Abstract
Given the widespread use of bisphenols and parabens in consumer products, the assessment of their intake is crucial and represents the first step towards the assessment of the potential risks that these compounds may pose to human health. In the present study, a total of 98 samples of food items commonly consumed by the Spanish population were collected from different national supermarkets and grocery stores for the determination of parabens and bisphenols. Our analysis demonstrated that 56 of the 98 food samples contained detectable levels of parabens with limits of quantification (LOQ) between 0.4 and 0.9 ng g−1. The total concentration of parabens (sum of four parabens: ∑parabens) ranged from below the LOQ to 281.7 ng g−1, with a mean value of 73.86 ng g−1. A total of 52% of the samples showed detectable concentrations of bisphenols. Bisphenol A (BPA) was the most frequently detected bisphenol in the food samples analysed, followed by bisphenol S (BPS) and bisphenol E (BPE). Bisphenol AF (BPAF), bisphenol B (BPB) and bisphenol P (BPP) were not found in any of the analysed samples. LOQ for these bisphenols were between 0.4 and 4.0 ng g−1.
Keywords: parabens, bisphenols, food, Spain
1. Introduction
The antimicrobial activity of parabens has been known since 1924 and for this reason these alkyl esters of p-hydroxybenzoic acid have been extensively used as preservatives in many consumer products such as health and personal care product and foodstuffs [1,2,3]. Parabens are regulated as preservatives in Commission Regulation (EU) No 1004/2014 on cosmetic products that sets a maximum limit of 0.4% and 0.8% for single esters and mixtures of esters, respectively [4].
There are growing concerns about the presence of these preservatives in pharmaceuticals and cosmetic products associated with their estrogenic effects demonstrated by in vivo and in vitro studies [5]. This disrupting hormone activity seems to be linked to the length of the alkyl chain, with long-chain parabens like propyl 4-hydroxybenzoate (PropPB) and butyl 4-hydroxybenzoate (ButPB) being those of highest concern [6]. In 2010, the EU Scientific Committee on Consumer Safety (SCCS) considered that the use of methyl 4-hydroxybenzoate (MetPB) and ethyl 4-hydroxybenzoate (EthPB) at the maximum authorized concentrations is safe but due to the lack of scientific data, the Committee cannot ascertain that the use of PropPB and ButPB at the maximum concentrations is completely safe [7]. With respect to the use of MetPB and EthPB (food additives E218 and E214, respectively) and their sodium salts (E219 and E215, respectively) in foodstuffs, the maximum permitted levels (MPLs) are between 300 and 1000 mg kg−1. No other parabens are approved for use in food. The European Food Safety Authority (EFSA) concluded that an ADI (Acceptable Daily Intake) of 0–10 mg kg−1 body weight (bw) day−1 could be set for MetPB and EthPB and their sodium salts [8]. In 2015, the European Medicines Agency (EMA) reported evidence of adverse health effects related to the intake of PropPB and ADI at 1.25 mg kg−1 bw day−1 [9]. Despite having been used as food preservatives for many decades, little information is available about parabens concentration in certain foods and dietary exposure [10,11].
Bisphenol A (BPA) is also known to disrupt hormone function. This chemical is produced in large volumes and it is used primarily to harden polycarbonate plastics and epoxy resins [12] used in a wide variety of consumer products including beverage bottles, food can coatings, plastic tableware, thermal paper, and medical devices. The most common route of human exposure is food and beverage consumption [13]. The harmful effects on the reproductive, cardiovascular, immune and metabolic systems related to human exposure to BPA have been extensively described [14]. In 2017, BPA was included in the European Chemical Agency (ECHA) Candidate List of substances of very high concern. In view of the recent limitations on the use of BPA in food contact materials [15,16,17,18], the food packaging industry is exploring alternatives to replace BPA in these materials [19,20,21].
In this regard, BPA substitutes such as BPS [4,4′-sulfonyldiphenol], BPF [4,4′-dihydroxydiphenylmethane], BPB [2,2-bis(4-hydroxyphenyl) butane], BPE [1,1-Bis (4-hydroxyphenyl) ethane], and BPAF [4,4′-(hexafluoroisopropylidene) diphenol] are being used as alternatives to BPA in some industrial applications for manufacturing polycarbonate resins [22,23,24,25,26]. As with BPA, these replacement chemicals have a structure similar to BPA and therefore also exhibit endocrine disrupting properties [19,27,28]. However, studies on the occurrence of bisphenols, other than BPA, in foodstuffs are limited. In 2015, the EFSA re-examined BPA exposure and toxicity issues, reducing the BPA tolerable dietary intake (TDI), previously set at 50 μg kg−1 bw day−1 [18], to 4 μg kg−1 bw day−1 [29]. No specific limits were indicated for other types of bisphenols.
In children, the food chain is the main exposure route to parabens and bisphenols. In addition, children are especially vulnerable to developmental exposure and it has been reported that exposure levels in infants and children in relation to their body weight are higher than in adults [30]. Recently, we have developed an analytical method to determine BPB, BPS, BPE and BPP concentration in food products for children [19].
We conducted an exhaustive literature review that showed that the analytical techniques most commonly used for the extraction of the analytes included in the present study are solid phase extraction (SPE) and QuEChERs (Quick, Easy, Cheap, Effective, Rugged, and Safe), and QuEChERs and liquid–liquid extraction (LLE) for the clean-up phase. The main separation techniques are liquid chromatography and gas chromatography coupled with mass spectrometry, used for analyte detection. Other studies have used an alternative method that uses liquid chromatography coupled with fluorescence detection (data available at “Supplementary Material”, Tables S1 and S2).
Bisphenols have been the focus of extensive research over the last years; however the available studies are mainly focused on BPA. Since BPA and its substitutes show similar endocrine disrupting properties and effects, further study of these compounds used to replace BPA is warranted. In this work, the concentration of parabens and bisphenols in 98 samples of food items commonly consumed by Spanish population is determined, which are collected from different supermarkets and grocery stores. Although several studies have addressed the presence of bisphenols in food items in Spain, they are very limited regarding the number of samples of food and food packaging analysed. In addition, there are very few studies on the presence of parabens in food and to our knowledge, no studies have been conducted on paraben concentrations in such a wide range of food products commonly consumed by the Spanish population. Moreover, existing studies focus on one class of endocrine disruptors only and none of them include the analysis of both bisphenols and parabens.
2. Material and Methods
2.1. Chemicals
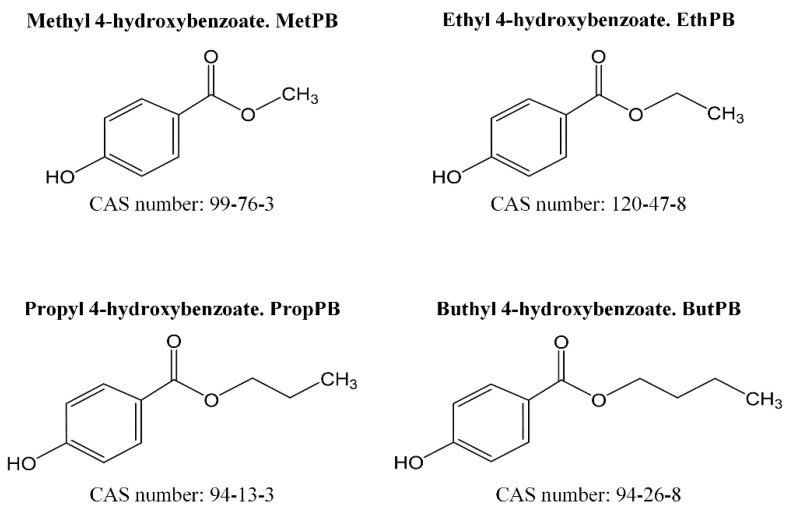
All reagents were of analytical grade. Ultrapure water (18.2 MΩ-cm) was prepared with the in-house Milli-Q Plus® system from Merck Millipore. MetPB, EthPB, PropPB and ButPB (≥99% purity) were supplied by Alfa Aesar (Thermo Fisher Scientific, Kandel, Germany) (Figure 1). BPA, BPF, BPS, BPAF, BPP (≥99% purity), BPE, BPB (≥98% purity), and deuterium labelled bisphenol A (BPA-d16, ≥99% purity) were supplied by Sigma-Aldrich (Madrid, Spain) (Figure 2). Ethyl-d5-paraben (EthPB-d5) was obtained from Toronto Research Chemicals (Toronto Research Chemicals, NY, Canada). Stock solutions of bisphenols (100 mg L−1), parabens (100 mg L−1) and internal standards BPA-d16 and EthPB-d5 (10 mg L−1) were prepared in methanol (MeOH). The working standard solution was prepared by diluting the stock solutions of the 11 analytes investigated (10 mg L−1) with MeOH and was stored in amber glass vials at −20 °C until analysis. Calibration standards were prepared by spiking food matrix with working standard solution. Liquid chromatography-mass spectrometry (LC-MS) solvents methanol and acetonitrile were provided by VWR Chemicals (VWR international, Barcelona, Spain). Ammonia solution 25% for LC-MS used as mobile phase modifier and sodium hydroxide (NaOH) pellets of reagent grade (≥98% purity) were from Sigma-Aldrich (Madrid, Spain). Sodium chloride (NaCl) and magnesium sulphate (MgSO₄) were from Panreac (Barcelona, Spain). Sorbents, silica functionalized with octadecyl groups (C18) and primary-secondary amine (PSA) were from Scharlab (Barcelona, Spain).
Figure 1.
Parabens analysed in the present work.
Figure 2.
Bisphenols analysed in the present work.
2.2. Instrumentation and Software
A Waters Acquity Ultra-high Performance Liquid Chromatography™ I-Class system (Waters Corporation, Milford, CT, USA) was used for the determination of seven bisphenols and four parabens. A Waters Xevo® TQ-XS (Waters Corporation, Milford, CT, USA) with an orthogonal Z-Spray™ electrospray ionization (ESI) source (Waters Corporation, Milford, CT, USA) was used for the spectrometric measurements. The column was a Waters UPLC® BEH C18 (2.1 mm × 50 mm, 1.7 μm particle size). A ScanVac CoolSafe™ lyophilizer (Lynge, Denmark) was used for lyophilization of food samples. Other laboratory equipment was a vortex-mixer (IKA, Staufen, Germany), a GX400 laboratory balance (Mettler-Toledo, Columbus, OH, USA), a Universal 32 centrifuge (Hettich, Tuttlingen, Germany), a Spectrafuge™ 24D centrifuge (Labnet International, Inc., Edison, NJ, USA) and a SBHC0NC sample concentrator (Stuart, Staffordshire, UK), and an Ultrasons-HD series ultrasonic bath (Selecta, Barcelona, Spain). For the treatment and analysis of data and for equipment control, MassLynx 4.1 software (Waters Corporation, Milford, CT, USA) from Waters was used.
