Abstract
Objective
The objectives of this study were to determine whether patients reporting symptoms are more likely to develop lymphedema and to describe the temporal relationship between symptom onset and lymphedema.
Methods
This was a prospective longitudinal cohort study of 647 women treated for breast cancer and screened for lymphedema using arm volume measurements and subjective questionnaires (n = 647; 2284 questionnaires [median 3.5 per patient, range = 1–24]). Primary study outcome was lymphedema (relative volume change ≥10%). The Kaplan–Meier method was used to estimate cumulative lymphedema incidence. Cox proportional hazards models were used to assess the relationship between symptoms, other risk factors, and lymphedema.
Results
A total of 64 patients (9.9%) developed lymphedema. On multivariable analysis, patients reporting increased arm size (hazard ratio = 3.09, 95% CI = 1.62–5.89) were more likely to progress to lymphedema than those who did not report this symptom. Of those who developed lymphedema, 37 (58%) reported an increased arm size a median of 6.1 months before lymphedema onset (range = 68.6 months before to 50.2 months after lymphedema onset).
Conclusion
Patients at risk of lymphedema who report increased arm size might do so prior to lymphedema onset and are at 3 times the risk of lymphedema as patients not reporting this symptom. Even without objective or observable edema, these patients should be followed vigilantly and considered for early intervention. Symptoms should be incorporated into screening and diagnostic criteria for lymphedema.
Impact
This study shows that patients at risk for breast cancer–related lymphedema who report increased arm size should be considered at high risk for progression to lymphedema—even without edema on measurement or clinical examination—and should be followed vigilantly, with consideration of early intervention.
Lay summary
If you are at risk of lymphedema and you feel as though your arm size has increased, you might develop lymphedema, and you are at 3 times the risk of lymphedema as patients not reporting this symptom. Even without measurable or observable edema, you should be followed vigilantly and consider early intervention.
As treatment for breast cancer (BC) has evolved over the past 2 decades, the 5-year survival rate has improved and is currently approximately 90%.1 Patients who have been treated for BC may encounter a myriad of health problems and challenges throughout their survivorship, which may include peripheral neuropathy, cancer-related fatigue, unintended weight fluctuations, cardiovascular complications, induced menopausal symptoms, osteoporosis, or BC-related lymphedema (BCRL), the focus of this manuscript.2–4 Optimizing patient care through prospective surveillance and medical and lifestyle risk reduction throughout survivorship is paramount.3,4
Approximately 20% of the 3.5 million women in the United States who have been treated for BC will develop BCRL, a chronic disease characterized by impaired lymphatic function and lymphatic fluid accumulation in the affected upper extremity, breast, or trunk.5 BCRL is but 1 sequela of BC treatment; however, it is highly feared amongst those treated for BC,6–8 and its treatment is burdensome and stressful. Established risk factors for BCRL include axillary lymph node dissection (ALND),5,9–13 nodal radiation, body mass index ≥30 kg/m2 at time of BC diagnosis,14–18 low-volume edema,19 and lack of breast reconstruction.20–23 Other potential risk factors evolving in the literature include chemotherapy (neoadjuvant, taxane-based, trastuzumab),11,24–27 tumor-specific features (lympho-vascular invasion and extranodal extension)27 and genetic predisposition.28,29 The risk of BCRL remains for a lifetime and there is no cure.
Historically, BCRL has been diagnosed via an impairment-based model, that is, when a patient presents with clinically apparent swelling and symptoms. There is momentum toward a prospective screening model for BCRL aimed at early diagnosis in which patients receive a preoperative baseline measurement and are followed at regular intervals postoperatively. In addition to objective measures, BCRL screening should integrate clinical examination and patient-reported symptoms.30–36 Although BCRL screening is recommended by the National Comprehensive Cancer Network, the International Society of Lymphology, the American Society of Breast Surgeons, and the National Lymphedema Network,4,34,37–39 it is not yet standard of care.
Symptoms may reflect developing or existing BCRL. Armer et al found that BCRL was predicted by reports of heaviness and numbness in the past year and also by swelling at the time of symptom survey.32 They concluded that symptoms might indicate early BCRL and recommended symptom monitoring as part of screening.32 A further prospective study compared 4 BCRL diagnostic criteria (200 mL, 10% volume, 2-cm circumference change from baseline, and symptoms) over 24 months, in which symptoms were the earliest predictor of BCRL.40 Two additional groups found patients reporting symptoms were more likely to develop BCRL than those who did not.33,41 Limitations of these studies include using patient self-report for BCRL diagnosis33,42,43; diagnostic criteria lacking a baseline30–32,42 which results in 40% to 50% misdiagnosis44; limited follow-up time32,41; and small sample size.30–32 Incorporation of symptoms into screening and diagnostic criteria for BCRL has not been well established as there is not yet 1 symptom or group of symptoms that indicate BCRL. In addition, there is lack of agreement between incidences of arm swelling via patient report versus objective measurement.10 The relationship between symptoms and BCRL must be better defined. Given that most facilities are not screening for BCRL, identifying evidence-based methods that may predict BCRL in absence of a formal screening program is imperative. Utilizing symptoms in this manner would allow for patients to self-screen in the community setting as part of an informal screening program.
The aims of this study are to quantify increased risk of progression to BCRL for patients reporting specific symptoms compared with those who do not report those symptoms and to determine the temporal relationship between symptom onset and increased arm volume by objective measurement. This study also aims to make recommendations regarding incorporation of symptoms into BCRL screening and diagnostic criteria.
Methods
Patient Population
This is a prospective longitudinal cohort study of symptoms and arm volumes obtained throughout BCRL screening at Massachusetts General Hospital (MGH) (ClinicalTrials.gov Number: NCT01521741).45 Patients are screened for BCRL with perometry (Perometer 350 NT, Pero-System Messgeräte, Wuppertal, Germany) and patient-reported outcome measure (PROM) at BC diagnosis, during active treatment, every 6 to 12 months for the first 5 years after surgery and yearly thereafter, or upon any signs or symptoms of swelling.45 Perometry is a valid, reliable, and diagnostically accurate arm volume measure.46–49 The PROM utilized for this study is a hybrid of 4 validated outcome measures, including the Disabilities of the Arm, Shoulder and Hand,50 the Functional Assessment of Cancer Therapy—Breast Cancer +4,51–54 the Arm Activity Survey,55 and the Lymphedema Breast Cancer Questionnaire,32,56 which are all reliable and valid outcome measures of function, symptoms, and quality of life (QOL) for women who have been treated for BC and/or are at risk of BCRL.
From 2009 to 2018, we screened more than 5500 patients at risk for BCRL starting at preoperative baseline. The inclusion criteria for this study included unilateral BC surgery with lymph node staging [sentinel lymph node biopsy (SLNB) or ALND], preoperative perometry, and ≥1 PROM filled out on the same day of perometry measurement ≥12 weeks postoperatively. There were 647 patients who met the inclusion criteria and completed 2284 PROM (median 3.5 per patient, range 1–24). Patient demographics and treatment-related variables were obtained by medical record review (Tab. 1). Verbal consent of all participants was obtained in accordance with institutional policy.
