Abstract
The ubiquitin–proteasome system (UPS) is the major intracellular and non-lysosomal protein degradation system. Thanks to its unique capacity of eliminating old, damaged, misfolded, and/or regulatory proteins in a highly specific manner, the UPS is virtually involved in almost all aspects of eukaryotic life. The critical importance of the UPS is particularly visible in immune cells which undergo a rapid and profound functional remodelling upon pathogen recognition. Innate and/or adaptive immune activation is indeed characterized by a number of substantial changes impacting various cellular processes including protein homeostasis, signal transduction, cell proliferation, and antigen processing which are all tightly regulated by the UPS. In this review, we summarize and discuss recent progress in our understanding of the molecular mechanisms by which the UPS contributes to the generation of an adequate immune response. In this regard, we also discuss the consequences of UPS dysfunction and its role in the pathogenesis of recently described immune disorders including cancer and auto-inflammatory diseases.
Keywords: ubiquitin–proteasome system, proteostasis, immunity, autoinflammation, cancer
1. The Ubiquitin–Proteasome System (UPS): Structure and Function
Four decades ago, it was discovered that conjugation of intracellular proteins with the small highly conserved ubiquitin polypeptide leads to their subsequent degradation by 26S proteasomes [1,2,3,4]. Since then, our understanding of the function of the ubiquitin–proteasome system (UPS) has progressed continuously. It is now well established that ubiquitination is mediated by an enzyme cascade (i.e., E1, E2, and E3) which transfers ubiquitin molecules to cellular targets in a sequential manner [5,6]. As illustrated in Figure 1, a ubiquitin-activating enzyme (E1) first activates ubiquitin in an ATP-hydrolyzing reaction which is then transferred to a cysteine residue of the active site of a ubiquitin-conjugating enzyme (E2). Then, in a two-step reaction, E2 enzymes promote ubiquitin conjugation to substrates with the help of ubiquitin-ligases (E3) which recognize specific targets. In this process, substrate specificity is ensured by an estimated number of 500–1000 putative E3 ligases encoded by the human genome [7]. Canonical ubiquitination is defined by the formation of isopeptide bonds between lysine residues of target proteins and the C-terminal glycine (glycine 76) of ubiquitin [8]. Non-canonical ubiquitination is less frequent and may occur on serine/threonine or cysteine residues of substrates via ester or thioester bonds, respectively [9,10,11,12,13]. Proteins may be marked with one single ubiquitin moiety on one single site resulting in mono-ubiquitination which is widely recognized as a major molecular signal regulating chromatin remodeling as well as protein sorting and/or trafficking [14,15]. However, the attached ubiquitin molecule itself exhibits eight potential ubiquitination sites (M1, K6, K11, K27, K29, K33, K48 and K63) which may be used for further ubiquitination cycles, thereby giving rise to polyubiquitin chains.
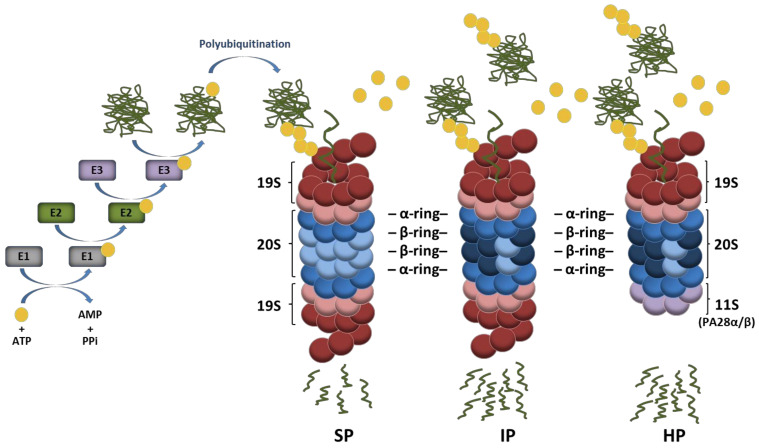
Figure 1.
Ubiquitin conjugation pathway of protein substrates and their subsequent degradation by proteasome complexes. Ubiquitin activation requires its transfer to an E1 ubiquitin-activating enzyme under ATP hydrolysis. In a second step, the activated ubiquitin is transferred to an E2 ubiquitin-conjugating enzyme and used for the covalent modification of intracellular proteins with the support of E3 ubiquitin ligases. Protein substrates may be marked with one single ubiquitin molecule on one acceptor site resulting in mono-ubiquitination. The ubiquitin molecule may also be subjected to ubiquitination, thereby leading to the formation of various polyubiquitin chains, among which the K48-linkages represent the most prevalent proteasome-targeting signals. Major proteasome complexes include standard proteasomes (SP), immunoproteasomes (IP), and hybrid proteasomes (HP) which distinguish themselves by the nature of their catalytic subunits and/or regulators (19S, 11S). It is understood that both IP and HP exhibit a higher capacity of degrading ubiquitin-modified proteins than SP.
Depending on the ubiquitin residue used for poly-ubiquitination, the assembled ubiquitin chains present various topologies which behave differently and carry distinct biological fates for the modified substrate. It is well appreciated that ubiquitin moieties linked to each other via lysine at position 48 (K48) represent the canonical signal for proteasomal degradation [16], while those linked via K63 allow the clearance of damaged organelles (i.e., ribosomes, endoplasmic reticulum, and/or mitochondria) by the autophagy-lysosome pathway [17]. Other ubiquitin linkages are primarily viewed as non-proteolytic signals involved in the regulation of various cellular processes with DNA repair and cell signaling being the most prominent ones [18]. Proteins modified with K48-linked polyubiquitin chains are typically degraded by the 26S proteasome which is a 2.5 MDa complex consisting of two sub-complexes, namely, the 20S core particle (CP) and the 19S regulatory particle (RP) [19,20]. The 19S RP plays a crucial role in the recognition of ubiquitin-modified proteins as well as their deubiquitination and subsequent unfolding before degradation by the 20S CP [21,22]. As shown in Figure 1, the 20S CP exhibits a barrel-like structure made out of four heptameric rings which are themselves composed of α- and β-subunits. The two outer rings comprise seven α-subunits whose N-termini are oriented towards seven β-subunits forming the two inner rings. The catalytic activity of the 20S complex is ensured by the incorporation of the β1, β2, and β5 subunits which are threonine proteases exhibiting caspase-, trypsin-, and chymotrypsin-like activities, respectively [23,24]. Under certain conditions, alternative catalytic subunits may assemble into newly synthetized proteasomes to build so-called immunoproteasomes (IP) that carry β1i, β2i, and β5i inducible subunits instead of the β1, β2, and β5 standard subunits [25,26]. IP distinguish themselves from their standard counterparts by exhibiting higher proteolytic activities which make them, under stress conditions, more efficient at degrading ubiquitin-modified proteins [27,28,29,30,31]. It is widely accepted that IP are constitutively expressed in immune cells, while they are induced in non-immune tissues in response to type I and/or II interferons (IFN) [26]. Mixed-type (also referred to as intermediate-type) proteasomes are defined as partially assembled IP bearing one or two out of the three inducible subunits and may be found under normal conditions in some tissues with high protein turnover rates such as the liver [32,33,34,35]. Complexity to this system is further added by the observation that 20S CP may be reversely capped at either one or both ends with proteasome regulators such as an 11S complex composed of PA28αβ or PA28γ subunits (Figure 1), PA200 or PI31 [36,37,38,39,40]. Because of these many possible combinations, an enormous diversity of proteasomes arises, whose biological relevance is, however, not fully understood. The majority of proteasomes resides in both the cytosol and nucleus, while damaged proteasomes are targeted to lysosomes through macro-autophagy [41] or removed extracellularly by means of exosomes [42]. Intriguingly, it was recently suggested that proteasomes are present in the lumen of endocytic and phagocytic organelles of professional antigen-presenting cells [43].
2. The UPS Is a Major Regulator of Cell Signalling in Response to Pathogens
Thanks to its ability to specifically eliminate intracellular proteins in response to diverse stimuli, the UPS is involved in almost all aspects of cell physiology and development [44]. The broad range of functions of the UPS is particularly well exemplified in immune cells of myeloid origin whereby it regulates key features of both innate and adaptive immunity including signal transduction, protein homeostasis, and MHC class I antigen presentation. Myeloid cells are critical components of the immune system and include monocytes, macrophages, (DC), and granulocytes whose main function resides in patrolling the body for potential invaders [45,46]. Recognition of microbial products by myeloid cells occurs through the binding of pathogen-associated molecular patterns (PAMP) to their pattern recognition receptor (PRR). Depending on their cellular localization and ligand specificity, PRR are traditionally subdivided into Toll-like receptors (TLR), retinoic acid-induced gene-I (RIG-I)-like receptors (RLR), nucleotide-binding oligomerization domain (NOD)-like receptors, and cytosolic DNA or RNA sensors inducing the cyclic-GMP-AMP synthase (cGAS) and protein kinase R (PKR), respectively [46]. The sensing of PAMP by PRR initiates a series of signalling cascades which ultimately leads to the activation of major transcription factors driving inflammation, namely, the nuclear factor-κB (NF-κB) as well as the interferon regulatory factors (IRF) 3 and 7 [47,48]. Following their translocation into the nucleus, NF-κB and IRF3/IRF7 induce the transcription of proinflammatory cytokines (i.e., TNF-α, IL-1β, and IL-6) and interferons (IFN), respectively, to counteract the invading pathogens. Strikingly, a large body of work, notably by the groups of Karin and Dixit, has shown that the intracellular signalling pathways triggered by PRR in response to danger signals heavily relies on the UPS which tightly controls key intersections of the initiated cascades [49,50,51,52].
In this process, the breakdown of signalling components by the UPS is intricately associated with the generation of non-proteolytic ubiquitin chains, among which are K63-linkages that regulate protein activities and/or interactions. For instance, as shown in Figure 2, during infection with gram-negative bacteria, binding of LPS to TLR-4 on myeloid cells results in the activation of the E3 ubiquitin ligase TRAF6 which generates K63-linked polyubiquitin chains, thereby activating the downstream TAK1 complex [53,54]. One major role of TAK1 is to activate the canonical heterotrimeric IκB kinase (IKK) complex which itself consists of the related protein kinases IKKα and IKKβ as well as IKKγ, a regulatory component also referred to as NF-κB essential modulator (NEMO) [55,56]. Importantly, TRAF6 is also implicated in the activation of the so-called linear ubiquitin chain assembly complex (LUBAC) comprising the proteins HOIP, HOIL-1, and sharpin [57,58]. Linear ubiquitination of NEMO by LUBAC promotes a NEMO conformational change that facilitates IKK assembly [59,60]. Full activation of IKK requires phosphorylation of IKKα (ser 176 and 180) and IKKβ (ser 177 and 181) by TAK1 [61]. One major substrate for IKK (i.e., IKKβ) is IκBα, the inhibitory component of NF-κB. Indeed, IκBα phosphorylation at Ser32 and Ser36 by IKK unmasks a lysine residue that is used by the E3 ubiquitin ligase SCFTRCP, thereby catalysing the K48-linked ubiquitination of IκBα and promoting its proteasomal degradation [62,63]. Interestingly, it has been suggested that IκBα is faster degraded by IP [27,64,65] than standard proteasomes (SP). Likewise, hybrid (immuno)-proteasomes (HP) asymmetrically capped at each end with 19S and PA28 complexes (Figure 1) have been shown to increase IκBα protein turnover [66,67]. Given that both IP and PA28 are constitutively present in myeloid cells [26,68], these observations propose a seductive rationale for the rapidity of these cells to release proinflammatory cytokines in response to pathogens. This assumption is in line with a number of reports showing that blocking the β5i inducible subunit with the small molecule inhibitor ONX 0914 (formerly PR-957) results in decreased production of proinflammatory cytokines in various disease models [69,70,71,72,73,74,75]. Similar observations were made in knockout mice lacking β5i in other various inflammation models [65,70,76,77]. Whether the beneficial effects detected on inflammation following IP inhibition really rely on impaired NF-κB signalling remains, however, a matter of debate. Some studies have highlighted the decreased efficiency of proteasomes devoid of functional β5i to degrade IκBα [27,64,65], while other investigations not [70,78]. The reasons for these discrepancies are unclear but may reflect distinct experimental conditions. Importantly, besides inducing proinflammatory genes, NF-κB also promotes the upregulation of the ubiquitin hydrolases TNFAIP3 (TNFα-induced protein 3, also known as A20), CYLD, and OTULIN which hydrolyse K63- and Met1-ubiquitin chains with varying efficiencies (Figure 2) [79,80,81,82,83]. By removing ubiquitin chains from TRAF6 and IKK, these deubiquitinating enzymes ensure a negative feedback loop in NF-κB signalling.
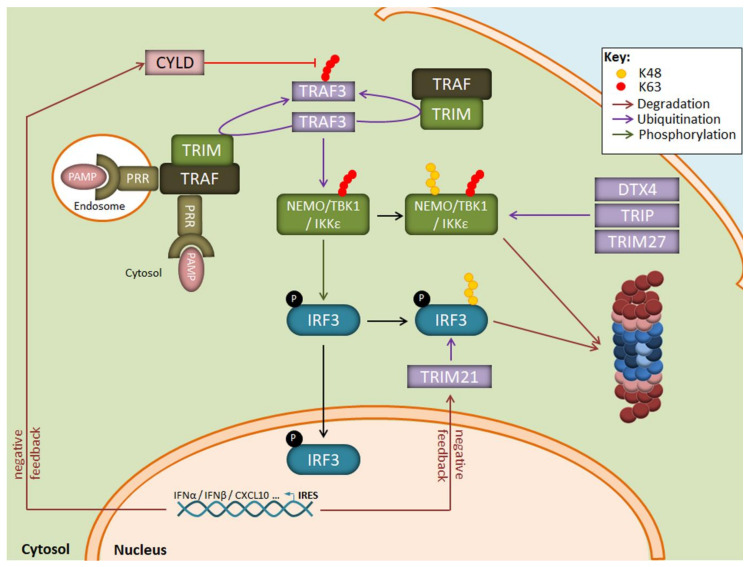
Figure 2.
Key contributions of the UPS to the regulation of the NF-κB signaling pathway in response to LPS. Depicted is the NF-B pathway emanating from the cell surface receptor TLR4 upon LPS binding. NF-κB signalling relies on the upstream E3 ubiquitin ligase TRAF6 which catalyzes K63-linked polyubiquitin chains which themselves activate the TAK kinase complex. Activated TAK1 phosphorylates the downstream heterotrimeric IKK kinase complex whose full activation further requires the generation of Met1-linked linear polyubiquitin chains by LUBAC. Once activated, IKK promotes the phosphorylation of κBα and its subsequent proteasomal degradation following its K48-ubiquitination by SCFTRCP, thereby allowing NF-κB nuclear translocation. Genes induced by NF-κB include those coding for deubiquitinating enzymes Otulin and A20 which ensure a negative feedback loop by trimming linear and K63-polyubiquitin chains.
Apart from the NF-κB transduction pathway, ubiquitination is also a key regulatory mechanism for IFN production in response to viruses. As illustrated in Figure 3, one central component in this signalling cascade is the NEMO-TBK1/IKKε complex which catalyses the phosphorylation of the transcription factors IRF3 and/or IRF7 for nuclear translocation. The assembly of NEMO-TBK1/IKKε requires the generation of multiple non-proteolytic ubiquitin chains. These include notably K63-linked polyubiquitin chains catalysed by the E3 ubiquitin ligase TRAF3 following recognition of viral products by some intracellular TLR, such as TLR3. TLR7, TLR8 or TLR9, and/or RIG-I-like receptors [84,85,86].
