Abstract
Characterization of growth and survival of mice displaying early onset hypertonic symptoms is critical as these animals are important for research investigating mechanisms and treatments of pediatric conditions associated with hypertonia, such as cerebral palsy. Currently, most animal models of cerebral palsy reproduce risk factors for developing this condition, with most failing to develop the physical symptoms or failing to survive in the postnatal period. The B6.Cg‐Glrbspa/J (Gly receptor mutation) transgenic mouse (spa mouse), displays symptoms of early onset hypertonia, though little has been reported on growth and survival, with no reports of growth and survival since genotyping became available. We found that the majority of spa mice display symptoms by P14‐P16. Of mice surviving to weaning, only ~9% were spa mice. By weaning age, spa mice had significantly lower weights than their heterozygote and wild‐type littermates. Of mice that died after weaning and prior to use in experiments or being culled, 48% were spa mice. The poor growth and decreased survival of spa mice across multiple developmental and adult ages resembled the varied survival rates observed in humans with mild or severe cerebral palsy. The understanding of the expected survival of these mice is helpful for planning breeding and animal numbers for experiments. Due to the symptoms and timing of symptom onset, spa mice will be valuable in uncovering mechanisms and long‐term effects of early onset hypertonia in order to move toward interventions for these conditions.
Keywords: animal models, cerebral palsy, mice, reproduction, survival rate
1. INTRODUCTION
Over the years there has been the emergence of a variety of spontaneous glycine (Gly) receptor mutations in c57 black 6 mice. One of these, the B6.Cg‐Glrbspa/J (Gly receptor mutation) transgenic mouse (spa mouse), displays early onset hypertonia (spasticity). 1 , 2 Spa mice have a homozygous insertion of LINE‐1 in the beta subunit of the Gly receptor gene resulting in a splicing error of this subunit. 3 This autosomal recessive mutation affects glycine receptors in both the brain and spinal cord. 2 , 4 Mice that have mutations in both sets of genes are termed “spa” mice, short for spastic. Since the recognition and original description of this mouse in 1961, most work has focused on the glycine receptor abnormality with little attention paid to the growth and developmental or onset of symptoms of spa mice. 1 , 2 , 3 , 4
Characterization of growth and survival of these mice is critical as animals displaying early onset hypertonia are important for research investigating mechanisms and treatments of pediatric conditions associated with hypertonia, such as cerebral palsy. Currently, most animal models of cerebral palsy are based on reproducing risk factors for developing this condition, with most animal models failing to develop the physical symptoms. 5 For conditions of early onset hypertonia, such as cerebral palsy, surprisingly little is known about the mechanisms underlying the development of hypertonia. 5 With the NIH encouraging pursuit of animal models for the study of cerebral palsy, a greater understanding of these mice and their survival is important. 6 To study the mechanisms underlying early onset hypertonia, animals which consistently display the appropriate symptoms are crucial. Very few animals display a hypertonic phenotype that emerges in the developmental period with husbandry of these animals often being complex and survival poor. 5 Therefore, longitudinal reporting of the growth and survival of spa mice is important for planning studies involving these animals while also respecting the 3Rs (replacement, reduction, and refinement) of animal research.
2. MATERIALS AND METHODS
2.1. Animals and breeding
The mouse colony was bred, maintained, and underwent experimental procedures under the Institutional Animal Care and Use Committee at Mayo Clinic (Protocols #A23215‐15, A00003598‐18, A00003622‐18) which is in compliance the American Veterinary Medical Association, US National Research Council's Guide for the Care and Use of Laboratory Animals, and US Public Health Service Policy on Care and Use of Laboratory Animals. 7 , 8 , 9 B6.Cg‐Glrbspa/J mice (C57 background) were obtained from Jackson Laboratories (Jax stock #000066; Bar Harbor, ME, USA) in 2015. Due to mice that are homozygous for the mutation (spa mice) having impaired ability of sperm to fertilize an egg 10 and concern for spa mouse females ability to rear pups due to physical symptoms, a heterozygote × heterozygote breeding scheme was used with matings starting when animals reached ~3 months of age and continuing until ~6 months of age (usually allowing for 3‐5 litters). At 2 time points, c57 mice were purchased from Jackson Laboratories for mating with a heterozygote mouse from the colony (2 pairs) with 1 or 2 litters per pair. The heterozygote mice offspring from these matings were then used for breeding. The mouse colony was tracked using the SoftMouse (Toronto, Ontario, CA) online digital platform.
