Abstract
Background
Percutaneous peripheral nerve stimulation (PNS) is an analgesic modality involving the insertion of a lead through an introducer needle followed by the delivery of electric current after needle withdrawal. This modality has been used extensively to treat chronic pain, but only small series have been published involving postoperative pain. The ultimate objective of this study is to determine the postoperative effects of percutaneous PNS following moderately to severely painful ambulatory surgery within a real-world clinical practice setting. The primary hypothesis is that surgical pain and opioid consumption during the initial 7 days after surgery will be reduced by percutaneous PNS compared with usual and customary analgesia (dual primary outcome measures).
Design
A multicenter pragmatic effectiveness trial. We are randomizing participants having painful orthopedic surgical procedures of the upper and lower extremity to receive 14 days of either 1) electrical stimulation or 2) sham in a double-masked fashion. End points are being assessed at various time points over 12 postoperative months.
Summary
The postoperative experience will be much improved if percutaneous PNS provides potent analgesia while concurrently decreasing opioid requirements following painful surgery. Because this modality can be administered for up to 60 days at home, it may provide postoperative analgesia that outlasts surgical pain yet has relatively few risks and, unlike opioids, has no systemic side effects or potential for abuse, addiction, and overdose. Percutaneous PNS has the potential to revolutionize postoperative analgesia as it has been practiced for the past century. This study will inform key stakeholders regarding an evidence-based nonpharmacologic approach to the management of postoperative pain.
Keywords: Percutaneous Peripheral Nerve Stimulation, Postoperative Pain, Postoperative Analgesia, Neuromodulation
Background and Rationale
There are tens of millions of ambulatory surgical procedures performed in the United States annually [1]. Over 80% of patients experience inadequate pain relief following surgery, with negative consequences for both individuals and society [2, 3]. For patients, inadequate postoperative analgesia results not only in suffering but also in increased risk of comorbidity (e.g., perioperative myocardial infarction), inferior rehabilitation [4], and a greater likelihood of transition from acute pain to persistent (“chronic”) postsurgical pain (incidence, 10–50%) [5]. The latter frequently results in decreased productivity and a strain on personal relationships as well as an increased risk of depression, chronic low back and joint pain, obesity, and accelerated onset of cardiovascular disease [6].
Clearly, inadequately controlled postsurgical pain is a substantial problem that is intimately related to a reliance on perioperative opioid use—the foundation of postoperative analgesia for over a century. Unfortunately, opioids have significant undesirable consequences for both individuals and society. Frequent systemic side effects such as nausea, vomiting, and pruritus are irritants [7, 8], but some effects may be fatal, such as cognitive impairment and respiratory depression. Even minor ambulatory surgical procedures can lead to chronic opioid use [9], with significant negative consequences such as hyperalgesia, dependence, decreased quality of life, and substance use disorder.
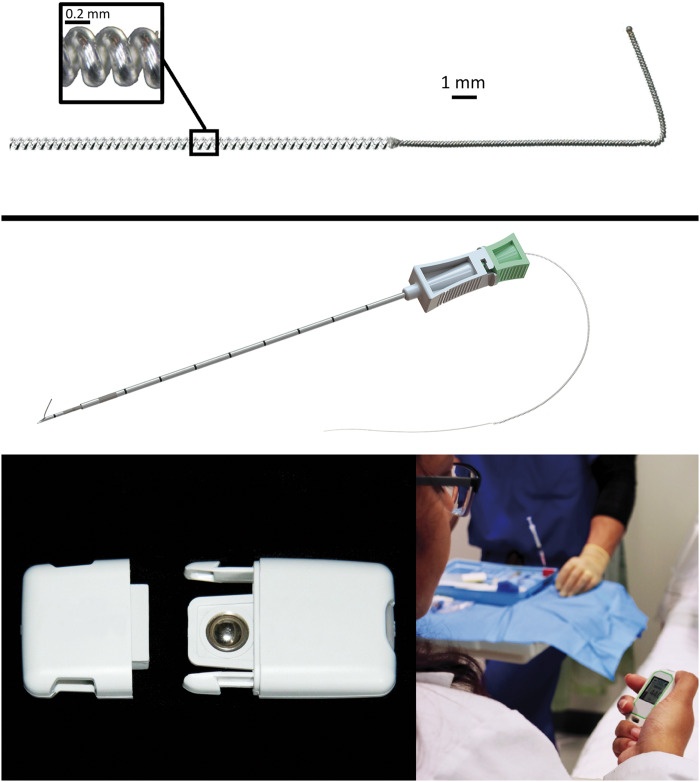
One analgesic alternative—percutaneous peripheral nerve stimulation (PNS)—may improve postsurgical analgesia while simultaneously decreasing or obviating opioid requirements. Furthermore, this modality is without risk of adverse systemic side effects [10]. Small-diameter, insulated electrical leads are available that permit relatively rapid percutaneous insertion through a needle, obviating the need for a surgical incision (Figure 1) [10]. Using ultrasound to guide placement, a lead may be reliably inserted approximately 1 cm from a peripheral nerve using landmarks and established techniques for peripheral nerve block administration [10]. An external stimulator is small enough to be directly adhered to the skin and subsequently delivers a small electric current through the insulated lead to the target nerve (Figure 1) [10].
