Abstract
In artisanal and small‐scale gold mining, occupational exposure to mercury (Hg) vapor is related to harmful effects on several organs, including the kidneys. We previously reported significantly increased levels of Hg in blood and urine despite normal kidney function in individuals from Colombia occupationally exposed to Hg compared with those nonexposed. We evaluated the contribution of 4 genetic variants in key genes encoding the transporters solute carrier (SLC; rs4149170 and rs4149182) and ATP-binding cassette(ABC; rs1202169 and rs1885301) in the pathogenesis of nephrotoxicity due to Hg exposure in these groups. Regression analysis was performed to determine the association between the blood- and urine-Hg concentration with SLC and ABC polymorphisms in 281 Colombian individuals (160 exposed and 121 nonexposed to Hg). We found an enrichment of ABCB1 rs1202169-T allele in the exposed group (p = .011; OR= 2.05; 95% CI = 1.18–3.58) compared with the nonexposure group. We also found that carriers of SLC22A8 rs4149182-G and ABCB1 rs1202169-T alleles had a higher urinary clearance rate of Hg than noncarriers (β = 0.13, p = .04), whereas carriers of SLC22A6 rs4149170-A and ABCB1 rs1202169-C alleles showed abnormal levels of estimated glomerular filtration rate (β = −84.96, p = .040) and beta-2-microglobulin (β = 743.38, p < .001). Our results suggest that ABCB1 rs1202169 and its interaction with SLC22A8 rs4149182 and SLC22A6 rs4149170 could mitigate Hg nephrotoxicity by controlling the renal proximal tubule cell accumulation of inorganic Hg. This will be useful to estimate the risk of kidney toxicity associated to Hg and the genetic selection to aid adaptation to Hg-rich environments.
Keywords: mercury, gold mining, kidney, transporters, polymorphism
Elemental mercury (Hg0) is widely used in artisanal and small-scale gold mining, a long-standing operation widespread around the world (Esdaile and Chalker, 2018). Mercury (Hg) emission due to the burning of mercury-gold amalgams is a source of serious health concerns in miners and nearby residents. Colombia is one of the main per capita Hg polluters in the world because of the use of Hg-amalgamation technique to extract gold from ores, which has been practiced since the colonial period (De Miguel et al., 2014; Güiza and Aristizábal, 2013). Hg0 is readily metabolized to inorganic mercury (Hg1+ or Hg2+), which can produce harmful effects on several organs, including the kidneys—one of the main target organs with accumulation particularly in the areas of the proximal tubules (Afrifa et al., 2019). The pathological effects of inorganic Hg on the kidneys typically include a broad spectrum of conditions ranging from proteinuria to nephritic syndromes, reaching hematuria and renal failure (Kobal et al., 2000).
In the kidney, the proximal tubule region of the nephron is the site where the excretory transport from blood to urine of a vast number of small molecules takes place. The transcellular transport across proximal tubule cells involves transporter proteins (Zhuo and Li, 2013). There are 2 major transporter families in charge of this transcellular flux: the ATP-binding cassettes (ABCs) family and the solute carriers (SLCs) family (Ivanyuk et al., 2017; Launay-Vacher et al., 2006), but no specific Hg transporter proteins have been identified yet. However, since inorganic Hg can form complexes with small molecules such as amino acids, it can be recognized by some transporter proteins due to molecular mimicry (Bridges and Zalups, 2005). Therefore, it has been proposed that inorganic Hg influx from the peritubular blood into proximal tubule cells is primarily done by SLC transporters, whereas inorganic Hg efflux from proximal tubule cells into the lumen involves the ABC transporters (Bridges and Zalups, 2017).
Genetic variants in genes encoding potential Hg-transporters may play a significant role in Hg toxicokinetics. Some single-nucleotide polymorphisms (SNPs) in putative Hg-transporter genes have been associated with total urine Hg concentrations in gold mining settings (Engström et al., 2013), as well as with methyl-Hg during early development (Engström et al., 2016; Llop et al., 2014). SNPs in detoxification genes have also been associated with human adaptation for tolerance to toxic agents (Schlebusch et al., 2015). In this context, we carried out an epidemiological study on occupational exposure to Hg0 on kidney function in a historically gold-mining town in Colombia. In a previous study, we reported that despite higher levels of total Hg in blood and urine in miners compared with a control group, their kidney function was unaltered (Sanchez Rodriguez et al., 2017). Therefore, we hypothesized that genetic variants in candidate Hg-transporters could mitigate negative impact of inorganic Hg on the kidneys.
In this study, we investigated whether genetic variants in ABCB1, ABCC2, SCL22A6, and SLC22A8 with potential role for Hg toxicokinetics (Andreoli and Sprovieri, 2017) could modify Hg nephrotoxicity in our gold mining population in Colombia.
MATERIALS AND METHODS
Study design, population, and sample collection
A cross-sectional study was performed in mining and nonmining communities located in the northeastern of Colombia with similar sociodemographic features. A sample size of 258 individuals was calculated using QUANTO software, version 1.2.4, assuming a log-additive inheritance model at 80% power and 5% level of significance according to the following parameters: we estimated, based on our previous study (Sanchez Rodriguez et al., 2017), an environmental exposure to Hg of 2.5%, a population risk of reduced estimated glomerular filtration rate (eGFR; <76.4 ml/min per 1.73 m2) of 25%, and an environmental effect of reduced eGFR per 10-fold increase in total blood Hg level of 1.3. We also estimated a genetic effect of 1.8, an gene-environment interaction effect of 1.2, and a minor-allele frequency of 0.2 using Colombian-ancestry individuals (CLM) data from the 1000 Genomes Project (Siva, 2008).