2.3. Food Sampling
The food items included were selected among the most consumed items by the Spanish population. Most of the selected food was packaged in plastic, cans, paper trays, paperboard, foil and carton packages. The food items included in this study were collected from different national supermarkets and grocery stores and were segregated into the categories defined by the NOVA food classification system based on their nature, extent and purpose of the industrial processes they undergo (unprocessed or minimally processed foods, processed food and ultra-processed foods) [31]. A singular feature of the NOVA classification is the definition of ultra-processed food, which are not modified foods, but formulations obtained by the processing of cheap industrial ingredients that usually also include additives to make them more durable and tastier [31].
2.4. Analytical Methods
Solid foods and dairy products were lyophilized prior to the treatment of the samples and their subsequent analysis. For sample treatment the method developed by García-Córcoles et al. (2018) [19] with some modifications was used. Briefly, 2 g of each sample were weighed into a 10 mL glass tube and 5 μL of a solution of internal standards in MeOH, BPA-d₁₆ and EthPB-d5 (10 ppm) was added. Samples were homogenized in 2 mL ultrapure water and 6 mL acetonitrile in a vortex-mixer for one minute, and subsequently bath sonicated for 30 min at 15 °C. NaCl 1 g was added to each food sample and centrifuged for 5 min at 4000 rpm (2594× g). The upper organic layer was transferred to a 10 mL glass tube and 600 mg MgSO₄ and 150 mg PSA were added to remove proteins, carbohydrates, and lipids. The mixture was stirred in a vortex-mixer for one minute and centrifuged for 5 min at 4000 rpm (2594× g). The supernatant was evaporated to dryness into a centrifugal evaporator (2000 rpm, 50 °C). For the chromagraphic analysis a new method based on ultrahigh performance liquid chromatography-tandem mass spectrometry was optimized. The extracts were reconstituted by adding 250 μL of a MeOH/H2O mixture (50:50, v/v) and stirring in a vortex mixer until homogenization. After centrifugation for 5 min at 4000 rpm (2594× g), the extract obtained was transferred to a glass vial and directly injected into the ultra-high performance liquid chromatography-tandem mass spectrometric (UHPLC-MS/MS) system.
2.5. Ultrahigh Performance Liquid Chromatography—Tandem Mass Spectrometry Analysis
The chromatographic conditions optimized for the analysis are summarized next. An Acquity UPLC® BEH C18 column (2.1 mm × 50 mm, 1.7 μm particle size) was used for analyte separation at 40 °C column temperature. Based on our previous experience, 0.025% (v/v) ammonium in water (solvent A) and MeOH (solvent B) were used as mobile phase [32]. Flow rate was 0.35 mL min−1 and injection volume was 10 μL. The mobile phase gradient in the food samples was: 0.0 to 1.0 min, 10% solvent B; 1.0 to 6.0 min, 10 to 90% B; 6.0 to 6.1 min, 90 to 100% B; 6.1 to 6.6 min back to 10% B (run time 10 min). For sensitive and selective quantification of the target compounds in food samples with the highest specificity and sensitivity, negative electrospray ionization in multiple-reaction-monitoring (MRM) mode was used. The most abundant transition monitored was used for quantification and the second to confirm identification. In two cases (BPA and BPE) only one transition was sensitive and useful. Table 1 shows the optimized parameters for the UHPLC-MS/MS analysis.
Table 1.
Optimized parameters for the Ultra-high performance liquid chromatography-tandem mass spectrometry (UHPLC-MS/MS) analysis of compounds.
| tR (min) | Transitions | CV | CE | tR (min) | Transitions | CV | CE | ||
|---|---|---|---|---|---|---|---|---|---|
| BPS | 0.5 | 249.1 → 107.5 b | −4 | −26 | MetPB | 0.9 | 151.0 → 91.4 a | −12 | −18 |
| 249.1 → 155.5 a | −4 | −20 | 151.0 → 135.5 b | −12 | −14 | ||||
| BPF | 4.2 | 199.1 → 76.4 b | −14 | −24 | EthPB | 2 | 165.1→ 91.6 a | −14 | −22 |
| 199.1 → 92.4 a | −14 | −20 | 165.1 → 136.3 b | −14 | −14 | ||||
| BPE | 4.6 | 213.1 → 197.7 | −46 | −18 | PropPB | 3 | 179.1 → 91.5 a | −26 | −22 |
| 179.1 → 114.5 b | −26 | −16 | |||||||
| BPA | 4.9 | 227.2 → 132.9 | −50 | −26 | ButPB | 3.9 | 193.1 → 91.5 a | −18 | −26 |
| 193.1 → 135.9 b | −18 | −16 | |||||||
| BPAF | 5 | 335.2 → 196.7 b | −4 | −36 | BPA-d16 | 4.8 | 241.3 → 141.7 | −24 | −26 |
| 335.2 → 264.9 a | −4 | −22 | |||||||
| BPB | 5.2 | 241.2 → 211.8 b | −10 | −16 | EthPB-d5 | 2 | 170.2 → 91.3 a | −24 | −16 |
| 241.2 → 225.9 a | −10 | −20 | 170.2 → 137.6 b | −24 | −14 | ||||
| BPP | 6.1 | 345.2 → 315.1 b | −18 | −40 | |||||
| 345.2 → 330.1 a | −18 | −24 | |||||||
| Voltage of capilar | 3 kV | Nebulizer gas pressure | 7.0 bar | ||||||
| Source temperature | 150 °C | Cone/desolvation gas | N2 (≥99.995%) | ||||||
| Desolvation temperature | 600 °C | Collision gas | Ar (99.999%) | ||||||
| Cone gas flow | 150 L h−1 | Dwell time | 25 ms | ||||||
| Desolvation gas flow | 500 L h−1 | Inter-scan delay | 3 ms | ||||||
| Collision gas flow | 0.15 mL min−1 | ||||||||
CV: Cone voltage (V); CE: Collision energy (eV). tR: retention time. a SRM transition used for quantification. b SRM transition used for confirmation.
2.6. Method Validation
Validation and quality parameters of the method, selectivity, linearity, and sensitivity (limit of detection (LOD) and limit of quantification (LOQ)) and accuracy (precision and trueness), were evaluated for method validation. A non-contaminated sample was used as a blank to verify method selectivity, and after spiking to confirm a good sensitivity and accuracy.
To minimize the influence of the matrix, quantitative analyses were performed based on matrix-matched calibration curves. The calibration curves were obtained from the analyses of each analyte in blank samples spiked with different concentrations of the analytes: 0 ng g−1, 1 ng g−1, 5 ng g−1, 10 ng g−1, 25 ng g−1, 50 ng g−1, 100 ng g−1 and 250 ng g−1. Figure 3 shows a chromatogram of a standard (1 ng g−1).
Figure 3.
Chromatogram of the lower calibration level.
Method selectivity was determined by analysing the signal of the blanks and verifying the absence of peaks in the retention times of the target analytes (Figure S1). LODs and LOQs were defined as the analyte concentration producing an analytical signal of three (LOD) and ten (LOQ) times the signal-to-noise ratio. Table 2 shows the parameters evaluated for calibration, LODs, LOQs and linearity for each analyte. Finally, method accuracy in terms of precision and trueness was evaluated by spiking blank samples at three concentration levels (1, 100, 250 ng g−1) of each compound investigated. Three replicates per day in six different days were obtained. Recovery data (trueness confirmation) were between 91% and 106% for all the analytes, with a standard relative deviation (precision confirmation) lower than 12% in all cases.
Table 2.
Validation and quality parameters of the method.
| b | Linearity | LOD | LOQ | Recovery Assay | ||||
|---|---|---|---|---|---|---|---|---|
| g ng−1 | R2 | %Plof | ng g−1 | ng g−1 | Added (ng g−1) | %Rec | %RSD | |
| MetPB | 0.8556 | 0.9535 | 15.2 | 0.1 | 0.4 | 1 | 95 | 10 |
| 100 | 102 | 5.1 | ||||||
| 250 | 96 | 6.3 | ||||||
| EthPB | 0.9615 | 0.9273 | 22.1 | 0.1 | 0.4 | 1 | 106 | 8.2 |
| 100 | 98 | 7.1 | ||||||
| 250 | 103 | 4.8 | ||||||
| PropPB | 0.1064 | 0.9742 | 15.3 | 0.3 | 0.9 | 1 | 94 | 12 |
| 100 | 98 | 6.2 | ||||||
| 250 | 96 | 4.1 | ||||||
| ButPB | 0.2612 | 0.982 | 35.4 | 0.2 | 0.7 | 1 | 96 | 7.2 |
| 100 | 103 | 3.2 | ||||||
| 250 | 98 | 4.6 | ||||||
| BPS | 0.0626 | 0.9665 | 22.1 | 0.3 | 1 | 1 | 91 | 11.1 |
| 100 | 92 | 7.4 | ||||||
| 250 | 103 | 9.4 | ||||||
| BPE | 0.1108 | 0.9945 | 60.2 | 0.3 | 1 | 1 | 93 | 8.2 |
| 100 | 105 | 2.3 | ||||||
| 250 | 97 | 6 | ||||||
| BPF | 0.1996 | 0.9915 | 48.1 | 0.1 | 0.5 | 1 | 103 | 8.6 |
| 100 | 104 | 7.2 | ||||||
| 250 | 94 | 5.6 | ||||||
| BPAF | 0.314 | 0.9851 | 20.3 | 0.1 | 0.4 | 1 | 95 | 8.5 |
| 100 | 103 | 4.1 | ||||||
| 250 | 96 | 10.3 | ||||||
| BPA | 0.0446 | 0.9755 | 12.1 | 0.3 | 0.9 | 1 | 104 | 7.2 |
| 100 | 104 | 4.5 | ||||||
| 250 | 94 | 10.7 | ||||||
| BPB | 0.0989 | 0.9848 | 20.3 | 0.3 | 0.9 | 1 | 104 | 6.2 |
| 100 | 97 | 7 | ||||||
| 250 | 93 | 5.5 | ||||||
| BPP | 0.0019 | 0.9723 | 16.2 | 1 | 4 | 5 | 104 | 8.6 |
| 100 | 92 | 5.2 | ||||||
| 250 | 94 | 4.2 | ||||||
b: Slope of the calibration curve; R2: R-squared correlation coefficient; %P: p-value of the lack-of-fit test; LOD: Limit of detection; LOQ: Limit of quantification; %Rec: Recovery; %RSD: Relative standard deviation in percentage.
2.7. Estimation of Dietary Exposure in Spanish Children
Dietary intakes of parabens and bisphenols were calculated for children aged 6–9 years. This age group is one of the most vulnerable groups to endocrine disrupting chemicals and extensive information about their food consumption, dietary habits and anthropometric measures is available from different surveys conducted by the Spanish Agency for Food Safety and Nutrition (AESAN) in collaboration with European Agencies such as EFSA.
Total daily exposure was calculated by multiplying the daily intake of food groups (g day−1) by the mean bisphenol or paraben concentrations (μg kg−1) and dividing this value by body weight in kg. The daily intake of food groups was obtained from the ENALIA study, a cross-sectional survey conducted under the umbrella of AESAN and EFSA on a nationally-representative sample of children and adolescents aimed at collecting data on food consumption. [33]. The bisphenol and paraben mean concentrations used were those calculated separating the analysed foods into the categories defined by ENALIA study (meat and derivatives, fish and derivatives, cereals and derivatives, vegetables and derivatives, fruits and derivatives, dairy and derivatives, eggs, salty snacks and pre-cooked foods) (https://www.aesan.gob.es/AECOSAN/web/seguridadalimentaria/subdetalle/enalia.htm#4).
