Abstract
Hemodialysis (HD) patients are highly susceptible to COVID-19 infection. However, comprehensive assessments of current evidence regarding COVID-19 in HD patients remain incomplete. We systematically searched PUBMED and EMBASE for articles published on incidence or mortality of COVID-19 infection in HD patients until September 2020. Two independent researchers extracted data and study-level risk of bias across studies. We conducted meta-analysis of proportions for incidence and mortality rate. Study heterogeneity and publication bias were assessed. A total of 29 articles with 3261 confirmed COVID-19 cases from a pool of 396,062 HD patients were identified. Incidence of COVID-19 in these HD patients was 7.7% (95% CI: 5.0–10.9%; study heterogeneity: I2 = 99.7%, p < 0.001; risk of publication bias, Egger’s test, p < 0.001). Overall mortality rate was 22.4% (95% CI: 17.9–27.1%; study heterogeneity: I2 = 87.1%, p < 0.001; risk of publication bias, Egger’s test: p = 0.197) in HD patients with COVID-19. Reported estimates were higher in non-Asian than Asian countries. Quality of study may affect the reported incidence but not the mortality among studies. Both incidence and mortality of COVID-19 infection were higher in HD patients. Available data may underestimate the real incidence of infection. International collaboration and standardized reporting of epidemiological data should be needed for further studies.
Keywords: COVID-19, hemodialysis, incidence, meta-analysis, mortality, systematic review
1. Introduction
The novel coronavirus, COVID-19, continues to generate a tremendous global burden with 62,363,527 confirmed cases and 1,456,687 deaths worldwide, as of the end of November 2020 [1]. Many risk factors related to the incidence of COVID-19 infection have been identified; for example, advanced age, diabetes mellitus, hypertension and smoking [2,3]. In addition, the mortality rate of COVID-19 infection varies from country to country, ranging from 35.4 per million population (South-east Asia) to 904.4 per million population (America) [1]. Higher risks of mortality caused by COVID-19 are found in patients with older age, more comorbidities, and immune dysfunction [3,4]. However, a comprehensive assessment of published literature with regard to the epidemiology in patients with renal impairment infected by COVID-19 remains scarce.
The prevalence of chronic kidney disease (CKD) is about 9.1% worldwide [5], and end-stage renal disease (ESRD) is associated with higher comorbidity, mortality risks, and socioeconomic impacts, affecting 2,859,750 patients globally [6]. In-center hemodialysis (HD) is the predominant renal replacement modality across different countries, except in Hong Kong, Mexico, and Guatemala [7]. HD patients are immune-dysregulated patients on account of uremia, comorbidities, and dialysis procedure-related bio-compatibility [8]. Furthermore, frequent personnel contact in crowded areas for their in-center facility treatment may hamper effective protective measures (such as social distancing, reducing personal contact, staying home) against COVID-19 infection. CKD has previously been associated with increased risk for COVID-19 infection [9], but the published observational studies have identified varying incidence and clinical impacts of COVID-19 in HD patients. To the best of our knowledge, there is no comprehensive understanding of incidence and clinical outcomes of COVID-19 in HD patients, but this information could be beneficial for the development of proper screening or preventive strategies against COVID-19 infection in this vulnerable population. The aim of the present study is to fill this knowledge gap, and systematically quantify the incidence and clinical impacts of COVID-19 infection in HD patients, identity methodological bias, and estimate the influence of bias on the results of studies.
2. Materials and Methods
2.1. Design and Search Strategy
We conducted a systematic review and meta-analysis of observational studies in published literature from PUBMED and EMBASE until 9 September 2020. The MeSH or Emtree terms included COVID-19, hemodialysis, incidence, prevalence, mortality, or prognosis (Supplementary Table S1). Studies were required to provide data on either incidence of proven COVID-19 infection (such as nucleic acid testing by polymerase chain reaction, serology, or image study) or related mortality of patients receiving maintenance HD therapy. The reference lists of included articles were also hand-searched. References were managed using EndNote version X8, Clarivate Analytics, PA, USA. This study adhered to the reporting guidelines of Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) [10] (Supplementary Table S2), and it has been recorded in the International Prospective Register of Systematic Reviews (PROSPERO) database (CRD42020209134).
2.2. Literature Selection
Two independent researchers (CYC and IWW) screened titles and abstracts to identify potentially eligible studies for full-text review. Original articles and case series with over 2 cases in English language were reviewed. We excluded the articles reporting incomplete data on incidence or mortality.
2.3. Data Extraction and Study Quality
Data extraction was completed in duplicate by 2 independent reviewers (CYC, WIW). When multiple articles reporting data from the same study population were identified, the most comprehensive data were used. Information including country, study design, settings, age, gender, presenting symptoms or signs, laboratory findings, treatment, and preventive strategies were extracted. The raw numbers of total, infected, and deceased cases were accurately recorded. Incidence rates were estimated by calculating the affected cases from the overall HD patients infected by COVID-19. Mortality was expressed as case fatality rate. We contacted the study authors regarding possible incomplete data on incidence or mortality presented in selected publications.
The methodological quality of included studies was assessed independently by 2 authors (YTC, CKH) based on a 20-item critical appraisal checklist for case-series studies developed by the Institute of Health Economics (IHE) [11]. If we answered ‘yes’ on an item of the checklist, then the item scored 1 point. If we answered ‘no’ or ‘unclear’ on a checklist item, then 0 points were scored. We considered studies scoring 14 or more points (≥70%) as “good quality” [12]. When the reviewers’ assessments differed with regard to data extractions or study quality evaluations, the additional reviewer (SCS) were drawn in on a case by case basis to discuss and make the final judgments.
2.4. Data Synthesis and Statistical Analysis
We performed meta-analysis of the proportions (with 95% confidential interval, CI) for the incidence and mortality rates of COVID-19 infection in patients receiving HD. Statistical heterogeneity among the studies was measured by Cochran’s Q test with the p-value, and the extent of heterogeneity attributable to heterogeneity was measured by the I2 statistic. We planned subgroup analyses based on geographic area (e.g., Asia or non-Asia) and quality of study (e.g., good or poor). We also used Egger’s test to determine potential publication bias. Statistical analyses were performed using MedCalc for Windows, version 15.0 (MedCalc Software, Ostend, Belgium).
3. Results
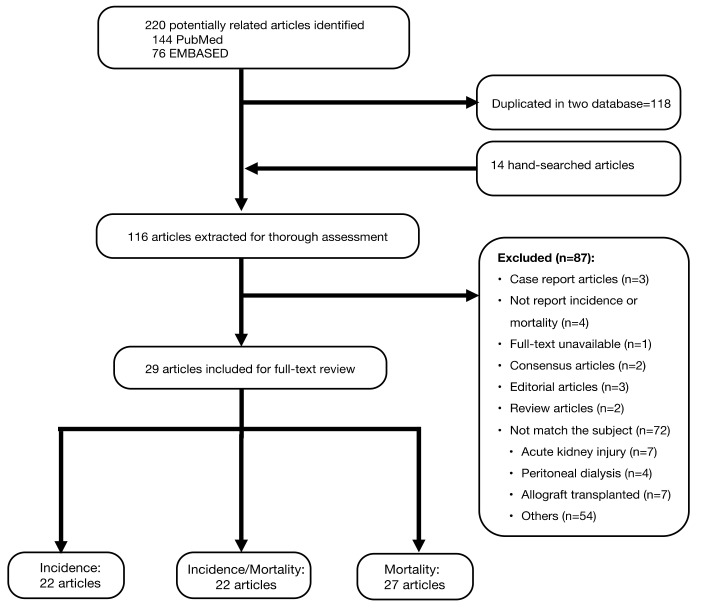
We identified 220 records form PUBMED (n = 144) and EMBASE (n = 76) databases for the initial assessment, and only 29 articles were included for full-text review (Figure 1). This systematic review and meta-analysis evaluated 29 international studies with 3261 confirmed COVID-19 cases from a pool of 396,062 HD patients. The mean age of COVID-19 infected HD patients was 64.9 years and 64.5% were men. The characteristics of the included studies are described in Table 1. Due to emergency and the uncertainty of this novel disease, articles were emerging into the literature after short observation periods. The mean observation time was 46.6 days (ranging from 13 to 121). We identified 22 HD cohorts (Asian countries: 47.8%; good study quality: 43.5%) for assessment of incidence and 27 HD cohorts (Asian countries: 42.9%; good study quality: 35.7%) for analysis of mortality related to COVID-19 infection (Table 2).