Table 1.
Demographic and Treatment-Related Data (N = 647)a
| Patient | Variable | n | % |
|---|---|---|---|
| Age at diagnosis (range: 27–83, mean 56.6 y) | <50 y | 188 | 29.1% |
| 50–69 y | 405 | 62.6% | |
| ≥ 70 y | 54 | 8.3% | |
| Breast surgery | Mastectomy | 150 | 23.2% |
| Lumpectomy | 497 | 76.8% | |
| Chemotherapy | Neoadjuvant chemo | 18 | 2.8% |
| Adjuvant chemo | 200 | 30.9% | |
| No chemo | 429 | 66.3% | |
| Body mass index (range 16.1–58.9, mean 27.7) | BMI ≥ 25 | 399 | 61.7% |
| BMI < 25 | 248 | 38.3% | |
| Axillary surgery | ALND | 120 | 18.6% |
| SLNB | 461 | 71.3% | |
| No axillary surgery | 66 | 10.2% | |
| Regional lymph node radiation | RLNR | 151 | 23.3% |
| No RLNR | 496 | 76.7% |
a BMI = body mass index; ALND = axillary lymph node dissection; SLNB = sentinel lymph node biopsy; RLNR = regional lymph node radiation.
Arm Volume Quantification and BCRL Treatment
Postoperative arm volume changes were calculated for patients undergoing unilateral BC surgery using the relative volume change (RVC) formula:34 RVC =  , where A1 and A2, U1 and U2 are arm volumes of the affected and unaffected arm at preoperative baseline and follow-up, respectively.57 BCRL is defined as an RVC ≥ 10% occurring >3 months after surgery40,58, and we assessed the outcome of RVC ≥ 5% given varied definitions of early BCRL. Of note, at MGH, patients are referred to a certified lymphedema therapist for individualized evaluation and treatment of BCRL at RVC ≥ 10%. Treatment will vary based on individual need and includes some or all of the following: patient education, compression bandaging or garments, manual or self-lymphatic drainage, kinesiotaping, skin care, and exercise. Historically, patients at MGH were not treated at 5% to <10%; therefore, patients in this study with RVC 5% to <10% did not receive treatment.
, where A1 and A2, U1 and U2 are arm volumes of the affected and unaffected arm at preoperative baseline and follow-up, respectively.57 BCRL is defined as an RVC ≥ 10% occurring >3 months after surgery40,58, and we assessed the outcome of RVC ≥ 5% given varied definitions of early BCRL. Of note, at MGH, patients are referred to a certified lymphedema therapist for individualized evaluation and treatment of BCRL at RVC ≥ 10%. Treatment will vary based on individual need and includes some or all of the following: patient education, compression bandaging or garments, manual or self-lymphatic drainage, kinesiotaping, skin care, and exercise. Historically, patients at MGH were not treated at 5% to <10%; therefore, patients in this study with RVC 5% to <10% did not receive treatment.
Symptoms
Eleven symptoms regarding the arm ipsilateral to surgery were analyzed: swelling; firmness/tightness; heaviness; stiffness; aching; increased arm size, shoulder size, or neck size; tighter sleeve fit, cuff fit, and ring fit. All symptoms are found in the Lymphedema and Breast Cancer Questionnaire,32,59 which has been validated in patients treated for BC, with and without BCRL. Patients were asked if they had either experienced the symptom in the past month or if there had been a change over the past month.32,59
Statistical Methods
The Kaplan–Meier method was used to estimate the 1- and 2-year cumulative incidence of BRCL outcomes (RVC ≥ 10%, RVC ≥ 5%). Univariate and multivariable Cox proportional hazards models were used to assess the relationship between symptoms, other risk factors, and BCRL risk. These models used the counting process approach to incorporate longitudinal symptoms measurements. Models evaluated the association between symptom status (present vs absent) at current measurement and risk of subsequent BRCL. Non-symptom risk factors included age at diagnosis, body mass index (BMI), type of breast and axillary surgery, type of radiotherapy, and current RVC. A multivariable model was derived for each BRCL outcome using backward selection among symptoms that were significant (P < .2) in the univariate models. All non-symptom risk factors were included in the multivariable models regardless of statistical significance. RStudio Version 1.2.1335 (RStudio, Boston, MA, USA) and SAS 9.4 (SAS Institute Inc., Cary, NC, USA) were used for statistical analysis.
Role of the Funding Source
The funders played no role in the design, conduct, or reporting of this study. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Results
Sample Characteristics
Of 647 patients, 150 (23.2%) and 497 (76.8%) underwent mastectomy and lumpectomy, respectively; 18 (2.8%) and 200 (30.9%) had neoadjuvant and adjuvant chemotherapy, respectively; 399 (61.7%) had a preoperative BMI ≥25 kg/m2, 120 (18.6%) underwent ALND, and 151 (23.3%) underwent RLNR (Tab. 1). The cumulative incidence of RVC ≥ 5% at 12 and 24 months was 22.1% and 36.4% and for RVC ≥ 10% it was 4.0% and 8.2%, respectively (Tab. 2).
Table 2.
Maximum Volume Reached, Time to Onset and Symptoms, Cumulative Incidence of BCRLa
| Maximum Volume Reached (RVC) | |||
|---|---|---|---|
| <5% | ≥5% | ≥10% | |
| Median time to onset of max volume (mo) | – | 11.48 | 16.86 |
| Total no. of patients surveyed (N = 647b) | 393 (60.7%) | 254 (39.3%) | 64 (9.9%) |
| No. of patients with reported symptoms (n = 547) | 313 (79.6%) | 234 (92.1%) | 61 (95.3%) |
| Cumulative incidence of BRCL (no. progressions before timepoint) 12 mo 24 mo |
– | 22.1% (134) 36.4% (203) |
4.0% (24) 8.2% (42) |
a BCRL = breast cancer-related lymphedema; RVC = relative volume change.
b Row totals do not sum to these values because patients with RVC ≥ 10% are included in both the ≥10% and ≥5% columns.
Symptoms in Patients With Edema During Study Follow-up
Of 647 patients, 393 (60.7%) never experienced an RVC of ≥5%; however, many (313, 79.6%) reported symptoms during follow-up. There were 64 (9.9%) and 254 (39.3%) patients who progressed to RVC ≥ 10% and RVC ≥ 5%, respectively, during the study period. Of these, 61 patients (95.3%) with RVC ≥ 10% and 234 (92.1%) with RVC ≥ 5% reported symptoms. (Tab. 2). Figure 1 illustrates the timing of symptom report stratified by RVC. Note that group size is not consistent across RVC levels. While many symptoms are reported within the first year postoperatively, patients at all edema levels reported symptoms more than 6 years postoperatively.
