Abstract
Objective
The objective of our study was to investigate the comparative effectiveness of antipsychotics for the risk of attempted or completed suicide among all patients with schizophrenia in Finland and Sweden.
Methods
Two nationwide register-based cohort studies were conducted, including all individuals with schizophrenia in Finland (n = 61 889) and Sweden (n=29 823). The main exposure was 10 most commonly used antipsychotic monotherapies; also, adjunctive pharmacotherapies were investigated. The main outcome measure was attempted or completed suicide, which was analyzed with within-individual models by comparing use and nonuse periods in the same individual to minimize selection bias. Sensitivity analyses included attempted suicide (hospitalization only) as an outcome.
Results
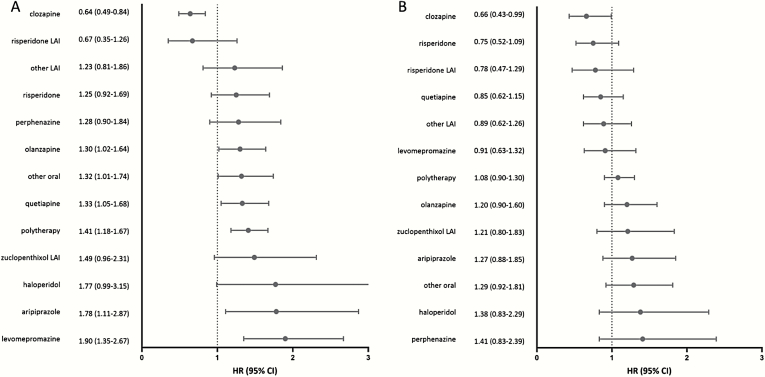
Compared with no use of antipsychotics, clozapine use was the only antipsychotic consistently associated with a decreased risk of suicidal outcomes. Hazard ratios (HRs) and 95% CIs for attempted or completed suicide were 0.64 (0.49–0.84) in the Finnish cohort and 0.66 (0.43–0.99) in the Swedish cohort. No other antipsychotic was associated with a reduced risk of attempted and/or completed suicide. Benzodiazepines and Z-drugs were associated with an increased risk of attempted or completed suicide (HRs: 1.29–1.30 for benzodiazepines and 1.33–1.62 for Z-drugs).
Conclusion
Clozapine was the only antipsychotic associated with decreased risk of attempted or completed suicide among patients with schizophrenia, and it should be considered as first-line treatment for high-risk patients.
Keywords: schizophrenia, antipsychotic, suicide, suicide attempt
Introduction
Suicide attempts and suicides are rather common phenomena in schizophrenia. Previous epidemiological estimates on persons with schizophrenia state that 25%–50% attempt suicide1 and 4%–13% end their life by suicide.2 The rate for completed suicide among patients with schizophrenia seems to be decreasing, as previous estimates show higher figures (above 10%) than more recent ones (around 5%).3 However, still, in Finland and Sweden, persons with schizophrenia are 6–14 times more likely to die due to suicide than individuals of the general population.3,4
Clinical factors predicting suicidal behavior or completed suicide have been thoroughly studied.5 A history of suicidal behavior (ie, ideation and/or attempt) is one of the strongest predictors for both completed suicide and future suicide attempts.2,5 Other predictors for attempted suicide are a history of alcohol use and family history of psychiatric illness, and for completed suicide male gender and younger age.5 However, a third of the patients who reported no suicidal ideation at the time of treatment initiation in first-episode psychosis developed suicidal ideation or engaged in suicidal behaviors during the first year of treatment.6 Thus, it is important for clinicians to be aware that transitions between suicidal stages occur frequently, especially among first-episode patients. It is often emphasized that the risk of suicide is highest within the first year after discharge from the initial hospitalization in first-episode psychosis and shortly after discharge among general psychiatric inpatients.7
A recent meta-analysis of placebo-controlled randomized controlled trials (RCTs) did not find an association for antipsychotic use and risk of suicide,8 possibly because suicidal patients are typically excluded from RCTs. Previous observational studies have reported that antipsychotic use in schizophrenia is associated with a lower risk of completed suicide than nonuse.9–11 A nationwide forensic toxicological study on suicide deaths found that partial and full nonadherence to antipsychotics were associated with 6.7- and 12.4-fold risks for completed suicide.12 In general, second-generation antipsychotics (SGA) are considered to be more effective than first-generation antipsychotics (FGAs) in reducing suicides,13 which is in line with a recent review on potential antisuicidal effects of SGAs.14 A previous Swedish case-control study among persons with schizophrenia found a lower suicide risk among patients who had been prescribed oral SGAs (clozapine, olanzapine, risperidone, or ziprasidone) than those who did not use these medications.10 However, the study found no significant associations for long-acting injectable antipsychotics (LAIs), antidepressants, or lithium.