Figure 3.
Regulation of the IRF3 signaling pathway by the UPS following LPS exposure. Binding of PAMP to intracellular PRR results in the autoactivation of the E3 ubiquitin ligase TRAF3 which activates the downstream TBK1/IKK1ε kinase complex by generating K63-linked polyubiquitin chains. The TBK1/IKK1ε complex catalyzes the phosphorylation of IRF3, thereby inducing its nuclear translocation and the transcription of genes coding for type I IFN. A prolonged activation of TBK1/IKK1ε by TRAF3 is prevented by the E3 ubiquitin ligases DTX4, TRIP, and TRIM27 which modify TBK1/IKK1ε with K48-linked polyubiquitin chains and target it for proteasomal degradation. Negative feedback mechanisms also include the upregulation of the CYLD and TRIM21 proteins which remove K63-linkages and target IRF3 for proteasomal degradation, respectively.
Suppression of TLR/RLR signalling in response to viral products involves the generation of K48-linked polyubiquitin chains which target key components of the cascade for proteasomal degradation. Such modifications affect notably IRF3/IRF7 and TBK1 by the E3 ubiquitin ligases RAUL [87,88] as well as DTX4 [89], TRIM27 [90] or TRIP [91]. In addition, target genes of IRF3/IRF7 include ubiquitin hydrolases such as CYLD, USP21, OTUD5, and USP3 which trim non-proteolytic chains [92,93,94], thereby deactivating key signalling nodes and preventing sustained type I IFN production (Figure 3). Another negative regulatory mechanism in the absence of danger signals is the constitutive K48-ubiquitination of cGAS which results in its degradation by autophagy [95]. Upon DNA virus infection, cGAS becomes rapidly stabilised thanks to the recruitment of the ubiquitin hydrolase USP14 which removes K48-linked ubiquitin chains. The role of IP in the regulation of the signalling events leading to type I IFN responses is poorly understood. It has been shown that IP deficiency resulted in decreased phosphorylation of IRF3 in response to LPS, although the underlying mechanisms remain obscure [96]. In line with this, data from our group suggest that microglia lacking β5i result in the stabilisation of various key components of the signalling pathways engaged by LPS (unpublished observations).
3. The UPS as a Major Guardian of Proteostasis during Activation of the Immune System
Proteostasis is a mechanism of cellular homeostasis ensuring the sensitive balance between synthesis, folding, trafficking, and degradation of proteins [97,98]. In the protein life cycle, trafficking is of particular importance, because all proteins are sorted into their distinct destinations to ensure their correct function. One major sorting pathway in the cell is the cytosolic pathway including nuclear, peroxisomal, mitochondrial, and cytosolic proteins. However, about 30% up to 60% of all proteins follow the secretory pathway and are sorted into ER, Golgi, lysosomal, plasma membrane or extracellular compartments depending on cell function [99]. Because immune cells massively use this pathway for the production of humoral immune factors such as cytokines or immunoglobulins, they are particularly sensitive to perturbations in protein transport. Thus, immune cell function heavily relies on proteostatic mechanisms to avoid unbalanced immune responses or immune cell death [100,101].
Proteostasis is highly challenged by ageing and many pathological situations such as infection, inflammation or oxidative stress that result in protein damage and/or misfolding [102,103,104]. Accumulation of protein aggregates causes cellular proteotoxic stress that triggers several cellular responses for adaptation including the unfolded protein response (UPR), the integrated stress response, and immune responses (ISR) as well as the upregulation of alternative proteasome isoforms to rebalance the system for adaptation [105]. Such proteostasis imbalances cause specific pathologies, so-called proteinopathies, that include neurodegenerative diseases characterized by the accumulation of ubiquitin–protein conjugates [106,107]. The proteostasis network thus represents adaptation mechanisms to increased proteotoxic burden and enables a functional proteome to maintain the health of the living cell.
Both the UPR and ISR have evolved as essential protein quality control (PQC) systems to counteract disrupted proteostasis and prevent the development of proteinopathies. These programs sense proteostatic imbalances by receptors in the cytosol (for the ISR) and the ER (for the UPR) and initiate the production of rescue factors as well as a global translational arrest. As illustrated in Figure 4, the phosphorylation of the translation initiation factor eIF2α by the two cytosolic kinases general control non-derepressible 2 (GCN2) [108], and double-stranded RNA-dependent protein kinase (PKR) [109] or protein kinase RNA-like endoplasmic reticulum kinase (PERK) [110] is a central event at the intersection of these pathways.
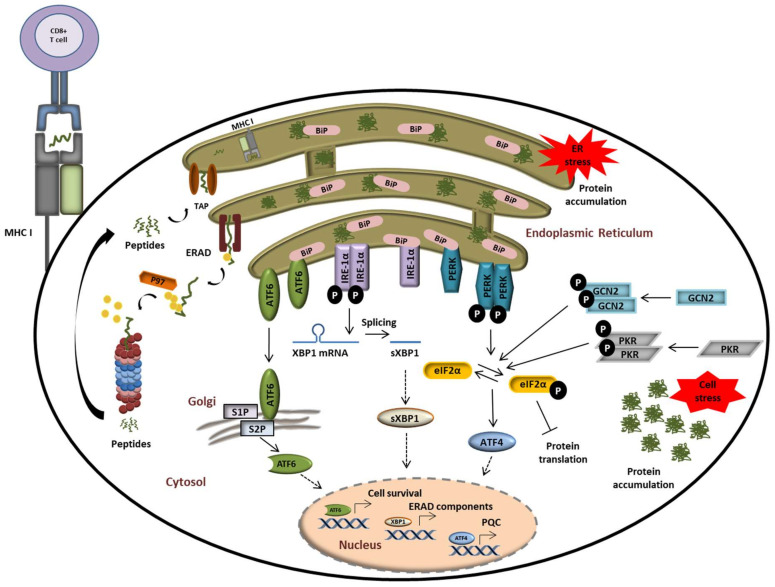
Figure 4.
The UPS plays a central role in alerting and resolving imbalances of protein homeostasis. Proteostasis disruption is typically characterized by the accumulation of misfolded and/or damaged proteins in the ER lumen which fail to be retro-translocated to the cytosol by ERAD for proteasomal degradation. Protein aggregation in the ER is sensed by the three ER receptors IRE1α, ATF6, and PERK which then undergo activation to trigger the UPR. This ultimately results in the activation of transcription factors ATF6, sXBP1, and ATF4, thereby promoting the transcription of genes coding for proteins destined to compensate the burden of unfolded/damaged proteins. Perturbed protein homeostasis is also sensed in the cytosol by GCN2 and PKR which undergo autoactivation following amino acid deficiency and stress granules, respectively. Both of these kinases phosphorylate eIF2α, thereby intersecting with the PERK-mediated pathway of the UPR.
All eIF2α kinases share similar kinase catalytic domains, dimerize, and autophosphorylate for full activation. Reflecting their unique regulatory mechanisms, each kinase responds to distinct environmental and physiological stimuli. While the heme-regulated inhibitor (HRI) is mainly expressed in erythroid cells to synchronize the availability of heme and iron with globin translation and the production of haemoglobin, all other kinases are ubiquitously expressed [111]. GCN2 is activated by metabolic stimuli such as amino acid starvation to coordinate translation with the availability of amino acids. Interestingly, it has been shown that proteasome inhibition also promotes the activation of GCN2 due to missing amino acid delivery from protein decay and recycling mechanisms [112,113,114]. PKR appears to be the most versatile kinase in terms of inducers. Although double-stranded RNA and virus infection represent the main activating agents for PKR, it has been shown that oxidative, ER-, or ribotoxic stress, growth factor deprivation, cytokines, or stress granules activate PKR as well [115]. By contrast, PERK is activated by misprocessed ER proteins in the lumen or membrane to catalyse eIF2α phosphorylation and the concomitant downstream events [110,116]. The global translational arrest upon eIF2α phosphorylation results in focused translation of ATF4 and other transcription factors. Phosphorylation of eIF2α can be counteracted by eIF2α phosphatases such as the protein phosphatase 1 (PP1) complex that recruits a PP1 catalytic subunit (PP1c) and one of the two regulatory subunits PPP1R15A or GADD34 [117,118]. As shown in Figure 4, perturbations of protein homeostasis in the ER lumen are sensed by three ER-transmembrane receptors: inositol-requiring protein 1α (IRE-1α), activating transcription factor 6 (ATF6), and PERK. Under physiological conditions, IRE-1α, ATF6, and PERK are associated with the molecular chaperone binding immunoglobulin protein BiP/GRP78 and reside in the inactive form [119,120]. In response to ER stress, BiP/GRP78 disassociates from the IRE-1α, PERK, and ATF6 to activate the three branches of the UPR. In addition to activating the splicing of the transcription factor Xbp1 to sXBP1, IRE-1 has been linked to the degradation of various cytosolic mRNAs, a process known as regulated IRE-1-dependent decay (RIDD) [121,122]. ATF6 is an ER membrane-bound transcription factor itself that is processed by the Golgi proteases S1P and S2P to become activated [123,124]. Transcription factors induced by all branches of the UPR and/or the ISR trigger the upregulation of stress genes. These genes encode rescue factors such as chaperones to increase the protein folding capacity, components of the ER-associated degradation (ERAD) for removal of irreversibly damaged proteins, and factors that control amino acid and lipid metabolism, cell death, or autophagy (Figure 4) [118,125,126]. Thus, reprogramming of the proteostasis network by downregulation of the global protein synthesis is a common downstream process of ISR and UPR.
Another adaptation mechanism to combat proteotoxic stress represents the upregulation of proteasome isoforms upon cytokine signalling or infection. IP are in particular important for rapid clearance of damaged or misfolded proteins to sustain cellular protein homeostasis under interferon-induced oxidative stress [27]. Unlike SP, IP have a high affinity for the interferon-γ (IFN-γ)-inducible PA28α/β regulatory particle [127]. As a response to cellular stress, e.g., oxidative stress, IP can assemble with both 19S and PA28α/β regulators to form a hybrid proteasome (HP, 19S-20S-PA28α/β) [128,129], thereby enhancing the rapid degradation of oxidant-damaged proteins in a ubiquitin-dependent manner. As such, it prevents the accumulation of protein aggregates and produces antigenic peptides for MHC class I antigen presentation [130,131]. Cytokine or TLR signalling causes an increase in ubiquitin–protein conjugates in different immune cells [68,132,133]. Importantly, IP formation prevents the accumulation of ubiquitin-modified proteins, as evidenced by increased aggregation of ubiquitin-positive inclusions in IP-lacking or IP specific ONX-0914 inhibitor-treated [27,69,134,135]. Proteostasis disruption caused by UPS impairment is especially challenging for myeloid cells. Our recent study revealed that inhibition of proteasomes (both SP and IP) by bortezomib results in accumulation of ubiquitin–protein conjugates and subsequent activation of the UPR, thereby resulting in the generation of a type I IFN response in myeloid cells [135]. Specifically, the activation of the IRE1α-dependent arm of UPR was mainly responsible for the induction of type I IFN signalling in microglia as well as the human monocytic cell line THP-1 following proteasome impairment. As alluded to earlier, the accumulation of proteins following disrupted protein homeostasis and neuroinflammation is a very common feature of most of the neurodegenerative diseases such as Alzheimer’s Disease (AD), Parkinson’s Disease (PD), and Huntington Disease (HD) [106,136,137]. Thibaudeau et al. have shown that toxic oligomers, e.g., amyloid-beta (Aβ), alpha-synuclein (α-syn), and mutant Huntington (Htt), which share a common 3D structure, inhibit proteasome activity and thereby sustain protein aggregation in vitro [138]. Nevertheless, further investigation of the UPS-mediated proteostasis in myeloid cells is needed to better understand the pathogenesis behind the proteasome impairment and its contribution to the development of neurodegenerative diseases.
4. Role of the UPS in MHC Class I Antigen Processing and Presentation
Antigen presentation is a key process for the initiation of primary adaptive immune responses, in the course of which foreign antigens in the form of short peptide fragments are presented on the surface of cells to cytotoxic T cells (CTL). Antigens are presented by two main classes of major histocompatibility complexes (MHCs). MHC class II molecules are expressed exclusively on the surface of professional antigen-presenting cells to present extracellular foreign antigens, which are processed in the lysosome (e.g., from phagocytosed bacteria or viruses) [139,140]. MHC class I molecules are expressed by all nucleated cells and bind self-peptides derived from cellular proteins as well as foreign peptides derived from proteins arising from intracellular pathogens such as viruses. Peptides to be presented by MHC class I molecules are typically 8–10 amino acids in length and originate from proteasome-dependent protein breakdown. Although both SP and IP are able to generate peptides for MHC class I presentation, the incorporation of the inducible subunits into proteasomes may be either beneficial or detrimental in this process. Hence, a substantial number of studies highlighted a positive role of IP in the initiation of CTL responses directed against viral and bacterial antigens [141,142,143,144,145,146]. However, IP have also been reported to abrogate the presentation of tumour epitopes [147,148,149,150]. The differences observed between SP and IP in the supply of MHC class I-restricted peptides may be easily explained by the fact that IP subunits exhibit a higher cleavage rate than their standard counterparts [151] which, depending on peptide sequence, may result in increased epitope generation or destruction.
In addition, both SP and IP are also capable of generating neoepitopes composed of two different fragments from peptide sequences which are not contiguous in the parent antigen [152,153,154,155,156,157,158,159]. Such peptides emerge from the fusion of proteasomal cleavage products in a process referred to as proteasome catalysed peptide splicing (PCPS) [160,161]. Because of the technical difficulties to detect such spliced peptides, the contribution of PCPS to the MHC class I peptidome has long been underestimated or even ignored [162]. However, a study reporting that one third of cell surface MHC class I ligands are represented by PCPS-generated peptides has recently brought PCPS into focus of immunology research [163]. As shown in Figure 4, proteasome-generated peptides are subsequently transported from the cytosol into the ER via transporter associated with antigen processing (TAP) and loaded onto the MHC class I molecules bound to the ER membrane [164,165,166,167,168]. Peptide-loaded MHC class I molecules in turn dissociate from the ER and are translocated to the cell surface for presentation to CTL which can directly eliminate infected cells [169]. Moreover, dendritic cells and macrophages can also present extracellular antigens by cross-presentation on MHC class I molecules in proteasome-dependent [43,170,171,172,173,174] and -independent [175,176,177,178,179,180] mechanisms. As initially postulated by Yewdell and colleagues [181], the major peptide source for MHC class I antigen presentation derives from defective ribosomal products (DRIPs), which appear due to errors during protein synthesis [182,183]. DRIPs are immediately ubiquitinated polypeptides at the ribosome and are degraded by proteasomes in situ. The usage of DRIPs as antigenic source ensures the presentation of peptides derived from almost all proteins irrespective of their localization [184,185,186,187,188,189,190]. Protein synthesis and thus, DRIP formation, is induced by infection or inflammation triggered by IFN signalling [27,132,191,192]. Enhanced degradation capacities of IP assure efficient generation of peptides for MHC class I antigen presentation as shown in IP-deficient mice [142,193]. Thus, the IP function relies on the clearance of inflammation-induced DRIPs and inflammation-damaged proteins, thereby improving peptide supply for antigen presentation [27].