Figure 1.
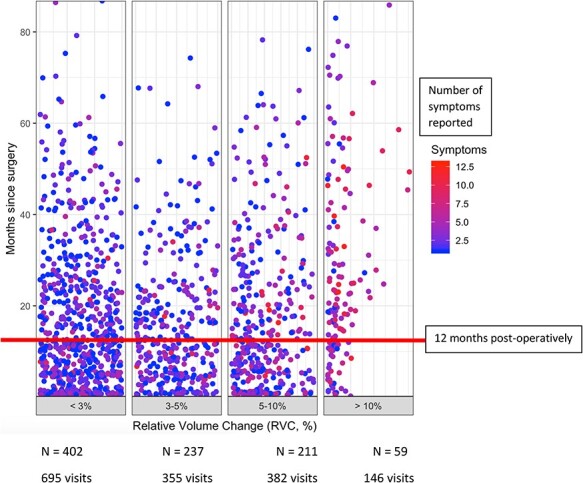
Timing and number of symptoms reported after breast cancer surgery stratified by RVC. Patients reported symptoms at all levels of objective edema (RVC < 3%, 3% to <5%, 5% to <10%, and ≥10%), with many reporting these symptoms within the first 12 months postoperatively, likely secondary to BC treatments during this time. Patients, however, reported symptoms up to >7 years postoperatively, with many patients reporting several symptoms at a time (red and purple data points).
Symptoms in Patients Without Edema During Study Follow-up
The patients who did not develop edema (ie, never had RVC ≥ 5%) reported a median of 2 symptoms in any given survey (range 0, 10) and reported symptoms up to 86.4 months postoperatively (median time of symptom report 12.1 months postoperatively, range 0.2–86.4 months). The most commonly reported symptoms in this group included tenderness, aching, and firmness/tightness (reported by 58.7%, 43.2%, and 35.9%, respectively).
Association of Symptoms and Other Risk Factors With Progression to RVC ≥ 10%
On univariate analysis, 5 patient-reported symptoms, including increased arm size, tighter sleeve fit, tighter sleeve cuff fit, or tighter ring fit as well as swelling, were associated with risk of subsequent progression to RVC ≥ 10% (P < .2; Tab. 3). In addition, preoperative BMI > 30 kg/m2 and mastectomy, ALND, RLNR, and RVC exceeding 3%–5% and >5% were moderately associated with subsequent progression. On multivariable analysis, change in arm size was the only symptom that remained significantly associated (P < .05) with progression to RVC ≥ 10%. Patients reporting an increase in arm size were at over 3-fold increased risk of progression to RVC ≥ 10% after adjusting for non-symptom risk factors (HR = 3.09, 95% CI = 1.62–5.89, P = .001). RVC exceeding 5% was also significantly associated with increased risk of subsequent progression to RVC > 10% in the multivariable model (HR = 5.64, 95% CI = 2.74–11.61, P < .001).
Table 3.
Association Between Progression to RVC ≥ 5% and ≥10% and Presence of Symptoms and Other Risk Factors
| RVC ≥ 10% | RVC ≥ 5% | |||||
|---|---|---|---|---|---|---|
| Multivariable Results | Multivariable Results | |||||
| Symptoms and Other Risk Factors | Univariate Results for Hazard Ratio a | Hazard Ratio a (95% CI) | P | Univariate Results for Hazard Ratio a | Hazard Ratio a (95% CI) | P |
| Symptom | ||||||
| Change in arm size | 6.44b | 3.09 (1.62–5.89) | .001 | 2.20b | 1.91 (1.21–3.04) | .006 |
| Change in sleeve size | 4.17b | – | – | 1.67c | – | – |
| Change in sleeve cuff size | 6.11d | – | – | 2.32 | – | – |
| Change in ring size | 1.82c | – | – | 1.29 | – | – |
| Heaviness | 1.57 | – | – | 0.58c | – | – |
| Swelling | 3.24b | – | – | 1.19 | – | – |
| Firmness/tightness | 1.28 | – | – | 0.89 | – | – |
| Stiffness | 1.19 | – | – | 0.61d | – | – |
| Aching | 1.22 | – | – | 0.80c | – | – |
| Tenderness | 1.03 | – | – | 1.10 | – | – |
| Numbness | 1.48 | – | – | 1.13 | – | – |
| Other risk factors | ||||||
| Age at diagnosis <50 y | 1.19 | 1.15 (0.60–2.20) | .684 | 1.49d | 1.59 (1.12–2.26) | .009 |
| BMI ≥ 30 | 1.75d | 1.59 (0.89–2.83) | .118 | 1.33c | 1.21 (0.89–1.65) | .233 |
| Mastectomy vs lumpectomy | 1.57c | 1.12 (0.57–2.18) | .749 | 0.85 | 0.68 (0.45–1.01) | .053 |
| ALND vs SLNB/none | 3.14b | 2.18 (0.96–4.99) | .064 | 1.46d | 1.75 (1.08–2.83) | .023 |
| RLNR vs other/none | 2.62b | 1.25 (0.54–2.91) | .601 | 1.29c | 1.18 (0.75–1.86) | .475 |
| RVC 3–5% vs <3% >5% vs <3% |
2.03c 8.64b |
1.86 (0.83–4.18) 5.64 (2.74–11.61) |
.132 < .001 |
n/a | n/a | n/a |
| RVC > 3% vs <3% | n/a | n/a | n/a | 2.00b | 1.88 (1.42–2.51) | <.001 |
a Hazard ratios for the development of lymphedema (RVC ≥ 10% or RVC ≥ 5%) in patients with a given symptom/risk factor vs those without it are shown. For univariate hazard ratios, the level of significance (P) was determined with the Wald test. Multivariable results in bold type were significant at P < .05. ALND = axillary lymph node dissection; BMI = body mass index; RLNR = regional lymph node radiation; RVC = relative volume change; SLNB = sentinel lymph node biopsy.
b P < .01.
c .05 ≤ P < .2.
d .01 ≤ P < .05.
Association of Symptoms and Other Risk Factors With Progression to RVC ≥ 5%
On univariate analysis, patient-reported symptoms (while at an RVC < 5%), including increased arm size, tighter sleeve fit, heaviness, stiffness, and aching, were at least moderately associated with risk of subsequent progression to RVC ≥ 5% (P < 0.2; Tab. 3). Non-symptom risk factors at least moderately associated with progression (P < 0.2) included being younger than 50 years of age at BC diagnosis, preoperative BMI > 30 kg/m2, ALND, RLNR, and elevated RVC > 3%. Consistent with the RVC ≥ 10% outcome, report of increased arm size remained significantly associated (P < .05) with risk of subsequent RVC ≥ 5% on multivariable analysis. When adjusting for non-subjective risk factors, a report of increased arm size was associated with a nearly 90% increased risk of progression to RVC ≥ 5% (HR = 1.91, 95% CI = 1.21–3.04, P = .006). Non-subjective risk factors significantly associated with increased risk of subsequent RVC ≥ 5% included age younger than 50 years at BC diagnosis (HR = 1.59, 95% CI = 1.12–2.26, P = .009), ALND (HR = 1.75, 95% CI = 1.08–2.83, P = .023), and RVC > 3% (HR = 1.88, 95% CI = 1.42–2.51, P < .001).