The body weight used for the calculations of total daily exposure was estimated at 29.8 kg based on the data provided by a survey on weight and height conducted on a nationally-representative sample of Spanish children aged 6–9 years [34].
2.8. Statistics
SPSS v.23 (version 23, IBM® SPSS® Statistics, Armonk, NY, USA) and Statgraphics plus 5.0 (version 5, Statpoint Technologies Inc., Warrenton, VA, USA) packages were used for the statistical treatment of analytical data. For calculations, the non-detected and non-quantified values/compounds were excluded from data treatment. The strength of the association between bisphenol and paraben concentration in food samples was measured by Spearman’s correlation. A p < 0.05 value was considered significant.
3. Results and Discussion
3.1. Parabens in Food Samples
Our results found detectable levels of parabens in 56 out of the 98 food samples analysed. The most frequently detected paraben was MetPB (49%), followed by EthPB (15.5%), and PropPB (10.2%) (Table 3). The detection frequency of ButPB was lower than for the rest of parabens (8.1%) (Table 3). This frequency pattern found in our food samples is consistent with the fact that generally the lower esters are the are the preferred parabens in foods [35] and is similar to that reported in human urine, blood and breast milk from the US population, where the most frequently detected paraben is MetPB [36].
Table 3.
Frequencies (%) and mean (ng g−1) of parabens and bisphenols in food samples.
| Parabens | Bisphenols | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MetPB | EthPB | ButPB | PropPB | ∑PBs | BPS | BPE | BPF | BPAF | BPA | BPB | BPP | ∑BPs | |
| Unprocessed or Minimally Processed Foods (n = 32) | |||||||||||||
| Frecuency (%) | 70 | 23.3 | 3.3 | 10 | 73.3 | 46.88 | 6.3 | 0 | 0 | 21.88 | 0 | 0 | 63 |
| Mean (ng g−1) | 106.90 (95.37) | 29.54 (40.06) | 2.5 (1.0) | 34.95 (48.0) | 80.7 (81.56) | 17.27 (13.7) | <LOQ | 0 | 0 | 6 (3.8) | 0 | 0 | 18.35 (15.47) |
| Processed Foods (n = 21) | |||||||||||||
| Frecuency (%) | 42.86 | 23.81 | 9.52 | 4.76 | 52 | 38.1 | 0 | 0 | 0 | 38.1 | 0 | 0 | 67 |
| Mean (ng g−1) | 142.05 (120.2) | 60.28 (56.2) | 55.35 (42.2) | 65.5 (26.2) | 130.7 (87.3) | 39.49 (67.0) | 0 | 0 | 0 | 86.3 (158.6) | 0 | 0 | 35 (119.5) |
| Ultra-Processed Foods (n = 47) | |||||||||||||
| Frecuency (%) | 38.3 | 6.38 | 10.64 | 12.77 | 49 | 6.38 | 4.26 | 0 | 0 | 27.66 | 0 | 0 | 36 |
| Mean (ng g−1) | 41.75 (47.18) | 28.6 (12.0) | 39.87 (60.07) | 3.15 (2.0) | 40.31 (48.46) | 47.48 (73.29) | <LOQ | 0 | 0 | 35.3 (42.4) | 0 | 0 | 38.34 (48.14) |
| All (n = 100) | |||||||||||||
| Frecuency (%) | 49 | 15 | 8.1 | 10.2 | 57.14 | 26.5 | 4.1 | 0 | 0 | 28.6 | 0 | 0 | 52 |
| Mean (ng g−1) | 84.6 (88.5) | 42.19 (44.71) | 39.07 (50.83) | 21.1 (31.5) | 73.86 (76.76) | 28.99 (46.29) | <LOQ | 0 | 0 | 43.28 (91.45) | 0 | 0 | 30.4 (41.9) |
∑PBs, ∑Parabens; ∑BPs, ∑Bisphenols; LOQ. limit of quantification. (_). Standard deviation.
The total concentration of parabens (∑parabens) ranged from below the LOQ to 281.7 ng g−1, with a mean value of 73.86 ng g−1 (Table 4, Table 5 and Table 6). Some of the samples such as chicken burger (281.7 ng g−1), frozen chopped onion (231.9 ng g−1), eggs (229.9 ng g−1), and milk bread with chocolate (145.3 ng g−1), contained remarkably high concentrations of MetPB. The highest concentration of EthPB was found in canned tuna in oil (146.9 ng g−1) and in anchovy stuffed olives (86.9 ng g−1), and of PropPB in milk bread with chocolate (145.3 ng g−1) and in olives (85.2 ng g−1). ButPB was found in lower concentrations than the other parabens, with its highest levels found in pineapple in plastic packaging (68.9 ng g−1). An example of a chromatogram of one of the food samples analysed in this study is shown in Figure S2.
Table 4.
Concentrations (ng g−1 fresh weight) of parabens and bisphenol analogues in unprocessed or minimally processed foods.
| Sample | Packaging | Parabens | Bisphenols | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MetPB | EthPB | ButPB | PropPB | ∑PBs | BPS | BPE | BPF | BPAF | BPA | BPB | BPP | ∑BPs | ||
| Chicken | Plastic and porex tray | ND | ND | ND | D | ND | ND | ND | ND | 2.1 (1.0) | ND | ND | 2.1 (1.0) | |
| Eggs | Plastic and paperboard | 229.9 (29.2) | ND | ND | ND | 229.9 (29.2) | ND | ND | ND | ND | ND | ND | ND | |
| Whole milk | Plastic | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ||
| Whole milk | Carton | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ||
| Whole milk | Carton | ND | ND | ND | ND | ND | D | ND | ND | ND | ND | ND | ||
| Whole milk | Carton | ND | ND | ND | ND | ND | D | ND | ND | ND | ND | ND | ||
| Frozen hake | Plastic and paperboard | ND | ND | ND | ND | ND | ND | ND | ND | D | ND | ND | ||
| Lentils | Plastic | ND | ND | ND | ND | ND | ND | ND | ND | D | ND | ND | ||
| Grape | Plastic | D | ND | ND | ND | D | ND | ND | ND | ND | ND | ND | ||
| Blueberries | Plastic | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ||
| Pineapple | Plastic | D | 5.8 (4.2) | 2.5 (1.0) | 68.9 (4.0) | 77.2 (37.4) | 44.3 (2.8) | ND | ND | ND | 11.3 (4.6) | ND | ND | 55.6 (23.3) |
| Raspberry | Plastic | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ||
| Melon | Plastic | 125.9 | ND | ND | ND | 125.9 | 4.22 (2.13) | ND | ND | ND | 7.86 (4.21) | ND | ND | 12.08 (2.57) |
| Apple | Not packed | 7.4 (3.2) | ND | ND | ND | 7.4 (3.2) | 12.7 (1.6) | ND | ND | ND | 6.0 (9.1) | ND | ND | 18.7 (4.7) |
| Apple | Plastic | 12.1 (9.2) | ND | ND | ND | 12.1 (9.2) | 8.5 (3.3) | ND | ND | ND | 2.9 (2.4) | ND | ND | 11.4 (3.96) |
| Pear | Not packed | 101.6 (84.7) | ND | ND | ND | 101.6 (84.7) | ND | ND | ND | ND | ND | ND | ND | |
| Frozen red fruit mix | Plastic | D | 100.2 (8.2) | ND | ND | 100.2 (8.2) | ND | ND | ND | ND | ND | ND | ND | |
| Frozen mango | Plastic | 39.5 | ND | ND | ND | 39.5 | D | ND | ND | ND | ND | ND | ND | |
| Frozen chopped garlic | Plastic | D | 13.6 (1.3) | ND | ND | 13.6 (1.3) | 1.36 (0.77) | ND | ND | ND | ND | ND | ND | 1.36 (0.77) |
| Frozen chopped onion | Plastic | 231.9 (18.9) | ND | ND | ND | 231.9 (18.9) | ND | ND | ND | ND | ND | ND | ND | |
| Frozen chopped parsley | Plastic | D | ND | ND | ND | 33.3 (13.9) | ND | ND | ND | ND | ND | ND | 33.3 (13.9) | |
| Frozen spinach | Plastic | D | ND | ND | ND | D | ND | ND | ND | ND | ND | ND | ||
| Tomato | Not packed | D | ND | ND | ND | 4.7 (2.2) | ND | ND | ND | ND | ND | ND | 4.7 (2.2) | |
| Tomato | Plastic | D | ND | ND | ND | 25.9 (10.7) | ND | ND | ND | ND | ND | ND | 25.9 (10.7) | |
| Striped carrot | Plastic | D | D | ND | ND | 11.5 (5.3) | ND | ND | ND | ND | ND | ND | 11.5 (5.3) | |
| Carrod | Plastic | D | 6 (3.6) | ND | ND | 6 (3.6) | D | ND | ND | ND | ND | ND | ND | |
| Lettuce | Plastic | D | D | ND | ND | ND | ND | ND | ND | ND | ND | ND | ||
| Pumpkin | Plastic | D | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ||
| Mushrooms | Plastic | D | 22.1 (10.4) | ND | 1.0 (0.1) | 23.1 (14.9) | 16.0 (6.9) | ND | ND | ND | ND | ND | ND | 16.0 (6.9) |
| Green pepper | Not packed | D | ND | ND | ND | 27.5 (6.3) | ND | ND | ND | ND | ND | ND | 27.5 (6.3) | |
| Mean | 106.90 (95.37) | 29.5 (40.6) | 2.5 (1.0) | 34.95 (48.0) | 73.17 (79.9) | 17.27 (13.7) | 6 (3.8) | 18.35 (15.47) | ||||||
∑PBs, ∑Parabens; ∑BPs, ∑Bisphenols; ND. not detected (<LOD); D. detected (>LOD and <LOQ). (_). Standard deviation.
Table 5.