5. The UPS in Disease
5.1. Autoinflammatory Diseases
As described above, proteasome dysfunction has been mainly correlated with proteinopathies of the central nervous system. Therefore, it was somehow unexpected that genetic alterations (e.g., loss of function mutations) in genes encoding proteasome components or their assembly factors have been found in patients suffering from autoinflammation. Autoinflammatory disorders represent sterile inflammatory conditions characterized by episodes of early-onset fever and disease-specific patterns of organ inflammation including neuroinflammation [194] called CANDLE/PRAAS (Chronic Atypical Neutrophilic Dermatosis with Lipodystrophy and Elevated Temperature/Proteasome Associated Autoinflammatory Syndromes) [195,196]. Depending on the laboratory in which such alterations were characterized, these syndromes have also been referred to as joint contractures, muscle atrophy, microcytic anaemia, and panniculitis-induced lipodystrophy (JMP), Nakajo-Nishimura syndrome (NKJO), POMP-related autoinflammation and immune dysregulation disease (PRAID), and CANDLE/PRAAS [197,198,199,200,201]. As shown in Table 1, mutations in the PSMB8 gene, encoding the IP subunit β5i/LMP7, were the first identified to cause CANDLE/PRAAS [197,199,200]. Therefore, it was proposed that CANDLE/PRAAS might be an IP deficiency disease. However, in 2015 Brehm et al. identified other mutations in genes coding for IP as well as SP 20S subunits: PSMA3 (encodes proteasome subunit α7), PSMB4 (encodes proteasome subunit β7), PSMB9 (encodes proteasome subunit β1i/LMP2), as well as POMP (encodes proteasome maturation protein, POMP) [201].
Table 1.
List of disorders associated with genetic alterations of genes coding for UPS components.
| Disease | Altered UPS Gene | Component |
|---|---|---|
| CANDLE/PRAAS | PSMB8 (β5i/LMP7) | 20S |
| PSMA3 (α7) | 20S | |
| PSMB4 (β7) | 20S | |
| PSMB9 (β1i/LMP2) | 20S | |
| POMP (POMP) | Proteasome assembly | |
| PSMG2 (PAC2) | Proteasome assembly | |
| PSMB10 (β2i/MECL-1) | 20S | |
| Neurodevelopmental Disorders | PSMC3 (Rpt5) | 19S |
| PSMD12 (Rpn5) | 19S | |
| PSMB1 (β6) | 20S | |
| A20 Haploinsufficiency | TNFAIP3 (A20) | DUB |
| Syndromic Multisystem Autoimmune Disease |
ITCH (ITCH) | E3 ligase |
A couple of years later, further mutations were identified in POMP [202], in the proteasome assembly chaperone 2 gene, PSMG2 [203], as well as in the third IP gene PSMB10 encoding the β2i/MECL1 subunit [204]. Regardless of the genomic alteration and subunit, the major molecular consequence of alterations in cells of CANDLE/PRAAS patients is a decreased proteasome assembly and activity of proteasomes leading to an accumulation of ubiquitinated proteins [198,199,201,202,203,204]. A common clinical feature of CANDLE/PRAAS is the systemic inflammation including neuroinflammation. Consistent with the phenotype observed in cells upon proteasome impairment, CANDLE/PRAAS patients are characterized by a chronic secretion of pro-inflammatory cytokines and type I IFN [196,201,203], thereby placing these conditions into the category of interferonopathies [194]. Intriguingly, proteasome loss-of-function mutations are not always associated with systemic autoinflammation. Herein, subjects with alterations in PSMD12, PSMC3 or PSMB1 suffer from neurodevelopmental disorders rather than typical CANDLE/PRAAS syndromes [205,206,207,208]. The reason for these varying phenotypes is still unclear and warrants further investigation.
Apart from proteasome genes, further components of the UPS may also play a role in the activation of inflammatory signalling pathways, when altered. For example, mutations in A20, an enzyme with dual DUB/E3 function [81,209], has been shown to cause dysregulation of NF-κB signalling in patients suffering autoinflammatory disorders (AIDs) [210,211]. Further examples of UPS alterations outside proteasome genes include mutations in the E3 ubiquitin ligase Itch, which results in truncation of the ITCH protein and ITCH deficiency. They are identified as a cause of syndromic multisystem autoimmune disease whose major phenotype is chronic lung disease [212]. Importantly, Itch has been shown to cooperate with other E3 ligases including WWP2 and Cbl-b [213,214] and its deficiency may compromise the breakdown of multiples targets in immune cells [215,216,217,218,219,220,221].
5.2. Manipulation of the UPS by Pathogens
In the above chapters, the UPS was outlined as an important player in the regulation of immune cell function and immune responses. Therefore, it is not very surprising that many pathogens have evolved sophisticated mechanisms to manipulate the UPS to ensure immune escape and survival in the host. In this chapter, two distinct examples of pathogenic mechanisms of UPS manipulation will be explained in more detail.
In 1976 an outbreak of an unknown disease occurred during a convention of war veterans in Philadelphia, USA. The causative agent was later identified as the gram-negative bacterium Legionella pneumophila causing severe lung inflammation known as Legionnaire’s diseases which are typically caught following inhalation of infected droplets and/or water from air conditioning systems. L. pneumophila provides an interesting example of pathogens capable of evading the immune system by hijacking the host UPS. Herein, L. pneumophila has been shown to exploit host proteasomes by expressing ubiquitin ligases that target other bacterial proteins for degradation when these are no longer required. One of these E3 ligases encoded by L. pneumophila include the U-box protein LubX which mediates the ubiquitination and subsequent proteasome-mediated degradation of its own effector protein SidH which is beneficial only during the early phase of infection [222,223,224].
In addition to influencing protein breakdown, L. pneumophila also impacts vesicle trafficking through the ubiquitination of the host ER-associated Rab small GTPases Rab33b and Rab1 by the ubiquitin ligase SidE [225,226]. Strikingly, the ubiquitin-conjugation system initiated by SidE differs fundamentally from the traditional one employed by eukaryotic cells. Indeed, instead of using ATP and the canonical three-enzyme cascade, it uses NAD to activate ubiquitin and form an ADP-ribose-ubiquitin intermediate whose ubiquitin moiety can then be directly transferred to intracellular substrates in an E1/E2-independent manner [226,227]. In addition, the phosphoribosyl moiety of the ADP-ribose-ubiquitin intermediate can also be used to modify further latent ubiquitin molecules so that these cannot be used for conjugation anymore, thereby impairing critical cellular processes of the innate immune response, inducing protein breakdown and signal transduction [227]. Besides SidE, another “all-in-one” ubiquitin ligase of L. pneumophila includes the transglutaminase MavC, which attaches ubiquitin via glutamine (Gln40) to UBE2N using two possible target lysine’s (Lys92 and Lys94) [228]. Mono-ubiquitination of UBE2N by MavC results in its inactivation, thereby abolishing the formation of K63-polyubiquitin chains required for NF-κB signalling. Importantly, L. pneumophila also expresses a significant number of DUBs which counteract the ubiquitination of intracellular host proteins [229]. These include SdeA that damps NF-κB signalling by preferentially trimming ubiquitin K63-linkages [230].
Since the recent outbreak of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) β-coronaviruses are back in the focus of the scientific community. Similar to many other viruses, β-coronaviruses interfere with the host UPS to evade immune recognition and ensure viral replication. Of particular interest is the viral papain-like protease (PLpro), which acts as a DUB with broad specificity in SARS-CoV-1 and -2 as well as in the Middle East respiratory syndrome-related coronavirus (MERS-CoV) [231,232,233,234]. By removing the K48-linked polyubiquitin chains from IκBα, PLpro prevents IκBα proteasomal degradation and therefore dampens NF-κB activation [235]. Interestingly, beside trimming ubiquitin chains, PLpro is capable of removing the ubiquitin-like modifier ISG15 from intracellular proteins [231]. In this regard, the de-ISGylation of IRF3 by PLpro has been shown to destabilize IRF3 and thus contribute to the evasion of coronaviruses to type I IFN responses [236].
5.3. Tumour Diseases
As previously stated, immune cell function strongly relies on a balanced proteostasis and a functional UPS. Thus, it is not surprising that the complete hematopoietic system and stem cell function in general are dependent on the timely degradation of signalling molecules during haematopoiesis and differentiation [237,238,239,240]. Consequently, the UPS and the proteostatic potential of hematopoietic cells represent targets of cancer therapy in hematopoietic malignancies [241]. Leukaemia is a group of blood cancers originating from transformation of haematopoietic stem or progenitor cells. Many leukaemia treatment strategies rely on the generation of genotoxic stress in highly proliferating cancer cells [242]. We will focus on two alternative strategies targeting components of the UPS, e.g., the proteasome or the cereblon (CRBN) E3-ubiquitin ligase.
Since its approval in 2003, pharmacological inhibition of proteasome activity by small molecules such as bortezomib (BTZ), also known under the brand name Velcade®, has become the first line therapy of multiple myeloma and mantle cell lymphoma. This led to significant improvement of survival rates of myeloma patients [243]. Meanwhile, because of side effects and BTZ resistance, a couple of additional proteasome inhibitors (Carfilzomib, Ixazomib, Delanzomib, Oprozomib, and Marizomib) with broader anti-cancer activities and altered pharmacological properties have been approved for clinical use. These also show improved clinical outcome in myeloma patients who relapsed upon initial BTZ treatment [242,244,245,246,247]. Thanks to the good efficacy of proteasome inhibitors in multiple myeloma or mantle cell lymphoma, several clinical trials have been launched to test responsiveness of other leukaemia types to proteasome inhibitors. Molecular mechanisms of BTZ resistance are not fully understood; however, three major features are observed in patients with relapses, namely, (i) genetic alterations in β5, (ii) altered proteasome subunit compositions, and (iii) induction of proteasome subunits. Genetic analysis of BTZ-resistant cells revealed a hot spot for mutations in exon 2 of PSMB5 encoding the β5 subunit. All mutations affect the BTZ binding site of β5, alanine in position 49, directly or in close proximity to, and prevent efficient binding of BTZ [242]. Another resistance mechanism relies on alteration of immuno-/constitutive proteasome ratio either by up- or down-regulation of immunoproteasomes [248]. Upregulation of the β5 subunit partly accompanied by the induction of other subunits represents the third resistance mechanism.
Proteasome abundance in cells is regulated by the transcription factor TCF11/Nrf1 encoded by the NFE2L1 gene [249]. TCF11/Nrf1 is an ER-membrane-resident protein, which is ubiquitin-modified by the E3 ligase Hrd1 and permanently degraded by ERAD under non-stressed conditions. Upon proteotoxic stress triggered by proteasome inhibitors, TCF11/Nrf1 is deglycosylated by NGLY1, pulled out of the ER-membrane by the AAA-ATPase p97 and cleaved by the aspartyl protease DDI2 (DNA-damage inducible 1 homolog 2). The cleaved form of TCF11/Nrf1 is translocated into the nucleus, where it binds to antioxidant response elements (AREs) in the promoter regions of almost all proteasome genes, and induces their expression [250,251,252] in a process referred to as bounce back response [253]. The complete NGLY1-DDI2-TCF11/Nrf1 axis is discussed as a new drug target for myeloproliferative neoplasms [254,255,256].
Thalidomide, also known under its brand name Contergan, initiated one of the biggest pharmacological disasters and scandals in Germany. More than 50 years ago, this drug was advertised as a harmless sleeping and tranquillizing agent that helps against morning sickness in pregnancy, but caused embryonic damage to thousands of children because of its teratogenic potential [257,258]. Half a decade later, thalidomide or its derivatives lenalidomide, and pomalidomide experience a revival as anticancer drugs for treatment of hematologic malignancies. The direct target of such thalidomide-like drugs is the protein cereblon, which together with the damaged DNA binding protein 1 (DBB1), cullin-4A (CUL4A), and regulator of cullins 1 (ROC1) forms an E3 ubiquitin ligase complex [259]. This ubiquitin ligase complex controls pathways involved in limb outgrowth of embryos and other developmental pathways [257]. Binding of thalidomide to the substrate recognition protein cereblon alters the substrate portfolio of the cereblon containing the E3 ubiquitin ligase complex. It has been demonstrated that the zinc finger-containing transcription factors Ikaros (IKZF 1) and Aiolos (IKZF3) are selectively bound by cereblon upon thalidomide treatment and target these transcription factors for degradation. Consequently, this causes cytotoxicity to multiple myeloma cells. In addition, Ikaros and Aiolos control cytokine production. Therefore, thalidomide and its derivatives also exert immunomodulatory effects [258,260,261]. In conclusion, components of the UPS turned out to be very important drug targets in the fight against cancer, and the future certainly will bring up some more promising candidates.