Symptoms and Timeline of Progression to BCRL
Median time to report of any symptom(s) indicative of a change in size of a body part that may be attributed to swelling, such as increased arm size, increased shoulder size, increase in neck size, increase in sleeve tightness, increase in sleeve cuff tightness, increase in ring tightness, or heaviness, was >12 months post-surgery (15.8, 16.2, 12.9, 18.9, 21.8, 17.9, and 15.8 months post-surgery, respectively). Timing to first report of these symptoms was 9.6, 9.7, 12.1, 11.3, 11.8, 11.1, and 7.6 months, respectively. Alternatively, median time to any report of symptoms associated with BC treatment or BCRL, such as swelling, firmness/tightness, stiffness, aching, tenderness, and numbness was ≤ 13.4 months post-surgery (11.5, 12.8, 13.4, 13.1, 10.9, and 12.4 months, respectively). Median time to first report of these symptoms was 4.6, 5.8, 6.6, 5.3, 3.0, and 5.1 months, respectively.
Report of increased arm size was associated with progression to RVC ≥ 10% in the multivariable model; therefore, it is important to understand how this symptom was reported by those who developed BCRL. The temporal relationship between the report of this symptom and onset of BCRL is illustrated in Figure 2. Of the 64 patients who developed BCRL (RVC ≥ 10%), 37 (58%) initially reported an increase in arm size a median of 6.1 months before BCRL onset (range 68.6 months before to 50.2 months after BCRL onset). Of the 254 patients who developed RVC ≥ 5% (39.3% of the cohort), the median time to report an increased arm size was at the date of development of RVC ≥ 5% (range 68.8 months before to 51.1 months after onset of RVC ≥ 5%).
Figure 2.
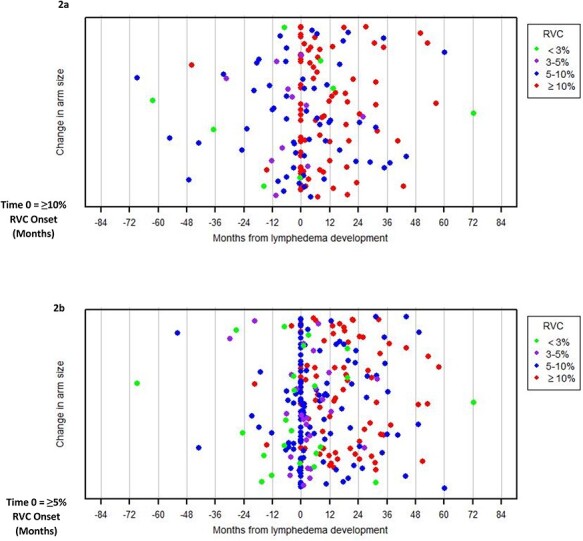
Temporal relationship between patient-reported change in arm size at varying edema levels and onset of BCRL. (a) Of the 64 patients who developed BCRL (RVC ≥ 10%), 37 (58%) initially reported an increase in arm size a median of 6.1 months before BCRL onset (range 68.6 months before to 50.2 months after BCRL onset). Red dots prior to time 0 indicate a report of change in arm size at an RVC ≥ 10% within 3 months postoperatively. (b) Of the 254 patients who developed RVC ≥ 5% (39.3% of the cohort), the median time to report an increased arm size was at the date of development of RVC ≥ 5% (range 68.8 months before to 51.1 months after onset of RVC ≥ 5%). Red and blue dots prior to time 0 indicate a report of change in arm size at RVC ≥ 10% and RVC ≥ 5%, respectively, within 3 months postoperatively.
Discussion
A patient cohort prospectively screened for BCRL is imperative to isolate the role of symptoms in BCRL development. This study cohort reflects the current trend toward prospective surveillance and the transition toward SLNB (for clinically node-negative and 1–2 positive nodes) and away from ALND (which introduces higher BCRL risk) to assess lymph node status in clinically node-negative BC, with RLNR being non-inferior to ALND for patients with 1–2 positive nodes on SLNB in a phase III trial, in terms of 5-year recurrence.60 In this study, 5.9% and 23.3% of those who underwent SLNB ± RLNR and ALND ± RLNR, respectively, progressed to BCRL (RVC ≥ 10%), consistent with previous findings.19
Many patients develop low-volume edema (RVC 5 to <10%) after BC treatment without subsequent progression to RVC ≥ 10%. Specht et al.19 found that 81.3% of patients who developed low-volume edema (RVC 5% to <10%) did not progress to BCRL at 24 month follow-up in absence of treatment. Over 29% of this study cohort developed low-volume edema (RVC 5 to ≤10%) but did not progress to RVC ≥ 10% despite more than 2 years of follow-up. This is an important distinction, as there has been a trend toward treatment of BCRL at RVC ≥ 5%. There is preliminary evidence that has found benefit of early intervention (compression sleeve, education, or exercise) in limiting progression from low-volume edema to BCRL (RVC ≥ 10%).61–66 However, these studies carry significant limitations such as small sample size, lack of a control group, varied measurements between the control and intervention groups, or use of 2-cm circumference difference to define BCRL. In addition, the vast majority of patients with low-volume swelling do not progress to BCRL. For example, in a cohort of 1173 patients prospectively screened for BCRL with a median follow-up of 27 months, only 18.7% of patients with low-volume swelling (RVC 5 to <10%) progressed to BCRL (RVC ≥ 10%) in absence of treatment. It is imperative to identify which patients in this group are at risk of progression and which are not to avoid BCRL overdiagnosis and overtreatment. Consideration for individualized conservative treatment for patients with low-volume edema (eg, compression sleeve, stretching, patient education) may be considered based on integration of risk factors, symptoms, and clinical examination. Beyond low-volume edema, prospective BCRL screening is imperative to capture BCRL at 10%, at which point intensive complete decongestive therapy, including compression bandaging, manual lymphatic drainage, exercise, and self-care, is indicated.