Figure 1.
Flow chart of literature search and selection.
Table 1.
Characteristics of studies reporting COVID-19 infection in hemodialysis patients.
| First Author | Country | Total HD, n | COVID-19, n | Age (Covid-19) | Male, % (Covid-19) | Presenting Symptoms/Signs, n (%) | Laboratory Findings, Median (IQR) or Mean ( ± SD ), 10⁹/L | Treatment, n (%) | Location of Treatment, n (%) | ARDS | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fever | Fatigue | Cough | Dyspnea | GI | Myalgia | WBC | Lymphocytes | Neutrophils | Platelet | Antiviral | HCQ | Tocilizumab | CS | In | Out | ICU | n, % | ||||||
| Yau K | Canada | 237 | 11 | 66 (63–72) | 6 (55) | 1 (9) | N/A | 3 (27) | 0 (0) | N/A | N/A | 4.72 (3.1–21.8) | 0.54 (0.05–1.38) | N/A | N/A | N/A | N/A | N/A | N/A | 5 (45) | 6 (55) | 2 (18) | 0 |
| Wang H | China | 230 | 37 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | 37 (100) | 0 | 0 | 0 |
| Su K | China | 230 | 37 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | 0 |
| Xiong F | China | 7154 | 154 | 63.2 (13.1) | 75 (57.3) | 68 (52) a | 59 (45) a | 49 (37) a | 34 (26) a | 35 (27) a | NA | 5.0 (3.8–7.3) | 0.7 (0.5–1.1) | 3.9 (3.0–6.1) | 144.2 (107–186) | 92 (80) c | N/A | N/A | 19 (17.3) d | N/A | N/A | N/A | 16 (10) |
| Wu J * | China | 49 | 49 | 62 (54–71) | 31 (63) | 23 (47) | 29 (59) | 24 (49) | 22 (45) | 6 (12) | NA | 5.6 (4.7–7.6) | 0.8 (0.5–1.0) | 4.0 (3.1–5.6) | 169 (120–234) | 47 (96) | N/A | N/A | 8 (16) | N/A | N/A | N/A | 10 (20) |
| Li J (1) | China | 244 | 7 | 59 (39–66) | 4 (57) | 1 (14) | 0 (0) | 0 (0) | 0 | 0 | 0 | 5.4 (2.6–6.4) | 0.5 (0.4–0.9) | 4.4 (1.9–5.0) | 134.0 (23.3) | 0 | 0 | 0 | 0 | 7 (100) | 0 (0) | N/A | N/A |
| Li J (2) | China | 6377 | 109 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Wang R | China | 201 | 5 | 57.6 (47–67) | 3 (60) | 3 (60) | 3 (60) | 1 (20) | 2 (40) | 2 (40) | 0 | 7.50 (5.94–9.25) | 0.80 (0.56–0.88) | 5.69 (4.97–7.76) | NA | N/A | N/A | N/A | N/A | 5 (100) | 0 | 0 | 0 |
| Ma Y | China | 230 | 42 | 64.57 (47–76) | 25 (60) | 4 (10) | 3 (7) | 3 (7) | N/A | 2 (5) | N/A | N/A | 1.42 (0.85–1.56) | 4.92 (4.23–7.06) | 154 (140–200) | N/A | N/A | N/A | N/A | 32 (76) | 10 (24) | 3 (7) | 2 (5) |
| Tang H ^ | China | 1027 | 99 | 61.3 ± 13.8 | 55 (56) | 27 (27) | N/A | 27 (27) | 14 (14) | 11 (11) | N/A | 4.9 (4.04–6.51) | 0.86 (0.66–1.15) | 3.45 (2.87–4.52) | 161 (117–200) | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Wang R | China | 202 | 7 | 59.43 (47–67) | 4 ( 57) | 5 (71) | 5 (71) | 3 (43) | 4 (57) | 6 (86) | NA | 7.5 (5.03–8.02) | 0.80 (0.49–0.92) | 5.65 (4.29–6.28) | 141 (114–213) | 5 (71) | N/A | N/A | 3 (43) | N/A | N/A | N/A | 1 (14) |
| Tortonese S * | France | 44 | 44 | 61 (51.5–72.5) | 29 (66) | 35 (80) | N/A | 19 (43) | 13 (30) | 6 (14) | N/A | N/A | 0.6 (0.46–1.04) | 3.91 (2.39–6.00) | 196.5 (136–249) | 0 (0) | 3 (7) | 4 (9.1) | N/A | 41 (93) | 3(7) | 15 (34) | 12 (28) |
| Alberici F * | Italy | 21 | 21 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | 17 (81) e | 17 (81) | 1 (5) | 4 (19) | 21 (100) | 0 (0) | 0( 0) | N/A |
| Manganaro M ^ | Italy | 2893 | 98 | 70 | 58 (59.3) | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Scarpioni R | Italy | 257 | 41 | 73 (52–90) | 31 (76) | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Esposito P | Italy | 260 | 17 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | 6 (35) | 11 (64) | 1 (6) | N/A |
| Alberici F | Italy | 643 | 94 | 72 (62–79) | 62 (66) | 64 (68) | NA | 22 (23) | 24 (25) | 6 (6) | 16 (17) | 5.08 (3.94–6.48) | 0.75 (0.55–1.09) | 3.51 (2.69–4.77) | 162 (126–230) | 60 (64) f | 72 (77) | 19 (20) | N/A | 57 (61) | 37 (40) | N/A | 57 (61) |
| Quintaliani G | Italy | 30,821 | 1093 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| La Milia V | Italy | 209 | 55 | 72.26 | N/A | 21 (38) | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | 25 (46) e | 25 (46) | 0 | 0 | 25 | 30 | 8 (15) | |
| Kikuchi K | Japan | 339,841 | 99 | 70–90 | 69 (70) | 79 (95) b | N/A | 47 (64) b | N/A | N/A | N/A | N/A | N/A | N/A | N/A | 31 (31) g | N/A | N/A | 15 (15) | 99 (100) | 0 (0) | N/A | N/A |
| Cho JH | Korea | 1175 | 11 | 57 (29–63) | 7 (64) | 6 (55) | 0 | 2 (18) | 0 | 0 | 0 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | 11 (100) | 0 (0) | 0( 0) | 0 |
| Jung HY * | Korea | 14 | 14 | 63.5 (40.0–88.0) | 6 (43) | N/A | 4 (29) | 7 (50) | 5 (36) | 2 (14) | 2 (14) | 5.8 (4–10) | 1.1 (1.0–4.5) | N/A | 162.1 (130–400) | 14 (100) h | 7 (50) | 0 (0) | 3 (21) | 14 (100) | 0 (0) | 4 (29) | 2 (14) |
| Sánchez-A JE * | Spain | 548 | 548 | 71 ± 15 | 359 (66) | 416 (76) | N/A | 372 (68) | 236 (43) | 13 (2.3) | N/A | N/A | N/A | N/A | N/A | 176 (32) h | 396 (72) | 12 (2) | 80 (14) | 444 (81) | 104 (19) | 33 (6) | N/A |
| Albalate M | Spain | 90 | 37 | 67.79 (17–100) | 23 (62) | 16 (43) | N/A | 10 (27) | 3 (8) | 0 (0) | 3 (8) | N/A | 0.919 (0.2–1.9) | N/A | N/A | 23 (62) i | 29 (78) | N/A | N/A | 16 (43) | 21 (57) | 0 (0) | N/A |
| Goicoechea M | Spain | 282 | 36 | 71 ± 12 | 23 (64) | 24 (67) | 9 (25) | 16 (44) | N/A | 6(17) | N/A | N/A | 0.79 ± 0.47 | NA | 164 ± 66 | 2 (5) h | 35 (97) | 27 (75) | 17 (47) | 36 (100) | 0 | 1 (2.7) | 11 (31) |
| Sánchez-P P | Spain | 478 | 16 | 79.5 (73.2–85) | 11 (69) | 16 (100) | 11 (38) | 11 (38) | 3 (19) | 5 (31) | 11 (38) | N/A | 8.4 (7.3–11.5) | N/A | N/A | 0 (0) | 12 (75) | 2 (13) | 4 (33) | 16 (100) | 0 (0) | 2 (13) | N/A |