2.2. Animal genotyping
Genotyping was performed from a 2‐ to 5‐mm tail snip obtained at the time of weaning of pups or after euthanasia of fetuses and pups of preweaning age. Genotypes were determined by PCR using the following primers. Wild‐type forward, 5′‐GCAACTTGAGAGC‐TGTATGT‐3′, and wild‐type reverse, 5′‐ACTTGGCTGGGCTTACATAT‐3′; wild‐type allele, 348 bp; spa forward, 5′‐TTCCTAAGTTCCGGT‐CTGTG‐3′, and spa reverse, 5′‐CAATTATCAAGGCTGATGGC‐3′; spa allele, 358 bp. 11 , 12 Mice who had only wild‐type alleles were identified as “wild type.” Mice that had both a wild‐type allele and spa allele were identified as “heterozygotes.” Mice that had only spa alleles were identified as “spa.”
2.3. Housing and husbandry
Mice were housed in identical conditions, following recommended housing and care guidelines, 7 in a pathogen‐free facility and in an area separate from other mice colonies. Mice were exposed to 12:12 hours light:dark cycle year round. The room was kept at ~21‐23°C. Cages were cleaned and changed weekly by veterinary technicians. Mouse chow (PicoLab® Rodent Diet 5053, LabDiet, St. Louis, MO) and tap water (via water bottles) were freely accessible. For mated pairs, breeder chow (PicoLab® Rodent Diet 5058, LabDiet, St. Louis, MO) was provided. Weaning of pups was performed between 21 and 28 days of age. Litters were not culled, regardless of size, due to lack of spa phenotype at birth. At weaning, if a pup displayed more severe spastic symptoms (ie, spasms that required manually placing animal in an upright position or disturbance by another mouse elicited symptoms), Boost Diet Gel® (Portland, ME) was made available on the cage floor and the animal was housed individually. Social housing (up to 5 mice of same sex and litter) was utilized for all other mice. All mice were housed in Jag 75 (Allentown, Inc, Allentown NJ) cages with 484 cm2 of floor area. For cages with spa mice with severe phenotypes, a paper mat and preconfigured nesting material was placed in the cage. For all other mice, including breeder pairs, standard shavings with paper material used for nesting were provided. 13
2.4. Animal weights
Animals used for experimental purposes were weighed prior to experimental procedures. Age groups were selected based on generalized grouping including preweaning mice (P14‐P16), weaning to 3 months of age (immature adult), 3‐6 months of age (early mature adult), and >6 months of age (late mature adult to old). 14 All animals were weighed using Entris Top Loading Scale (Sartorius Lab Instruments, Goettingen, Germany).
2.5. Animal survival
Mice in the colony were not bred for survival studies, but rather for specific experiments, which were performed at ages targeting key developmental times 15 or at mature adult stages (~3 to 8 months old). 16 , 17 Mice not needed for experiments or breeding were euthanized following confirmation of genotype (~6 to 8 weeks of age) in accordance with American Veterinary Medical Association guidelines. 8 Genotypes were determined by PCR, 11 , 12 with mice designated as wild type, heterozygote, or spa depending on the expression of wild‐type and/or spa alleles. Animals were weighed with Entris Top Loading Scale (Sartorius Lab Instruments, Goettingen, Germany) prior to experimental procedures. Ages of animals were selected based on developmental time points of interest for planned experiments including preweaning mice (P14‐P16), weaning to 3 months of age (immature adult), 3‐6 months of age (early mature adult), and >6 months of age (late mature adult to old). 14 , 15
2.6. Data analysis and statistics
All statistical analyses were performed using Prism 7.0 (Graphpad, La Jolla, CA). With respect to continuous variables, differences between groups were examined using unpaired t‐tests when data were normally distributed according to D’Agostino and Pearson normality tests. Two‐way ANOVA was used when comparing 2 factors, with Bonferroni post hoc tests where appropriate. Chi‐squared tests were used for evaluating relationships between variables. Statistical significance was established at the P < .05 level. All experimental data are presented as mean ± 95% confidence intervals, unless otherwise specified.
