Abstract
Background
Endoscopic submucosal dissection (ESD) is gaining enormous popularity in the treatment of early gastric cancers (EGCs) in many institutions across the world. However, appropriate selection of candidates for endoscopic resection is crucial to sufficiently mitigate non-e-curative (NEC) resection. This study aims at identifying the various clinico-pathologic factors that independently predict the NEC outcome and depth of submucosal invasion following ESD procedure in patients with EGC.
Methods
Multiple logistic regression analysis was applied to investigate factors that independently predict both non-curability phenomenon and the level of submucosal invasion in patients with early gastric neoplasia. Statistical Packages for the Social Sciences version 23 was used for analysis.
Results
A total of 153 patients (162 EGC lesions) underwent en-bloc ESD after which the rate of complete resection and non-e-curative outcome were 95% and 22.2%, correspondingly. Multivariate analysis depicted that tumor location in the upper two third of stomach (odds ratio [OR], 5.46; 95% confidence interval [95% CI], 1.65–18.12; p = 0.006), tumor size > 2 cm (OR, 7.63; 95% CI, 2.29–25.42; p = 0.001), histologically undifferentiated tumor (OR, 15.54; 95% CI, 1.65–146.22; p = 0.001), and tumors with 0-IIa/0-IIc or their mixed variants with predominant 0-IIa/0-IIc (OR, 9.77; 95% CI, 1.23–77.65; p = 0.031) were all independent predictors of NEC resection for early gastric tumors. Additionally, location in the upper two third of the stomach (OR, 8.88; 95% CI, 2.90–27.17; p < 0.001), ulcerated lesions (OR, 3.70; 95% CI, 1.15–11.90; p = 0.028), lesions with > 2 cm (OR, 2.94; 95% CI, 1.08–8.02; p = 0.036) and those with poor differentiation (OR, 6.51; 95% CI, 2.23–18.98; p = 0.001) were found to have significant association with submucosal invasion.
Conclusions
Tumors located in the upper two third of the stomach having a larger size (> 2 cm), poor histo-differentiation and a gross type of 0-IIa/0-IIc or their mixed variants with predominant 0-IIa/0-IIc were significantly associated with a risk of NEC after ESD procedure. Thus, early gastric tumors displaying these features need to be handled carefully during endoscopic resection. Our findings may shed light on the pre-procedural detection of clinicopathologic factors that determine non-e-curability in patients with EGC.
Keywords: Early gastric cancer, ESD, Clinico-pathologic factors, Non-e-curative resection
Background
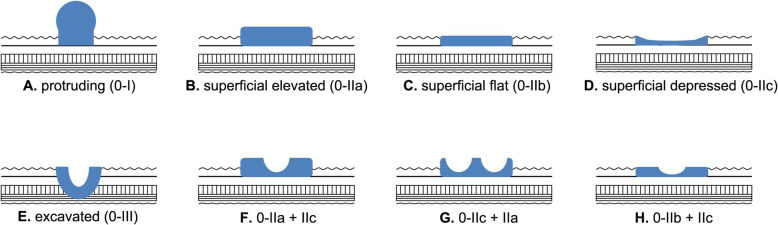
Gastric cancer remains to be the fifth most common malignancy and the third leading cause of cancer-related deaths across the globe, and exhibits significant epidemiological variation as a function of age and geography [1]. Cancers of the stomach are two times higher in males than in females and the East Asian countries possess a comparatively higher burden of cases than North American, European and African countries [1, 2]. A subset of these cancers confined to the submucosal layer are called early gastric cancers (EGCs) irrespective of lymph node metastasis [3]. According to Japanese Gastric Cancer Association (JGCA) [3] classification scheme, EGCs belong to class zero (‘0’), which are further subdivided into specific subtypes. These class ‘0’ lesions include protruding type (0-I), superficial lesions with elevation (0-IIa), superficial flat (0-IIb), superficial depressed (0-IIc) and the last type encompasses excavated lesions (0-III). In practice, however, the occurrence of combined macroscopic features is not uncommon, where the more dominant type is written first followed by the other [4].
Despite being a gold standard method in the treatment of early stage gastric cancers for the last many decades, gastrectomy has nowadays been replaced by a relatively less invasive therapeutic endoscopy that has promising clinical outcomes [5, 6]. Endoscopic approach of tissue resection has witnessed dramatic changes over the last four decades. It started in the form of endoscopic mucosal resection (EMR) in the early 1980s and, subsequently, endoscopic submucosal dissection (ESD) came into play which has now proven itself as a very popular means of resecting EGCs [7].
In accordance with the Japanese guidelines, patients with EGC without lymph node involvement are given specific criteria for diagnosis [8, 9]. To that end, absolute indication is considered in patients with EGCs which: are less than 2 cm in size, have differentiated morphology with intra-mucosal location, and are devoid of ulcer. On the other hand, indications are expanded for early stage gastric lesions which: measure greater than 2 cm, hardly extend beyond the mucosal layer, have differentiated histology and lack ulceration or if size is less than 3 cm in presence of ulceration, and undifferentiated lesions where size is less than 2 cm. Lympho-vascular involvement, however, should not be present in both absolute and expanded indications [8, 9]. The above-mentioned indications were established after evaluating the likelihood of metastasis to lymph nodes in specimens obtained from gastrectomy [10].
There is a general consensus that during selection of patients with superficial gastric neoplasia for endoscopic treatment, complete removal of a lesion has to be assured with no lymph nodes involved and the margins’ status has to be clear [3, 11]. However, the definition applied for EGCs at the present fails to take account of lymph node status [12–14] and, hence, nothing is mentioned about lymph node involvement after microscopic evaluation of specimens obtained from endoscopic resection [15, 16]. To make matters worse, there is no any single imaging modality to date that can help experts to preoperatively decide on lymph node status with full confidence [17–19]. Yet, metastasis to lymph nodes is seen in patients with EGCs that varies according to the extent of invasion (intra-mucosal vs submucosal) and the risk is lower in the former (less than 3%) as compared to the latter (up to 20%) [10, 20–22]. If regional lymph nodes remain involved after endoscopic resection, it implies that the endoscopic treatment is suboptimal and patients would end up having additional interventions on top of ESD [23]. The ultimate goal of ESD, nevertheless, is to attain curability which can be assessed by microscopic evaluation of resected specimens using a set of inclusion criteria (absolute and expanded) as stated above [9]. And the post-ESD clinical results are similar for all patients not exceeding the expanded criteria as could be indicated by many studies conducted in the Asian continent [24–28].
It is worthy to adopt an en-bloc means of resection for EGCs in order to obtain a specimen that has appropriate histologic details for reliable assessment and potential reduction in the emergence of recurrent lesions [3, 29]. The attainment of complete resection is declared when an en-bloc resected lesion lacks both margin positivity and lympho-vascular invasion. Following ESD procedure, patients who do not conform to the inclusion criteria are assumed to have non-e-curative (NEC) outcomes and may require further intervention depending on certain cicumstances such as fear of metastasis to lymph nodes as well as for having bad prognostic behavior [10, 13]. Nonetheless, the risk of lymph node metastasis in patients who received NEC resection treatment after undergoing gastrectomy was found to be less than 10% [3, 30–32]. In addition, there is a growing number of elderly patients with or without concomitant illnesses who are unfit for operation [33]. Hence, the need to identify tumor-associated factors which determine oncologic outcome in ESD is indispensable in order to have appropriate selection of candidates for subsequent surgical intervention. Of note, there is an ever-changing trend in both technical aspects as well as indications for patients with early gastric neoplasia in the setting of ESD. Thus, the goal of our study was to investigate the potential clinico-pathologic parameters that lead to NEC endoscopic resection following ESD. Moreover, we performed analysis of various tumor-associated factors to unveil their role in submucosal invasion in patients with EGC.