The study population comprised 281 adult participants (aged ≥18): 160 in the exposed group and 121 in the nonexposed group who provided samples for DNA testing. Participants in the exposed group were residents of communities where they practiced mining activities for a median of 19.5 years (range 9–25 years), the last year of which they had direct contact with Hg. Participants in the nonexposed group were permanent residents of communities with no mining activities and no life history of contact with Hg. Similar sociodemographic characteristics were found in the 2 communities (Table 1). Both clinical and epidemiological information was collected for each participant through a detailed personal interview. We excluded participants with a self-reporting degree of consanguinity lower than the fourth. Moreover, the overall within population inbreeding estimate FIS value was −0.03613 ± 0.06321 (p = .88), which is in agreement with excess of heterozygosity and, thus, suggests an absence of endogamy in this population. All relevant information about study design, population, and sample collection characteristics was previously described in detail by our group (Sanchez Rodriguez et al., 2015). All subjects provided written informed consent to participate in the study as approved by the Scientific Research Committee of the Universidad Industrial de Santander. This study complied with the Colombian Medical Code of Ethics and it has been performed in accordance with the ethical standards of the 1964 Helsinki Declaration.
Table 1.
Sociodemographic Characteristics, Exposure, and Effect Biomarkers of Study Population
| Variable | Exposure (n = 160) | Nonexposure (n = 121) | p-value |
|---|---|---|---|
| n (%) or median (IQR) | n (%) or median (IQR) | ||
| Sexa | |||
| Male | 101 (63.12) | 59 (48.76) | .022 |
| Female | 59 (36.88) | 62 (51.24) | |
| Age (years)b | 40 (18–62) | 47 (22–59) | <.001 |
| Hg exposure biomarker | |||
| Blood-Hg (µg Hg/l)b | 7.0 (3.4–11.0) | 2.5 (2.5–4.7) | <.001 |
| Urine-Hg (µg Hg/g creatinine)b | 3.8 (2.9–10.1) | 2.9 (2.9–3.0) | <.001 |
| Hair-Hg (µg Hg/g hair)b | 0.8 (0.5–1.3) | 0.4 (0.2–0.7) | <.001 |
| Kidney effect biomarkers | |||
| eGFR (ml/min/1.73 m2)b | 82.6 (74.5–89.6) | 75.7 (69.3–84.1) | <.001 |
| B2M (ng/ml)b | 41.1 (23.1–62.9) | 36.6 (22.3–64.6) | .988 |
Abbreviations: Hg, mercury; B2M, β-2-microglobulin; eGFR, estimated glomerular filtration rate; IQR, interquartile range.
Pearson’s Chi-square test.
Mann-Whitney U-test/Wilcoxon rank-sum test for differences between groups.
Mercury analysis
Total Hg concentrations of blood-Hg and urine-Hg were measured using a S4 Atomic Absorption Spectrometer equipped with a VP100 hydride generation system (Thermo Electron Co., Cambridge, UK). Total hair-Hg concentration was quantified using an RA-915+ atomic absorption spectrometer mercury analyzer with Zeeman background correction and coupled to a RP-91C pyrolysis chamber (Lumex, St Petersburg, Russia). Total Hg was used as biomonitoring data since it can give precise information on the total internal exposure of an individual at a given point in time, whereas total hair-mercury was used as a confounder for the effects of methyl-Hg due to fish consumption (Angerer et al., 2007; Boerleider et al., 2017).
Total hair-mercury adjustment was supported by our previously reported findings, where significant differences in the geometric means of hair-mercury concentration were showed between the exposed and nonexposed group (0.8 vs 0.4 µg Hg/g hair, p < .001, Table 1; Sanchez Rodriguez et al., 2017). In addition, while blood-Hg and urine-Hg are reflective of Hg0 exposures, fish-derived Hg contributes significantly to the total Hg concentration in blood as methylated Hg (Yard et al., 2012) and in urine as demethylated methyl-Hg (Sherman et al., 2013), which might result in the overestimation of Hg0 exposure. All reagents and protocols for the quantification of Hg, including the determinations of the limits of detection and quantification, and total Hg concentrations have previously been described in detail (Sanchez Rodriguez et al., 2015). Testing was carried out at the Industrial Consultation Laboratory of the Universidad Industrial de Santander that has been accredited according to ISO/IEC 17025:2005, performing well in international quality control programs.
Kidney function testing
Glomerular function was evaluated by determining serum and 24-h urine creatinine, and then estimating of the eGFR with the CKD-EPI formula: eGFR (ml/min/1.73 m2) = K1 × (serum creatinine/K2)−1.209 × 0.993age; where K1 = 144 and K2 = 0.7 if female or K1 = 141 and K2 = 0.9 if male (Levey et al., 2009). Creatinine was measured by Jaffé method using the Selectra JR Clinical Chemistry Analyzer (Vital Scientific, France). Tubular function was evaluated by determining urinary excretion of the beta-2-microglobulin (B2M) by an immunometric assay using the Siemens Immulite One analyzer (Siemens Healthcare Diagnostics, Germany). The experimental details were published previously (Sanchez Rodriguez et al., 2017).
DNA isolation and genotyping
Total genomic DNA was extracted from 5 ml of EDTA-treated peripheral whole blood using the standard salting-out method (Miller et al., 1988). DNA concentration was determined with a Nanodrop One Spectrophotometer (Thermo Fisher Scientific, USA) and adjusted to 20 ng/μl with TE buffer. A total of 4 SNPs (ABCB1 rs1202169, ABCC2 rs1885301, SLC22A6 rs4149170, and SLC22A8 rs4149182) were genotyped by TaqMan-based allelic discrimination assay (Applied Biosystems, USA). These SNPs were selected because they had previously been associated with Hg clearance (Engström et al., 2013, 2016). PCR amplification and allelic discrimination were performed according to product specifications using a CFX96 Touch Real-Time PCR Detection System (Bio-Rad, USA).
Statistical analysis
Both genotype and allele frequencies were determined by the gene-counting method. Hardy-Weinberg equilibrium was assessed for each SNP using the R package “Hardy-Weinberg.” To get insight into the potential mechanisms underlying the significantly associated SNPs in our population, we performed a linkage disequilibrium (LD) analysis with the most widely studied SNPs in the same gene. The LD structure was based on the Admixed American populations (AMR) from the 1000 Genomes Project (Siva, 2008) using LDlink application (Machiela and Chanock, 2015). To determine the presence of population genetic structure an analysis of molecular variance (AMOVA) was performed with Arlequin, version 3.5 (Excoffier and Lischer, 2010). The Pearson Chi-square test or the Fisher’s exact test was used to examine the differences between the genotypic and allelic frequencies of the study groups, and odds ratios (ORs) were calculated with a 95% CI. A multivariable generalized linear regression model was used to assess the effect of SNP on Hg levels and on 2 kidney function biomarkers: eGFR and B2M, while adjusting for possible confounders such as sex and age as well as hair-Hg levels. Each SNP was coded as 0, 1, and 2 according to the count of their minor allele. The p values < .05 (p < .05) were considered statistically significant. The nominal significance level was retained when significant empirical p-values were obtained through 5000 replicate permutations (Clarke et al., 2011; Knijnenburg et al., 2009). Interactions between SNPs were examined by logistic regression to analyze their combined effects on Hg levels and kidney function biomarkers. The significant interactions terms were decomposed by a simple slope analysis. Statistical analysis was performed with R programming language, version 3.5.1.