The majority (>79%) of patients in all RVC groups, including those with no edema (0% to <3% and 3% to <5%), reported symptoms after BC treatment. Many of these symptoms are not indicative of BCRL but may be secondary to BC treatment, especially during the first year postoperatively (Fig. 1). Although frequency of symptom report decreases, patients report symptoms for years and, in most cases, these symptoms are in absence of edema progression. This underlines the importance of identifying symptoms that may predict BCRL and to distinguish between symptoms related to BC treatment and those associated with BCRL. In this study, symptoms associated with perceived change in limb size (increased arm size; sleeve, sleeve cuff, or ring tightness; heaviness) were reported on average later than those associated with edema or BC treatment (swelling, firmness/tightness, stiffness, aching, tenderness, or numbness). This makes clinical sense, as the median time to onset of BCRL in this cohort is 16.6 months. Although there is no clear demarcation between symptoms related to BC surgery and those related to BCRL, most patients with and without edema report symptoms. Clinicians should pay attention to patients newly reporting these symptoms as part of screening for BCRL, especially ≥1-year post-surgery, as these are less likely to be associated with BC surgery itself.
Although many symptoms are reported by patients after BC treatment, only report of an increase in arm size was associated with progression to BCRL (RVC ≥ 5% or RVC ≥ 10%) on multivariate analysis. Accordingly, individual risk factors for these patients should be considered, and close ongoing screening and/or early treatment is warranted.
It has been shown that low-volume edema (RVC 3 to < 5%) within 3 months after surgery, or RVC 5% to <10% at any point post-surgery, are risk factors for progression to RVC ≥ 10%.19 In this study, those with low-volume edema (RVC 5% to <10%) had an increased risk of RVC progression to ≥10%, and those with RVC > 3% had an increased risk of RVC progression to ≥5%. This speaks to the importance of a screening program; patients with any elevation in RVC should be closely monitored with consideration of individual risk factors.
Patients with BCRL report poorer QOL than those without BCRL,30,42,43 likely secondary to the symptoms they endure. Patients with BCRL may experience up to 10 distressing symptoms daily (eg, aching, heaviness).31 They may also experience a cluster of symptoms, including altered sensation, loss of body confidence, decreased physical activity, fatigue, and psychological distress, which impact QOL.30 However, the relationship between limb volume and symptoms is not direct: patients with significant arm edema may not experience severe symptoms.3,67 Therefore, it may be the presence, not severity, of BCRL that impacts QOL, and treatment aimed at volume reduction alone may be inadequate in improving patient QOL.30 This nonlinear relationship has made it difficult to understand how symptoms should be incorporated into BCRL screening and diagnosis. Furthermore, early treatment for BCRL may prevent declines in QoL experienced by many patients with later-stage BCRL. For example, 1 study demonstrated improvements in depression and anxiety following treatment for BCRL.68
There is no universal agreement on diagnostic criteria for BCRL, and evidence-based integration of objective data, clinical examination, and symptoms into such criteria is needed. Based on available data, we call on the National Lymphedema Network, the International Lymphedema Framework, and the International Society of Lymphology to collaborate towards developing universal diagnostic criteria for BCRL. Based on this study’s results, we recommend that patient report of increased arm size be included.
Health care providers must be aware of treatment-related risk factors for BCRL and symptoms associated with risk of progression to BCRL. In this study, patients reported symptoms at all RVCs, including RVC < 3% (absence of edema). Clinicians should not rule out a BCRL diagnosis when patients with RVC < 10% and negative clinical exam report symptoms, especially increased arm size. For these patients, there may be a role for segmental edema evaluation and analysis to allow for early diagnosis, as these patients may present with focal swelling in absence of whole arm volume changes, which may represent developing BCRL.69
The traditional model of BC care focuses on disease management and ongoing surveillance for recurrence; however, this approach does not address the myriad of sequelae encountered by patients physically and emotionally throughout survivorship.4 A prospective surveillance model for physical rehabilitation and exercise may be integrated with disease management to improve care throughout survivorship.4 If impairments and functional limitations are identified early, rehabilitative interventions may be implemented and healthy lifestyle behaviors such as weight management, physical activity, and exercise may be prescribed and encouraged.4 This is no different in the case of BCRL after BC treatment. As previously discussed, prospective BCRL screening is a recommendation of several organizations,4,34,37–39 yet it is not standard of care. Data from this study indicate that within a BCRL screening program utilizing appropriate diagnostic criteria for BCRL, specific symptoms indicate increased risk BCRL. In the absence of a formal screening program, patients should be educated regarding the role of symptoms in BCRL development, and through patient vigilance, early diagnosis may be possible. Patients should be educated that symptoms are subtle; BCRL is insidious and progressive, and any symptom(s) should be reported to the treatment team. Given that patients in this study reported increased arm size up to >5 years before BCRL onset, and this symptom is associated with progression to BCRL, symptoms should be incorporated into diagnosis alongside arm volume changes and/or clinical exam. The relationship between edema severity and symptoms is nonlinear; therefore, treatment aimed at volume reduction alone is likely insufficient.30 Symptom resolution should be central to the goals of BCRL treatment. In addition, the practitioner should approach patients at risk for and with BCRL. A myriad of impairments may result in decreased function and limitations in participation for patients after BC treatment, further impacting their QOL. These must continue to be addressed throughout survivorship alongside BCRL.
Role of the Funding Source
The funding source had no role in the study's design, conduct, and reporting.
Limitations
This study is not without limitations. BCRL screening visits are incorporated into oncology follow-up appointments to minimize need for isolated screening visits. Symptoms or increased RVC occurring temporarily between visits are not captured. It would be helpful to quantify which patients present with symptoms between screening visits and identify barriers in seeking medical attention. In addition, perometry is but 1 mode of measurement for BCRL screening, and diagnostic criteria vary. Symptoms should be correlated similarly with other modes of measurement for BCRL screening, such as bio-impedance or tissue tonometry, for example. Finally, we have defined BCRL as RVC ≥ 10%, consistent with previous literature. 32,40 However, as there are no universal diagnostic criteria for BCRL and the role of edema <10% is not defined, we included RVC ≥ 5%.
Conclusion
In this study, many patients reported an increase in arm size months before developing BCRL, and these patients were at 3 times the risk of BCRL than those who did not report this symptom. When patients report symptoms in the absence of RVC ≥ 10%, lymphedema diagnosis should not be ruled out. These patients should be considered at high risk for BCRL development and therefore be followed vigilantly and longitudinally; furthermore, such patients should be under consideration for early intervention. Patients treated for BC should be screened longitudinally to identify new symptoms or those which change as patients get further out from surgery and may be indicative of developing BCRL, which has a median time of onset >1 year after surgery. Early detection of symptoms is vital for patients at risk of BCRL; it should be incorporated longitudinally for patients treated for BC and integrated into diagnostic criteria for BCRL. There is much known about risk factors, role of symptoms, clinical exam, and objective changes in early BCRL. We therefore recommend integration of the following data into early detection and treatment of BCRL. Patients reporting a new symptom of change in arm size, subtle focal pitting edema on clinical examination, or RVC 5% to <10% should be considered as potentially having BCRL. Clinical examination can now be quantified using a new tool, the Cancer-Related Lymphedema of the Upper Extremity tool,70 which can be used to monitor subtle changes in clinical examination. Objective measurements (perometry, bioimpedance, circumference converted to volume) should be consistent, and patients presenting with potential new BCRL should be monitored closely according to strict measurement protocol, incorporating baseline measures. Patients should be risk-stratified when presenting with potential new BCRL, with the highest risk patients (ie, those who underwent ALND and RLNR) considered for immediate treatment vs lower risk patients (SLNB only) more appropriate for a short period of close attention looking for any progression in swelling through clinical exam or objective measures. We should not underestimate the burden of even conservative BCRL treatment such as a compression sleeve, and we must ensure we are not over- or under-treating this population. We call on the National Lymphedema Network, the International Lymphedema Framework, and the International Society of Lymphology to collaborate towards developing universal diagnostic criteria for early BCRL, which should incorporate much greater specificity of symptoms, clinical exam, and objective measures.