Methods
Inclusion and exclusion criteria
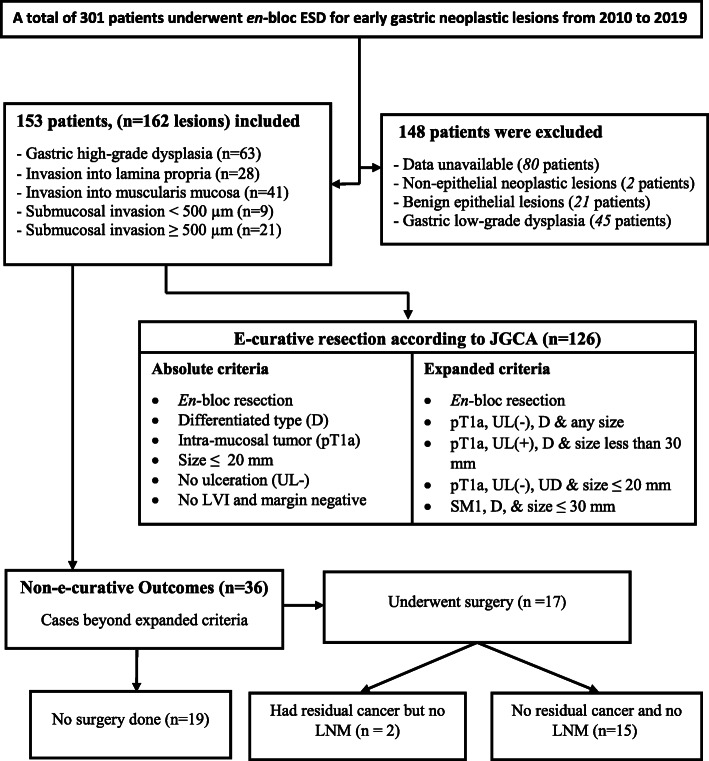
Figure 1, illustrates the general enrolment process of patients with early gastric neoplasia into our study. Accordingly, there were a total of 301 patients who received en-bloc ESD after fulfilling absolute or expanded indications. Briefly, we excluded 148 patients who had the following features: detailed data were unavailable (n=80), neoplastic lesions with non-epithelial origin (n=2), non-neoplastic epithelial lesions (n=21), and gastric low-grade dysplasia (n=45). Notably, we did not consider those patients who underwent endoscopic mucosal resection or piecemeal type of resection. After exclusion of the above-mentioned cases, a total of 162 early gastric neoplastic lesions (from 153 patients) were retained for subsequent descriptive, comparative and logistic regression analysis. In this study, we made analyses of risk factors based on individual neoplastic lesions as some of the study subjects had multiple tumors resected by en-bloc ESD.
Fig. 1.
Flowchart for patients with early gastric neoplasia who underwent ESD. ESD = Endoscopic submucosal Dissection, EGC = early gastric cancer, JGCA = Japanese Gastric Cancer Association, UD = Undifferentiated, U = Ulcer, SM1= Submucosal invasion < 500 μm LVI = Lympho-vascular invasion, UL= Ulceration, LNM = lymph node metastasis type
ESD procedure and specimen preparation
All enrolled patients underwent proper endoscopic evaluation, and ESD procedure was in turn performed by experienced endoscopist. The types of ESD knives and electrocautery used were DualKnife (KD-650 L; Olympus, Tokyo, Japan), ITknife2 (KD-611 L; Olympus), Coagrasper (FD-410LR; Olympus), and Gastroscope (GIF-Q260J; Olympus). The margin of resection was marked using electrocoagulation knife with inclusion of nearly 0.5 cm normal mucosal tissue around the lesion. A solution of normal saline was injected into the submucosa to elevate the lesion off the muscularis propria. The mucosa and submucosa on the outer edge of the lesion were incised circumferentially along the previously marked margin. Then, the submucosa was dissected until the mucosal lesion had been completely resected. Preventive coagulation was implemented for exposed blood vessels in the post-ESD resection defects. The measurements of the resected lesion and mucosal defects were recorded. The resected specimens were immediately stretched and pinned on a flat polystyrene board to prevent folding, and fixed in 10% formalin to facilitate optimal orientation during paraffin embedding. For histologic evaluation, the fixed specimens were serially sectioned at 2-mm intervals.
Study design and period
The present study is retrospective in design which involved patients diagnosed as early gastric epithelial neoplasia on whom ESD resection was consecutively performed at a tertiary hospital (Tongji Hospital, Wuhan, China). The study period ranges from January 2010 to October 2019.
Study setting
This study was conducted in the Institute of Pathology at Tongji Hospital, Tongji Medical College of Huazhong University of Science and Technology, Wuhan, China.
Data collection
The demographic information of patients including age and gender was obtained from previous medical records. Similarly, data related to endoscopic tumor characteristics such as macroscopic type, location, tumor size, and ulcerative findings were gleaned from previous records of endoscopic examination done by experts in the field. The processed tissue specimens in the form of slides were searched in an archive according to the identification number of each recruited patient in its respective shelf. After collecting all the slides, we arranged them serially in accordance with the corresponding year of ESD resection. After that, we began microscopic evaluation of all the slides and data pertaining to each parameter were recorded.
For difficult cases, proper consultation to senior experts was done to re-evaluate cases under multi-headed microscope to avoid discordance in the final histopathologic assessment. A new slide preparation was requested from reserved tissue blocks (formalin fixed paraffin embedded) as needed, especially for older slides in which case evaluation was difficult owing to poor slide quality. In such instances, a thin slice of specimen (4 μm thick) was prepared using a sectioning microtome, and routine hematoxylin and eosin staining was carried out followed by microscopic evaluation of slides. We made meticulous histopathologic evaluation of ESD-resected specimens with necessary discussions and consultations to minimize intra-observer bias.
Definitions and clinico-pathologic assessment
On the whole, 153 patients with early gastric neoplasia were recruited into our study, all of whom received an en-bloc type ESD based on the indications established by JGCA [3, 9]. However, subsequent histopathologic evaluation of all lesions was performed by adopting the JGCA guideline established in 2014 [9]. Accordingly, the lesions were grouped as e-curative and NEC based on their respective clinical and pathological characteristics. As per the JGCA [9] (Fig. 1), curative resection is declared for EGCs that conform to both absolute and expanded criteria after ESD procedure. Patients with absolute indication should have a differentiated morphology, size of two centimeters or below, and tumors have to be confined to the mucosal region. Additionally, curative resection is considered for patients with expanded indications that include: 1) differentiated intra-mucosal tumor of any size that has no ulceration, 2) ulcerated intra-mucosal cancer with differentiated histology having maximum diameter of ≤ 30 mm, 3) tumor size of ≤ 30 mm with submucosal invasion of < 500 μm as measured from the muscularis mucosa, 4) an intra-mucosal tumor of ≤ 20 mm diameter with undifferentiated histology and is devoid of ulcer. On top of satisfying the absolute and expanded indications, the type of resection has to be en-bloc, both horizontal and vertical margins negative, and lympho-vascular involvement should also be negative. The NEC group are those who do not fulfill the above-mentioned criteria.
Three intra-gastric tumor locations namely upper, middle and lower one-thirds were considered during our analysis. Tumors seen during endoscopic inspection were classified based on macroscopic features as: protruding (0-I), purely superficial elevated (0-IIa) or mixed type with predominant superficial elevated, purely superficial flat (0-IIb) or mixed with predominant superficial flat type and purely superficial depressed (0-IIc) or mixed with predominant superficial depressed types (see Fig. 2). There were no lesions belonging to excavated (0-III) macroscopic variant in this study. For the purpose of analysis, tumors were divided into two groups with respect to their macroscopic type. ‘Group 1’ constituted protruding (0-I), purely superficial flat (0-IIb) and mixed variants of superficial flat lesions, and ‘Group 2’ included purely superficial elevated or depressed lesions (0-IIa or IIc) and/or their corresponding mixed variants where the superficial elevated or depressed is a predominant feature. The maximum diameter of each lesion (in centimeter) was taken into account during microscopic visualization, and ulceration status was thoroughly evaluated with dichotomous result (absence vs presence). Diagnostic decision on ulcerative findings was dependent on histologic evaluation of a tissue but information from endoscopic examination was also incorporated to provide a final conclusion. Further evaluation of the remaining parameters depended on histopathological examination of the ESD-resected specimen. The degree of histologic tumor differentiation was divided as differentiated (D) type in which case a well or moderately differentiated tubular or papillary adenocarcinoma is identified. On the contrary, if a tumor showed poor differentiation or a signet ring cell type of carcinoma or mucinous type adenocarcinoma, it was regarded as having undifferentiated (UD) histology. If a single tumor lesion displays both histologic variants of differentiated and undifferentiated histology, the type that is quantitatively predominant would be considered.
Fig. 2.
Endoscopy-based macroscopic sub-classification of type ‘0’ early gastric lesions. A) Protruding [0-I], with ≥ 3 mm elevation. B) Superficial elevated [0-IIa], with < 3 mm elevation. C) Superficial flat lesions [0-IIb], tumors with no elevation or depression. D) Superficial depressed [0-IIc], tumors with slight depression. E) Excavated [0-III], tumors which are deeply depressed. F) Superficial elevated and depressed lesions with predominant elevated type [0-IIa + IIc]. G) Superficial elevated and depressed lesions with predominant superficial depressed type [0-IIc + IIa]. H) Superficial flat and depressed lesions with predominant superficial flat type [0-IIb + IIc].
The Lauren’s classification scheme was also used to classify EGCs as intestinal, diffuse and mixed. The extent of tumor invasion for the superficial gastric lesions was assessed by taking the muscularis mucosa as landmark (shown in Fig. 3). Hence, there were three groups of patients in relation to tumor invasion as follows: in one group, the tumor is confined to the mucosa (M), in the second group, invasion extends deep into the submucosa but hardly exceeds 500 μm as measured from the muscularis mucosa (SM1), and the last set included patients with greater or equal to 500 μm submucosal penetration from the muscularis mucosa (SM2). But during our analyses, we grouped patients into two as mucosal and submucosal (SM1 and SM2) with respect to the depth of tumor invasion as the number of lesions with submucosal invasion was low. In addition, we assessed the lymphatic and vascular invasion characteristics, where the expected outcome fell into either absent or present. Furthermore, we evaluated margin clearance status (both horizontal and vertical margins) with end results falling into two-tier class as positive or negative.