RESULTS
Sociodemographic characteristics of the study participants as well as differences in Hg and kidney function biomarkers are presented in Table 1. Both genotypic and allelic frequencies analyzed in groups occupationally exposed and nonexposed to Hg are listed in Table 2. The distribution of all 4 SNPs under study was consistent with Hardy-Weinberg equilibrium in the specific groups, as well as in the combined sample (Table 2). According to the hierarchy AMOVA, no genetic structure was evidenced (FST = 0.00521; p = .10182 ± .00091) between the exposure and nonexposure groups.
Table 2.
Distribution of Genetic Variants in Transporter Genes in Hg Exposure and Nonexposure Groups
| Gene | SNP | Genotype/Allele | Exposure (n = 160) | Nonexposure (n = 121) | HWE p-value | p-valuea | p-valueb | OR (95% CI) |
|---|---|---|---|---|---|---|---|---|
| n (%) | n (%) | |||||||
| ABCB1 | rs1202169 | TT | 54 (33.7) | 24 (19.8) | 0.3884c | .0259 | .0145 | 2.05 (1.18–3.58) |
| TC | 83 (51.9) | 70 (57.9) | 0.1097d | .3503 | ||||
| CC | 23 (14.4) | 27 (22.3) | 0.1131e | .1099 | ||||
| T | 191 (59.7) | 118 (48.8) | .0115 | 1.55 (1.11–2.17) | ||||
| C | 129 (40.3) | 124 (51.2) | 0.64 (0.45–0.90) | |||||
| ABCC2 | rs1885301 | GG | 52 (32.5) | 43 (35.5) | 0.3526c | .3843 | ||
| GA | 84 (52.5) | 54 (44.6) | 0.4271d | |||||
| AA | 24 (15.0) | 24 (19.8) | 0.9297e | |||||
| G | 188 (58.7) | 140 (57.9) | .8616 | |||||
| A | 132 (41.2) | 102 (42.1) | ||||||
| SLC22A6 | rs4149170 | GG | 129 (80.6) | 100 (82.6) | 0.8785c | .8246 | ||
| GA | 30 (18.7) | 20 (16.5) | 0.6067d | |||||
| AA | 1 (0.6) | 1 (0.8) | 0.9085e | |||||
| G | 288 (90.0) | 220 (90.9) | .7676 | |||||
| A | 32 (10.0) | 22 (9.1) | ||||||
| SLC22A8 | rs4149182 | CC | 121 (75.6) | 90 (74.4) | 0.6452c | .7966 | ||
| CG | 35 (21.9) | 29 (24.0) | 0.8660d | |||||
| GG | 4 (2.5) | 2 (1.7) | 0.8252e | |||||
| C | 277 (86.6) | 209 (86.4) | 1.000 | |||||
| G | 43 (13.4) | 33 (13.6) |
The statistical significance was determined using Pearson’s Chi-square test with p value simulated from 2000 permutations.
p-value for allele and genotype associations.
p-value for each genotype versus the other 2 genotypes.
p-value for Hardy-Weinberg Equilibrium in exposure group.
p-value for Hardy-Weinberg Equilibrium in nonexposure group.
p-value for Hardy-Weinberg Equilibrium in the whole sample.
Statistically significant differences in the distribution of genotypes and alleles of ABCB1 rs1202169 were observed between the studied groups, where an enrichment of both T allele (p = .011; OR = 2.05; 95% CI = 1.18–3.58) and TT genotype (p = .025; OR = 1.55; 95% CI = 1.11–2.17) was detected in the exposure group compared with the nonexposure group (Table 2). Several SNPs have been studied intensively in the ABCB1 encoding regions with important functional implications, including rs1128503, rs2032582, and rs1045642 (Soranzo et al., 2004). A strong LD was revealed between rs1202169 and rs1128503 in the AMR populations (R2 = 0.93, D’ = 0.97) and slightly stronger in Colombians population (R2 = 0.96, D’ = 0.98).
To test the effect of SNP on total Hg levels and kidney function biomarkers, a multivariable linear regression model was used. In the combined sample none of the SNPs investigated showed association with total blood- or urine-Hg levels under an additive model of inheritance controlled by exposure status and other relevant confounders including sex, age, eGFR, and hair-Hg levels (Table 3). However, statistically significantly increased total urine-Hg levels were found in relation to SLC22A6 rs4149170 in the recessive model (β = 0.433; p = .045 and pperm = .020) but not in the dominant model (Table 3). No associations were found with any of the kidney function biomarkers assessed (Table 4).
Table 3.