Figure 1.
A percutaneous peripheral nerve stimulation system approved by the US Food and Drug Administration to treat acute pain (OnePass, SPR Therapeutics, Cleveland, OH). The insulated lead (MicroLead, SPR Therapeutics) is 0.2 mm in diameter wrapped into a helical coil 0.6 mm in diameter (top panel), which is percutaneously inserted using a preloaded introducer (middle panel). The rechargeable battery snaps into the pulse generator (SPRINT PNS System, SPR Therapeutics) and is controlled with a handheld remote control (bottom panels). Used with permission from Brian M. Ilfeld, MD, MS.
Ultrasound-guided percutaneous PNS was first reported in situ by Huntoon and Burgher in 2009 [11] using an epidural neurostimulation electrode for the treatment of neuropathic pain. Although multiple different lead designs and percutaneous approaches have been subsequently reported, they were used nearly exclusively for chronic pain conditions [10]. More recently, the US Food and Drug Administration (FDA) cleared the first percutaneous PNS lead and pulse generator system for use treating acute (postoperative) pain (Figure 1) [10]. The pulse generators have a mass (30 g) and footprint (6.2 × 3.7 × 1.4 cm) small enough to allow the unit to be adhered directly to the patient. Replaceable or rechargeable batteries permit prolonged application, with an FDA-defined maximum of 60 indwelling days. The 2-month duration offers the possibility of a perioperative analgesic modality that, for most patients, should significantly outlast the surgical pain being treated while also offering an option for patients whose pain has become chronic, lasting past the time of normal tissue healing [12].
Since receiving regulatory approval, multiple case reports and small series have suggested significant analgesic- and opioid-sparing benefits following painful surgical procedures [10]. The ultimate objective of this study is to determine the postoperative effects of percutaneous PNS following moderately to severely painful ambulatory surgery within a real-world clinical practice setting. We therefore initiated a randomized controlled pilot study applying percutaneous PNS for ambulatory orthopedic surgical procedures to 1) determine the feasibility of and optimize a study protocol and 2) estimate the treatment effect of this intervention to power a subsequent definite trial (NCT03481725). At the time of this writing, the temporary cessation of elective ambulatory surgery due to the COVID-19 pandemic limited enrollment to 61 of an anticipated 64 participants. Nevertheless, following eventual completion of the pilot study, we will undertake the multicenter pragmatic clinical trial described in this article, with the ultimate objective being to determine the postoperative effects of percutaneous PNS following moderately to severely painful ambulatory surgery.
Methods
The ultimate objective of the proposed research is to determine the postoperative effects of percutaneous PNS following moderately to severely painful ambulatory surgery. The primary specific aim of the trial is to determine the effect of percutaneous PNS on postoperative opioid requirements and analgesia following moderately to severely painful ambulatory surgery. The secondary specific aims are to determine the effect of percutaneous PNS on physical and emotional functioning, chronic pain, and quality of life following moderately to severely painful ambulatory surgery.
This will be a multicenter, randomized, quadruple-masked, sham-controlled, parallel-arm, pragmatic (Figure 2) clinical trial with human participants. The study will be overseen by a data safety monitoring board composed of two physicians familiar with the ethical conduct of clinical research and one biostatistician. The details of the following description may change prior to enrollment initiation based on the findings of a currently ongoing pilot study (ClinicalTrials.gov Id: NCT03481725). We will include a diverse group of seven recruitment sites that will provide a broad, representative patient sample of active-duty military members and civilians with ethnic, racial, and socioeconomic diversity within a wide geographic range (Table 1). The trial will be prospectively registered at ClinicalTrials.gov, and the protocol will be approved by the institutional review board at each of the enrolling centers as well as the US Army Medical Research and Development Command Human Research Protection Office. An independent, three-person data safety monitoring board consisting of two physician subject matter experts and a biostatistician will be responsible for the conduct and oversight of all aspects of the investigation from the planning phase through data analysis. Written informed consent will be obtained from all participants.
Figure 2.
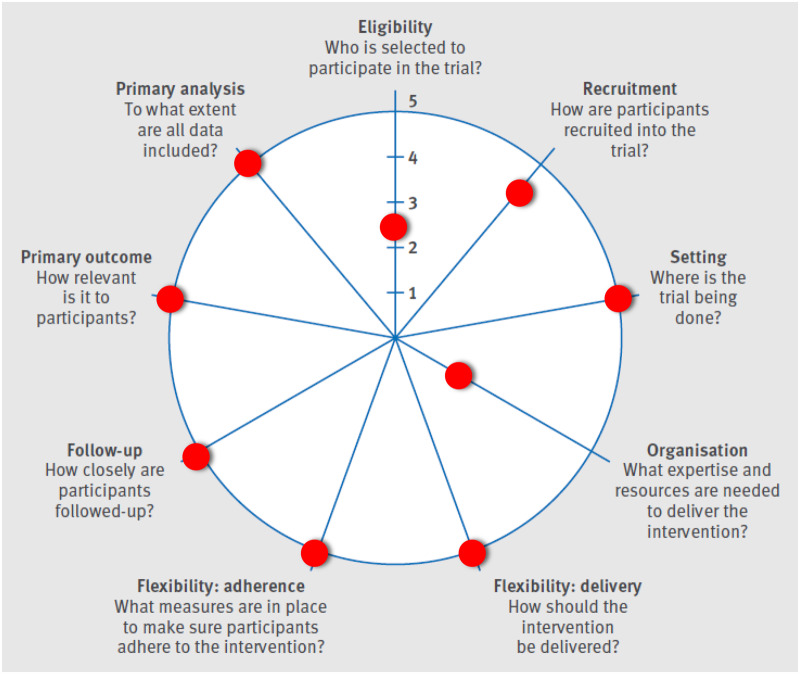
The Pragmatic-Explanatory Continuum Indicator Summary 2 (PRECIS-2) wheel. Adapted by permission from BMJ Publishing Group Limited. [The PRECIS-2 tool: designing trials that are fit for purpose, Loudon K, Treweek S, Sullivan F, Donnan P, Thorpe KE, Zwarenstein M. BMJ 2015;350:h2147].