| Arslan H | Turkey | 602 | 7 | 62 (25–79) | 3 ( 43) | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | 0 |
| Corbett R.W. | UK | 1530 | 300 | 67 (57–77) | 180 (60) | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Valeri AM * | USA | 59 | 59 | 63 (56–78) | 33 (56) | 29 (49) | 13 (22) | 23 (39) | 21 (36) | 9 (15) | 4 (7) | 6 (4.5–7.8) | 0.8 (0.58–1.23) | N/A | N/A | N/A | 34 (58) | 3 (5) | N/A | 59 (100) | 0 (0) | 8 (14) | N/A |
| Fisher M * | USA | 114 | 114 | 64.5 | 68 (61) | 51 (45) | N/A | 15 (13) | 57 (50) | 6 (5) | N/A | 5.83 | N/A | N/A | N/A | 0 (0) | 87 (76) | 0 (0) | 0 | 114 (100) | 0 (0) | 15 (13) | 14 (12) |
Abbreviation: HD: hemodialysis; GI: gastrointestinal symptoms; WBC: White blood cells; HCQ: hydroxychloroquine; CS: Corticosteroids; In: in-patient wards; Out: out-patient clinics; ICU: intensive care units; ARDS: acute respiratory distress syndrome. Age and gender reported statistics of COVID-19 cases. Age was expressed in mean ± SD or median (IQR). The study of Li J et al [13]. reported 2 independent cohorts that were analyzed separately for meta-analysis. * Not included for incidence assessment because the denominator cannot be ascertained from literature or author queries. ^ Not included for mortality assessment because of incomplete data. a. This study reported 154 confirmed COVID-19 cases. Analyses were conducted from 131 cases because 23 patients did not provide consent. The percentages were calculated using 131 cases as denominator. b. This study reported 99 confirmed COVID-19 cases. “Fever” recordings were missing in 16 cases (the percentages were calculated using 83 cases as denominator). "Cough" recordings were missing in 25 cases (the percentages were calculated using 74 cases as denominator). Antiviral therapy was reported as: c. not specified, used 115 patients as denominator; d. used 110 patients as denominator; e. lopinavir/ritonavir or remdesivir; f, lopinavir/ritonavir or darunavir/ritonavir g. famipiravir; h. lopinavir/ritonavir; i. 17 cases using azithromycin and 6 cases using lopinavir/ritonavir.
Table 2.
Characteristics of studies related to COVID-19 infection in hemodialysis patients.
| Authors | Year | Journal | Date (M/D) | Country | City | Study Design | Setting | Duration | Confirmatory Test | Preventive Strategies | Quality Score | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yau K, et al | 2020 | Am J Kidney Dis | 07/19 | Canada | Toronto | Prospective | HD centers | 15 | RT−PCR | PPE, quarantine, and isolation gowns | 13 | [14] |
| Wang H, et al | 2020 | Kidney Med | 04/16 | China | Wuhan | Retrospective | Hospital | 34 | RT-PCR + serology + CT | Timely upgrading of personal protection measures, quarantine and isolation | 8 | [15] |
| Su K, et al | 2020 | Infect Control Hosp Epidemiol | 04/24 | China | Wuhan | Retrospective | HD centers | 26 | RT-PCR + serology + CT | Isolation ward, quarantine | 9 | [16] |
| Xiong F, et al | 2020 | J Am Soc Nephrol | 05/10 | China | Wuhan | Retrospective | Hospital | 69 | RT−PCR | Medical mask, isolation | 14 | [17] |
| Wu J, et al | 2020 | Clin J Am Soc Nephrol | 05/24 | China | Wuhan | Retrospective | Hospital | 40 | RT−PCR | Isolation | 13 | [18] |
| Li J, et al (1) | 2020 | Kidney Disease | 05/25 | China | Wuhan | Retrospective | Hospital | 17 | RT−PCR + CT | Isolation in a dedicated area | 12 | [13] |
| Li J, et al (2) | 2020 | Kidney Disease | 05/25 | China | Wuhan | Retrospective | HD centers | 13 | RT−PCR + CT | N/A | 12 | [13] |
| Wang R, et al | 2020 | Am J Kidney Dis | 05/31 | China | Wuhan | Case series | HD centers | N/A | RT−PCR | Surgical or N95 masks | 12 | [19] |
| Ma Y, et al | 2020 | Kidney Int Rep | 06/09 | China | Wuhan | Retrospective | Hospital | 58 | RT−PCR+ CT | Patients: N95 mask, quarantine or Isolation. Staff: PPE | 12 | [20] |
| Tang H, et al | 2020 | Am J Kidney Dis | 07/03 | China | Wuhan | Retrospective | HD centers | 121 | RT−PCR, serology | N/A | 15 | [21] |
| Wang R, et al | 2020 | Clin Kidney J | 07/23 | China | Wuhan | Retrospective | Hospital | 86 | RT−PCR, RT−PCR + CT | Patient: mask. Staff: Waterproof disposable gown, cap, gloves, face shield and N95 face mask | 11 | [22] |
| Tortonese S, et al | 2020 | Kidney Int Rep | 07/18 | France | Paris | Retrospective | Hospital | 61 | RT−PCR + CT | Mask | 12 | [23] |
| Alberici F, et al | 2020 | Kidney Int Rep | 04/04 | Italy | Brescia | Case series | Hospital | N/A | RT−PCR | N/A | 11 | [24] |
| Manganaro M, et al | 2020 | J Nephrol | 04/12 | Italy | Piedmont/ Aosta Valley | Retrospective | Hospital | 35 | CXR * | Surgical masks, hand disinfection | 8 | [25] |
| Scarpioni R, et al | 2020 | G Ital Nefrol | 04/14 | Italy | Piacenza | Retrospective | Hospital | N/A | RT−PCR, CT | Mask, alcohol-based sanitizer, changing clothes and shoes | 3 | [26] |
| Esposito P, et al | 2020 | Hemodial Int | 05/05 | Italy | Genoa | Retrospective | HD centers | N/A | RT−PCR | Handwashing, use of PPE | 9 | [27] |
| Alberici F, et al | Kidney Int | 05/08 | Italy | Brescia | Retrospective | HD centers | 33 | RT−PCR | N/A | 14 | [28] | |
| Quintaliani G, et al | 2020 | J Nephrol | 07/03 | Italy | Nationwide | Retrospective | HD centers | 59 | RT−PCR | N/A | 10 | [29] |