Fig. 3.
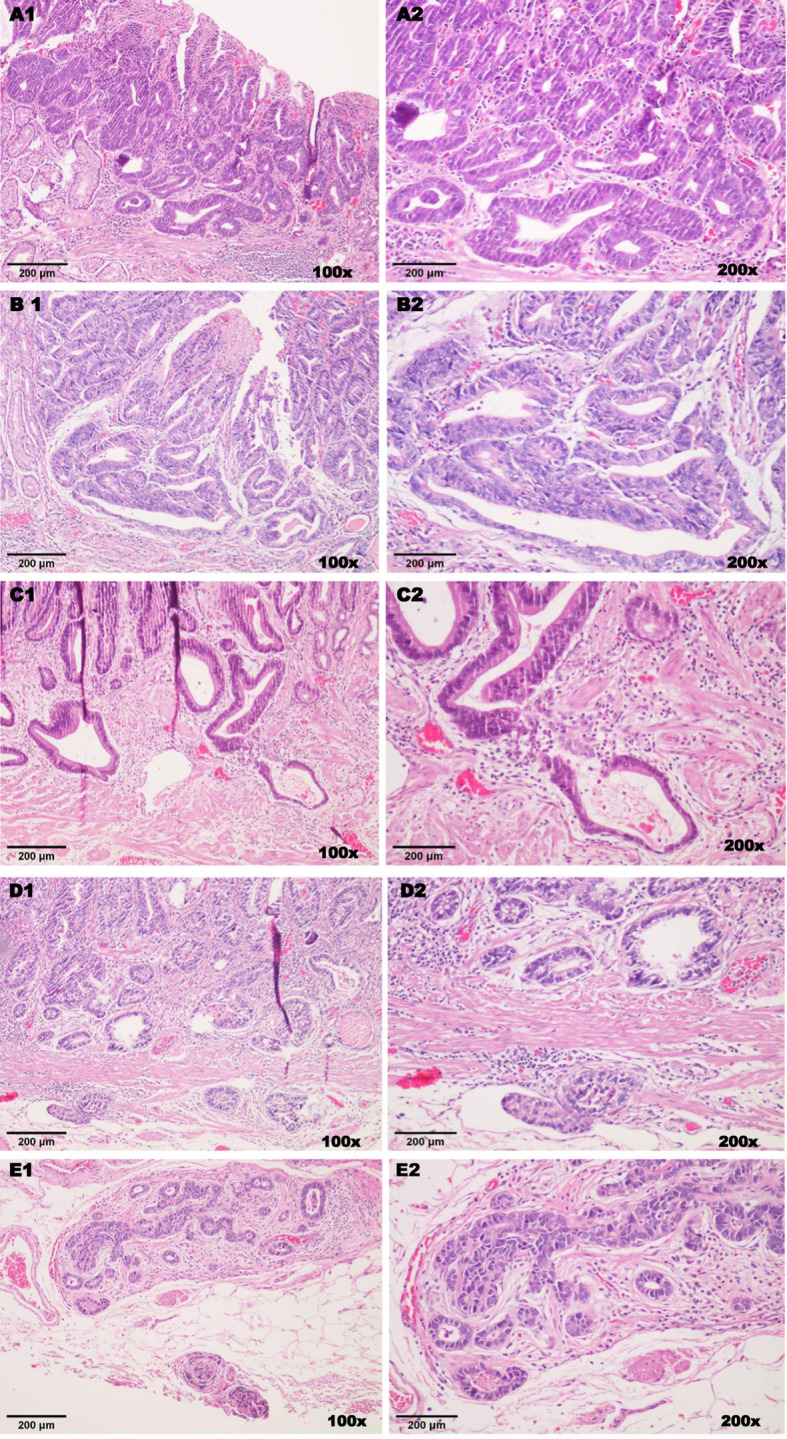
Different stages of early gastric neoplasia according to the depth of invasion (total magnification = 100x & 200x, scale = 100 & 200 μm). Hematoxylin and Eosin staining. (A1/A2) Gastric high grade dysplasia displaying complex glandular structure, significant cellular atypia, nuclear pseudo-stratification, hyperchromasia and poor nuclear polarization. (B1/B2) Cancer invading lamina propria. (C1/C2) Invasion involving the muscularis mucosae layer. (D1/D2) Gastric cancer that infiltrates into the submucosal layer but less than 500 μm depth from the muscularis mucosae, there is also associated lymphatic invasion. (E1/E2) Submucosal invasion greater than 500 μm from the muscularis mucosae with positive vertical margin
Statistical analysis
Relevant data was initially entered into an Excel Spreadsheet and then imported into Statistical Package for the Social Sciences version 23 for final analysis. While executing descriptive analysis, continuous variables were calculated as mean ± standard deviation, and categorical variables were expressed using frequency and percentages. A Chi-squared test or Fisher’s exact probability test (as appropriate) and student’s t-test were used to investigate possible differences among categorical and continuous variables, respectively. In an attempt to investigate whether tumor-related factors had predictive effect on curability and depth of tumor invasion, a logistic regression model was applied. To that end, each variable was first tested using univariate analysis. Subsequently, all the variables that showed significance in the univariate analysis were retained for multivariate analysis in order to sort out the factors that were independently associated with NEC resection and submucosal infiltration after ESD. Two-tailed p < 0.05 was considered statistically significant. Calculation of both odds ratios (OR) and the 95% confidence intervals (95% CI) were made in order to estimate the relative risk of NEC outcomes and depth of submucosal invasion following ESD resection as well as to measure the degree of association among different clinico-pathologic factors.
Results
Baseline characteristics of study patients following ESD procedure
A total of 162 early gastric neoplastic lesions (153 patients) received an en-bloc type of ESD resection. Table 1 provides a summarized baseline information of recruited patients including frequency distribution and their corresponding clinical parameters. The rate of completeness of resection and NEC outcome were 95% and 22.2%, respectively. Three fourth of the participants (74.1%) were males and the mean age (± standard deviation) of the study population was 61.10 ± 8.76 years.
Table 1.
Overall frequency and percentage distribution of patients with early gastric neoplasia after ESD procedure
| Clinico-pathologic parameter | Total number of EGC lesions (n=162) | Percentage |
|---|---|---|
| Mean Age in years (±SD) | 61.10 ± 8.76 | – |
| Gender | ||
| Female | 42 | 25.9% |
| Male | 120 | 74.1% |
| Tumor location | ||
| Lower one third | 95 | 58.6% |
| Middle or Upper third | 67 | 41.4% |
| Gross tumor type | ||
| Group 1 | 46 | 28.4% |
| Group 2 | 116 | 71.6% |
| Ulceration status | ||
| Absent | 135 | 83.% |
| Present | 27 | 16.7% |
| Size of tumor | ||
| ≤ 2 cm | 111 | 68.5% |
| > 2 cm | 51 | 31.5% |
| Differentiation | ||
| Differentiated | 129 | 79.6% |
| Undifferentiated | 33 | 20.4% |
| Lauren’s category | ||
| Intestinal | 129 | 79.6% |
| Diffuse/Mixed | 33 | 20.4% |
| Level of tumor invasion | ||
| Intra-mucosal tumor | 132 | 81.5% |
| Submucosal tumor | 30 | 18.5% |
| Horizontal margin | ||
| Negative | 159 | 98.1% |
| Positive | 3 | 1.9% |
| Vertical margin | ||
| Negative | 158 | 97.5% |
| Positive | 4 | 2.5% |
| Lympho-vascular invasion | ||
| Absent | 159 | 98.1% |
| Present | 3 | 1.9% |
| Resection type (en-bloc) | 162 | 100% |
| Curability outcome (non-e-curative) | 36 | 22.2% |
| Complete resection | 154 | 95% |
ESD Endoscopic Submucosal Dissection, EGC Early Gastric Cancer, SD Standard Deviation, Group 1 = lesions with Protruding (0-I), Purely superficial flat (0-IIb) and Mixed variants with 0-IIb predominant, Group2 = Purely superficial elevated (0-IIa) or depressed (0-IIc) or mixed variants with either 0-IIa or 0-IIc predominant
Majority of the neoplastic lesions (58.6%) were located in the lower one third of the stomach and 71.6% of all the tumors had a macroscopic type belonging to the group with purely superficial elevated or depressed lesions (0-IIa or 0-IIc) and/or their corresponding mixed variants where the superficial elevated or depressed is a predominant feature (Group 2). All other macroscopic lesions such as protruding (0-I), purely superficial flat (0-IIb) and mixed variants of superficial flat constituted for 28.4% (Group 1). A large proportion of gastric lesions had a size ≤ 2 cm (68.5%, 111 lesions), absence of ulceration (83.0%, 135 lesions), and differentiated type of histology (79.6%, 129 lesions). According to Lauren’s classification of gastric tumors, a total of 129 lesions (79.6%) displayed an intestinal-type morphology whereas nearly 20% were non-intestinal (diffuse/mixed histology). In terms of depth of tumor invasion, there were higher number of intra-mucosal lesions as compared to the submucosa, 81.5% and 18.5%, respectively. The horizontal margin was positive in three lesions (1.9%), and four lesions (2.5%) had positive vertical margin. Positive lympho-vascular invasion was identified in three lesions (1.9%).