On specific antipsychotics, clozapine is the only drug with approval from the US Food and Drug Administration for preventing suicide in patients with schizophrenia.13 In most observational studies, a decreased risk of suicide has been reported for clozapine.9,10,15 In the InterSePT trial, clozapine was associated with a lower risk of suicidal behavior than olanzapine (hazard ratio [HR]: 0.76, 0.58–0.97) and showed superiority in all key measures of suicidality.16 Contrary to this, a previous Swedish case-control study showed that olanzapine (compared with haloperidol) showed similar lower risks of suicide and attempted suicide as clozapine.15 Previous studies have mainly focused on suicidal death, and studies assessing the risk of attempted suicide are scarce. Whether all specific SGAs are effective in preventing completed suicides also remains unclear. In addition, confounding may be present in previous observational studies due to selection bias (eg, illness and symptom severity). As treatment practices may vary between countries and selective prescribing may impact on the results, we utilized data from 2 countries to minimize these effects. The objective of our study was to investigate the comparative effectiveness of antipsychotics for the risk of attempted or completed suicide among persons with schizophrenia in Finland and Sweden by applying within-individual design in order to minimize selection bias.
Methods
Study Cohorts
Two nationwide cohorts of persons with schizophrenia were identified from Finnish and Swedish registers. All residents of both countries have been assigned a unique personal identification number, which was utilized in the linkage of country-specific registers.
The Finnish cohort included all persons (n = 61 889) treated due to schizophrenia (the International Classification of Diseases [ICD] codes F20 and F25 [ICD-10] and 295 [ICD-8 and ICD-9]) in inpatient care during 1972–2014 in Finland.17 To keep the study cohorts homogenous, we have included in the definition of schizophrenia only schizophrenia and schizoaffective disorder, a definition often used in previous literature, and excluded delusional disorder (F22) and psychosis NAS (F29). The cohort was identified from the Hospital Discharge Register (HDR) maintained by the National Institute of Health and Welfare. Data from the HDR (all hospital care periods with diagnoses, 1972–2017), Prescription Register (reimbursed prescription drug purchases, 1995–2017), and causes of death from Statistics Finland (1972–2017) were collected for the cohort. The cohort included 61 889 community-dwelling persons with schizophrenia. The incident cohort (first-episode patients) was identified as persons having their first-ever diagnoses of schizophrenia during 1996–2014 and not having used antipsychotics during a preceding year (n = 8342). The follow-up started on January 1, 1996 for persons diagnosed before that and at the first discharge from inpatient care for persons diagnosed during 1996–2014. The follow-up time ended at death or December 31, 2017, whichever occurred first.
The Swedish cohort included all persons aged 16–64 with schizophrenia diagnoses (ICD-10 F20 and F25) and registered schizophrenia treatment contact between July 1, 2006 until December 31, 2013 in Sweden.18 Schizophrenia diagnoses were derived from the National Patient Register (NPR, maintained by the National Board of Health and Welfare, inpatient and specialized outpatient care), disability pensions and sickness absences from the MiDAS register (maintained by the Swedish Social Insurance Agency). Data from NPR (all hospital care periods and specialized outpatient visits with diagnoses, July 2005–December 2016), the Prescribed Drug Register (PDR, maintained by the National Board of Health and Welfare, prescription drug purchases July 2005–December 2016), the Causes of Death Register (maintained by the National Board of Health and Welfare, causes of death 2005–2016), and the LISA register (maintained by Statistics Sweden, demographic characteristics) were collected for the cohort. The cohort included 29 823 community-dwelling persons with schizophrenia. The incident cohort was identified as persons having their first-ever diagnoses of schizophrenia during July 2005–December 2013 and not having used antipsychotics during a preceding year (n = 4603). The follow-up started on July 1, 2006 for persons diagnosed before that and at the first recorded diagnoses for persons diagnosed during July 2006–December 2013. The follow-up time ended at death or December 31, 2016, whichever occurred first.
Exposure
Antipsychotics were defined as Anatomical Therapeutic Chemical (ATC) classification (ATC) codes N05A (lithium excluded). Antipsychotics were further categorized into oral vs LAIs according to their drug formulation. Polytherapy refers to the concomitant use of 2 or more antipsychotics. Adjunctive pharmacotherapy categories included antidepressants (N06A), mood stabilizers (carbamazepine N03AF01, valproic acid N03AG01, and lamotrigine N03AX09), lithium (N05AN01), benzodiazepines (N05BA, N05CD), and benzodiazepine-related so-called Z-drugs (N05CF). The PRE2DUP method was utilized to construct drug use periods, ie, when drug use started and ended, based on purchase dates, amounts of drugs dispensed, and personal drug use patterns.19 Each drug for each person was modeled separately, and modeling was further conducted separately for oral antipsychotics and LAIs. The method is based on the calculation of sliding averages of the daily dose in DDDs according to individual drug use patterns and it takes time periods of hospital care into account (when drugs are provided by the caring unit and not recorded in the registers). Each drug package (based on Nordic Article Number, vnr) is controlled in the modeling by expert-defined parameters, which assign upper and lower limits for the dose that can be used in clinical practice based on drug form, whether tablets can be divided or not, and according to drug form (eg, administration interval for different LAIs). The PRE2DUP method has been validated in several studies.19–22
Outcomes
The main outcome measure was attempted or completed suicide, defined as inpatient care and causes of death diagnoses ICD-10 X60-84 and Y10-34. Diagnoses with undetermined intent (Y10-34) were included to compensate underreporting of suicides and regional differences in ascertainment practices. Secondary outcome measure was suicide attempt, defined as inpatient care diagnoses only. Both outcomes were treated as recurrent events.