3. RESULTS
3.1. Animal survival
To assess the influence of genotype on mouse attrition prior to weaning, litter numbers were recorded for 105 litters. The mean litter size across 105 litters was 6 (range 1‐12) pups. With regard to the fetal mice (embryonic day (E) 15‐E18), a total of 47 mice from 6 litters were euthanized for experimental purposes. Genotyping of the fetal mice (E15‐E18) revealed a roughly Mendelian distribution of genotypes as expected from a heterozygote by heterozygote breeding scheme, 10 (21.3%) mice were spa, 25 (53.2%) mice were heterozygotes, and 12 (25.5%) mice were wild type (Figure 1A). Of the P14‐P16 mice, 8 (9.8%) were spa, 52 (63.4%) were heterozygotes, 22 (26.8%) were wild type (Figure 1B). Approximately 67% of spa mice had the hypertonic phenotype by P14‐P16. All spa mice displayed a hypertonic phenotype by weaning which persisted. No spa mice had spontaneous resolution of hypertonic symptoms. Of mice that survived weaning, 71 (9.4%) were spa, 417 were heterozygotes (56.7%), and 249 (33.9%) were wild type (Figure 1C). Overall, these results show a remarkable attrition in spa mice compared to both wild‐type and heterozygote mice during postnatal maturation. Between birth and P14‐16 only ~40% of spa pups survive, with minimal additional attrition by weaning. The reduced survival of spa mice compared to both wild‐type and heterozygote mice was considered statistically significant (Chi‐square, P = .0048 at P14‐16 and P < .0001 at weaning).
FIGURE 1.
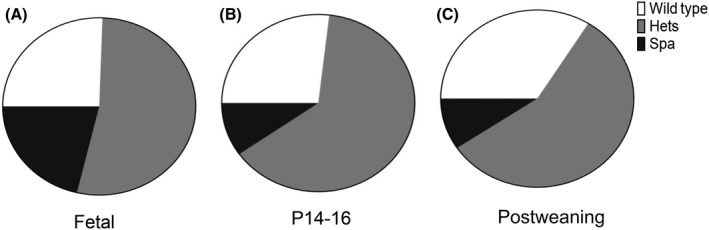
Proportion of mice based on genotypes. Proportion of mice with wild‐type (white), heterozygote (gray), and spa (black) genotypes in the (A) fetal, (B) ~ 2 weeks postnatal, and (C) postweaning. Based on Mendelian inheritance pattern, it would be expected that ~25% of mice would be wild type and 25% spa with 50% heterozygotes. This similar pattern is seen in the fetal mice with 21% being spa mice. However, by P14/P16, the percentage of spa mice decreased to 10% with relative expansion of heterozygote mice percentage. In the postweaning period, percentage of spa mice had a slight decrease to about 9% with a relative expansion of both heterozygotes (57%) and wild‐type (34%) mice percentages
For the 735 mice that survived weaning, 40 (5.4%) mice died of causes unrelated to experiments or being culled for the colony. Of these 40 mice, 19 were spa (47.5%), 17 were heterozygotes (42.5%), and 4 were wild type (10.0%). The increased likelihood of death of spa mice compared to heterozygote and wild‐type mice was considered statistically significant (Chi‐square, P < .0001).
3.2. Body weight gain
Body weights were obtained when mice were used for experimental purposes or routinely at ages from P14 through approximately 1 year of age. At all ages, spa mice had lower weights when compared to heterozygote and wild‐type mice, with this difference being statistically significant at all time points from weaning onward (Table 1, Figure 2). There was no significant difference in body weight between heterozygote mice and wild‐type mice at any time point (Table 1, Figure 2). When comparing body weights of spa mice across ages, the only significant increase in body weight compared to the previous weight occurred between weaning and 3 months of age (P < .0001, Table 1).
Table 1.