The baseline characteristics of patients with regard to depth of tumor invasion (mucosal vs submucosal) are shown in Table 4. Out of 162 lesions, 18.5% (30 lesions) showed invasion into the submucosal layer. Considering tumor location and macroscopic endoscopic features, tumors in the upper two third of the stomach and those having 0-IIa/0-IIc or their mixed types with 0-IIa/0-IIc predominant had submucosal invasion of 80% and 90%, correspondingly. In terms of histologic differentiation, the number of cases in both differentiated and undifferentiated groups exhibiting submucosal invasion were equal (50% each).
Table 4.
Baseline characteristics of patients with EGC and their difference in terms of depth of invasion following ESD procedure
| Patient Parameters | All EGCs lesions (n = 162) [No. (%)] |
Mucosal lesion (n = 132) [No. (%)] |
Submucosal (n = 30) [No. (%)] |
p*-value |
|---|---|---|---|---|
| Mean age in years (±SD) | 61.10 (± 8.76) | 60.33 (± 8.38) | 64.53 (± 9.70) | 0.563 |
| Gender | 0.981 | |||
| Female | 42 (25.9) | 34 (25.8) | 8 (26.7) | |
| Male | 120 (74.1) | 98 (74.2) | 22 (73.3) | |
| Tumor location | < 0.001 | |||
| Lower third | 95 (58.6) | 89 (67.4) | 6 (20.0) | |
| Middle or Upper third | 67 (41.4) | 43 (32.6) | 24 (80.0) | |
| Gross tumor type | 0.013 | |||
| Group 1 | 46 (28.4) | 43 (32.6) | 3 (10.0) | |
| Group 2 | 116 (71.6) | 89 (67.4) | 27 (90.0) | |
| Ulceration status | 0.001 | |||
| Absent | 135 (83.3) | 116 (87.9) | 19 (63.3) | |
| Present | 27 (16.7) | 16 (12.1) | 11 (36.7) | |
| Size of tumor in | 0.001 | |||
| ≤2 cm | 111 (68.5) | 98 (74.2) | 13 (43.3) | |
| > 2 cm | 51 (31.5) | 34 (25.8) | 17 (56.7) | |
| Differentiation | < 0.001 | |||
| Differentiated | 129 (79.6) | 114 (86.4) | 15 (50.0) | |
| Undifferentiated | 33 (20.4) | 18 (13.6) | 15 (50.0) | |
| Horizontal margin | 0.088 | |||
| Negative | 159 (98.1) | 131 (99.2) | 28 (91.7) | |
| Positive | 3 (1.9) | 1 (0.8) | 2 (8.3) | |
| Vertical margin | 0.001 | |||
| Negative | 158 (97.5) | 132 (100) | 26 (86.7) | |
| Positive | 4 (2.5) | 0 (0.0) | 4 (13.3) | |
| Lympho-vascular invasion | < 0.001 | |||
| Absent | 159 (98.1) | 132 (100) | 27 (90.0) | |
| Present | 3 (1.9) | 0 (0.0) | 3 (10.0) |
ESD Endoscopic Submucosal Dissection, EGC Early Gastric Cancers, Group 1 = lesions with Protruding (0-I), Purely superficial flat (0-IIb) and Mixed variants with 0-IIb predominant, Group 2 = Purely superficial elevated (0-IIa) or depressed (0-IIc) or mixed variants with either 0-IIa or 0-IIc predominant
*Chi-squared independent test/Fisher’s exact test of probability or Student t-test
Factors that contributed to NEC resection after ESD procedure
Table 2 summarizes the demographic and clinico-pathologic factors of patients with early gastric neoplasia in relation to the post-ESD curability outcome. Accordingly, all patients were divided into two groups (e-curative vs non-e-curative). Overall, there were 126 early gastric lesions (77.8%) that received curative ESD resection whereas 36 lesions had a NEC resection (22.2%). We tried to compare e-curative and NEC groups in terms of their demographic and clinico-pathologic features using a Chi-squared independent test or Fisher’s exact test and student t-test applied as appropriate. Hence, there was a significant difference in curability outcome among tumor-associated factors such as tumor location, differentiation, size of tumor, and depth of tumor invasion (all with p < 0.001). Likewise, a significant difference was found between these groups of patients with regard to macroscopic tumor type (p = 0.002), ulcerative finding (p = 0.011), horizontal margin (p = 0.010), vertical margin (p = 0.002) and lympho-vascular invasion (p = 0.010) following endoscopic resection. On the other hand, no significant difference was observed with respect to gender of patients (p = 0.472) and the difference in age was only marginally significant (p = 0.046).
Table 2.
Baseline characteristics of patients and their difference in curability outcomes following ESD
| Patient Parameter | All EGCs lesions (n = 162) [No. (%)] |
E-curative (n = 126) [No. (%)] |
Non-e-curative (n = 36) [No. (%)] |
p*-value |
|---|---|---|---|---|
| Mean age in years (±SD) | 61.10 (± 8.76) | 60.37 (± 8.27) | 63.67 (± 10.0) | 0.046 |
| Gender | 0.472 | |||
| Female | 42 (25.9) | 31 (24.6) | 11 (30.6) | |
| Male | 120 (74.1) | 95 (75.4) | 25 (69.4) | |
| Tumor location | < 0.001 | |||
| Lower third | 95 (58.6) | 84 (66.7) | 11 (30.6) | |
| Middle or Upper third | 67 (41.4) | 42 (33.3) | 25 (69.4) | |
| Gross tumor type | 0.002 | |||
| Group 1 | 46 (28.4) | 43 (34.1) | 3 (8.3) | |
| Group 2 | 116 (71.6) | 83 (65.9) | 33 (91.7) | |
| Ulceration status | 0.011 | |||
| Absent | 135 (83.3) | 110 (87.3) | 25 (69.4) | |
| Present | 27 (16.7) | 16 (12.7) | 11 (30.6) | |
| Size of tumor | < 0.001 | |||
| ≤ 2 cm | 111 (68.5) | 98 (77.8) | 13 (36.1) | |
| > 2 cm | 51 (31.5) | 28 (22.2) | 23 (63.9) | |
| Differentiation | < 0.001 | |||
| Differentiated | 129 (79.6) | 115 (91.3) | 14 (38.9) | |
| Undifferentiated | 33 (20.4) | 12 (9.5) | 21 (60.0) | |
| Lauren’s category | < 0.001 | |||
| Intestinal | 129 (79.6) | 115 (91.3) | 14 (38.9) | |
| Diffuse/ Mixed | 33 (20.4) | 11 (8.7) | 22 (61.1) | |
| Level of tumor invasion | < 0.001 | |||
| Intra-mucosal tumor | 132 (81.5) | 124 (98.4) | 8 (22.2) | |
| Submucosal tumor | 30 (18.5) | 2 (1.6) | 28 (77.8) | |
| Horizontal margin | 0.010 | |||
| Negative | 159 (98.1) | 126 (100) | 33 (91.7) | |
| Positive | 3 (1.9) | 0 (0.0) | 3 (8.3) | |
| Vertical margin | 0.002 | |||
| Negative | 158 (97.5) | 126 (100) | 32 (88.9) | |
| Positive | 4 (2.5) | 0 (0.0) | 4 (11.1) | |
| Lympho-vascular invasion | 0.010 | |||
| Absent | 159 (98.1) | 126 (100) | 33 (91.7) | |
| Present | 3 (1.9) | 0 (0.0) | 3 (8.3) |
ESD Endoscopic Submucosal Dissection, EGC Early Gastric Cancers, SD Standard Deviation, Group 1 = lesions with Protruding (0-I), Purely superficial flat (0-IIb) and Mixed variants with 0-IIb predominant, Group2 = Purely superficial elevated (0-IIa) or depressed (0-IIc) or mixed variants with either 0-IIa or 0-IIc predominant.
*Chi-squared independent test/Fisher’s exact test of probability or Student t-test as appropriate
The predictive impact of all clinico-pathologic factors on patients with early gastric cancer following ESD procedure was investigated using logistic regression analysis as depicted in Table 3.
Table 3.