Statistical Analyses
The analyses were conducted separately in the Finnish and Swedish cohorts (supplementary figure 1). The primary analyses were conducted with within-individual design.23 Within-individual design was analyzed with stratified Cox proportional hazard regression models, in which each individual formed his or her own stratum, and the follow-up time for each individual was reset to 0 after each outcome event. In within-individual design, all time-invariant covariates (such as sex) were controlled for in the design and analyses were adjusted for time-varying covariates, ie, sequential order of treatments, use of antidepressants, benzodiazepines, Z-drugs, mood stabilizers and lithium, and time since cohort entry. Only persons having an outcome event contributed to within-individual analyses (these subsets of cohorts are described in table 1). As we noticed in a previous study, an increasing number of changes in drug regimen happening within a month before completed suicide,12 we conducted all analyses also by censoring the first 30 days of exposure time from the beginning of each exposure period as this may represent a time period of suboptimal pharmacological effect. All results are presented with and without 30 days censoring.
Table 1.
Characteristics of Study Cohort, Those Who Had Suicide Attempt or Death, and Those Who Had Suicide Attempt During the Follow-up in Finnish and Swedish Individuals with Schizophrenia
| Finnish schizophrenia cohort | Swedish schizophrenia cohort | |||||
|---|---|---|---|---|---|---|
| All N = 61 889 % (n) |
With suicide attempt/death n = 4315 % (n) |
With suicide attempt n = 3135 % (n) |
All n = 29 823 % (n) |
With suicide attempt/death n = 2203 % (n) |
With suicide attempt n = 1793 % (n) |
|
| At the beginning of follow-upa | ||||||
| Age, mean (SD) | 46.2 (16.0) | 37.3 (12.5) | 36.3 (12.1) | 44.9 (12.0) | 40.0 (12.5) | 39.2 (12.6) |
| Male gender | 50.3 | 42.3 | 47.0 | 57.0 | 51.7 | 47.9 |
| Time (years) since first schizophrenia diagnoses | ||||||
| ≤1 | 38.1 | 48.4 | 50.2 | 34.0 | 43.4 | 45.2 |
| 1–5 | 9.0 | 12.4 | 12.2 | 14.9 | 16.7 | 16.2 |
| >5 | 52.9 | 39.2 | 37.6 | 51.0 | 40.0 | 38.5 |
| The number of previous psychiatric hospitalizations | ||||||
| 0–1 | 48.8 | 40.4 | 39.9 | 31.4 | 33.7 | 34.8 |
| 2–3 | 27.1 | 30.4 | 30.1 | 18.9 | 17.8 | 17.2 |
| ≥4 | 24.1 | 29.2 | 30.0 | 49.7 | 48.5 | 48.0 |
| Previous suicide attempt | 9.1 | 28.1 | 30.9 | 9.1 | 31.2 | 34.7 |
| Substance abuse | 14.6 | 31.2 | 33.7 | 22.3 | 42.7 | 44.3 |
| Prior use of LAI | 16.1 | 15.6 | 15.8 | 24.1 | 25.5 | 25.3 |
| Prior use of clozapine | 6.7 | 7.3 | 7.3 | 12.1 | 9.9 | 9.7 |
| Antidepressant use | 23.7 | 41.9 | 44.5 | 34.0 | 49.7 | 52.3 |
| Benzodiazepine and related drug use | 31.6 | 47.1 | 48.2 | 46.9 | 62.4 | 64.0 |
| Mood stabilizer use | 7.1 | 12.4 | 14.2 | 10.2 | 16.3 | 18.0 |
| Lithium use | 5.0 | 7.9 | 8.7 | 8.2 | 11.6 | 11.9 |
| During the follow-upb | ||||||
| The number of psychiatric hospitalizations | ||||||
| 0–1 | 53.1 | 33.1 | 23.4 | 63.4 | 35.5 | 29.0 |
| 2–3 | 16.0 | 14.1 | 13.3 | 63.4 | 18.5 | 18.4 |
| ≥4 | 30.8 | 52.8 | 63.4 | 21.9 | 46.1 | 52.6 |
| Suicide attempt | 5.1 | 72.6 | 100.0 | 6.0 | 81.4 | 100.0 |
| Substance abuse | 12.3 | 35.2 | 41.9 | 14.4 | 42.3 | 46.6 |
| Clozapine use | 22.8 | 30.3 | 35.6 | 19.4 | 22.5 | 24.4 |
| Antidepressant use | 49.6 | 73.3 | 81.0 | 52.6 | 73.9 | 79.7 |
| Benzodiazepine and related drug use | 63.5 | 81.9 | 88.5 | 70.8 | 86.9 | 90.4 |
| Mood stabilizer use | 24.6 | 42.3 | 50.8 | 22.0 | 38.1 | 43.7 |
| Lithium use | 9.5 | 17.2 | 20.7 | 13.1 | 23.4 | 25.8 |
Note: LAI, long-acting injectable antipsychotics.
aDuring 1 year before start of follow-up.
bDuring the entire follow-up period.