Contributor Information
Cheryl L Brunelle, Department of Physical and Occupational Therapy, Massachusetts General Hospital, Boston, Massachusetts.
Sacha A Roberts, Department of Radiation Oncology, Massachusetts General Hospital.
Nora K Horick, Biostatistics Center, Massachusetts General Hospital.
Tessa C Gillespie, Department of Radiation Oncology, Massachusetts General Hospital.
Jamie M Jacobs, Center for Psychiatric Oncology and Behavioral Sciences, Massachusetts General Hospital.
Kayla M Daniell, Department of Radiation Oncology, Massachusetts General Hospital.
George E Naoum, Department of Radiation Oncology, Massachusetts General Hospital.
Alphonse G Taghian, Department of Radiation Oncology, Massachusetts General Hospital, 55 Fruit St, Boston, MA 02114 (USA).
Author Contributions
Concept/idea/research design: C.L. Brunelle, S.A. Roberts, T.C. Gillespie, K.M. Daniell, G.E. Naoum, A.G. Taghian
Writing: C.L. Brunelle, S.A. Roberts, N.K. Horick, T.C. Gillespie, J.M. Jacobs, K.M. Daniell, A.G. Taghian
Data collection: C.L. Brunelle, S.A. Roberts, T.C. Gillespie, K.M. Daniell
Data analysis: C.L. Brunelle, S.A. Roberts, N.K. Horick, T.C. Gillespie, J.M. Jacobs, G.E. Naoum, A.G. Taghian
Project management: C.L. Brunelle, T.C. Gillespie, K.M. Daniell, A.G. Taghian
Fund procurement: T.C. Gillespie, A.G. Taghian
Providing facilities/equipment: A.G. Taghian
Clerical/secretarial support: T.C. Gillespie
Consultation (including review of manuscript before submitting): C.L. Brunelle, J.M. Jacobs, G.E. Naoum
Funding
This study was supported by awards from the National Cancer Institute (nos. R01CA139118 and P50CA08393 to A.G. Taghian). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health. This program is supported by the Adele McKinnon Research Fund for Breast Cancer-Related Lymphedema (A.G. Taghian), the Olayan-Xefos Family Fund for Breast Cancer Research (A.G. Taghian), and the Heinz Family Foundation (A.G. Taghian).
Ethics Approval
The protocol for lymphedema screening has been previously published and approved by the Institutional Review Board (2008P000540).
Clinical Trial
This study is registered at Clinicaltrials.gov (NCT01521741).
Disclosures
The authors completed the ICMJE Form for Disclosure of Potential Conflicts of Interest and reported the following: A.G. Taghian is on the Scientific Advisory Board of Puretech Health and is a previous consultant in VisionRT. A.G. Taghian was loaned equipment from ImpediMed for use in investigator-initiated clinical trials. C. Brunelle is on the Scientific Advisory Board of Puretech Health. These associations are unrelated to this study. All other authors declare no conflicts of interest.
References
- 1. Surveillance, Epidemiology, and End Results (SEER) Program. NCI . Cancer Stat Facts: Female Breast Cancer. Bethesda, Maryland: National Cancer Institute. Accessed August 24, 2020. https://seer.cancer.gov/statfacts/html/breast.html
- 2. Bodai BI, Tuso P. Breast cancer survivorship: a comprehensive review of long-term medical issues and lifestyle recommendations. Perm J. 2015;19:48–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Melisko, ME, Gradishar, WJ, Moy B. American Society of Clinical Oncology Educational Book 36. Alexandria, VA: American Society of Clinical Oncology (ASCO). 2018. [DOI] [PubMed] [Google Scholar]
- 4. Stout NL, Binkley JM, Schmitz KH, et al. A prospective surveillance model for rehabilitation for women with breast cancer. Cancer. 2012;118:2191–2200. [DOI] [PubMed] [Google Scholar]
- 5. DiSipio T, Rye S, Newman B, Hayes S. Incidence of unilateral arm lymphoedema after breast cancer: a systematic review and meta-analysis. Lancet Oncol. 2013;14:500–515. [DOI] [PubMed] [Google Scholar]
- 6. Erickson VS, Pearson ML, Ganz PA, Adams J, Kahn KL. Arm edema in breast cancer patients. JNCI J Natl Cancer Inst. 2001;93:96–111. [DOI] [PubMed] [Google Scholar]
- 7. McLaughlin SA, Bagaria S, Gibson T, et al. Trends in risk reduction practices for the prevention of lymphedema in the first 12 months after breast cancer surgery. J Am Coll Surg. 2013;216:380–389. [DOI] [PubMed] [Google Scholar]
- 8. Collins LG, Nash R, Round T, Newman B. Perceptions of upper-body problems during recovery from breast cancer treatment. Support Care Cancer. 2004;12:106–113. [DOI] [PubMed] [Google Scholar]
- 9. Tsai RJ, Dennis LK, Lynch CF, Snetselaar LG, Zamba GKD, Scott-conner C. The risk of developing arm lymphedema among breast cancer survivors: a meta-analysis of treatment factors. Ann Surg Oncol. 2009;16:1959–1972. [DOI] [PubMed] [Google Scholar]
- 10. McLaughlin SA, Wright MJ, Morris KT, et al. Prevalence of lymphedema in women with breast cancer 5 years after sentinel lymph node biopsy or axillary dissection: objective measurements. J Clin Oncol. 2008; 26:5213–5219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kilbreath SL, Refshauge KM, Beith JM, et al. Risk factors for lymphoedema in women with breast cancer: a large prospective cohort. Breast. 2016;28:29–36. [DOI] [PubMed] [Google Scholar]
- 12. Warren LEG, Miller CL, Horick N, et al. The impact of radiation therapy on the risk of lymphedema after treatment for breast cancer: a prospective cohort study. Int J Radiat Oncol Biol Phys. 2014;88:565–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shaitelman SF, Chiang Y-J, Griffin KD, et al. Radiation therapy targets and the risk of breast cancer-related lymphedema: a systematic review and network meta-analysis. Breast Cancer Res Treat. 2016;162:201–215. [DOI] [PubMed] [Google Scholar]