Factors that contributed to non-e-curative outcome in patients with early gastric cancers after ESD
| Clinico-pathologic parameters | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Age (in years) | 1.05 (1.00–1.09) | 0.049 | 1.08 (1.01–1.17) | 0.029 |
| Tumor location | ||||
| Lower one third | 1 (Reference) | 1 (Reference) | ||
| Middle or Upper third | 4.55 (2.04–10.12) | < 0.001 | 5.46 (1.65–18.12) | 0.006 |
| Gross tumor type | ||||
| Group 1 | 1 (Reference) | 1 (Reference) | ||
| Group 2 | 5.70 (1.65–19.65) | 0.006 | 9.77 (1.23–77.65) | 0.031 |
| Ulceration status | ||||
| Absent | 1 (Reference) | 1 (Reference) | ||
| Present | 3.03 (1.25–7.31) | 0.014 | 2.39 (0.59–9.65) | 0.223 |
| Size of tumor | ||||
| ≤ 2 cm | 1 (Reference) | 1 (Reference) | ||
| > 2 cm | 6.19 (2.79–13.77) | < 0.001 | 7.63 (2.29–25.42) | 0.001 |
| Differentiation | ||||
| Differentiated | 1 (Reference) | 1 (Reference) | ||
| Undifferentiated | 16.43 (6.60–40.89) | < 0.001 | 15.54 (1.65–146.22) | 0.016 |
| Lauren’s category | ||||
| Intestinal | 1 (Reference) | 1 (Reference) | ||
| Non-intestinal | 16.43 (6.60–40.89) | < 0.001 | 3.29 (0.36–31.40) | 0.300 |
ESD Endoscopic Submucosal Dissection, OR Odds Ratio, CI Confidence Interval, Group 1 = lesions with Protruding (0-I), Purely superficial flat (0-IIb) and Mixed variants with 0-IIb predominant, Group2 = Purely superficial elevated (0-IIa) or depressed (0-IIc) or mixed variants with either 0-IIa or 0-IIc predominant
Initially, the role of each tumor-associated variable was examined separately for curability outcome in a univariate analysis. As a result, significant effect on non-e-curability was obtained in association with tumors located in the upper two third of the stomach, larger tumor size (> 2 cm), undifferentiated histology and tumors that belonged to diffuse or mixed by Lauren’s classification (all with p < 0.001). In addition, univariate analysis revealed significant associations with NEC resection when lesions: were ulcerated (p = 0.014), had purely superficial elevated or depressed or their mixed counterparts (p = 0.006), and when patients had advanced age (p = 0.049).
Finally, a multivariate logistic regression analysis was carried out (Table 3) in which all the clinico-pathologic factors were considered together in order to further investigate factors that independently predict a NEC outcome. Thus, tumor location in the upper two third of the stomach (odds ratio [OR], 5.46; 95% confidence interval [95% CI], 1.65–18.12; p = 0.006), tumor size greater than 2 cm (OR, 7.63; 95% CI, 2.29–25.42; p = 0.001), microscopically undifferentiated tumor (OR, 15.54; 95% CI, 1.65–146.22; p = 0.001), and old age (OR, 1.08; 95% CI, 1.01–1.17; p = 0.029) were found to have independent predictive influence on NEC resection of early gastric tumors.
In a similar fashion, purely superficial elevated or depressed or mixed gross type lesions with either elevated or depressed component predominating, independently contributed to a NEC outcome (OR, 9.77; 95% CI, 1.23–77.65; p = 0.031). The presence of ulceration (OR, 2.39; 95% CI, 0.59–9.65; p = 0.223) and non-intestinal (diffuse or mixed) tumors by Lauren’s classification (OR, 3.29; 95% CI, 0.36–31.40; p = 0.300) did not show any significant contribution to no-curability after multivariate analysis.
Factors associated with submucosal invasion in patients with EGC after ESD
Herein, we conducted both comparative and multiple logistic regression analysis of clinico-pathologic factors with respect to the depth of invasion as illustrated in Table 4 and Table 5, respectively. A remarkably significant difference was obtained in tumors located in the upper two third vs lower third of the stomach (p < 0.001), macroscopic type of 0-IIa/0-IIc or their mixed types with 0-IIa/0-IIc predominant gross type vs 0-I/0-IIb and mixed with 0-IIb dominant (p = 0.013), lesions with ulcerative finding vs non-ulcerated (p = 0.001), size ≥ 2 cm vs size < 2 cm (p = 0.001), and undifferentiated vs differentiated histology (p < 0.001).
Table 5.
Risk factors for submucosal invasion in patients with early gastric cancer following Endoscopic Submucosal Dissection
| Clinico-pathologic parameters | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Tumor location | ||||
| Lower one third | 1 (Reference) | 1 (Reference) | ||
| Middle or Upper third | 8.28 (3.15–21.75) | < 0.001 | 8.88 (2.90–27.17) | < 0.001 |
| Gross tumor type | ||||
| Group 1 | 1 (Reference) | 1 (Reference) | ||
| Group 2 | 4.35 (1.25–15.13) | 0.021 | 3.59 (0.84–15.45) | 0.086 |
| Ulceration status | ||||
| Absent | 1 (Reference) | 1 (Reference) | ||
| Present | 4.20 (1.69–10.41) | 0.002 | 3.70 (1.15–11.90) | 0.028 |
| Size of tumor | ||||
| ≤ 2 cm | 1 (Reference) | 1 (Reference) | ||
| > 2 cm | 3.77 (1.66–8.57) | 0.002 | 2.94 (1.08–8.02) | 0.036 |
| Differentiation | ||||
| Differentiated | 1 (Reference) | 1 (Reference) | ||
| Undifferentiated | 6.33 (2.65–15.14) | < 0.001 | 6.51 (2.23–18.98) | 0.001 |
OR Odds Ratio, CI Confidence Interval, Group 1 = lesions with Protruding (0-I), Purely superficial flat (0-IIb) and Mixed variants with 0-IIb predominant, Group2 = Purely superficial elevated (0-IIa) or depressed (0-IIc) or mixed variants with either 0-IIa or 0-IIc predominant
Later on, univariate and multivariate logistic regression analyses were carried out on the factors where a statistically significant difference was observed (Table 5). In the univariate analysis, submucosal infiltration was significantly related with tumors of upper two third location, gross type 0-IIa/0-IIc or their mixed types with 0-IIa/0-IIc predominant, ulcerated lesions, lesions with ≥ 2 cm size and undifferentiated histology (all with p < 0.05). Multivariate logistic regression analysis revealed that the following tumor-related factors were independent predictors of submucosal invasion: location in the upper two third of the stomach (OR, 8.88; 95% CI, 2.90–27.17; p < 0.001), ulcerated lesions (OR, 3.70; 95% CI, 1.15–11.90; p = 0.028), lesions with greater than 2 cm (OR, 2.94; 95% CI, 1.08–8.02; p = 0.036) and those with poor differentiation (OR, 6.51; 95% CI, 2.23–18.98; p = 0.001).
Results of gastrectomy specimens for patients who had NEC outcome following ESD
Parallel to evaluation of ESD resected specimens, we looked at some of the gastrectomy specimens together with their lymph node dissections for patients who had a NEC with an attempt to determine the presence of residual tumor or/and lymph node metastasis (Fig. 1). Hence, out of the total 36 lesions which fulfilled the criteria for NEC outcome, 17 patients received additional surgical treatment. Fifteen of those patients who received additional surgery showed evidence of mucosal defect with chronic ulcerative and inflammatory changes or/and granulation tissue. But no cancerous cells were detected in the resected lymph nodes and surrounding tissues. Residual cancer was found in two patients who underwent surgery but none of them exhibited metastatic disease in the dissected lymph nodes.
Discussion
A timely detection of early gastric cancers and appropriate selection of their means of treatment is very crucial as it results in favorable prognostication and reduced mortalities [11]. A remarkably high rate (95%) of 5-year survival has been reported in patients with early gastric cancer who underwent ESD fulfilling the indications for expanded criteria [34, 35]. Despite the curative intent of ESD procedure, there is an inevitable NEC resection in about 11.9%–21.4% of EGC patients who might require additional surgical treatment depending on their overall situation [36–41]. Unlike those tumor-associated factors such as location, size and macroscopic type, it is difficult to accurately predict the depth of tumor invasion and lympho-vascular involvement using endoscopy-based pre-ESD evaluations [42]. Recent studies indicated that conventional endoscopic methods had about 72%–78% accuracy in predicting the level of tumor infiltration in EGCs. Likewise, a similar precision rate (67% to 85%) in extent of invasion has been reported for ultra-sonographic endoscopy, which is a common method of evaluation in several institutions [17–19].
Whatsoever the case, appropriate selection of candidates for subsequent additional surgery is essential in the setting of non-curability following ESD resection. This is in order to obviate the possibility of local recurrence and metastasis to lymph nodes that are potentially associated with NEC endoscopic resection. Additionally, it helps to eliminate unnecessary ESD procedure on patients if they finally have to end up doing surgery following NEC outcome. The present study indicated that the rate of complete resection and non-curability were 95% and 22.2%, respectively, which is in harmony with several previous reports [36–41]. Of note, our study identified 36 patients who had NEC resection because their conditions went beyond the absolute and expanded criteria following en-bloc ESD (Fig. 1). Other factors such as involvement of lympho-vascular invasion and positive margin status (horizontal or/and vertical margins) were the reasons for non-curative resection in these patients.
