Abstract
Background
Possible human immunodeficiency virus (HIV)-1 clearance has rarely been reported. In this study, we describe a unique case of an HIV-positive, combination antiretroviral therapy (cART)-experienced woman with prior acquired immunodeficiency syndrome (AIDS) who has not experienced viral rebound for over 12 years since discontinuing cART.
Methods
Leukapheresis, colonoscopy, and lymph node excision were performed for detailed examination of virologic (including HIV reservoir) and immunologic features. Comparisons were made with chronically infected patients and healthy controls.
Results
No HIV-specific antibodies were detected in serum. Plasma HIV ribonucleic acid (RNA) levels were <0.2 copies/mL, and, except for low-frequency HIV deoxyribonucleic acid (DNA)+ cells in lymph node tissue (1 copy/3 × 106 cells), HIV antigen could not be detected by quantitative virus outgrowth (<0.0025 infectious units/106 CD4+ T cells) or by most measurements of HIV RNA or DNA in blood, lymph node, or gut-associated mononuclear cells. Human immunodeficiency virus-specific T-cell responses were detectable but low. Brain imaging revealed a prior biopsy site and persistent white matter disease since 1996. Human immunodeficiency virus DNA+ cells in the 1996 brain biopsy specimen confirmed her identity and initial HIV diagnosis.
Conclusions
This represents the first report of complete seroreversion, prolonged posttreatment virus suppression, a profoundly small HIV reservoir, and persistent HIV-specific T cells in an adult with prior AIDS.
Keywords: functional cure, HIV-1 seroreversion, HIV-specific T cells
Despite years of suppressive combination antiretroviral therapy (cART), plasma viremia rebounds from the latent reservoir within 3–4 weeks after cART discontinuation in most human immunodeficiency virus (HIV)-1-infected individuals [1–3]. However, there are some, referred to as posttreatment controllers (PTCs), in whom viremic control persists to ≤400 HIV-1 ribonucleic acid (RNA) copies/mL for years after cART cessation [4–7]. Prolonged posttreatment control appears to occur more frequently with cART initiation during acute/early versus chronic infection [4–9].
Insight into the mechanisms responsible for the lack of detectable virus replication may be gained from examination of the contemporaneous immune response. In long-term nonprogressors/elite controllers (LTNP/ECs), high-frequency, highly functional HIV-specific CD8+ T cells primarily target epitopes restricted by protective human leukocyte antigen (HLA) class I proteins, such as B57 and B58, and are maintained for decades with very low-level virus replication in the absence of cART (reviewed in [10]). Conversely, in progressors in whom virus replication is passively controlled by cART rather than actively controlled by an effective immune response, the frequency of HIV-specific CD8+ T cells contracts significantly [11]. In this setting, defective CD8+ T-cell proliferation and killing persist [12]. In PTCs, low-frequency and poor-quality T-cell responses remain after cART interruption [6]. This is similar to the low-frequency T-cell responses described in the Berlin and London patients, 2 cases of sterilizing HIV cure after hematopoietic stem cell transplantation (HSCT) with grafts from CCR5 ∆32/∆32 donors [13–16], suggesting that virus replication is not being actively contained by the immune response posttreatment in these instances.
Although the T-cell response rapidly contracts when viral replication is suppressed by other means, the relationship with the HIV-specific antibody (Ab) response is more complex. In chronic infection, most LTNP/ECs and cART-suppressed progressors maintain full seroreactivity [17–20]. Incomplete HIV antibody evolution and/or seroreversion has been reported in patients who initiated cART early in acute infection [21–25]. However, cART interruption typically led to rebounding viremia and seroconversion, even with extremely small reservoirs [2, 21, 23, 24]. The evolution of HIV serologies in PTCs has not been described. In contrast, partial seroreversion in the absence of cART has only been reported in rare cases of demonstrated or suspected HIV-1 clearance. Among these are the Berlin and London patients [13–16, 26] and a few extreme cases of spontaneous control (referred to here as ExtreCs) with disproportionately high virus-specific T-cell responses and reservoirs below the lower detection limits [17, 27, 28]. Therefore, significant seroreversion in non-immunodeficient adults without cART is limited to rare examples of profound reduction in antigenic stimulation in vivo.
In this study, we describe the first case of non-rebounding viremia for over 12 years posttreatment interruption and complete HIV-1 seroreversion in a patient who had initiated cART during an illness compatible with acquired immune deficiency syndrome (AIDS).
A 56-year-old Argentine woman was referred to the National Institutes of Health (NIH) in 2015 for evaluation of suppressed HIV-1 RNA levels for 8 years after discontinuing cART. Nineteen years prior, in 1996, she had been hospitalized in Buenos Aires with a 5-week history of weight loss, blurred vision, left-sided weakness, and fever. She denied previous unusual or recurrent infections, major surgeries, prior hospitalizations, and all HIV risk factors. Cachexia, nystagmus, and left hemiparesis were noted on examination. Magnetic resonance imaging (MRI) revealed numerous confluent subcortical white matter and patchy brainstem T2 fluid-attenuated inversion recovery (FLAIR)-hyperintense, nonenhancing lesions. Brain biopsy demonstrated nonspecific inflammation. Leukopenia was noted. Based on the clinical picture, HIV testing was performed on November 25, 1996. Human immunodeficiency virus enzyme-linked immunosorbent assay (ELISA) was reactive, and HIV-1 Western blot (WB) was confirmatory with gp160, gp41, and p25 bands. Serologies for syphilis and Chagas disease were negative, but Toxoplasma immunoglobulin (Ig)G was positive. For presumed toxoplasmic encephalitis (TE), she received clindamycin, pyrimethamine, and leucovorin. Two weeks after initiating zidovudine, didanosine, and nevirapine, CD4 count was 164 cells/mm3 and HIV-1 RNA was 2200 copies/mL (reverse-transcription polymerase chain reaction [RT-PCR]) (Figure 1). After improving clinically, she was discharged home and has been followed since 1997 at Helios Salud.