| La Milia V, et al | 2020 | Kidney Int Rep | 07/10 | Italy | Lombardy | Prospective | Hospital/ HD centers | 22 | RT−PCR | Upgrade of PPE | 11 | [30] |
| Kikuchi K, et al | 2020 | Ther Apher Dial | 06/09 | Japan | Nationwide | Prospective | Hospital/ HD centers | 89 | RT−PCR, CT | Mask, sufficient distance | 15 | [31] |
| Cho JH, et al | 2020 | J Am Soc Nephrol | 06/01 | Korea | Daegu | Retrospective | HD centers | 24 | RT−PCR | Mask, hand sanitizer, cohort isolation, notify first | 15 | [32] |
| Jung HY, et al | 2020 | J Clin Med | 06/02 | Korea | Daegu | Prospective | Hospital | 89 | RT−PCR | Mask, isolation in negative pressure room | 17 | [33] |
| Sánchez-A JE, et al | 2020 | Nefrologia | 04/06 | Spain | Nationwide | Prospective | HD centers | 24 | RT−PCR | N/A | 17 | [34] |
| Albalate M, et al | 2020 | Nefrologia | 04/30 | Spain | Madrid | Retrospective | Hospital | 35 | RT−PCR | Mask, alcohol-based sanitizer | 10 | [35] |
| Goicoechea M, et al | 2020 | Kidney Int | 05/11 | Spain | Madrid | Retrospective | Hospital | 29 | RT−PCR | N/A | 12 | [36] |
| Sánchez-P P, et al | 2020 | Nefrologia | 07/06 | Spain | Valencia | Prospective | Hospital/HD centers | 45 | RT−PCR | PPE, isolation | 17 | [37] |
| Arslan H, et al | 2020 | Exp Clin Transplant | 06/11 | Turkey | Ankara | Retrospective | HD centers | N/A | RT−PCR + CT | N/A | 13 | [38] |
| Corbett RW, et al | 2020 | J Am Soc Nephrol | 06/19 | UK | London | Prospective | HD centers | 42 | RT−PCR | Mask, isolation units | 16 | [39] |
| Valeri AM, et al | 2020 | J Am Soc Nephrol | 05/28 | USA | New York | Retrospective | Hospital | 30 | RT−PCR | Staff: Mask, PPE | 15 | [40] |
| Fisher M, et al | 2020 | Kidney360 | 06/17 | USA | New York | Retrospective | Hospital | 44 | RT−PCR | N/A | 14 | [41] |
Abbreviation: PPE: Personal protective equipment (including masking gloves, face shields, masks, disposable gowns, caps); RT-PCR, Reverse-transcriptase polymerase chain reaction; CT: Chest computed tomography; CXR, Chest X-ray; Ref, reference. * COVID-19 infection was confirmed if signs of interstitial pneumonia presented on chest radiography, nucleic acid testing was performed only in person having contact history with suspected or confirmed cases of COVID-19. Duration: denoted observation period, expressed in days. Quality score indicates the number of positive answers in the case-series appraisal sheets. The study of Li J et al. reported two independent cohorts, which were analyzed separately for meta-analysis.
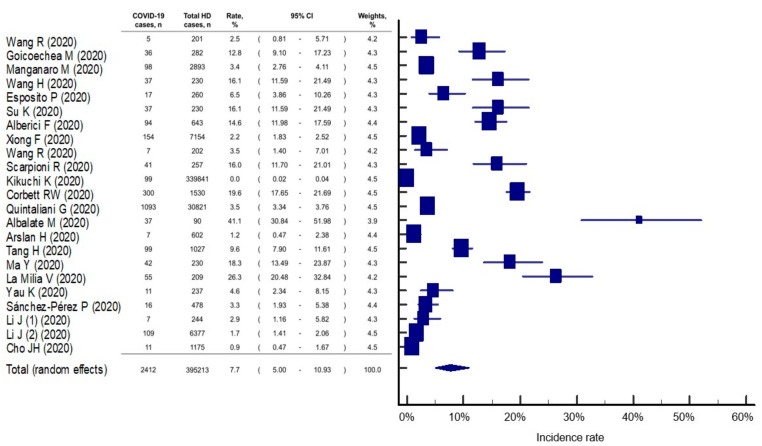
We found that the incidence of COVID-19 infection in patients receiving HD therapy was 7.7% (95% CI: 5.0–10.9%), but there was evidence of statistical heterogeneity among the studies (I2 = 99.7%, p < 0.001) (Figure 2). Egger’s test (p < 0.001) indicated a high risk of publication bias. Our meta-analysis also showed that the incidence of COVID-19 in HD patients was 5.0% (95% CI: 2.5–8.4%) and 10.5% (95% CI: 6.6–15.3%) in Asian and in non-Asian countries, respectively. In studies with good quality, the incidence was estimated at 5.2% (95% CI: 1.2–11.8%) of HD patients infected by COVID-19, but it was lower than in those studies with poor quality (8.7%, 95% CI: 6.4–11.2%).
Figure 2.
Incidence rate of COVID-19 infection in patients with hemodialysis. The study of Li J et al [13]. reported two independent cohorts that were analyzed separately for meta-analysis. HD, hemodialysis; CI, confidential interval.
Fever was the most predominant clinical manifestation (reported in 19 studies) and was observed in 889 of 1448 COVID-19 infected HD patients (61.4%, 95% CI: 40.2–65.5%), followed by cough (19 studies, 654 of 1398 patients, 46.8%, 95% CI: 25.7–44.7%), dyspnea (16 studies, 438 of 1246 patients, 35.2%, 95% CI: 16.9–36.6%) and fatigue (12 studies, 136 of 471 patients, 35.2%, 95% CI: 14.6–49.9%). Eleven studies reported hematological parameters of infected patients; however, most of them had white blood cells, lymphocytes, neutrophils and platelets within normal ranges (Table 1). From 16 studies reporting the presence of dyspnea or 11 studies reporting the use of chest radiography or tomography, only 12 studies have provided image descriptions. Bilateral lungs involvements with ground-glass opacities were the most common finding (11 studies, ranged from 43 to 100% of study population). However, negative radiological findings can also be found in 10–50% of HD patients [33,41].
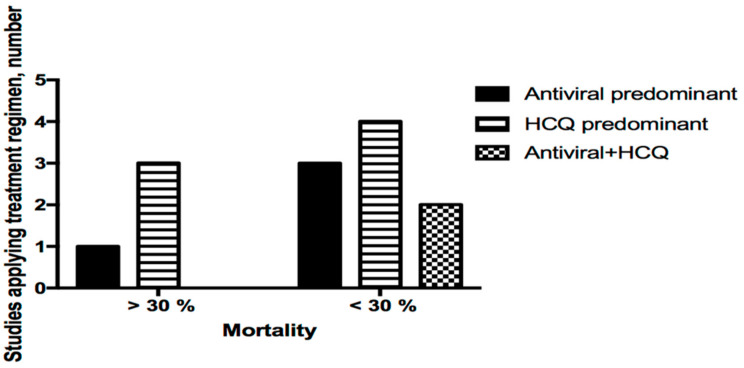
The treatment regimen for COVID-19 infection in HD patients is largely empirical and has been incomplete in most of the studies. Comparison of therapeutic effects between different treatment regimens was extremely difficult because information regarding the dosing, duration, and indication of prescription has been largely unknown among studies. Eleven studies reported the use of antiviral agents and hydroxychloroquine, seven studies reported the use of tocilizumab, and nine studies reported the use of corticosteroid. From the reported data, we identified three main treatment regimens: antiviral agent predominant, hydroxychloroquine predominant, or combination of antiviral agent plus hydroxychloroquine. Although reduced use of these regimens was more likely to have higher mortality (mortality > 30%, Figure 3), the exact effect remains to be proven in well-designed clinical trials.
Figure 3.