Mice weight for age and genotype
| Age | Genotype | Body weight (g) | P value vs wild type | P value heterozygote vs Spa | P value of same genotype vs previous age |
|---|---|---|---|---|---|
| P14‐P16 | Wild type | 8.6 (±0.6) | — | — | — |
| Heterozygote | 9.3 (±0.5) | P > .999 | — | — | |
| Spa | 6.9 (±1.2) | P > .999 | P = .495 | — | |
| Weaning | Wild type | 13.5 (±0.6) | — | — | P = .012 |
| Heterozygote | 14.0 (±0.9) | P > .999 | — | P = .0001 | |
| Spa | 9.1 (±2.9) | P = .034 | P = .015 | P > .999 | |
| Postweaning to <3 mo | Wild type | 24.2 (±1.1) | — | — | P < .0001 |
| Heterozygote | 24.9 (±2.4) | P > .999 | — | P < .0001 | |
| Spa | 18.9 (±1.7) | P < .0001 | P < .0001 | P < .0001 | |
| 3 mo to <6 mo | Wild type | 27.4 (±1.9) | — | — | P = .007 |
| Heterozygote | 28.0 (±2.5) | P > .999 | — | P = .203 | |
| Spa | 20.3 (±1.6) | P < .0001 | P < .0001 | P > .999 | |
| ≥6 mo | Wild type | 33.4 (±2.2) | — | — | P < .0001 |
| Heterozygote | 34.7 (±2.3) | P = .872 | — | P < .0001 | |
| Spa | 21.9 (±4.1) | P < .0001 | P < .0001 | P > .999 |
Mean (CI) weight of mice by genotype across ages. There was no significant difference in body weights of all mice at postnatal day 14‐16 (P14‐P16). However, at all ages from weaning onward, spa mice weighed significantly less than wild‐type and heterozygote mice. There was no significant difference in body weights between wild‐type and heterozygote mice at each age. When comparing body weights across ages within genotypes, weight gain occurred as age increased. For wild‐type mice, body weight increased significantly at all ages. For heterozygote mice, body weight increased significantly at all ages, except between the ages of postweaning to <3 mo and 3 mo to <6 mo. For spa mice, the only significant increase in body weight occurred between weaning and postweaning to <3 mo of age. Two‐way ANOVA with post hoc Bonferroni testing.
FIGURE 2.

Body weights of mice based on age and genotype. Spa mice (black) had a significantly lower body weight at all time points postweaning as compared to wild‐type (white) and heterozygote (gray) mice (mean ± CI, Two‐way ANOVA with post hoc Bonferroni testing, *P < .0001, **P = .034 (spa vs wild type), ***P = .015 (spa vs heterozygote)). There was no significant difference between body weights of heterozygote and wild‐type mice at each time point
As expected, at every time point assessed, female mice had significantly lower body weights than male mice (data not shown). At each age, there were no differences in body weights between female heterozygote and wild‐type mice nor between male heterozygote and wild‐type mice (Table 2). From 3 months of age and older, female spa mice weighed significantly less than both heterozygote and wild‐type female mice (Table 2). In contrast to females, male spa mice weighed significantly less than both male heterozygote and wild‐type mice for all postweaning ages assessed (Table 2).
Table 2.
Mice body weights across postweaning ages for males and females
| Age | Sex |
Wild‐type Body weight (g) |
Heterozygote Body weight (g) |
Spa Body weight (g) |
P value wild type vs Spa | P value heterozygote vs Spa |
|---|---|---|---|---|---|---|
| Postweaning to <3 mo | Female | 20.6 (±0.6) | 20.8 (±2.2) | 18.3 (±2.0) | P > .999 | P > .999 |
| Male | 26.9 (±0.7) | 29.0 (±1.6) | 19.4 (±3.0) | P < .0001 | P < .0001 | |
| 3 mo to <6 mo | Female | 23.7 (±1.0) | 25.2 (±2.39) | 18.0 (±1.6) | P < .0001 | P < .0001 |
| Male | 32.7 (±2.1) | 32.7 (±1.7) | 22.5 (±2.4) | P < .0001 | P < .0001 | |
| ≥6 mo | Female | 30.4 (±4.1) | 32.4 (±3.44) | 20.6 (±4.4) | P < .0001 | P < .0001 |
| Male | 35.4 (±2.9) | 36.6 (±3.1) | 26.8 (±10.5) | P = .0013 | P = .0003 |
Mean (CI) body weight of mice by sex and genotype across mature ages. At all ages postweaning in males and over 3 mo of age in females, spa mice weighed significantly less than wild‐type and heterozygote mice of the same sex. Wild‐type and heterozygote mice of the same sex and age had no significant difference in body weight. Two‐way ANOVA with post hoc Bonferroni testing.