Table 1.
Enrolling centers and local principal investigators
| Facility | Location | Investigator |
|---|---|---|
| United States Military Treatment Facilities | ||
| Brooke Army Medical Center | San Antonio, TX | Sandeep Dhanjal, MD |
| Naval Medical Center San Diego | San Diego, CA | Robert Hackworth, MD |
| Walter Reed National Military Medical Center | Bethesda, MD | Harold Gelfand, MD |
| Womack Army Medical Center | Fayetteville, NC | Anthony Plunkett, MD |
| Civilian University Medical Centers | ||
| Cedars-Sinai Medical Center | Los Angeles, CA | Alice Vijjeswarapu, MD |
| Cleveland Clinic | Cleveland, OH | Alparslan Turan, MD |
| University of California, San Diego | San Diego, CA | John Finneran IV, MD |
Participants
Enrollment will be offered to adult patients at least 18 years of age undergoing ambulatory orthopedic surgery with a planned single-injection peripheral nerve block for postoperative analgesia. The surgical procedures included will be rotator cuff repair, hallux valgus correction, and ankle arthrodesis or arthroplasty. Patients will be excluded for any of the following:
Chronic analgesic use, including opioids (daily use within the 2 weeks prior to surgery and duration of use >4 weeks).
Neuromuscular deficit of the target nerve(s).
Compromised immune system based on medical history (e.g., immunosuppressive therapies such as chemotherapy, radiation, sepsis, infection) or other conditions that place the participant at increased risk.
Implanted spinal cord stimulator, cardiac pacemaker or defibrillator, deep brain stimulator, or other implantable neurostimulator whose stimulus current pathway may overlap.
History of bleeding disorder.
Antiplatelet or anticoagulation therapies other than aspirin due to the risk of bleeding with a 20-gauge insertion needle.
Allergy to skin-contact materials (occlusive dressings, bandages, tape, and so on).
Incarceration.
Pregnancy.
Chronic pain of greater than 3 months of any severity in an anatomic location other than the surgical site.
Anxiety disorder.
History of substance abuse.
Inability to contact the investigators during the treatment period and vice versa (e.g., lack of telephone access).
The study will be offered to potential participants within 2 weeks prior to surgery (institutional review board partial waiver to allow adherence to Health Insurance Portability and Accountability Act regulations). Individuals wishing to participate will have a percutaneous lead inserted within 7 days prior to surgery (but usually within 24 hours of the procedure). Participants will have a brachial plexus (shoulder) or a subgluteal sciatic (foot and ankle) lead inserted under ultrasound guidance using procedures described previously (OnePass and MicroLead, SPR Therapeutics, Cleveland, OH) [14, 15]. Immediately prior to surgery, each participant will receive either an ultrasound-guided interscalene (shoulder) or a popliteal sciatic (foot and ankle) nerve block with ropivacaine 0.5% and epinephrine (20 mL).
Treatment Group Assignment
Only after lead insertion confirmation will each participant be randomly allocated to one of two possible treatments: either receiving electric current (experimental group) or not (control group). Randomization will be stratified by institution and anatomic lead location (Table 1) in a 1:1 ratio and in randomly chosen block sizes. Randomization lists for each enrolling center will be created by the informatics group at the Cleveland Clinic using computer-generated lists. Treatment group assignment will be conveyed to the enrolling sites via the same secure web-based system used to collect and collate all post-intervention outcomes. Stimulators are capable of being programmed to either pass electrical current or not pass electrical current. These two modes (active and sham) are indistinguishable in appearance, and therefore investigators, participants, and all clinical staff will be masked to treatment group assignment, with the only exception being the unmasked individual who programs the stimulator (and who will not have direct contact with the participant following programming). The unmasked individual will have access to the randomization list on REDCap, will program the stimulator appropriately (sham vs active), and will provide the individual interacting with the study participant with the device without indicating the treatment group.