Concentrations (ng g−1 fresh weight) of parabens and bisphenol analogues in processed foods.
| Sample | Packaging | Parabens | Bisphenols | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MetPB | EthPB | ButPB | PropPB | ∑PBs | BPS | BPE | BPF | BPAF | BPA | BPB | BPP | ∑BPs | ||
| Cooked ham | Plastic | ND | ND | ND | ND | ND | ND | ND | ND | 6.6 (3.4) | ND | ND | 6.6 (3.4) | |
| Spicy Sausage | Plastic | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ||
| Spicy Sausage | Plastic | D | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ||
| Chicken burguer | Plastic | 191.1(118.3) | ND | ND | ND | 191.1 (118.3) | ND | ND | ND | ND | ND | ND | ND | |
| Chicken burguer | Plastic | 281.7 (88.9) | ND | ND | ND | 281.7 (88.9) | ND | ND | ND | ND | ND | ND | ND | |
| Sausage (Chorizo) | Plastic | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ||
| Serrano ham | Plastic | ND | ND | ND | ND | 39.3 (21.3) | ND | ND | ND | 17.3 (14.9) | ND | ND | 56.6 (15.56) | |
| Plain yogurt (sweetened) | Plastic | ND | ND | ND | ND | ND | ND | ND | ND | 29.88 (18.6) | ND | ND | 29.88 (18.6) | |
| Plain yogurt (sweetened) | Plastic | ND | ND | ND | ND | ND | ND | ND | ND | 12.3 (6.0) | ND | ND | 12.3 (6.0) | |
| Guacamole | Plastic | D | ND | ND | ND | D | ND | ND | ND | ND | ND | ND | ||
| Olives | Plastic | 5.2 (1.7) | 29.2 (14.5) | ND | ND | 34.4 (16.97) | 8.5 (5.4) | ND | ND | ND | ND | ND | ND | 8.5 (5.4) |
| Olives | Plastic | D | 13.6 (5.5) | 85.2 (39.5) | 65.5 (26.2) | 164.3 (36.99) | 30.2 (7.7) | ND | ND | ND | ND | ND | ND | 30.2 (7.7) |
| Anchovy stuffed olives | Can | ND | 86.9 (17.5) | ND | ND | 86.9 (17.5) | ND | ND | ND | ND | ND | ND | ND | |
| Semi-cured cheese | Plastic | ND | 24.8 (7.2) | ND | ND | 24.8 (7.2) | ND | ND | ND | ND | D | ND | ND | |
| Semi-cured cheese (slice) | Plastic | D | ND | ND | ND | 5.6 (1.9) | ND | ND | ND | ND | ND | ND | 5.6 (1.9) | |
| Pasta | Plastic | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ||
| Rice (for microwave) | Plastic and paperboard | 90.2 (28.6) | ND | ND | ND | 90.2 (28.6) | 3.3 (1.4) | ND | ND | ND | ND | ND | ND | 3.3 (1.4) |
| Canned tuna in oil | Can | ND | ND | ND | ND | ND | ND | ND | ND | 409.0 (31.0) | ND | ND | 409.0 (31.0) | |
| Canned tuna in oil | Can | D | 146.9 (8.5) | 25.5 (14.9) | ND | 172.4 (85.8) | 187.8 (14.2) | ND | ND | ND | D | ND | ND | 187.8 (14.2) |
| Canned sweet corn | Can | ND | ND | ND | ND | ND | ND | ND | ND | 42.7 (6.2) | ND | ND | 42.7 (6.2) | |
| Cake | Not packed | ND | ND | ND | ND | 1.7 (0.7) | ND | ND | ND | ND | ND | ND | 1.7 (0.7) | |
| Mean | 142.05 (120.2) | 60.28 (56.2) | 55.35 (42.2) | 65.5 (26.2) | 130.7 (87.3) | 39.49 (67.0) | 86.3 (158.6) | 35 (119.5) | ||||||
∑PBs, ∑Parabens; ∑BPs, ∑Bisphenols; ND. not detected (<LOD); D. detected (>LOD and <LOQ). (_). Standard deviation.
Table 6.
Concentrations (ng g−1 dw) of parabens and bisphenol analogues in ultra-processed foods.
| Sample | Package Type | Parabens | Bisphenols | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MetPB | EthPB | ButPB | PropPB | ∑PBs | BPS | BPE | BPF | BPAF | BPA | BPB | BPP | ∑BPs | ||
| Sausage (Hot dogs) | Plastic | 6.8 (7.2) | ND | ND | ND | 6.8 (7.2) | ND | ND | ND | ND | ND | ND | ND | |
| Turkey cold cut | Plastic | D | ND | 2.05 (0.06) | ND | 2.05 (0.06) | ND | ND | ND | ND | ND | ND | ND | |
| Sausage (Turkey cold) | Plastic | D | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ||
| Mortadella (Bologna) | Plastic | D | ND | 16.5 (11.2) | ND | 16.5 (11.2) | 5.43 (3.34) | ND | ND | ND | ND | ND | ND | 5.43 (3.34) |
| Chocolate milkshake | Carton | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ||
| Chocolate milkshake | Carton | D | ND | ND | ND | ND | D | ND | ND | ND | ND | ND | ||
| Chocolate milkshake | Carton | D | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ||
| Chocolate milkshake | Carton | ND | ND | ND | ND | ND | D | ND | ND | ND | ND | ND | ||
| Semi-fermented milk | Plastic and foil | 6.1 (3.67) | ND | ND | ND | 6.1 (3.67) | ND | ND | ND | ND | ND | ND | ND | |
| Semi-fermented milk | Plastic and foil | 41.08 (12.55) | ND | ND | ND | 41.08 (12.55) | ND | ND | ND | ND | ND | ND | ND | |
| Semi-fermented milk | Plastic and foil | 88.38 (34.9) | ND | ND | ND | 88.38 (34.9) | ND | ND | ND | ND | ND | ND | ND | |
| Flavoured Yogurt | Plastic | 26.6 (6.1) | ND | ND | ND | 26.6 (6.1) | ND | ND | ND | ND | 60.85 (17.2) | ND | ND | 60.85 (17.2) |
| Flavoured Liquid Yogurt | Plastic | 145.66 (8.86) | ND | ND | ND | 145.66 (8.86) | ND | ND | ND | ND | 115.4 (65.96) | ND | ND | 115.4 (65.96) |
| Flavoured Liquid Yogurt | Plastic | 42.49 (20.46) | ND | ND | ND | 42.49 (20.46) | ND | ND | ND | ND | ND | ND | ND | |
| Spread cheese | Foil and paperboard | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ||
| Melted cheese | Plastic | ND | ND | ND | ND | 4.9 (0.9) | ND | ND | ND | D | ND | ND | 4.9 (0.9) | |
| Melted cheese | Plastic | ND | ND | ND | ND | ND | ND | ND | ND | 2 (0.3) | ND | ND | 2 (0.3) | |
| Breadsticks for cheese | Plastic | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ||
| Tuna dumplings | Can | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ||
| Battered hake sticks | Plastic | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ||
| Pizza (cooked ham and cheese) | Plastic | ND | ND | ND | ND | ND | ND | ND | ND | 4.3 (1.8) | ND | ND | 4.3 (1.8) | |
| Pizza (4 cheese) | Plastic | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ||
| Pizza (bolognese) | Plastic | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ||
| Ketchup | Plastic | ND | ND | ND | D | ND | ND | ND | ND | ND | ND | ND | ||
| Ketchup | Plastic | ND | ND | ND | D | ND | ND | ND | ND | D | ND | ND | ||
| Tomato sauce | Carton | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ||
| Corn snacks | Plastic | ND | ND | ND | 1.4 (0.3) | 1.4 (0.3) | ND | ND | ND | ND | ND | ND | ND | |
| Corn snacks | Plastic | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ||
| Nachos | Plastic | ND | ND | ND | ND | ND | ND | ND | ND | 42.1 (4.2) | ND | ND | 42.1 (4.2) | |
| Chips | Plastic | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ||
| Chips | Plastic | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ||
| Chips | Plastic | D | ND | ND | ND | 132.1 (21.2) | ND | ND | ND | ND | ND | ND | 132.1 (21.2) | |
| Chips (Sour Cream & Onion) | Plastic, foil and paperboard | 7.7 (0.7) | ND | ND | ND | 7.7 (0.7) | ND | ND | ND | ND | 8.8 (10.7) | ND | ND | 8.8 (10.7) |
| Gummy candy | Plastic | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ||
| Gummy candy | Plastic | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ||
| Chocolate doughnuts | Plastic | ND | D | ND | ND | ND | ND | ND | ND | 82 (10.2) | ND | ND | 82 (10.2) | |
| Milk bread | Plastic | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ||
| Croissants | Plastic | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ||
| Croissants | Plastic | D | ND | 4.3 (2.6) | 1.5 (0.8) | 5.8 (1.98) | ND | ND | ND | ND | ND | ND | ND | |
| Chocolate puff pastry | Plastic | ND | ND | ND | ND | ND | ND | ND | ND | 1 | ND | ND | 1 | |
| Cacao-filled roll | Plastic | ND | ND | ND | ND | ND | ND | ND | ND | D | ND | ND | ||
| Muffins | Plastic | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ||
| Muffins | Plastic | D | ND | ND | 4.2 (0.8) | 4.2 (0.8) | ND | ND | ND | ND | ND | ND | ND | |
| Burger bun | Plastic | 10.9 (9.6) | 20.1 (7.7) | ND | ND | 31.0 (6.5) | ND | ND | ND | ND | 1.2 (1.6) | ND | ND | 1.2 (1.6) |
| Sandwich bread | Plastic | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ||
| Milk bread with chocolate chips | Plastic | ND | ND | 145.3 (10.2) | ND | 145.3 (10.2) | ND | ND | ND | ND | D | ND | ND | |
| Puffed rice cake with chocolate | Plastic | D | 37.1 (5.5) | 31.2 (8.3) | 5.5 (3.0) | 73.8 (16.8) | ND | ND | ND | ND | ND | ND | ND | |
| Mean | 41.75 (47.18) | 28.6 (12.0) | 39.87 (60.07) | 3.15 (2.0) | 40.31 (48.46) | 47.48 (73.29) | 35.3 (42.4) | 38.34 (48.14) | ||||||
∑PBs, ∑Parabens; ∑BPs, ∑Bisphenols; ND. not detected (<LOD); D. detected (>LOD and <LOQ). (_). Standard deviation.
The literature review revealed only a small number of studies about the presence of parabens in food, maybe related to the fact that the source of parabens in food is not known with certainty (use as antimicrobial agents and in food packaging) [2,3,37]. We compared the concentrations of parabens detected in this study with those reported in other studies carried out in the European Union (EU) (Table S1) as the levels should comply with EU legislation on food safety assessment. Moreover, a LOD from 0.1 to 0.3 ng g−1 and a LOQ from 0.4 to 0.9 ng g−1 found for the studied analytes mean that this method can be consider sensitive as other authors have reported similar values for parabens (information available at “Supplementary Material”, Table S1).
The concentration of parabens found in this study is higher than that found in other works (Table S1). A possible explanation for this is that the foods analysed in this study are different and come in different packages than those analysed in previous works, which makes comparison difficult. In addition, food categorization into specific groups is vague/unclear/unspecific in previous works. Lastly, most of the foods selected for the present study are processed foods, where parabens are extensively used as additives, and come in plastic packaging, from where parabens may leach into the food inside, hence the higher concentrations found. The concentrations of parabens varied widely even within the same category of foodstuff, and processed foods generally contained higher paraben concentrations than unprocessed/fresh foods [2,3].
As in this study, the most frequently detected paraben in European studies was MetPB, which is also the one detected in the highest concentrations in food. MetPB concentrations previously reported in European studies were similar to the concentrations found in our food samples, ranging from below the LOD to 84.69 ng g−1 [38,39,40,41,42,43,44]. The European studies have also reported that other parabens frequently detected were EthPB (ranging from <LOD to 0.82 ng g−1) and PropPB (ranging from <LOD to 7.43 ng g−1) [38,40,41,42,43,44].
On the other hand, ButPB was not detected in the European studies (<LOD) [41,42], but we found detectable concentrations of this paraben (ranging from <LOD to 145.3 ng g−1) although with the lowest frequency (8%) in the food samples analysed (Table 3).