Treatment regimen used and proportion of high mortality (>30%) in different studies. Three main patterns were identified among 11 studies. Chi-square test, p = 0.587.
Inpatient care was needed in 1045 (82.5%) of 1267 patients, from 19 studies pooled. Moreover, 11 of these 19 studies required absolute hospitalization (100.0%) of their infected patients. Admission to intensive care unit occurred in 84 (6.6%) of patients. The mean in-hospital length of stay was 14.5 ± 8.8 days (data available for analysis from 8 studies). Sixteen studies described presence of the acute respiratory distress syndrome (ARDS). The syndrome was found in 133 of 717 infected cases (18.5%, 95% CI 4.5–21.7%). However, only six studies reported the number of patients using mechanical ventilator supports. The proportion of patients needing support ranged from 0 to 93% according to different studies.
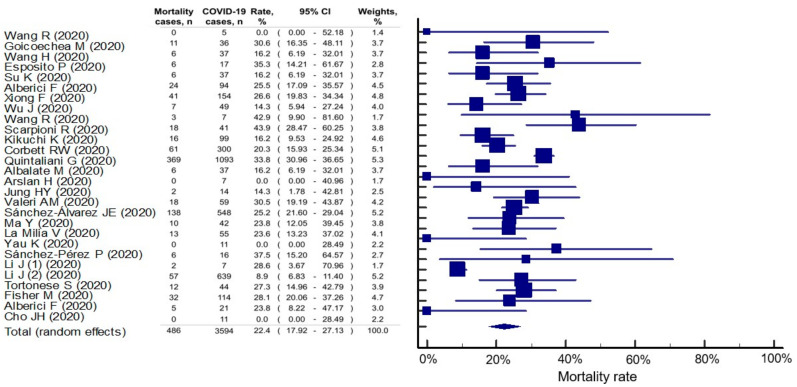
The overall mortality rate in HD patients with COVID-19 was 22.4% (95% CI: 17.9–27.1%), but significant statistical heterogeneity among the studies was found (I2 = 87.1%, p < 0.001) (Figure 4). However, based on the results of Egger’s test (P = 0.197), there was no publication bias in this outcome. Compared with those in Asian countries (17.0%, 95% CI: 11.4–23.5%), COVID-19-infected HD patients in non-Asian countries had a higher mortality rate (26.7%, 95% CI: 22.5–31.0%). In the studies with good quality, mortality was estimated at 23.8% (95% CI: 20.2–27.6%), which was similar in those studies with poor quality (21.6%, 95% CI: 14.5–29.6%).
Figure 4.
Mortality rate in hemodialysis patients with COVID-19 infection. The study of Li J et al [13]. reported two independent cohorts that were analyzed separately for meta-analysis. The second cohort of Li J et al. reported mortality in 57 deaths from 639 COVID-19 cases (all cases showed feature of viral pneumonitis and 109 were further confirmed by nuclear acid testing). HD, hemodialysis; CI, confidential interval. HD, hemodialysis; CI, confidential interval.
The causes of mortality were unreported in most of the studies. Only seven studies reported cause of mortality. The respiratory failure secondary to ARDS (63–100%) was the main cause of mortality, followed by cardia arrest, sepsis, and hyperkalemia.
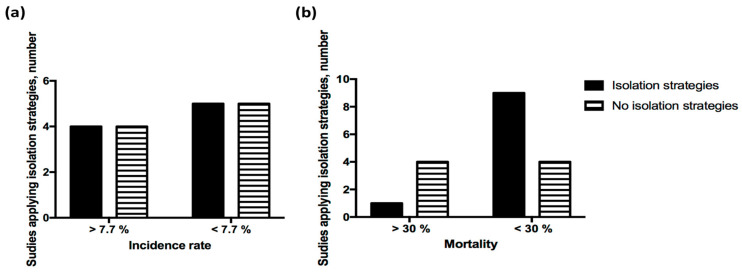
Twenty studies described the preventive strategies implemented for their HD patients. Ample alerts were observed in these studies regarding the use of protective measures. Masking was mandatory in the vast majority of HD facilities. Other preventive methods included the use of gloves, face shields, disposable gowns, caps, or alcohol sanitizer (Table 2). Isolation in independent areas of treatment was instructed rather than social distancing in some studies. However, the implementation of isolation of patients in dedicated areas had small influence in the incidence (Figure 5a) or mortality (Figure 5b) of HD patients.
Figure 5.
Impact of use of isolation strategies on the outcome of COVID-19 infection in HD patients. The impacts of applying isolation strategies at HD facilities on the (a) incidence (p = 1.0) and (b) mortality (p = 0.088) of COVID-19 was not significant. p value between groups by using Fisher’s exact test.
4. Discussion
The COVID-19 infection has been declared a global emergency affecting 0.7% of the 7.8 billion worldwide human population, with the burden still growing [1]. In spite of universal precautions adopted to prevent this infection in the HD community, the incidence of this novel viral infection remains high among HD patients. This systematic review and meta-analysis of 29 international studies, including 3261 confirmed COVID-19 cases, drawn from a pool of 396,062 HD patients, found that the incidence of COVID-19 infection was 7.7% and the mortality rate was 22.4%, i.e., higher than in the general population. Understanding of incidence, clinical presentation, and mortality related to COVID-19 in HD patients may help to design appropriate interventions for prevention, timely diagnosis, and treatment of this global challenge in this vulnerable population.
HD patients are more susceptible to COVID-19 infection because of greater age, coexistence of comorbidities, and immune-suppressed status [15,16]. Necessary, frequent visits to areas of high population density (public transportation or HD facilities) and close personal contacts (with medical, nursing, or caregiver staff) make effective strategies to prevent viral infection, such as social distancing or stay-home orders, difficult to implement for this select population of patients [28]. Accordingly, a 15.4-fold increase was noted in the incidence of COVID-19 in our study, with patients also being older compared to the general population [39]. The mean ages of patients was slightly greater (63.5 years) in patients of non-Asian studies than those of Asian studies (61.8 years). Variations in both criteria for viral screening and confirmatory methods of COVID-19 infection may also explain the difference in the incidence observed between studies of the two geographic areas. The difference in incidence observed between Asian and non-Asian populations may be greater than expected. Asian countries adopted universal screening using a nucleic acid test, serology, or computed tomography. The serologic antibody response is detectable 7 to 10 days or later after the onset of symptoms of COVID-infection in the general population [42]; however, the humoral response may extend from 14 to 55 days in HD patients [21]. By contrast, non-Asian countries, except for Canada [14], conducted viral screening only in symptomatic patients or patients at high risk of exposure, using mainly the nucleic acid test. The latter approach may mitigate the overwhelming burden on testing facilities; however, subclinical cases increase the difficulty of identifying COVID-19-infected HD patients and controlling outbreaks in the dialysis centers, which may lead to underestimation of the exact incidence of COVID-19 infection in asymptomatic HD patients. Manganos et al., at a very early stage of the disease outbreak, used radiographic signs suggestive of interstitial pneumonia as surrogate criteria for COVID-19 disease [25]. A nationwide serology screening involving 28,503 HD patients in the US found that seroprevalence was 8.3%, standardizing with the US dialysis population [43]; however, serology data were largely unreported in non-Asian studies. All these differences may confer heterogeneity to the global incidence observed in the HD population.