Following surgery, stimulators (SPRINT, SPR Therapeutics, Cleveland, OH) will be attached to the leads and initiated within the recovery room. Participants and their caretakers will be educated on lead and stimulator care and functioning and will be informed that patients frequently do not have the sensations postoperatively that were experienced during preoperative lead insertion as therapeutic benefit with subthreshold stimulation occurs [16]. In other words, once proper lead placement is confirmed with comfortable sensations during insertion, therapeutic levels of stimulation may be delivered subthreshold—below the intensity required for sensation yet still providing relief—following surgery. Stimulation will be delivered with a square waveform at 100 Hz. Although the frequency will be fixed following the initial programming, the amplitude and pulse duration may be adjusted by the patient within a provider-defined range with a small Bluetooth-connected remote control (Figure 1). Participants will be provided with two rechargeable batteries, will be instructed to keep one in the charger with the other in the functioning unit, and will need to exchange these two batteries at the same time once a day. A carryover analgesic effect of more than 2 hours allows showering following temporary stimulator disconnection and removal.
Prior to discharge, participants and their caretakers will be provided with verbal and written stimulator and lead instructions and the telephone and pager numbers of a local health care provider who is available at all times. Participants will be discharged home with their leads in situ and with a prescription for immediate-release oral opioid tablets (oxycodone 5 mg; 1–2 tablets every 4–6 hours; No. 40). Participants will be contacted by telephone for end-point collection. Health care providers will remove the lead on postoperative day 14 (±3 days). Similar to perineural catheters [4] , this procedure encompasses simply removing the occlusive dressing and gently pulling on the lead. Following study completion, the results will be provided to all enrolled participants using nontechnical language.
Outcome Measurements (End Points)
We have selected outcome measures that have established reliability and validity, have minimal interrater discordance, and are recommended for pain-related clinical trials by the World Health Organization and the Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT) consensus statement [17]. Outcomes will be evaluated at baseline and up to 12 months following surgery (Table 2).
Table 2.
Summary of post-enrollment assessments
| Time Point | Postoperative Days |
Postoperative Months |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 7 | 10 | 18 | 1 | 3 | 6 | 12 | |
| Opioid consumption, previous 24 h | • | • | • | • | • | • | • | • | • | • | • | • |
| Nausea and vomiting | • | • | • | • | • | • | • | • | ||||
| Average pain (NRS) | • | • | • | • | • | • | • | • | • | • | • | • |
| Worst pain (NRS) | • | • | • | • | • | • | • | • | • | • | • | • |
| Brief Pain Inventory, Short Form | • | • | • | • | • | • | • | • | ||||
| World Health Organization Quality of Life Instrument | • | • | • | • | • | |||||||
| Posttraumatic Stress Disorder Checklist (PCL-C) | • | |||||||||||
| Masking assessment | • | |||||||||||
NRS = Numeric Rating Scale.
Participants will have demographic and anthropomorphic data collected as well as comorbidities and medications. In addition, because posttraumatic stress disorder (PTSD) may be associated with the severity of pain [18], at baseline we will apply the PTSD Checklist (PCL-C), a 20-item self-report measure reflecting symptoms of PTSD validated in military [19], veteran [20–22], and civilian populations [23]. Postoperatively, surgical end points will be recorded such as surgical duration, tourniquet duration (if applicable), analgesic administration, and anesthetic administered. Participant demographic, surgical, and percutaneous PNS administration data will be uploaded from each enrolling center via the internet to a secure [24], password-protected, encrypted central server (REDCap, Department of Outcomes Research, Cleveland Clinic, Cleveland, OH) [25]. All data collection following the day of enrollment (postoperative day 0)—regardless of enrolling center—will be collected by telephone by blinded staff from the University of California, San Diego, minimizing interrater discordance. This study-specific data will not be available in the standard electronic health records, as participants will be outpatients.
Primary Outcome Measures
We will test the two hypotheses that 1) opioid consumption and 2) surgical pain will be significantly decreased within the first 4 days following surgery with percutaneous PNS compared with usual and customary analgesia. The first co-primary outcome will be cumulative opioid use from discharge until postoperative day 4 (opioid consumption will be self-reported for the previous 24 hours at each time point). The second co-primary outcome is designated as the mean value of the “average” pain scores for postoperative days 0–7. Current, worst, least, and average pain at the surgical site will be assessed using a numeric rating scale (NRS) as part of the Brief Pain Inventory (Short Form) [26]. The use of single items (e.g., average pain) in addition to the composite score is supported by the IMMPACT recommendations for assessing pain in clinical trials [27–29]. The NRS will be recorded at all time points (Table 2), with the primary end point being the mean value of the “average” pain scores for postoperative days 0–7. The NRS is a highly sensitive measure of pain intensity with numbers ranging from 0 to 10, where 0 is equivalent to no pain and 10 is equivalent to the worst imaginable pain. The NRS has been demonstrated to be a valid and reliable measure following analgesic interventions [30]. In addition, NRS scores correlate well with other measures of pain intensity [31] and demonstrate high test-retest reliability [32]. These NRS characteristics led to recent IMMPACT consensus recommendations for use of the 10-point NRS of pain intensity for pain trials [17]. In order to claim that percutaneous PNS is superior to usual and customary analgesia, at least one of hypotheses 1 and 2 must be superior with the other being either superior or at least noninferior.
