma A., Kotoky J. Dual delivery of chloramphenicol and essential oil by poly-ε-caprolactone–Pluronic nanocapsules to treat MRSA-Candida co-infected chronic burn wounds. RSC Adv. 2017;7:1749–1758. doi: 10.1039/C6RA26561H. [DOI] [Google Scholar]
- 48.Flores F.C., De Lima J.A., Ribeiro R.F., Alves S.H., Rolim C.M.B., Beck R.C.R., Da Silva C.B. Antifungal Activity of Nanocapsule Suspensions Containing Tea Tree Oil on the Growth of Trichophyton rubrum. Mycropatology. 2013;175:281–286. doi: 10.1007/s11046-013-9622-7. [DOI] [PubMed] [Google Scholar]
- 49.Napoli E.M., Curcuruto G., Ruberto G. Screening of the essential oil composition of wild Sicilian thyme. Biochem. Syst. Ecol. 2010;38:816–822. doi: 10.1016/j.bse.2010.08.008. [DOI] [Google Scholar]
- 50.NIST, National Institute of Standard and Technology, Mass Spectral Library. [(accessed on 6 December 2019)];1998 Available online: http://chemdata.nist.gov.
- 51.Adams R.P. Identification of Essential Oil Components by Gas Chromatographic/Quadrupole Mass Spectrometry. Allured Publishing Corporation; Carol Stream, IL, USA: 2007. [Google Scholar]
- 52.Mota K.S.D.L., Pereira F.D.O., De Oliveira W.A., Lima I.O., Lima E.D.O. Antifungal Activity of Thymus vulgaris L. Essential Oil and Its Constituent Phytochemicals against Rhizopus oryzae: Interaction with Ergosterol. Molecules. 2012;17:14418–14433. doi: 10.3390/molecules171214418. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available in this article.