Secondary analyses were conducted with traditional multivariate-adjusted Cox regression models. These analyses were adjusted for sex, age at cohort entry, number of previous hospitalizations due to psychosis, time since first schizophrenia diagnosis, continuously updated variables for current vs no use of medications (lipid-modifying agents, opioid analgesics, nonopioid analgesics, anticholinergic anti-Parkinson drugs, and prior use of LAI), and continuously updated variables for the following diagnoses: substance abuse, cardiovascular disease, diabetes, asthma/chronic obstructive pulmonary disease, previous cancer, renal disease, or previous suicide attempt. Definitions of comorbidities and other medications are described in supplementary table 1. As sensitivity analyses, the main outcome measure was also assessed among incident patients, defined as having their first diagnoses of schizophrenia during follow-up and not having used antipsychotics a year prior to their index hospitalization. Other sensitivity analyses were conducted among those who had used olanzapine during the follow-up, with olanzapine as reference (the most commonly used antipsychotic in these cohorts).
The results are presented for the 10 most commonly used antipsychotics, namely (oral if not otherwise stated) clozapine, olanzapine, quetiapine, risperidone, risperidone LAI, aripiprazole, perphenazine, zuclopenthixol LAI, haloperidol, and levomepromazine. In addition to these, the rest of the oral medications were grouped into the category “other orals” and LAIs into “other LAIs” and, finally, all combinations were coded as polytherapy. The results are presented as adjusted hazard ratios with 95% CIs.
The Regional Ethics Board of Stockholm approved this research project (decision 2007/762–31). Permissions were granted by pertinent institutional authorities at the Finnish National Institute for Health and Welfare (permission THL/847/5.05.00/2015), the Social Insurance Institution of Finland (65/522/2015), and Statistics Finland (TK53-1042-15).
Results
Mean age at the beginning of follow-up was 46.2 years (SD 16.0) in the Finnish cohort and 44.9 (SD 12.0) in the Swedish cohort, with an equal gender distribution in the Finnish cohort (males 50.3%) and somewhat more men in the Swedish cohort (57.0%) (table 1).
The median follow-up time was 15.3 (interquartile range [IQR]: 7.9–22.0) years in the Finnish cohort and 9.8 (IQR 6.9–10.5) years in the Swedish cohort (corresponding to maximum follow-up times 22 and 11 years, respectively). Suicide attempt/death was recorded for 7.0% (n = 4315) and 7.4% (n = 2203) of the Finnish and Swedish cohorts, respectively (table 1). Compared to the entire cohorts, persons with suicide attempt/death were somewhat younger (5 years in the Swedish cohort and 9 years in the Finnish cohort), more likely to be women, and presented higher prevalence of previous substance abuse and suicide attempts. During the follow-up, the persons with suicidality continued having more substance abuse and had more use of all medication classes as compared to the total cohorts. The use of benzodiazepines and related drugs and lithium was more frequent and other mood stabilizer use less frequent in Sweden than in Finland.
Compared with no use of antipsychotics, clozapine use was the only antipsychotic consistently associated with a decreased risk of suicidal outcomes in within-individual analyses: HR for risk of attempted or completed suicide was 0.64 (95% CI: 0.49–0.84) in the Finnish cohort, and 0.66 (0.43–0.99) in the Swedish cohort in within-individual models (figure 1). When censoring the first 30 days from the beginning of use, clozapine was associated with a decreased risk of suicide attempt/death in the Finnish cohort (HR: 0.74, 0.56–0.99) but did not reach statistical significance in the Swedish cohort (HR: 0.67, 0.42–1.06; supplementary figure 2). Risperidone LAI was the second best performing antipsychotic, although not reaching statistical significance in any analyses (eg, for the main analyses, HR: 0.67, 0.35–1.26 in the Finnish cohort, HR: 0.78, 0.47–1.29 in the Swedish cohort). Incidence rates of the main outcome are presented in supplementary tables 2–3.
Fig. 1.
Hazard ratios (HRs) and 95% CIs of attempted or completed suicide associated with the use of specific antipsychotic monotherapies and combinations of antipsychotics (polytherapy) in within-individual model: (A) Finnish and B) Swedish schizophrenia cohort. The reference is no antipsychotic use. LAI, long-acting injectable antipsychotic.
Clozapine was also the only antipsychotic associated with decreased risk of attempted suicide, HR being 0.60 (0.46–0.79) in the Finnish cohort and 0.62 (0.40–0.95) in the Swedish cohort (supplementary figure 3). Sensitivity analyses with censoring the first 30 days from the beginning of use showed similar results (clozapine HR: 0.68, 0.51–0.92 in the Finnish cohort and HR: 0.63, 0.39–1.02 in the Swedish cohort; supplementary figure 4).