Association of Genetic Variants in Transporter Genes With Hg Levels in Blood and Urine
| Gene | SNP | n | Model | Blood-Hg (µg Hg/l) |
Urine-Hg (µg Hg/g creatinine) |
||||
|---|---|---|---|---|---|---|---|---|---|
| β | p-valuea | p-valueb | β | p-valuea | p-valueb | ||||
| ABCB1 | rs1202169 | 281 | Additive | −0.029 | .221 | .070 | −0.025 | .368 | .824 |
| Dominant | −0.048 | .180 | .078 | −0.015 | .710 | .541 | |||
| Recessive | −0.023 | .581 | .258 | −0.056 | .257 | .667 | |||
| ABCC2 | rs1885301 | 281 | Additive | −0.002 | .931 | 1.000 | 0.007 | .789 | .655 |
| Dominant | −0.030 | .366 | .573 | −0.014 | .730 | 1.000 | |||
| Recessive | 0.041 | .329 | .194 | 0.046 | .354 | .110 | |||
| SLC22A6 | rs4149170 | 281 | Additive | 0.039 | .306 | 1.000 | 0.041 | .359 | 1.000 |
| Dominant | 0.033 | .414 | 1.000 | 0.025 | .591 | 1.000 | |||
| Recessive | 0.229 | .219 | 1.000 | 0.433 | .045 | .020 | |||
| SLC22A8 | rs4149182 | 281 | Additive | 0.031 | .334 | .222 | 0.033 | .389 | .206 |
| Dominant | 0.037 | .309 | .206 | 0.026 | .549 | .451 | |||
| Recessive | 0.024 | .824 | .594 | 0.140 | .267 | .115 | |||
Each SNP was examined using multivariable linear regression models assuming additive, dominant, and recessive models of inheritance adjusted by exposure status, age, sex, eGFR, and hair-Hg levels (µg Hg/g hair).
p-value from generalized linear model.
Empirical p-value determined based on 5000 permutations from generalized linear model.
Table 4.
Association of Genetic Variants in Transporter Genes With Kidney Function Biomarkers
| Gene | SNP | Biomarker | Total sample (n = 272) |
Exposure (n = 155) |
Nonexposure (n = 117) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| β | p-valuea | p-valueb | β | p-valuea | p-valueb | β | p-valuea | p-valueb | |||
| ABCB1 | rs1202169 | eGFR | −0.26 | .74 | .66 | 0.44 | .66 | .39 | −1.05 | .43 | .92 |
| B2M | 10.65 | .06 | .08 | 14.94 | .07 | .20 | 2.59 | .74 | .45 | ||
| ABCC2 | rs1885301 | eGFR | −0.11 | .87 | .92 | 0.79 | .42 | .42 | −0.87 | .46 | .22 |
| B2M | −0.65 | .90 | 1.00 | −11.67 | .16 | .06 | 10.78 | .12 | .05 | ||
| SLC22A6 | rs4149170 | eGFR | 1.46 | .24 | .10 | 0.21 | .89 | .98 | 2.77 | .19 | .09 |
| B2M | 9.41 | .30 | .43 | 5.56 | .68 | .64 | 9.40 | .44 | .28 | ||
| SLC22A8 | rs4149182 | eGFR | 0.29 | .78 | 1.00 | −0.96 | .46 | 1.00 | 1.99 | .30 | .66 |
| B2M | 2.16 | .78 | .98 | 9.46 | .39 | .18 | −11.63 | .29 | .47 | ||
Each SNP was examined using multivariable linear regression models assuming additive model of inheritance adjusted by age, sex, blood-Hg levels (µg Hg/l), urine-Hg levels (µg Hg/g creatinine), and hair-Hg levels (µg Hg/g hair). Total sample was also adjusted by exposure status.
p-value from generalized linear model.
Empirical p-value determined based on 5000 permutations from generalized linear model.
In order to identify marginal associations that might help shed additional light on Hg toxicogenetics, the interaction between all pairs of individual SNPs was tested by generalized linear regression models. A statistically significant SNP-SNP interaction was found between the SNPs ABCB1 rs1202169 and SLC22A8 rs4149182 on total urine-Hg levels (p = .047 and pperm = .025). A simple slope analysis showed that the effect of rs4149182-G allele on an increase in total urine-Hg levels only exists when individuals carry the rs1202169-T allele (β = 0.13, p = .04, and pperm = .021; Figure 1).
Figure 1.
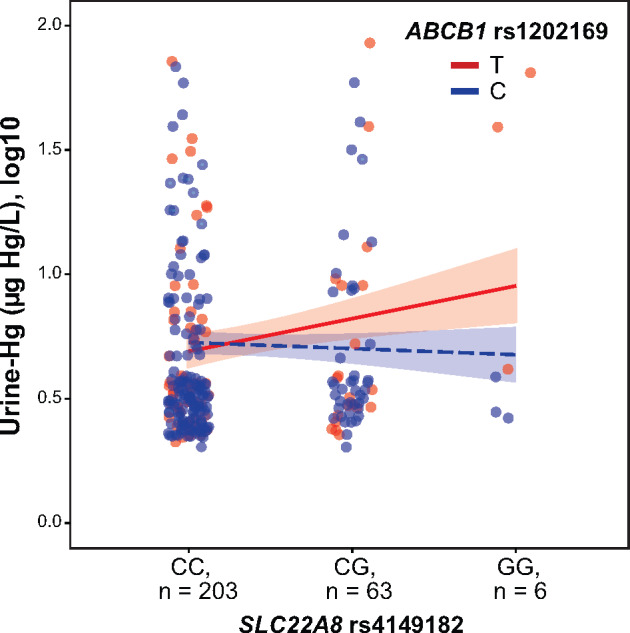
Interaction effects of genetic variants in a multivariable model of urine-Hg. The urine-Hg concentration was defined as a function of interaction between SLC22A8 rs4149182 under an additive model and ABCB1 rs1202169 under a dominant model in the total sample. The interaction plot shows a significantly increasing trend of urine-Hg levels when individuals carry both rs4149182-G and rs1202169-T alleles (β = −0.15, p = .047, and pperm = .025). A simple slope analysis showed that the association between urine-Hg and SLC22A8 rs4149182 was stronger in individuals with rs1202169-T allele (β = 0.13, p = .04, and pperm = .021) compared with individuals with rs1202169-C allele (β = −0.02, p = .60, and pperm = .89). All interaction analyses were adjusted by exposure status, age, sex, estimated glomerular filtration rate, and hair-Hg levels (µg Hg/g hair).