Author Contributions
G.Ç. and S.K. conceived the study, collected the data and draft the manuscript. M.S.-T., E.K. and F.E. helped conceived the study, analyzed the data and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by German Research Foundation: SFB/Transregio 167 Project A04 and the Comprehensive Cancer Center Mecklenburg-Vorpommern (CCC-MV). We acknowledge support for Article Processing Charge from the DFG (German Research Foundation, 393148499) and the Open Access Publication Fund of the University of Greifswald.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ciehanover A., Hod Y., Hershko A. A heat-stable polypeptide component of an ATP-dependent proteolytic system from reticulocytes. Biochem. Biophys. Res. Commun. 1978;81:1100–1105. doi: 10.1016/0006-291x(78)91249-4. [DOI] [PubMed] [Google Scholar]
- 2.Ciechanover A., Elias S., Heller H., Ferber S., Hershko A. Characterization of the heat-stable polypeptide of the ATP-dependent proteolytic system from reticulocytes. J. Biol. Chem. 1980;255:7525–7528. [PubMed] [Google Scholar]
- 3.Hershko A., Ciechanover A., Rose I.A. Resolution of the ATP-dependent proteolytic system from reticulocytes: A component that interacts with ATP. Proc. Natl. Acad. Sci. USA. 1979;76:3107–3110. doi: 10.1073/pnas.76.7.3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilkinson K.D., Urban M.K., Haas A.L. Ubiquitin is the ATP-dependent proteolysis factor I of rabbit reticulocytes. J. Biol. Chem. 1980;255:7529–7532. [PubMed] [Google Scholar]
- 5.Pickart C.M. Mechanisms underlying ubiquitination. Annu. Rev. Biochem. 2001;70:503–533. doi: 10.1146/annurev.biochem.70.1.503. [DOI] [PubMed] [Google Scholar]
- 6.Pickart C.M., Eddins M.J. Ubiquitin: Structures, functions, mechanisms. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2004;1695:55–72. doi: 10.1016/j.bbamcr.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 7.Li W., Bengtson M.H., Ulbrich A., Matsuda A., Reddy V.A., Orth A., Chanda S.K., Batalov S., Joazeiro C.A.P. Genome-wide and functional annotation of human E3 ubiquitin ligases identifies MULAN, a mitochondrial E3 that regulates the organelle’s dynamics and signaling. PLoS ONE. 2008;3:e1487. doi: 10.1371/journal.pone.0001487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berndsen C.E., Wolberger C. New insights into ubiquitin E3 ligase mechanism. Nat. Struct. Mol. Biol. 2014;21:301–307. doi: 10.1038/nsmb.2780. [DOI] [PubMed] [Google Scholar]
- 9.Swatek K.N., Komander D. Ubiquitin modifications. Cell Res. 2016;26:399–422. doi: 10.1038/cr.2016.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McDowell G., Kucerova R., Philpott A. Non-canonical ubiquitylation of the proneural protein Ngn2 occurs in both Xenopus embryos and mammalian cells. Biochem. Biophys. Res. Commun. 2010;400:655–660. doi: 10.1016/j.bbrc.2010.08.122. [DOI] [PubMed] [Google Scholar]
- 11.Golnik R., Lehmann A., Kloetzel P.M., Ebstein F. Major histocompatibility complex (MHC) class I processing of the NY-ESO-1 antigen is regulated by Rpn10 and Rpn13 proteins and immunoproteasomes following non-lysine ubiquitination. J. Biol. Chem. 2016;291:8805–8815. doi: 10.1074/jbc.m115.705178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tait S.W., De Vries E., Maas C., Keller A.M., D’Santos C.S., Borst J. Apoptosis induction by Bid requires unconventional ubiquitination and degradation of its N-terminal fragment. J. Cell Biol. 2007;179:1453–1466. doi: 10.1083/jcb.200707063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vosper J.M.D., McDowell G.S., Hindley C.J., Fiore-Heriche C.S., Kucerova R., Horan I., Philpott A. Ubiquitylation on canonical and non-canonical sites targets the transcription factor neurogenin for ubiquitin-mediated proteolysis. J. Biol. Chem. 2009;284:15458–15468. doi: 10.1074/jbc.m809366200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oh E., Akopian D., Rape M. Principles of ubiquitin-dependent signaling. Annu. Rev. Cell Dev. Biol. 2018;34:137–162. doi: 10.1146/annurev-cellbio-100617-062802. [DOI] [PubMed] [Google Scholar]
- 15.Sadowski M., Suryadinata R., Tan A.R., Roesley S.N.A., Sarcevic B. Protein monoubiquitination and polyubiquitination generate structural diversity to control distinct biological processes. IUBMB Life. 2012;64:136–142. doi: 10.1002/iub.589. [DOI] [PubMed] [Google Scholar]
- 16.Kravtsova-Ivantsiv Y., Ciechanover A. Non-canonical ubiquitin-based signals for proteasomal degradation. J. Cell Sci. 2012;125:539–548. doi: 10.1242/jcs.093567. [DOI] [PubMed] [Google Scholar]
- 17.Grumati P., Dikic I. Ubiquitin signaling and autophagy. J. Biol. Chem. 2018;293:5404–5413. doi: 10.1074/jbc.tm117.000117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Akutsu M., Dikic I., Bremm A. Ubiquitin chain diversity at a glance. J. Cell Sci. 2016;129:875–880. doi: 10.1242/jcs.183954. [DOI] [PubMed] [Google Scholar]
- 19.Dahlmann B. Proteasomes. Essays Biochem. 2005;41:31–48. doi: 10.1042/eb0410031. [DOI] [PubMed] [Google Scholar]
- 20.Tanaka K., Mizushima T., Saeki Y. The proteasome: Molecular machinery and pathophysiological roles. Biol. Chem. 2012;393:217–234. doi: 10.1515/hsz-2011-0285. [DOI] [PubMed] [Google Scholar]
- 21.Smith D.M., Chang S.-C., Park S., Finley D., Cheng Y., Goldberg A.L. Docking of the proteasomal ATPases’ Carboxyl termini in the 20S proteasome’s α ring opens the gate for substrate entry. Mol. Cell. 2007;27:731–744. doi: 10.1016/j.molcel.2007.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Finley D. Recognition and processing of ubiquitin-protein conjugates by the proteasome. Annu. Rev. Biochem. 2009;78:477–513. doi: 10.1146/annurev.biochem.78.081507.101607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bard J.A., Goodall E.A., Greene E.R., Jonsson E., Dong K.C., Martin A. Structure and function of the 26S proteasome. Annu. Rev. Biochem. 2018;87:697–724. doi: 10.1146/annurev-biochem-062917-011931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Finley D., Chen X., Walters K.J. Gates, channels, and switches: Elements of the proteasome machine. Trends Biochem. Sci. 2016;41:77–93. doi: 10.1016/j.tibs.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murata S., Takahama Y., Kasahara M., Tanaka K. The immunoproteasome and thymoproteasome: Functions, evolution and human disease. Nat. Immunol. 2018;19:923–931. doi: 10.1038/s41590-018-0186-z. [DOI] [PubMed] [Google Scholar]
- 26.Ebstein F., Kloetzel P.-M., Krüger E., Seifert U., Krüger E. Emerging roles of immunoproteasomes beyond MHC class I antigen processing. Cell. Mol. Life Sci. 2012;69:2543–2558. doi: 10.1007/s00018-012-0938-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seifert U., Bialy L.P., Ebstein F., Bech-Otschir D., Voigt A., Schröter F., Prozorovski T., Lange N., Steffen J., Rieger M., et al. Immunoproteasomes preserve protein homeostasis upon interferon-induced oxidative stress. Cell. 2010;142:613–624. doi: 10.1016/j.cell.2010.07.036. [DOI] [PubMed] [Google Scholar]
- 28.Yun Y.S., Kim K.H., Tschida B., Sachs Z., Noble-Orcutt K.E., Moriarity B.S., Ai T., Ding R., Williams J., Chen L., et al. mTORC1 coordinates protein synthesis and immunoproteasome formation via PRAS40 to prevent accumulation of protein stress. Mol. Cell. 2016;61:625–639. doi: 10.1016/j.molcel.2016.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Niewerth D., Kaspers G.J.L., Assaraf Y.G., Van Meerloo J., Kirk C.J., Anderl J., Blank J.L., Van de Ven P.M., Zweegman S., Jansen G., et al. Interferon-γ-induced upregulation of immunoproteasome subunit assembly overcomes bortezomib resistance in human hematological cell lines. J. Hematol. Oncol. 2014;7:7. doi: 10.1186/1756-8722-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Opitz E., Koch A., Klingel K., Schmidt F., Prokop S., Rahnefeld A., Sauter M., Heppner F.L., Völker U., Kandolf R., et al. Impairment of immunoproteasome function by β5i/LMP7 subunit deficiency results in severe enterovirus myocarditis. PLoS Pathog. 2011;7:e1002233. doi: 10.1371/journal.ppat.1002233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krüger E., Kloetzel P.-M. Immunoproteasomes at the interface of innate and adaptive immune responses: Two faces of one enzyme. Curr. Opin. Immunol. 2012;24:77–83. doi: 10.1016/j.coi.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 32.Guillaume B., Chapiro J., Stroobant V., Colau D., Van Holle B., Parvizi G., Bousquet-Dubouch M.-P., Théate I., Parmentier N., Eynde B.J.V.D. Two abundant proteasome subtypes that uniquely process some antigens presented by HLA class I molecules. Proc. Natl. Acad. Sci. USA. 2010;107:18599–18604. doi: 10.1073/pnas.1009778107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guillaume B., Stroobant V., Bousquet-Dubouch M.-P., Colau D., Chapiro J., Parmentier N., Dalet A., Eynde B.J.V.D. Analysis of the processing of seven human tumor antigens by intermediate proteasomes. J. Immunol. 2012;189:3538–3547. doi: 10.4049/jimmunol.1103213. [DOI] [PubMed] [Google Scholar]
- 34.Dahlmann B. Mammalian proteasome subtypes: Their diversity in structure and function. Arch. Biochem. Biophys. 2016;591:132–140. doi: 10.1016/j.abb.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 35.Gohlke S., Kloss A., Tsokos M., Textoris-Taube K., Keller C., Kloetzel P.M., Dahlmann B. Adult human liver con-tains intermediate-type proteasomes with different enzymatic properties. Ann. Hepatol. 2014;13:429–438. doi: 10.1016/S1665-2681(19)30850-6. [DOI] [PubMed] [Google Scholar]
- 36.Ma C.P., Slaughter C.A., DeMartino G.N. Identification, purification, and characterization of a protein activator (PA28) of the 20 S proteasome (macropain) J. Biol. Chem. 1992;267:10515–10523. [PubMed] [Google Scholar]
- 37.Wójcik C., Tanaka K., Paweletz N., Naab U., Wilk S. Proteasome activator (PA28) subunits, α, β and γ (Ki antigen) in NT2 neuronal precursor cells and HeLa S3 cells. Eur. J. Cell Biol. 1998;77:151–160. doi: 10.1016/S0171-9335(98)80083-6. [DOI] [PubMed] [Google Scholar]
- 38.Ustrell V., Hoffman L., Pratt G., Rechsteiner M. PA200, a nuclear proteasome activator involved in DNA repair. EMBO J. 2002;21:3516–3525. doi: 10.1093/emboj/cdf333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McCutchen-Maloney S.L., Matsuda K., Shimbara N., Binns D.D., Tanaka K., Slaughter C.A., DeMartino G.N. cDNA cloning, expression, and functional characterization of PI31, a proline-rich inhibitor of the proteasome. J. Biol. Chem. 2000;275:18557–18565. doi: 10.1074/jbc.M001697200. [DOI] [PubMed] [Google Scholar]
- 40.Zaiss D.M., Standera S., Kloetzel P.-M., Sijts A.J.A.M. PI31 is a modulator of proteasome formation and antigen processing. Proc. Natl. Acad. Sci. USA. 2002;99:14344–14349. doi: 10.1073/pnas.212257299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cohen-Kaplan V., Livneh I., Avni N., Fabre B., Ziv T., Kwon Y.T., Ciechanover A. p62- and ubiquitin-dependent stress-induced autophagy of the mammalian 26S proteasome. Proc. Natl. Acad. Sci. USA. 2016;113:E7490–E7499. doi: 10.1073/pnas.1615455113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bochmann I., Ebstein F., Lehmann A., Wohlschlaeger J., Sixt S.U., Kloetzel P., Dahlmann B. T lymphocytes export proteasomes by way of microparticles: A possible mechanism for generation of extracellular proteasomes. J. Cell. Mol. Med. 2013;18:59–68. doi: 10.1111/jcmm.12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sengupta D., Graham M., Liu X., Cresswell P. Proteasomal degradation within endocytic organelles mediates antigen cross-presentation. EMBO J. 2019;38:e99266. doi: 10.15252/embj.201899266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.DeMartino G.N., Gillette T.G. Proteasomes: Machines for all reasons. Cell. 2007;129:659–662. doi: 10.1016/j.cell.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 45.Laiosa C.V., Stadtfeld M., Graf T. Determinants of lymphoid-myeloid lineage diversification. Annu. Rev. Immunol. 2006;24:705–738. doi: 10.1146/annurev.immunol.24.021605.090742. [DOI] [PubMed] [Google Scholar]
- 46.Owens T., Benmamar-Badel A., Wlodarczyk A., Marczynska J., Mørch M.T., Dubik M., Arengoth D.S., Asgari N., Webster G., Khorooshi R. Protective roles for myeloid cells in neuroinflammation. Scand. J. Immunol. 2020;92:e12963. doi: 10.1111/sji.12963. [DOI] [PubMed] [Google Scholar]
- 47.Hiscott J. Convergence of the NF-κB and IRF pathways in the regulation of the innate antiviral response. Cytokine Growth Factor Rev. 2007;18:483–490. doi: 10.1016/j.cytogfr.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 48.Hiscott J., Grandvaux N., Sharma S., Tenoever B.R., Servant M.J., Lin R. Convergence of the NF-κB and interferon signaling pathways in the regulation of antiviral defense and apoptosis. Ann. N. Y. Acad. Sci. 2003;1010:237–248. doi: 10.1196/annals.1299.042. [DOI] [PubMed] [Google Scholar]
- 49.Heaton S.M., Borg N.A., Dixit V.M. Ubiquitin in the activation and attenuation of innate antiviral immunity. J. Exp. Med. 2016;213:1–13. doi: 10.1084/jem.20151531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu X.-F., Karin M. Emerging roles of Lys63-linked polyubiquitylation in immune responses. Immunol. Rev. 2015;266:161–174. doi: 10.1111/imr.12310. [DOI] [PubMed] [Google Scholar]
- 51.Karin M., Ben-Neriah Y. Phosphorylation meets ubiquitination: The control of NF-κB activity. Annu. Rev. Immunol. 2000;18:621–663. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- 52.Wertz I.E., Dixit V.M. Ubiquitin-mediated regulation of TNFR1 signaling. Cytokine Growth Factor Rev. 2008;19:313–324. doi: 10.1016/j.cytogfr.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 53.Ye H., Arron J.R., Lamothe B., Cirilli M., Kobayashi T., Shevde N.K., Segal D., Dzivenu O.K., Vologodskaia M., Yim M., et al. Distinct molecular mechanism for initiating TRAF6 signalling. Nature. 2002;418:443–447. doi: 10.1038/nature00888. [DOI] [PubMed] [Google Scholar]
- 54.Yin Q., Lin S.-C., Lamothe B., Lu M., Lo Y.-C., Hura G., Zheng L., Rich R.L., Campos A.D., Myszka D.G., et al. E2 interaction and dimerization in the crystal structure of TRAF6. Nat. Struct. Mol. Biol. 2009;16:658–666. doi: 10.1038/nsmb.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rothwarf D.M., Zandi E., Natoli G., Karin M. IKK-γ is an essential regulatory subunit of the IκB kinase complex. Nature. 1998;395:297–300. doi: 10.1038/26261. [DOI] [PubMed] [Google Scholar]