COVID-19-related mortality estimates range from 1.4% to 8% in the general population and are higher (25.5–39%) in hospitalized patients [2,3,36,44,45]. The prognosis of HD patients with COVID-19 remains unclear. We found overall mortality of 22.4% in HD patients infected with COVID-19. Previous literature has indicated several risk factors for high mortality in HD, including greater age, male gender, underlying cardiac or pulmonary disease, diabetes and hypertension and the use of mechanical ventilation [15,16,25]. Cough was associated with risk of mortality in French and Italian HD patients [23,34], fever predicted mortality in an Italian HD cohort [28]. Other prognostic factors have included dialysis vintage, thrombocytopenia, lymphopenia, and increased LDH or CRP level [23,28,36]. However, most studies have reported less severe clinical symptoms in HD patients compared with the general population [13,19,20,36]. In a Chinese series, the most common symptoms were fever, cough, and bilateral ground-glass or patchy opacity of the lungs [21]. However, a retrospective Chinese study comparing 49 HD vs. 52 non-renal failure patients having similar baseline characteristics found that fever, fatigue, and dry cough were more predominant in controls but less frequent in HD infected patients. In this series, fatigue and anorexia were the most common symptoms among HD-infected patients [18]. In addition, 25% of infected patients confirmed by nucleic acid test and 79% of those identified by serologic testing were asymptomatic during the whole clinical course [21]. Further large prospective studies, including different ethnicities, should be conducted to inform risk stratification with the ultimate goal of improving the outcome of HD patients with COVID-19 infection.
This viral infection can trigger severe immune cytokine storm, ARDS, and respiratory failure, leading to high risk of mortality [46]. Increased serum concentration of interleukin (IL)-2, IL-6, IL-7, granulocyte-colony stimulating factor, interferon-γ inducible protein 10, monocyte chemoattractant protein-1, macrophage inflammatory protein 1-α, tumor necrosis factor (TNF)-α, and ferritin have been observed in individuals infected with COVID-19 [46,47]. This hyper-inflammatory storm may play a role in the tissue damage and death of patients [4,48]; however, this response is blunted in infected HD patients. Several studies have revealed leukopenia, lymphopenia, lower serum calcium concentration, and elevated CRP levels in HD patients; however, other researchers have failed to find changes in numbers of granulocytes or lymphocytes in infected HD patients [17,18,19,21,22]. Ma Y et al. found that the counts of T cells, CD4 T cells, CD8 T cells, natural killer cells, and B lymphocytes were reduced in the peripheral blood of infected HD patients compared with non-HD patients. In contrast, the serum levels of IL-4, IL-6, IL-10, interferon-γ, and TNF-α were lowest in infected HD patients compared to non-infected HD patients or COVID-19-infected patients with normal renal function [20]. Further evidence of attenuated cytokine reaction in HD patients could be manifest in the low proportion of ARDS reported in various studies. Our meta-analysis indicated an overall incidence of ARDS of 18.5%, which is significantly lower than the reported incidence from hospitalized patients (33%) [49]. On the other hand, the reduction of activity of angiotensin-converting enzyme 2 (ACE2) was observed in HD patients compared to CKD or renal transplant recipients [50]. This transmembrane protein is the key factor for the COVID-19 virus entering the host cell [51]. Theoretically, the binding of viral particle to ACE2 induces the downregulation of this enzyme, increasing levels of bradykinin and its metabolite, the des-Arg(9)-bradykinin. In turn, this dysregulated bradykinin signaling upregulates pro-inflammatory genes and nuclear death signaling [52]. It is unknown whether the immuno-compromised status per se, the dysregulated bradykinin axis, or the hemodiafiltration/hemoperfusion may have facilitated cytokine clearance. Although these findings may prove beneficial for patient survival, they also imply protracted duration in eliminating the virus and hence persistent shedding in HD patients, which must be considered from a public health perspective. Studies investigating the dynamics of viral load in HD patients remain limited. Appropriate duration for quarantine or treatment course should be designed in future trials to avoid an inadvertent transmission of COVID-19 among HD patients.
Again, studies among HD patients from Asian countries have reported lower mortality (17.0%) than from non-Asian countries (26.7%). Asian patients are more likely to be young and have milder symptomatology than their non-Asian counterparts. The ubiquitous deployment of CT scan, especially in China, may have allowed better detection of severe lung condition feasible to timely intervention [22]. The optimal antiviral therapy for HD patients is largely unknown. Current consensus recommends the use of antiviral therapy in the first stage for viral clearance, followed up by immune-suppressive strategies (glucocorticoids or anti-cytokine drugs) to ameliorate cytokine injury [24]. Combinations of antibiotics or Chinese herbal medicine administrations were observed in Chinese studies [17,18,19]. We could not draw any solid conclusion from comparison of treatment regimen to patient outcome in our study. Further randomized controlled trials comparing the effectiveness and safety of different therapies should be undertaken in HD patients.
The findings of our study have several implications for clinical practice and also preventive medicine. The high incidence and subclinical presentation may prevent the timely identification of infected patients and may result in extensive spreading of virus in highly loaded medical areas. Universal testing to stop the dissemination of COVID-19 should be leveraged with the appropriate testing capacity. For infected HD patients, cautions regarding prolonged viral shedding and prudence in the use of immuno-suppressive agents should be considered, taking into account the blunted immune reaction of HD patients. Ultimately, given the multiple coexistent high-risk conditions, vaccination, if proven safe, should be prioritized for HD patients.
The results of our study provide a panoramic understanding of COVID-19 infection in HD patients. However, several limitations should be addressed. First, COVID-19 infection is unlikely to be eliminated in the near future, and more studies related to the epidemiology in HD patients with COVID-19 infection will be published after the presented work. Therefore, a regularly updated systematic review and meta-analysis is suggested to confirm our findings. Second, all included studies report mortality with COVID-19 infection in HD patients after short follow-up periods, while the long-term outcomes in this population are yet to be determined. Third, we could not derive all the important information from the included studies, even if we did contact the study authors for those data. To reduce the effect of possible reporting bias on our result estimates, we conducted subgroup analyses using the study quality, which showed similar findings to the overall analyses. Finally, we included studies reporting data of patients receiving in-center HD treatment. Data of dialysis patients undergoing different modalities, such as home-HD or peritoneal dialysis, remain unknown. We suggest the introduction of standardized international registry of COVID-19 infected dialysis patients to collect detailed patient characteristics and prognosis data, which would be beneficial for the fight against the current pandemic and for the further development of optimal management for dialysis patients.
5. Conclusions
This systematic review and meta-analysis of international studies has demonstrated a higher incidence of COVID-19 infection and related mortality among HD patients compared to the general population. Available data may underestimate the real incidence of infection because of subclinical presentation and screening criteria used for diagnosis. In spite of differences in incidence and mortality observed between Asian and non-Asian infected HD patients, the present data may provide insight for the design of surveillance and diagnosis strategies specific to HD patients. International collaboration and standardized reporting should be urgently initiated to refine consensus on the optimal management of this novel infection in dialysis patients.
Acknowledgments
We thank all researchers and clinicians involved in the individual trials.
Supplementary Materials
The following are available online at https://www.mdpi.com/2227-9032/9/1/47/s1, Table S1: Search strategy, Table S2: PRISMA checklist.
Author Contributions
I.-W.W. and S.-C.S. designed the study; C.-Y.C., S.-C.S., Y.-T.C. and C.-K.H. carried out the study; C.-Y.C., S.-C.S. and I.-W.W. analyzed the data; S.-C.S. made the figures; I.-W.W. and S.-C.S. drafted the paper; H.-J.H., C.-C.L., C.-Y.S., Y.-C.C. and M.-J.H. revised the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Chang Gung Memorial Hospital, grant number CGRPG2F0081 and CRRPG2H0121.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.WHO Coronavirus Disease (COVID-19) Dashboard. [(accessed on 30 November 2020)];2020 Available online: https://covid19.who.int.