The utilization of ESD for management of early gastric lesions has attained tremendous popularity and the need for expansion of the indication criteria for resection is also increasing. Therefore, sufficient and reliable data on factors that are associated with non-curability following endoscopy-based gastric tumor resection is vital for better clinical outcomes. In terms of respectability, the en-bloc means of resection provides a better curative outcome as compared to a piecemeal method [43]. Therefore, in this particular study, we aimed to investigate the demographic and tumor-associated parameters that predict submucosal infiltration and a NEC outcome in patients with early gastric neoplasia who received en-bloc ESD resection.
Overall, the results of our multivariate analysis indicated that the following factors were independently associated with both submucosal invasion and NEC outcomes: 1) upper tumor location (upper two third of the stomach), 2) larger tumor size (> 2 cm), and 3) tumors with undifferentiated histologic pattern. Tumors with purely superficial elevated (0-IIa) or depressed (0-IIc) or mixed gross type with predominant superficial elevated or depressed component (mixed with 0-IIa or 0-IIc dominant) and advanced age were also identified as independent predictors of non-e-curability. In addition to that, ulcerated lesions showed significant association with submucosal infiltration.
Many studies have shown that upper tumor location, enlarged tumor size and undifferentiated microscopic feature independently predict for NEC outcomes, which is consistent with the present study [39, 40, 44–46]. Others reported that tumors located in the upper two third of stomach had higher rate of submucosal invasion and hence a greater frequency of NEC resection as opposed to tumors of the lower third (antrum), which is in line with our study [47, 48]. Similarly, it was clearly described that proximally located gastric tumors behave more aggressively and have a worse prognosis compared to distal counterparts [48–53]. EGCs located in the middle and upper third are not detected as early, owing to the technical difficulty faced during endoscopic evaluation and thus compromising diagnostic yield from forceps biopsy. This is because maintaining a front view of endoscopy is challenging and it has to be used in its retroflexed fashion [54]. Additionally, there is a difference in thickness of submucosal layer between body and antrum, with the former being thinner as compared to the latter [55]. Last but not least, relatively abundant lymphatic vessels are found in the lamina propria of cardia region as opposed to lower regions of the stomach [56]. By combining all these evidences, EGCs situated in the upper portion of the stomach are likely to result in NEC resection and hence, special caution is needed while dealing with tumors in this location.
The real impact of tumor size on ESD outcome has been debated by many researchers. One large-scale study [24] indicated that the size of tumor has nothing to do with curability outcome after ESD. On the contrary, the present study has shown a significant contribution of larger tumor size (> 2 cm) to NEC outcome in a multivariate analysis. In line with our study, Imagawa et al. reported a significant difference (p < 0.0001) in curability outcome between lesions of ≤ 2 cm and > 2 cm, 59% vs 89%, respectively. Lee et al. indicated that there was a significant correlation between depth of tumor invasion and its size (p < 0.001). As a rule of thumb, the bigger the lesion is, the more extensive the vascular bed will be, and excess bleeding is anticipated that might interfere with ESD procedure. Moreover, data on a large series of cases [10] demonstrated that tumors with larger than 3 cm are significantly correlated with an increased risk of metastasis to lymph nodes. For the aforementioned reasons, the post-ESD outcome is likely to be influenced by large size EGCs.
Our study demonstrated that EGCs with undifferentiated histology are significantly associated with NEC outcome in a multivariate analysis. The morphologic patterns of both differentiated and undifferentiated gastric adenocarcinoma are somewhat dissimilar. According to several recent studies, the rate of curative resection in EGCs was low with tumors exhibiting undifferentiated microscopic pattern as opposed to differentiated tumors [34, 57–59]. Another peculiar behavior of gastric adenocarcinoma displaying undifferentiated histology (mainly the signet-ring cell carcinoma) is the tendency to extend along the sub-epithelial plane [60]. Hence, a wider safety margin of the surrounding mucosa has to be considered for tumors with poor differentiation.
Regarding macroscopic type of tumors, majority of published data consider the three-tier grouping system of Paris classification: elevated, flat and depressed gross types [61]. But in reality, lesions with mixed endoscopic appearances are commonly seen in clinical practice as shown in Fig. 2. The Japanese classification system [3] provides a better and wider macroscopic description for early gastric neoplasia that includes all pure and mixed variants in their respective category (0-I, 0-II [a,b,c], 0-III and mixed with any one of the types predominating).
In our study, we adopted the Japanese guidelines for macroscopic classification of the EGCs to investigate their association with non-curability after ESD. In concordance with a study done by Ohara et al. [62], our multivariate analysis depicted that EGCs with purely superficial elevated (0-IIa) or depressed (0-IIc) or their mixed gross types with predominant elevated or depressed component (Mixed with 0-IIa or 0-IIc dominant) were significantly associated with NEC resection. In a univariate analysis, Abe et al. [63] recently reported that superficial depressed and/or elevated lesions were found to be indicative of submucosal invasion ≥ 500 μm, even though, their finding was lacking significance in a multivariate regression analysis. Similarly, Yamada et al. found that EGCs with superficial elevated and depressed endoscopic features were greatly linked with submucosal as well as invasion of lympho-vascular structures [64]. The above-mentioned studies are consistent with our results in that superficial elevated or depressed macroscopic types have a predilection to invade the submucosal layer and hence leading to non-curability after endoscopic resection.
It is well known that metastasis to lymph nodes is a very critical condition in determining curability following resection of EGCs. Several previous researchers had also confirmed that EGCs invading deep into the submucosal layer and lympho-vascular structures are independently associated with metastasis to lymph nodes [20, 65–68]. Thus, taking all these ideas together, we can infer that EGCs with superficial elevated or depressed gross type are likely to have metastasis to lymph nodes. And great care is needed while encountering EGCs with purely superficial elevated (0-IIa) or depressed (0-IIc) or their mixed gross types with predominant elevated or depressed component (mixed with 0-IIa or 0-IIc dominant).
In the present study, presence of ulcerative finding was associated with NEC resection in a univariate analysis and it was found to be an independent predictor of submucosal infiltration in patients with EGCs after ESD as shown in multivariate analysis (Table 5). In a multivariate analysis, Ohnita et al. [44] reported that absence of ulceration significantly predicted curative outcomes following ESD (p = 0.002). In a similar way, other studies demonstrated the interference of ulcer in curability of endoscopically resected lesions [69, 70]. It has been reported that presence of ulceration, mainly in those with greater than 2 cm size, caused technical difficulty during removal procedure leading to incomplete resection of EGCs [71]. Hence, the procedure of ESD might not be smooth in the setting of ulceration, thus, hindering proper dissection along the submucosal plane resulting in NEC resection.
Our study is not without shortcomings. Firstly, it was a retrospective study and thus a large-scale multi-center prospective cohort data is needed for external validation. Secondly, the number of enrolled subjects was relatively small, especially those cases who had ulceration, positive margins and positive lympho-vascular structures. Lastly, data related to patients’ follow up and lymph node status were unavailable. Consequently, we could not deal with post-ESD tumor recurrences and disease survival conditions.
Conclusions
We demonstrated that certain tumor-associated factors such as location in upper two third of the stomach, larger tumor size (> 2 cm) and undifferentiated histology are significantly associated with submucosal invasion and NEC outcomes. Therefore, great care is needed while handling EGCs with these clinico-pathologic features. There is very little description in the literature regarding endoscopic macroscopic feature of tumors and their role in curability after ESD by using the JGCA classification. In this study, we discovered that tumors with macroscopic type of purely superficial elevated (0-IIa) or depressed (0-IIc) or mixed gross type with predominant 0-IIa or 0-IIc are highly linked with non-e-curability. Therefore, the findings of our study may shed light on the consideration of certain clinico-pathologic features such as location and macroscopic types in the indication criteria for ESD.
Acknowledgements
We thank Professor Mei Liu for providing the patients’ clinical information.
Abbreviations
- CI
Confidence interval
- D
Differentiated
- EGC
Early gastric cancer
- EMR
Endoscopic mucosal resection
- ESD
Endoscopic submucosal dissection
- JGCA
Japanese gastric cancer association
- M
Mucosal
- NEC
Non-e-curative
- OR
Odds ratio
- SD
Standard deviation
- SM
Submucosal
- T1a
Intra-mucosal tumor
- T1b
Submucosal tumor
- U
Ulcer
- UD
Undifferentiated
Authors’ contributions
KSE, CZ and XW conceived and designed the study. KSE performed the microscopic evaluation, analyzed data, interpreted results, and drafted the manuscript. CZ performed the microscopic evaluation, provided the HE images and revised the manuscript. MAG, ZW, FZ, LL, SQ, LQ and JW participated in the acquisition, analysis and interpretation of data. All authors reviewed the manuscript and approved the submitted version.