Isopropylparaben and benzylparaben have been detected in European studies in food samples [38,40,41,43,44,45], but these parabens have not been analysed in the present study; therefore, they should be included in future studies for their determination in food.
Pearson’s correlations revealed that EthPB concentration was positively associated with PropPB (p = 0.0025; Spearman’s coefficient 0.335) and ButPB (p = 0.0001; Spearman’s coefficient 0.506) concentrations. These correlations found in the food samples analysed show that EthPB and PropPB originate from the same sources. However, the source of parabens in foods is not completely understood but their use as broad-spectrum antimicrobial preservatives used in processed foods may be a potential source [2,3] as well as the use of certain food packaging materials where parabens are added as antimicrobials from where they can be released into the food inside [46,47,48]. We found no differences in the concentrations and frequency of paraben occurrence among the food in different packaging (Table 4, Table 5 and Table 6). We also found detectable concentrations of MetPB in two different brands of chocolate milkshake samples packed in carton. However, in a recent study conducted in Spain [49] reported MetPB concentrations ranging from nonquantifiable to 155.359 ng g−1 in milk carton samples.
Parabens were also detected in eggs, which could be explained by the ingestion of paraben-contaminated feed or soil that penetrate into chicken tissues and are subsequently transferred into eggs [50]. Parabens were also detected in non-packaged fruit and vegetables as parabens may naturally occur in some fruits and vegetables and may contribute to disease resistance through their antimicrobial and antifungal properties [51,52,53]. Furthermore, EthPB has been reported to have allelopathic functions [54,55] and MetPB has been also found in a wide variety of plant species and it could be applied in the preparation of bio-based poly (ether ester) materials [56].
3.2. Bisphenols in Food Samples
Diet accounts for up to 99% of BPA exposure [57]. Table 4, Table 5 and Table 6 show the concentrations of bisphenol analogues and the sum of their concentrations in the food samples analysed. A total of 52% of the samples showed detectable concentrations of bisphenols. BPA was the most frequently detected bisphenol in ultra-processed food (mean = 43.28 ng g−1 fresh weight). BPS was the second most frequently detected bisphenol in the food samples (26.5%). BPE was found in 4.1% of food samples. However, BPF, BPAF, BPB and BPP were not found in any of the samples analysed.
The concentration of ∑bisphenols ranged from below LOQ to 409 ng g−1. The highest ∑bisphenols were found in processed food, in canned tuna samples, with a mean value of 409 ng g−1 of BPA and 187.8 ng g−1 of BPS. A special concern is that canned and raw tuna is one of the most consumed fish products [25]. Moreover, bisphenol bioaccessibility is higher in canned seafood than in other food matrices with values ranging from 80 to 99% [25,58]. These results are consistent with other studies reporting higher concentrations of individual and total bisphenols in canned food than in foods sold in glass, paper, or plastic containers [21,59]. In an EFSA comprehensive report regarding the levels of BPA in foodstuff in the EU, the ratio of BPA concentrations between canned and non-canned foods ranged from 3 to 500 times, meaning that the contamination could also occur during food processing and manufacture [60].
This might be due to the leaching of bisphenols from the epoxy resins that line the cans into the food. The highest level of bisphenols (132.10 ng g−1 BPS) in the processed food group was found in potato chips. High concentrations of BPS were also found in pineapple samples sold in plastic packaging (44.3 ng g−1 BPS) included in the unprocessed or minimally processed food categories. The lowest bisphenol concentrations were found in frozen chopped garlic (mean = 1.36 ng g−1 BPS), cake (mean = 1.7 ng g−1 BPS) and burger bun (mean = 1.36 ng g−1 BPA). LODs and LOQs for these compounds are in the range of 0.1 to 1 ng g−1 and 0.4 to 4.0 ng g−1 respectively, which is in agreement with the results reported by similar works (information available at “Supplementary Material”, Table S2).
However, relevant bisphenol concentrations were also found in nonpackaged food, which could be explained by the potential contamination during primary production activities [61,62]. In addition, the ubiquity of plastics could also be related to the unexpected presence of bisphenols in food [63].
In recent decades the harmful effects on human health related the use of BPA in food packaging have raised much controversy [64,65], with a large number of studies dealing with BPA determination in food [45,66,67,68]. More recently, there has also been an increase in research that focuses not only on BPA but also on BPA analogues, as they have been reported to exhibit similar adverse health effects to BPA [27,69,70].
There is extensive literature available about BPA levels in food, but the studies assess different types of food and different BPA analogues. For this reason, comparison between studies conducted in different countries can be difficult. We performed a literature review and selected those studies conducted in the EU that determined the presence of BPA and BPA analogues in food samples (Table S2). Large variations in bisphenol concentrations were found in the present study, similar to those reported in previous studies due to methodological differences [21,71,72,73]. The results published in the European literature show concentrations ranging from <LOD to 835 ng g−1 (Table S2).
BPA is the most frequently detected bisphenol in the analysed food (Table S2), as in the present study (28.6%) (Table 3). Other bisphenols frequently detected in European studies were BPF (<LOD to 139.26 ng g−1) [20,25,26,59,74,75,76,77], followed by BPB (<LOD to 183.20 ng g−1) [25,63,71,76,78,79,80]. However, BPAF, BPS and BPE have a lower detection frequency in the food samples analysed from EU countries [25,26,59,63,71,77]. In contrast, in our work BPS and BPE were detected in 26.5% and 4.1% of the food samples analysed, respectively (Table 3). González et al. (2020) found BPE in two of 40 samples analysed [63], which could be explained by the use of BPE as a replacement for BPA in many products as a result of recent regulation limiting the presence of BPA. BPS is one of the most widely BPA substitutes used in the manufacturing of polycarbonate plastics and epoxy resins [81]. In addition, BPS has been frequently found in human biological samples, with detection rates of 81% [21], 65% and 30% [82,83], 70% [84], 40% [85], and 78% [86] in urine and 3% in breast milk [87]. Lastly, BPS has shown endocrine disrupting activity similar to BPA in in vivo and in vitro assays [88].
Other BPA analogues that have been analysed in EU studies are bisphenol AP (BPAP), bisphenol Z (BPZ), and bisphenol M (BPM), but their presence in foods is scarce [25,59,78]. In the present study, these bisphenols were not determined in our food samples, but given their reported estrogenic activity, it will be interesting to include them in future analyses.
In the present study, the association between BPA and BPS concentrations was measured with the Spearman’s correlation and a significant correlation was found between the two bisphenols in the analysed food samples (r = 0.825, p < 0.043). These results suggest that BPS is one of the main BPA analogues used in food-contact material and that is used together with BPA in different food-contact materials.
We found no marked differences between the concentrations of bisphenols in food packed in different materials, but the concentration of bisphenols was higherin canned tuna in oil. In contrast, in other canned foodstuff such as anchovy stuffed olives and tuna dumplings no detectable levels of bisphenols were found. Surprisingly, only detectable concentrations of BPE were found in food samples in carton packages. In contrast, a recent study conducted in Spain [49] found BPA in milk cartons at concentrations ranging from 0.0018 to 0.059 ng g−1. It is surprising that the inner surface of carton packages is made of four layers of low-density polyethylene. More studies should be conducted to describe the presence of bisphenols other than BPA in this kind of packaging material. BPA was also detected in non-packed apples and pears, which that could be explained by contamination during the primary production [63].
Some of the analysed samples (pineapple, canned tuna in oil, yogurt and chips) exceeded the migration limit for BPA recently established by the European Commission at 50 µg kg−1 [18]. However, this does not represent a health risk these food products are not consumed in excess in Spain [89]. Nonetheless, migration of bisphenols should be explored in other brands of these products.
3.3. Estimated Dietary Intake in Children
Tables S3 and S4 show the estimated dietary bisphenol and paraben intake in children aged 6–9 years for each foodstuff category.
The estimated intake of ∑PBs was 2.28 µg kg−1 bw day¯1 and 1.25 µg kg−1 bw day−1 for ∑BPs, which were higher than those calculated by Liao et al. [2,21] for United States children. However, these estimated intake values did not exceed the limit of 10 mg kg−1 bw day¯1 and 4 mg kg−1 bw day¯1 set by the EFSA for parabens and bisphenols, respectively [8,29]. The evaluation of other BPA substitutes and their TDI values was not possible because no limits have been set yet by international organizations.
This study has some limitations including the fact that several food items were not included and that the foods analysed were mainly packaged in plastic containers, hence higher concentrations of parabens and bisphenols, and therefore higher estimated daily intakes, are expected. Lastly, even though the estimated dietary intake of parabens and bisphenols are below the TDI, other routes of exposure such as household dust, air, and dental fillings must be considered. Lastly, the cumulative effect of parabens and bisphenols together with other endocrine disruptors present in food such as heavy metals, pesticides, and polybrominated diphenyl ethers could pose a risk to human health and should be studied.
4. Conclusions
Our findings confirm significant amounts of parabens and bisphenols detected in daily consumed products by the Spanish population. MetPB was the most frequently detected paraben in the analysed samples. Although BPA is being gradually replaced by its analogues in many food-contact materials, it is still the most frequently bisphenol detected in food, followed by BPS. The estimated dietary exposure to bisphenols and parabens did not exceed the TDIs established by the EFSA. However, because other bisphenols in addition to BPA are found in foods, a risk assessment of their presence and the establishment of limits such as TDIs for each bisphenol individually and for the sum of bisphenols are necessary. This study shows the importance of collecting more data on the occurrence of parabens and bisphenols in food to assess dietary exposure and possible health impact, especially for the more vulnerable populations.
Acknowledgments
The results presented in this article are part of a doctoral thesis by Yolanda Gálvez-Ontiveros, Nutrition and Food Sciences Doctorate Program of the University of Granada. The authors are grateful to the Spanish Ministry of Education, Culture and Sports for the pre-doctoral fellowship granted to Yolanda Gálvez-Ontiveros (FPU19/05989).