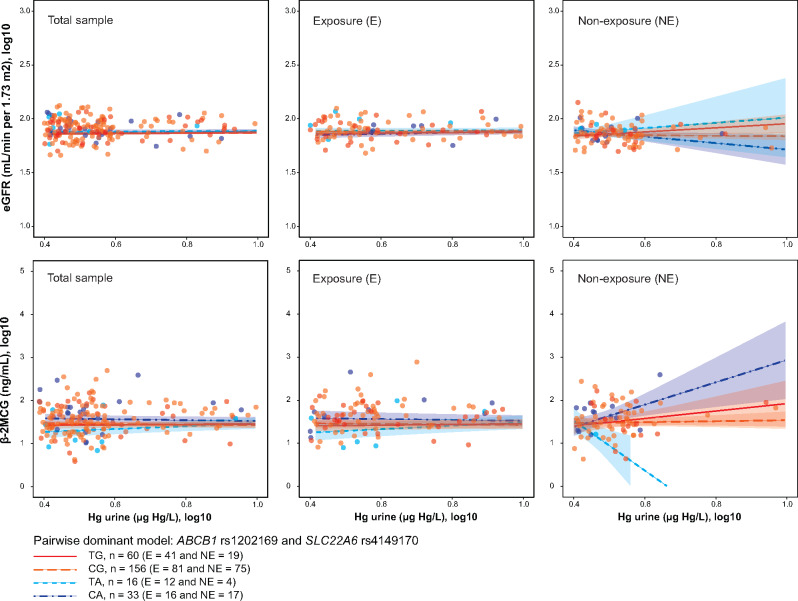
Regarding kidney function assessment, statistically significant associations were found for a SNP-SNP interaction between total urine-Hg levels and the epistatic pairs SLC22A6 rs4149170 and ABCB1 rs1202169 with both eGFR and B2M. Thus, when total urine-Hg level increases, individuals with rs4149170-A and rs1202169-C alleles showed lower levels of eGFR (β = −84.96, p = 0.040, and pperm = .001) and higher levels of B2M (β = 743.38, p < .001, and pperm = 0.003). This correlation was observed in the nonexposure group but not in the exposure group. When a simple slope analysis was conducted to determine whether the slope for each epistatic pair differed from zero, we found that the effect of total urine-Hg levels on B2M, but not on eGFR, only exists when individuals carry both rs4149170-A and rs1202169-C alleles (Figure 2).
Figure 2.
Pairwise interaction effects of genetic variants and urine-Hg on kidney function biomarkers. Estimated glomerular filtration rate (eGFR) and beta-2-microglobulin (B2M) were defined as a function of pairwise interaction between urine-Hg and ABCB1 rs1202169 and SLC22A6 rs4149170 under a dominant model in relation to the minor allele: ABCB1 rs1202169 (TT = 78 vs TC + CC = 203) and SLC22A6 rs4149170 (GG = 229 vs AA + AG = 52). The interaction plots show that when nonexposure individuals but not exposure individuals carry both rs4149170-A and rs1202169-C alleles, eGFR levels significantly decreased (upper panel, β = −84.96, p = .40, and pperm = .014), whereas B2M levels significantly increased (lower panel, β = 743.38, p < .001, and pperm = .003) with the excretion rate of urine-Hg. Both lower eGFR levels and higher B2M levels are indicative of kidney injury. No significant interactions were observed when the analysis was performed in the total sample. All interaction analyses were adjusted by exposure status, age, sex, eGFR, and hair-Hg levels (µg Hg/g hair).
DISCUSSION
In this study, total Hg in blood and urine concentrations, kidney function biomarkers, and genetic variants in multispecific transporter genes were examined in Hg-exposed and nonexposed groups. We observed significant associations with renal clearance of total Hg and glomerular and tubular functions of the kidney. We suggest that the epistatic interaction between the ABCB1, but not the ABCC2, and the SLC22A6 and SLC22A8 genes could mitigate negative impact of inorganic Hg on the kidneys. To the best of our knowledge, this is the first study carried out to evaluate the role of genetic variation in Hg toxicity in Colombia.
In the kidneys, inorganic Hg (Hg1+ or Hg2+) accumulate mainly in the proximal tubule cells, causing renal injury by oxidative stress mechanisms (Zalups, 2000). Inorganic Hg influx from the peritubular blood into proximal tubule cells occurs primarily due to function of the SLC transporters, whereas inorganic Hg efflux from proximal tubule cells into the lumen involves the ABC transporters (Bridges and Zalups, 2017). Considering that Hg0 is readily metabolized to inorganic Hg, we hypothesized that SNPs in genes encoding SLC and ABC transporters might influence inorganic Hg nephrotoxic effects in individuals occupationally exposed to Hg0.
Both ABCB1 and ABCC2 efflux transporters are mainly expressed in the apical membrane of renal proximal tubule cells (Dean et al., 2001). Regarding Hg exposure, the ABCB1 rs1202169 has been associated with hair-Hg concentrations in a cohort with methyl-Hg exposure due to fish consumption (Engström et al., 2016), whereas the ABCC2 rs1885301 has been associated with urine-Hg concentrations in populations with occupational exposure to Hg0 (Engström et al., 2013). Interestingly, our genetic epidemiological evidence suggests that ABCB1 rs1202169, but not ABCC2 rs1885301, may modify the risk of kidney injury by affecting the renal clearance of total Hg since there is an enrichment of the T allele (p = .011; OR = 2.05) in the exposure group compared with the nonexposure group (Table 2).
Since a phenotype-associated SNP may have an indirect effect by being linked to a causal variant, we tested the LD between this Hg-associated SNP and the most widely studied SNPs in the ABCB1 to understand the effect of the ABCB1 rs1202169 on Hg nephrotoxicity. A strong LD was revealed between rs1202169 and rs1128503 in Colombians (CLM; R2 = 0.96, D’ = 0.98). The synonymous ABCB1 rs1128503, located at exon 12—one of the intracellular loops of the protein—can change the protein structure and function (Fung and Gottesman, 2009; Kimchi-Sarfaty et al., 2007). In vitro evaluation of this SNP as part of a common haplotype was carried out in cell models; demonstrating that the use of a rare codon (ie, from GGC to GGT[U], Gly412Gly) has an effect on the timing of cotranslational folding of the transmembrane domain, thereby altering the transporter activity (Kimchi-Sarfaty et al., 2007).