- 14. Ridner SH, Dietrich MS, Stewart BR, Armer JM. Body mass index and breast cancer treatment-related lymphedema. Support Care Cancer. 2011;19:853–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fu M, Axelrod D, Guth A, et al. Patterns of obesity and lymph fluid level during the first year of breast cancer treatment: a prospective study. J Pers Med. 2015;5:326–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Helyer LK, Varnic M, Le LW, Leong W, McCready D. Obesity is a risk factor for developing postoperative lymphedema in breast cancer patients. Breast J. 2010;16:48–54. [DOI] [PubMed] [Google Scholar]
- 17. Jammallo LS, Miller CL, Singer M, et al. Impact of body mass index and weight fluctuation on lymphedema risk in patients treated for breast cancer. Breast Cancer Res Treat. 2013;142: 59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bevilacqua JLB, Kattan MW, Changhong Y, et al. Nomograms for predicting the risk of arm lymphedema after axillary dissection in breast cancer. Ann Surg Oncol. 2012;19: 2580–2589. [DOI] [PubMed] [Google Scholar]
- 19. Specht MC, Miller CL, Russell TA, et al. Defining a threshold for intervention in breast cancer-related lymphedema: what level of arm volume increase predicts progression? Breast Cancer Res Treat 2013;140:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Miller CL, Colwell AS, Horick N, et al. Immediate implant reconstruction is associated with a reduced risk of lymphedema compared to mastectomy alone: a prospective cohort study. Ann Surg. 2016;263:399–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Crosby M, Card A, Liu J, Lindstrom W, Chang D. Immediate breast reconstruction and lymphedema incidence. Plast Reconstr Surg. 2012;129:789e–795e. [DOI] [PubMed] [Google Scholar]
- 22. Card A, Crosby M, Liu J, Lindstrom W, Lucci A, Chang D. Reduced incidence of breast cancer-related lymphedema following mastectomy and breast reconstruction versus mastectomy alone. Plast Reconstr Surg. 2012;130:1169–1178. [DOI] [PubMed] [Google Scholar]
- 23. Lee K-T, Mun G-H, Lim S-Y, Pyon J-K, Oh K-S, Bang S-I. The impact of immediate breast reconstruction on post-mastectomy lymphedema in patients undergoing modified radical mastectomy. The Breast. 2013;22:53–57. [DOI] [PubMed] [Google Scholar]
- 24. Armer JM, Ballman KV, McCall L, et al. Factors associated with lymphedema in women with node-positive breast cancer treated with neoadjuvant chemotherapy and axillary dissection. JAMA Surg. 2019;154:800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhu W, Li D, Li X, et al. Association between adjuvant docetaxel-based chemotherapy and breast cancer-related lymphedema. Anticancer Drugs. 2017;28:350–355. [DOI] [PubMed] [Google Scholar]
- 26. Ohsumi S, Shimozuma K, Ohashi Y, et al. Subjective and objective assessment of edema during adjuvant chemotherapy for breast cancer using taxane-containing regimens in a randomized controlled trial: the national surgical adjuvant study of breast cancer 02. Oncology. 2012;82:131–138. [DOI] [PubMed] [Google Scholar]
- 27. Invernizzi M, Michelotti A, Noale M, et al. Breast cancer systemic treatments and upper limb lymphedema: a risk assessment platform encompassing tumor-specific pathological features reveals the potential role of trastuzumab. JCM. 2019;8:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Newman B, Lose F, Kedda MA, et al. Possible genetic predisposition to lymphedema after breast cancer. Lymphat Res Biol. 2012;10:2–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Visser J, Van Geel M, Cornelissen AJM, Van Der Hulst RRWJ, Qiu SS. Breast cancer-related lymphedema and genetic predisposition: a systematic review of the literature. Lymphat Res Biol. 2019;17:288–293. [DOI] [PubMed] [Google Scholar]
- 30. Ridner SH. Quality of life and a symptom cluster associated with breast cancer treatment-related lymphedema. Support Care Cancer. 2005;13:904–911. [DOI] [PubMed] [Google Scholar]
- 31. Fu MR, Rosedale M. Breast cancer survivors’ experiences of lymphedema-related symptoms. J Pain Symptom Manage. 2009;38:849–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Armer JM, Radina ME, Porock D, Culbertson SD. Predicting breast cancer-related lymphedema using self-reported symptoms. Nurs Res. 2003;52:370–379. [DOI] [PubMed] [Google Scholar]
- 33. Norman SA, Localio AR, Potashnik SL, et al. Lymphedema in breast cancer survivors: incidence, degree, time course, treatment, and symptoms. J Clin Oncol. 2009;27:390–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. McLaughlin SA, Staley AC, Vicini F, et al. Considerations for clinicians in the diagnosis, prevention, and treatment of breast cancer-related lymphedema: recommendations from a multidisciplinary expert asbrs panel: part 1: Definitions, assessments, education, and future directions. Ann Surg Oncol. 2017;24:2818–2826. [DOI] [PubMed] [Google Scholar]
- 35. National Lymphedema Network . Screening and early detection of breast cancer-related lymphedema: the imperative. 2011. Accessed October 22, 2020. https://lymphnet.org/position-papers [Google Scholar]
- 36. Ostby P, Armer J, Dale P, Van Loo M, Wilbanks C, Stewart B. Surveillance recommendations in reducing risk of and optimally managing breast cancer-related lymphedema. J Pers Med. 2014;4:424–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Executive Committee of the International Society of Lymphology . The diagnosis and treatment of peripheral lymphedema: 2016 consensus document of the International Society of Lymphology. Lymphology. 2016;49:170–184. [PubMed] [Google Scholar]
- 38. National Lymphedema Network . 2011 Position statement of the National Lymphedema Network: screening and measurement for early detection of breast cancer-related symptoms. 2011. Accessed October 22, 2020. https://lymphnet.org/position-papers
- 39. National Comprehensive Cancer Network - NCCN . 2020. Plymouth Meeting, PA: NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): survivorship.