Funding
This research was supported by the National Natural Science Foundation of China (81700012 and 81600015) in the collection, analysis, and interpretation of data and in writing the manuscript.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
This study was approved by the Institutional Review Board (Medical Ethics Committee of Tongji Hospital) of Tongji Medical College, Huazhong University of Science and Technology. No specific IRB reference number is available. There were no administrative barriers, and basic statistical data related to this work were obtained from the hospital’s registry as part of patients’ care and support. Because of the retrospective nature of the study, and all the direct personal identifiers were anonymously kept, the need to obtain informed consent was waived by the ethics committee.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflicts of interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Kidane Siele Embaye and Chao Zhang contributed equally to this work.
References
- 1.Bray F, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Leung WK, et al. Screening for gastric cancer in Asia: current evidence and practice. Lancet Oncol. 2008;9(3):279–287. doi: 10.1016/S1470-2045(08)70072-X. [DOI] [PubMed] [Google Scholar]
- 3.Japanese Gastric Cancer, A Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14(2):101–112. doi: 10.1007/s10120-011-0041-5. [DOI] [PubMed] [Google Scholar]
- 4.Schlemper RJ, Hirata I, Dixon MF. The macroscopic classification of early neoplasia of the digestive tract. Endoscopy. 2002;34(2):163–168. doi: 10.1055/s-2002-19855. [DOI] [PubMed] [Google Scholar]
- 5.Yada T, Yokoi C, Uemura N. The current state of diagnosis and treatment for early gastric cancer. Diagn Ther Endosc. 2013;2013:241320. doi: 10.1155/2013/241320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gotoda T, et al. Gastric ESD: current status and future directions of devices and training. Gastrointest Endosc Clin N Am. 2014;24(2):213–233. doi: 10.1016/j.giec.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 7.Takizawa K, Ono H, Muto M. Current indications of endoscopic submucosal dissection for early gastric cancer in Japan. Jpn J Clin Oncol. 2019;49(9):797–802. doi: 10.1093/jjco/hyz100. [DOI] [PubMed] [Google Scholar]
- 8.Ono H, et al. Guidelines for endoscopic submucosal dissection and endoscopic mucosal resection for early gastric cancer. Dig Endosc. 2016;28(1):3–15. doi: 10.1111/den.12518. [DOI] [PubMed] [Google Scholar]
- 9.Association, J.G.C Japanese gastric cancer treatment guidelines 2014 (ver. 4) Gastric Cancer. 2017;20(1):1–19. doi: 10.1007/s10120-016-0622-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gotoda T, et al. Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer. 2000;3(4):219–225. doi: 10.1007/PL00011720. [DOI] [PubMed] [Google Scholar]
- 11.Soetikno R, et al. Endoscopic mucosal resection for early cancers of the upper gastrointestinal tract. J Clin Oncol. 2005;23(20):4490–4498. doi: 10.1200/JCO.2005.19.935. [DOI] [PubMed] [Google Scholar]
- 12.Murakami T. Early cancer of the stomach. World J Surg. 1979;3(6):685–692. doi: 10.1007/BF01654788. [DOI] [PubMed] [Google Scholar]
- 13.Gotoda T. Endoscopic resection of early gastric cancer: the Japanese perspective. Curr Opin Gastroenterol. 2006;22(5):561–569. doi: 10.1097/01.mog.0000239873.06243.00. [DOI] [PubMed] [Google Scholar]
- 14.Kitaoka H, et al. Surgical treatment of early gastric cancer. Jpn J Clin Oncol. 1984;14(2):283–293. [PubMed] [Google Scholar]
- 15.Yokota T, et al. Significant prognostic factors in patients with early gastric cancer. Int Surg. 2000;85(4):286–290. [PubMed] [Google Scholar]
- 16.Coburn NG, et al. Significant regional variation in adequacy of lymph node assessment and survival in gastric cancer. Cancer. 2006;107(9):2143–2151. doi: 10.1002/cncr.22229. [DOI] [PubMed] [Google Scholar]
- 17.Choi J, et al. Comparison of endoscopic ultrasonography and conventional endoscopy for prediction of depth of tumor invasion in early gastric cancer. Endoscopy. 2010;42(9):705–713. doi: 10.1055/s-0030-1255617. [DOI] [PubMed] [Google Scholar]
- 18.Choi J, et al. Endoscopic prediction of tumor invasion depth in early gastric cancer. Gastrointest Endosc. 2011;73(5):917–927. doi: 10.1016/j.gie.2010.11.053. [DOI] [PubMed] [Google Scholar]
- 19.Tsujii Y, et al. Integrated diagnostic strategy for the invasion depth of early gastric cancer by conventional endoscopy and EUS. Gastrointest Endosc. 2015;82(3):452–459. doi: 10.1016/j.gie.2015.01.022. [DOI] [PubMed] [Google Scholar]
- 20.Yamao T, et al. Risk factors for lymph node metastasis from intramucosal gastric carcinoma. Cancer. 1996;77(4):602–606. doi: 10.1002/(SICI)1097-0142(19960215)77:4<602::AID-CNCR3>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 21.Ohashi S, et al. Clinicopathological variables associated with lymph node metastasis in submucosal invasive gastric cancer. Gastric Cancer. 2007;10(4):241–250. doi: 10.1007/s10120-007-0442-7. [DOI] [PubMed] [Google Scholar]
- 22.Tajima Y, et al. Risk factors for lymph node metastasis from gastric cancers with submucosal invasion. Ann Surg Oncol. 2010;17(6):1597–1604. doi: 10.1245/s10434-010-0930-6. [DOI] [PubMed] [Google Scholar]
- 23.Barreto SG, Windsor JA. Redefining early gastric cancer. Surg Endosc. 2016;30(1):24–37. doi: 10.1007/s00464-015-4184-z. [DOI] [PubMed] [Google Scholar]
- 24.Isomoto H, et al. Endoscopic submucosal dissection for early gastric cancer: a large-scale feasibility study. Gut. 2009;58(3):331–336. doi: 10.1136/gut.2008.165381. [DOI] [PubMed] [Google Scholar]
- 25.Shin KY, et al. Clinical outcomes of the endoscopic submucosal dissection of early gastric cancer are comparable between absolute and new expanded criteria. Gut Liver. 2015;9(2):181–187. doi: 10.5009/gnl13417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu L, et al. Early gastric Cancer: current advances of endoscopic diagnosis and treatment. Gastroenterol Res Pract. 2016;2016:9638041. doi: 10.1155/2016/9638041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tanabe S, et al. Gastric cancer treated by endoscopic submucosal dissection or endoscopic mucosal resection in Japan from 2004 through 2006: JGCA nationwide registry conducted in 2013. Gastric Cancer. 2017;20(5):834–842. doi: 10.1007/s10120-017-0699-4. [DOI] [PubMed] [Google Scholar]
- 28.Neuhaus H. ESD around the world: Europe. Gastrointest Endosc Clin N Am. 2014;24(2):295–311. doi: 10.1016/j.giec.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 29.Bourke MJ, Neuhaus H, Bergman JJ. Endoscopic Submucosal Dissection: Indications and Application in Western Endoscopy Practice. Gastroenterology. 2018;154(7):1887–1900.e5. doi: 10.1053/j.gastro.2018.01.068. [DOI] [PubMed] [Google Scholar]
- 30.Kunisaki C, et al. Surgical outcomes for early gastric cancer in the upper third of the stomach. J Am Coll Surg. 2005;200(1):15–19. doi: 10.1016/j.jamcollsurg.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 31.Oda I, et al. Treatment strategy after non-curative endoscopic resection of early gastric cancer. Br J Surg. 2008;95(12):1495–1500. doi: 10.1002/bjs.6305. [DOI] [PubMed] [Google Scholar]
- 32.Ryu KW, et al. Surgical indication for non-curative endoscopic resection in early gastric cancer. Ann Surg Oncol. 2007;14(12):3428–3434. doi: 10.1245/s10434-007-9536-z. [DOI] [PubMed] [Google Scholar]
- 33.Park JW, et al. Predictive factors for lymph node metastasis in early gastric cancer with lymphatic invasion after endoscopic resection. Surg Endosc. 2017;31(11):4419–4424. doi: 10.1007/s00464-017-5490-4. [DOI] [PubMed] [Google Scholar]
- 34.Ahn JY, et al. Endoscopic and oncologic outcomes after endoscopic resection for early gastric cancer: 1370 cases of absolute and extended indications. Gastrointest Endosc. 2011;74(3):485–493. doi: 10.1016/j.gie.2011.04.038. [DOI] [PubMed] [Google Scholar]
- 35.Min BH, et al. Surveillance strategy based on the incidence and patterns of recurrence after curative endoscopic submucosal dissection for early gastric cancer. Endoscopy. 2015;47(9):784–793. doi: 10.1055/s-0034-1392249. [DOI] [PubMed] [Google Scholar]