Superiority of clozapine was also demonstrated in traditional between-individual analyses, HR 0.35 (0.30–0.42) in the Finnish cohort and HR 0.41 (0.33–0.53) in the Swedish cohort (supplementary figure 5). In these between-individual models, other antipsychotic monotherapies were associated with decreased risk of suicide attempt/death, including risperidone LAI, other LAIs, oral risperidone, oral perphenazine, oral olanzapine, and polytherapy in the Finnish cohort and oral aripiprazole, oral risperidone, oral olanzapine, other LAI, quetiapine, and other orals in the Swedish cohort. These associations changed somewhat when the first 30 days of use were censored, although the clozapine results remained the same (supplementary figure 6).
Sensitivity analyses in the incident cohorts showed low numbers of events, and limited statistical power as presented with wide CIs: HR for clozapine was 0.62 (0.32–1.20) in the Finnish cohort and 0.41 (0.13–1.28) in the Swedish cohort. HR for risperidone LAI was 0.72 (0.16–3.12) in the Finnish cohort and 0.09 (0.01–0.81) in the Swedish cohort, with all other results, including the value of 1.00 in the CI. Sensitivity analyses among those persons who had used olanzapine also were in line with the primary analyses, clozapine being the only antipsychotic associated with a decreased risk in head-to-head comparison with olanzapine (HRs 0.45, 0.30–0.67 and 0.33, 0.14–0.80 in the Finnish and Swedish cohorts, respectively) and other antipsychotics being equal to olanzapine (supplementary figure 7).
Regarding adjunctive pharmacotherapies, benzodiazepines and Z-drugs were associated with an increased risk of attempted or completed suicides (HRs for benzodiazepines, 1.29–1.30, and, for Z-drugs, 1.33–1.62, not reaching statistical significance in the Swedish cohort; supplementary figure 8). In sensitivity analyses with censoring the first 30 days of use, Z-drug use was associated with an 80% and 58% increased risk of suicide attempt/death (HR 1.80, 1.12–2.90 in the Finnish cohort and HR 1.58, 1.09–2.30 in the Swedish cohort; supplementary figure 9), whereas a significant association for benzodiazepines was only noted in the Finnish cohort (1.26, 1.01–1.56 vs 1.17, 0.81–1.70). No significant associations were observed for antidepressants, mood stabilizers, or lithium in either of the cohorts.
Discussion
To our knowledge, this is the first study investigating the risk of attempted/completed suicide among persons with schizophrenia utilizing the within-individual comparison. We present the results from 2 nationwide cohorts of persons with schizophrenia, both showing clozapine as the only antipsychotic associated with a decreased risk of suicidal outcomes. Adjunctive pharmacotherapies either presented no association or were associated with a higher risk of suicidal outcomes, namely benzodiazepines and Z-drugs.
According to our results, for persons with suicide attempts, clozapine should be the preferred choice of antipsychotic. The main results and sensitivity analyses from both cohorts support this notion. Clozapine has also been shown to be associated with the lowest risk of completed suicide in previous studies.9,10,15 However, our results are contrary to previous reviews on potential antisuicidal effects of all SGAs13,14 as no other antipsychotic than clozapine was associated with a decreased risk of suicidal outcomes in the within-individual analyses. Some results showed a promising trend for risperidone LAI but, in almost all analyses, results for other SGAs remained insignificant. However, in general, the results are not purely explained by the lack of statistical power as the most frequently used antipsychotics, such as oral olanzapine, showed poor results in within-individual models. Frequent changes may be made in medication regimens within the month prior to the suicidal event, as the symptoms of suicidality worsen and require more intensive treatment. This phenomenon was observed in a previous study based on forensic toxicology of suicide victims.12 However, the effectiveness of most pharmacotherapies does not reach optimal levels during the first days or even weeks of use and may lead to a faulty association of medication use and suicidality, if this protopathic bias is not corrected for. However, our results did not show major differences between the main analysis and the analysis in which the first 30 days of treatment were censored.
By within-individual design,23 we were able to compare exposure periods of specific antipsychotics to time periods when no antipsychotic was used. This adjusts for patient-related, time-invariant factors, such as sex, genetics, and many life-style behaviors, which remain similar in longer follow-ups. Whereas, for a relatively rare outcome, such as the suicide attempt/death used in this study (present in for about 7% of both cohorts), the majority of observation time in traditional between-individual analyses comes from persons not having the outcome event (no suicidal behavior or at least no severe suicidal attempts), the within-individual results shown here demonstrate the real-world effectiveness among those individuals actually engaging in severe enough suicidal behavior leading to hospitalization or death.
The mechanisms behind the effectiveness of clozapine in preventing suicides and suicide attempts are not entirely clear, but it is known that clozapine’s ability to bind central nervous system receptors is distinct from other SGAs24 and, in RCTs, clozapine is substantially more efficacious than other antipsychotics for overall symptoms and, especially, for depressive symptoms that may predispose patients to suicidal behavior.25 Clozapine has effects on the norepinephrine system, which is linked to the emergence of depression26 and this may contribute to its effectiveness in suicide prevention. Clozapine is also known to be effective in reducing substance use behavior (or inhibiting initiation of substance use),27 and substance abuse is a risk factor for suicide.5 Clozapine is also known to have independent antiaggressive properties in patients suffering from different psychiatric disorders.28 As suicidality is often considered an act of aggression toward oneself, this might also be behind the protective properties. It could be argued that the superior effect of clozapine is due to frequent follow-ups by health care staff demanded by the safety blood draws included in the clozapine treatment regimen. However, if this was the case, we should see a similar beneficial effect with LAIs, since they are often administered weekly, biweekly, or monthly, often at intervals shorter than the follow-ups performed for clozapine users. As no such effect was observed with LAIs, it is unlikely that the results are only due to more frequent follow-ups.