- 56.Yamaoka S., Courtois G., Bessia C., Whiteside S.T., Weil R., Agou F., Kirk H.E., Kay R.J., Israël A. Complementation cloning of NEMO, a component of the IκB kinase complex essential for NF-κB activation. Cell. 1998;93:1231–1240. doi: 10.1016/S0092-8674(00)81466-X. [DOI] [PubMed] [Google Scholar]
- 57.Cohen P., Strickson S. The role of hybrid ubiquitin chains in the MyD88 and other innate immune signalling pathways. Cell Death Differ. 2017;24:1153–1159. doi: 10.1038/cdd.2017.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Emmerich C.H., Ordureau A., Strickson S., Arthur J.S.C., Pedrioli P.G.A., Komander D., Cohen P. Activation of the canonical IKK complex by K63/M1-linked hybrid ubiquitin chains. Proc. Natl. Acad. Sci. USA. 2013;110:15247–15252. doi: 10.1073/pnas.1314715110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lo Y.-C., Lin S.-C., Rospigliosi C.C., Conze D.B., Wu C.-J., Ashwell J.D., Eliezer D., Wu H. Structural basis for recognition of diubiquitins by NEMO. Mol. Cell. 2009;33:602–615. doi: 10.1016/j.molcel.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rahighi S., Ikeda F., Kawasaki M., Akutsu M., Suzuki N., Kato R., Kensche T., Uejima T., Bloor S., Komander D., et al. Specific recognition of linear ubiquitin chains by NEMO is important for NF-κB activation. Cell. 2009;136:1098–1109. doi: 10.1016/j.cell.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 61.Häcker H., Karin M. Regulation and function of IKK and IKK-related kinases. Sci. STKE. 2006;2006:re13. doi: 10.1126/stke.3572006re13. [DOI] [PubMed] [Google Scholar]
- 62.Winston J.T., Strack P., Beer-Romero P., Chu C.Y., Elledge S.J., Harper J.W. The SCFbeta-TRCP-ubiquitin ligase complex associates specifically with phosphorylated destruction motifs in IkappaBalpha and beta-catenin and stimulates IkappaBalpha ubiquitination in vitro. Genes Dev. 1999;13:270–283. doi: 10.1101/gad.13.3.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Suzuki H., Chiba T., Kobayashi M., Takeuchi M., Suzuki T., Ichiyama A., Ikenoue T., Omata M., Furuichi K., Tanaka K. IκBα ubiquitination is catalyzed by an SCF-like complex containing Skp1, cullin-1, and two F-box/WD40-repeat proteins, βTrCP1 and βTrCP2. Biochem. Biophys. Res. Commun. 1999;256:127–132. doi: 10.1006/bbrc.1999.0289. [DOI] [PubMed] [Google Scholar]
- 64.Visekruna A., Joeris T., Seidel D., Kroesen A., Loddenkemper C., Zeitz M., Kaufmann S.H., Schmidt-Ullrich R., Steinhoff U. Proteasome-mediated degradation of IκBα and processing of p105 in Crohn disease and ulcerative colitis. J. Clin. Investig. 2006;116:3195–3203. doi: 10.1172/JCI28804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schmidt N., Gonzalez E., Visekruna A., Kühl A.A., Loddenkemper C., Mollenkopf H., Kaufmann S.H.E., Steinhoff U., Joeris T. Targeting the proteasome: Partial inhibition of the proteasome by bortezomib or deletion of the immunosubunit LMP7 attenuates experimental colitis. Gut. 2010;59:896–906. doi: 10.1136/gut.2009.203554. [DOI] [PubMed] [Google Scholar]
- 66.Mitchell S., Mercado E.L., Adelaja A., Ho J.Q., Cheng Q.J., Ghosh G., Hoffmann A. An NFκB activity calculator to delineate signaling crosstalk: Type I and II interferons enhance NFκB via distinct mechanisms. Front. Immunol. 2019;10:1425. doi: 10.3389/fimmu.2019.01425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xu J., Zhou L., Ji L., Chen F., Fortmann K., Zhang K., Liu Q., Li K., Wang W., Wang H., et al. The REGγ-proteasome forms a regulatory circuit with IκBɛ and NFκB in experimental colitis. Nat. Commun. 2016;7:10761. doi: 10.1038/ncomms10761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ebstein F., Lange N., Urban S., Seifert U., Krüger E., Kloetzel P.-M. Maturation of human dendritic cells is accompanied by functional remodelling of the ubiquitin-proteasome system. Int. J. Biochem. Cell Biol. 2009;41:1205–1215. doi: 10.1016/j.biocel.2008.10.023. [DOI] [PubMed] [Google Scholar]
- 69.Muchamuel T., Basler M., Aujay M.A., Suzuki E., Kalim K.W., Lauer C., Sylvain C., Ring E.R., Shields J., Jiang J., et al. A selective inhibitor of the immunoproteasome subunit LMP7 blocks cytokine production and attenuates progression of experimental arthritis. Nat. Med. 2009;15:781–787. doi: 10.1038/nm.1978. [DOI] [PubMed] [Google Scholar]
- 70.Basler M., Dajee M., Moll C., Groettrup M., Kirk C.J. Prevention of experimental colitis by a selective inhibitor of the immunoproteasome. J. Immunol. 2010;185:634–641. doi: 10.4049/jimmunol.0903182. [DOI] [PubMed] [Google Scholar]
- 71.Basler M., Mundt S., Muchamuel T., Moll C., Jiang J., Groettrup M., Kirk C.J. Inhibition of the immunoproteasome ameliorates experimental autoimmune encephalomyelitis. EMBO Mol. Med. 2014;6:226–238. doi: 10.1002/emmm.201303543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu H., Wan C., Ding Y., Han R., He Y., Xiao J., Hao J. PR-957, a selective inhibitor of immunoproteasome subunit low-MW polypeptide 7, attenuates experimental autoimmune neuritis by suppressing Th17-cell differentiation and regulating cytokine production. FASEB J. 2017;31:1756–1766. doi: 10.1096/fj.201601147R. [DOI] [PubMed] [Google Scholar]
- 73.Vachharajani N., Joeris T., Luu M., Hartmann S., Pautz S., Jenike E., Pantazis G., Prinz I., Hofer M.J., Steinhoff U., et al. Prevention of colitis-associated cancer by selective targeting of immunoproteasome subunit LMP7. Oncotarget. 2017;8:50447–50459. doi: 10.18632/oncotarget.14579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Althof N., Goetzke C.C., Kespohl M., Voss K., Heuser A., Pinkert S., Kaya Z., Klingel K., Beling A. The immunoproteasome-specific inhibitor ONX 0914 reverses susceptibility to acute viral myocarditis. EMBO Mol. Med. 2018;10:200–218. doi: 10.15252/emmm.201708089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Guo Y., Chen X., Li D., Liu H., Ding Y., Han R., Shi Y., Ma X. PR-957 mediates neuroprotection by inhibiting Th17 differentiation and modulating cytokine production in a mouse model of ischaemic stroke. Clin. Exp. Immunol. 2018;193:194–206. doi: 10.1111/cei.13132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Volkov A., Hagner S., Löser S., Alnahas S., Raifer H., Hellhund A., Garn H., Steinhoff U. β5i subunit deficiency of the immunoproteasome leads to reduced Th2 response in OVA induced acute asthma. PLoS ONE. 2013;8:e60565. doi: 10.1371/journal.pone.0060565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kimura H., Usui F., Karasawa T., Kawashima A., Shirasuna K., Inoue Y., Komada T., Kobayashi M., Mizushina Y., Kasahara T., et al. Immunoproteasome subunit LMP7 deficiency improves obesity and metabolic disorders. Sci. Rep. 2015;5:15883. doi: 10.1038/srep15883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bitzer A., Basler M., Krappmann D., Groettrup M. Immunoproteasome subunit deficiency has no influence on the canonical pathway of NF-κB activation. Mol. Immunol. 2017;83:147–153. doi: 10.1016/j.molimm.2017.01.019. [DOI] [PubMed] [Google Scholar]
- 79.Heger K., Wickliffe K.E., Ndoja A., Zhang J., Murthy A., Dugger D.L., Maltzman A., Melo F.D.S.E., Hung J., Zeng Y., et al. OTULIN limits cell death and inflammation by deubiquitinating LUBAC. Nature. 2018;559:120–124. doi: 10.1038/s41586-018-0256-2. [DOI] [PubMed] [Google Scholar]
- 80.Harhaj E.W., Dixit V.M. Deubiquitinases in the regulation of NF-κB signaling. Cell Res. 2010;21:22–39. doi: 10.1038/cr.2010.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wertz I.E., O’Rourke K.M., Zhou H., Eby M., Aravind L., Seshagiri S., Wu P., Wiesmann C., Baker R.T., Boone D.L., et al. De-ubiquitination and ubiquitin ligase domains of A20 downregulate NF-κB signalling. Nature. 2004;430:694–699. doi: 10.1038/nature02794. [DOI] [PubMed] [Google Scholar]
- 82.Wertz I.E., Newton K., Seshasayee D., Kusam S., Lam C., Zhang J., Popovych N., Helgason E., Schoeffler A., Jeet S., et al. Phosphorylation and linear ubiquitin direct A20 inhibition of inflammation. Nature. 2015;528:370–375. doi: 10.1038/nature16165. [DOI] [PubMed] [Google Scholar]
- 83.Martin F., Dixit V.M. A20 edits ubiquitin and autoimmune paradigms. Nat. Genet. 2011;43:822–823. doi: 10.1038/ng.916. [DOI] [PubMed] [Google Scholar]
- 84.Häcker H., Tseng P.-H., Karin M. Expanding TRAF function: TRAF3 as a tri-faced immune regulator. Nat. Rev. Immunol. 2011;11:457–468. doi: 10.1038/nri2998. [DOI] [PubMed] [Google Scholar]
- 85.Häcker H., Redecke V., Blagoev B., Kratchmarova I., Hsu L.-C., Wang G.G., Kamps M.P., Raz E., Wagner H., Häcker G., et al. Specificity in Toll-like receptor signalling through distinct effector functions of TRAF3 and TRAF6. Nature. 2005;439:204–207. doi: 10.1038/nature04369. [DOI] [PubMed] [Google Scholar]
- 86.Tseng P.-H., Matsuzawa A., Zhang W., Mino T., Vignali D.A.A., Karin M. Different modes of ubiquitination of the adaptor TRAF3 selectively activate the expression of type I interferons and proinflammatory cytokines. Nat. Immunol. 2009;11:70–75. doi: 10.1038/ni.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lei C.-Q., Zhang Y., Xia T., Jiang L.-Q., Zhong B., Shu H.-B. FoxO1 negatively regulates cellular antiviral response by promoting degradation of IRF3. J. Biol. Chem. 2013;288:12596–12604. doi: 10.1074/jbc.M112.444794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yu Y., Hayward G.S. The ubiquitin E3 ligase RAUL negatively regulates type i interferon through ubiquitination of the transcription factors IRF7 and IRF3. Immunity. 2010;33:863–877. doi: 10.1016/j.immuni.2010.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cui J., Li Y., Zhu L., Liu D., Songyang Z., Wang H.Y., Wang R.-F. NLRP4 negatively regulates type I interferon signaling by targeting the kinase TBK1 for degradation via the ubiquitin ligase DTX4. Nat. Immunol. 2012;13:387–395. doi: 10.1038/ni.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zheng Q., Hou J., Zhou Y., Yang Y., Xie B., Cao X. Siglec1 suppresses antiviral innate immune response by inducing TBK1 degradation via the ubiquitin ligase TRIM27. Cell Res. 2015;25:1121–1136. doi: 10.1038/cr.2015.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang M., Wang L., Zhao X., Zhao K., Meng H., Zhao W., Gao C. TRAF-interacting protein (TRIP) negatively regulates IFN-β production and antiviral response by promoting proteasomal degradation of TANK-binding kinase 1. J. Exp. Med. 2012;209:1703–1711. doi: 10.1084/jem.20120024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fan Y., Mao R., Yu Y., Liu S., Shi Z., Cheng J., Zhang H., An L., Zhao Y., Xu X., et al. USP21 negatively regulates antiviral response by acting as a RIG-I deubiquitinase. J. Exp. Med. 2014;211:313–328. doi: 10.1084/jem.20122844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Friedman C.S., O’Donnell M.A., Legarda-Addison D., Ng A., Cárdenas W.B., Yount J.S., Moran T.M., Basler C.F., Komuro A., Horvath C.M., et al. The tumour suppressor CYLD is a negative regulator of RIG-I-mediated antiviral response. EMBO Rep. 2008;9:930–936. doi: 10.1038/embor.2008.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cui J., Song Y., Li Y., Zhu Q., Tan P., Qin Y., Wang H.Y., Wang R.-F. USP3 inhibits type I interferon signaling by deubiquitinating RIG-I-like receptors. Cell Res. 2014;24:400–416. doi: 10.1038/cr.2013.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chen M., Meng Q., Qin Y., Liang P., Tan P., He L., Zhou Y., Chen Y., Huang J., Wang R.F., et al. TRIM14 Inhibits cGAS degradation mediated by selective autophagy receptor p62 to promote innate immune responses. Mol. Cell. 2016;64:105–119. doi: 10.1016/j.molcel.2016.08.025. [DOI] [PubMed] [Google Scholar]
- 96.Reis J., Hassan F., Guan X.Q., Shen J., Monaco J.J., Papasian C.J., Qureshi A.A., Van Way C.W., III, Vogel S.N., Morrison D.C., et al. The immunoproteasomes regulate LPS-induced TRIF/TRAM signaling pathway in murine macrophages. Cell Biophys. 2011;60:119–126. doi: 10.1007/s12013-011-9183-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Morimoto R.I., Cuervo A.M. Proteostasis and the aging proteome in health and disease. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2014;69:S33–S38. doi: 10.1093/gerona/glu049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bett J.S. Proteostasis regulation by the ubiquitin system. Essays Biochem. 2016;60:143–151. doi: 10.1042/ebc20160001. [DOI] [PubMed] [Google Scholar]
- 99.Gomez-Navarro N., Miller E.A. Protein sorting at the ER–Golgi interface. J. Cell Biol. 2016;215:769–778. doi: 10.1083/jcb.201610031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Maseda D., Meister S., Neubert K., Herrmann M., Voll R.E. Proteasome inhibition drastically but reversibly impairs murine lymphocyte development. Cell Death Differ. 2008;15:600–612. doi: 10.1038/sj.cdd.4402297. [DOI] [PubMed] [Google Scholar]
- 101.Obeng E.A., Carlson L.M., Gutman D.M., Harrington W.J., Jr., Lee K.P., Boise L.H. Proteasome inhibitors induce a terminal unfolded protein response in multiple myeloma cells. Blood. 2006;107:4907–4916. doi: 10.1182/blood-2005-08-3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Haigis M.C., Yankner B.A. The aging stress response. Mol. Cell. 2010;40:333–344. doi: 10.1016/j.molcel.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Olzscha H. Posttranslational modifications and proteinopathies: How guardians of the proteome are defeated. Biol. Chem. 2019;400:895–915. doi: 10.1515/hsz-2018-0458. [DOI] [PubMed] [Google Scholar]
- 104.Hipp M.S., Kasturi P., Hartl F.U. The proteostasis network and its decline in ageing. Nat. Rev. Mol. Cell Biol. 2019;20:421–435. doi: 10.1038/s41580-019-0101-y. [DOI] [PubMed] [Google Scholar]
- 105.Miles J., Scherz-Shouval R., Van Oosten-Hawle P. Expanding the organismal proteostasis network: Linking systemic stress signaling with the innate immune response. Trends Biochem. Sci. 2019;44:927–942. doi: 10.1016/j.tibs.2019.06.009. [DOI] [PubMed] [Google Scholar]