- 2.McMichael T.M., Currie D.W., Clark S., Pogosjans S., Kay M., Schwartz N.G., Lewis J., Baer A., Kawakami V., Lukoff M.D., et al. Epidemiology of Covid-19 in a Long-Term Care Facility in King County, Washington. N. Engl. J. Med. 2020;382:2005–2011. doi: 10.1056/NEJMoa2005412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rao K.S., Suryaprakash V., Senthilkumar R., Preethy S., Katoh S., Ikewaki N., Abraham S.J.K. Role of immune dysregulation in increased mortality among a specific subset of COVID-19 patients and immune-enhancement strategies for combatting through nutritional supplements. Front. Immunol. 2020;11:1548. doi: 10.3389/fimmu.2020.01548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.GBD Chronic Kidney Disease Collaboration Global, regional, and national burden of chronic kidney disease, 1990-2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020;395:709–733. doi: 10.1016/S0140-6736(20)30045-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carney E.F. The impact of chronic kidney disease on global health. Nat. Rev. Nephrol. 2020;16:251. doi: 10.1038/s41581-020-0268-7. [DOI] [PubMed] [Google Scholar]
- 7.USRDS US renal data system 2018 annual data report. Am. J. Kidney Dis. 2019;73:S1. doi: 10.1053/j.ajkd.2019.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Betjes M.G. Immune cell dysfunction and inflammation in end-stage renal disease. Nat. Rev. Nephrol. 2013;9:255–265. doi: 10.1038/nrneph.2013.44. [DOI] [PubMed] [Google Scholar]
- 9.Henry B.M., Lippi G. Chronic kidney disease is associated with severe coronavirus disease 2019 (COVID-19) infection. Int. Urol. Nephrol. 2020;52:1193–1194. doi: 10.1007/s11255-020-02451-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PloS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alqahtani J.S., Oyelade T., Aldhahir A.M., Alghamdi S.M., Almehmadi M., Alqahtani A.S., Quaderi S., Mandal S., Hurst J.R. Prevalence, severity and mortality associated with copd and smoking in patients with COVID-19: A rapid systematic review and meta-analysis. PLoS ONE. 2020;15:e0233147. doi: 10.1371/journal.pone.0233147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu C., He Y., Liu L., Li F., Shi Y. Children with COVID-19 behaving milder may challenge the public policies: A systematic review and meta-analysis. BMC Pediatr. 2020;20:410. doi: 10.1186/s12887-020-02316-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li J., Yang Y., Gong M., Shi J., Zhou X., Xing X., Pan H., Guo S., Chang X., Cheng A., et al. Aggressive Quarantine Measures Reduce the High Morbidity of COVID-19 in Patients on Maintenance Hemodialysis and Medical Staff of Hemodialysis Facilities in Wuhan, China. Kidney Dis. 2020;6:271–283. doi: 10.1159/000508579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yau K., Muller M.P., Lin M., Siddiqui N., Neskovic S., Shokar G., Fattouh R., Matukas L.M., Beaubien-Souligny W., Thomas A., et al. COVID-19 Outbreak in an Urban Hemodialysis Unit. Am. J. Kidney Dis. 2020;76:690–695. doi: 10.1053/j.ajkd.2020.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang H. Maintenance hemodialysis and COVID-19: Saving lives with caution, care, and courage. Kidney Med. 2020;2:365–366. doi: 10.1016/j.xkme.2020.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Su K., Ma Y., Wang Y., Song Y., Lv X., Wei Z., Shi M., Ding G., Shen B., Wang H. How we mitigated and contained the COVID-19 outbreak in a hemodialysis center: Lessons and experience. Infect. Control Hosp. Epidemiol. 2020;41:1240–1242. doi: 10.1017/ice.2020.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xiong F., Tang H., Liu L., Tu C., Tian J.B., Lei C.T., Liu J., Dong J.W., Chen W.L., Wang X.H., et al. Clinical Characteristics of and Medical Interventions for COVID-19 in Hemodialysis Patients in Wuhan, China. J. Am. Soc. Nephrol. 2020;31:1387–1397. doi: 10.1681/ASN.2020030354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu J., Li J., Zhu G., Zhang Y., Bi Z., Yu Y., Huang B., Fu S., Tan Y., Sun J., et al. Clinical Features of Maintenance Hemodialysis Patients with 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. Clin. J. Am. Soc. Nephrol. 2020;15:1139–1145. doi: 10.2215/CJN.04160320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang R., Liao C., He H., Hu C., Wei Z., Hong Z., Zhang C., Liao M., Shui H. COVID-19 in Hemodialysis Patients: A Report of 5 Cases. Am. J. Kidney Dis. 2020;76:141–143. doi: 10.1053/j.ajkd.2020.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma Y., Diao B., Lv X., Zhu J., Chen C., Liu L., Zhang S., Shen B., Wang H. Epidemiological, Clinical, and Immunological Features of a Cluster of COVID-19-Contracted Hemodialysis Patients. Kidney Int. Rep. 2020;5:1333–1341. doi: 10.1016/j.ekir.2020.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tang H., Tian J.B., Dong J.W., Tang X.T., Yan Z.Y., Zhao Y.Y., Xiong F., Sun X., Song C.X., Xiang C.G., et al. Serologic Detection of SARS-CoV-2 Infections in Hemodialysis Centers: A Multicenter Retrospective Study in Wuhan, China. Am. J. Kidney Dis. 2020;76:490–499.e491. doi: 10.1053/j.ajkd.2020.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang R., He H., Liao C., Hu H., Hu C., Zhang J., Gao P., Wu X., Cheng Z., Liao M., et al. Clinical outcomes of hemodialysis patients infected with severe acute respiratory syndrome coronavirus 2 and impact of proactive chest computed tomography scans. Clin. Kidney J. 2020;13:328–333. doi: 10.1093/ckj/sfaa086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tortonese S., Scriabine I., Anjou L., Loens C., Michon A., Benabdelhak M., Ouali S., Morin G., Laifi M., Dobosziewicz H., et al. COVID-19 in Patients on Maintenance Dialysis in the Paris Region. Kidney Int. Rep. 2020;5:1535–1544. doi: 10.1016/j.ekir.2020.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alberici F., Delbarba E., Manenti C., Econimo L., Valerio F., Pola A., Maffei C., Possenti S., Piva S., Latronico N., et al. Management of patients on dialysis and with kidney transplantation during the SARS-CoV-2 (COVID-19) pandemic in Brescia, Italy. Kidney Int. Rep. 2020;5:580–585. doi: 10.1016/j.ekir.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Manganaro M., Baldovino S., Working Group of the Piedmont. Aosta Valley Section of the SI.N First considerations on the SARS-CoV-2 epidemic in the Dialysis Units of Piedmont and Aosta Valley, Northern Italy. J. Nephrol. 2020;33:393–395. doi: 10.1007/s40620-020-00732-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scarpioni R., Manini A., Valsania T., De Amicis S., Albertazzi V., Melfa L., Ricardi M., Rocca C. Covid-19 and its impact on nephropathic patients: The experience at Ospedale "Guglielmo da Saliceto" in Piacenza. G. Ital. Nefrol. 2020;37:2020-vol2022. [PubMed] [Google Scholar]