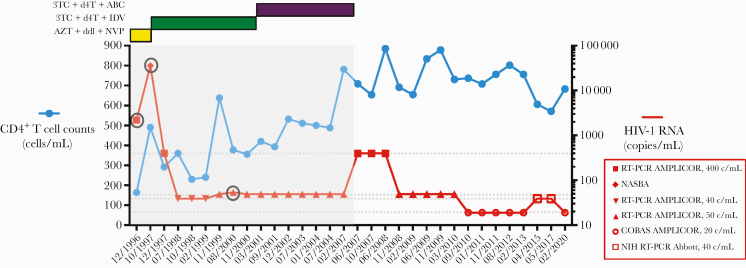
Figure 1.
CD4+ T-cell counts and human immunodeficiency virus (HIV)-1 ribonucleic acid (RNA) levels over time since 1996 HIV-1 diagnosis. Gray area designates period of treatment with combination antiretroviral therapy (cART). Specific regimens, including zidovudine (azidothymidine [AZT]), didanosine (ddI), nevirapine (NVP), lamivudine (3TC), stavudine (d4T), indinavir (IDV), and abacavir (ABC), are shown at the top left. Human immunodeficiency virus-1 RNA assays are specified by different symbols according to the right y-axis key. Circled are detectable HIV-1 RNA levels.
In October 1997, CD4 count was 490 cells/mm3 (32%), but viral load had rebounded to 36 000 copies/mL (NASBA), attributed to erratic cART adherence (Figure 1). Concern for drug resistance prompted a change to lamivudine, stavudine, and indinavir. Viral load was reduced to <50 copies/mL (RT-PCR AMPLICOR assay) in July 1998 and remained so through 2001 except for a “blip” to 54 copies/mL in 2000. In 2001, due to worsening fat redistribution and hypertriglyceridemia, abacavir was substituted for indinavir. In 2007, she discontinued cART due to treatment fatigue and worsening lipodystrophy. However, she continued to receive regular follow up, remained clinically well, and had stable CD4 counts and no rebounding virus, which prompted re-evaluation in 2013. Human immunodeficiency virus-1 ELISA/WB was negative on 2 occasions. CCR5 genotype was wild type. Human immunodeficiency virus deoxyribonucleic acid (DNA) by real-time PCR was negative. The patient was positive for the protective HLA allele B*58.
The patient was seen at NIH in 2015 and 2017. She reported no complaints, was working full time as an artisanal seamstress, and her only medications were losartan and atorvastatin. Her clinical course and laboratory results remained stable between 2015 and 2017. Numerous specialists noted a mostly benign physical examination. Pertinent positive findings included mild facial and extremity wasting; a protuberant abdomen without hepatosplenomegaly; shotty inguinal adenopathy bilaterally; minimally saccadic ocular pursuits; slightly brisk 3+ patellar reflexes; an up-going, left-sided plantar response; slight sway with Romberg testing; and minimal difficulty with tandem gait.
MATERIALS AND METHODS
Patient Consent Statement
Written consent of the case patient, chronic HIV-infected patients, and healthy volunteers was obtained for NIH evaluations conducted under National Institute of Allergy and Infectious Diseases Institutional Review Board-approved protocols, including clinical evaluations, phlebotomy, leukapheresis, excisional lymph node biopsy, and endoscopy with biopsies.
Subjects
Human immunodeficiency virus infection was determined by Multispot HIV-1/2 Rapid Test (Bio-Rad Laboratories, Redmond, VA) and Cambridge Biotech HIV-1 Western Blot (Maxim Biomedical, Inc., Rockville, MD). Human immunodeficiency virus-1 RNA levels were measured using RealTime HIV-1 Viral Load Assay (lower threshold, 40 copies/mL; Abbott Laboratories). An HIV-1 single copy assay done in triplicate with a 0.2 copy/mL lower threshold and CCR5 Δ32 genotyping were performed [29, 30]. The LTNP/ECs, viremic progressors, and ART recipients with HIV RNA levels of <40 copies/mL have been defined previously [12]. The HLA class I/II typing was performed by sequence-specific hybridization [31].
Cellular Human Immunodeficiency Virus-1 Deoxyribonucleic Acid/Ribonucleic Acid Polymerase Chain Reaction and Quantitative Virus Outgrowth Assay
To collect colonic tissue, subjects underwent endoscopy with moderate sedation. Approximately 30 biopsies were randomly taken from gut mucosa and processed for further analysis [32]. Human immunodeficiency virus-1 DNA and cell-associated RNA in mononuclear cells from peripheral blood, gut-associated lymphoid tissue, and lymph node were amplified over 40 cycles by semi-quantitative PCR (qPCR) with primers specific for HIV-1 Gag, Env, and long terminal repeat (LTR), as described [33]. Detection limit was 1 copy per 2 × 105 cells. Measurements of total HIV DNA in enriched CD4+ T cells with a single copy sensitivity were performed by droplet digital PCR (ddPCR) according to published protocols [34]. To determine the frequency of CD4+ T cells carrying replication-competent HIV, quantitative coculture assays were conducted using serially diluted (1 × 106, 200 000, 40 000, 8000, 1600, and 320 in duplicate) and 39 replicates of 10 × 106 CD4+ T cells [34]. The cultures were incubated with irradiated peripheral blood mononuclear cells (PBMCs) of HIV-seronegative donors and anti-CD3 antibody. Subsequently, 1 × 106 CD8-depleted, anti-CD3-stimulated PBMCs from HIV-seronegative donors were added to each well on days 2 and 8. Cell suspensions were periodically removed and replenished with fresh interleukin (IL)-2 medium to maintain optimal cell density. Human immunodeficiency virus p24 ELISA was performed on culture supernatants between days 14 and 21. The infectious units per million cells were determined as described [35].
Human Immunodeficiency Virus-Specific Antibody Activity
Neutralizing activity in serum was measured using a TZM-bl assay and pseudo-viruses derived from primary isolates [36]. The luciferase immunoprecipitation systems (LIPS) assay was used to generate highly quantitative antibody response profiles [37].
Cerebrospinal Fluid Analysis
In addition to routine studies for cell counts, glucose, and protein, JC and BK viruses were tested in cerebrospinal fluid (CSF) using a multiplex qPCR assay; IL-6, tumor necrosis factor-α, and HIV p24 using ultrasensitive single molecule assays (Simoa); and HIV RNA using the COBAS Ampliprep/COBAS TaqMan (Roche Diagnostics, Indianapolis, IN). Cytomegalovirus (CMV) DNA from total nucleic acids isolated from CSF and plasma was quantified by real-time PCR [38]. For determination of near full-length HIV-1 DNA PCR, genomic DNA was subjected to limiting dilution before amplification as previously described [39]. Single-molecule sequencing was performed by the Sanger method.