Abbreviations
| BPA | Bisphenol A |
| AESAN | Spanish Agency for Food Safety and Nutrition |
| BPA-d16 | Deuterium labelled bisphenol A |
| BPAF | Bisphenol AF |
| BPAP | Bisphenol AP |
| BPB | Bisphenol B |
| BPE | Bisphenol E |
| BPF | Bisphenol F |
| BPM | Bisphenol M |
| BPP | Bisphenol P |
| BPS | Bisphenol S |
| BPZ | Bisphenol Z |
| ButPB | Butyl 4-hydroxybenzoate |
| bw | Body weight |
| C18 | Octadecyl-functionalized silica |
| ECHA | European Chemical Agency |
| EFSA | European Food Safety Authority |
| ESI | Electrospray ionization source |
| EthPB | Ethyl 4-hydroxybenzoate |
| EthPB-d5 | Deuterium labelled ethylparaben |
| LC-MS | Liquid chromatography-mass spectrometry |
| LLE | Liquid-liquid extraction |
| LOD | Limit of detection |
| LOQ | Limit of quantification |
| MeOH | Methanol |
| MetPB | Methyl 4-hydroxybenzoate |
| MgSO4 | Magnesium sulphate |
| MPLs | Maximum permitted levels |
| MRM | Multiple-reaction-monitoring |
| NaCl | Sodium chloride |
| PropPB | Propyl 4-hydroxybenzoate |
| PSA | Primary Secondary Amine |
| QuEChERs | Quick, Easy, Cheap, Effective, Rugged, and Safe |
| SCCS | Scientific Committee on Consumer Safety |
| SPE | Solid phase extraction |
| TDI | Tolerable dietary intake |
| UHPLC-MS/MS | Ultra-high performance liquid chromatography-tandem mass spectrometry |
Supplementary Materials
The following are available online at https://www.mdpi.com/2304-8158/10/1/92/s1, Figure S1: Chromatogram of the blank. Figure S2: Chromatogram from one of the samples (canned tuna in oil), which contains remarkable levels of BPA, BPS, MetPB, EthPB and ButPB. Table S1: Concentrations (ng g−1 or ng mL−1 dw) of parabens found in food from countries of the European Union. Table S2: Concentrations (ng g−1 or ng mL−1 dw) of bisphenols found in food samples from European Union countries. Table S3: Estimated dietary intake of parabens in Spanish children aged 6–9 years. Table S4: Estimated dietary intake of bisphenols in Spanish children aged 6–9 years.
Author Contributions
Conceptualization, A.Z.-G., M.A. and A.R.; Methodology, all authors; Laboratory analysis, Y.G.-O., I.M.-R., L.R.; Writing—Original Draft Preparation, A.Z.-G., M.A. and A.R.; Writing—Review and Editing; A.Z.-G., M.A. and A.R.; Supervision, A.R. and A.Z.-G.; Project Administration, A.R.; Funding Acquisition, A.R. All authors have read and agreed to the published version of the manuscript.
Funding
This work was carried out within the frame of GP/EFSA/ENCO/380 2018/03/G04: OBEMIRISK: Knowledge platform for assessing the risk of Bisphenols on gut microbiota and its role in obesogenic phenotype: looking for biomarkers. This research was also funded by Plan Estatal de I + D + I 2013-2016, Proyecto cofinanciado FEDER-ISCIII PI17/01758, Proyecto cofinanciado FEDER-Consejería de Salud y Familias, Junta de Andalucía PE-0250-2019, Proyecto cofinaciado FEDER/Junta de Andalucía-Consejería de Transformación Económica, Industria, Conocimiento y Universidades/ Proyecto (P18-RT-4247) and by Fundación Mapfre MAPFRE2018.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the article or supplementary material.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Fransway A.F., Fransway P.J., Belsito D.V., Warshaw E.M., Sasseville D., Fowler J.F., Jr., DeKoven J.G., Pratt M.D., Maibach H.I., Taylor J.S., et al. Parabens. Dermatitis. 2019;30:3–31. doi: 10.1097/DER.0000000000000429. [DOI] [PubMed] [Google Scholar]
- 2.Liao C., Kannan K. Occurrence of and Dietary Exposure to Parabens in Foodstuffs from the United States. Environ. Sci. Technol. 2013;47:3918–3925. doi: 10.1021/es400724s. [DOI] [PubMed] [Google Scholar]
- 3.Liao C., Chen L., Kannan K. Occurrence of Parabens in Foodstuffs from China and its Implications for Human Dietary Exposure. Environ. Int. 2013;57–58:68–74. doi: 10.1016/j.envint.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 4.European Commission Commission Regulation (EU) No 1004/2004. of 18 September 2014 amending Annex V to Regulation (EC) No 1223/2009 of the European Parliament and of the Council on cosmetic products. Off. J. Eur. Union. 2014;L282:5–8. [Google Scholar]
- 5.Golden R., Gandy J., Vollmer G. A Review of the Endocrine Activity of Parabens and Implications for Potential Risks to Human Health. Crit. Rev. Toxicol. 2005;35:435–458. doi: 10.1080/10408440490920104. [DOI] [PubMed] [Google Scholar]
- 6.Boberg J., Taxvig C., Christiansen S., Hass U. Possible Endocrine Disrupting Effects of Parabens and their Metabolites. Reprod. Toxicol. 2010;30:301–312. doi: 10.1016/j.reprotox.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 7.European Commission . [(accessed on 10 December 2020)]. Scientific Committee on Consumer Safety (SCCS). Opinion on Parabens. SCCS/1348/10, Revision 22 March 2011.36p. Available online: https://ec.europa.eu/health/scientific_committees/consumer_safety/docs/sccs_o_041.pdf. [Google Scholar]
- 8.European Food Safety Authority (EFSA) European Union; Sep 29, 2004. [(accessed on 10 December 2020)]. EFSA Advises on the Safety of Paraben Usage in Food. Available online: https://www.efsa.europa.eu/en/news/efsa-advises-safety-paraben-usage-food. [Google Scholar]
- 9.European Medicines Agency . Sep 8, 2015. [(accessed on 10 December 2020)]. European Public MRL Assessment Report (EPMAR) Propyl 4-Hydroxybenzoate and Its Sodium Salt (All Food Producing Species). EMA/CVMP/632934/2014.9p. Available online: https://www.ema.europa.eu/en/documents/mrl-report/propyl-4-hydroxybenzoate-its-sodium-salt-all-food-producing-species-european-public-mrl-assessment_en.pdf. [Google Scholar]
- 10.Nobile M., Arioli F., Pavlovic R., Ceriani F., Lin S.K., Panseri S., Villa R., Chiesa L.M. Presence of emerging contaminants in baby food. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2020;37:131–142. doi: 10.1080/19440049.2019.1682686. [DOI] [PubMed] [Google Scholar]
- 11.Pradhan R., Hejmady S., Taliyan R., Khadgawat R., Gupta T., Kachhawa G., Kumar R., Singhvi G., Dubey S.K. Simultaneous Estimation of Parabens and Bisphenol a in Ready-to-Eat Foodstuffs by using QbD-Driven High-Pressure Liquid Chromatography Method. Int. J. Environ. Anal. Chem. 2020 doi: 10.1080/03067319.2020.1756272. [DOI] [Google Scholar]
- 12.Huang Y.Q., Wong C.K., Zheng J.S., Bouwman H., Barra R., Wahlström B., Neretin L., Wong M.H. Bisphenol A (BPA) in China: A review of sources, environmental levels, and potential human health impacts. Environ. Int. 2012;42:91–99. doi: 10.1016/j.envint.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 13.Juan-García A., Gallego C., Font G. Toxicidad Del Bisfenol A: Revisión. Rev. Toxicol. 2015;32:144–160. [Google Scholar]
- 14.Rochester J.R. Bisphenol A and Human Health: A Review of the Literature. Reprod. Toxicol. 2013;42:132–155. doi: 10.1016/j.reprotox.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 15.Food and Drug Administration (FDA). Indirect Food Additives: Polymers. Fed. Regist. 2012;77:41899–41902. [Google Scholar]
- 16.Food and Drug Administration (FDA) Indirect Food Additives: Adhesives and Components of Coatings. Fed. Regist. 2013;78:41840–41843. [Google Scholar]
- 17.French Republic Regulation No. 1442/2012 of 24 December 2012 Aiming at Banning the Manufacture, Import, Export and Commercialisation of all Forms of Food Packaging Containing Bisphenol A. Off. J. Fr. Repub. 2012 text 2 of 154. [Google Scholar]
- 18.European Commission Commission Regulation (EU) 2018/213 of 12 February 2018 on the use of bisphenol A in varnishes and coatings intended to come into contact with food and amending Regulation (EU) No 10/2011 as regards the use of that substance in plastic food contact materials. Off. J. Eur. Union. 2018;L41:6–12. [Google Scholar]
- 19.Garcia-Córcoles M.T., Cipa M., Rodriguez-Gomez R., Rivas A., Olea-Serrano F., Vilchez J.L., Zafra-Gomez A. Determination of Bisphenols with Estrogenic Activity in Plastic Packaged Baby Food Samples using Solid-Liquid Extraction and Clean-Up with Dispersive Sorbents Followed by Gas Chromatography Tandem Mass Spectrometry Analysis. Talanta. 2018;178:441–448. doi: 10.1016/j.talanta.2017.09.067. [DOI] [PubMed] [Google Scholar]
- 20.Gallo P., Di Marco Pisciottano I., Esposito F., Fasano E., Scognamiglio G., Mita G.D., Cirillo T. Determination of BPA, BPB, BPF, BADGE and BFDGE in Canned Energy Drinks by Molecularly Imprinted Polymer Cleaning up and UPLC with Fluorescence Detection. Food Chem. 2017;220:406–412. doi: 10.1016/j.foodchem.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 21.Liao C., Kannan K. Concentrations and Profiles of Bisphenol A and Other Bisphenol Analogues in Foodstuffs from the United States and their Implications for Human Exposure. J. Agric. Food Chem. 2013;61:4655–4662. doi: 10.1021/jf400445n. [DOI] [PubMed] [Google Scholar]
- 22.Danzl E., Sei K., Soda S., Ike M., Fujita M. Biodegradation of Bisphenol A, Bisphenol F and Bisphenol S in Seawater. Int. J. Environ. Res. Public Health. 2009;6:1472–1484. doi: 10.3390/ijerph6041472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feng Y., Yin J., Jiao Z., Shi J., Li M., Shao B. Bisphenol AF may Cause Testosterone Reduction by Directly Affecting Testis Function in Adult Male Rats. Toxicol. Lett. 2012;211:201–209. doi: 10.1016/j.toxlet.2012.03.802. [DOI] [PubMed] [Google Scholar]
- 24.Kitamura S., Suzuki T., Sanoh S., Kohta R., Jinno N., Sugihara K., Yoshihara S., Fujimoto N., Watanabe H., Ohta S. Comparative Study of the Endocrine-Disrupting Activity of Bisphenol A and 19 Related Compounds. Toxicol. Sci. 2005;84:249–259. doi: 10.1093/toxsci/kfi074. [DOI] [PubMed] [Google Scholar]