- 40. Armer JM, Stewart BR. A comparison of diagnostic criteria for lymphedema in a post-breast cancer population. Lymphat Res Biol. 2005;3:208–217. [DOI] [PubMed] [Google Scholar]
- 41. Hayes SC, Rye S, Battistutta D, Newman B. Prevalence of upper-body symptoms following breast cancer and its relationship with upper-body function and lymphedema. Lymphology. 2010;43:178–187. [PubMed] [Google Scholar]
- 42. Bell RJ, Robinson PJ, Barallon R, Fradkin P, Schwarz M, Davis SR. Lymphedema: experience of a cohort of women with breast cancer followed for 4 years after diagnosis in Victoria, Australia. Support Care Cancer. 2013;21:2017–2024. [DOI] [PubMed] [Google Scholar]
- 43. Clough-Gorr KM, Ganz PA, Silliman R. Older breast cancer survivors: factors associated with self-reported symptoms of persistent lymphedema over 7 years of follow up. Breast J. 2010;16:147–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sun F, Skolny MN, Swaroop MN, et al. The need for preoperative baseline arm measurement to accurately quantify breast cancer-related lymphedema. Breast Cancer Res Treat. 2016;157:229–240. [DOI] [PubMed] [Google Scholar]
- 45. Brunelle C, Skolny M, Ferguson C, Swaroop M, O’Toole J, Taghian AG. Establishing and sustaining a prospective screening program for breast cancer-related lymphedema at the Massachusetts General Hospital: lessons learned. J Pers Med. 2015;5:153–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Stanton AW, Northfield JW, Holroyd B, Mortimer PS, Levick JR. Validation of an optoelectronic limb volumeter (Perometer). Lymphology. 1997;30:77–97. [PubMed] [Google Scholar]
- 47. Lee M-J, Boland RA, Czerniec S, Kilbreath SL. Reliability and concurrent validity of the perometer for measuring hand volume in women with and without lymphedema. Lymphat Res Biol. 2011;9:13–18. [DOI] [PubMed] [Google Scholar]
- 48. Schonholz SM. Preoperative assessment enables the early detection and successful treatment of lymphedema. Cancer. 2009;115:909. [DOI] [PubMed] [Google Scholar]
- 49. Hidding JT, Viehoff PB, Beurskens CHG, van Laarhoven HWM, Nihjhuis-van der Sanden MWG, van der Wees P. Measurement properties of instruments for measuring of lymphedema: systematic review. Phys Ther. 2016;96:1965–1981. [DOI] [PubMed] [Google Scholar]
- 50. Gummesson C, Atroshi I, Ekdahl C. The disabilities of the arm, shoulder and hand (DASH) outcome questionnaire: longitudinal construct validity and measuring of self-rated health change after surgery. BMC Musculoskelet Disord. 2004;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Brady MJ, Cella DF, Mo F, et al. Reliability and validity of the functional assessment of cancer therapy-breast quality-of-life instrument. J Clin Oncol. 1997;15:974–986. [DOI] [PubMed] [Google Scholar]
- 52. Cella DF, Tulsky DS, Gray G. The functional assessment of cancer therapy (FACT) scale: development and validation of the general measure. J Clin Oncol. 1993;11:570–579. [DOI] [PubMed] [Google Scholar]
- 53. Cella D. F.A.C.T. Manual: manual for the Functional Assessment of Cancer Therapy (Fact) Scales and the Functional Assessment Of HIV Infection (FAHI) Scale. Chicago, IL: Rush-Presbyterian-St. Luke’s Medical Center. 1994.
- 54. Coster S, Poole K, Fallowfield L. The validation of a quality of life scale to assess the impact of arm morbidity in breast cancer patients post-operatively. Breast Cancer Res Treat. 2001;68:273–282. [DOI] [PubMed] [Google Scholar]
- 55. Lee T, Kilbreath S, Sullivan G, Refshauge K. The development of an arm activity survey for breast cancer survivors using the protection motivation theory. BMC Cancer. 2007;7:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Armer JM, Ballman KV, McCall L, et al. Lymphedema symptoms and limb measurement changes in breast cancer survivors treated with neoadjuvant chemotherapy and axillary dissection: results of American College of Surgeons oncology group (ACOSOG) Z1071 (alliance) substudy. Support Care Cancer. 2019;27:495–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ancukiewicz M, Russell TA, Otoole J, et al. Standardized method for quantification of developing lymphedema in patients treated for breast cancer. Int J Radiat Oncol Biol Phys. 2011;79:1436–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Armer JM, Henggeler MH, Brooks CW, Zagar EA, Homan SH, Stewart B. The health deviation of post-breast cancer lymphedema: symptom assessment and impact on self-care agency. Self Care Depend Care Nurs. 2008;16:14–21. [PMC free article] [PubMed] [Google Scholar]
- 59. Armer JM, Whitman M. The problem of lymphedema following breast cancer treatment: prevalence, symptoms and self-management. Lymphology. 2002;35:153–159.12570324 [Google Scholar]
- 60. Donker M, van Tienhoven G, Straver ME, et al. Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer (EORTC 10981-22023 AMAROS): a randomised, multicentre, open-label, phase 3 non-inferiority trial. Lancet Oncol. 2014;15:1303–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Stout Gergich NL, Pfalzer LA, McGarvey C, Springer B, Gerber LH, Soballe P. Preoperative assessment enables the early diagnosis and successful treatment of lymphedema. Cancer. 2008;112:2809–2819. [DOI] [PubMed] [Google Scholar]
- 62. Soran A, Ozmen T, McGuire KP, et al. The importance of detection of subclinical lymphedema for the prevention of breast cancer-related clinical lymphedema after axillary lymph node dissection: a prospective observational study. Lymphat Res Biol. 2014;12:289–294. [DOI] [PubMed] [Google Scholar]
- 63. Lacomba MT, Sánchez MJY, Goñi ÁZ, et al. Effectiveness of early physiotherapy to prevent lymphoedema after surgery for breast cancer: randomised, single blinded, clinical trial. BMJ. 2010;340:140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Whitworth PW, Shah C, Vicini F, Cooper A. Preventing breast cancer-related lymphedema in high-risk patients: the impact of a structured surveillance protocol using bioimpedance spectroscopy. Front Oncol. 2018;8:197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Temkin SM, Mcguire KP, Bellavance EC, et al. Preventing breast cancer-related lymphedema in high-risk patients: the impact of a structured surveillance protocol using bioimpedance spectroscopy. Front Oncol. 2018;8:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ozsoy-Unubol T, Sanal-Toprak C, Bahar-Ozdemir Y, Akyuz G. Efficacy of kinesio taping in early stage breast cancer associated lymphedema: a randomized single blinded study. Lymphology. 2019;52:166–176. [PubMed] [Google Scholar]
- 67. Sierla R, Lee TSM, Black D, Kilbreath SL. Lymphedema following breast cancer: regions affected, severity of symptoms, and benefits of treatment from the patients’ perspective. Clin J Oncol Nurs. 2013;17:325–331. [DOI] [PubMed] [Google Scholar]
- 68. Can AG, Eksioglu E, Çakçı FA. Early detection and treatment of subclinical lymphedema in patients with breast cancer. Lymphat Res Biol. 2019;368–373. [DOI] [PubMed] [Google Scholar]
- 69. Daniell KD, Roberts SA, Gillespie TG, Brunelle CL, Taghian A. Assessing Segmental Limb Volume Changes for Early Detection of BCRL. Chicago, IL, USA: International Lymphedema Framework. [Google Scholar]
- 70. Spinelli B, Kallan MJ, Zhang X, et al. Intra- and interrater reliability and concurrent validity of a new tool for assessment of breast cancer–related lymphedema of the upper extremity. Arch Phys Med Rehabil. 2019;100:315–326. [DOI] [PMC free article] [PubMed] [Google Scholar]