RNAScope/DNAScope
Next-generation in situ hybridization (ISH) for both viral RNA and viral DNA with a lower detection threshold of 2 copies was performed on formalin-fixed paraffin-embedded brain biopsies as previously described [40]. Probes targeted gag, pol, vif, vpr, tat, rev, vpu, env, and nef regions for a total of 78 probe pairs (78ZZ).
T-Cell Frequency, Proliferation, and Cytotoxic Capacity in Response to HIVSF162-Infected CD4+ T-Cell Targets or Gag Peptides
CD4+ T cells were positively selected, polyclonally stimulated, and infected with magnetized HIVSF162 as reported previously [12]. CD8+ T-cell capacity to produce interferon (IFN)-γ and kill was assessed in response to LIVE/DEAD Fixable Violet Stain (Invitrogen Molecular Probes, Eugene, OR)-labeled autologous uninfected or HIVSF162-infected CD4+ T-cell targets as described previously [12]. 5,6-Carboxyfluorescein diacetate, succinimidyl ester (Molecular Probes, Eugene, OR)-labeled PBMCs were incubated with HIV-1 Consensus B Gag 15-mer peptides (NIH AIDS Reagent Program, Germantown, MD) or CD4+ T-cell targets for 6 days to measure proliferation [12, 31].
Flow Cytometry
Multiparameter flow cytometry was performed according to standard protocols. All staining occurred at 4°C for 30 minutes with the following antibodies from BD Biosciences (unless indicated otherwise): FITC-conjugated anti-CD3; PE-conjugated anti-CD8; RDI-conjugated anti-p24 (Kc57; Beckman Coulter, Inc., Fullerton, CA); PerCP-conjugated anti-CD3 and anti-CD8; APC-conjugated anti-CD4 and anti-IFN-γ; and AmCyan-conjugated anti-CD3. Samples were analyzed on a FACSAria multilaser cytometer (Becton-Dickinson) with FACSDiva software. In cytotoxicity experiments, at least 5000 events gated on live CD4+ T-cell targets were collected. Data were analyzed using FlowJo software (TreeStar, San Carlos, CA).
Statistical Analysis
All comparisons between groups were made using the unequal variances t test.
RESULTS
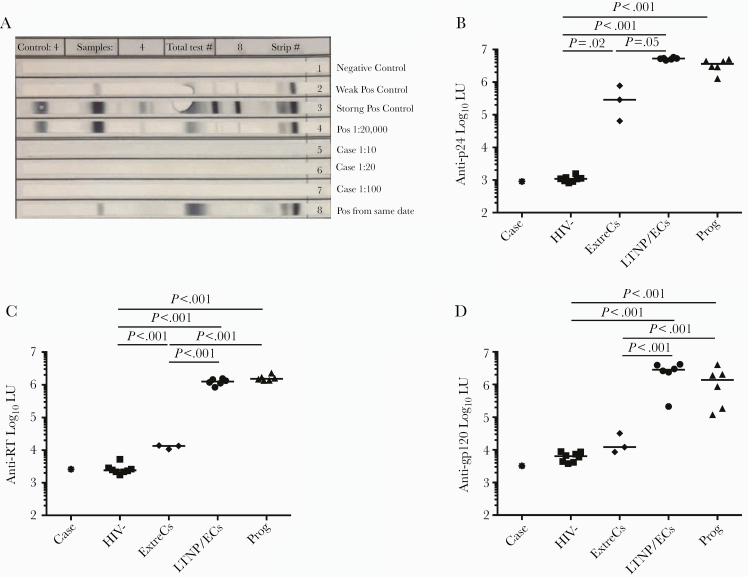
At the 2015 and 2017 NIH visits, the patient’s CD4 counts were 606 cells/mm3 (35%) and 571 cells/mm3 (38%), respectively (Figure 1). Human immunodeficiency virus RNA levels were <40 copies/mL in a clinical assay and <0.2 copies/mL in an ultrasensitive single-copy assay, similar to levels found in ExtreCs and many conventional LTNP/ECs [17]. The patient was confirmed to carry the protective B*58:01 allele [41]. Excisional lymph node biopsy in 2015 revealed follicular and paracortical hyperplasia. Excision of a palpable node from an adjacent site in 2017 demonstrated only adipose tissue without lymph node elements. Normal-appearing mucosa without abnormalities was noted on pathologic examination of samples from colonoscopy. Quantification of mononuclear subsets in colonic mucosa demonstrated 50.4% CD4+ T cells, 1.2 × 106 CD4+ T cells/gram, and a 1.2 CD4/CD8 ratio, comparable to those of healthy HIV-negative controls and non-immunodeficient HIV-positive patients [17]. Consistent with 2013 results, Multispot was negative for HIV-1 and HIV-2. Human immunodeficiency virus-1 WB was negative at 1:10, 1:20, and 1:100 serum dilutions (Figure 2A). Human immunodeficiency virus-specific antibody profile in the LIPS assay against a p24, RT, and gp120 panel was most consistent with HIV-seronegative individuals and distinguishable from LTNP/ECs and ExtreCs (Figure 2B–D). In addition, serum from this case exhibited no neutralizing antibody activity against a panel of A, B, and C clade viruses. Positive serologies included anti-HBV surface Ab, anti-HAV Ab total, anti-CMV IgG (5.7 U/mL), and anti-VZV IgG (287 Index). Quantitative Ig analysis revealed normal IgG (911 mg/dL), IgA (182 mg/dL), and IgM (58 mg/dL) levels. Surreptitious cART use was excluded by undetectable serum levels of antiretrovirals available in Argentina in 2015 and 2017, including efavirenz, rilpivirine, lopinavir, atazanavir, darunavir, raltegravir, elvitegravir, and dolutegravir.
Figure 2.