- 106.Limanaqi F., Biagioni F., Gambardella S., Familiari P., Frati A., Fornai F. Promiscuous roles of autophagy and proteasome in neurodegenerative proteinopathies. Int. J. Mol. Sci. 2020;21:3028. doi: 10.3390/ijms21083028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Golde T.E., Borchelt D.R., Giasson B.I., Lewis J. Thinking laterally about neurodegenerative proteinopathies. J. Clin. Investig. 2013;123:1847–1855. doi: 10.1172/JCI66029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dever T.E., Feng L., Wek R.C., Cigan A., Donahue T.F., Hinnebusch A.G. Phosphorylation of initiation factor 2α by protein kinase GCN2 mediates gene-specific translational control of GCN4 in yeast. Cell. 1992;68:585–596. doi: 10.1016/0092-8674(92)90193-G. [DOI] [PubMed] [Google Scholar]
- 109.Kostura M., Mathews M.B. Purification and activation of the double-stranded RNA-dependent eIF-2 kinase DAI. Mol. Cell. Biol. 1989;9:1576–1586. doi: 10.1128/MCB.9.4.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Harding H.P., Zhang Y., Ron D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature. 1999;397:271–274. doi: 10.1038/16729. [DOI] [PubMed] [Google Scholar]
- 111.Jiang H.-Y., Wek R.C., Anthony T. Coping with stress: eIF2 kinases and translational control. Biochem. Soc. Trans. 2006;34:7–11. doi: 10.1042/BST0340007. [DOI] [PubMed] [Google Scholar]
- 112.Rousseau A., Bertolotti A. Regulation of proteasome assembly and activity in health and disease. Nat. Rev. Mol. Cell Biol. 2018;19:697–712. doi: 10.1038/s41580-018-0040-z. [DOI] [PubMed] [Google Scholar]
- 113.Harding H.P., Novoa I., Zhang Y., Zeng H., Wek R., Schapira M., Ron D. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol. Cell. 2000;6:1099–1108. doi: 10.1016/S1097-2765(00)00108-8. [DOI] [PubMed] [Google Scholar]
- 114.Pakos-Zebrucka K., Koryga I., Mnich K., Ljujic M., Samali A., Gorman A.M. The integrated stress response. EMBO Rep. 2016;17:1374–1395. doi: 10.15252/embr.201642195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lee Y.S., Kunkeaw N., Lee Y. Protein kinase R and its cellular regulators in cancer: An active player or a surveillant? Wiley Interdiscip. Rev. RNA. 2020;11:e1558. doi: 10.1002/wrna.1558. [DOI] [PubMed] [Google Scholar]
- 116.Harding H.P., Zhang Y., Bertolotti A., Zeng H., Ron D. Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol. Cell. 2000;5:897–904. doi: 10.1016/S1097-2765(00)80330-5. [DOI] [PubMed] [Google Scholar]
- 117.Novoa I., Zeng H., Harding H.P., Ron D. Feedback inhibition of the unfolded protein response by GADD34-mediated dephosphorylation of eIF2α. J. Cell Biol. 2001;153:1011–1022. doi: 10.1083/jcb.153.5.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ma Y., Hendershot L.M. Delineation of a negative feedback regulatory loop that controls protein translation during endoplasmic reticulum stress. J. Biol. Chem. 2003;278:34864–34873. doi: 10.1074/jbc.M301107200. [DOI] [PubMed] [Google Scholar]
- 119.Bertolotti A., Zhang Y., Hendershot L.M., Harding H.P., Ron D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat. Cell Biol. 2000;2:326–332. doi: 10.1038/35014014. [DOI] [PubMed] [Google Scholar]
- 120.Sommer T., Jarosch E. BiP binding keeps ATF6 at bay. Dev. Cell. 2002;3:1–2. doi: 10.1016/S1534-5807(02)00210-1. [DOI] [PubMed] [Google Scholar]
- 121.Hollien J., Weissman J.S. Decay of endoplasmic reticulum-localized mRNAs during the unfolded protein response. Science. 2006;313:104–107. doi: 10.1126/science.1129631. [DOI] [PubMed] [Google Scholar]
- 122.Maurel M., Chevet E., Tavernier J., Gerlo S. Getting RIDD of RNA: IRE1 in cell fate regulation. Trends Biochem. Sci. 2014;39:245–254. doi: 10.1016/j.tibs.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 123.Haze K., Yoshida H., Yanagi H., Yura T., Mori K. Mammalian transcription factor ATF6 is synthesized as a transmembrane protein and activated by proteolysis in response to endoplasmic reticulum stress. Mol. Biol. Cell. 1999;10:3787–3799. doi: 10.1091/mbc.10.11.3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ye J., Rawson R.B., Komuro R., Chen X., Davé U.P., Prywes R., Brown M.S., Goldstein J.L. ER stress induces cleavage of membrane-bound ATF6 by the same proteases that process SREBPs. Mol. Cell. 2000;6:1355–1364. doi: 10.1016/S1097-2765(00)00133-7. [DOI] [PubMed] [Google Scholar]
- 125.Harding H.P., Zhang Y., Zeng H., Novoa I., Lu P.D., Calfon M., Sadri N., Yun C., Popko B., Paules R., et al. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol. Cell. 2003;11:619–633. doi: 10.1016/S1097-2765(03)00105-9. [DOI] [PubMed] [Google Scholar]
- 126.Vattem K.M., Wek R.C. Reinitiation involving upstream ORFs regulates ATF4 mRNA translation in mammalian cells. Proc. Natl. Acad. Sci. USA. 2004;101:11269–11274. doi: 10.1073/pnas.0400541101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Fabre B., Lambour T., Garrigues L., Amalric F., Vigneron N., Menneteau T., Stella A., Monsarrat B., Eynde B.V.D., Burlet-Schiltz O., et al. Deciphering preferential interactions within supramolecular protein complexes: The proteasome case. Mol. Syst. Biol. 2015;11:771. doi: 10.15252/msb.20145497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tanahashi N., Murakami Y., Minami Y., Shimbara N., Hendil K.B., Tanaka K. Hybrid proteasomes. J. Biol. Chem. 2000;275:14336–14345. doi: 10.1074/jbc.275.19.14336. [DOI] [PubMed] [Google Scholar]
- 129.Jung T., Grune T. The proteasome and the degradation of oxidized proteins: Part I—Structure of proteasomes. Redox Biol. 2013;1:178–182. doi: 10.1016/j.redox.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kriegenburg F., Poulsen E.G., Koch A., Krüger E., Hartmann-Petersen R. Redox control of the ubiquitin-proteasome system: From molecular mechanisms to functional significance. Antioxid. Redox Signal. 2011;15:2265–2299. doi: 10.1089/ars.2010.3590. [DOI] [PubMed] [Google Scholar]
- 131.Respondek D., Voss M., Kühlewindt I., Klingel K., Krüger E., Beling A. PA28 modulates antigen processing and viral replication during coxsackievirus B3 infection. PLoS ONE. 2017;12:e0173259. doi: 10.1371/journal.pone.0173259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lelouard H., Gatti E., Cappello F., Gresser O., Camosseto V., Pierre P. Transient aggregation of ubiquitinated proteins during dendritic cell maturation. Nature. 2002;417:177–182. doi: 10.1038/417177a. [DOI] [PubMed] [Google Scholar]
- 133.Szeto J., Kaniuk N.A., Canadien V., Nisman R., Mizushima N., Yoshimori T., Bazett-Jones D.P., Brumell J.H. ALIS are stress-induced protein storage compartments for substrates of the proteasome and autophagy. Autophagy. 2006;2:189–199. doi: 10.4161/auto.2731. [DOI] [PubMed] [Google Scholar]
- 134.Schmidt C., Berger T., Groettrup M., Basler M. Immunoproteasome inhibition impairs T and B cell activation by restraining ERK signaling and proteostasis. Front. Immunol. 2018;9:2386. doi: 10.3389/fimmu.2018.02386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Studencka-Turski M., Çetin G., Junker H., Ebstein F., Krüger E. Molecular insight into the IRE1α-mediated type I interferon response induced by proteasome impairment in myeloid cells of the brain. Front. Immunol. 2019;10:2900. doi: 10.3389/fimmu.2019.02900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Jansen A.H.P., Reits E.A.J., Hol E.M. The ubiquitin proteasome system in glia and its role in neurodegenerative diseases. Front. Mol. Neurosci. 2014;7:73. doi: 10.3389/fnmol.2014.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Cianciulli A., Porro C., Calvello R., Trotta T., Lofrumento N.E., Panaro M.A. Microglia mediated neuroinflammation: Focus on PI3K modulation. Biomolecules. 2020;10:137. doi: 10.3390/biom10010137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Thibaudeau T.A., Anderson R.T., Smith D.M. A common mechanism of proteasome impairment by neurodegenerative disease-associated oligomers. Nat. Commun. 2018;9:1–14. doi: 10.1038/s41467-018-03509-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Roche P.A., Furuta K. The ins and outs of MHC class II-mediated antigen processing and presentation. Nat. Rev. Immunol. 2015;15:203–216. doi: 10.1038/nri3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wieczorek M., Abualrous E.T., Sticht J., Álvaro-Benito M., Stolzenberg S., Noé F., Freund C. Major histocompatibility complex (MHC) class I and MHC class II proteins: Conformational plasticity in antigen presentation. Front. Immunol. 2017;8:292. doi: 10.3389/fimmu.2017.00292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Van Kaert L., Ashton-Rickardt P.G., Eichelberger M., Gaczynska M., Nagashima K., Rock K.L., Goldberg A.L., Doherty P.C., Tonegawa S. Altered peptidase and viral-specific T cell response in LMP2 mutant mice. Immunity. 1994;1:533–541. doi: 10.1016/1074-7613(94)90043-4. [DOI] [PubMed] [Google Scholar]
- 142.Kincaid E.Z., Che J.W., York I., Escobar H., Reyes-Vargas E., Delgado J.C., Welsh R.M., Karow M.L., Murphy A.J., Valenzuela D.M., et al. Mice completely lacking immunoproteasomes show major changes in antigen presentation. Nat. Immunol. 2011;13:129–135. doi: 10.1038/ni.2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hutchinson S., Sims S., O’Hara G., Silk J., Gileadi U., Cerundolo V., Klenerman P. A dominant role for the immunoproteasome in CD8+ T cell responses to murine cytomegalovirus. PLoS ONE. 2011;6:e14646. doi: 10.1371/journal.pone.0014646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sijts A.J.A.M., Standera S., Toes R.E.M., Ruppert T., Beekman N.J.C.M., van Veelen P.A., Ossendorp F.A., Melief C.J.M., Kloetzel P.M. MHC class I antigen processing of an adenovirus CTL epitope is linked to the levels of immunoproteasomes in infected cells. J. Immunol. 2000;164:4500–4506. doi: 10.4049/jimmunol.164.9.4500. [DOI] [PubMed] [Google Scholar]
- 145.De Graaf N., Van Helden M.J.G., Textoris-Taube K., Chiba T., Topham D.J., Kloetzel P.-M., Zaiss D., Sijts A.J.A.M. PA28 and the proteasome immunosubunits play a central and independent role in the production of MHC class I-binding peptides in vivo. Eur. J. Immunol. 2011;41:926–935. doi: 10.1002/eji.201041040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Guimarães G., Gomes M.T.R., Campos P.C., Marinho F.V., De Assis N.R.G., Silveira T.N., Oliveira S.C. Immunoproteasome subunits are required for CD8+T Cell function and host resistance to Brucella abortus infection in mice. Infect. Immun. 2017;86:86. doi: 10.1128/IAI.00615-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Morel S., Lévy F., Burlet-Schiltz O., Brasseur F., Probst-Kepper M., Peitrequin A.-L., Monsarrat B., Van Velthoven R., Cerottini J.-C., Boon T., et al. Processing of some antigens by the standard proteasome but not by the immunoproteasome results in poor presentation by dendritic cells. Immunity. 2000;12:107–117. doi: 10.1016/S1074-7613(00)80163-6. [DOI] [PubMed] [Google Scholar]
- 148.Chapatte L., Ayyoub M., Morel S., Peitrequin A.-L., Lévy N., Servis C., Eynde B.J.V.D., Valmori D., Lévy F. Processing of tumor-associated antigen by the proteasomes of dendritic cells controls in vivo T-Cell responses. Cancer Res. 2006;66:5461–5468. doi: 10.1158/0008-5472.CAN-05-4310. [DOI] [PubMed] [Google Scholar]
- 149.Chapiro J., Claverol S., Piette F., Ma W., Stroobant V., Guillaume B., Gairin J.-E., Morel S., Burlet-Schiltz O., Monsarrat B., et al. Destructive cleavage of antigenic peptides either by the immunoproteasome or by the standard proteasome results in differential antigen presentation. J. Immunol. 2006;176:1053–1061. doi: 10.4049/jimmunol.176.2.1053. [DOI] [PubMed] [Google Scholar]
- 150.Keller M., Ebstein F., Bürger E., Textoris-Taube K., Gorny X., Urban S., Zhao F., Dannenberg T., Sucker A., Keller C., et al. The proteasome immunosubunits, PA28 and ER-aminopeptidase 1 protect melanoma cells from efficient MART-126-35-specific T-cell recognition. Eur. J. Immunol. 2015;45:3257–3268. doi: 10.1002/eji.201445243. [DOI] [PubMed] [Google Scholar]
- 151.Mishto M., Liepe J., Textoris-Taube K., Keller C., Henklein P., Weberruß M., Dahlmann B., Enenkel C., Voigt A., Kuckelkorn U., et al. Proteasome isoforms exhibit only quantitative differences in cleavage and epitope generation. Eur. J. Immunol. 2014;44:3508–3521. doi: 10.1002/eji.201444902. [DOI] [PubMed] [Google Scholar]
- 152.Hanada K.-I., Yewdell J.W., Yang J.C. Immune recognition of a human renal cancer antigen through post-translational protein splicing. Nature. 2004;427:252–256. doi: 10.1038/nature02240. [DOI] [PubMed] [Google Scholar]
- 153.Vigneron N., Stroobant V., Chapiro J., Ooms A., DeGiovanni G., Morel S., Van der Bruggen P., Boon T., Eynde B.J.V.D. An antigenic peptide produced by peptide splicing in the proteasome. Science. 2004;304:587–590. doi: 10.1126/science.1095522. [DOI] [PubMed] [Google Scholar]
- 154.Warren E.H., Vigneron N., Gavin M.A., Coulie P.G., Stroobant V., Dalet A., Tykodi S.S., Xuereb S.M., Mito J.K., Riddell S.R., et al. An antigen produced by splicing of noncontiguous peptides in the reverse order. Science. 2006;313:1444–1447. doi: 10.1126/science.1130660. [DOI] [PubMed] [Google Scholar]
- 155.Dalet A., Vigneron N., Stroobant V., Hanada K.-I., Eynde B.J.V.D. Splicing of distant peptide fragments occurs in the proteasome by transpeptidation and produces the spliced antigenic peptide derived from fibroblast growth factor-5. J. Immunol. 2010;184:3016–3024. doi: 10.4049/jimmunol.0901277. [DOI] [PubMed] [Google Scholar]
- 156.Ebstein F., Textoris-Taube K., Keller C., Golnik R., Vigneron N., Eynde B.J.V.D., Schuler-Thurner B., Schadendorf D., Lorenz F.K.M., Uckert W., et al. Proteasomes generate spliced epitopes by two different mechanisms and as efficiently as non-spliced epitopes. Sci. Rep. 2016;6:24032. doi: 10.1038/srep24032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Michaux A., Larrieu P., Stroobant V., Fonteneau J.-F., Jotereau F., Eynde B.J.V.D., Moreau-Aubry A., Vigneron N. A spliced antigenic peptide comprising a single spliced amino acid is produced in the proteasome by reverse splicing of a longer peptide fragment followed by trimming. J. Immunol. 2014;192:1962–1971. doi: 10.4049/jimmunol.1302032. [DOI] [PubMed] [Google Scholar]
- 158.Platteel A.C.M., Mishto M., Textoris-Taube K., Keller C., Liepe J., Busch D.H., Kloetzel P.M., Sijts A.J.A.M. CD8+T cells of Listeria monocytogenes-infected mice recognize both linear and spliced proteasome products. Eur. J. Immunol. 2016;46:1109–1118. doi: 10.1002/eji.201545989. [DOI] [PubMed] [Google Scholar]