- 27.Esposito P., Russo R., Conti N., Falqui V., Massarino F., Moriero E., Peloso G., Traverso G.B., Garibotto G., Viazzi F. Management of COVID-19 in hemodialysis patients: The Genoa experience. Hemodial. Int. 2020;24:423–427. doi: 10.1111/hdi.12837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alberici F., Delbarba E., Manenti C., Econimo L., Valerio F., Pola A., Maffei C., Possenti S., Lucca B., Cortinovis R., et al. A report from the Brescia Renal COVID Task Force on the clinical characteristics and short-term outcome of hemodialysis patients with SARS-CoV-2 infection. Kidney Int. 2020;98:20–26. doi: 10.1016/j.kint.2020.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quintaliani G., Reboldi G., Di Napoli A., Nordio M., Limido A., Aucella F., Messa P., Brunori G., Italian Society of Nephrology, C.-R.G. Exposure to novel coronavirus in patients on renal replacement therapy during the exponential phase of COVID-19 pandemic: Survey of the Italian Society of Nephrology. J. Nephrol. 2020;33:725–736. doi: 10.1007/s40620-020-00794-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.La Milia V., Bacchini G., Bigi M.C., Casartelli D., Cavalli A., Corti M., Crepaldi M., Limardo M., Longhi S., Manzoni C., et al. COVID-19 Outbreak in a Large Hemodialysis Center in Lombardy, Italy. Kidney Int. Rep. 2020;5:1095–1099. doi: 10.1016/j.ekir.2020.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kikuchi K., Nangaku M., Ryuzaki M., Yamakawa T., Hanafusa N., Sakai K., Kanno Y., Ando R., Shinoda T., Nakamoto H., et al. COVID-19 of dialysis patients in Japan: Current status and guidance on preventive measures. Ther. Apher. Dial. 2020;24:361–365. doi: 10.1111/1744-9987.13531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cho J.H., Kang S.H., Park H.C., Kim D.K., Lee S.H., Do J.Y., Park J.W., Kim S.N., Kim M.S., Jin K., et al. Hemodialysis with Cohort Isolation to Prevent Secondary Transmission during a COVID-19 Outbreak in Korea. J. Am. Soc. Nephrol. 2020;31:1398–1408. doi: 10.1681/ASN.2020040461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jung H.Y., Lim J.H., Kang S.H., Kim S.G., Lee Y.H., Lee J., Chang H.H., Kim S.W., Choi J.Y., Cho J.H., et al. Outcomes of COVID-19 among Patients on In-Center Hemodialysis: An Experience from the Epicenter in South Korea. J. Clin. Med. 2020;9:1688. doi: 10.3390/jcm9061688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sánchez-Álvarez J.E., Pérez Fontán M., Jiménez Martín C., Blasco Pelícano M., Cabezas Reina C.J., Sevillano Prieto Á.M., Melilli E., Crespo Barrios M., Macía Heras M., Del Pino Y.P.M.D. SARS-CoV-2 infection in patients on renal replacement therapy. Report of the COVID-19 Registry of the Spanish Society of Nephrology (SEN) Nefrologia. 2020;40:272–278. doi: 10.1016/j.nefro.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Albalate M., Arribas P., Torres E., Cintra M., Alcazar R., Puerta M., Ortega M., Procaccini F., Martin J., Jimenez E., et al. High prevalence of asymptomatic COVID-19 in haemodialysis: Learning day by day in the first month of the COVID-19 pandemic. Nefrologia. 2020;40:279–286. doi: 10.1016/j.nefro.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goicoechea M., Sanchez Camara L.A., Macias N., Munoz de Morales A., Rojas A.G., Bascunana A., Arroyo D., Vega A., Abad S., Verde E., et al. COVID-19: Clinical course and outcomes of 36 hemodialysis patients in Spain. Kidney Int. 2020;98:27–34. doi: 10.1016/j.kint.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sánchez-Pérez P., González-Calero P., Poma-Saavedra F.H., Orero-Calvé E., Devesa-Such R., Soldevila-Orient A., Henningsmeyer-Utrera B., Lacueva-Moya J. Results of a healthcare organization model for COVID-19 on hemodialysis in a tertiary hospital and its subsidized centers. Nefrologia. 2020;40:453–460. doi: 10.1016/j.nefro.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arslan H., Musabak U., Ayvazoglu Soy E.H., Kurt Azap O., Sayin B., Akcay S., Haberal K.M., Akdur A., Yildirim S., Haberal M. Incidence and Immunologic Analysis of Coronavirus Disease (COVID-19) in Hemodialysis Patients: A Single-Center Experience. Exp. Clin. Transplant. 2020;18:275–283. doi: 10.6002/ect.2020.0194. [DOI] [PubMed] [Google Scholar]
- 39.Corbett R.W., Blakey S., Nitsch D., Loucaidou M., McLean A., Duncan N., Ashby D.R., West London R., Transplant C. Epidemiology of COVID-19 in an Urban Dialysis Center. J. Am. Soc. Nephrol. 2020;31:1815–1823. doi: 10.1681/ASN.2020040534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Valeri A.M., Robbins-Juarez S.Y., Stevens J.S., Ahn W., Rao M.K., Radhakrishnan J., Gharavi A.G., Mohan S., Husain S.A. Presentation and Outcomes of Patients with ESKD and COVID-19. J. Am. Soc. Nephrol. 2020;31:1409–1415. doi: 10.1681/ASN.2020040470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fisher M., Yunes M., Mokrzycki M.H., Golestaneh L., Alahiri E., Coco M. Chronic hemodialysis patients hospitalized with COVID-19: Short-term outcomes in the bronx, New York. Kidney360. 2020;1:755–762. doi: 10.34067/KID.0003672020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.To K.K.-W., Tsang O.T.-Y., Leung W.-S., Tam A.R., Wu T.-C., Lung D.C., Yip C.C.-Y., Cai J.-P., Chan J.M.-C., Chik T.S.-H., et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: An observational cohort study. Lancet Infect. Dis. 2020;20:565–574. doi: 10.1016/S1473-3099(20)30196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Anand S., Montez-Rath M., Han J., Bozeman J., Kerschmann R., Beyer P., Parsonnet J., Chertow G.M. Prevalence of SARS-CoV-2 antibodies in a large nationwide sample of patients on dialysis in the USA: A cross-sectional study. Lancet. 2020;396:1335–1344. doi: 10.1016/S0140-6736(20)32009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mehra M.R., Desai S.S., Kuy S., Henry T.D., Patel A.N. Cardiovascular Disease, Drug Therapy, and Mortality in Covid-19. N. Engl. J. Med. 2020;382:e102. doi: 10.1056/NEJMoa2007621. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 45.Cummings M.J., Baldwin M.R., Abrams D., Jacobson S.D., Meyer B.J., Balough E.M., Aaron J.G., Claassen J., Rabbani L.E., Hastie J., et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: A prospective cohort study. Lancet. 2020;395:1763–1770. doi: 10.1016/S0140-6736(20)31189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. HLH Across Speciality Collaboration, UK. COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wiersinga W.J., Rhodes A., Cheng A.C., Peacock S.J., Prescott H.C. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review. JAMA. 2020;324:782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 48.Henry B.M., de Oliveira M.H.S., Benoit S., Plebani M., Lippi G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): A meta-analysis. Clin. Chem. Lab. Med. 2020;58:1021–1028. doi: 10.1515/cclm-2020-0369. [DOI] [PubMed] [Google Scholar]
- 49.Tzotzos S.J., Fischer B., Fischer H., Zeitlinger M. Incidence of ARDS and outcomes in hospitalized patients with COVID-19: A global literature survey. Crit. Care. 2020;24:516. doi: 10.1186/s13054-020-03240-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roberts M.A., Velkoska E., Ierino F.L., Burrell L.M. Angiotensin-converting enzyme 2 activity in patients with chronic kidney disease. Nephrol. Dial. Transplant. 2013;28:2287–2294. doi: 10.1093/ndt/gft038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bourgonje A.R., Abdulle A.E., Timens W., Hillebrands J.L., Navis G.J., Gordijn S.J., Bolling M.C., Dijkstra G., Voors A.A., Osterhaus A.D., et al. Angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 and the pathophysiology of coronavirus disease 2019 (COVID-19) J. Pathol. 2020;251:228–248. doi: 10.1002/path.5471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Roche J.A., Roche R. A hypothesized role for dysregulated bradykinin signaling in COVID-19 respiratory complications. FASEB J. 2020;34:7265–7269. doi: 10.1096/fj.202000967. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.