Human immunodeficiency virus (HIV)-1 Western blot (WB) and HIV antibody response profiles by luciferase immunoprecipitation systems (LIPS) assay of the case were most consistent with an uninfected individual. (A) HIV-1 WB was performed at 3 serum dilutions, including standard (1:100). Control sera results are also shown. (B–D) LIPS antibody response profiles specific for HIV-1 p24 (B), reverse-transcriptase ([RT] C), and glycoprotein (gp)-120 (D) reported in log10 luminometer units (LU) are shown for the case (asterisk), HIV-uninfected controls (HIV-, squares), extreme controllers ([ExtreCs] diamonds), conventional long-term nonprogressors/elite controllers ([LTNP/ECs] circles) and viremic progressors ([Prog] triangles). Horizontal lines represent median values. Only significant P values referring to comparisons among uninfected controls and chronically infected patients are shown.
Human immunodeficiency virus reservoir was not detectable by end-point HIV DNA and cell-associated RNA PCR (Gag, Env, and LTR) in mononuclear cells derived from the patient’s peripheral blood (107), colon (2 × 106), or lymph node (3 × 106). Real-time PCR was also negative for HIV RNA in PBMCs, colon, and lymph node and for DNA in PBMCs and colon. However, cellular DNA from lymph node was positive for HIV Env (negative for LTR and Gag) at a frequency of 1 copy/3 × 106 cells. Further analysis identified a sequence that was not consistent with contamination by a laboratory strain or a recent patient isolate, but it was homologous with 4 patient sequences reported in the LANL HIV sequence database. By quantitative ddPCR, HIV DNA was not detected in purified CD4+ T cells from PBMCs. In addition, the frequency of cells carrying replication competent virus was <0.0025 infectious units/million CD4+ T cells from 3.9 × 108 total CD4+ T cells, which had been purified from 2.5 × 109 PBMCs. Therefore, in contrast to most PTCs and LTNP/ECs, but similar to 2 ExtreCs and the Berlin and London patients, the HIV reservoir size in this patient was profoundly small [14, 16, 17, 27, 28].
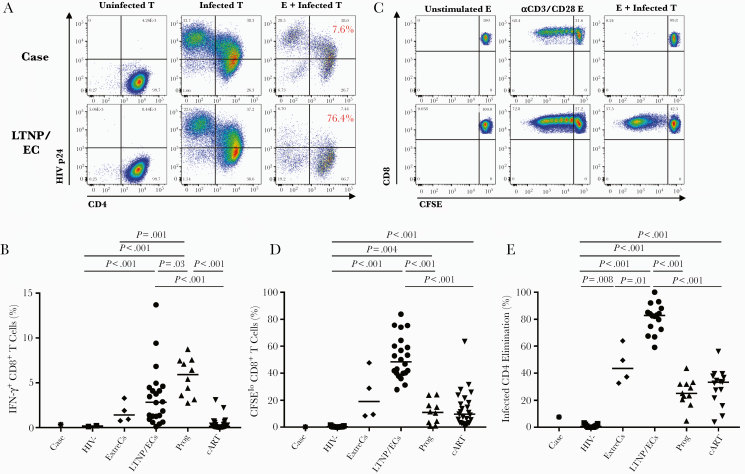
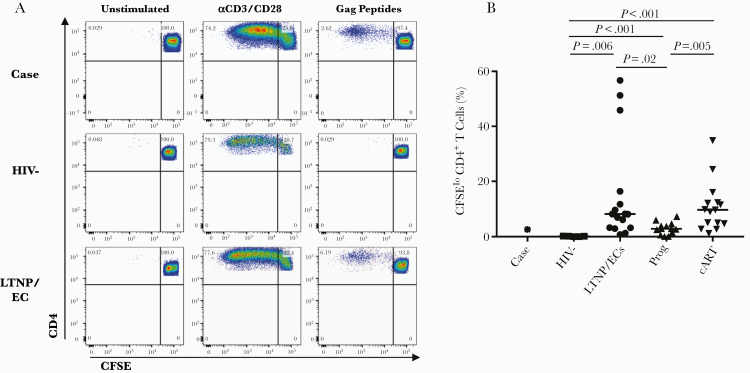
Activated CD4+ T cells from this patient were fully susceptible to infection with R5-tropic HIV-1 (Figure 3A, left and middle columns). In response to these targets, the net frequency of HIV-specific CD8+ T cells was barely detectable, comparable to HIV-negative controls and some chronic patients with prolonged HIV suppression (Figure 3B) [10, 17]. It is interesting to note that, after 6-day stimulation, HIV-specific CD8+ T-cell proliferative and cytotoxic responses to infected targets remained low (Figure 3A, middle and right columns, C-E). CD4+ T-cell proliferation after 6-day stimulation with HIV Gag peptides was higher than responses in HIV-negative controls and similar to those in chronically infected patients (Figure 4) [12]. These results were confirmed in repeat experiments. In summary, low but detectable virus-specific CD4+ and CD8+ T-cell responses in this case-patient provided evidence of HIV infection, but these were strikingly different from the robust T-cell responses in most LTNP/ECs and ExtreCs [17, 31, 42–46].
Figure 3.
The case’s CD8+ T-cell responses to human immunodeficiency virus (HIV)-infected targets differed from those of spontaneous HIV controllers. (A) Flow plots gated on autologous CD4+ T-cell targets (T) from the case (top row) and a conventional long-term nonprogressors/elite controller ([LTNP/EC] bottom row) depict high-level HIVSF162 infection (middle column) in contrast to non-superinfected CD4+ T cells (left column). Cytotoxic responses, measured by percentage elimination of infected CD4+ T-cell targets by day 6 CD8+ T-cell effectors (E), are shown on representative plots as red values (right column). (B) Summary data of interferon-γ + CD8+ T-cell responses after 6-hour stimulation with autologous infected targets are shown for the case (asterisk), uninfected controls (squares), extreme controllers ([ExtreCs] diamonds), LTNP/ECs (circles), viremic progressors (upturning triangles), and combination antiretroviral therapy-suppressed progressors (downturning triangles). Background responses to uninfected targets have been subtracted. (C) Flow plots show CD8+ T-cell proliferation to HIV-infected targets (right column) along with medium (left column) and anti-CD3/anti-CD28-stimulated (middle column) controls of the case (top row) and representative LTNP/EC (bottom row). (D and E) Summary data of CD8+ T-cell proliferation (D) and cytotoxic capacity (E) are depicted for the case (asterisk) and other groups as shown in (B). Background cytotoxic responses to uninfected targets have been subtracted. Horizontal lines represent median values. Only significant P values referring to comparisons among uninfected controls and chronically infected patients are shown. CFSE, 5,6-carboxyfluorescein diacetate, succinimidyl ester.