4. DISCUSSION
Little has been reported on growth and survival of spa mice since they were originally described in 1961, 2 which was prior to availability of genotyping. 18 Subsequently, the specific genetic abnormality for spa mice was identified in 1994. 18 Despite the phenotypic reevaluation of spa mice in conjunction with genotyping in 1997, 4 the growth and survival of spa mice was not reevaluated. Furthermore, despite the cerebral palsy‐like phenotype of spa mice which prompted their use in sentinel work on botulinum toxin in hypertonic muscles, 19 use of these mice for exploring the developmental changes in the central nervous system and motor units in conditions of early onset hypertonia remain virtually unexplored.
In spa mice, we identified that: (a) there is a remarkable attrition prior to weaning and before the phenotypic onset; (b) the majority of spa mice have a spa phenotype detectable at P14‐P16, prior to previously described onset at ~P21; and (iii) the developmental body weight gain of spa mice is impaired compared to wild‐type and heterozygote littermates. These observations have important implications for experimental design considerations in spa studies, including humane endpoints for body weight reduction. 20
Spa mice have the highest attrition between birth and P16. This may be related to maternal difference in rearing of the mutant pups, though maternal‐pup interactions were not evaluated as part of this study. 21 In mice with a genetic mutation resulting in a lack of gephyrin (a protein required for Gly receptor clustering) death occurs by P1 due to failure to suckle with these pups showing a rapid development of hyperextension limb posturing prior to death. 22 It is also possible that spa mice pups have difficulty with suckling, as spa mice have an alteration in Gly signaling. In our study, all homozygous spa mice exhibited the spastic phenotype by P28, with a majority displaying a detectable phenotype by ~P14‐16. Although we did not evaluate ventilation in these mice, hypertonic activation of the diaphragm or upper airway muscles may result in an apnea. The frequency and duration of apneic events in spa mice younger than P14 and older than P14 will be a key indicator of whether respiratory dysfunction is the cause of spa deaths prior to weaning. It may be the case that a fraction of spa mutants are symptomatic before weaning and die before overt signs are readily observable. Additional loss of spa mice occurred postweaning with the number of incidental deaths compared to heterozygote or wild‐type mice being disproportionately greater in spa mice. This survival difference in the postweaning period has not been explicitly evaluated. 4 Death of spa mice has notable implications for estimating colony breeding and number of spa mice needed for experiments, particularly if older spa mice are needed. While death of spa mice is high, this corresponds with death in inhuman conditions of early onset hypertonia, like cerebral palsy, where mortality rates before age 2 years is up to 10% and mortality by maturity up to 60%, depending on the severity of symptoms. 23 , 24
Spa mice show a significant impairment in body weight gain across all ages postweaning. This observation was consistent in both female and male mice, though the pattern of body weight gain differed between males and females. This impaired body weight gain makes spa mice distinctly smaller than their heterozygote and wild‐type sex‐matched littermates, different than previously reported. 4 It is not clear if this difference is due to the spa mice physical symptoms alone, impaired feeding, or if there is some maternal stress in rearing of spa pups that contributes to their lower body weight. 25 Interestingly, the impaired growth of spa mice mirrors the lower growth rates observed clinically in conditions of early onset hypertonia, like cerebral palsy. Specifically, in children with cerebral palsy, the differences in weight and height as compared to age‐matched peers are more pronounced with age, with children with cerebral palsy being lighter and shorter than their age‐matched peers. 26
We know that by adulthood, spa mice have reduced motor neuron numbers in a variety of motor pools 16 , 17 and a reduced response of muscle to neuromuscular transmission (ie, neuromuscular transmission failure). 27 , 28 Current efforts within the laboratory are aimed at determining the developmental time course of motor neuron loss and muscle weakness in relation to symptom onset (P14‐28) and weaning (P28). Other groups have established that both motor neurons and the associated spinal interneuronal network exhibit reduced glycinergic inhibition in 2‐ to 6‐week‐old mice. 11 , 29 , 30 , 31 Importantly, the pathology of spa mice may be a contingent on the transition from the depolarizing to hyperpolarizing effect of chloride channel activation in response to glycine and/or GABA that occurs prior to P14 in rodents. 32 The present work, establishing the gross phenotype, weight, and survival throughout development of the spa mice, will enable us to rationalize the experimental design and timeline to assess the motor system‐ and molecular‐level pathology. This critical work is needed for furthering the understanding of potential mechanisms underlying human conditions of early onset hypertonia. Thus, understanding growth and survival of spa mice is important for investigators and animal care facilities working with these or similar genetically modified mice. Provision of appropriate husbandry of these animals and the accurate estimation of survival for adequate experimental animal numbers is essential for rigorous, robust, and repeatable experiments.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHOR CONTRIBUTIONS
JEB, MJF, and GCS contributed to the design of the study, the interpretation of the data, drafting, and critical revisions of the manuscript. MJF and JEB also contributed to the acquisition of the data. All the authors approved the final version of the manuscript.