Aripiprazole is one of the medications that showed varying results in within-individual vs between-individual comparisons and also between Finnish and Swedish cohorts. This could reflect possible selection of patients when comparing these analysis results. Aripiprazole use may be avoided in patients at acute risk for suicidal behavior, since one of the side effects of aripiprazole treatment is an initial increase in agitation and anxiety, especially if started at low doses, which clinicians may fear could result in the worsening of suicidal behavior, as reported by some case studies.29 Package information leaflet of aripiprazole also carries a warning of possible worsening of suicidal ideation, unlike, eg, olanzapine. Although this fear has later shown to likely not be valid,30 it may still have guided clinical prescribing during our study period. Also, as the CIs with aripiprazole are very wide due to a small number of outcome events, these results should be interpreted with caution.
Benzodiazepines and related drugs, so-called Z-drugs, were associated with an increased risk of attempted or completed suicide in most analyses. The fact that these associations did not weaken but strengthened when the first 30 days of use was censored from the analyses suggests that longer-term use is hazardous for suicidality outcomes. This is in line with a previous study on the cumulative use of benzodiazepines in schizophrenia, which found an up to 70% increased risk of suicidal death for benzodiazepine use.11 The use of benzodiazepines and related drugs has been linked with reduced impulse control, even after their hypnotic effect wears off, which increases the translation of suicidal ideation to suicidal actions.31,32 Our results likely reflect actual risks associated with benzodiazepine use, as our within-individual analysis eliminates selection bias.
Other adjunctive pharmacotherapies, namely antidepressants, lithium, and other mood stabilizers, did not show any impact on the risk of attempted or completed suicide. This is in line with previous studies, such as Reutfors et al,10 where no impact was observed for antidepressant or lithium use. Some previous studies have demonstrated a decreased overall mortality associated with antidepressant use11,33 but not with suicide mortality. Both a previous Finnish study and meta-analyses by Cipriani et al concluded that lithium use in mood disorders was associated with a decreased risk of suicide but not with suicide attempts34 or deliberate self-harm.35 In line with this, a previous study found that current use of antidepressants was associated with an increased risk of attempted suicide but decreased risk of completed suicide among cohorts of persons ever using antidepressants.36
The main strengths of our study include 2 large nationwide cohorts of persons with schizophrenia from 2 countries and, thus, the results are generalizable to countries that have a state-funded health care system, which provides patients with schizophrenia universal health care and antipsychotics with very low copayments. The long follow-up times, up to 22 years with Finnish and 11 years with Swedish data, are also strengths of this study. This is the first study analyzing suicidal outcomes by using each individual as their own control person, limiting the impact of selection bias and adjusting for all time-invariant features within the design. Antipsychotic and other medication use was modeled with the PRE2DUP method, which provides reliable estimates of duration and timing of drug exposure.19–22
The time-dependent fluctuation of clinical state could not fully be controlled for and, since treatments are started when the clinical state deteriorates, the effectiveness of treatments may be underestimated for all treatments in the primary analysis. However, a sensitivity analysis excluding the first month of treatment showed basically the same rank order for treatments as did the main analysis, which indicates that protopathic bias does not explain the differences in comparative effectiveness between the medications. Since within-individual analysis does not include those patients without outcome or variation in the drug exposure, we did also a secondary analysis by using between-individual design, which includes all patients. Also, results from this analysis were consistent with the results from the primary analysis. Early discontinuation of medications and short periods of nonadherence cannot be observed in register-based data, which is a limitation of our study. As there has generally been shown to be a substantial lag in setting a diagnosis of schizophrenia after the first onset of prodromal symptoms, it is possible that setting the inclusion criteria at schizophrenia diagnosis rather than the onset of prodromal symptoms may include some survival bias into the analysis. Patients committing suicides before receiving their schizophrenia diagnosis have not been included in the cohort.
Conclusion
The results from 2 large nationwide cohorts provide evidence on the comparative real-world effectiveness of specific antipsychotics in the prevention of severe suicidal behavior. Clozapine is the only pharmacological treatment associated with a substantially decreased risk of attempted or completed suicide and should be considered as first-line treatment for patients with suicidal ideation or behavior.
Supplementary Material
Funding
This study was funded by the Finnish Ministry of Social Affairs and Health through the developmental fund for Niuvanniemi Hospital. H.T. was funded by the Academy of Finland (grants 315969 and 320107). M.L. was partly funded by personal grants from the Finnish Medical Foundation and Emil Aaltonen Foundation. The funders of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding authors had full access to all of the data and the final responsibility to submit for publication.