Figure 4.
The case’s human immunodeficiency virus (HIV)-specific CD4+ T-cell proliferative response overlapped with those of chronically infected patients. (A) Flow plots gated on 5,6-carboxyfluorescein diacetate, succinimidyl ester (CFSE)-labeled CD4+ T cells from the case (top row), an uninfected, healthy control (middle row), and a conventional long-term nonprogressors/elite controller ([LTNP/EC] bottom row) depict proliferation over 6 days in response to medium control (left column), anti-CD3/anti-CD28 monoclonal antibodies (middle column), and pooled overlapping peptides spanning the Gag protein (right column). (B) Summary data of Gag-specific CD4+ T-cell proliferative responses are shown for the case (asterisk) uninfected controls (squares), LTNP/ECs (circles), viremic progressors (upturning triangles), and combination antiretroviral therapy-suppressed progressors (downturning triangles). Horizontal lines represent median values. Only significant P values referring to comparisons among uninfected controls and chronically infected patients are shown.
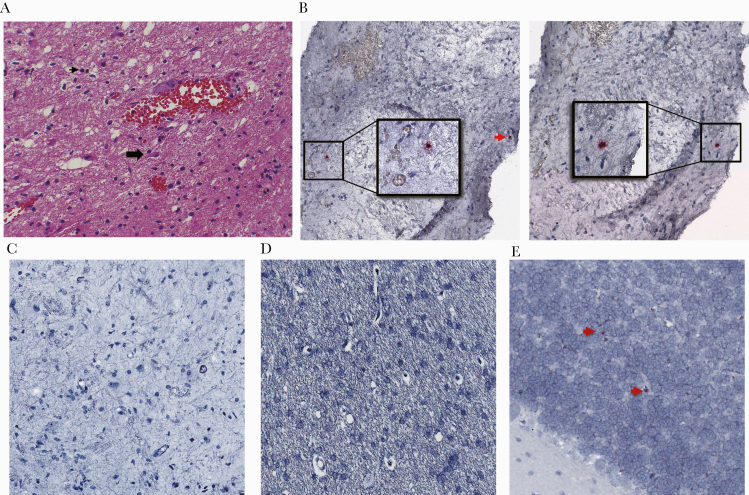
Negative HIV-1 serology, nearly undetectable antigen levels, and low HIV-specific immune responses raised questions about this patient’s identity and diagnosis. Her current and previous doctors at Helios Salud, who had overlapped and had reviewed her inpatient and outpatient results, confirmed her identity. They also shared the original report of 3 positive HIV-1 WB bands. Absence of specimens from early time points precluded repeat HIV-1 antigen or antibody testing. However, pathologic examination of a formaldehyde-fixed, paraffin-embedded block from the 1996 brain biopsy revealed brain parenchyma with mild lymphocytic infiltrate and reactive gliosis. Toxoplasma immunostains were negative (Figure 5A). It is interesting to note that rare cells harboring HIV-1 DNA were detected by ISH on different sections (Figure 5B). No vRNA+ cells were detected (Figure 5C). Human immunodeficiency virus DNA/RNA in ISH results were as expected in control normal and HIV-infected brain tissues (Figure 5D and E). These findings supported intracerebral infection with HIV-1 in 1996.
Figure 5.
Brain biopsy specimen from 1996 revealed human immunodeficiency virus (HIV) deoxyribonucleic acid (DNA)+ cells by in situ hybridization (ISH). (A) Mild lymphocytic infiltrate and reactive gliosis was identified by routine hematoxylin and eosin and immunohistochemistry staining of formaldehyde-fixed, paraffin-embedded brain specimen. Black arrowhead points to a lymphocyte; block arrow idenitifes a reactive astrocyte. (B and C) By ultrasensitive DNAscope (B) and RNAscope (C) ISH using the HIV-1 clade B probe covering gag, pol, vif, vpr, tat, rev, vpu, env, and nef regions, cells bearing HIV DNA in 2 sections (insets and red arrow, left and right columns, B), but not ribonucleic acid (C), were detected. (D and E) Uninfected (D) and HIV-infected brain specimens (E) were used as controls.
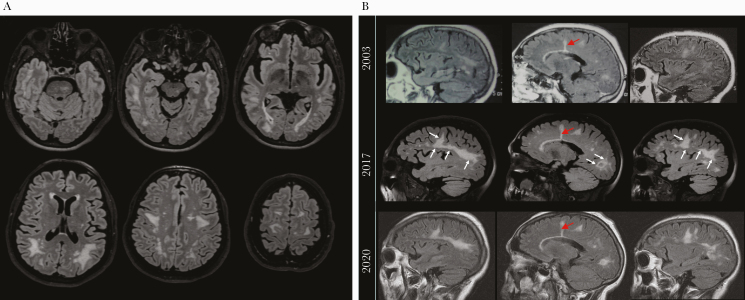
Because the neurologic syndrome precipitated the patient’s original evaluation, follow-up studies were performed in 2017. The MRI showed extensive T2 FLAIR signal abnormality predominantly in deep and subcortical white matter, with relative sparing of the subcortical U-fibers, which appeared stable since at least 2003, accounting for differences in MRI technique (Figure 6). A linear T2 hyperintensity from the 1996 biopsy tract could still be appreciated (Figure 6B). There was no evidence of abnormal enhancement. Investigation into the etiology of these nonspecific white matter lesions yielded negative or normal results, including erythrocyte sedimentation rate, C-reactive protein, antineutrophil cytoplasmic antibodies, C3/C4 levels, long chain fatty acid profile, plasma amino acids, tuberculin skin testing, and serologies for human T-lymphotropic virus-I, hepatitis C virus, and syphilis. The CSF analysis was unremarkable, and BK/JC virus, CMV, and HIV-1 RNA (<20 copies/mL) were not detected by PCR. The CSF cytokine profile was not suggestive of significant neuroinflammation. Also not detected were CSF HIV p24 (<0.01 pg/mL) and HIV-specific antibodies by LIPS analysis. Genomic DNA equivalent to 164 000 cells from the CSF cell pellet was negative by PCR for near full-length HIV-1 DNA (nested PCR sensitivity, 10 copies/reaction). Therefore, the MRI pattern of persistent white matter abnormalities without recent CSF abnormalities was suggestive of prior HIV-related encephalitis.