ACKNOWLEDGMENTS
This work was supported by the National Institutes of Health grants R01‐AG044615 (GCS), R01‐HL96750 (GCS), Mayo Clinic Children's Research Center Pediatric Team Science Award (JEB), Mayo Clinic Office of Research Diversity and Inclusion Career Support and Advancement Award (JEB), Mayo Clinic CTSA UL1 TR000135 (JEB), Mr and Mrs Richard and Rosemary Crandall (JEB), and an NHMRC CJ Martin fellowship (MJF). We thank Jeffrey Bailey and Rebecca Macken for their technical assistance in the completion of this project. We acknowledge the expert care and assistance that the veterinary staff and animal care technicians provided for our colony.
Brandenburg JE, Fogarty MJ, Sieck GC. Growth and survival characteristics of spa mice. Anim Models Exp Med. 2020;3:319–324. 10.1002/ame2.12137
REFERENCES
- 1. Heller AH, Hallett M. Electrophysiological studies with the spastic mutant mouse. Brain Res. 1982;234(2):299‐308. [DOI] [PubMed] [Google Scholar]
- 2. Chai CK. Hereditary Spasticity in Mice. J Hered. 1961;52(5):241‐243. [Google Scholar]
- 3. White WF, Heller AH. Glycine receptor alteration in the mutant mouse spastic. Nature. 1982;298(5875):655‐657. [DOI] [PubMed] [Google Scholar]
- 4. Simon ES. Phenotypic heterogeneity and disease course in three murine strains with mutations in genes encoding for alpha 1 and beta glycine receptor subunits. Mov Disord. 1997;12(2):221‐228. [DOI] [PubMed] [Google Scholar]
- 5. Brandenburg JE, Fogarty MJ, Sieck GC. A critical evaluation of current concepts in cerebral palsy. Physiology (Bethesda). 2019;34(3):216‐229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. NINDS, NICHD . NINDS/NICHD Strategic Plan for Cerebral Palsy Research. 2017.
- 7. National Research Council . Guide for the care and use of laboratory animals. Washington, DC: The National Academies Press; 1996. [Google Scholar]
- 8. Leary S, Underwood W, Anthony R, et al. AVMA guidelines for the euthanasia of animals: 2020 edition. Schaumburg, IL: American Veterinary Medical Association; 2020. [Google Scholar]
- 9. National Institutes of Health Office of Laboratory Animal Welfare . PHS Policy on Humane Care and Use of Laboratory Animals. Accessed May 1, 2020.
- 10. Sato Y, Son JH, Tucker RP, Meizel S. The zona pellucida‐initiated acrosome reaction: defect due to mutations in the sperm glycine receptor/Cl(‐) channel. Dev Biol. 2000;227(1):211‐218. [DOI] [PubMed] [Google Scholar]
- 11. Graham BA, Schofield PR, Sah P, Margrie TW, Callister RJ. Distinct physiological mechanisms underlie altered glycinergic synaptic transmission in the murine mutants spastic, spasmodic, and oscillator. J Neurosci. 2006;26(18):4880‐4890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Muller E, Le Corronc H, Scain AL, Triller A, Legendre P. Despite GABAergic neurotransmission, GABAergic innervation does not compensate for the defect in glycine receptor postsynaptic aggregation in spastic mice. Eur J Neurosci. 2008;27(10):2529‐2541. [DOI] [PubMed] [Google Scholar]
- 13. Leidinger CS, Thöne‐Reineke C, Baumgart N, Baumgart J. Environmental enrichment prevents pup mortality in laboratory mice. Lab Anim. 2018;53(1):53‐62. [DOI] [PubMed] [Google Scholar]
- 14. Flurkey KM, Currer J, Harrison DE. Chapter 20 ‐ mouse models in aging research In: Fox JG, Davisson MT, Quimby FW, Barthold SW, Newcomer CE, Smith AL, eds. The Mouse in Biomedical Research. 2nd ed. Burlington, VT: Academic Press; 2007:637‐672. [Google Scholar]
- 15. Mantilla CB, Fahim MA, Brandenburg JE, Sieck GC. Functional development of respiratory muscles In: Polin RA, Abman SH, Rowitch DH, Beneithz WE, Fox WW, eds. Fetal and Neonatal Physiology. 5th ed. Philadelphia, PA: Elsevier; 2017. [Google Scholar]