- 159.Dalet A., Robbins P.F., Stroobant V., Vigneron N., Li Y.F., El-Gamil M., Hanada K.-I., Yang J.C., Rosenberg S.A., Eynde B.J.V.D. An antigenic peptide produced by reverse splicing and double asparagine deamidation. Proc. Natl. Acad. Sci. USA. 2011;108:E323–E331. doi: 10.1073/pnas.1101892108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Mishto M., Liepe J. Post-translational peptide splicing and T cell responses. Trends Immunol. 2017;38:904–915. doi: 10.1016/j.it.2017.07.011. [DOI] [PubMed] [Google Scholar]
- 161.Platteel A.C.M., Liepe J., Van Eden W., Mishto M., Sijts A.J.A.M. An unexpected major role for proteasome-catalyzed peptide splicing in generation of T Cell epitopes: Is there relevance for vaccine development? Front. Immunol. 2017;8:1441. doi: 10.3389/fimmu.2017.01441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Mishto M. What we see, what we do not see, and what we do not want to see in HLA Class I immunopeptidomes. Proteomics. 2020;20:2000112. doi: 10.1002/pmic.202000112. [DOI] [PubMed] [Google Scholar]
- 163.Liepe J., Marino F., Sidney J., Jeko A., Bunting D.E., Sette A., Kloetzel P.M., Stumpf M.P.H., Heck A.J.R., Mishto M. A large fraction of HLA class I ligands are proteasome-generated spliced peptides. Science. 2016;354:354–358. doi: 10.1126/science.aaf4384. [DOI] [PubMed] [Google Scholar]
- 164.Parham P. Transporters of delight. Nature. 1990;348:674–675. doi: 10.1038/348674a0. [DOI] [PubMed] [Google Scholar]
- 165.Spies T., Bresnahan M., Bahrain S., Arnold D., Blanck G., Mellins E., Pious D., Demars R. A gene in the human major histocompatibility complex class II region controlling the class I antigen presentation pathway. Nature. 1990;348:744–747. doi: 10.1038/348744a0. [DOI] [PubMed] [Google Scholar]
- 166.Trowsdale J., Hanson I., Mockridge I., Beck S., Townsend A.R., Kelly A. Sequences encoded in the class II region of the MHC related to the ‘ABC’ superfamily of transporters. Nature. 1990;348:741–744. doi: 10.1038/348741a0. [DOI] [PubMed] [Google Scholar]
- 167.Deverson E.V., Gow I.R., Coadwell W.J., Monaco J.J., Butcher G.W., Howard J.C. MHC class II region encoding proteins related to the muKidrug resistance family of transmembrane transporters. Nature. 1990;348:738–741. doi: 10.1038/348738a0. [DOI] [PubMed] [Google Scholar]
- 168.Monaco J.J., Cho S., Attaya M. Transport protein genes in the murine MHC: Possible implications for antigen processing. Science. 1990;250:1723–1726. doi: 10.1126/science.2270487. [DOI] [PubMed] [Google Scholar]
- 169.Van De Weijer M.L., Luteijn R.D., Wiertz E.J.H.J. Viral immune evasion: Lessons in MHC class I antigen presentation. Semin. Immunol. 2015;27:125–137. doi: 10.1016/j.smim.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 170.Kovacsovics-Bankowski M., Rock K.L. A phagosome-to-cytosol pathway for exogenous antigens presented on MHC class I molecules. Science. 1995;267:243–246. doi: 10.1126/science.7809629. [DOI] [PubMed] [Google Scholar]
- 171.Ackerman A.L., Kyritsis C., Tampé R., Cresswell P. Early phagosomes in dendritic cells form a cellular compartment sufficient for cross presentation of exogenous antigens. Proc. Natl. Acad. Sci. USA. 2003;100:12889–12894. doi: 10.1073/pnas.1735556100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Palmowski M.J., Gileadi U., Salio M., Gallimore A., Millrain M., James E., Addey C., Scott D., Dyson J., Simpson E., et al. Role of immunoproteasomes in cross-presentation. J. Immunol. 2006;177:983–990. doi: 10.4049/jimmunol.177.2.983. [DOI] [PubMed] [Google Scholar]
- 173.Guermonprez P., Saveanu L., Kleijmeer M.J., Davoust J., Van Endert P., Amigorena S. ER-phagosome fusion defines an MHC class I cross-presentation compartment in dendritic cells. Nature. 2003;425:397–402. doi: 10.1038/nature01911. [DOI] [PubMed] [Google Scholar]
- 174.Houde M., Bertholet S., Gagnon E., Brunet S., Goyette G., Laplante A., Princiotta M.F., Thibault P., Sacks D., Desjardins M. Phagosomes are competent organelles for antigen cross-presentation. Nature. 2003;425:402–406. doi: 10.1038/nature01912. [DOI] [PubMed] [Google Scholar]
- 175.Ma W., Stroobant V., Heirman C., Sun Z., Thielemans K., Mulder A., Van der Bruggen P., Eynde B.J.V.D. The vacuolar pathway of long peptide cross-presentation can be TAP dependent. J. Immunol. 2018;202:451–459. doi: 10.4049/jimmunol.1800353. [DOI] [PubMed] [Google Scholar]
- 176.Shen L., Sigal L.J., Boes M., Rock K.L. Important role of cathepsin S in generating peptides for TAP-independent MHC class I crosspresentation in vivo. Immunity. 2004;21:155–165. doi: 10.1016/j.immuni.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 177.Pfeifer J.D., Wick M.J., Roberts R.L., Findlay K., Normark S.J., Harding C.V. Phagocytic processing of bacterial antigens for class I MHC presentation to T cells. Nature. 1993;361:359–362. doi: 10.1038/361359a0. [DOI] [PubMed] [Google Scholar]
- 178.Liu T., Chambers B., Diehl A.D., Van Kaer L., Jondal M., Ljunggren H.G. TAP peptide transporter-independent presentation of heat-killed Sendai virus antigen on MHC class I molecules by splenic antigen-presenting cells. J. Immunol. 1997;159:5364–5371. [PubMed] [Google Scholar]
- 179.Campbell D.J., Serwold T., Shastri N. Bacterial proteins can be processed by macrophages in a transporter associated with antigen processing-independent, cysteine protease-dependent manner for presentation by MHC class I molecules. J. Immunol. 2000;164:168–175. doi: 10.4049/jimmunol.164.1.168. [DOI] [PubMed] [Google Scholar]
- 180.Bertholet S., Goldszmid R., Morrot A., Debrabant A., Afrin F., Collazo-Custodio C., Houde M., Desjardins M., Sher A., Sacks D. Leishmania antigens are presented to CD8+T cells by a transporter associated with antigen processing-independent pathway in vitro and in vivo. J. Immunol. 2006;177:3525–3533. doi: 10.4049/jimmunol.177.6.3525. [DOI] [PubMed] [Google Scholar]
- 181.Yewdell J.W., Antón L.C., Bennink J.R. Defective ribosomal products (DRiPs): A major source of antigenic peptides for MHC class I molecules? J. Immunol. 1996;157:1823–1826. [PubMed] [Google Scholar]
- 182.Schubert U.S., Antón L.C., Gibbs J., Norbury C.C., Yewdell J.W., Bennink J.R. Rapid degradation of a large fraction of newly synthesized proteins by proteasomes. Nature. 2000;404:770–774. doi: 10.1038/35008096. [DOI] [PubMed] [Google Scholar]
- 183.Reits E.A.J., Vos J.C., Grommé M., Neefjes J. The major substrates for TAP in vivo are derived from newly synthesized proteins. Nature. 2000;404:774–778. doi: 10.1038/35008103. [DOI] [PubMed] [Google Scholar]
- 184.Yewdell J.W. DRiPs solidify: Progress in understanding endogenous MHC class I antigen processing. Trends Immunol. 2011;32:548–558. doi: 10.1016/j.it.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Antón L.C., Yewdell J.W. Translating DRiPs: MHC class I immunosurveillance of pathogens and tumors. J. Leukoc. Biol. 2014;95:551–562. doi: 10.1189/jlb.1113599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Wei J., Yewdell J.W. Immunoribosomes: Where’s there’s fire, there’s fire. Mol. Immunol. 2019;113:38–42. doi: 10.1016/j.molimm.2017.12.026. [DOI] [PubMed] [Google Scholar]
- 187.Wei J., Yewdell J.W. Flu DRiPs in MHC class I immunosurveillance. Virol. Sin. 2018;34:162–167. doi: 10.1007/s12250-018-0061-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Yewdell J.W., Hollý J. DRiPs get molecular. Curr. Opin. Immunol. 2020;64:130–136. doi: 10.1016/j.coi.2020.05.009. [DOI] [PubMed] [Google Scholar]
- 189.Yewdell J.W., Reits E.A.J., Neefjes J. Making sense of mass destruction: Quantitating MHC class I antigen presentation. Nat. Rev. Immunol. 2003;3:952–961. doi: 10.1038/nri1250. [DOI] [PubMed] [Google Scholar]
- 190.Rock K.L., Reits E., Neefjes J. Present Yourself! By MHC class I and MHC class II molecules. Trends Immunol. 2016;37:724–737. doi: 10.1016/j.it.2016.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Wei J., Kishton R.J., Angel M., Conn C.S., Dalla-Venezia N., Marcel V., Vincent A., Catez F., Ferré S., Ayadi L., et al. Ribosomal proteins regulate MHC class I peptide generation for immunosurveillance. Mol. Cell. 2019;73:1162–1173.e5. doi: 10.1016/j.molcel.2018.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Lelouard H., Ferrand V., Marguet D., Bania J., Camosseto V., David A., Gatti E., Pierre P. Dendritic cell aggresome-like induced structures are dedicated areas for ubiquitination and storage of newly synthesized defective proteins. J. Cell Biol. 2004;164:667–675. doi: 10.1083/jcb.200312073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Fehling H.J., Swat W., LaPlace C., Kuhn R., Rajewsky K., Muller U., Von Boehmer H. MHC class I expression in mice lacking the proteasome subunit LMP-7. Science. 1994;265:1234–1237. doi: 10.1126/science.8066463. [DOI] [PubMed] [Google Scholar]
- 194.Kim H., Sanchez G.A.M., Jesus A.A. Insights from mendelian interferonopathies: Comparison of CANDLE, SAVI with AGS, monogenic lupus. J. Mol. Med. 2016;94:1111–1127. doi: 10.1007/s00109-016-1465-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Torrelo A., Patel S., Colmenero I., Gurbindo D., Lendínez F., Hernández A., López-Robledillo J.C., Dadban A., Requena L., Paller A.S. Chronic atypical neutrophilic dermatosis with lipodystrophy and elevated temperature (CANDLE) syndrome. J. Am. Acad. Dermatol. 2010;62:489–495. doi: 10.1016/j.jaad.2009.04.046. [DOI] [PubMed] [Google Scholar]
- 196.Torrelo A. CANDLE syndrome as a paradigm of proteasome-related autoinflammation. Front. Immunol. 2017;8:927. doi: 10.3389/fimmu.2017.00927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Agarwal A.K., Xing C., DeMartino G.N., Mizrachi D., Hernandez M.D., Sousa A.B., De Villarreal L.M., Dos Santos H.G., Garg A. PSMB8 encoding the β5i proteasome subunit is mutated in joint contractures, muscle atrophy, microcytic anemia, and panniculitis-induced lipodystrophy syndrome. Am. J. Hum. Genet. 2010;87:866–872. doi: 10.1016/j.ajhg.2010.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Arima K., Kinoshita A., Mishima H., Kanazawa N., Kaneko T., Mizushima T., Ichinose K., Nakamura H., Tsujino A., Kawakami A., et al. Proteasome assembly defect due to a proteasome subunit beta type 8 (PSMB8) mutation causes the autoinflammatory disorder, Nakajo-Nishimura syndrome. Proc. Natl. Acad. Sci. USA. 2011;108:14914–14919. doi: 10.1073/pnas.1106015108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Kitamura A., Maekawa Y., Uehara H., Izumi K., Kawachi I., Nishizawa M., Toyoshima Y., Takahashi H., Standley D.M., Tanaka K., et al. A mutation in the immunoproteasome subunit PSMB8 causes autoinflammation and lipodystrophy in humans. J. Clin. Investig. 2011;121:4150–4160. doi: 10.1172/JCI58414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Liu Y., Ramot Y., Torrelo A., Paller A.S., Si N., Babay S., Kim P.W., Sheikh A., Lee C.-C.R., Chen Y., et al. Mutations in proteasome subunit β type 8 cause chronic atypical neutrophilic dermatosis with lipodystrophy and elevated temperature with evidence of genetic and phenotypic heterogeneity. Arthritis Rheum. 2011;64:895–907. doi: 10.1002/art.33368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Brehm A., Liu Y., Sheikh A., Marrero B., Omoyinmi E., Zhou Q., Montealegre G., Biancotto A., Reinhardt A., De Jesus A.A., et al. Additive loss-of-function proteasome subunit mutations in CANDLE/PRAAS patients promote type I IFN production. J. Clin. Investig. 2015;125:4196–4211. doi: 10.1172/JCI81260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Poli M.C., Ebstein F., Nicholas S.K., De Guzman M.M., Forbes L.R., Chinn I.K., Mace E.M., Vogel T.P., Carisey A.F., Benavides F., et al. Heterozygous truncating variants in POMP escape nonsense-mediated decay and cause a unique immune dysregulatory syndrome. Am. J. Hum. Genet. 2018;102:1126–1142. doi: 10.1016/j.ajhg.2018.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.De Jesus A.A., Brehm A., VanTries R., Pillet P., Parentelli A.-S., Sanchez G.A.M., Deng Z., Paut I.K., Krüger E. Novel proteasome assembly chaperone mutations in PSMG2/PAC2 cause the autoinflammatory interferonopathy CANDLE/PRAAS4. J. Allergy Clin. Immunol. 2019;143:1939–1943.e8. doi: 10.1016/j.jaci.2018.12.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Sarrabay G., Méchin D., Salhi A., Boursier G., Rittore C., Crow Y., Rice G., Tran T.-A., Cezar R., Duffy D., et al. PSMB10, the last immunoproteasome gene missing for PRAAS. J. Allergy Clin. Immunol. 2020;145:1015–1017.e6. doi: 10.1016/j.jaci.2019.11.024. [DOI] [PubMed] [Google Scholar]
- 205.Wahl C., Kautzmann S., Krebiehl G., Strauss K., Woitalla D., Müller T., Bauer P., Riess O., Krüger R. A comprehensive genetic study of the proteasomal subunit S6 ATPase in German Parkinson’s disease patients. J. Neural Transm. 2008;115:1141–1148. doi: 10.1007/s00702-008-0054-3. [DOI] [PubMed] [Google Scholar]
- 206.Küry S., Besnard T., Ebstein F., Khan T.N., Gambin T., Douglas J., Bacino C.A., Sanders S.J., Lehmann A., Latypova X., et al. De Novo disruption of the proteasome regulatory subunit PSMD12 causes a syndromic neurodevelopmental disorder. Am. J. Hum. Genet. 2017;100:352–363. doi: 10.1016/j.ajhg.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Ansar M., Ebstein F., Özkoç H., Paracha S.A., Iwaszkiewicz J., Gesemann M., Zoete V., Ranza E., Santoni F.A., Sarwar M.T., et al. Biallelic variants in PSMB1 encoding the proteasome subunit β6 cause impairment of proteasome function, microcephaly, intellectual disability, developmental delay and short stature. Hum. Mol. Genet. 2020;29:1132–1143. doi: 10.1093/hmg/ddaa032. [DOI] [PubMed] [Google Scholar]
- 208.Liepe J., Ovaa H., Mishto M. Why do proteases mess up with antigen presentation by re-shuffling antigen sequences? Curr. Opin. Immunol. 2018;52:81–86. doi: 10.1016/j.coi.2018.04.016. [DOI] [PubMed] [Google Scholar]
- 209.Bosanac I., Wertz I.E., Pan B., Yu C., Kusam S., Lam C., Phu L., Phung Q., Maurer B., Arnott D., et al. Ubiquitin binding to A20 ZnF4 is required for modulation of NF-κB signaling. Mol. Cell. 2010;40:548–557. doi: 10.1016/j.molcel.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 210.Rigante D. Phenotype variability of autoinflammatory disorders in the pediatric patient: A pictorial overview. J. Evid. Based Med. 2020;13:227–245. doi: 10.1111/jebm.12406. [DOI] [PubMed] [Google Scholar]