Secondary Outcome Measures
We will also test the hypothesis that physical and emotional functioning will be significantly improved with percutaneous PNS as compared with usual and customary analgesia as measured with the Brief Pain Inventory (Short Form). This instrument includes—in addition to pain intensity scales—seven measures evaluating the ability of pain to interfere with physical and emotional functioning, such as sleep, relations with others, and enjoyment of life (the Interference subscale) [33, 34]. Finally, we will test the two hypotheses that 1) the incidence and intensity of chronic pain will be significantly decreased 6 months following surgery and 2) quality of life will be significantly improved 1 month following surgery with percutaneous PNS compared with usual and customary analgesia. Chronic pain will be evaluated using the “worst” pain measured with the NRS, and quality of life will be measured with the World Health Organization Quality of Life-BREF [35–37]. This instrument was developed by the World Health Organization to focus on those aspects of life most important to patients and is composed of 24 questions assessing four dimensions: 1) physical health; 2) psychological health; 3) social relationships; and 4) environment [38]. The first two dimensions will be of greater interest than the remaining two.
Known possible adverse events include infection (approximately 1 for every 32,000 indwelling days) [39]; bleeding; discomfort due to overstimulation; and lead dislodgement, migration, and fracture. No nerve injury due to percutaneous PNS has been reported, but it is always a possibility with perineural manipulation. All adverse events will be reported to the institutional review boards, the data safety monitoring boards, and the Army Human Research Protections Office.
Statistical Analysis
At the time of this writing, the pilot study to determine the treatment effect size estimation has not been completed due to the COVID-19 pandemic and the resulting temporary cessation of elective surgical procedures. When the study is ultimately completed, data from this investigation will be used to determine the sample size of the definitive trial. The statistical plan will be created, approved by regulatory oversight bodies, and posted to ClinicalTrials.gov prior to initiation of enrollment in the definitive trial. At the time of this writing—although this may be revised following analysis of the pilot study data—we plan to first test for noninferiority of PNS to usual care on each of the two primary outcomes using one-tailed noninferiority tests. The noninferiority deltas will be 1 point (worse) in pain score and 20% higher in opioid consumption. Noninferiority will be assessed at the overall 0.05 significance level with no adjustment to the significance criterion for testing two outcomes because noninferiority is required on both outcomes—i.e., an intersection union test. A noninferiority delta of 1 point in pain score is conservative, as receiver operating characteristic curve analysis has demonstrated that changes from baseline of at least 1.7 along a 10-point NRS accurately identified patients who rated improvements as “much improved” or higher compared with those who perceived no change or worsening pain following analgesic interventions [28, 31, 40–42].
Discussion
Current treatment for postsurgical pain overwhelmingly involves opioid analgesics. For ambulatory surgery, prescriptions for opioids are provided that in nearly all cases exceed the required number of tablets. This is because it is impossible to predict how many tablets each individual will require [43]. The results are inadequate analgesia due to the relatively low potency of oral analgesics [2, 3]; undesired and sometimes dangerous side effects [7, 8]; and literally billions of unused opioid tablets, of which approximately 500 million doses are diverted and abused annually [44]. Alternative medications such as gabapentinoids and tricyclic antidepressants also have significant drawbacks, such as a lack of efficacy for inflammatory pain, a negative impact on reaction time, and impairment of the ability to work and function [45].
An alternative non-opioid analgesic class—local anesthetic—has been used for over a century to provide potent, targeted perioperative anesthesia and analgesia. However, the duration of even the longest-acting clinically available local anesthetic—bupivacaine—provides analgesia for no more than 18 hours when either infiltrated or included in a single-injection peripheral nerve block. The duration of a peripheral nerve block may be increased using “perineural local anesthetic infusion”: a catheter is percutaneously inserted adjacent to a peripheral nerve, followed by prolonged administration of local anesthetic [46]. Unfortunately, such “continuous blocks” have their own set of limitations, including a duration usually limited to less than 4 days due to the risk of infection and, for ambulatory patients, the burden of carrying an infusion pump and local anesthetic reservoir that also limits duration to usually less than 3 days. Additionally, catheters frequently dislodge and leak fluid, and local anesthetic toxicity with the risk of cardiac fibrillation is possible. Moreover, catheters can be challenging to accurately insert—even with the use of ultrasound—because the precise tissue plane adjacent to the target nerve (usually a potential space) must be precisely cannulated. Most significantly, perineural infusion induces motor, sensory, and proprioception blocks that limit ambulation and physical therapy and increase the risk of falling [47].
In sharp contrast, percutaneous PNS negates nearly all of these substantial issues. This novel technique induces no proprioceptive, motor, or sensory deficits, permitting unconstrained participation in physical therapy without increasing the risk of falling. Unlike with continuous blocks, there is no risk of local anesthetic leakage or toxicity, the latter allowing the concurrent use of multiple leads [12]. Helically coiled leads minimize the risks of migration, dislodgement, and infection, permitting a dramatically long duration of lead retention—in some cases, well over a year [39]. The footprint of new electrical generators is so small that these may be adhered directly to the patient (Figure 1), thus avoiding the patient burden of carrying a heavy local anesthetic reservoir and portable infusion pump [48]. Combined, these characteristics permit a far longer duration of use for percutaneous PNS compared with continuous peripheral nerve blocks. With up to 60 days of indwelling use, this modality essentially outlasts the pain resulting from nearly all surgical procedures, potentially negating the need for a large (or perhaps any) opioid prescription. Percutaneous PNS has the potential to revolutionize postoperative analgesia as it has been practiced for the past century by improving pain control without any adverse systemic side effects and by decreasing or possibly negating opioid requirements [49].