In cancer, a meta-analysis showed a convincing significant association of the ABCB1 rs1128503 with chemotherapy, where patients carrying ABCB1 rs1128503 TT genotype and T allele were more likely to have a good response to chemotherapy (Zhou et al., 2015). The rs1128503 C/T is reported to be in strong LD with rs2032582 G/T and rs1045642 C/T; accounting for important interethnic differences in haplotypes diversity, being TTT and CGC the 2 major haplotypes (Kim et al., 2001; Tang et al., 2002). Although the haplotype TTT was associated with minimizing ABCB1 activity (Salama et al., 2006), it also has been suggested that the effect on drug treatment improvement associated with the ABCB1 rs1128503-T is independent of rs2032582-T and rs1045642-T (Levran et al., 2008). Although the mechanism underlying the association between the ABCB1 SNPs and drug treatment is uncertain, this association could be related to the clearance of the drugs. Therefore, since the rs1128503-C allele correlates with the rs1202169-T allele, the strong LD suggests that the ABCB1 could exhibit a lower Hg-related nephrotoxicity due to a higher clearance rate of this metal in the kidney because ABCB1 is more likely to be properly folded in the exposure group than in the nonexposure group.
Although we could not find evidence for a direct association of the ABCB1 with the Hg levels, we found that the ABCB1 may interplay with SLC22A6 and SLC22A8 to modify both total Hg clearance and nephrotoxicity (Figs. 1 and 2). SLC22A6 and SLC22A8 influx transporters are both expressed in the basolateral membrane of renal proximal tubule cells (Koepsell, 2013), and they have been considered as major mediators for the uptake of inorganic Hg into cells (Bridges and Zalups, 2005) as well as key players in the handling of several toxins, including those derived from the gut microbiome (Wu et al., 2017). The SNPs SLC22A6 rs4149170 and SLC22A8 rs4149182 have been associated with total urine-Hg in populations exposed to Hg vapor from gold mining, where individuals with the rs4149170 AA or the rs4149182 CC genotype showed lower urine-Hg concentrations (Engström et al., 2013).
Considering that coexpressed genes in a tissue are more likely to act together, a comprehensive in silico analysis on the Human Protein Atlas dataset (Uhlen et al., 2015) revealed strong interconnections between SLCs and ABCs transporters in the kidney, including classic metabolic pathways that contributing to regulation of electrolyte transport (Rosenthal et al., 2019). In this context, we tested whether SLC-ABC interactions could regulate Hg body burden and nephrotoxicity. Although the study population had a low frequencies of SLC22A8 rs4149182 GG and SLC22A6 rs4149170 AA genotypes (Table 2), our results suggest interesting links between SLC and ABC transporters. On the one hand, carriers of the SLC22A8 rs4149182-G and ABCB1 rs1202169-T alleles might have a clearance of total urine-Hg at a higher rate than noncarriers (Figure 1). On the other hand, glomerular and tubular functions of the kidney might both be affected in presence of total Hg when individuals carry SLC22A6 rs4149170-A and ABCB1 rs1202169-C alleles, an effect that could be more relevant at tubular than glomerular level (Figure 2). This noteworthy observation was not evident when using the combined sample, but by exposure status; where significant association were found in the nonexposure group but not in the exposure group. This suggests that ABCB1 rs1202169 might be playing an important role since it showed statistically significant differences in the distribution in the exposure group compared with the nonexposure group (Table 2).
The SNPs SLC22A8 rs4149182 and SLC22A6 rs4149170, located in the 5′-flanking regions of their respective genes, might affect gene expression at a transcriptional level. In fact, in the Genotype-Tissue Expression (GTEx) dataset (GTEx Consortium, 2013), there is expression quantitative trait loci evidence for rs4149182-G but not for rs4149170-A with a reduced gene expression in several tissues; including a small set of kidney samples (n = 73, β = −0.25, and p = .02). An in silico analysis suggested that the C/G transversion results in a loss of the metal-regulatory transcription factor 1-DNA binding site (Engström et al., 2013).
Taken together, our pieces of evidence suggest that a lower expression of SLC22A8 associated with the rs4149182-G allele would be limiting Hg influx rate at the basolateral (blood) membrane, whereas a higher activity of ABCB1 associated with the rs1202169-T allele would be promoting Hg efflux rate at the luminal (urine) membrane (Figure 1). Since approximately 40–60% of the inorganic Hg that accumulates in proximal tubular cells is transported by the systemic circulation (Bridges and Zalups, 2017), the functional genetic variants in these multispecific transporters would be playing a protective role by controlling the renal proximal tubule cell accumulation of inorganic Hg and thereby toxicity in these target epithelial cells. This can be particularly applicable in the exposure group than in the nonexposure group. Although the kidney function was found unaltered in both groups (Sanchez Rodriguez et al., 2017), the tubular and glomerular biomarkers were found with a significant trend toward indicating injury of this organ in the nonexposure group—where most individuals do not carry the potentially protective alleles—compared with the exposure group (Figure 2).
In our study, the main difference on the genetic variants distribution was observed on the ABCB1 rs1202169-T allele, which was significantly enriched in the exposure group compared with the nonexposure group (Table 2). Therefore, we propose that this SNP may be being selected to aid adaptation to Hg-rich environments. Indeed, human adaptation for tolerance to toxic agents has been associated with SNPs in detoxification genes (Schlebusch et al., 2015).
There are some limitations in this association study. First, although the study is limited by a small sample size and modest power despite representing almost the entire population from the mining and nonmining communities, our findings may contribute to a future meta-analysis that can potentially enlighten the role of SNPs in multispecific transporters genes in Hg toxicity. Second, since the SNPs in the genes of interest were selected based on their involvement in Hg metabolism, some other relevant genetic variants might be missed by our analysis. Finally, the complex pathogenesis of Hg toxicity suggests that other genetic and environmental factors may be contributing to the associations found in our study. In humans, for instance, selenium might reduce the overall intoxication induced by Hg in individuals exposed to this metal (Bjorklund, 2015; Chen et al., 2006).
In conclusion, the ABCB1 rs1202169-T allele and -TT genotype were statistically significantly enriched in the exposure group compared with the nonexposure group. We also found that the combination of ABCB1 rs1202169-T and SLC22A8 rs4149182-G alleles was significantly associated with level of total urine-Hg, whereas the combination ABCB1 rs1202169-C and SLC22A6 rs4149170-A alleles was significantly associated with biomarkers of kidney function. Our genetic epidemiological findings suggest that in our historically artisanal and small‐scale gold mining population in Colombia, the ABCB1 rs1202169 may modulate the pathogenesis of inorganic Hg nephrotoxicity due to occupational exposure to Hg0 by interplaying with the SLC22A6 rs4149170 and SLC22A8 rs4149182 polymorphism. Replication studies with genes involved in Hg toxicokinetics, and with more SNPs in these and other genes are necessary to clarify the role of genetic variation in Hg toxicity.