- 25.Russo G., Barbato F., Mita D.G., Grumetto L. Occurrence of Bisphenol A and its analogues in some foodstuff marketed in Europe. Food Chem. Toxicol. 2019;131:110575. doi: 10.1016/j.fct.2019.110575. [DOI] [PubMed] [Google Scholar]
- 26.Gallart-Ayala H., Moyano E., Galceran M.T. Fast Liquid Chromatography-Tandem Mass Spectrometry for the Analysis of Bisphenol A-Diglycidyl Ether, Bisphenol F-Diglycidyl Ether and their Derivatives in Canned Food and Beverages. J. Chromatogr. A. 2011;1218:1603–1610. doi: 10.1016/j.chroma.2011.01.026. [DOI] [PubMed] [Google Scholar]
- 27.Andujar N., Galvez-Ontiveros Y., Zafra-Gomez A., Rodrigo L., Jesus Alvarez-Cubero M., Aguilera M., Monteagudo C., Rivas A. Bisphenol A Analogues in Food and their Hormonal and Obesogenic Effects: A Review. Nutrients. 2019;11:2136. doi: 10.3390/nu11092136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rivas A., Lacroix M., Olea-Serrano F., Laios I., Leclercq G., Olea N. Estrogenic Effect of a Series of Bisphenol Analogues on Gene and Protein Expression in MCF-7 Breast Cancer Cells. J. Steroid Biochem. Mol. Biol. 2002;82:45–53. doi: 10.1016/S0960-0760(02)00146-2. [DOI] [PubMed] [Google Scholar]
- 29.European Food Safety Authority (EFSA) Scientific Opinion. Scientific Opinion on the risk to public health related to the presence of bisphenol A (BPA) in foodstuffs: Executive summary. EFSA panel on food contact materials, enzymes, flavourings and processing aids (CEF) EFSA J. 2015;13:3978. doi: 10.2903/j.efsa.2015.3978. [DOI] [Google Scholar]
- 30.Beszterda M., Franski R. Endocrine Disruptor Compounds in Environment: As a Danger for Children Health. Pediatr. Endocrinol. Diabetes Metab. 2018;24:88–95. doi: 10.18544/PEDM-24.02.0107. [DOI] [PubMed] [Google Scholar]
- 31.Monteiro C.A., Cannon G., Moubarac J., Levy R.B., Louzada M.L.C., Jaime P.C. The UN Decade of Nutrition, the NOVA Food Classification and the Trouble with Ultra-Processing. Public Health Nutr. 2018;21:5–17. doi: 10.1017/S1368980017000234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martín-Pozo L., Cantarero-Malagón S., Hidalgo F., Navalón A., Zafra-Gómez A. Determination of endocrine disrupting chemicals in human nails using an alkaline digestion prior to ultra-high performance liquid chromatography–tandem mass spectrometry. Talanta. 2020;208:120429. doi: 10.1016/j.talanta.2019.120429. [DOI] [PubMed] [Google Scholar]
- 33.López-Sobaler A.M., Aparicio A., Rubio J., Marcos V., Sanchidrián R., Santos S., Pérez-Farinós N., Dal-Re M.Á., Villar-Villalba C., Yusta-Boyo M.J., et al. Adequacy of usual macronutrient intake and macronutrient distribution in children and adolescents in Spain: A National Dietary Survey on the Child and Adolescent Population, ENALIA 2013–2014. Eur. J. Nutr. 2019;58:705–719. doi: 10.1007/s00394-018-1676-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perez-Farinos N., Lopez-Sobaler A.M., Angeles Dal Re M., Villar C., Labrado E., Robledo T., Ortega R.M. The ALADINO Study: A National Study of Prevalence of Overweight and Obesity in Spanish Children in 2011. Biomed Res. Int. 2013;2013:163687. doi: 10.1155/2013/163687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Soni M.G., Carabin I.G., Burdock G.A. Safety Assessment of Esters of P-Hydroxybenzoic Acid (Parabens) Food Chem. Toxicol. 2005;43:985–1015. doi: 10.1016/j.fct.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 36.Quiros-Alcala L., Buckley J.P., Boyle M. Parabens and Measures of Adiposity among Adults and Children from the US General Population: NHANES 2007-2014. Int. J. Hyg. Environ. Health. 2018;221:652–660. doi: 10.1016/j.ijheh.2018.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maher H.M., Alzoman N.Z., Almeshal M.A., Alotaibi H.A., Alotaibi N.N., Al-Showiman H. Quantitative Screening of Parabens in Ready-to-Eat Foodstuffs Available in the Saudi Market using High Performance Liquid Chromatography with Photodiode Array Detection. Arab. J. Chem. 2020;13:2897–2911. doi: 10.1016/j.arabjc.2018.07.019. [DOI] [Google Scholar]
- 38.Alvarez-Muñoz D., Rodriguez-Mozaz S., Maulvault A.L., Tediosi A., Fernandez-Tejedor M., Van den Heuvel F., Kotterman M., Marques A., Barcelo D. Occurrence of Pharmaceuticals and Endocrine Disrupting Compounds in Macroalgaes, Bivalves, and Fish from Coastal Areas in Europe. Environ. Res. 2015;143:56–64. doi: 10.1016/j.envres.2015.09.018. [DOI] [PubMed] [Google Scholar]
- 39.Alvarez-Muñoz D., Rodriguez-Mozaz S., Jacobs S., Serra-Compte A., Caceres N., Sioen I., Verbeke W., Barbosa V., Ferrari F., Fernandez-Tejedor M., et al. Pharmaceuticals and Endocrine Disruptors in Raw and Cooked Seafood from European Market: Concentrations and Human Exposure Levels. Environ. Int. 2018;119:570–581. doi: 10.1016/j.envint.2018.07.006. [DOI] [PubMed] [Google Scholar]
- 40.Azzouz A., Palacios Colon L., Souhail B., Ballesteros E. A Multi-Residue Method for GC-MS Determination of Selected Endocrine Disrupting Chemicals in Fish and Seafood from European and North African Markets. Environ. Res. 2019;178:108727. doi: 10.1016/j.envres.2019.108727. [DOI] [PubMed] [Google Scholar]
- 41.Chiesa L.M., Pavlovic R., Panseri S., Arioli F. Evaluation of Parabens and their Metabolites in Fish and Fish Products: A Comprehensive Analytical Approach using LC-HRMS. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2018;35:2400–2413. doi: 10.1080/19440049.2018.1544721. [DOI] [PubMed] [Google Scholar]
- 42.Villaverde-de-Sáa E., Rodil R., Quintana J.B., Cela R. Matrix Solid-Phase Dispersion Combined to Liquid Chromatography–tandem Mass Spectrometry for the Determination of Paraben Preservatives in Mollusks. J. Chromatogr. A. 2016;1459:57–66. doi: 10.1016/j.chroma.2016.06.070. [DOI] [PubMed] [Google Scholar]
- 43.Jakimska A., Huerta B., Barganska Z., Kot-Wasik A., Rodriguez-Mozaz S., Barcelo D. Development of a Liquid Chromatography-Tandem Mass Spectrometry Procedure for Determination of Endocrine Disrupting Compounds in Fish from Mediterranean Rivers. J. Chromatogr. A. 2013;1306:44–58. doi: 10.1016/j.chroma.2013.07.050. [DOI] [PubMed] [Google Scholar]
- 44.Pico Y., Belenguer V., Corcellas C., Diaz-Cruz M.S., Eljarrat E., Farre M., Gago-Ferrero P., Huerta B., Navarro-Ortega A., Petrovic M., et al. Contaminants of Emerging Concern in Freshwater Fish from Four Spanish Rivers. Sci. Total Environ. 2019;659:1186–1198. doi: 10.1016/j.scitotenv.2018.12.366. [DOI] [PubMed] [Google Scholar]
- 45.Abril C., Martin J., Luis Malvar J., Luis Santos J., Aparicio I., Alonso E. Dispersive Liquid-Liquid Microextraction as a New Clean-Up Procedure for the Determination of Parabens, Perfluorinated Compounds, UV Filters, Biocides, Surfactants, and Plasticizers in Root Vegetables. Anal. Bioanal. Chem. 2018;410:5155–5163. doi: 10.1007/s00216-018-1165-9. [DOI] [PubMed] [Google Scholar]
- 46.Žnideršič L., Mlakar A., Prosen H. Development of a SPME-GC-MS/MS Method for the Determination of some Contaminants from Food Contact Material in Beverages. Food Chem. Toxicol. 2019;134:110829. doi: 10.1016/j.fct.2019.110829. [DOI] [PubMed] [Google Scholar]
- 47.Chung D.W., Papadakis S.E., Yam K.L. Release of Propyl Paraben from a Polymer Coating into Water and Food Simulating Solvents for Antimicrobial Packaging Applications. J. Food Process. Preserv. 2001;25:71–87. doi: 10.1111/j.1745-4549.2001.tb00444.x. [DOI] [Google Scholar]
- 48.Lu L., Xiong W., Li X., Lv S., Tang X., Chen M., Zou Z., Lin Z., Qiu B., Chen G. Determination of the Migration of Eight Parabens from Antibacterial Plastic Packaging by Liquid Chromatography-Electrospray Ionization-Tandem Mass Spectrometry. Anal. Methods. 2014;6:2096–2101. doi: 10.1039/c3ay42080a. [DOI] [Google Scholar]
- 49.Herrero L., Quintanilla-Lopez J.E., Fernandez M.A., Gomara B. Plasticisers and Preservatives in Commercial Milk Products: A Comprehensive Study on Packages used in the Spanish Market. Food Chem. 2021;338:128031. doi: 10.1016/j.foodchem.2020.128031. [DOI] [PubMed] [Google Scholar]
- 50.Pajurek M., Pietron W., Maszewski S., Mikolajczyk S., Piskorska-Pliszczynska J. Poultry eggs as a source of PCDD/Fs, PCBs, PBDEs and PBDD/Fs. Chemosphere. 2019;223:651–658. doi: 10.1016/j.chemosphere.2019.02.023. [DOI] [PubMed] [Google Scholar]
- 51.Hagel J.M., Chen X., Facchini P.J. Production of Methylparaben in Escherichia coli. J. Ind. Microbiol. Biotechnol. 2019;46:91–99. doi: 10.1007/s10295-018-2102-9. [DOI] [PubMed] [Google Scholar]
- 52.Martínez J.A. Natural Fungicides Obtained from Plants. IntechOpen Access Publisher; London, UK: 2012. pp. 3–28. [Google Scholar]
- 53.Blažević I., Radonic A., Mastelie J., Zekic M., Skocibusic M., Maravic A. Hedge Mustard (Sisymbrium Officinale): Chemical Diversity of Volatiles and their Antimicrobial Activity. Chem. Biodivers. 2010;7:2023–2034. doi: 10.1002/cbdv.200900234. [DOI] [PubMed] [Google Scholar]
- 54.Liu Y., Zhang C., Wei S., Cui H., Huang H. Compounds from the Subterranean Part of Johnsongrass and their Allelopathic Potential. Weed Biol. Manag. 2011;11:160–166. doi: 10.1111/j.1445-6664.2011.00416.x. [DOI] [Google Scholar]
- 55.Huang H., Liu C., Wei S., Wang J., Zhang C. Dynamic Root Exudation of Phenolic Allelochemicals from Johnson Grass (Sorghum Halepense) Weed Biol. Manag. 2015;15:133–137. [Google Scholar]
- 56.Hu K., Zhao D., Wu G., Ma J. Aromatic poly(ether ester)s derived from a naturally occurring building block nipagin and linear aromatic α,ω-diols. RSC Adv. 2017;7:32989–33000. [Google Scholar]
- 57.Martinez M.A., Rovira J., Prasad Sharma R., Nadal M., Schuhmacher M., Kumar V. Comparing Dietary and Non-Dietary Source Contribution of BPA and DEHP to Prenatal Exposure: A Catalonia (Spain) Case Study. Environ. Res. 2018;166:25–34. doi: 10.1016/j.envres.2018.05.008. [DOI] [PubMed] [Google Scholar]