Pragmatism vs Efficacy
As Loudon and colleagues [13] explained, “there are few purely explanatory or pragmatic trials; rather than dichotomy there is a continuum.” The current study contains aspects of both types of trials. The setting of the trial, intervention delivery and adherence, primary outcome, and primary analysis are strongly associated with pragmatic trials (Figure 2). However, there are aspects of the trial that are not clearly consistent with the intent of pragmatic trials, such as multiple exclusion criteria, inclusion of a sham treatment arm, and additional data collection at more time points than the current standard of care. These aspects will increase internal validity at the expense of external validity. We have attempted to balance the requirements for these two sometimes-conflicting aspects to produce a pragmatic effectiveness trial that will inform key stakeholders regarding this nonpharmacologic approach to the management of postoperative pain and that will concurrently provide evidence for the efficacy of this relatively new postoperative analgesic modality.
Acknowledgments
The authors appreciate the invaluable assistance of Baharin Abdullah (Clinical Translational Research Center, University of California, San Diego, La Jolla, CA).
Funding sources: The US Army Medical Research Acquisition Activity, 820 Chandler Street, Fort Detrick, MD 21702-5014, is the awarding and administering acquisition office. This study was supported by the Assistant Secretary of Defense for Health Affairs endorsed by the Department of Defense through the Pain Management Collaboratory Pragmatic Clinical Trials Demonstration Projects under Awards No. W81XWH-18-2-0003, W81XWH-18-2-0007, W81XWH-18-2-0008, and W81XWH-18-2-0009. The research reported in this article was supported by the National Center for Complementary and Integrative Health of the National Institutes of Health under Award No. U24AT009769. The opinions, interpretations, conclusions, and recommendations are those of the authors and are not necessarily endorsed by the funding agencies. This article is a product of the National Institutes of Health–Department of Defense–Veterans Affairs Pain Management Collaboratory. For more information about the Collaboratory, visit https://painmanagementcollaboratory.org.
Conflicts of interest: James C. Eisenach, MD, provides consulting to Adynxx (San Francisco, CA). Harold Gelfand, MD, is participating in research funded by the Henry Jackson Foundation (Bethesda, MD) through a grant from Pacira Pharmaceuticals (Parsippany, NJ). The institution of Brian Ilfeld, MD, MS, has received funding from SPR Therapeutics (Cleveland, OH) for other research studies. The institution has also received funding for other research from Heron Pharmaceuticals (San Diego, CA), Infutronix (Natick, MA), Epimed International (Farmers Branch, TX), Myoscience (Fremont, CA), and Ferrosan Medical (Szczecin, Poland). Daniel I. Sessler, MD, is a consultant for Pacira Pharmaceuticals (Parsippany, NJ). The author’s institution receives funding from Pacira Pharmaceuticals and Heron Therapeutics (San Diego, CA). The institution of Alparslan Turan, MD, receives funding from Pacira Pharmaceuticals (Parsippany, NJ). The remaining authors report no conflicts of interest.
Supplement sponsorship: This article appears as part of the supplement entitled “NIH-DOD-VA Pain Management Collaboratory (PMC)”. This supplement was made possible by Grant Number U24 AT009769 from the National Center for Complementary and Integrative Health (NCCIH), and the Office of Behavioral and Social Sciences Research (OBSSR). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NCCIH, OBSSR, and the National Institutes of Health.