- 36.Lee SH, et al. Risk factors and clinical outcomes of non-curative resection in patients with early gastric cancer treated with endoscopic submucosal dissection: a retrospective multicenter study in Korea. Clin Endosc. 2019;53(2):196–205. doi: 10.5946/ce.2019.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim SG. Treatment strategy after incomplete endoscopic resection of early gastric cancer. Clin Endosc. 2016;49(4):332. doi: 10.5946/ce.2016.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hatta W, et al. Is radical surgery necessary in all patients who do not meet the curative criteria for endoscopic submucosal dissection in early gastric cancer? A multi-center retrospective study in Japan. J Gastroenterol. 2017;52(2):175–184. doi: 10.1007/s00535-016-1210-4. [DOI] [PubMed] [Google Scholar]
- 39.Hirasawa K, et al. Risk assessment chart for curability of early gastric cancer with endoscopic submucosal dissection. Gastrointest Endosc. 2011;74(6):1268–1275. doi: 10.1016/j.gie.2011.07.067. [DOI] [PubMed] [Google Scholar]
- 40.Toyokawa T, et al. Risk factors for non-curative resection of early gastric neoplasms with endoscopic submucosal dissection: analysis of 1,123 lesions. Exp Ther Med. 2015;9(4):1209–1214. doi: 10.3892/etm.2015.2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Suzuki H, et al. Clinical outcomes of early gastric cancer patients after noncurative endoscopic submucosal dissection in a large consecutive patient series. Gastric Cancer. 2017;20(4):679–689. doi: 10.1007/s10120-016-0651-z. [DOI] [PubMed] [Google Scholar]
- 42.Choi YY, et al. Risk factors of submucosal or Lymphovascular invasion in early gastric Cancer <2 cm. Medicine (Baltimore) 2016;95(22):e3822. doi: 10.1097/MD.0000000000003822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saito Y, et al. Endoscopic treatment of large superficial colorectal tumors: a case series of 200 endoscopic submucosal dissections (with video) Gastrointest Endosc. 2007;66(5):966–973. doi: 10.1016/j.gie.2007.02.053. [DOI] [PubMed] [Google Scholar]
- 44.Ohnita K, et al. Factors related to the curability of early gastric cancer with endoscopic submucosal dissection. Surg Endosc. 2009;23(12):2713–2719. doi: 10.1007/s00464-009-0473-8. [DOI] [PubMed] [Google Scholar]
- 45.Park JC, et al. Predictive factors for local recurrence after endoscopic resection for early gastric cancer: long-term clinical outcome in a single-center experience. Surg Endosc. 2010;24(11):2842–2849. doi: 10.1007/s00464-010-1060-8. [DOI] [PubMed] [Google Scholar]
- 46.Goto A, et al. Outcomes of endoscopic submucosal dissection for early gastric cancer and factors associated with incomplete resection. Hepatogastroenterology. 2013;60(121):46–53. doi: 10.5754/hge12533. [DOI] [PubMed] [Google Scholar]
- 47.Kang DH, et al. Location characteristics of early gastric cancer treated with endoscopic submucosal dissection. Surg Endosc. 2017;31(11):4673–4679. doi: 10.1007/s00464-017-5534-9. [DOI] [PubMed] [Google Scholar]
- 48.Huang Q, et al. Differences in Clinicopathology of early gastric carcinoma between proximal and distal location in 438 Chinese patients. Sci Rep. 2015;5:13439. doi: 10.1038/srep13439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim DY, et al. Clinicopathological characteristics of patients with proximal third gastric carcinoma. Acta Chir Belg. 2004;104(6):677–682. doi: 10.1080/00015458.2004.11679642. [DOI] [PubMed] [Google Scholar]
- 50.Park JC, et al. Clinicopathological features and prognostic factors of proximal gastric carcinoma in a population with high helicobacter pylori prevalence: a single-center, large-volume study in Korea. Ann Surg Oncol. 2010;17(3):829–837. doi: 10.1245/s10434-009-0785-x. [DOI] [PubMed] [Google Scholar]
- 51.Amini N, et al. Clinicopathological features and prognosis of gastric cardia adenocarcinoma: a multi-institutional US study. J Surg Oncol. 2015;111(3):285–292. doi: 10.1002/jso.23799. [DOI] [PubMed] [Google Scholar]
- 52.Piso P, et al. Proximal versus distal gastric carcinoma--what are the differences? Ann Surg Oncol. 2000;7(7):520–525. doi: 10.1007/s10434-000-0520-0. [DOI] [PubMed] [Google Scholar]
- 53.Bosman FT, et al. WHO classification of tumours of the digestive system. Geneva: World Health Organization; 2010. https://www.cabdirect.org/cabdirect/abstract/20113051318. Accessed 12 Aug 2019.
- 54.Nam HS, et al. Preprocedural prediction of non-curative endoscopic submucosal dissection for early gastric cancer. PLoS One. 2018;13(10):e0206179. doi: 10.1371/journal.pone.0206179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Park S, et al. Stretching causes extensive changes of gastric submucosa: is it acceptable to define 500 microm as the safe margin? Gut Liver. 2008;2(3):199–204. doi: 10.5009/gnl.2008.2.3.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Akashi Y, et al. Cytoarchitecture of the lamina muscularis mucosae and distribution of the lymphatic vessels in the human stomach. Med Mol Morphol. 2011;44(1):39–45. doi: 10.1007/s00795-010-0503-6. [DOI] [PubMed] [Google Scholar]
- 57.Jeon HK, et al. Endoscopic submucosal dissection for undifferentiated-type early gastric cancer: short- and long-term outcomes. Surg Endosc. 2018;32(4):1963–1970. doi: 10.1007/s00464-017-5892-3. [DOI] [PubMed] [Google Scholar]
- 58.Kim J-H, et al. Follow-up outcomes of endoscopic resection for early gastric cancer with undifferentiated histology. Surg Endosc. 2014;28(9):2627–2633. doi: 10.1007/s00464-014-3514-x. [DOI] [PubMed] [Google Scholar]
- 59.Bang CS, et al. Therapeutic outcomes of endoscopic resection of early gastric Cancer with undifferentiated-type histology: a Korean ESD registry database analysis. Clin Endosc. 2017;50(6):569–577. doi: 10.5946/ce.2017.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim H, et al. Growth patterns of signet ring cell carcinoma of the stomach for endoscopic resection. Gut Liver. 2015;9(6):720–726. doi: 10.5009/gnl14203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Workshop, P.i.t.P The Paris endoscopic classification of superficial neoplastic lesions: Esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc. 2003;58(6 Suppl):S3–43. doi: 10.1016/s0016-5107(03)02159-x. [DOI] [PubMed] [Google Scholar]
- 62.Ohara Y, et al. The superficial elevated and depressed lesion type is an independent factor associated with non-curative endoscopic submucosal dissection for early gastric cancer. Surg Endosc. 2016;30(11):4880–4888. doi: 10.1007/s00464-016-4825-x. [DOI] [PubMed] [Google Scholar]
- 63.Abe S, et al. Depth-predicting score for differentiated early gastric cancer. Gastric Cancer. 2011;14(1):35–40. doi: 10.1007/s10120-011-0002-z. [DOI] [PubMed] [Google Scholar]
- 64.Yamada T, et al. Risk factors for submucosal and lymphovascular invasion in gastric cancer looking indicative for endoscopic submucosal dissection. Gastric Cancer. 2014;17(4):692–696. doi: 10.1007/s10120-013-0323-1. [DOI] [PubMed] [Google Scholar]
- 65.Kim DY, et al. Factors related to lymph node metastasis and surgical strategy used to treat early gastric carcinoma. World J Gastroenterol. 2004;10(5):737–740. doi: 10.3748/wjg.v10.i5.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nasu J, et al. Predictive factors of lymph node metastasis in patients with undifferentiated early gastric cancers. J Clin Gastroenterol. 2006;40(5):412–5. doi: 10.1097/00004836-200605000-00009. [DOI] [PubMed] [Google Scholar]
- 67.Lee IS, et al. Suitability of endoscopic submucosal dissection for treatment of submucosal gastric cancers. Br J Surg. 2013;100(5):668–673. doi: 10.1002/bjs.9051. [DOI] [PubMed] [Google Scholar]
- 68.Kim JY, et al. Lymph node metastasis in early gastric cancer: evaluation of a novel method for measuring submucosal invasion and development of a nodal predicting index. Hum Pathol. 2013;44(12):2829–2836. doi: 10.1016/j.humpath.2013.07.037. [DOI] [PubMed] [Google Scholar]
- 69.Lee TH, et al. Appropriate indications for endoscopic submucosal dissection of early gastric cancer according to tumor size and histologic type. Gastrointest Endosc. 2010;71(6):920–926. doi: 10.1016/j.gie.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 70.Yokoi C, et al. Endoscopic submucosal dissection allows curative resection of locally recurrent early gastric cancer after prior endoscopic mucosal resection. Gastrointest Endosc. 2006;64(2):212–218. doi: 10.1016/j.gie.2005.10.038. [DOI] [PubMed] [Google Scholar]
- 71.Oka S, et al. Endoscopic submucosal dissection for residual/local recurrence of early gastric cancer after endoscopic mucosal resection. Endoscopy. 2006;38(10):996–1000. doi: 10.1055/s-2006-944780. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.