- 211.Beck D.B., Aksentijevich I. Biochemistry of autoinflammatory diseases: Catalyzing monogenic disease. Front. Immunol. 2019;10:101. doi: 10.3389/fimmu.2019.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Lohr N.J., Molleston J.P., Strauss K.A., Torres-Martinez W., Sherman E.A., Squires R.H., Rider N.L., Chikwava K.R., Cummings O.W., Morton D.H., et al. Human ITCH E3 ubiquitin ligase deficiency causes syndromic multisystem autoimmune disease. Am. J. Hum. Genet. 2010;86:447–453. doi: 10.1016/j.ajhg.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Aki D., Li H., Zhang W., Zheng M., Elly C., Lee J.H., Zou W., Liu Y.-C. The E3 ligases Itch and WWP2 cooperate to limit TH2 differentiation by enhancing signaling through the TCR. Nat. Immunol. 2018;19:766–775. doi: 10.1038/s41590-018-0137-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Bachmaier K., Krawczyk C.M., Kozieradzki I., Kong Y.-Y., Sasaki T., Oliveira-Dos-Santos A., Mariathasan S., Bouchard D., Wakeham A., Itie A., et al. Negative regulation of lymphocyte activation and autoimmunity by the molecular adaptor Cbl-b. Nature. 2000;403:211–216. doi: 10.1038/35003228. [DOI] [PubMed] [Google Scholar]
- 215.Ahmed N., Zeng M., Sinha I., Polin L., Wei W.-Z., Rathinam C., Flavell R., Massoumi R., Venuprasad K. The E3 ligase Itch and deubiquitinase Cyld act together to regulate Tak1 and inflammation. Nat. Immunol. 2011;12:1176–1183. doi: 10.1038/ni.2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Theivanthiran B., Kathania M., Zeng M., Anguiano E., Basrur V., Vandergriff T., Pascual V., Wei W.-Z., Massoumi R., Venuprasad K. The E3 ubiquitin ligase Itch inhibits p38α signaling and skin inflammation through the ubiquitylation of Tab1. Sci. Signal. 2015;8:ra22. doi: 10.1126/scisignal.2005903. [DOI] [PubMed] [Google Scholar]
- 217.Layman A.A.K., Sprout S.L., Phillips D., Oliver P.M. Ndfip1 restricts Th17 cell potency by limiting lineage stability and proinflammatory cytokine production. Sci. Rep. 2017;7:39649. doi: 10.1038/srep39649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Kathania M., Khare P., Zeng M., Cantarel B., Zhang H., Ueno H., Venuprasad K. Itch inhibits IL-17-mediated colon inflammation and tumorigenesis by ROR-γt ubiquitination. Nat. Immunol. 2016;17:997–1004. doi: 10.1038/ni.3488. [DOI] [PubMed] [Google Scholar]
- 219.Meuwissen M.E., Schot R., Buta S., Oudesluijs G., Tinschert S., Speer S.D., Li Z., Van Unen L., Heijsman D., Goldmann T., et al. Human USP18 deficiency underlies type 1 interferonopathy leading to severe pseudo-TORCH syndrome. J. Exp. Med. 2016;213:1163–1174. doi: 10.1084/jem.20151529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Zhang X., Bogunovic D., Payelle-Brogard B., Francois-Newton V., Speer S.D., Yuan C., Volpi S., Li Z., Sanal O., Mansouri D., et al. Human intracellular ISG15 prevents interferon-α/β over-amplification and auto-inflammation. Nature. 2015;517:89–93. doi: 10.1038/nature13801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Kang J.A., Jeon Y.J. Emerging roles of USP18: From biology to pathophysiology. Int. J. Mol. Sci. 2020;21:6825. doi: 10.3390/ijms21186825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Kubori T., Hyakutake A., Nagai H. Legionella translocates an E3 ubiquitin ligase that has multiple U-boxes with distinct functions. Mol. Microbiol. 2008;67:1307–1319. doi: 10.1111/j.1365-2958.2008.06124.x. [DOI] [PubMed] [Google Scholar]
- 223.Kubori T., Shinzawa N., Kanuka H., Nagai H. Legionella metaeffector exploits host proteasome to temporally regulate cognate effector. PLoS Pathog. 2010;6:e1001216. doi: 10.1371/journal.ppat.1001216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Qiu J., Luo Z.-Q. Hijacking of the host ubiquitin network by Legionella pneumophila. Front. Cell. Infect. Microbiol. 2017;7:487. doi: 10.3389/fcimb.2017.00487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Qiu J., Sheedlo M.J., Yu K., Tan Y., Nakayasu E.S., Das C., Liu X., Luo Z.-Q. Ubiquitination independent of E1 and E2 enzymes by bacterial effectors. Nature. 2016;533:120–124. doi: 10.1038/nature17657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Kotewicz K.M., Ramabhadran V., Sjoblom N., Vogel J.P., Haenssler E., Zhang M., Behringer J., Scheck R.A., Isberg R.R. A single Legionella effector catalyzes a multistep ubiquitination pathway to rearrange tubular endoplasmic reticulum for replication. Cell Host Microbe. 2017;21:169–181. doi: 10.1016/j.chom.2016.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Bhogaraju S., Dikic I. Ubiquitination without E1 and E2 enzymes. Nature. 2016;533:43–44. doi: 10.1038/nature17888. [DOI] [PubMed] [Google Scholar]
- 228.Gan N., Nakayasu E., Hollenbeck P.J., Luo Z.-Q. Legionella pneumophila inhibits immune signalling via MavC-mediated transglutaminase-induced ubiquitination of UBE2N. Nat. Microbiol. 2018;4:134–143. doi: 10.1038/s41564-018-0282-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Kubori T., Kitao T., Nagai H. Emerging insights into bacterial deubiquitinases. Curr. Opin. Microbiol. 2019;47:14–19. doi: 10.1016/j.mib.2018.10.001. [DOI] [PubMed] [Google Scholar]
- 230.Sheedlo M.J., Qiu J., Tan Y., Paul L.N., Luo Z.-Q., Das C. Structural basis of substrate recognition by a bacterial deubiquitinase important for dynamics of phagosome ubiquitination. Proc. Natl. Acad. Sci. USA. 2015;112:15090–15095. doi: 10.1073/pnas.1514568112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Lindner H.A., Lytvyn V., Qi H., Lachance P., Ziomek E., Ménard R. Selectivity in ISG15 and ubiquitin recognition by the SARS coronavirus papain-like protease. Arch. Biochem. Biophys. 2007;466:8–14. doi: 10.1016/j.abb.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Klemm T., Ebert G., Calleja D.J., Allison C.C., Richardson L.W., Bernardini J.P., Lu B.G., Kuchel N.W., Grohmann C., Shibata Y., et al. Mechanism and inhibition of the papain-like protease, PLpro, of SARS-CoV-2. EMBO J. 2020;39:e106275. doi: 10.15252/embj.2020106275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Kilianski A., Mielech A.M., Deng X., Baker S.C. Assessing activity and inhibition of Middle East respiratory syndrome coronavirus papain-like and 3C-like proteases using luciferase-based biosensors. J. Virol. 2013;87:11955–11962. doi: 10.1128/JVI.02105-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Yang X., Chen X., Bian G., Tu J., Xing Y., Wang Y., Chen Z. Proteolytic processing, deubiquitinase and interferon antagonist activities of Middle East respiratory syndrome coronavirus papain-like protease. J. Gen. Virol. 2014;95:614–626. doi: 10.1099/vir.0.059014-0. [DOI] [PubMed] [Google Scholar]
- 235.Frieman M., Ratia K., Johnston R.E., Mesecar A.D., Baric R.S. Severe acute respiratory syndrome coronavirus papain-like protease ubiquitin-like domain and catalytic domain regulate antagonism of IRF3 and NF-κB signaling. J. Virol. 2009;83:6689–6705. doi: 10.1128/JVI.02220-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Shin D., Mukherjee R., Grewe D., Bojkova D., Baek K., Bhattacharya A., Schulz L., Widera M., Mehdipour A.R., Tascher G., et al. Papain-like protease regulates SARS-CoV-2 viral spread and innate immunity. Nature. 2020;587:657–662. doi: 10.1038/s41586-020-2601-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Hamilton A.M., Zito K. Breaking it down: The ubiquitin proteasome system in neuronal morphogenesis. Neural Plast. 2013;2013:196848. doi: 10.1155/2013/196848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Boukhalfa A., Miceli C., Ávalos Y., Morel E., Dupont N. Interplay between primary cilia, ubiquitin-proteasome system and autophagy. Biochimie. 2019;166:286–292. doi: 10.1016/j.biochi.2019.06.009. [DOI] [PubMed] [Google Scholar]
- 239.Gerhardt C., Wiegering A., Leu T., Rüther U. Control of Hedgehog signalling by the cilia-regulated proteasome. J. Dev. Biol. 2016;4:27. doi: 10.3390/jdb4030027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Baloghova N., Lidak T., Cermak L. Ubiquitin ligases involved in the regulation of Wnt, TGF-β, and notch signaling pathways and their roles in mouse development and homeostasis. Genes. 2019;10:815. doi: 10.3390/genes10100815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Micel L.N., Tentler J.J., Smith P.G., Eckhardt G.S. Role of ubiquitin ligases and the proteasome in oncogenesis: Novel targets for anticancer therapies. J. Clin. Oncol. 2013;31:1231–1238. doi: 10.1200/JCO.2012.44.0958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Cloos J., Roeten M.S., Franke N.E., Van Meerloo J., Zweegman S., Kaspers G.J., Jansen G. (Immuno)proteasomes as therapeutic target in acute leukemia. Cancer Metastasis Rev. 2017;36:599–615. doi: 10.1007/s10555-017-9699-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Raedler L. Velcade (Bortezomib) receives 2 new FDA indications: For retreatment of patients with multiple myeloma and for first-line treatment of patients with mantle-cell lymphoma. Am. Health Drug Benefits. 2015;8:135–140. [PMC free article] [PubMed] [Google Scholar]
- 244.Horton T.M., Perentesis J.P., Gamis A.S., Alonzo T.A., Gerbing R.B., Ballard J., Adlard K., Howard D.S., Smith F.O., Jenkins G., et al. A phase 2 study of bortezomib combined with either idarubicin/cytarabine or cytarabine/etoposide in children with relapsed, refractory or secondary acute myeloid leukemia: A report from the Children’s Oncology Group. Pediatr. Blood Cancer. 2014;61:1754–1760. doi: 10.1002/pbc.25117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Kraus M., Müller-Ide H., Rückrich T., Bader J., Overkleeft H., Driessen C. Ritonavir, nelfinavir, saquinavir and lopinavir induce proteotoxic stress in acute myeloid leukemia cells and sensitize them for proteasome inhibitor treatment at low micromolar drug concentrations. Leuk. Res. 2014;38:383–392. doi: 10.1016/j.leukres.2013.12.017. [DOI] [PubMed] [Google Scholar]
- 246.Wartman L.D., Fiala M.A., Fletcher T., Hawkins E.R., Cashen A., DiPersio J.F., Jacoby M.A., Stockerl-Goldstein K.E., Pusic I., Uy G.L., et al. A phase I study of carfilzomib for relapsed or refractory acute myeloid and acute lymphoblastic leukemia. Leuk. Lymphoma. 2015;57:728–730. doi: 10.3109/10428194.2015.1076930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Dou Q.P., Zonder J.A. Overview of proteasome inhibitor-based anti-cancer therapies: Perspective on bortezomib and second generation proteasome inhibitors versus future generation inhibitors of ubiquitin-proteasome system. Curr. Cancer Drug Targets. 2014;14:517–536. doi: 10.2174/1568009614666140804154511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Zhang X.-D., Baladandayuthapani V., Lin H., Mulligan G., Li B., Esseltine D.-L.W., Qing Z., Xu J., Hunziker W., Barlogie B., et al. Tight junction protein 1 modulates proteasome capacity and proteasome inhibitor sensitivity in multiple myeloma via EGFR/JAK1/STAT3 signaling. Cancer Cell. 2016;29:639–652. doi: 10.1016/j.ccell.2016.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Hamazaki J., Murata S. ER-resident transcription factor Nrf1 regulates proteasome expression and beyond. Int. J. Mol. Sci. 2020;21:3683. doi: 10.3390/ijms21103683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Steffen J., Seeger M., Koch A., Krüger E. Proteasomal degradation is transcriptionally controlled by TCF11 via an ERAD-dependent feedback loop. Mol. Cell. 2010;40:147–158. doi: 10.1016/j.molcel.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 251.Meiners S., Heyken D., Weller A., Ludwig A., Stangl K., Kloetzel P.-M., Krüger E. Inhibition of proteasome activity induces concerted expression of proteasome genes and de novo formation of mammalian proteasomes. J. Biol. Chem. 2003;278:21517–21525. doi: 10.1074/jbc.M301032200. [DOI] [PubMed] [Google Scholar]
- 252.Vangala J.R., Sotzny F., Krüger E., Deshaies R.J., Radhakrishnan S.K. Nrf1 can be processed and activated in a proteasome-independent manner. Curr. Biol. 2016;26:R834–R835. doi: 10.1016/j.cub.2016.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Radhakrishnan S.K., Lee C.S., Young P., Beskow A., Chan J.Y., Deshaies R.J. Transcription factor Nrf1 mediates the proteasome recovery pathway after proteasome inhibition in mammalian cells. Mol. Cell. 2010;38:17–28. doi: 10.1016/j.molcel.2010.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Northrop A., Byers H.A., Radhakrishnan S.K. Regulation of NRF1, a master transcription factor of proteasome genes: Implications for cancer and neurodegeneration. Mol. Biol. Cell. 2020;31:2158–2163. doi: 10.1091/mbc.E20-04-0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Gu Y., Wang X., Wang Y., Wang Y., Li J., Yu F.-X. Nelfinavir inhibits human DDI2 and potentiates cytotoxicity of proteasome inhibitors. Cell. Signal. 2020;75:109775. doi: 10.1016/j.cellsig.2020.109775. [DOI] [PubMed] [Google Scholar]
- 256.Tomlin F.M., Gerling-Driessen U.I.M., Liu Y.-C., Flynn R.A., Vangala J.R., Lentz C.S., Clauder-Muenster S., Jakob P., Mueller W.F., Ordoñez-Rueda D., et al. Inhibition of NGLY1 inactivates the transcription factor Nrf1 and potentiates proteasome inhibitor cytotoxicity. ACS Cent. Sci. 2017;3:1143–1155. doi: 10.1021/acscentsci.7b00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Ito T., Ando H., Suzuki T., Ogura T., Hotta K., Imamura Y., Yamaguchi Y., Handa H. Identification of a primary target of thalidomide teratogenicity. Science. 2010;327:1345–1350. doi: 10.1126/science.1177319. [DOI] [PubMed] [Google Scholar]
- 258.Stewart A.K. How thalidomide works against cancer. Science. 2014;343:256–257. doi: 10.1126/science.1249543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Angers S., Li T., Yi X., MacCoss M.J., Moon R.T., Zheng N. Molecular architecture and assembly of the DDB1-CUL4A ubiquitin ligase machinery. Naure. 2006;443:590–593. doi: 10.1038/nature05175. [DOI] [PubMed] [Google Scholar]
- 260.Krönke J., Udeshi N.D., Narla A., Grauman P., Hurst S.N., McConkey M., Svinkina T., Heckl D., Comer E., Li X., et al. Lenalidomide causes selective degradation of IKZF1 and IKZF3 in multiple myeloma cells. Science. 2014;343:301–305. doi: 10.1126/science.1244851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Lu G., Middleton R.E., Sun H., Naniong M., Ott C.J., Mitsiades C.S., Wong K.-K., Bradner J.E., Kaelin W.G., Jr. The myeloma drug lenalidomide promotes the cereblon-dependent destruction of ikaros proteins. Science. 2014;343:305–309. doi: 10.1126/science.1244917. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.