Trial registration: ClinicalTrials.gov Id: NCT03481725
References
- 1. Cullen KA, Hall MJ, Golosinskiy A.. Ambulatory surgery in the United States, 2006. Natl Health Stat Report 2009;28(11):1–25. [PubMed] [Google Scholar]
- 2. Correll DJ, Vlassakov KV, Kissin I.. No evidence of real progress in treatment of acute pain, 1993-2012: Scientometric analysis. J Pain Res 2015;7:199–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Apfelbaum JL, Chen C, Mehta SS, Gan TJ.. Postoperative pain experience: Results from a national survey suggest postoperative pain continues to be undermanaged. Anesth Analg 2003;97(2):534–40. [DOI] [PubMed] [Google Scholar]
- 4. Ilfeld BM, Vandenborne K, Duncan PW, et al. Ambulatory continuous interscalene nerve blocks decrease the time to discharge readiness after total shoulder arthroplasty: A randomized, triple-masked, placebo-controlled study. Anesthesiology 2006;105(5):999–1007. [DOI] [PubMed] [Google Scholar]
- 5. Kehlet H, Jensen TS, Woolf CJ.. Persistent postsurgical pain: Risk factors and prevention. Lancet 2006;367(9522):1618–25. [DOI] [PubMed] [Google Scholar]
- 6. Robbins CB, Vreeman DJ, Sothmann MS, Wilson SL, Oldridge NB.. A review of the long-term health outcomes associated with war-related amputation. Mil Med 2009;174(6):588–92. [DOI] [PubMed] [Google Scholar]
- 7. Dahan A, Aarts L, Smith TW.. Incidence, reversal, and prevention of opioid-induced respiratory depression. Anesthesiology 2010;112(1):226–38. [DOI] [PubMed] [Google Scholar]
- 8. Chidambaran V, Olbrecht V, Hossain M, Sadhasivam S, Rose J, Meyer MJ.. Risk predictors of opioid-induced critical respiratory events in children: Naloxone use as a quality measure of opioid safety. Pain Med 2014;15(12):2139–49. [DOI] [PubMed] [Google Scholar]
- 9. Sun EC, Darnall BD, Baker LC, Mackey S.. Incidence of and risk factors for chronic opioid use among opioid-naive patients in the postoperative period. JAMA Intern Med 2016;176(9):1286–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ilfeld BM, Finneran JJ IV. Cryoneurolysis and percutaneous peripheral nerve stimulation to treat acute pain in the time of the opioid crisis: A narrative review. Anesthesiology. In press. [DOI] [PubMed] [Google Scholar]
- 11. Huntoon MA, Burgher AH.. Ultrasound-guided permanent implantation of peripheral nerve stimulation (PNS) system for neuropathic pain of the extremities: Original cases and outcomes. Pain Med 2009;10(8):1369–77. [DOI] [PubMed] [Google Scholar]
- 12. Ilfeld BM, Grant SA.. Ultrasound-guided percutaneous peripheral nerve stimulation for postoperative analgesia: Could neurostimulation replace continuous peripheral nerve blocks? Reg Anesth Pain Med 2016;41(6):720–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Loudon K, Treweek S, Sullivan F, Donnan P, Thorpe KE, Zwarenstein M.. The PRECIS-2 tool: Designing trials that are fit for purpose. BMJ 2015;350:h2147. [DOI] [PubMed] [Google Scholar]
- 14. Ilfeld BM, Finneran JJ IV, Gabriel RA, et al. Ultrasound-guided percutaneous peripheral nerve stimulation: Neuromodulation of the suprascapular nerve and brachial plexus for postoperative analgesia following ambulatory rotator cuff repair. A proof-of-concept study. Reg Anesth Pain Med 2019;44(3):310–8. [DOI] [PubMed] [Google Scholar]
- 15. Ilfeld BM, Gabriel RA, Said ET, et al. Ultrasound-guided percutaneous peripheral nerve stimulation: Neuromodulation of the sciatic nerve for postoperative analgesia following ambulatory foot surgery, a proof-of-concept study. Reg Anesth Pain Med 2018;43(6):580–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shamji MF, De Vos C, Sharan A.. The advancing role of neuromodulation for the management of chronic treatment—Refractory pain. Neurosurgery 2017;80(suppl 3):S108–13. [DOI] [PubMed] [Google Scholar]
- 17. Dworkin RH, Turk DC, Peirce-Sandner S, et al. Research design considerations for confirmatory chronic pain clinical trials: IMMPACT recommendations. Pain 2010;149(2):177–93. [DOI] [PubMed] [Google Scholar]
- 18. Buchheit T, Van de Ven T, John Hsia HL, et al. Pain phenotypes and associated clinical risk factors following traumatic amputation: Results from Veterans Integrated Pain Evaluation Research (VIPER). Pain Med 2015;17(1):149–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bliese PD, Wright KM, Adler AB, Cabrera O, Castro CA, Hoge CW.. Validating the Primary Care Posttraumatic Stress Disorder Screen and the Posttraumatic Stress Disorder Checklist with soldiers returning from combat. J Consult Clin Psychol 2008;76(2):272–81. [DOI] [PubMed] [Google Scholar]
- 20. Yeager DE, Magruder KM, Knapp RG, Nicholas JS, Frueh BC.. Performance characteristics of the Posttraumatic Stress Disorder Checklist and SPAN in Veteran Affairs primary care settings. Gen Hosp Psychiatry 2007;29(4):294–301. [DOI] [PubMed] [Google Scholar]
- 21. Dobie DJ, Kivlahan DR, Maynard C, et al. Screening for post-traumatic stress disorder in female Veteran’s Affairs patients: Validation of the PTSD checklist. Gen Hosp Psychiatry 2002;24(6):367–74. [DOI] [PubMed] [Google Scholar]
- 22. Keen SM, Kutter CJ, Niles BL, Krinsley KE.. Psychometric properties of PTSD Checklist in sample of male veterans. J Rehabil Res Dev 2008;45(3):465–74. [DOI] [PubMed] [Google Scholar]