ACKNOWLEDGMENTS
The authors want to thank all people who participated in the study by allowing access to the samples. Dr Ludmila Prokunina-Olsson, Laboratory of Translational Genomics, DCEG, NCI is acknowledged for critical comments. The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article.
DISCLAIMER
The funder of this study had no role in study design; the collection, analysis, and interpretation of data; the writing of the report or the decision to submit the report for publication.
FUNDING
The Administrative Department of Science, Technology and Innovation (COLCIENCIAS; Grant No. 1102-744-55577-2016); Universidad Industrial de Santander, Bucaramanga, Colombia; Universidad de Santander, Bucaramanga, Colombia.
DECLARATION OF CONFLICTING INTERESTS
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
DATA AVAILABILITY
The data that support the findings of this study are available from the corresponding author upon reasonable request. The data are not publicly available due to the nature of the questions asked in this study due to them containing information that could compromise research participant consent.
The authors certify that all research involving human subjects was done under full compliance with all government policies and the Helsinki Declaration.
REFERENCES
- Afrifa J., Opoku Y. K., Gyamerah E. O., Ashiagbor G., Sorkpor R. D. (2019). The clinical importance of the mercury problem in artisanal small-scale gold mining. Front. Public Health 7, 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreoli V., Sprovieri F. (2017). Genetic aspects of susceptibility to mercury toxicity: An overview. Int. J. Environ. Res. Public Health 14, 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angerer J., Ewers U., Wilhelm M. (2007). Human biomonitoring: State of the art. Int. J. Hyg. Environ. Health 210, 201–228. [DOI] [PubMed] [Google Scholar]
- Bjorklund G. (2015). Selenium as an antidote in the treatment of mercury intoxication. Biometals 28, 605–614. [DOI] [PubMed] [Google Scholar]
- Boerleider R. Z., Roeleveld N., Scheepers P. T. J. (2017). Human biological monitoring of mercury for exposure assessment. Aims Environ. Sci. 4, 251–276. [Google Scholar]
- Bridges C. C., Zalups R. K. (2005). Molecular and ionic mimicry and the transport of toxic metals. Toxicol. Appl. Pharmacol. 204, 274–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges C. C., Zalups R. K. (2017). Mechanisms involved in the transport of mercuric ions in target tissues. Arch. Toxicol. 91, 63–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Yu H., Zhao J., Li B., Qu L., Liu S., Zhang P., Chai Z. (2006). The roles of serum selenium and selenoproteins on mercury toxicity in environmental and occupational exposure. Environ. Health Perspect. 114, 297–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke G. M., Anderson C. A., Pettersson F. H., Cardon L. R., Morris A. P., Zondervan K. T. (2011). Basic statistical analysis in genetic case-control studies. Nat. Protoc. 6, 121–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Miguel E., Clavijo D., Ortega M. F., Gomez A. (2014). Probabilistic meta-analysis of risk from the exposure to hg in artisanal gold mining communities in Colombia. Chemosphere 108, 183–189. [DOI] [PubMed] [Google Scholar]
- Dean M., Rzhetsky A., Allikmets R. (2001). The human ATP-binding cassette (ABC) transporter superfamily. Genome Res. 11, 1156–1166. [DOI] [PubMed] [Google Scholar]
- Engström K., Ameer S., Bernaudat L., Drasch G., Baeuml J., Skerfving S., Bose-O’Reilly S., Broberg K. (2013). Polymorphisms in genes encoding potential mercury transporters and urine mercury concentrations in populations exposed to mercury vapor from gold mining. Environ. Health Perspect. 121, 85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engström K., Love T. M., Watson G. E., Zareba G., Yeates A., Wahlberg K., Alhamdow A., Thurston S. W., Mulhern M., McSorley E. M., et al. (2016). Polymorphisms in ATP-binding cassette transporters associated with maternal methylmercury disposition and infant neurodevelopment in mother-infant pairs in the Seychelles Child Development Study. Environ. Int. 94, 224–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esdaile L. J., Chalker J. M. (2018). The mercury problem in artisanal and small-scale gold mining. Chemistry 24, 6905–6916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Excoffier L., Lischer H. E. (2010). Arlequin suite ver 3.5: A new series of programs to perform population genetics analyses under Linux and Windows. Mol. Ecol. Resour. 10, 564–567. [DOI] [PubMed] [Google Scholar]
- Fung K. L., Gottesman M. M. (2009). A synonymous polymorphism in a common MDR1 (ABCB1) haplotype shapes protein function. Biochim. Biophys. Acta 1794, 860–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GTEx Consortium. (2013). The genotype-tissue expression (GTEx) project. Nat. Genet. 45, 580–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Güiza L., Aristizábal J. D. (2013). Mercury and gold mining in Colombia: A failed state. Univ. Sci. 18, 33–39. [Google Scholar]
- Ivanyuk A., Livio F., Biollaz J., Buclin T. (2017). Renal drug transporters and drug interactions. Clin. Pharmacokinet. 56, 825–892. [DOI] [PubMed] [Google Scholar]
- Kim R. B., Leake B. F., Choo E. F., Dresser G. K., Kubba S. V., Schwarz U. I., Taylor A., Xie H. G., McKinsey J., Zhou S., et al. (2001). Identification of functionally variant MDR1 alleles among European Americans and African Americans. Clin. Pharmacol. Ther. 70, 189–199. [DOI] [PubMed] [Google Scholar]
- Kimchi-Sarfaty C., Oh J. M., Kim I. W., Sauna Z. E., Calcagno A. M., Ambudkar S. V., Gottesman M. M. (2007). A “silent” polymorphism in the MDR1 gene changes substrate specificity. Science 315, 525–528. [DOI] [PubMed] [Google Scholar]