Conflict of interest: J.T., E.M.-R., H.T., and A.T. have participated in research projects funded by grants from Janssen-Cilag and Eli Lilly to their employing institution. H.T. reports personal fees from Janssen-Cilag. J.T. reports personal fees from the Finnish Medicines Agency, European Medicines Agency, Eli Lilly, Janssen-Cilag, Lundbeck, and Otsuka, is a member of advisory board for Lundbeck, and has received grants from the Stanley Foundation and Sigrid Jusélius Foundation. M.L. is a board member of Genomi Solutions Ltd, has received honoraria from Sunovion Ltd and Orion Pharma Ltd, and research funding from the Finnish Medical Foundation and Emil Aaltonen Foundation.
References
- 1. Meltzer HY Treatment of suicidality in schizophrenia. Ann NY Acad Sci. 2001;932:44–58; discussion 58. [DOI] [PubMed] [Google Scholar]
- 2. Hor K, Taylor M. Suicide and schizophrenia: a systematic review of rates and risk factors. J Psychopharmacol. 2010;24(4 suppl):81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tanskanen A, Tiihonen J, Taipale H. Mortality in schizophrenia: 30-year nationwide follow-up study. Acta Psychiatr Scand. 2018;138(6):492–499. [DOI] [PubMed] [Google Scholar]
- 4. Westman J, Eriksson SV, Gissler M, et al. Increased cardiovascular mortality in people with schizophrenia: a 24-year national register study. Epidemiol Psychiatr Sci. 2018;27(5):519–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cassidy RM, Yang F, Kapczinski F, Passos IC. Risk factors for suicidality in patients with schizophrenia: a systematic review, meta-analysis, and meta-regression of 96 studies. Schizophr Bull. 2018;44(4):787–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bertelsen M, Jeppesen P, Petersen L, et al. Suicidal behaviour and mortality in first-episode psychosis: the OPUS trial. Br J Psychiatry. 2007;191(suppl 51):140–146. doi: 10.1192/bjp.191.51.s140. [DOI] [PubMed] [Google Scholar]
- 7. Nordentoft M, Madsen T, Fedyszyn I. Suicidal behavior and mortality in first-episode psychosis. J Nerv Ment Dis. 2015;203(5):387–392. [DOI] [PubMed] [Google Scholar]
- 8. Schneider-Thoma J, Efthimiou O, Huhn M, et al. Second-generation antipsychotic drugs and short-term mortality: a systematic review and meta-analysis of placebo-controlled randomised controlled trials. Lancet Psychiatry. 2018;5(8):653–663. [DOI] [PubMed] [Google Scholar]
- 9. Tiihonen J, Lönnqvist J, Wahlbeck K, et al. 11-year follow-up of mortality in patients with schizophrenia: a population-based cohort study (FIN11 study). Lancet. 2009;374(9690):620–627. [DOI] [PubMed] [Google Scholar]
- 10. Reutfors J, Bahmanyar S, Jönsson EG, et al. Medication and suicide risk in schizophrenia: a nested case-control study. Schizophr Res. 2013;150(2-3):416–420. [DOI] [PubMed] [Google Scholar]
- 11. Tiihonen J, Mittendorfer-Rutz E, Torniainen M, Alexanderson K, Tanskanen A. Mortality and cumulative exposure to antipsychotics, antidepressants, and benzodiazepines in patients with schizophrenia: an observational follow-up study. Am J Psychiatry. 2016;173(6):600–606. [DOI] [PubMed] [Google Scholar]
- 12. Forsman J, Taipale H, Masterman T, Tiihonen J, Tanskanen A. Adherence to psychotropic medication in completed suicide in Sweden 2006–2013: a forensic-toxicological matched case-control study. Eur J Clin Pharmacol. 2019;75:1421–1430. doi: 10.1007/s00228-019-02707-z. [DOI] [PubMed] [Google Scholar]
- 13. Kasckow J, Felmet K, Zisook S. Managing suicide risk in patients with schizophrenia. CNS Drugs. 2011;25(2):129–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pompili M, Baldessarini RJ, Forte A, et al. Do atypical antipsychotics have antisuicidal effects? A hypothesis-generating overview. Int J Mol Sci. 2016;17(10):1700. doi: 10.3390/ijms17101700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ringbäck Weitoft G, Berglund M, Lindström EA, Nilsson M, Salmi P, Rosén M. Mortality, attempted suicide, re-hospitalisation and prescription refill for clozapine and other antipsychotics in Sweden-a register-based study. Pharmacoepidemiol Drug Saf. 2014;23(3):290–298. [DOI] [PubMed] [Google Scholar]
- 16. Meltzer HY, Alphs L, Green AI, et al. Clozapine treatment for suicidality in schizophrenia: International Suicide Prevention Trial (InterSePT). Arch Gen Psychiatry. 2003;60(1):82–91. [DOI] [PubMed] [Google Scholar]