Figure 6.
Brain magnetic resonance imaging (MRI) showed persistent diffuse white matter disease since 1996. (A). Axial T2 fluid-attenuated inversion recovery (FLAIR) images show multiple bilateral confluent hyperintensities most prominently affecting the white matter of the posterior frontal, parietal, and temporal lobes with relative sparing of the subcortical U-fibers. Additional abnormal signal intensity lesions were seen in the midbrain and pons. (B) Sagittal T2-FLAIR images (midsagittal and parasagittal) obtained in 2003, 2017 (National Institutes of Health), and 2020 show persistent white matter lesions on sequential scans, with possible worsening between 2003 and 2017 (white arrows) that likely reflects varying MRI technique. Also shown is the linear hyperintensity remaining from the 1996 biopsy (red arrows).
DISCUSSION
This case is the first, to our knowledge, of an individual with AIDS who subsequently experienced prolonged post-cART HIV-1 RNA suppression, complete seroreversion, and low HIV-specific T-cell responses. The durability of posttreatment HIV suppression for 12 years is rare considering that only 2 other PTCs, both treated during early infection, have been reported to sustain posttreatment control for over 10 years [6, 7]. In our case, continuous care since 1997 by providers at a single ambulatory care center and persistence on sequential MRIs of a similar pattern of central nervous system (CNS) disease and findings indicative of the brain biopsy tract since 1996 decreased the possibility of mistaken identity. Although it is possible that HIV was misdiagnosed in 1996, several factors suggested otherwise, including the following: CD4 lymphocytopenia during a compatible illness; clinical and immunologic improvement with anti-Toxoplasma and antiretroviral therapies; detectable plasma HIV-1 RNA levels on 3 separate specimens tested by 2 assays at 2 medical centers; rare HIV-1 Env DNA+ lymph node mononuclear cells; detectable HIV-specific T-cell responses; HIV-1 DNA+ cells on the 1996 brain biopsy; and absence of conditions associated with false-positive HIV-1 ELISA/WB results [47]. Therefore, the bulk of the data supported a history of advanced HIV infection in this case.
In 2.5 billion PBMCs, we detected no HIV-1 DNA by ddPCR and no outgrowth of replication-competent virus in our case. Low-frequency HIV Env DNA+ cells were detected in a palpable lymph node that exhibited hyperplasia, as was observed in the London patient [16]. Whether the positive signal represented expansion of a defective clone or intact provirus is unclear. In any event, the reservoir size of our case contrasts with most PTCs, spontaneous controllers, and cART-suppressed progressors and is most reminiscent of rare cases of putative cure [2, 14, 16, 17, 27, 28]. Similar techniques and cell numbers were used in our prior investigation of the B*57+ ExtreC C4, who also had a reservoir below the level of detection [17]. In a recent paper that included C4 (referred to as EC2), no single genome-intact proviral sequences were detected in 1.5 billion PBMCs [28]. As in C4/EC2, determination of the landscape of integrated HIV-1 DNA would likely not be possible in our PTC, but it could be attempted along with other assessments to further characterize her reservoir if additional biospecimens become available.
The complete absence of HIV-specific antibodies in our case was unexpected and distinguished her from other rare cases of seroreversion in which virus eradication has been proposed [17, 27]. The weak HIV antibody profile of C4 was comparable to that of a B*57+ controller from the Sydney Blood Bank Cohort (C135) in whom clearance was suspected of an attenuated nef/3’ LTR-deleted HIV-1 acquired in 1981 [17, 27]. Our case is even distinct from the Berlin patient who had maintained low-level Env-specific antibodies for many years after sterilizing cure, and other individuals with declining HIV-specific antibodies after HSCT [15, 26, 48]. Normal Ig levels and detectable antibodies specific for hepatitis viruses and human herpesviruses supported her ability to maintain appropriate humoral responses to vaccination or persistent latent infections. The reasons for complete seroreversion in our case are unknown, but they might relate to greater clearance of HIV antigens from lymphoid tissue-based follicular dendritic cells that typically drive the persistence and affinity maturation of HIV-specific antibodies [49]. In any event, loss of HIV-specific antibodies in this case without rebounding viremia supported that remarkably low in vivo antigen levels had occurred for some time.
Given our case’s lack of neutralizing antibodies, possession of a protective HLA allele, and the known association between spontaneous control and robust T-cell responses, it was of interest to characterize her HIV-specific T-cell responses. Detectable HIV-specific CD4+ T-cell proliferation was consistent with antigen-driven expansion of low-frequency virus-specific memory cells. Her low-quality HIV-specific CD8+ T-cell responses, even after 6 days of restimulation, contrasted with most spontaneous controllers [17, 31, 42–46] and suggested a mechanism that might be associated with cART as has been proposed in PTCs [6]. Alternatively, the mechanism of control in our case could be the same as in spontaneous controllers, but a markedly lower T-cell precursor frequency yielded minimal responses upon restimulation. Still further, it is possible that she possesses a novel mechanism of secondary control. Therefore, these virus-specific T-cell analyses provided evidence, similar to that shown for the Berlin and London patients [14, 16], of only remote T-cell stimulation by HIV in vivo and suggest that ongoing active immune control, as observed in LTNP/ECs, is not occurring.
Equally enigmatic was our case’s significant cerebral white matter disease. Negative JC virus PCR in CSF and immunostains for TE recently, and lack of contrast enhancement and mass effect on MRI scans spanning 23 years are not supportive of TE, CNS lymphoma, or progressive multifocal leukoencephalopathy. Along with normal activities of daily living and a stable neurologic examination, the clinical picture is most consistent with a remote process that left residual abnormalities on MRI. Recent pathologic examination of her 1996 biopsy specimen was consistent with, but not pathognomonic for, HIV encephalitis. The HIV-1 DNA+ cells by ISH supported CNS involvement by HIV infection. A false-positive result due to contamination with blood is unlikely considering the presence of vDNA+ cells on multiple sections. Therefore, pathologic findings and HIV-1 DNA+ cells by ISH corroborated the original HIV/AIDS diagnosis.