- 16. Brandenburg JE, Gransee HM, Fogarty MJ, Sieck GC. Differences in lumbar motor neuron pruning in an animal model of early onset spasticity. J Neurophysiol. 2018;120(2):601‐609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brandenburg JE, Fogarty MJ, Brown AD, Sieck GC. Phrenic motor neuron loss in an animal model of early onset hypertonia. J Neurophysiol. 2020;123(5):1682‐1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kingsmore SF, Giros B, Suh D, Bieniarz M, Caron MG, Seldin MF. Glycine receptor beta‐subunit gene mutation in spastic mouse associated with LINE‐1 element insertion. Nat Genet. 1994;7(2):136‐141. [DOI] [PubMed] [Google Scholar]
- 19. Cosgrove AP, Graham HK. Botulinum toxin A prevents the development of contractures in the hereditary spastic mouse. Dev Med Child Neurol. 1994;36(5):379‐385. [DOI] [PubMed] [Google Scholar]
- 20. Talbot SR, Biernot S, Bleich A, et al. Defining body‐weight reduction as a humane endpoint: a critical appraisal. Lab Anim. 2019;54(1):99‐110. [DOI] [PubMed] [Google Scholar]
- 21. Shoji H, Kato K. Maternal care affects the development of maternal behavior in inbred mice. Dev Psychobiol. 2009;51(4):345‐357. [DOI] [PubMed] [Google Scholar]
- 22. Feng G, Tintrup H, Kirsch J, et al. Dual requirement for gephyrin in glycine receptor clustering and molybdoenzyme activity. Science. 1998;282(5392):1321‐1324. [DOI] [PubMed] [Google Scholar]
- 23. Durufle‐Tapin A, Colin A, Nicolas B, Lebreton C, Dauvergne F, Gallien P. Analysis of the medical causes of death in cerebral palsy. Ann Phys Rehabil Med. 2014;57(1):24‐37. [DOI] [PubMed] [Google Scholar]
- 24. Hutton JL, Pharoah POD. Life expectancy in severe cerebral palsy. Arch Dis Child. 2006;91(3):254‐258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Curley J, Davidson S, Bateson P, Champagne F. Social enrichment during postnatal development induces transgenerational effects on emotional and reproductive behavior in mice. Front Behav Neurosci. 2009;3:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Krick J, Murphy‐Miller P, Zeger S, Weight E. Pattern of growth in children with cerebral palsy. J Am Diet Assoc. 1996;96(7):680‐685. [DOI] [PubMed] [Google Scholar]
- 27. Brandenburg JE, Fogarty MJ, Sieck GC. Neuromuscular transmission failure in an animal model of early onset spasticity. Society for Neuroscience Annual Meeting; 2018; Program #675.11. https://www.abstractsonline.com/pp8/#!/4649/presentation/23187
- 28. Fogarty MJ, Sieck GC, Brandenburg JE. Impaired neuromuscular transmission of the tibialis anterior in a rodent model of hypertonia. J Neurophysiol. 2020;123(5):1864‐1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Graham BA, Schofield PR, Sah P, Callister RJ. Altered inhibitory synaptic transmission in superficial dorsal horn neurones in spastic and oscillator mice. J Physiol. 2003;551(Pt 3):905‐916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tadros MA, Farrell KE, Schofield PR, et al. Intrinsic and synaptic homeostatic plasticity in motoneurons from mice with glycine receptor mutations. J Neurophysiol. 2014;111(7):1487‐1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. von Wegerer J, Becker K, Glockenhammer D, Becker CM, Zeilhofer HU, Swandulla D. Spinal inhibitory synaptic transmission in the glycine receptor mouse mutant spastic. Neurosci Lett. 2003;345(1):45‐48. [DOI] [PubMed] [Google Scholar]
- 32. Wong‐Riley MT, Liu Q. Neurochemical and physiological correlates of a critical period of respiratory development in the rat. Respir Physiol Neurobiol. 2008;164(1‐2):28‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]