- Knijnenburg T. A., Wessels L. F., Reinders M. J., Shmulevich I. (2009). Fewer permutations, more accurate p-values. Bioinformatics 25, i161–i168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobal A. B., Flisar Z., Miklavcic V., Dizdarevic T., Sesek-Briski A. (2000). Renal function in miners intermittently exposed to elemental mercury vapour. Arh. Hig. Rada. Toksikol. 51, 369–380. [PubMed] [Google Scholar]
- Koepsell H. (2013). The slc22 family with transporters of organic cations, anions and zwitterions. Mol. Aspects Med. 34, 413–435. [DOI] [PubMed] [Google Scholar]
- Launay-Vacher V., Izzedine H., Karie S., Hulot J. S., Baumelou A., Deray G. (2006). Renal tubular drug transporters. Nephron Physiol. 103, p97–106. [DOI] [PubMed] [Google Scholar]
- Levey A. S., Stevens L. A., Schmid C. H., Zhang Y. L., Castro A. F. 3rd, Feldman H. I., Kusek J. W., Eggers P., Van Lente F., Greene T., et al. ; for the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). (2009). A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 150, 604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levran O., O’Hara K., Peles E., Li D., Barral S., Ray B., Borg L., Ott J., Adelson M., Kreek M. J. (2008). ABCB1 (MDR1) genetic variants are associated with methadone doses required for effective treatment of heroin dependence. Hum. Mol. Genet. 17, 2219–2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llop S., Engstrom K., Ballester F., Franforte E., Alhamdow A., Pisa F., Tratnik J. S., Mazej D., Murcia M., Rebagliato M., et al. (2014). Polymorphisms in ABC transporter genes and concentrations of mercury in newborns–evidence from two Mediterranean birth cohorts. PLoS One 9, e97172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machiela M. J., Chanock S. J. (2015). LDlink: A web-based application for exploring population-specific haplotype structure and linking correlated alleles of possible functional variants. Bioinformatics 31, 3555–3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S. A., Dykes D. D., Polesky H. F. (1988). A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 16, 1215–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal S. B., Bush K. T., Nigam S. K. (2019). A network of SLC and ABC transporter and DME genes involved in remote sensing and signaling in the gut-liver-kidney axis. Sci. Rep. 9, 11879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salama N. N., Yang Z., Bui T., Ho R. J. (2006). MDR1 haplotypes significantly minimize intracellular uptake and transcellular P-gp substrate transport in recombinant LLC-PK1 cells. J. Pharm. Sci. 95, 2293–2308. [DOI] [PubMed] [Google Scholar]
- Sanchez Rodriguez L. H., Florez-Vargas O., Rodriguez-Villamizar L. A., Vargas Fiallo Y., Stashenko E. E., Ramirez G. (2015). Lack of autoantibody induction by mercury exposure in artisanal gold mining settings in Colombia: Findings and a review of the epidemiology literature. J. Immunotoxicol. 12, 368–375. [DOI] [PubMed] [Google Scholar]
- Sanchez Rodriguez L. H., Rodriguez-Villamizar L. A., Florez-Vargas O., Fiallo Y. V., Ordonez A., Gutierrez M. D. (2017). No effect of mercury exposure on kidney function during ongoing artisanal gold mining activities in Colombia. Toxicol. Ind. Health 33, 67–78. [DOI] [PubMed] [Google Scholar]
- Schlebusch C. M., Gattepaille L. M., Engstrom K., Vahter M., Jakobsson M., Broberg K. (2015). Human adaptation to arsenic-rich environments. Mol. Biol. Evol. 32, 1544–1555. [DOI] [PubMed] [Google Scholar]
- Sherman L. S., Blum J. D., Franzblau A., Basu N. (2013). New insight into biomarkers of human mercury exposure using naturally occurring mercury stable isotopes. Environ. Sci. Technol. 47, 3403–3409. [DOI] [PubMed] [Google Scholar]
- Siva N. (2008). 1000 genomes project. Nat. Biotechnol. 26, 256. [DOI] [PubMed] [Google Scholar]
- Soranzo N., Cavalleri G. L., Weale M. E., Wood N. W., Depondt C., Marguerie R., Sisodiya S. M., Goldstein D. B. (2004). Identifying candidate causal variants responsible for altered activity of the ABCB1 multidrug resistance gene. Genome Res. 14, 1333–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang K., Ngoi S. M., Gwee P. C., Chua J. M., Lee E. J., Chong S. S., Lee C. G. (2002). Distinct haplotype profiles and strong linkage disequilibrium at the MDR1 multidrug transporter gene locus in three ethnic Asian populations. Pharmacogenetics 12, 437–450. [DOI] [PubMed] [Google Scholar]
- Uhlen M., Fagerberg L., Hallstrom B. M., Lindskog C., Oksvold P., Mardinoglu A., Sivertsson A., Kampf C., Sjostedt E., Asplund A., et al. (2015). Proteomics. Tissue-based map of the human proteome. Science 347, 1260419. [DOI] [PubMed] [Google Scholar]
- Wu W., Bush K. T., Nigam S. K. (2017). Key role for the organic anion transporters, OAT1 and OAT3, in the in vivo handling of uremic toxins and solutes. Sci. Rep. 7, 4939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yard E. E., Horton J., Schier J. G., Caldwell K., Sanchez C., Lewis L., Gastaňaga C. (2012). Mercury exposure among artisanal gold miners in Madre de Dios, Peru: A cross-sectional study. J. Med. Toxicol. 8, 441–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalups R. K. (2000). Molecular interactions with mercury in the kidney. Pharmacol. Rev. 52, 113–143. [PubMed] [Google Scholar]
- Zhou Z., Chen Q., Zuo D., Wang H., Hua Y., Cai Z. (2015). Abcb1 (rs1128503) polymorphism and response to chemotherapy in patients with malignant tumors-evidences from a meta-analysis. Int. J. Clin. Exp. Med. 8, 265–272. [PMC free article] [PubMed] [Google Scholar]
- Zhuo J. L., Li X. C. (2013). Proximal nephron. Compr. Physiol. 3, 1079–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request. The data are not publicly available due to the nature of the questions asked in this study due to them containing information that could compromise research participant consent.