- 17. Taipale H, Mehtälä J, Tanskanen A, Tiihonen J. Comparative effectiveness of antipsychotic drugs for rehospitalization in schizophrenia—a nationwide study with 20-year follow-up. Schizophr Bull. 2018;44(6):1381–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tiihonen J, Mittendorfer-Rutz E, Majak M, et al. Real-world effectiveness of antipsychotic treatments in a nationwide cohort of 29 823 patients with schizophrenia. JAMA Psychiatry. 2017;74(7):686–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tanskanen A, Taipale H, Koponen M, et al. From prescription drug purchases to drug use periods—a second generation method (PRE2DUP). BMC Med Inform Decis Mak. 2015;15:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Taipale H, Tanskanen A, Koponen M, Tolppanen AM, Tiihonen J, Hartikainen S. Agreement between PRE2DUP register data modeling method and comprehensive drug use interview among older persons. Clin Epidemiol. 2016;8:363–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tanskanen A, Taipale H, Koponen M, et al. Drug exposure in register-based research-An expert-opinion based evaluation of methods. PLoS One. 2017;12(9):e0184070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Forsman J, Taipale H, Masterman T, Tiihonen J, Tanskanen A. Comparison of dispensed medications and forensic-toxicological findings to assess pharmacotherapy in the Swedish population 2006 to 2013. Pharmacoepidemiol Drug Saf. 2018;27(10):1112–1122. doi: 10.1002/pds.4426. [DOI] [PubMed] [Google Scholar]
- 23. Lichtenstein P, Halldner L, Zetterqvist J, et al. Medication for attention deficit-hyperactivity disorder and criminality. N Engl J Med. 2012;367(21):2006–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mauri MC, Paletta S, Maffini M, et al. Clinical pharmacology of atypical antipsychotics: an update. EXCLI J. 2014;13:1163–1191. http://www.excli.de/vol13/Mauri_13102014_proof.pdf%5Cnhttp://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=emed12&NEWS=N&AN=2014834064. Accessed October 19, 2019. [PMC free article] [PubMed] [Google Scholar]
- 25. Huhn M, Nikolakopoulou A, Schneider-Thoma J, et al. Comparative efficacy and tolerability of 32 oral antipsychotics for the acute treatment of adults with multi-episode schizophrenia: a systematic review and network meta-analysis. Lancet. 2019;394(10202):939–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Khokhar JY, Henricks AM, Sullivan EDK, Green AI.. Unique Effects of Clozapine: A Pharmacological Perspective. Vol 82 1st ed. Elsevier Inc. Adv Pharmacol.2018;82:137–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Krause M, Huhn M, Schneider-Thoma J, Bighelli I, Gutsmiedl K, Leucht S. Efficacy, acceptability and tolerability of antipsychotics in patients with schizophrenia and comorbid substance use. A systematic review and meta-analysis. Eur Neuropsychopharmacol. 2019;29(1):32–45. doi: 10.1016/j.euroneuro.2018.11.1105. [DOI] [PubMed] [Google Scholar]
- 28. Kraus JE, Sheitman BB. Clozapine reduces violent behavior in groups. J Neuropsychiatr. 2005;17(1):36–44. [DOI] [PubMed] [Google Scholar]
- 29. Padder T, Skodnek K, Hashmi S, et al. Acute akathisia with suicidal ideation associated with low dose aripiprazole. Psychiatry (Edgmont). 2006;3(4):40–43. http://www.ncbi.nlm.nih.gov/pubmed/21103170%0Ahttp://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC2990567. Accessed October 19, 2019. [PMC free article] [PubMed] [Google Scholar]
- 30. Ulcickas Yood M, Delorenze G, Quesenberry CP Jr, et al. Epidemiologic study of aripiprazole use and the incidence of suicide events. Pharmacoepidemiol Drug Saf. 2010;19(11):1124–1130. [DOI] [PubMed] [Google Scholar]
- 31. Dodds TJ Prescribed benzodiazepines and suicide risk: a review of the literature. Prim Care Companion CNS Disord. 2017;19(2). doi: 10.4088/PCC.16r02037. [DOI] [PubMed] [Google Scholar]
- 32. Sun Y, Lin CC, Lu CJ, Hsu CY, Kao CH. Association between zolpidem and suicide: a nationwide population-based case-control study. Mayo Clin Proc. 2016;91(3):308–315. [DOI] [PubMed] [Google Scholar]
- 33. Tiihonen J, Suokas JT, Suvisaari JM, Haukka J, Korhonen P. Polypharmacy with antipsychotics, antidepressants, or benzodiazepines and mortality in schizophrenia. Arch Gen Psychiatry. 2012;69(5):476–483. [DOI] [PubMed] [Google Scholar]
- 34. Toffol E, Hätönen T, Tanskanen A, et al. Lithium is associated with decrease in all-cause and suicide mortality in high-risk bipolar patients: a nationwide registry-based prospective cohort study. J Affect Disord. 2015;183:159–165. [DOI] [PubMed] [Google Scholar]
- 35. Cipriani A, Hawton K, Stockton S, Geddes JR. Lithium in the prevention of suicide in mood disorders: updated systematic review and meta-analysis. BMJ. 2013;346:f3646. [DOI] [PubMed] [Google Scholar]
- 36. Tiihonen J, Lönnqvist J, Wahlbeck K, Klaukka T, Tanskanen A, Haukka J. Antidepressants and the risk of suicide, attempted suicide, and overall mortality in a nationwide cohort. Arch Gen Psychiatry. 2006;63(12):1358–1367. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.