- 58.Cunha S.C., Alves R.N., Fernandes J.O., Casal S., Marques A. First Approach to Assess the Bioaccessibility of Bisphenol A in Canned Seafood. Food Chem. 2017;232:501–507. doi: 10.1016/j.foodchem.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 59.Cesen M., Lambropoulou D., Laimou-Geraniou M., Kosjek T., Blaznik U., Heath D., Heath E. Determination of Bisphenols and Related Compounds in Honey and their Migration from Selected Food Contact Materials. J. Agric. Food Chem. 2016;64:8866–8875. doi: 10.1021/acs.jafc.6b03924. [DOI] [PubMed] [Google Scholar]
- 60.EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF) Scientific opinion on the risks to public health related to the presence of bisphenol A (BPA) in foodstuffs. EFSA J. 2013;13:3978. [Google Scholar]
- 61.Mercogliano R., Santonicola S. Investigation on Bisphenol A Levels in Human Milk and Dairy Supply Chain: A Review. Food Chem. Toxicol. 2018;114:98–107. doi: 10.1016/j.fct.2018.02.021. [DOI] [PubMed] [Google Scholar]
- 62.Santonicola S., Ferrante M.C., di Leo G., Murru N., Anastasio A., Mercogliano R. Study on Endocrine Disruptors Levels in Raw Milk from Cow’s Farms: Risk Assessment. Ital. J. Food Saf. 2018;7:158–161. doi: 10.4081/ijfs.2018.7668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.González N., Cunha S.C., Ferreira R., Fernandes J.O., Marquès M., Nadal M., Domingo J.L. Concentrations of Nine Bisphenol Analogues in Food Purchased from Catalonia (Spain): Comparison of Canned and Non-Canned Foodstuffs. Food Chem. Toxicol. 2020;136:110992. doi: 10.1016/j.fct.2019.110992. [DOI] [PubMed] [Google Scholar]
- 64.Darbre P.D. Endocrine Disruptors and Obesity. Curr. Obes. Rep. 2017;6:18–27. doi: 10.1007/s13679-017-0240-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rivas A., Monteagudo C., Heras-Gonzalez L., Mariscal-Arcas M., Luisa Lorenzo-Tovar M., Olea-Serrano F. Association of Bisphenol A Exposure with Dietary Quality Indices in Spanish Schoolchildren. Food Chem. Toxicol. 2016;94:25–30. doi: 10.1016/j.fct.2016.05.010. [DOI] [PubMed] [Google Scholar]
- 66.Casajuana N., Lacorte S. New Methodology for the Determination of Phthalate Esters, Bisphenol A, Bisphenol A Diglycidyl Ether, and Nonylphenol in Commercial Whole Milk Samples. J. Agric. Food Chem. 2004;52:3702–3707. doi: 10.1021/jf040027s. [DOI] [PubMed] [Google Scholar]
- 67.Errico S., Nicolucci C., Migliaccio M., Micale V., Mita D.G., Diano N. Analysis and Occurrence of some Phenol Endocrine Disruptors in Two Marine Sites of the Northern Coast of Sicily (Italy) Mar. Pollut. Bull. 2017;120:68–74. doi: 10.1016/j.marpolbul.2017.04.061. [DOI] [PubMed] [Google Scholar]
- 68.Ferrer E., Santoni E., Vittori S., Font G., Manes J., Sagratini G. Simultaneous Determination of Bisphenol A, Octylphenol, and Nonylphenol by Pressurised Liquid Extraction and Liquid Chromatography-Tandem Mass Spectrometry in Powdered Milk and Infant Formulas. Food Chem. 2011;126:360–367. [Google Scholar]
- 69.Gálvez-Ontiveros Y., Paez S., Monteagudo C., Rivas A. Endocrine Disruptors in Food: Impact on Gut Microbiota and Metabolic Diseases. Nutrients. 2020;12:1158. doi: 10.3390/nu12041158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Siracusa J.S., Yin L., Measel E., Liang S., Yu X. Effects of Bisphenol A and its Analogs on Reproductive Health: A Mini Review. Reprod. Toxicol. 2018;79:96–123. doi: 10.1016/j.reprotox.2018.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Alabi A., Caballero-Casero N., Rubio S. Quick and Simple Sample Treatment for Multiresidue Analysis of Bisphenols, Bisphenol Diglycidyl Ethers and their Derivatives in Canned Food Prior to Liquid Chromatography and Fluorescence Detection. J. Chromatogr. A. 2014;1336:23–33. doi: 10.1016/j.chroma.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 72.Doumas M., Rouillon S., Venisse N., Nadeau C., Eugene P.P., Farce A., Chavatte P., Dupuis A., Migeot V., Carato P. Chlorinated and Brominated Bisphenol A Derivatives: Synthesis, Characterization and Determination in Water Samples. Chemosphere. 2018;213:434–442. doi: 10.1016/j.chemosphere.2018.09.061. [DOI] [PubMed] [Google Scholar]
- 73.Fasano E., Cirillo T., Esposito F., Lacorte S. Migration of Monomers and Plasticizers from Packed Foods and Heated Microwave Foods using QuEChERS Sample Preparation and Gas Chromatography/Mass Spectrometry. LWT Food Sci. Technol. 2015;64:1015–1021. [Google Scholar]
- 74.Cacho J.I., Campillo N., Vinas P., Hernandez-Cordoba M. Stir Bar Sorptive Extraction Coupled to Gas Chromatography-Mass Spectrometry for the Determination of Bisphenols in Canned Beverages and Filling Liquids of Canned Vegetables. J. Chromatogr. A. 2012;1247:146–153. doi: 10.1016/j.chroma.2012.05.064. [DOI] [PubMed] [Google Scholar]
- 75.Cirillo T., Esposito F., Fasano E., Scognamiglio G., Di Marco Pisciottano I., Mita G.D., Gallo P. BPA, BPB, BPF, BADGE and BFDGE in Canned Beers from the Italian Market. Food Addit. Contam. Part B Surveill. 2019;12:268–274. doi: 10.1080/19393210.2019.1650835. [DOI] [PubMed] [Google Scholar]
- 76.Grumetto L., Gennari O., Montesano D., Ferracane R., Ritieni A., Albrizio S., Barbato F. Determination of Five Bisphenols in Commercial Milk Samples by Liquid Chromatography Coupled to Fluorescence Detection. J. Food Prot. 2013;76:1590–1596. doi: 10.4315/0362-028X.JFP-13-054. [DOI] [PubMed] [Google Scholar]
- 77.Regueiro J., Wenzl T. Determination of Bisphenols in Beverages by Mixed-Mode Solid-Phase Extraction and Liquid Chromatography Coupled to Tandem Mass Spectrometry. J. Chromatogr. A. 2015;1422:230–238. doi: 10.1016/j.chroma.2015.10.046. [DOI] [PubMed] [Google Scholar]
- 78.Barboza L.G.A., Cunha S.C., Monteiro C., Fernandes J.O., Guilhermino L. Bisphenol A and its Analogs in Muscle and Liver of Fish from the North East Atlantic Ocean in Relation to Microplastic Contamination. Exposure and Risk to Human Consumers. J. Hazard. Mater. 2020;393:122419. doi: 10.1016/j.jhazmat.2020.122419. [DOI] [PubMed] [Google Scholar]
- 79.Cunha S.C., Cunha C., Ferreira A.R., Fernandes J.O. Determination of Bisphenol A and Bisphenol B in Canned Seafood Combining QuEChERS Extraction with Dispersive Liquid-Liquid Microextraction Followed by Gas Chromatography-Mass Spectrometry. Anal. Bioanal. Chem. 2012;404:2453–2463. doi: 10.1007/s00216-012-6389-5. [DOI] [PubMed] [Google Scholar]
- 80.Fattore M., Russo G., Barbato F., Grumetto L., Albrizio S. Monitoring of Bisphenols in Canned Tuna from Italian Markets. Food Chem. Toxicol. 2015;83:68–75. doi: 10.1016/j.fct.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 81.Chen Y., Shu L., Qiu Z., Lee D.Y., Settle S.J., Hee S.Q., Telesca D., Yang X., Allard P. Exposure to the BPA-Substitute Bisphenol S Causes Unique Alterations of Germline Function. PLoS Genet. 2016;12:e1006223. doi: 10.1371/journal.pgen.1006223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vela-Soria F., Ballesteros O., Zafra-Gómez A., Ballesteros L., Navalón A. A Multiclass Method for the Analysis of Endocrine Disrupting Chemicals in Human Urine Samples. Sample Treatment by Dispersive Liquid–liquid Microextraction. Talanta. 2014;129:209–218. doi: 10.1016/j.talanta.2014.05.016. [DOI] [PubMed] [Google Scholar]
- 83.Vela-Soria F., Rodriguez I., Ballesteros O., Zafra-Gomez A., Ballesteros L., Cela R., Navalon A. Simplified Matrix Solid Phase Dispersion Procedure for the Determination of Parabens and Benzophenone-Ultraviolet Filters in Human Placental Tissue Samples. J. Chromatogr. A. 2014;1371:39–47. doi: 10.1016/j.chroma.2014.10.063. [DOI] [PubMed] [Google Scholar]
- 84.Xue J., Wu Q., Sakthivel S., Pavithran P.V., Vasukutty J.R., Kannan K. Urinary Levels of Endocrine-Disrupting Chemicals, Including Bisphenols, Bisphenol A Diglycidyl Ethers, Benzophenones, Parabens, and Triclosan in Obese and Non-Obese Indian Children. Environ. Res. 2015;137:120–128. doi: 10.1016/j.envres.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 85.Yang Y., Guan J., Yin J., Shao B., Li H. Urinary Levels of Bisphenol Analogues in Residents Living Near a Manufacturing Plant in South China. Chemosphere. 2014;112:481–486. doi: 10.1016/j.chemosphere.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 86.Zhou X., Kramer J.P., Calafat A.M., Ye X. Automated on-Line Column-Switching High Performance Liquid Chromatography Isotope Dilution Tandem Mass Spectrometry Method for the Quantification of Bisphenol A, Bisphenol F, Bisphenol S, and 11 Other Phenols in Urine. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2014;944:152–156. doi: 10.1016/j.jchromb.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 87.Deceuninck Y., Bichon E., Marchand P., Boquien C., Legrand A., Boscher C., Antignac J.P., Le Bizec B. Determination of Bisphenol A and Related Substitutes/Analogues in Human Breast Milk using Gas Chromatography-Tandem Mass Spectrometry. Anal. Bioanal. Chem. 2015;407:2485–2497. doi: 10.1007/s00216-015-8469-9. [DOI] [PubMed] [Google Scholar]
- 88.Rochester J.R., Bolden A.L. Bisphenol S and F: A Systematic Review and Comparison of the Hormonal Activity of Bisphenol A Substitutes. Environ. Health Perspect. 2015;123:643–650. doi: 10.1289/ehp.1408989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.AECOSAN Encuesta Nacional de Alimentación En La Población Infantil Y Adolescente. Resultados Sobre Datos de Consumo. Agencia Española de Consumo, Seguridad Alimentaria Y Nutrición. Ministry of Health, Madrid, Spain. [(accessed on 10 July 2010)]; Available online: Http://Www.Aecosan.Msssi.Gob.Es/AECOSAN/Web/Seguridad_alimentaria/Subdetalle/Enalia.Htm.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available in the article or supplementary material.