CONCLUSIONS
In summary, the findings of complete absence of HIV-specific antibodies and weak T-cell responses in this case with a history of advanced HIV infection and non-rebounding viremia for over 12 years after treatment interruption are extremely unique. With a reservoir of replicating virus below the level of detection and only extremely rare copies of HIV DNA, this case might represent the best example of posttreatment functional cure and, like very few other individuals, offers hope that durable remission might be possible without the need for excessively toxic interventions.
Acknowledgments
We want to thank the case patient and other volunteers for their generous donations of biospecimens.
Disclaimer. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
Financial support. This project has been funded in part by the Intramural Research Programs of National Institutes of Allergy and Infectious Diseases, National Institute of Dental and Craniofacial Research and National Cancer Institute, National Institutes of Health, under Contract No. HHSN261200800001E.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
Presented in part: HIV Vaccines (C9) Keystone Symposium, March 26–30, 2017, Steamboat Springs, CO.
References
- 1. Davey RT Jr, Bhat N, Yoder C, et al. HIV-1 and T cell dynamics after interruption of highly active antiretroviral therapy (HAART) in patients with a history of sustained viral suppression. Proc Natl Acad Sci U S A 1999; 96:15109–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chun TW, Justement JS, Murray D, et al. Rebound of plasma viremia following cessation of antiretroviral therapy despite profoundly low levels of HIV reservoir: implications for eradication. AIDS 2010; 24:2803–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Li JZ, Etemad B, Ahmed H, et al. The size of the expressed HIV reservoir predicts timing of viral rebound after treatment interruption. AIDS 2016; 30:343–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hocqueloux L, Prazuck T, Avettand-Fenoel V, et al. Long-term immunovirologic control following antiretroviral therapy interruption in patients treated at the time of primary HIV-1 infection. AIDS 2010; 24:1598–601. [DOI] [PubMed] [Google Scholar]
- 5. Lodi S, Meyer L, Kelleher AD, et al. Immunovirologic control 24 months after interruption of antiretroviral therapy initiated close to HIV seroconversion. Arch Intern Med 2012; 172:1252–5. [DOI] [PubMed] [Google Scholar]
- 6. Saez-Cirion A, Bacchus C, Hocqueloux L, et al. Post-treatment HIV-1 controllers with a long-term virological remission after the interruption of early initiated antiretroviral therapy ANRS VISCONTI Study. PLoS Pathog 2013; 9:e1003211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Namazi G, Fajnzylber JM, Aga E, et al. The control of HIV after antiretroviral medication pause (CHAMP) study: posttreatment controllers identified from 14 clinical studies. J Infect Dis 2018; 218:1954–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Van Gulck E, Bracke L, Heyndrickx L, et al. Immune and viral correlates of “secondary viral control” after treatment interruption in chronically HIV-1 infected patients. PLoS One 2012; 7:e37792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sneller MC, Justement JS, Gittens KR, et al. A randomized controlled safety/efficacy trial of therapeutic vaccination in HIV-infected individuals who initiated antiretroviral therapy early in infection. Sci Transl Med 2017; 9:eaan8848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Migueles SA, Connors M. Success and failure of the cellular immune response against HIV-1. Nat Immunol 2015; 16:563–70. [DOI] [PubMed] [Google Scholar]
- 11. Casazza JP, Betts MR, Picker LJ, Koup RA. Decay kinetics of human immunodeficiency virus-specific CD8+ T cells in peripheral blood after initiation of highly active antiretroviral therapy. J Virol 2001; 75:6508–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Migueles SA, Weeks KA, Nou E, et al. Defective human immunodeficiency virus-specific CD8+ T-cell polyfunctionality, proliferation, and cytotoxicity are not restored by antiretroviral therapy. J Virol 2009; 83:11876–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hütter G, Nowak D, Mossner M, et al. Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplantation. N Engl J Med 2009; 360:692–8. [DOI] [PubMed] [Google Scholar]
- 14. Yukl SA, Boritz E, Busch M, et al. Challenges in detecting HIV persistence during potentially curative interventions: a study of the Berlin patient. PLoS Pathog 2013; 9:e1003347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gupta RK, Abdul-Jawad S, McCoy LE, et al. HIV-1 remission following CCR5Δ32/Δ32 haematopoietic stem-cell transplantation. Nature 2019; 568:244–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gupta RK, Peppa D, Hill AL, et al. Evidence for HIV-1 cure after CCR5Δ32/Δ32 allogeneic haemopoietic stem-cell transplantation 30 months post analytical treatment interruption: a case report. Lancet HIV 2020; 7:e340–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mendoza D, Johnson SA, Peterson BA, et al. Comprehensive analysis of unique cases with extraordinary control over HIV replication. Blood 2012; 119:4645–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pereyra F, Palmer S, Miura T, et al. Persistent low-level viremia in HIV-1 elite controllers and relationship to immunologic parameters. J Infect Dis 2009; 200:984–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hatano H, Delwart EL, Norris PJ, et al. Evidence for persistent low-level viremia in individuals who control human immunodeficiency virus in the absence of antiretroviral therapy. J Virol 2009; 83:329–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Amor A, Toro C, Jiménez V, et al. Seroreversion of HIV antibodies in patients with prolonged suppression of viraemia under HAART. AIDS 2006; 20:1460–2. [DOI] [PubMed] [Google Scholar]
- 21. Jurriaans S, Sankatsing SU, Prins JM, et al. HIV-1 seroreversion in an HIV-1-seropositive patient treated during acute infection with highly active antiretroviral therapy and mycophenolate mofetil. AIDS 2004; 18:1607–8. [DOI] [PubMed] [Google Scholar]
- 22. Kassutto S, Johnston MN, Rosenberg ES. Incomplete HIV type 1 antibody evolution and seroreversion in acutely infected individuals treated with early antiretroviral therapy. Clin Infect Dis 2005; 40:868–73. [DOI] [PubMed] [Google Scholar]