- 23. Freedy JR, Steenkamp MM, Magruder KM, et al. Post-traumatic stress disorder screening test performance in civilian primary care. Fam Pract 2010;27(6):615–24. [DOI] [PubMed] [Google Scholar]
- 24. van Oostrom JH. Web-based data collection: Security is only as good as the weakest link. Anesth Analg 2005;17(1):149–61. [DOI] [PubMed] [Google Scholar]
- 25. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG.. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42(2):377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cleeland CS, Ryan KM.. Pain assessment: Global use of the Brief Pain Inventory. Ann Acad Med Singapore 1994;23(2):129–38. [PubMed] [Google Scholar]
- 27. Dworkin RH, Turk DC, Farrar JT, et al. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain 2005;113(1–2):9–19. [DOI] [PubMed] [Google Scholar]
- 28. Dworkin RH, Turk DC, McDermott MP, et al. Interpreting the clinical importance of group differences in chronic pain clinical trials: IMMPACT recommendations. Pain 2009;146(3):238–44. [DOI] [PubMed] [Google Scholar]
- 29. Turk DC, Dworkin RH, Burke LB, et al. Developing patient-reported outcome measures for pain clinical trials: IMMPACT recommendations. Pain 2006;125(3):208–15. [DOI] [PubMed] [Google Scholar]
- 30. Rowbotham M, Harden N, Stacey B, Bernstein P, Magnus ML.. Gabapentin for the treatment of postherpetic neuralgia: A randomized controlled trial. JAMA 1998;280(21):1837–42. [DOI] [PubMed] [Google Scholar]
- 31. Gilron I, Jensen MP.. Clinical trial methodology of pain treatment studies: Selection and measurement of self-report primary outcomes for efficacy. Reg Anesth Pain Med 2011;36(4):374–81. [DOI] [PubMed] [Google Scholar]
- 32. Lundeberg T, Lund I, Dahlin L, et al. Reliability and responsiveness of three different pain assessments. J Rehabil Med 2001;33(6):279–83. [DOI] [PubMed] [Google Scholar]
- 33. Broderick JE, Schwartz JE, Vikingstad G, Pribbernow M, Grossman S, Stone AA.. The accuracy of pain and fatigue items across different reporting periods. Pain 2008;139(1):146–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zelman DC, Gore M, Dukes E, Tai KS, Brandenburg N.. Validation of a modified version of the brief pain inventory for painful diabetic peripheral neuropathy. J Vasc Nurs 2005;23(3):97–104. [DOI] [PubMed] [Google Scholar]
- 35. Study protocol for the World Health Organization project to develop a Quality of Life assessment instrument (WHOQOL). Qual Life Res 1993;2:153–9. [PubMed] [Google Scholar]
- 36. The World Health Organization quality of life assessment (WHOQOL): Position paper from the World Health Organization. Soc Sci Med 1995;41:1403–9. [DOI] [PubMed] [Google Scholar]
- 37. Saxena S, Orley J, Group W.. Quality of life assessment: The world health organization perspective. Eur Psychiatry 1997;12:263s–6s. [DOI] [PubMed] [Google Scholar]
- 38.The WHOQOL Group. Development of the World Health Organization WHOQOL-BREF quality of life assessment. Psychol Med 1998;28:551–8. [DOI] [PubMed] [Google Scholar]
- 39. Ilfeld BM, Gabriel RA, Saulino MF, et al. Infection rates of electrical leads used for percutaneous neurostimulation of the peripheral nervous system. Pain Pract 2017;17(6):753–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Farrar JT, Young JP Jr., LaMoreaux L, Werth JL, Poole RM.. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain 2001;94:149–58. [DOI] [PubMed] [Google Scholar]
- 41. Jensen MP, Chen C, Brugger AM.. Interpretation of visual analog scale ratings and change scores: A reanalysis of two clinical trials of postoperative pain. J Pain 2003;4(7):407–14. [DOI] [PubMed] [Google Scholar]
- 42. Farrar JT, Portenoy RK, Berlin JA, Kinman JL, Strom BL.. Defining the clinically important difference in pain outcome measures. Pain 2000;88(3):287–94. [DOI] [PubMed] [Google Scholar]
- 43. Brummett CM, Waljee JF, Goesling J, et al. New persistent opioid use after minor and major surgical procedures in US adults. JAMA Surg 2017;152(6):e170504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Katz NP, Birnbaum HG, Castor A.. Volume of prescription opioids used nonmedically in the United States. J Pain Palliat Care Pharmacother 2010;24(2):141–4. [DOI] [PubMed] [Google Scholar]
- 45. Buvanendran A, Kroin JS, Della Valle CJ, Kari M, Moric M, Tuman KJ.. Perioperative oral pregabalin reduces chronic pain after total knee arthroplasty: A prospective, randomized, controlled trial. Anesth Analg 2010;110(1):199–207. [DOI] [PubMed] [Google Scholar]
- 46. Ilfeld BM. Continuous peripheral nerve blocks: An update of the published evidence and comparison with novel, alternative analgesic modalities. Anesth Analg 2017;124(1):308–35. [DOI] [PubMed] [Google Scholar]
- 47. Ilfeld BM, Duke KB, Donohue MC.. The association between lower extremity continuous peripheral nerve blocks and patient falls after knee and hip arthroplasty. Anesth Analg 2010;111(6):1552–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Deer TR, Levy RM, Rosenfeld EL.. Prospective clinical study of a new implantable peripheral nerve stimulation device to treat chronic pain. Clin J Pain 2010;26(5):359–72. [DOI] [PubMed] [Google Scholar]
- 49. van Zundert A, Helmstadter A, Goerig M, Mortier E.. Centennial of intravenous regional anesthesia. Bier’s Block (1908-2008). Reg Anesth Pain Med 2008;33(5):483–9. [DOI] [PubMed] [Google Scholar]