- 23. Hare CB, Pappalardo BL, Busch MP, et al. Seroreversion in subjects receiving antiretroviral therapy during acute/early HIV infection. Clin Infect Dis 2006; 42:700–8. [DOI] [PubMed] [Google Scholar]
- 24. Killian MS, Norris PJ, Rawal BD, et al. The effects of early antiretroviral therapy and its discontinuation on the HIV-specific antibody response. AIDS Res Hum Retroviruses 2006; 22:640–7. [DOI] [PubMed] [Google Scholar]
- 25. Manak MM, Jagodzinski LL, Shutt A, et al. Decreased seroreactivity in individuals initiating antiretroviral therapy during acute HIV infection. J Clin Microbiol 2019; 57:e00757–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Burbelo PD, Bayat A, Rhodes CS, et al. HIV antibody characterization as a method to quantify reservoir size during curative interventions. J Infect Dis 2014; 209:1613–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zaunders J, Dyer WB, Churchill M, et al. Possible clearance of transfusion-acquired nef/LTR-deleted attenuated HIV-1 infection by an elite controller with CCR5 Δ32 heterozygous and HLA-B57 genotype. J Virus Erad 2019; 5:73–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jiang C, Lian X, Gao C, et al. Distinct viral reservoirs in individuals with spontaneous control of HIV-1. Nature 2020; 585:261–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Palmer S, Wiegand AP, Maldarelli F, et al. New real-time reverse transcriptase-initiated PCR assay with single-copy sensitivity for human immunodeficiency virus type 1 RNA in plasma. J Clin Microbiol 2003; 41:4531–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Glass WG, McDermott DH, Lim JK, et al. CCR5 deficiency increases risk of symptomatic West Nile virus infection. J Exp Med 2006; 203:35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Migueles SA, Laborico AC, Shupert WL, et al. HIV-specific CD8+ T cell proliferation is coupled to perforin expression and is maintained in nonprogressors. Nat Immunol 2002; 3:1061–8. [DOI] [PubMed] [Google Scholar]
- 32. Ciccone EJ, Read SW, Mannon PJ, et al. Cycling of gut mucosal CD4+ T cells decreases after prolonged anti-retroviral therapy and is associated with plasma LPS levels. Mucosal Immunol 2010; 3:172–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Elbiek T, Dewar RL, Natarajan V. Isolation and detection of human immunodeficiency virus. In: Rose NR, De Macario EC, Folds JD, Lane HC, Nakamura RM, eds. Manual of Clinical Laboratory Immunology. 5th ed. Washington, DC: American Society for Microbiology Press; 1997: pp 781–7. [Google Scholar]
- 34. Clarridge KE, Blazkova J, Einkauf K, et al. Effect of analytical treatment interruption and reinitiation of antiretroviral therapy on HIV reservoirs and immunologic parameters in infected individuals. PLoS Pathog 2018; 14:e1006792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Myers LE, McQuay LJ, Hollinger FB. Dilution assay statistics. J Clin Microbiol 1994; 32:732–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Doria-Rose NA, Klein RM, Manion MM, et al. Frequency and phenotype of human immunodeficiency virus envelope-specific B cells from patients with broadly cross-neutralizing antibodies. J Virol 2009; 83:188–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Burbelo PD, Ching KH, Mattson TL, et al. Rapid antibody quantification and generation of whole proteome antibody response profiles using LIPS (luciferase immunoprecipitation systems). Biochem Biophys Res Commun 2007; 352:889–95. [DOI] [PubMed] [Google Scholar]
- 38. Yun Z, Lewensohn-Fuchs I, Ljungman P, et al. A real-time TaqMan PCR for routine quantitation of cytomegalovirus DNA in crude leukocyte lysates from stem cell transplant patients. J Virol Methods 2003; 110:73–9. [DOI] [PubMed] [Google Scholar]
- 39. Imamichi H, Dewar RL, Adelsberger JW, et al. Defective HIV-1 proviruses produce novel protein-coding RNA species in HIV-infected patients on combination antiretroviral therapy. Proc Natl Acad Sci U S A 2016; 113:8783–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Deleage C, Wietgrefe SW, Del Prete G, et al. Defining HIV and SIV reservoirs in lymphoid tissues. Pathog Immun 2016; 1:68–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Goulder PJ, Bunce M, Krausa P, et al. Novel, cross-restricted, conserved, and immunodominant cytotoxic T lymphocyte epitopes in slow progressors in HIV type 1 infection. AIDS Res Hum Retroviruses 1996; 12:1691–8. [DOI] [PubMed] [Google Scholar]
- 42. Betts MR, Nason MC, West SM, et al. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood 2006; 107:4781–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sáez-Cirión A, Lacabaratz C, Lambotte O, et al.; Agence Nationale de Recherches sur le Sida EP36 HIV Controllers Study Group HIV controllers exhibit potent CD8 T cell capacity to suppress HIV infection ex vivo and peculiar cytotoxic T lymphocyte activation phenotype. Proc Natl Acad Sci U S A 2007; 104:6776–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Migueles SA, Osborne CM, Royce C, et al. Lytic granule loading of CD8+ T cells is required for HIV-infected cell elimination associated with immune control. Immunity 2008; 29:1009–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sáez-Cirión A, Sinet M, Shin SY, et al.; ANRS EP36 HIV Controllers Study Group Heterogeneity in HIV suppression by CD8 T cells from HIV controllers: association with Gag-specific CD8 T cell responses. J Immunol 2009; 182:7828–37. [DOI] [PubMed] [Google Scholar]
- 46. Hersperger AR, Pereyra F, Nason M, et al. Perforin expression directly ex vivo by HIV-specific CD8 T-cells is a correlate of HIV elite control. PLoS Pathog 2010; 6:e1000917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kleinman S, Busch MP, Hall L, et al. False-positive HIV-1 test results in a low-risk screening setting of voluntary blood donation. Retrovirus Epidemiology Donor Study. JAMA 1998; 280:1080–5. [DOI] [PubMed] [Google Scholar]
- 48. Koelsch KK, Rasmussen TA, Hey-Nguyen WJ, et al. Impact of allogeneic hematopoietic stem cell transplantation on the hiv reservoir and immune response in 3 hiv-infected individuals. J Acquir Immune Defic Syndr 2017; 75:328–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Heesters BA, Myers RC, Carroll MC. Follicular dendritic cells: dynamic antigen libraries. Nat Rev Immunol 2014; 14:495–504. [DOI] [PubMed] [Google Scholar]